Abstract
Obese adolescents represent a particularly vulnerable group for vitamin D deficiency which appears to have negative consequences on insulin resistance and glucose homeostasis. Poor vitamin D status is also associated with future risk of type 2 diabetes and metabolic syndrome in the obese. The biological mechanisms by which vitamin D influences glycemic control in obesity are not well understood, but are thought to involve enhancement of peripheral/hepatic uptake of glucose, attenuation of inflammation and/or regulation of insulin synthesis/secretion by pancreatic β cells. Related to the latter, recent data suggest that the active form of vitamin, 1,25-dihydroxyvitamin D, does not impact insulin release in healthy pancreatic islets; instead they require an environmental stressor such as inflammation or vitamin D deficiency to see an effect. To date, a number of observational studies exploring the relationship between the vitamin D status of obese adolescents and markers of glucose homeostasis have been published. Most, although not all, show significant associations between circulating 25-hydroxyvitamn D concentrations and insulin sensitivity/resistance indices. In interpreting the collective findings of these reports, significant considerations surface including the effects of pubertal status, vitamin D status, influence of parathyroid hormone status and the presence of nonalcoholic fatty liver disease. The few published clinical trials using vitamin D supplementation to improve insulin resistance and impaired glucose tolerance in obese adolescents have yielded beneficial effects. However, there is a need for more randomized controlled trials. Future investigations should involve larger sample sizes of obese adolescents with documented vitamin D deficiency, and careful selection of the dose, dosing regimen and achievement of target 25-hydroxyvitamn D serum concentrations. These trials should also include clamp-derived measures of in vivo sensitivity and β-cell function to more fully characterize the effects of vitamin D replenishment on insulin resistance.
Keywords: adolescent obesity, diabetes, glucose tolerance, hypovitaminosis D, insulin resistance, insulin sensitivity, vitamin D deficiency, vitamin D insufficiency
Introduction
There is a growing awareness that obese adolescents represent a particularly vulnerable group for vitamin D deficiency [Garanty-Bogacka et al. 2011; Harel et al. 2011; Shin et al. 2013; Turer et al. 2013]. Evidence accumulated over the last several years has fueled the speculation that this vitamin D deficiency may be a major contributor to the obesity-associated complications of insulin resistance (IR) and type 2 diabetes (T2DM). Biological plausibility exists as the vitamin D receptor (VDR) has been identified in nearly every tissue type, including those important in glucose metabolism. Vitamin D deficiency has independently been linked to IR, impaired glucose tolerance (IGT) and T2DM as well as sharing similar risk factors including physical inactivity and non-white ethnicity. From a practice standpoint, the mounting research evokes potential for the improvement of vitamin D status to aid in the mitigation of these metabolic health problems.
The objectives of this review are to survey the research on the association between vitamin D status and IR as well as the effects of correcting vitamin D status on IR in adolescent obesity, to explore the biological mechanisms by which vitamin D influences glycemic control, and to present clinical considerations related to vitamin D and IR in the obese adolescent.
Adolescent obesity: definition and prevalence
Obesity is a condition of excess adiposity and the most common measurement used in its diagnosis is body mass index (BMI; weight in kilograms divided by the square of height in meters). The World Health Organization (WHO) [de Onis et al. 2007], US Centers for Disease Control and Prevention (CDC) [Ogden et al. 2012] and the International Obesity Task Force (IOTF) [Cole et al. 2000] each have slightly different BMI cutoffs in defining pediatric obesity. For example, CDC considers a BMI greater than or equal to the 95th percentile on the BMI-for-age growth charts to be indicative of obesity [Ogden et al. 2012], while WHO defines pediatric obesity as a BMI greater than two standard deviations above the WHO growth standard median [de Onis et al. 2007]. In adults, waist circumference (WC) is used as a sensitive indicator of abdominal obesity (AO) and is thought to be a better predictor of cardiovascular disease and diabetes risk, although there is no such consensus about methodology and criteria to be used for classifying AO in adolescents [de Moraes et al. 2011].
An examination of data collected from over 1700 published studies indicates that the worldwide prevalence of overweight and obesity rose by 47.1% in children and adolescents between 1980 and 2013 [Ng et al. 2014]. Distinct geographical patterns for child and adolescent obesity were also noted, with high rates in many Middle Eastern and North African countries, especially in girls; and in several Pacific Island and Caribbean nations for both boys and girls. Within western Europe, obesity rates for girls <20 years of age ranged from 13.5% in Luxemburg to 3.8% in the Netherlands, while in boys it ranged from 13.9% in Israel to 4.1% in the Netherlands [Ng et al. 2014]. In the United States, the latest report shows that the rates of all classes of obesity in adolescents have increased since 2000 with 2011–2012 prevalence ~21% in boys and ~20% in girls aged 12–19 years [Skinner and Skelton, 2014].
While there are methodological difficulties in determining global trends [Fazeli et al. 2013], the increasing prevalence and severity of pediatric obesity has been accompanied by an increase in the incidence of T2DM and IGT that has reached unprecedented proportions in the US adolescent population [Dabelea et al. 2014]. It has been estimated that a third to a half of obese children and adolescents display some clinical symptoms of abnormal glucose metabolism [Dabelea et al. 2014; Rodbard, 2008; Sinha et al. 2002; Viner et al. 2005]. Perhaps the most troubling aspects of altered glucose metabolism in obese children are the implications for chronic disease and early death in adulthood [Hillier and Pedula, 2003].
Vitamin D deficiency: definition and prevalence in obese adolescents
There is general agreement that circulating serum concentrations of 25-hydroxyvitamin D [25(OH)D] are the best available indicator of the net incoming contributions from cutaneous synthesis and total intake of food and supplements [Brannon et al. 2008]. However, what is not clearly established is the extent to which 25(OH)D concentrations relate to or serve as predictors of health outcomes. Moreover, the classifications of vitamin D status are controversial and can vary among laboratories. The Institute of Medicine (IOM) defines vitamin D deficiency, or hypovitaminosis D, as serum 25(OHD concentrations <50 nmol/l [Ross, 2011], whereas the consensus report from the 14th Vitamin D Workshop states that target levels of 25(OH)D should be above 75 nmol/l at a minimum [Henry et al. 2010]. This has led some experts to advocate a separate classification of vitamin D status, ‘insufficiency’, defined as a serum 25(OH)D concentration between ~50 and 75 nmol/l [Grant and Holick, 2005]. Thus, when reviewing the literature, it is essential to identify the 25(OH)D cutoffs used in classifying the vitamin D status of study participants.
Differing guidelines also exist regarding the proper definitions of vitamin D deficiency in clinical practice. The Endocrine Society has suggested that 25(OH)D levels of 75–250 nmol/l (30–100 ng/ml) are ‘sufficient’, 52–72 nmol/l (21–29 ng/ml) are ‘insufficient’ and less than 50 nmol/l (20 ng/ml) are ‘deficient’ [Holick et al. 2011]. The Society for Adolescent Health and Medicine [Harel et al. 2013] has a definition similar to the Endocrine Society but considers 25(OH)D levels 75–125 nmol/l (30–50 ng/ml) to be sufficient for the adolescent.
Hypovitaminosis D is considered to be a worldwide problem in both adults and children [van Schoor and Lips, 2011]. Interestingly, national surveys across three different continents reveal that among youth, vitamin D deficiency is more frequent in adolescents than younger children [Stoffman and Gordon, 2009]. In the US, data collected from the National Health and Nutrition Examination Survey (NHANES) 2003–2006 show that approximately 27% of adolescents age 12–18 years are vitamin D deficient [Turer et al. 2013], while in Europe one study showed that over 90% of teenage girls had 25(OH)D concentrations <50 nmol/l during the winter months [Andersen et al. 2005].
Excess adiposity has long been associated with vitamin D deficiency [Drincic et al. 2012] and it is estimated that 34–92% of obese children have suboptimal vitamin D status [Olson et al. 2012; Turer et al. 2013]. Epidemiological data also suggest that the prevalence of vitamin D deficiency in obese children is directly related to the degree of adiposity, with overweight children at 29%, obese at 34% and severely obese at 49% [Turer et al. 2013].
Vitamin D chemistry and physiology
Food sources and skin synthesis
Vitamin D, synonym calciferol, often referred to as the ‘sunshine vitamin’, is essential for life in all higher organisms. It is a secosteroid hormone which exists in two forms: ergocalciferol (vitamin D2), found in fungi; and cholecalciferol (vitamin D3), found in vertebrates. Only a few foods contain appreciable amounts of vitamin D (Table 1). Wild fish, fish liver, offal and egg yolk provide the highest concentrations of natural vitamin D [Schmid and Walther, 2013]. In the United States and Canada, fortified milk is the primary dietary source of vitamin D [Calvo et al. 2004; Keast et al. 2013]; in Europe, few countries have such a mandated food fortification program [Braegger et al. 2013]. Dietary supplements containing vitamin D also provide a significant source [Bailey et al. 2010; Macdonald, 2013].
Table 1.
Comparison of vitamin D sources.*
| Source | Approximate amount obtained | Advantages | Comments |
|---|---|---|---|
| Sun UVB | Up to 10,000 IU per day^ | ‘Natural way’; maintains vitamin D levels longer; toxicity unlikely | Not always available; risk of sunburn and skin cancers with overuse.¢ Exposure time required to significantly increase 25(OH)D concentrations for those with highly pigmented skin make this an impractical source. |
| Artificial UV | 2000–4000 IU§ per 10 minute sessionb | Generally available; toxicity unlikely | Risk of sunburn and skin cancers with overuse;¢ some tanning lamps high in UVA which is most associated with melanoma.¶ UVB only lamps are available for specific use of improving vitamin D status. Exposure time required to significantly increase 25(OH)D concentrations for those with highly-pigmented skin make this an impractical source. |
| Fish | IU per 85 gŦ | Fatty fish are good source of long-chain omega-3 fatty acids (DHA, EPA) | Some fish may contain mercury, PCBs, other contaminants.‡ Large variation in vitamin D content are found between species and between different species harvested from different locations. No significant correlation between fat and vitamin D content.Ŧ |
| Tilapia | 1540 | ||
| Wild salmon | 840 | ||
| Farmed salmon | 205 | ||
| Cod | 61–235 | ||
| Offal | IU per 85 gŦ | Good source of protein and other fat soluble vitamins, iron (liver) | High in cholesterol. Vitamin D supplementation of cattle can increase vitamin D content.Ŧ |
| Beef liver | 6–479 | ||
| Beef kidney | 4–92 | ||
| Whole chicken egg | 28–59 IUŦ in 50 g egg (in yolk only) | Good source of high quality protein | Vitamin D content of egg is dependent on vitamin D content of chicken feed. Whole eggs are also high in cholesterol.Ŧ |
| Fortified milk | 400 IU/l# | Good source of other ‘bone health’ nutrients (calcium, protein) | Vitamin D fortification mandated in USA and Canada.# Not suitable for those with lactose intolerances or milk allergies. |
| Fortified orange juice | 400 IU/l** | Natural source of vitamin C, folate, potassium. Some cofortified with other nutrients | Taste of juice may be altered. Found to significantly improve vitamin D status in children; however,‡‡ due to sugar content, intake should be limited in overweight or obese. |
| Dietary Supplement | 200–5000 IU cholecalciferol (D3) | Available OTC, convenient, inexpensive. | Risk of toxicity with very high doses. Preparations available with or without other nutrients. Fish liver-derived supplements may contain potentially toxic amounts of vitamin A. D3 content of OTC found to be highly variable; potency= 9–146%.¢¢ |
| Prescription-grade supplement | 50,000 IU ergocalciferol (D2) or D3 | High doses available; pill or Intramuscular injectable | Risk of toxicity. Requires medical supervision. Some data indicate D2 not as effective as D3, while others show no difference.¶¶ Slightly improved efficacy with oral route, but administration method should be based on patient’s choice, compliance and availability§§ |
Adapted from Grant and Holick [2005]
25(OH)D, 25-hydroxyvitamin D; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; OTC, over the counter; UV, ultraviolet.
Vitamin D can also be synthesized in human skin in response to sunlight exposure and is the strongest factor influencing vitamin D status [Borradale and Kimlin, 2009]. Solar ultraviolet-B (UVB) radiation (290–315 nm) initiates cutaneous synthesis of vitamin D by the photoconversion of 7-dehydrocholesterol to previtamin D3 [Webb and Engelsen, 2006]. Then, over a period of 1–2 days at body temperature, previtamin D3 isomerizes to D3; once formed, it is sterically unacceptable and ejected from the cell membrane into the extracellular space and then into circulation. It is important to note that prolonged exposure to UVB light does not increase previtamin D3 production, but rather is photodegraded to biologically inert isomers [Holick, 2006].
Activation of vitamin D
Once in the circulation, due either to absorption of dietary vitamin D or skin synthesis, vitamin D (both D2 and D3 forms) is transported to the liver where it undergoes its first hydroxylation at carbon-25 via 25-hydroxylase, making 25(OH)D, or calcidiol, the major form of vitamin D circulating in the blood yet biologically inactive [Holick, 2006]. For it to become active, 25(OH)D must undergo a second hydroxylation at carbon-1 by 1-α-hydroxylase, present primarily in the kidneys making 1,25-dihydroxyvitamin D [1,25(OH)2D] or calcitriol. Production of 25(OH)D is controlled via negative feedback by vitamin D, 25(OH)D and 1,25(OH)2D [Lips, 2006]. In addition to the classical endocrine role of vitamin D involving renal synthesis of 1,25(OH)2D, numerous other tissues possess enzyme systems capable of hydroxylating 25(OH)D to produce the active form for intracrine and autocrine/paracrine functions [Bikle, 2007]. Other metabolites of vitamin D, such as 24R,25-dihydroxyvitamin D3, have also been found to have biological activity but the functions are not well understood [Tuohimaa et al. 2013].
Transport of vitamin D and its metabolites
Once generated, 1,25(OH)2D is then transported systemically or locally to nuclear VDR in target cells, followed by the subsequent generation of appropriate biological responses. Another key component of this system is the group-specific protein known as vitamin D-binding protein (DBP) which carries vitamin D and its metabolites to their sites of metabolism and various target organs [Speeckaert et al. 2014]. Although >99% of 25(OH)D circulates bound to DBP or other serum proteins, the general assumption is that biological activity involves unbound or ‘free’ fractions even though this component in serum is very small [Chun et al. 2013]. This ‘free-hormone hypothesis’ has been proposed as a universal mechanism for cellular uptake of steroid hormones [Chun et al. 2013], though recent data challenge this assertion in vitamin D metabolism. In a randomized controlled trial of vitamin D repletion on changes in parathyroid hormone (PTH) and calcium levels, the abundance of DBP did not change in response to 25(OH)D concentrations [Ponda et al. 2014]. Additionally, the researchers found that 25(OH) concentrations were a significant predictor of PTH levels, while DBP and albumin levels were not and using calculated bioavailable 25(OH)D instead of total 25(OH)D weakened the model predicting change in PTH levels.
Role of VDR in biological functions
The genomic responses to 1,25(OH)2D result from its stereospecific interactions with its nuclear VDR. This protein is a member of the superfamily of the steroid hormone zinc finger receptors [Haussler et al. 2013]. Upon binding to 1,25(OH)2D, VDR forms a heterodimer with the retinoid-X receptor (RXR) and binds to vitamin D response elements (VDREs) on DNA sequences resulting in expression or transrepression of specific gene products [Haussler et al. 2013]. In humans, VDR is encoded by the VDR gene. VDR regulates the expression of numerous genes involved in calcium/phosphate homeostasis, cellular proliferation and differentiation, and immune response, largely in a ligand-dependent manner [Wang et al. 2012]. Germane to glucose tolerance and insulin sensitivity, VDR is present in pancreatic β cells [Mitri et al. 2011; Wang et al. 2012; Zeitz et al. 2003] and in the peripheral tissues of adipose tissue [Ding et al. 2012] and skeletal muscle [Ceglia, 2009].
Storage and excretion of vitamin D and its metabolites
Vitamin D is fat soluble and has a halflife of 4–6 weeks [Heaney et al. 2003]. Vitamin D can accumulate in the body, where it is distributed widely [Heaney et al. 2009], but is primarily stored in adipose tissue and released slowly [Blum et al. 2008; Mawer et al. 1972; Rosenstreich et al. 1971], so that higher doses of vitamin D lead to a long residence time [Rosenstreich et al. 1971]. For example, doses of 50,000 IU have been shown to increase the halflife to 90 days [Wu et al. 2003].
Both 25(OH)D and 1,25(OH)2D can undergo hydroxylation and oxidation to yield several metabolites that are related to deactivation and rapid clearance of 1,25(OH)2D. This is particularly true for the carbon-23 and -24 oxidations, which ultimately yield a biologically inactive water-soluble metabolite, 1-α-hydroxy-24,25,26,27-tetranor-23-COOH-vitamin D [Holick, 2006].
Factors affecting vitamin D status
Several factors can influence vitamin D status. Any barrier to the penetration of UVB radiation into the skin epidermis and dermis is inversely correlated with circulating 25(OH)D. These include: decreased solar zenith angle such as occurs during the winter months in temperate climates and at high latitudes year-round; the use of sunscreen or sunblock – even sun protection factor (SPF) as low as 7 can significantly block vitamin D production); high melanin skin pigmentation (which functions as a natural sunscreen) [Macdonald, 2013; Prentice, 2008]; and cultural clothing practices where little/no skin is exposed [Guzel et al. 2001]. Other factors associated with increased risk of vitamin D deficiency or insufficiency include avoidance of milk, fat malabsorption, and excess adiposity [Holick, 2007].
Relationship between vitamin D status, obesity and obesity-linked metabolic complications
Analysis of data from 21 European and North American adult cohorts predicts that each 1 kg/m2 higher BMI is associated with 1.15% lower 25(OH)D concentration [Vimaleswaran et al. 2013]. This relationship is theorized to be explained by vitamin D’s preferred deposition in body fat compartments, making it unavailable for use by other tissues [Blum et al. 2008; Mawer et al. 1972]. Early studies demonstrate that, compared with lean controls, obese individuals are only about half as efficient in converting the vitamin, whether taken orally or through cutaneous synthesis following UVB exposure, to 25(OH)D [Wortsman et al. 2000]. However, some recent investigations challenge this hypothesis, concluding that the low vitamin D status of obesity is simply a result of volumetric dilution in larger sized individuals. These newer studies show that when 25(OH)D concentrations are corrected for body mass, vitamin D bioavailability does not differ between normal weight and obese individuals [Drincic et al. 2012].
There are very few studies that examine the relationship between vitamin D status and body fat indexes exclusively in adolescents. In a multivariate analysis of data collected on 58 obese adolescents, 25(OH)D decreased by 1.15 ± 0.55 nmol/l per 1% increment in total body fat mass, whereas it was not significantly associated with AO as determined by computed tomography (CT) measured visceral adipose tissue mass [Lenders et al. 2009].
As AO is a known risk factor for IR and metabolic syndrome (cluster of risk factors for cardiovascular disease and T2DM) in adults and children [Alberti et al. 2009; Zimmet et al. 2007], little is known about the distribution or effects of vitamin D in specific fat compartments. An early animal radiotracer study found vitamin D in all fat compartments studied, including subcutaneous and visceral (epididymal, perirenal and mesenteric) depots [Rosenstreich et al. 1971]. A recent investigation involving Korean obese adolescents used receiver operation characteristic curve analysis to establish a serum 25(OH)D cutoff value of 43.9 nmol/l that reflected AO, meaning a vitamin D status less than this value is associated with increased risk for AO and metabolic syndrome in the population studied [Nam et al. 2012]. This value is below the <50 nmol/l cutoff used in the definition of vitamin D deficiency by both IOM and the Endocrine Society.
While there is a well-documented connection between vitamin D status and obesity, there is uncertainty over whether vitamin D deficiency contributes to, or is a consequence of, obesity. A newly published meta-analysis of 12 randomized controlled trials, including adult and adolescents, showed a possible small effect of vitamin D supplementation on reducing BMI [Pathak et al. 2014]. However, there is also evidence that weight loss alone can significantly improve vitamin D status [Coupaye et al. 2013; Lin et al. 2011]. In a weight intervention study of obese children, a reduction of 1 kg/m2 BMI was accompanied by an increase of ~12.5 nmol/l 25(OH)D serum concentration [Reinehr et al. 2007]. The beneficial effects of weight loss on vitamin D status are likely to be transient, however, as 25(OH)D concentrations are found to fall when measured over time [Coupaye et al. 2013].
Vitamin D deficiency has been independently linked to IGT, IR and T2DM associated with obesity. The first observations relating vitamin D status to T2DM in humans came from reports showing that both healthy and diabetic subjects have a seasonal variation in glycemic control [Chagas et al. 2012]. Since then, cross-sectional studies in adults have reported that low 25(OH)D concentrations are related to glucose intolerance, IR and metabolic syndrome [Pittas et al. 2007]. Likewise, an association of vitamin D status with IR and glucose intolerance has been found in children and adolescents [Alemzadeh et al. 2008; Chung et al. 2014; Jimenez-Pavon et al. 2014; Nsiah-Kumi et al. 2012; Olson et al. 2012]. Findings from prospective studies have also demonstrated that vitamin D status at baseline is inversely associated with future risk of T2DM and metabolic syndrome [Khan et al. 2013] and the latest data from the PROspective Metabolism and ISlet cell Evaluation (PROMISE) cohort study indicate that this inverse association with the metabolic syndrome risk is partly driven by vitamin D’s association with glucose homeostasis [Kayaniyil et al. 2014]. When under the scrutiny of a randomized controlled trial, however, this relationship does not always hold up [George et al. 2012]. Much of this discrepancy can be attributed to differences in methods employed, such as vitamin D dose and outcome measures, and participant characteristics, most notably body weight/fat status and age. The following section reviews the research on vitamin D specific to IR, focusing on a population known to be high risk for vitamin D deficiency: obese adolescents.
Studies on vitamin D and IR in obese adolescents
Observational studies
To date, a number of observational studies exploring the relationship between the vitamin D status of obese adolescents and markers of glucose homeostasis as the primary measurement have been published (Table 2a). Also in the literature are several observational studies comparing the vitamin D status of obese adolescents with multiple metabolic health measures including IR (Table 2b). Most, although not all, show significant associations between circulating 25(OH)D concentrations and insulin sensitivity/resistance indices. In interpreting the collective findings of these reports, a few themes surface including the effects of pubertal status, vitamin D status, influence of PTH status, and the presence of nonalcoholic fatty liver disease (NAFLD).
Table 2a.
Observational studies on vitamin D status and insulin resistance in obese adolescents.
| Study | Participants | Results |
|---|---|---|
| Alemzadeh et al. [2008] | 127 obese (BMI = 37 ± 8.5) | • 25(OH)D <75 nmol/l present in 74% of participants |
| 6–18 years62.2% female | • Vitamin D status significantly influenced by vitamin D intake, season, ethnicity/race and adiposity | |
| 30.7 % black | • 25(OH)D positively correlated with QUICKI and inversely correlated with HbA1c | |
| USA (WI) | ||
| Ashraf et al. [2009] | 51 obese (BMI = 43 ± 9.9) | • 25(OH)D <50 nmol/l present in 78.4%, <37 nmol/l in 60.8% of participants |
| 14 ± 2 years | • Vitamin D status not significantly correlated with BMI, fasting glucose or insulin, glucose or insulin AUC, HOMA-IR, Matsuda index | |
| black, females | • Participants with 25(OH)D <37 nmol/l had significantly lower insulin AUC and WBISI (Matsuda index) | |
| USA (AL) | ||
| Delvin et al. [2010] | 1745 obese and nonobese (males, BMI = 20.1 ± 4.2; females, BMI = 20.4 ± 4.5) | • 25(OH)D <75 nmol/l present in 93% of participants |
| 9–16 years | • Modest but significant inverse correlation (-2.8%) between HOMA-IR and each 10 nmol/l increase in 25(OH)D after adjustment for age and BMI | |
| 49.7% female | ||
| Canada | ||
| Erdonmez et al. [2011] | 310 obese and nonobese (BMI = 19.3–40.3) | • 25(OH)D = 25–50 nmol/l present in 53%, <25 nmol/l in 12% participants |
| 14 ± 2 years | • Prevalence of obesity and vitamin D deficiency higher in females | |
| 59% female | • No significant relationship between vitamin D status and obesity | |
| Turkey | • IFG and IGT rates at 8% and 5% respectively; frequency of metabolic syndrome was 12.3% | |
| • No significant associations between insulin sensitivity/resistance indices | ||
| Kelly et al. [2011] | 85 obese and nonobese (BMI-Z = -1.2–4.1) | • 25(OH)D <75 nmol/l present in 74%, <50 nmol/l in 47% of participants |
| 4–18 years | • Older age, higher BMI-Z and black race all negatively associated with 25(OH)D | |
| 45% female | • After adjusting for puberty, participants with 25(OH)D <50 nmol/l had significantly higher fasting glucose and insulin, and HOMA-IR | |
| 45% black | ||
| USA (PA) | ||
| Nunlee-Bland et al. [2011] | 19 obese (BMI = 37 ± 7) and 15 nonobese blacks | • 25(OH)D were 33.7 ± 10 and 51.9 ± 19 nmol/l for obese and nonobese, respectively and significantly different |
| 11–20 years | • 25(OH)D correlated with adiponectin; inversely correlated with HOMA-IR | |
| 59% female | • HbA1c not significantly different between obese and nonobese | |
| USA (DC) | ||
| Roth et al. [2011] | 125 obese (BMI-Z = 2.7 ± 0.6) and 31 nonobese | • 25(OH)D <75 nmol/l present in 96%, <50 nmol/l in 76% of participants |
| 6–16 years | • Vitamin D status not significantly different between obese and nonobese | |
| 51% female | • 25(OH)D positively correlated with QUICKI; inversely correlated with HOMA-IR, even after adjustments for age, gender, BMI | |
| 26% ‘migration background’ | • In obese only, 25(OH)D positively correlated with adiponectin and negatively correlated with HbA1c, but not resistin, | |
| Germany | ||
| Alemzadeh and Kichler [2012] | 133 obese (BMI-Z = 2.4 ± 0.4) | • 25(OH)D <50 nmol/l present in 45% of participants, with highest prevalence in Black participants |
| 3–18 years | • Fat mass negatively correlated with 25(OH)D and positively correlated with PTH | |
| 78% female | • 58% of cohort met diagnostic criteria for metabolic syndrome which also had higher PTH and PTH:25(OH)D ratio but lower 25(OH)D than those without metabolic syndrome | |
| 36% black | • Blacks displayed a higher PTH:25(OH)D ratio than other racial groups | |
| USA (WI) | • PTH level predicted chronic inflammation and dylipemia independent of 25(OH)D | |
| Buyukinan et al. [2012] | 106 obese (48 prepubertal BMI = 26 ± 3; 58 pubertal BMI = 30 ± 2) | • 25(OH)D <50 nmol/l present in 44%, 50–75 nmol/l in 52%, ≥75 nmol/l in 4% of prepubertal participants |
| 8–16 years | • 25(OH)D <50 nmol/l present in 78%, 50–75 nmol/l in 19%, ≥75 nmol/l in 3% of pubertal participants | |
| 51% female | • Prevalence of vitamin D deficiency higher in the pubertal group | |
| Turkey | • Fasting insulin and HOMA-IR significantly higher in those with 25(OH)D <50 nmol/l, although fasting and 120 min glucose and 12 min insulin levels were not | |
| Khadgawat et al. [2012] | 62 obese (BMI = 29 ± 4.8) | • All participants vitamin D deficient [25(OH)D = 21.2 ± 10.5 nmol/l] |
| 6–17 years | • Vitamin D status not correlated with insulin kinetics in prepubertal children, however, vitamin D status inversely correlated with HOMA-IR in postpubertal participants | |
| 44% female | ||
| India | ||
| Nsiah-Kumi et al. [2012] | 198 obese and nonobese Native American (BMI percentile for age = 77.7 ± 1.7) | • 25(OH)D <75 nmol/l present in 97% of participants (mean = 44.4 ± 1.0 nmol/l) |
| 5–18 years | • 25(OH)D inversely correlated with fasting glucose and insulin, 2 hour glucose and HOMA-IR | |
| 53% female | ||
| USA (NE) | ||
| Olson et al. [2012] | 411 obese (BMI-Z = 2.2–2.7) and 87 nonobese | • 25(OH)D <75 nmol/l present in 92%, <50 nmol/l in 50% of obese; frequencies were 68 and 22%, respectively, in nonobese |
| 6–16 years | • 25(OH)D inversely correlated with HOMA-IR and 2 hour glucose after adjustments for age, BMI | |
| 65% female | • 25(OH)D not correlated with HbA1c or systolic or diastolic blood pressure Z scores | |
| 25% black | ||
| USA (TX) | ||
| Poomthavorn et al. [2012] | 150 obese (BMI = 28.6 ± 4.8; 11.2 ± 2.6 years); 29 nonobese (BMI = 17 ± 2.7; 8.7 ± 1.5 years) | • (25(OH)D= 70.4 ± 16.5 nmol/l in obese; =68.9 ± 13.7 nmol/l in nonobese |
| 49.3% females in obese group; 86.2% females in nonobese group | • 25(OH)D <50 nmol/l present in 11.3% obese and 10.3% nonobese | |
| Thailand | • 25% obese had IGT, IFG and diabetes | |
| • No significant relationships among 25(OH)D, weight, height, BMI and insulin sensitivity indices | ||
| Rajakumar et al. [2012] | 92 obese and 91 nonobese (BMI = 26.7 ± 0.7) | • 25(OH)D <50 nmol/l present in 38% of white and 71% of black participants |
| 12.6 ± 0.2 years | • Among whites, no differences across 25(OH)D quartiles for fasting glucose and insulin, HbA1c, insulin sensitivity and DI | |
| 53% female | • In blacks, the observed significance of higher insulin sensitivity and DI in the highest 25(OH)D quartile vanished after adjusting for adiposity | |
| 46% black | ||
| USA (PA) | ||
| Chung et al. [2014] | 1466 obese (8%) and nonobese | • 25(OH)D <37.5 nmol/l present in 38%, 37.5–50 nmol/l in 37%, ≥50 nmol/l in 25% participants |
| 10–19 years | • 25(OH)D significantly related to markers of adiposity | |
| 47.5% female | • Significant inverse relationship between vitamin D status and HOMA-IR, fasting insulin, QUICKI, and risk of IFG, which was independent of adiposity | |
| Korea | ||
| Pirgon et al. 2013] | 87 obese (BMI-Z = 2.1 ± 0.3) with non- NAFLD and 30 nonobese | • 25(OH)D were lower in the NAFLD obese compared with non-NAFLD obese and nonobese |
| 51% femaleTurkey | • 25(OHD) inversely correlated with HOMA-IR and with alanine aminotransferase in the NAFLD obese, but not in the non-NAFLD obese | |
| de las Heras et al. [2013] | 175 obese (105 normal, BMI = 35 ± 5; 43 IGT, BMI = 37 ± 7; type 2 diabetes, BMI = 37 ± 6) | • Proportion of vitamin D deficient and insufficient participants did not differ between glucose tolerance groups |
| 9–20 years | • 25(OH)D not correlated with in vivo insulin sensitivity or DI in all groups combined or in each group separately | |
| 57.7% female | ||
| 46.3% black | ||
| USA (PA) | ||
| Jimenez-Pavon et al. [2014] | 1053 obese and nonobese (BMI = 21.4 ± 3.6) | • In females but not males, 25(OH)D was an independent risk factor for IR (HOMA-IR), after adjustment for pubertal status |
| 14.9 ± 1.2 years | ||
| 52.6% female | ||
| 9 European countries | ||
| Stanley et al. [2013] | 15 obese (BMI = 34.4 ± 7.1) and 15 matched nonobese females | • 25(OH)D not significantly different between obese and nonobese (56.4 ± 28.9 and 54.9 ± 20.9 nmol/l, respectively) |
| 12–18 years | • 25(OH)D not associated with measures of glucose homeostasis | |
| USA (MA) | • PTH and PTH:25(OH)D ratio inversely correlated with HOMA-IR and positively correlated with QUICKI | |
| Torun et al. [2013] | 118 obese (BMI = 29.6 ± 3.9) and 68 matched nonobese | • 25(OH)D significantly different between obese and nonobese (35.9 ± 20.2 and 46.4 ± 23.7 nmol/l, respectively) |
| 9–15 years | • Among obese, vitamin D status not correlated with HOMA-IR | |
| 50% female | • HOMA-IR correlated with measures of adiposity and metabolic health (triglycerides, LDL, alanine aminotransferase) | |
| Turkey |
25(OH)D, 25-hydroxyvitamin D; AUC, area under the curve; BMI, body mass index; BMI-Z, body mass index z-score; DI, disposition index; HOMA-IR, homeostasis model for assessment of insulin resistance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IR, insulin resistance; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; PTH, parathyroid hormone; QUICKI, quantitative insulin sensitivity check index; WBISI, whole body insulin sensitivity index.
Table 2b.
Observational studies on vitamin D status and metabolic health measurements in obese adolescents.
| Study | Objective | Participants | Comments |
|---|---|---|---|
| Ganji et al. [2011] | Investigate the relation between 25(OH)D and cardiometabolic risk factors in US adolescents | Epidemiological data from NHANES 2001–2006; includes 5867 12–19 year olds | 25(OH)D significantly associated with several cardiometabolic risk factors regardless of obesity (including negative correlations with waist circumference, systolic blood pressure, HOMA-IR, and positive correlation with HDL cholesterol). |
| Pacifico et al. [2011] | Examine association of vitamin D status with the metabolic syndrome, its individual components and early atherosclerotic abnormalities in a large sample of Italian overweight/obese and normal weight children and adolescents | 452 Caucasian children/adolescents, 11 ± 4 years old (304 overweight/obese and 148 nonobese) | 25(OH)D inversely associated with presence of metabolic syndrome. Obesity, central obesity, hypertension, hypertriglyceridemia, low-HL cholesterol, IR were all associated with increased odds of having low 25(OH)D, after adjustment for age, sex and tanner stage. No correlations between vitamin D status and subclinical antherosclerotic measurements. |
| Codoner-Franch et al. [2012] | Examine relationship of vitamin D status, parathyroid hormone, and serum calcium-phosphorus levels to biomarkers of oxidative stress, inflammation and endothelial activation in obese children. | 66 obese and 39 nonobese Caucasian children, 7–14 years | 25(OH)D <50 nmol/l detected in 5% of nonobese and 30% of obese. In obese, vitamin D status inversely correlated with nitrosative stress, inflammation, and endothelial dysfunction. |
| Parikh et al. [2012] | Study relation between vitamin D status and cardiometabolic risk factors in healthy black and white adolescents living in southern US | 701 healthy adolescents, BMI = 23 ± 5, 54% black, 14–18 years | Independent of adiposity, 25(OH)D is associated with various cardiometabolic risk factors (adiponectin, leptin, fibrinogen, fasting glucose, HOMA-IR, HDL cholesterol, systolic and diastolic blood pressure) in adolescents living in a sun-rich climate. |
| Jang et al. [2013] | Investigate the prevalence of vitamin D insufficiency and the association of 25(OH)D with metabolic risk factors in Korean girls | 320 12.4–14.5 year-old females, mean BMI = 19.9 (11.6% with BMI ≥85th percentile) | 25(OH)D <50 nmol/l present in 63.8% of participants; no differences in prevalence of vitamin D deficiency between overweight and nonoverweight. Vitamin D status positively correlated with milk intake and negatively with soft drink intake. 25(OH)D inversely associated with fasting glucose IR, after adjustment for activity and BMI. Participants with vitamin D deficiency had higher metabolic risk scores. |
| Kardas et al. [2013] | Elucidate the association of obesity with 25(OH)D and adiponectin levels in Turkish children | 63 obese (BMI = 28.5 ± 2.7) and 51 nonobese (BMI = 19.6 ± 3.6) children and adolescents, 10–16 years | 25(OH)D positively correlated with adiponectin and HDL-cholesterol and inversely correlated BMI, triglycerides, total cholesterol, LDL cholesterol, fasting glucose, HOMA-IR, and systolic and diastolic blood pressure. Adiponectin levels lower in the obese group and inversely correlated with BMI and positively correlated with fasting glucose and HOMA-IR. |
| Black et al. [2014] | Determine the relationship between vitamin D status and NAFLD in adolescents | Participants in the population-based West Australian Pregnancy Cohort including 994 17–year-olds non-NAFLD group (BMI=10-24) and NAFLD group (BMI=23-33) | 25(OH)D <75 nmol/l present in 68% of NAFLD group and 49% of non-NAFLD group. 25(OH)D inversely associated with risk of NAFLD, after adjusting for television/computer viewing, BMI and HOMA-IR. |
25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; HDL, high-density lipoprotein; HOMA-IR, homeostasis model for assessment of insulin resistance; IR, insulin resistance; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease.
Puberty is a known modulator of insulin sensitivity [Travers et al. 1995] and is thought to be due to the interaction of various hormones including growth hormone secretion [Hannon et al. 2006]. Two studies were designed to specifically examine the effects of puberty on the association between 25(OH)D serum concentrations and IR [Buyukinan et al. 2012; Khadgawat et al. 2012]. Both show that the association does not become significant until children reach puberty. Furthermore, the analysis by Kelly and colleagues of cross-sectional data of obese children required adjustments for puberty to reveal associations between vitamin D status and homeostatic model assessment (HOMA) IR [Kelly et al. 2011], and a recent report exploring the relationship between vitamin D status and IR and cardiovascular risks in very young obese children (2-6 years) saw no association [Creo et al. 2013]. Interestingly, one of the studies that did not show a correlation between vitamin D status and IR did not take into account pubertal status despite a wide age distribution of participants (9–15 years) [Torun et al. 2013].
Another plausible explanation for the lack of association observed in at least one of the reviewed studies is that the vitamin D status of the participants was not sufficiently compromised. The preponderance of evidence demonstrates that the majority of obese adolescents fall into the IOM definition of vitamin D deficiency. In the paper by Stanley and colleagues the mean serum 25(OH)D concentrations of the study participants was notably above 50 nmol/l [Stanley et al. 2013]. Yet, despite no correlation between IR and vitamin D status, there was a significant inverse correlation between HOMA-IR and PTH concentrations as well as the ratio of PTH: 25(OH)D. Almemzadeh and Kichler noted a similar relationship with IR and PTH in obese adolescents [Alemzadeh and Kichler, 2012]. It is speculated that the status of both vitamin D and PTH need to be considered for optimal evaluation of the impact of vitamin D status on glucose metabolism. Indeed, findings from a recent prospective investigation of adult women illustrate that vitamin D deficiency with increased serum PTH concentrations is independently associated with deterioration in insulin sensitivity, β-cell function and glycemia which is not observed in women with vitamin D deficiency in the context of lower PTH [Kramer et al., 2014].
Pediatric NAFLD, defined by hepatic fat infiltration >5% hepatocytes as assessed by liver biopsy in the absence of excessive alcohol intake, viral, autoimmune and drug-induced liver disease, is emerging as one of the most common complications of adolescent obesity [Marzuillo et al. 2014]. The main risk factors for pediatric NAFLD are obesity and IR; NAFLD is strongly associated with the clinical features of IR especially the metabolic syndrome and T2DM [Marzuillo et al. 2014]. Pirgon and colleagues, in the first study to look into the vitamin D status of obese adolescents with NAFLD, revealed that compared with non-NAFLD obese teens, those with NAFLD had significantly lower serum concentrations of 25(OH)D [Pirgon et al. 2013]. Moreover, vitamin D status was negatively correlated with HOMA-IR in those with NAFLD, but not in those without NAFLD [Pirgon et al. 2013]. The only other known investigation of vitamin D and NAFLD in adolescents showed that lower 25(OH)D concentrations are associated with NAFLD, independent of adiposity, physical activity and IR [Black et al. 2014]. It is unclear whether poor vitamin D status contributes directly to the risk of developing NAFLD or if this association is confounded by hepatic steatosis [Pirgon et al. 2013], as the liver is a primary site of vitamin D activation. Notwithstanding, it would be appropriate to screen for vitamin D deficiency in adolescents at risk for NAFLD.
Intervention studies
Only a few intervention studies involving the supplementation of obese adolescents exist, of which only two are randomized controlled trials (Table 3). Both of these were similar in duration (12 weeks) and in participant characteristics and both demonstrated favorable effects of vitamin D supplementation on glucose homeostasis and/or metabolic syndrome outcomes. What was different, however, was the dose of cholecalciferol. We used a daily dose of 4000 IU/day [Belenchia et al. 2013], whereas Kelishadi and colleagues used a weekly dose of 300,000 IU [Kelishadi et al. 2014]. In spite of the greater dose used in the latter trial, the daily regimen was more effective at increasing serum 25(OH)D concentrations (increase of ~47 nmol/l in the former versus 35 nmol/l in the latter), although both achieved a vitamin D status sufficient to see beneficial effects on IR (96 ± 23 nmol/l in the former and 79.9 ± 5.3 nmol/l in the latter), unlike the nonrandomized, nonplacebo controlled intervention studies of Ashraf and colleagues [Ashraf et al. 2011] and Harel and colleagues [Harel et al. 2011]. By comparison, the results of a 2012 meta-analysis of the evidence on vitamin D supplementation and glycemic control in adults suggested a weak effect of vitamin D supplementation in reducing fasting glucose and improving IR in patients with T2DM or IGT [George et al. 2012]. However, a major flaw of this meta-analysis is that the studies included used wide-ranging vitamin D forms and dosing regimens. In most trials where no effects were observed, the dose or duration of vitamin D supplementation were inadequate to increase serum 25(OH)D to sufficient concentrations, i.e. >75 nmol/l, the threshold for ‘sufficiency’ as advocated by the Endocrine Society and the Society for Adolescent Health and Medicine.
Table 3.
Vitamin D intervention studies in obese adolescents using insulin sensitivity/resistance as primary outcome.
| Study | Participants | Methods/Treatment | Results |
|---|---|---|---|
| Ashraf et al. [2011] | 80 obese postmenarchal adolescents | Objective 1: cross-sectional design. Participants evaluated for blood pressure and fasting 25(OH)D, lipid profile, C-reactive protein, and alanine transaminase followed by an oral glucose tolerance test. | • Objective 1: vitamin D status inversely associated with fasting glucose and positively associated with LDL cholesterol, independent of race and BMI. |
| 66% black (BMI = 43.5 ± 10; 25(OH)D = 35 ± 16.5 nmol/l) | Objective 2: subset of 14 (13 blacks) vitamin D deficient participants supplemented with 50,000 IU of ergocalciferol for 8 weeks | • Objective 2: 25(OH)D increased from 26.4 ± 11 to 63.6 ± 30.1 nmol/l with supplementation. Fasting glucose improved although no effects on HOMA-IR, insulin AUC, or glucose AUC. | |
| 34% white (BMI = 41.9 ± 8; 25(OH)D = 71 ± 25.7 nmol/l) | |||
| Harel et al. [2011] | 68 obese adolescents BMI = 38 ± 1 | Objective 1: retrospective chart review of male and female obese adolescents screened for vitamin D status and lipid abnormalities. | • Objective 1: prevalence of vitamin D deficiency/insufficiency was 100% in females and 91% in males. |
| 53% females | Objective 2: patients with 25(OH)D <50 nmol/l treated with 50,000 IU vitamin D for 6–8 weeks; those with 25(OH)D = 50–75 nmol/l treated with 800 IU vitamin D for 3 months. | • Objective 2: 25(OH)D >75nmol/l in only 28% of the participants after the initial course of vitamin D treatment. Repeat courses with same dosage in other 72% did not significantly change their low vitamin D status. | |
| 45% black | |||
| Belenchia et al. [2013] | 35 obese adolescents BMI = 39.8 ± 6.1 | Double-blind, randomized, placebo-controlled control trial. Participants randomized to 4000 IU/day cholecalciferol or placebo for 6 months. Anthropometrics, inflammatory markers, fasting glucose, fasting insulin, and HOMA-IR measured at baseline and two follow-up visits (3- and 6-month). | • At 3 months: |
| 14.1 ± 2.8 years | • 25(OH)D significantly increased in vitamin D group; no change in placebo group. | ||
| 50% female | • No change in IR/sensitivity | ||
| 30% black | • At 6 months: | ||
| 25(OH)D = 49 ± 17.8 nmol/l | • 93% of the participants in vitamin D group were sufficient status (25(OH)D = 96 ± 23.7 nmol/l); no changes in status with placebo | ||
| • Vitamin D group had significant decreases in fasting insulin, HOMA-IR and leptin to adiponectin ratio | |||
| • No significant differences in BMI, serum inflammatory markers or plasma glucose concentrations between groups | |||
| Kelishadi et al. [2014] | 43 obese adolescents | Triple-masked randomized placebo-controlled trial. Participants randomized to 300,000 IU/week cholecalciferol or placebo for 12 weeks. Cardiometabolic risk factors, IR and metabolic syndrome score were determined. | • After 12 weeks, vitamin D group significantly increased 25(OH)D (79.9 ± 5.3 nmol/l) |
| BMI = 28 ± 1 | • Fasting insulin and triglyceride, and HOMA-IR and metabolic syndrome score decreased significantly in vitamin D group compared with both baseline and placebo group. | ||
| 10–16 years | • No significant differences for total cholesterol, LDL cholesterol, HDL cholesterol, fasting glucose, and blood pressure. | ||
| 25(OH)D = 45 ± 5.5 nmol/l |
25(OH)D, 25-hydroxyvitamin D; AUC, area under the curve; BMI, body mass index; HDL, LDL, high-density lipoprotein; HOMA-IR, homeostasis model for assessment of insulin resistance; IR, insulin resistance; LDL, low-density lipoprotein.
Biological mechanisms by which vitamin D influences glycemic control in obesity
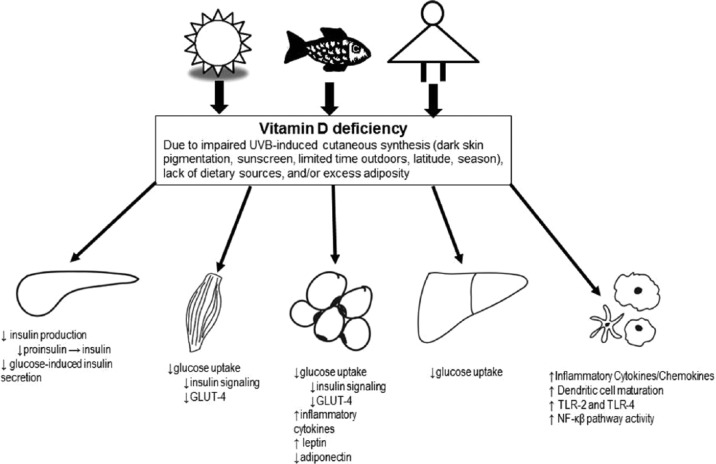
The biological mechanisms by which vitamin D influences glycemic control in obesity are not well understood. The proposed mechanisms include regulation of glucose-mediated synthesis/secretion of insulin by pancreatic β-cells, enhancing peripheral/hepatic uptake of glucose through both direct and indirect means, and reducing inflammation (Figure 1).
Figure 1.
Potential mechanistic links between vitamin D deficiency in obesity and insulin resistance/impaired glucose metabolism, including regulation of glucose-mediated synthesis and secretion of insulin by pancreatic beta cells, enhancing peripheral (skeletal muscle, adipose tissue) and/or hepatic uptake of glucose through both direct and indirect means, and reducing inflammation.
GLUT-4, glucose transporter 4; NF-κB, nuclear factor-κB; TLR, toll-like receptor; UVB, ultraviolet B.
Both VDR and the 1-α-hydroxylase enzyme are expressed in insulin-secreting pancreatic β-cells and there is mounting support for a role of 1,25(OH)2D in regulating insulin production and secretion [Billaudel et al. 1993]. However, the research suggests that calcitriol does not impact on insulin release in healthy pancreatic islets but does in those subjected to an environmental stressor such as inflammation or vitamin D deficiency [Wolden-Kirk et al. 2013]. In an animal study, it was observed that vitamin D deficiency results in calcium-independent pancreatic β-cell dysregulation that can be improved by correcting vitamin D deficiency [Labriji-Mestaghanmi et al. 1988]. Similar effects were seen in a human study, where vitamin D deficient adults were randomized to receive supplemental vitamin D, calcium, or vitamin D and calcium [Mitri et al. 2011]. Those supplemented with vitamin D saw improvements in β-cell function, whereas those who received calcium did not. In contrast, the cross-sectional study by de las Heras and colleagues of obese adolescents showed no relationship between 25(OH)D and in vivo insulin sensitivity or β-cell function relative to insulin sensitivity in any of the groups studied (normal glucose tolerance versus prediabetes, versus T2DM) [de las Heras et al. 2013]. This may be partly attributed to the majority of participants being vitamin D deficient; measuring β-cell function after correction for this vitamin D deficiency would be an important next step in understanding the role of vitamin D in insulin secretion. Lastly, while it is well-known that vitamin D is crucial in maintaining extracellular calcium concentrations and calcium influx into β-cells is necessary for insulin secretion to occur, VDR signaling may play a more direct role in glucose-induced insulin secretion [Lee et al. 1994].
In addition to regulating insulin production and release, there is evidence that VDR signaling facilitates insulin-stimulated glucose uptake in insulin-sensitive tissue [Huang et al. 2002]. In skeletal muscle, adipose tissue and the liver, 1,25(OH)2D has been shown to directly activate the transcription of the human insulin receptor gene and increase expression of the insulin receptor [Calle et al. 2008; Maestro et al. 2002, 2003]. Furthermore, there is evidence that calcitriol increases insulin signaling in skeletal muscle [Alkharfy et al. 2013]. Additionally, calcitriol has also been demonstrated, in vivo, to upregulate the expression of glucose transporter 4 (GLUT-4) in skeletal muscle and to stimulate GLUT-4 translocation in adipocytes [Castro et al. 2014; Manna and Jain, 2012]. Whether vitamin D deficiency influences insulin sensitivity and glucose uptake through calcium dependent or independent mechanisms remains unknown.
The chronic inflammation that accompanies obesity leads to hyperinsulinemia, IR and eventually β-cell dysfunction/death. These consequences are largely driven by the increased production of inflammatory cytokines, chemokines and adipokines by immune cells, such as macrophages and adipocytes. There is a wealth of data suggesting that calcitriol is a strong immunomodulator and improves systemic inflammation in a variety of manners. Vitamin D status has an inverse association with several of the pro-inflammatory biomarkers that are associated with the development of IR such as tumour necrosis factor-α (TNFα), interleukin 1β (IL-1β), IL-2, IL-6 and interferon γ (IFN-γ) [Flores, 2005]. Furthermore, improving vitamin D status has been shown to decrease general systemic inflammation [Hopkins et al. 2011; Shab-Bidar et al. 2012; Wamberg et al. 2013]. These effects on systemic and tissue-specific inflammation have been attributed to several factors including the inhibition of the NF-κβ pathway, shifting T-helper cells towards the anti-inflammatory TH2 subset, decreasing the expression of toll-like receptor 4 (TLR-4) and decreasing the maturation of dendritic cells [Cantorna et al. 2004; Chen et al. 2013; Ding et al. 2013; Du et al. 2009; Guillot et al. 2010].
Role of adiponectin in the link between vitamin D deficiency and pediatric obesity
A recent study proteonomically identified adiponectin as a key regulatory protein in the link between vitamin D deficiency and pediatric obesity [Walker et al. 2014]. Adiponectin is an adipocytokine that is secreted exclusively from adipose tissue in response to insulin [Motoshima et al. 2002]. As with 25(OH)D, circulating concentrations of the molecule are inversely proportional to fat mass and strongly associated with both IR and impaired glucose metabolism in adolescents [Buemann et al. 2005; Punthakee et al. 2006]. Furthermore, adiponectin has been demonstrated to have an insulin-sensitizing effect in peripheral tissue such as muscle and adipose, as well as regulatory effects on gluconeogenesis [Berg et al. 2001; Park et al. 2011]. Adiponectin receptors are also expressed in insulin producing pancreatic β-cells but little is known about their role in these cells [Kharroubi et al. 2003]. A few of the observational studies on vitamin D status and IR in obese adolescents measured circulating adiponectin concentrations and found that it was significantly correlated with 25(OH)D concentration [Kardas et al. 2013; Nunlee-Bland et al. 2011; Parikh et al. 2012; Roth et al. 2011]. Our vitamin D supplementation trial of obese adolescents did not find any changes in adiponectin, but observed a significant decrease in the ratio of leptin to adiponectin [Belenchia et al. 2013], which has recently been proposed as a potential clinical tool for the assessment of IR and found to be more strongly correlated with results from a hyperinsulinemic/euglycemic clamp than were HOMA-IR or QUICKI methods [Belenchia et al. 2013]. The mechanism by which vitamin D and adiponectin interact has not been elucidated. There is some evidence suggesting that vitamin D indirectly stimulates the production of adiponectin through its action interaction with peroxisome proliferator-activated receptor γ (PPARγ) and has a strong stimulatory effect on adiponectin production [Liu et al. 2009; Maeda et al. 2001; Nimitphong et al. 2009].
Clinical considerations related to vitamin D and IR in the obese adolescents
Treatment of obesity-associated IR and hyperglycemia
Standard approaches to the treatment of the IR and hyperglycemia associated with obesity include weight loss and drug therapy. A BMI reduction of just 5% has been estimated to lead to an improved health status in the obese [National Heart Forum, 2012]. While lifestyle changes are a cost-effective method to delay the progression of impaired fasting glucose to diabetes mellitus [Diabetes Prevention Program Research Group, 2012], implementation is often difficult in the clinical setting. The drug metformin is a biguanide that is thought to reduce hepatic glucose production and improve IR [Miller et al. 2013]. While its approved use is for the treatment of diabetes, it has been used off-label for the management of IR, prediabetes and polycystic ovary syndrome. A recent meta-analysis of 14 clinical trials studying the efficacy of metformin for weight loss among obese children and adolescents found a statistically significant reduction in BMI after 6 months, with the effect fading to non-significance by 12 months [McDonagh et al. 2014]. Common side effects include gastrointestinal side effects such as abdominal pain, cramping, bloating and diarrhea. Lactic acidosis, though rare, is the most common serious adverse reaction associated with metformin use.
Our laboratory showed that correcting the vitamin D status of obese adolescents produced an attenuation of IR similar to results involving metformin [Belenchia et al. 2013]. Metformin is reported to reduce the HOMA-IR score by ~2 units [Park et al. 2009]; by comparison in our study vitamin D decreased the HOMA-IR score by ~1.5 units. Remarkably, this improvement in IR was independent of changes in body weight and without the side effects of metformin.
Indications for testing of vitamin D status
No current guidelines support universal screening for vitamin D deficiency among the general population. While some have raised the question whether treatment can precede laboratory measurement to reduce the cost of testing on the healthcare system [Souberbielle et al. 2012], clinicians may wish to screen their obese adolescent patients as they are at greater risk for vitamin D deficiency. Other high-risk adolescent groups that may warrant vitamin D testing include those with darker skin pigmentation, populations from higher latitudes, night shift workers, and those with underlying medical conditions predisposing to vitamin D deficiency (Table 4). The clinician may also choose to screen patients from low-risk groups who present with potential symptoms of vitamin D deficiency, such as fatigue, weakness, or musculoskeletal heaviness or pain [Erkal et al. 2006].
Table 4.
Medical conditions associated with vitamin D deficiency among adolescents.*
| Obesity (BMI > 95th percentile) |
| Osteomalacia |
| Osteoporosis |
| Chronic kidney disease |
| Hepatic failure |
| Malabsorption syndromes |
| Cystic fibrosis |
| Inflammatory bowel disease (Crohn’s disease/ulcerative colitis) |
| Bariatric surgery |
| Radiation enteritis |
| Hyperparathyroidism |
| Medications |
| Antiseizure medications |
| Glucocorticoids |
| HIV medications |
| Antifungals, e.g. ketoconazole |
| Cholestyramine |
| African-American race or Hispanic ethnicity |
| Pregnant and lactating women |
| History of nontraumatic fractures |
| Granuloma-forming disorders |
| Sarcoidosis |
| Tuberculosis |
| Histoplasmosis |
| Coccidiomycosis |
| Berylliosis |
| Lymphomas |
Adapted from Holick et al. [2011].
BMI, body mass index.
Practice recommendations
As aforementioned, the vast majority of vitamin D is received from skin exposure to sunlight. It has been estimated that 15 minutes of unprotected sun exposure should provide sufficient vitamin D for a light-skinned person [Misra et al. 2008]. Patients with darker skin pigmentation or a high risk for vitamin D deficiency, in addition to persons who require continuous sunscreen such as those with very fair complexion or a family history of skin cancer, may need supplemental vitamin D from additional sources.
There is general agreement among IOM [Ross, 2011], the Endocrine Society [Holick et al. 2011], and the American Academy of Pediatrics (AAP) [American Academy of Pediatrics, 2012] to establish 600 IU (15 µg) of vitamin D as the minimum recommended daily allowance for healthy adolescents up to age of 18 years. The Endocrine Society has recommended that higher risk adolescents may need at least 1000 IU (25 µg) vitamin D daily. IOM, the Endocrine Society and the European Food Safety Authority [EFSA Panel on Dietetic Products, 2012] has set 4000 IU (100 µg) vitamin D3 daily as the upper limit for adolescent intake.
Aside from vitamin D fortified milk, many adolescents have diets that do not typically include food sources of vitamin D (Table 1). Clinicians should consider vitamin D supplementation among patients with vitamin D deficiency despite attempts to improve their status through exposure to the Sun and food sources.
Oral vitamin D supplements include D2 and D3 (Table 1). Controversy exists between these two forms in regards to the efficacy of increasing serum 25(OH)D levels. A meta-analysis has suggested that D3 is preferable to D2, especially in the context of weekly or monthly dosing regimens [Tripkovic et al. 2012], but the clinical significance of these differences may be less striking. Intramuscular (IM) vitamin D has been used to treat low vitamin D status; however IM preparations are not available in many countries.
After evaluating recommendations by IOM and the Endocrine Society, the Society for Adolescent Health and Medicine made the following recommendations regarding the treatment of suboptimal vitamin D levels among adolescents [Harel et al. 2013]:
Vitamin D deficiency [serum 25(OH)D concentration less than 50 nmol/l (20 ng/ml)] – treat with 50,000 IU vitamin D2 once weekly for 8 weeks (‘stoss therapy’).
Vitamin D insufficiency [serum 25(OH)D concentration 50–72.5 nmol/l (20–29 ng/ml)] – treat with vitamin D3 1000 IU/day for at least 3 months. Adolescents at higher risk for vitamin D deficiency may require longer therapy.
After treatment, 25(OH)D serum concentration may be monitored after 3–4 months and then twice yearly to assess for need for vitamin D dose optimization [Pludowski et al. 2013].
Most adolescents requiring vitamin D supplementation have sufficient calcium status and do not require supplemental calcium. However, patients who have hyperparathyroidism or frank rickets may require calcium supplemented in the context of vitamin D replacement to avoid ‘hungry bone syndrome’. This syndrome is characterized by hypocalcemia due to a suppression in bone reabsorption and increased bone mineralization, in the context of vitamin D replacement and a normalization of PTH [Witteveen et al. 2013]. Patients at risk for hungry bone syndrome require 30–75 mg/kg/day of elemental calcium given in three daily doses until vitamin D doses have been reduced to maintenance levels and 25(OH)D and PTH levels normalize [Misra et al. 2008].
Complications of vitamin D treatment
The tolerable upper level (UL) established by IOM is 4000 IU for children and adults [Ross, 2011], although research supports that the dose required to actually achieve a toxic concentration of serum 25(OH)D is significantly greater. Vitamin D intoxication is associated with several adverse effects including nephrolithiasis, hypertension, pain, conjunctivitis, anorexia, thirst, and vomiting and weight loss; all are due to hypercalcemia and occur only at very high vitamin D intakes [Jones, 2008]. Both the intoxication literature and more recent controlled dosing studies have been analyzed by Hathcock and colleagues [Hathcock et al. 2007]. These authors show that essentially no cases of confirmed intoxication have been reported at serum 25(OH)D levels below 500 nmol/l (200 ng/mL). Correspondingly, the oral intakes needed to produce such levels are in excess of 20,000 IU/day in otherwise healthy adults and, more usually, above 50,000 IU/day.
Case reports of vitamin D toxicity among children often describe dosing and pharmacologic errors as the reason for complications [Rajakumar et al. 2013]. Careful education of adolescents and their families in addition to periodic monitoring of 25(OH)D and calcium status are recommended to avoid preventable errors in vitamin D intake. It is worth noting that subjects who have significant vitamin D production from extensive sun exposure (e.g. lifeguards) may have serum 25(OH)D levels greater than 100 ng/ml (250 nmol/L) without the development of complications associated with vitamin D toxicity [Holick, 2009].
Conclusion
In conclusion, vitamin D deficiency is a common problem associated with adolescent obesity. Excess adiposity is linked with poor vitamin D status (and vice versa) and the effects of this deficiency during obesity seem to have negative consequences on IR and glucose homeostasis. The few published clinical trials using vitamin D supplementation to improve IR and IGT in obese adolescents have yielded beneficial effects. However, there is a need for more randomized controlled trials, involving larger sample sizes, focusing on obese adolescents with documented vitamin D deficiency and careful selection of the dose, dosing regimen, and achievement of target 25(OH)D concentrations. These trials should also include clamp-derived measures of in vivo sensitivity and β-cell function to more fully characterize the effects of vitamin D replenishment on IR.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest in preparing this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Catherine A. Peterson, University of Missouri, Department of Nutrition and Exercise Physiology, 204 Gwynn Hall, Columbia, MO 65211, USA
Aneesh K. Tosh, Department of Child Health, University of Missouri School of Medicine, University of Missouri, Columbia, MO, USA
Anthony M. Belenchia, Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, MO, USA
References
- Alberti K., Eckel R., Grundy S., Zimmet P., Cleeman J., Donato K., et al. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- Alemzadeh R., Kichler J. (2012) Parathyroid hormone is associated with biomarkers of insulin resistance and inflammation, independent of vitamin D status, in obese adolescents. Metab Syndr Relat Disord 10: 422–429. [DOI] [PubMed] [Google Scholar]
- Alemzadeh R., Kichler J., Babar G., Calhoun M. (2008) Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 57: 183–191. [DOI] [PubMed] [Google Scholar]
- Alkharfy K., Al-Daghri N., Yakout S., Hussain T., Mohammed A., Krishnaswamy S. (2013) Influence of vitamin D treatment on transcriptional regulation of insulin-sensitive genes. Metab Syndr Relat Disord 11: 283–288. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics. (2012) Dietary reference intakes for calcium and vitamin D. Pediatrics 130: e1424. [Google Scholar]
- Andersen R., Molgaard C., Skovgaard L., Brot C., Cashman K., Chabros E., et al. (2005) Teenage girls and elderly women living in northern Europe have low winter vitamin D status. Eur J Clin Nutr 59: 533–541. [DOI] [PubMed] [Google Scholar]
- Ashraf A., Alvarez J., Gower B., Saenz K., McCormick K. (2011) Associations of serum 25-hydroxyvitamin D and components of the metabolic syndrome in obese adolescent females. Obesity 19: 2214–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf A., Alvarez J., Saenz K., Gower B., McCormick K., Franklin F. (2009) Threshold for effects of vitamin D deficiency on glucose metabolism in obese female African-American adolescents. J Clin Endocrinol Metab 94: 3200–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R., Dodd K., Goldman J., Gahche J., Dwyer J., Moshfegh A., et al. (2010) Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr 140: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenchia A., Tosh A., Hillman L., Peterson C. (2013) Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr 97: 774–781. [DOI] [PubMed] [Google Scholar]
- Berg A., Combs T., Du X., Brownlee M., Scherer P. (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953. [DOI] [PubMed] [Google Scholar]
- Biancuzzo R., Clarke N., Reitz R., Travison T., Holick M. (2013) Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab 98: 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. (2007) What is new in vitamin D: 2006–2007. Curr Opin Rheumatol 19: 383–388. [DOI] [PubMed] [Google Scholar]
- Billaudel B., Delbancut A., Sutter B., Faure A. (1993) Stimulatory effect of 1,25-dihydroxyvitamin D3 on calcium handling and insulin secretion by islets from vitamin D3-deficient rats. Steroids 58: 335–341. [DOI] [PubMed] [Google Scholar]
- Black L., Jacoby P., She Ping-Delfos W., Mori T., Beilin L., Olynyk J., et al. (2014) Low serum 25-hydroxyvitamin D concentrations associate with non-alcoholic fatty liver disease in adolescents independent of adiposity. J Gastroenterol Hepatol 29: 1215–1222. [DOI] [PubMed] [Google Scholar]
- Blum M., Dolnikowski G., Seyoum E., Harris S., Booth S., Peterson J., et al. (2008) Vitamin D(3) in fat tissue. Endocrine 33: 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradale D., Kimlin M. (2009) Vitamin D in health and disease: an insight into traditional functions and new roles for the ‘sunshine vitamin’. Nutr Res Rev 22: 118-136. [DOI] [PubMed] [Google Scholar]
- Braegger C., Campoy C., Colomb V., Decsi T., Domellof M., Fewtrell M., et al. (2013) Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr 56: 692–701. [DOI] [PubMed] [Google Scholar]
- Brannon P., Yetley E., Bailey R., Picciano M. (2008) Overview of the conference ‘Vitamin D and Health in the 21st Century: an Update’. Am J Clin Nutr 88: 483S–490S. [DOI] [PubMed] [Google Scholar]
- Buemann B., Sorensen T., Pedersen O., Black E., Holst C., Toubro S., et al. (2005) Lower-body fat mass as an independent marker of insulin sensitivity – the role of adiponectin. Int J Obes 29: 624–631. [DOI] [PubMed] [Google Scholar]
- Buyukinan M., Ozen S., Kokkun S., Saz E. (2012) The relation of vitamin D deficiency with puberty and insulin resistance in obese children and adolescents. J Pediatr Endocrinol Metab 25: 83–87. [DOI] [PubMed] [Google Scholar]
- Calle C., Maestro B., Garcia-Arencibia M. (2008) Genomic actions of 1,25-dihydroxyvitamin D3 on insulin receptor gene expression, insulin receptor number and insulin activity in the kidney, liver and adipose tissue of streptozotocin-induced diabetic rats. BMC Mol Biol 9: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo M., Whiting S., Barton C. (2004) Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr 80: 1710S–1716S. [DOI] [PubMed] [Google Scholar]
- Cantorna M., Zhu Y., Froicu M., Wittke A. (2004) Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr 80: 1717S–1720S. [DOI] [PubMed] [Google Scholar]
- Castro A., Frederico M., Cazarolli L., Bretanha L., Tavares L., Buss Z., et al. (2014) Betulinic acid and 1,25(OH)(2) vitamin D(3) share intracellular signal transduction in glucose homeostasis in soleus muscle. Int J Biochem Cell Biol 48: 18–27. [DOI] [PubMed] [Google Scholar]
- Ceglia L. (2009) Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care 12: 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagas C., Borges M., Martini L., Rogero M. (2012) Focus on vitamin D, inflammation and type 2 diabetes. Nutrients 4: 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang J., Ge X., Du J., Deb D., Li Y. (2013) Vitamin D receptor inhibits nuclear factor kappaB activation by interacting with IkappaB kinase beta protein. J Biol Chem 288: 19450–19458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun R., Peercy B., Orwoll E., Nielson C., Adams J., Hewison M. (2013) Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol pii S0960-0760(13)00186-6. 10.1016/j.jsbmb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Lee Y., Hong H., Kang M., Kwon H., Shin C., et al. (2014) Inverse relationship between vitamin D status and insulin resistance and the risk of impaired fasting glucose in Korean children and adolescents: the Korean National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Public Health Nutr 17: 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codoner-Franch P., Tavarez-Alonso S., Simo-Jorda R., Laporta-Martin P., Carratala-Calvo A., Alonso-Iglesias E. (2012) Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. J Pediatr 161: 848–854. [DOI] [PubMed] [Google Scholar]
- Cole T., Bellizzi M., Flegal K., Dietz W. (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320: 1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupaye M., Breuil M., Riviere P., Castel B., Bogard C., Dupre T., et al. (2013) Serum vitamin D increases with weight loss in obese subjects 6 months after Roux-en-Y gastric bypass. Obes Surg 23: 486–493. [DOI] [PubMed] [Google Scholar]
- Creo A., Rosen J., Ariza A., Hidaka K., Binns H. (2013) Vitamin D levels, insulin resistance, and cardiovascular risks in very young obese children. J Pediatr Endocrinol Metab 26: 97–104. [DOI] [PubMed] [Google Scholar]
- Dabelea D., Mayer-Davis E., Saydah S., Imperatore G., Linder B., Divers J., et al. (2014) Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 311: 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de las Heras J., Rajakumar K., Lee S., Bacha F., Holick M., Arslanian S. (2013) 25-Hydroxyvitamin D in obese youth across the spectrum of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes Care 36: 2048–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moraes A., Fadoni R., Ricardi L., Souza T., Rosaneli C., Nakashima A., et al. (2011) Prevalence of abdominal obesity in adolescents: a systematic review. Obes Rev 12: 69–77. [DOI] [PubMed] [Google Scholar]
- Delvin E., Lambert M., Levy E., O’Loughlin J., Mark S., Gray-Donald K., et al. (2010) Vitamin D status is modestly associated with glycemia and indicators of lipid metabolism in French-Canadian children and adolescents. J Nutr 140: 987–991. [DOI] [PubMed] [Google Scholar]
- de Onis M., Onyango A., Borghi E., Siyam A., Nishida C., Siekmann J. (2007) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group (2012) The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care 35: 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C., Gao D., Wilding J., Trayhurn P., Bing C. (2012) Vitamin D signalling in adipose tissue. Br J Nutr 108: 1915–1923. [DOI] [PubMed] [Google Scholar]
- Ding C., Wilding J., Bing C. (2013) 1,25-dihydroxyvitamin D3 protects against macrophage-induced activation of NFkappaB and MAPK signalling and chemokine release in human adipocytes. PLoS One 8: e61707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drincic A., Armas L., Van Diest E., Heaney R. (2012) Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 20: 1444–1448. [DOI] [PubMed] [Google Scholar]
- Du T., Zhou Z., You S., Lin J., Yang L., Zhou W., et al. (2009) Regulation by 1, 25-dihydroxy-vitamin D3 on altered TLRs expression and response to ligands of monocyte from autoimmune diabetes. Clin Chim Acta 402: 133–138. [DOI] [PubMed] [Google Scholar]
- Economos C., Moore C., Hyatt R., Kuder J., Chen T., Meydani S., et al. (2014) Multinutrient-fortified juices improve vitamin D and vitamin E status in children: a randomized controlled trial. J Acad Nutr Diet 114: 709–717. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Dietetic Products, Nutritition and Allergies (2012) Scientific opinion on the tolerable upper intake level of vitamin D. EFSA J 10: 2813–2857. [Google Scholar]
- Erdonmez D., Hatun S., Cizmecioglu F., Keser A. (2011) No relationship between vitamin D status and insulin resistance in a group of high school students. J Clin Res Pediatr Endocrinol 3: 198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkal M., Wilde J., Bilgin Y., Akinci A., Demir E., Bodeker R., et al. (2006) High prevalence of vitamin D deficiency, secondary hyperparathyroidism and generalized bone pain in Turkish immigrants in Germany: identification of risk factors. Osteoporos Int 17: 1133–1140. [DOI] [PubMed] [Google Scholar]
- Fazeli F., van der Aa M., van der Vorst M., Knibbe C., de Boer A. (2013) Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: a systematic review and evaluation of methodological approaches. Diabetologia 56: 1471–1488. [DOI] [PubMed] [Google Scholar]
- Flores M. (2005) A role of vitamin D in low-intensity chronic inflammation and insulin resistance in type 2 diabetes mellitus? Nutr Res Rev 18: 175–182. [DOI] [PubMed] [Google Scholar]
- Ganji V., Zhang X., Shaikh N., Tangpricha V. (2011) Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001–2006. Am J Clin Nutr 94: 225–233. [DOI] [PubMed] [Google Scholar]
- Garanty-Bogacka B., Syrenicz M., Goral J., Krupa B., Syrenicz J., Walczak M., et al. (2011) Serum 25-hydroxyvitamin D (25-OH-D) in obese adolescents. Endokrynol Pol 62: 506–511. [PubMed] [Google Scholar]
- Garland C., Garland F., Gorham E. (2003) Epidemiologic evidence for different roles of ultraviolet A and B radiation in melanoma mortality rates. Ann Epidemiol 13: 395–404. [DOI] [PubMed] [Google Scholar]
- George P., Pearson E., Witham M. (2012) Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med 29: e142–e150. [DOI] [PubMed] [Google Scholar]
- Grant W., Holick M. (2005) Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev 10: 94–111. [PubMed] [Google Scholar]
- Guillot X., Semerano L., Saidenberg-Kermanac’h N., Falgarone G., Boissier M. (2010) Vitamin D and inflammation. Joint Bone Spine 77: 552–557. [DOI] [PubMed] [Google Scholar]
- Guzel R., Kozanoglu E., Guler-Uysal F., Soyupak S., Sarpel T. (2001) Vitamin D status and bone mineral density of veiled and unveiled Turkish women. J Womens Health Gend Based Med 10: 765–770. [DOI] [PubMed] [Google Scholar]
- Hannon T., Janosky J., Arslanian S. (2006) Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res 60: 759–763. [DOI] [PubMed] [Google Scholar]
- Harel Z., Cromer B., DiVasta A., Gordon C. (2013) Recommended vitamin D intake and management of low vitamin D status in adolescents: a position statement of the society for adolescent health and medicine. J Adolesc Health 52: 801–803. [DOI] [PubMed] [Google Scholar]
- Harel Z., Flanagan P., Forcier M., Harel D. (2011) Low vitamin D status among obese adolescents: prevalence and response to treatment. J Adolesc Health 48: 448–452. [DOI] [PubMed] [Google Scholar]
- Hathcock J., Shao A., Vieth R., Heaney R. (2007) Risk assessment for vitamin D. Am J Clin Nutr 85: 6–18. [DOI] [PubMed] [Google Scholar]
- Haussler M., Whitfield G., Kaneko I., Haussler C., Hsieh D., Hsieh J., et al. (2013) Molecular mechanisms of vitamin D action. Calcif Tissue Int 92: 77–98. [DOI] [PubMed] [Google Scholar]
- Heaney R., Davies K., Chen T., Holick M., Barger-Lux M. (2003) Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77: 204–210. [DOI] [PubMed] [Google Scholar]
- Heaney R., Horst R., Cullen D., Armas L. (2009) Vitamin D3 distribution and status in the body. J Am Coll Nutr 28: 252–256. [DOI] [PubMed] [Google Scholar]
- Henry H., Bouillon R., Norman A., Gallagher J., Lips P., Heaney R., et al. (2010) 14th Vitamin D Workshop consensus on vitamin D nutritional guidelines. J Steroid Biochem Mol Biol 121: 4–6. [DOI] [PubMed] [Google Scholar]
- Hillier T., Pedula K. (2003) Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care 26: 2999–3005. [DOI] [PubMed] [Google Scholar]
- Holick M. (2004) Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80: 1678S–1688S. [DOI] [PubMed] [Google Scholar]
- Holick M. (2006) Vitamin D. In: Shils M., Shike M., Ross A., Caballero B., Cousins R. (eds), Modern Nutrition in Health and Disease, 10th edn. Baltimore, MD: Lippincott Williams & Wilkins, pp. 376–395. [Google Scholar]
- Holick M. (2007) Vitamin D deficiency. N Engl J Med 357: 266–281. [DOI] [PubMed] [Google Scholar]
- Holick M. (2009) Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 19: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M., Binkley N., Bischoff-Ferrari H., Gordon C., Hanley D., Heaney R., et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96: 1911–1930. [DOI] [PubMed] [Google Scholar]
- Holick M., Jenkins M. (2003) The UV Advantage. New York: ibooks. [Google Scholar]
- Hopkins M., Owen J., Ahearn T., Fedirko V., Flanders W., Jones D., et al. (2011) Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: a randomized, controlled clinical trial. Cancer Prev Res 4: 1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Ishizuka T., Miura A., Kajita K., Ishizawa M., Kimura M., et al. (2002) Effect of 1 alpha,25-dihydroxy vitamin D3 and vitamin E on insulin-induced glucose uptake in rat adipocytes. Diabetes Res Clin Pract 55: 175–183. [DOI] [PubMed] [Google Scholar]
- Jang H., Lee H., Park J., Kang J., Song J. (2013) Association between serum vitamin D and metabolic risk factors in Korean schoolgirls. Osong Public Health Res Perspect 4: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Pavon D., Sese M., Valtuena J., Cuenca-Garcia M., Gonzalez-Gross M., Gottrand F., et al. (2014) Leptin, vitamin D, and cardiorespiratory fitness as risk factors for insulin resistance in European adolescents: gender differences in the HELENA Study. Appl Physiol Nutr Metab 39: 530–537. [DOI] [PubMed] [Google Scholar]
- Jones G. (2008) Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 88: 582S–586S. [DOI] [PubMed] [Google Scholar]
- Kardas F., Kendirci M., Kurtoglu S. (2013) Cardiometabolic risk factors related to vitamin D and adiponectin in obese children and adolescents. Int J Endocrinol 2013: 503270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaniyil S., Harris S., Retnakaran R., Vieth R., Knight J., Gerstein H., et al. (2014) Prospective association of 25(OH)D with metabolic syndrome. Clin Endocrinol 80: 502–507. [DOI] [PubMed] [Google Scholar]
- Keast D., Fulgoni V., III, Nicklas T., O’Neil C. (2013) Food sources of energy and nutrients among children in the United States: National Health and Nutrition Examination Survey 2003–2006. Nutrients 5: 283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelishadi R., Salek S., Salek M., Hashemipour M., Movahedian M. (2014) Effects of vitamin D supplementation on insulin resistance and cardiometabolic risk factors in children with metabolic syndrome: a triple-masked controlled trial. J Pediatr 90: 28–34. [DOI] [PubMed] [Google Scholar]
- Kelly A., Brooks L., Dougherty S., Carlow D., Zemel B. (2011) A cross-sectional study of vitamin D and insulin resistance in children. Arch Dis Child 96: 447–452. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Bajdik C., Willemze R., De Gruijl F., Bouwes Bavinck J. (2003) The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol 120: 1087–1093. [DOI] [PubMed] [Google Scholar]
- Khadgawat R., Thomas T., Gahlot M., Tandon N., Tangpricha V., Khandelwal D., et al. (2012) The effect of puberty on interaction between vitamin D status and insulin resistance in obese Asian-Indian children. Int J Endocrinol 2012: 173581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H., Kunutsor S., Franco O., Chowdhury R. (2013) Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proc Nutr Soc 72: 89–97. [DOI] [PubMed] [Google Scholar]
- Kharroubi I., Rasschaert J., Eizirik D., Cnop M. (2003) Expression of adiponectin receptors in pancreatic beta cells. Biochem Biophys Res Commun 312: 1118–1122. [DOI] [PubMed] [Google Scholar]
- Kramer C.K., Swaminathan B., Hanley A.J., Connelly P.W., Sermer M., Zinman B., et al. (2014) Prospective Associations of Vitamin D Status with Beta-cell function, Insulin Sensitivity and Glycemia: The Impact of Parathyroid Hormone Status. Diabetes. [DOI] [PubMed] [Google Scholar]
- Labriji-Mestaghanmi H., Billaudel B., Garnier P., Malaisse W., Sutter B. (1988) Vitamin D and pancreatic islet function. I. Time course for changes in insulin secretion and content during vitamin D deprivation and repletion. J Endocrinol Invest 11: 577–584. [DOI] [PubMed] [Google Scholar]
- LeBlanc E., Perrin N., Johnson J., Jr., Ballatore A., Hillier T. (2013) Over-the-counter and compounded vitamin D: is potency what we expect? JAMA Intern Med 173: 585–586. [DOI] [PubMed] [Google Scholar]
- Lee S., Clark S.A., Gill R.K., Christakos S. (1994) 1,25-Dihydroxyvitamin D3 and pancreatic beta-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology 134: 1602–1610. [DOI] [PubMed] [Google Scholar]
- Lenders C., Feldman H., Von S., Merewood A., Sweeney C., Wilson D., et al. (2009) Relation of body fat indexes to vitamin D status and deficiency among obese adolescents. Am J Clin Nutr 90: 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E., Armstrong-Moore D., Liang Z., Sweeney J., Torres W., Ziegler T., et al. (2011) Contribution of adipose tissue to plasma 25-hydroxyvitamin D concentrations during weight loss following gastric bypass surgery. Obesity 19: 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips P. (2006) Vitamin D physiology. Prog Biophys Mol Biol 92: 4–8. [DOI] [PubMed] [Google Scholar]
- Liu E., Meigs J., Pittas A., McKeown N., Economos C., Booth S., et al. (2009) Plasma 25-hydroxyvitamin D is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr 139: 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald H. (2013) Contributions of sunlight and diet to vitamin D status. Calcif Tissue Int 92: 163–176. [DOI] [PubMed] [Google Scholar]
- Maeda N., Takahashi M., Funahashi T., Kihara S., Nishizawa H., Kishida K., et al. (2001) PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50: 2094–2099. [DOI] [PubMed] [Google Scholar]
- Maestro B., Davila N., Carranza M., Calle C. (2003) Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol 84: 223–230. [DOI] [PubMed] [Google Scholar]
- Maestro B., Molero S., Bajo S., Davila N., Calle C. (2002) Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem Funct 20: 227–232. [DOI] [PubMed] [Google Scholar]
- Manna P., Jain S. (2012) Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-gamma-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J Biol Chem 287: 42324–42332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzuillo P., Del Giudice E., Santoro N. (2014) Pediatric non-alcoholic fatty liver disease: New insights and future directions. World J Hepatol 6: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawer E., Backhouse J., Holman C., Lumb G., Stanbury S. (1972) The distribution and storage of vitamin D and its metabolites in human tissues. Clin Sci 43: 413–431. [DOI] [PubMed] [Google Scholar]
- McDonagh M., Selph S., Ozpinar A., Foley C. (2014) Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr 168: 178–184. [DOI] [PubMed] [Google Scholar]
- Miller R., Chu Q., Xie J., Foretz M., Viollet B., Birnbaum M. (2013) Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 494: 256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M., Pacaud D., Petryk A., Collett-Solberg P., Kappy M. (2008) Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 122: 398–417. [DOI] [PubMed] [Google Scholar]
- Mitri J., Dawson-Hughes B., Hu F., Pittas A. (2011) Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr 94: 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofa K., Liu C., Vione D., Gao K., Ogawa H. (2013) Sources, factors, mechanisms and possible solutions to pollutants in marine ecosystems. Environ Pollut 182: 461–478. [DOI] [PubMed] [Google Scholar]
- Motoshima H., Wu X., Sinha M., Hardy V., Rosato E., Barbot D., et al. (2002) Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab 87: 5662–5667. [DOI] [PubMed] [Google Scholar]
- Nam G., Kim do H., Cho K., Park Y., Han K., Choi Y., et al. (2012) Estimate of a predictive cut-off value for serum 25-hydroxyvitamin D reflecting abdominal obesity in Korean adolescents. Nutr Res 32: 395–402. [DOI] [PubMed] [Google Scholar]
- National Heart Forum (2012) Bending the obesity cost curve in California: reducing the average body mass index in the state by 5 percent could lead to health care savings of more then $28 billion in 10 years and $81 billion in 20 years. Issue Brief. Washington DC and Princeton, NJ: Trust for America’s Health and Robert Wood Foundation. [Google Scholar]
- Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitphong H., Chanprasertyothin S., Jongjaroenprasert W., Ongphiphadhanakul B. (2009) The association between vitamin D status and circulating adiponectin independent of adiposity in subjects with abnormal glucose tolerance. Endocrine 36: 205–210. [DOI] [PubMed] [Google Scholar]
- Nsiah-Kumi P., Erickson J., Beals J., Ogle E., Whiting M., Brushbreaker C., et al. (2012) Vitamin D insufficiency is associated with diabetes risk in native American children. Clin Pediatr 51: 146–153. [DOI] [PubMed] [Google Scholar]
- Nunlee-Bland G., Gambhir K., Abrams C., Abdul M., Vahedi M., Odonkor W. (2011) Vitamin D deficiency and insulin resistance in obese African-American adolescents. J Pediatr Endocrinol Metab 24: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden C., Carroll M., Kit B., Flegal K. (2012) Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 307: 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M., Maalouf N., Oden J., White P., Hutchison M. (2012) Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J Clin Endocrinol Metab 97: 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifico L., Anania C., Osborn J., Ferraro F., Bonci E., Olivero E., et al. (2011) Low 25(OH)D3 levels are associated with total adiposity, metabolic syndrome, and hypertension in Caucasian children and adolescents. Eur J Endocrinol 165: 603–611. [DOI] [PubMed] [Google Scholar]
- Parikh S., Guo D., Pollock N., Petty K., Bhagatwala J., Gutin B., et al. (2012) Circulating 25-hydroxyvitamin D concentrations are correlated with cardiometabolic risk among American black and white adolescents living in a year-round sunny climate. Diabetes Care 35: 1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Kinra S., Ward K., White B., Viner R. (2009) Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 32: 1743–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Kim D., Kwon D., Yang H. (2011) Long-term central infusion of adiponectin improves energy and glucose homeostasis by decreasing fat storage and suppressing hepatic gluconeogenesis without changing food intake. J Neuroendocrinol 23: 687–698. [DOI] [PubMed] [Google Scholar]
- Pathak K., Soares M., Calton E., Zhao Y., Hallett J. (2014) Vitamin D supplementation and body weight status: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 15: 528–537. [DOI] [PubMed] [Google Scholar]
- Pirgon O., Cekmez F., Bilgin H., Eren E., Dundar B. (2013) Low 25-hydroxyvitamin D level is associated with insulin sensitivity in obese adolescents with non-alcoholic fatty liver disease. Obes Res Clin Pract 7: e275–e283. [DOI] [PubMed] [Google Scholar]
- Pittas A., Lau J., Hu F., Dawson-Hughes B. (2007) The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92: 2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pludowski P., Karczmarewicz E., Bayer M., Carter G., Chlebna-Sokol D., Czech-Kowalska J., et al. (2013) Practical guidelines for the supplementation of vitamin D and the treatment of deficits in central Europe – recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol 64: 319–327. [DOI] [PubMed] [Google Scholar]
- Ponda M., McGee D., Breslow J. (2014) Vitamin D binding protein levels do not influence the effect of vitamin D repletion on serum PTH and calcium: data from a randomized, controlled trial. J Clin Endocrinol Metab 99: 2494–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poomthavorn P., Saowan S., Mahachoklertwattana P., Chailurkit L., Khlairit P. (2012) Vitamin D status and glucose homeostasis in obese children and adolescents living in the tropics. Int J Obes 36: 491–495. [DOI] [PubMed] [Google Scholar]
- Prentice A. (2008) Vitamin D deficiency: a global perspective. Nutr Rev 66: S153–S164. [DOI] [PubMed] [Google Scholar]
- Punthakee Z., Delvin E., O’Loughlin J., Paradis G., Levy E., Platt R., et al. (2006) Adiponectin, adiposity, and insulin resistance in children and adolescents. J Clin Endocrinol Metab 91: 2119–2125. [DOI] [PubMed] [Google Scholar]
- Rajakumar K., de Las H., Lee S., Holick M., Arslanian S. (2012) 25-hydroxyvitamin D concentrations and in vivo insulin sensitivity and beta-cell function relative to insulin sensitivity in black and white youth. Diabetes Care 35: 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumar K., Reis E., Holick M. (2013) Dosing error with over-the-counter vitamin D supplement: a risk for vitamin D toxicity in infants. Clin Pediatr 52: 82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr T., de Sousa G., Alexy U., Kersting M., Andler W. (2007) Vitamin D status and parathyroid hormone in obese children before and after weight loss. Eur J Endocrinol 157: 225–232. [DOI] [PubMed] [Google Scholar]
- Rodbard H. (2008) Diabetes screening, diagnosis, and therapy in pediatric patients with type 2 diabetes. Medscape J Med 10: 184. [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich S., Rich C., Volwiler W. (1971) Deposition in and release of vitamin D3 from body fat: evidence for a storage site in the rat. J Clin Invest 50: 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A. (2011) The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr 14: 938–939. [DOI] [PubMed] [Google Scholar]
- Roth C., Elfers C., Kratz M., Hoofnagle A. (2011) Vitamin d deficiency in obese children and its relationship to insulin resistance and adipokines. J Obes 2011: 495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Walther B. (2013) Natural vitamin D content in animal products. Adv Nutr 4: 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shab-Bidar S., Neyestani T., Djazayery A., Eshraghian M., Houshiarrad A., Kalayi A., et al. (2012) Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes. Diabetes Metab Res Rev 28: 424–430. [DOI] [PubMed] [Google Scholar]
- Shin Y., Shin H., Lee Y. (2013) Vitamin D status and childhood health. Korean J Pediatr 56: 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Fisch G., Teague B., Tamborlane W., Banyas B., Allen K., et al. (2002) Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 346: 802–810. [DOI] [PubMed] [Google Scholar]
- Skinner A., Skelton J. (2014) Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr 168: 561–566. [DOI] [PubMed] [Google Scholar]
- Souberbielle J., Courbebaisse M., Cormier C., Pierrot-Deseilligny C., Viard J., Jean G., et al. (2012) When should we measure vitamin D concentration in clinical practice? Scand J Clin Lab Invest 243(Suppl.): 129–135. [DOI] [PubMed] [Google Scholar]
- Speeckaert R., Van Gele M., Speeckaert M., Lambert J., van Geel N. (2014) The biology of hyperpigmentation syndromes. Pigment Cell Melanoma Res 27: 512–524. [DOI] [PubMed] [Google Scholar]
- Stanley T., Bredella M., Pierce L., Misra M. (2013) The ratio of parathyroid hormone to vitamin D is a determinant of cardiovascular risk and insulin sensitivity in adolescent girls. Metab Syndr Relat Disord 11: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffman N., Gordon C. (2009) Vitamin D and adolescents: what do we know? Curr Opin Pediatr 21: 465–471. [DOI] [PubMed] [Google Scholar]
- Tangpricha V., Koutkia P., Rieke S., Chen T., Perez A., Holick M. (2003) Fortification of orange juice with vitamin D: a novel approach for enhancing vitamin D nutritional health. Am J Clin Nutr 77: 1478–1483. [DOI] [PubMed] [Google Scholar]
- Tangpricha V., Turner A., Spina C., Decastro S., Chen T., Holick M. (2004) Tanning is associated with optimal vitamin D status (serum 25-hydroxyvitamin D concentration) and higher bone mineral density. Am J Clin Nutr 80: 1645–1649. [DOI] [PubMed] [Google Scholar]
- Terenetskaya I. (2004) Two methods for direct assessment of the vitamin D synthetic capacity of sunlight and artificial UV sources. J Steroid Biochem Mol Biol 89–90: 623–626. [DOI] [PubMed] [Google Scholar]
- Torun E., Gonullu E., Ozgen I., Cindemir E., Oktem F. (2013) Vitamin D deficiency and insufficiency in obese children and adolescents and its relationship with insulin resistance. Int J Endocrinol 2013: 631845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers S., Jeffers B., Bloch C., Hill J., Eckel R. (1995) Gender and Tanner stage differences in body composition and insulin sensitivity in early pubertal children. J Clin Endocrinol Metab 80: 172–178. [DOI] [PubMed] [Google Scholar]
- Tripkovic L., Lambert H., Hart K., Smith C., Bucca G., Penson S., et al. (2012) Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr 95: 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohimaa P., Wang J., Khan S., Kuuslahti M., Qian K., Manninen T., et al. (2013) Gene expression profiles in human and mouse primary cells provide new insights into the differential actions of vitamin D3 metabolites. PLoS One 8: e75338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turer C., Lin H., Flores G. (2013) Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics 131: e152–e161. [DOI] [PubMed] [Google Scholar]
- van Schoor N., Lips P. (2011) Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab 25: 671–680. [DOI] [PubMed] [Google Scholar]
- Vimaleswaran K., Berry D., Lu C., Tikkanen E., Pilz S., Hiraki L., et al. (2013) Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 10: e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner R., Segal T., Lichtarowicz-Krynska E., Hindmarsh P. (2005) Prevalence of the insulin resistance syndrome in obesity. Arch Dis Child 90: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G., Ricotti R., Roccio M., Moia S., Bellone S., Prodam F., et al. (2014) Pediatric obesity and vitamin D deficiency: a proteomic approach identifies multimeric adiponectin as a key link between these conditions. PLoS One 9: e83685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamberg L., Cullberg K., Rejnmark L., Richelsen B., Pedersen S. (2013) Investigations of the anti-inflammatory effects of vitamin D in adipose tissue: results from an in vitro study and a randomized controlled trial. Horm Metab Res 45: 456–462. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhu J., DeLuca H. (2012) Where is the vitamin D receptor? Arch Biochem Biophys 523: 123–133. [DOI] [PubMed] [Google Scholar]
- Webb A., Engelsen O. (2006) Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol 82: 1697–1703. [DOI] [PubMed] [Google Scholar]
- Witteveen J., van, Thiel S., Romijn J., Hamdy N. (2013) Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. Eur J Endocrinol 168: R45–R53. [DOI] [PubMed] [Google Scholar]
- Wolden-Kirk H., Overbergh L., Gysemans C., Brusgaard K., Naamane N., Van L.L., et al. (2013) Unraveling the effects of 1,25OH2D3 on global gene expression in pancreatic islets. J Steroid Biochem Mol Biol 136: 68–79. [DOI] [PubMed] [Google Scholar]
- Wortsman J., Matsuoka L., Chen T., Lu Z., Holick M. (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72: 690–693. [DOI] [PubMed] [Google Scholar]
- Wu F., Staykova T., Horne A., Clearwater J., Ames R., Mason B., et al. (2003) Efficacy of an oral, 10-day course of high-dose calciferol in correcting vitamin D deficiency. N Z Med J 116: U536. [PubMed] [Google Scholar]
- Zabihiyeganeh M., Jahed A., Nojomi M. (2013) Treatment of hypovitaminosis D with pharmacologic doses of cholecalciferol, oral vs intramuscular; an open labeled RCT. Clin Endocrinol 78: 210–216. [DOI] [PubMed] [Google Scholar]
- Zeitz U., Weber K., Soegiarto D., Wolf E., Balling R., Erben R. (2003) Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J 17: 509–511. [DOI] [PubMed] [Google Scholar]
- Zimmet P., Alberti K., Kaufman F., Tajima N., Silink M., Arslanian S., et al. (2007) The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes 8: 299–306. [DOI] [PubMed] [Google Scholar]