Our results implicate reduced striatal and thalamic brain iron levels in attention deficit hyperactivity disorder pathophysiology before medication and suggest that reduced brain iron levels may normalize with psychostimulant treatment.
Abstract
Purpose
To comprehensively assess brain iron levels in typically developing control subjects and patients with attention deficit hyperactivity disorder (ADHD) when psychostimulant medication history is accounted for.
Materials and Methods
This prospective study was approved by the institutional review board, and informed consent was obtained. Brain iron was indexed noninvasively by using magnetic resonance (MR) imaging relaxation rates (R2, R2*, R2′) and magnetic field correlation (MFC) in the globus pallidus, putamen, caudate nucleus, and thalamus for 22 patients with ADHD (12 medication-naïve patients and 10 with a history of psychostimulant treatment) and 27 control subjects (age range, 8–18 years). Serum iron measures were also collected. Subgroup differences were analyzed with data-appropriate omnibus tests followed by post hoc pairwise comparisons; false discovery rate correction was conducted to control for multiple comparisons.
Results
Medication-naïve ADHD patients had significantly lower striatal and thalamic MFC indexes of brain iron than did control subjects (putamen, P = .012; caudate nucleus, P = .008; thalamus, P = .012) and psychostimulant-medicated ADHD patients (putamen, P = .006; caudate nucleus, P = .010; thalamus, P = .021). Conversely, the MFC indexes in medicated patients were comparable to those in control subjects. No significant differences were detected with R2, R2*, R2′, or serum measures.
Conclusion
Lower MFC indexes of striatal and thalamic brain iron in medication-naïve ADHD patients and lack of differences in psychostimulant-medicated patients suggest that MFC indexes of brain iron may represent a noninvasive diagnostic biomarker that responds to psychostimulant treatment.
© RSNA, 2014
Introduction
The first-line therapy for attention deficit hyperactivity disorder (ADHD) is treatment with psychostimulants that act primarily on the dopaminergic system in the striatum and on the noradrenergic system in the frontal cortex (1). Given that psychostimulants indirectly increase synaptic dopamine levels by blocking dopamine transporters (1), it is surmised that a reduction in dopamine is a critical component of ADHD pathophysiology (2). In a recent meta-analysis of molecular imaging studies in adults with ADHD, Fusar-Poli et al (3) reported that reduced dopamine biomarkers were consistently detected in medication-naïve patients whereas increased dopamine biomarkers were consistently detected in patients with previous psychostimulant treatment. This observation supports the hypotheses that dopamine deficiency characterizes ADHD before medication and that the dopaminergic system adapts in response to psychostimulants (4,5).
Along with an aberrant dopaminergic system, reports of reduced serum and brain iron in ADHD suggest that iron homeostasis may also be disrupted (6–8). However, psychostimulant medication history was unaccounted for in these studies. Rather than viewing atypical dopamine and iron homeostasis as separate pathologic mechanisms, we conjecture that abnormal brain iron levels in ADHD reflect iron’s involvement in the dopamine metabolic pathway that is targeted and potentially altered by psychostimulants (9,10). To test this possible relationship, we examined whether brain iron differences exist in pediatric ADHD patients according to their history of psychostimulant treatment. Brain iron was regarded as a potential dopamine-related biomarker because (a) it is a required cofactor for dopamine synthesis (9,10); (b) altered iron levels have been associated with cognitive and dopaminergic changes (11–13); and (c), in contrast to the radioactive tracers used as dopamine biomarkers in molecular imaging, brain iron can be probed noninvasively with use of magnetic resonance (MR) imaging methods (14,15).
Multimodal MR imaging indexes of brain iron consisting of conventional water proton relaxation rates (ie, R2, R2*, R2′) (14,15) and the recently developed magnetic field correlation (MFC) metric (16–18) were used to examine brain iron levels in medication-naïve and psychostimulant-medicated children and adolescents with ADHD and in typically developing control subjects. Although relaxation rates are sensitive to iron, their specificity is limited because they are also influenced by noniron molecular relaxation mechanisms (14,15). MFC was measured because of its insensitivity to these noniron mechanisms (16,17), its correlation with putative postmortem iron concentrations (17), and its ability to help detect gray matter intraregional iron differences due to normal aging (18) and pathologic conditions (19–21). Guided by the recent meta-analysis by Fusar-Poli et al (3), we hypothesized that reduced brain iron levels would be detected specifically in medication-naïve ADHD patients. We performed this study to comprehensively assess brain iron levels in typically developing control subjects and patients with ADHD when psychostimulant medication history is accounted for.
Materials and Methods
Participants
For this prospective study, patients with ADHD and control subjects (age range, 8–18 years) were recruited from the New York University Child Study Center, Medical Center, and the local community. Informed consent and child’s assent were obtained as approved by the institutional review board. Participant recruitment and MR imaging began in June 2009 and was completed in April 2011. Inclusion criteria consisted of an estimated full-scale intelligence quotient (IQ) greater than 79 measured with the Wechsler Abbreviated Scale of Intelligence (22), right-handedness measured with the Edinburgh Handedness Inventory (23), and absence of known neurologic, cognitive, or chronic medical diseases. For inclusion in the ADHD group, a diagnosis of ADHD using the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), text revision (24), was required and was assessed by licensed clinicians or supervised trainees on the basis of the Schedule of Affective Disorders and Schizophrenia for Children–Present and Lifetime Version (25), which was administered to each parent and child separately. In addition, DSM-IV Total Index of the Conners Parent and Teacher Rating Scale–Revised: Long Version (26) had to be greater than 1.5 standard deviation beyond the mean (T-score = 65), whereas the Behavioral Rating Inventory of Executive Function–Parent Version (27) Global Executive Composite T-scores had to be greater than 60. Inclusion also required a diagnosis of the combined type or predominantly inattentive type of ADHD with a previous DSM-IV combined type childhood diagnosis. Exclusion criteria consisted of diagnosis of psychotic, major depressive, conduct, tic, or pervasive developmental disorders. Inclusion criteria for the control group required absence of axis I disorders on the basis of the Schedule of Affective Disorders and Schizophrenia for Children–Present and Lifetime Version and T scores of 65 or less on the DSM-IV Total Index of the Conners Parent and Teacher Rating Scale–Revised: Long Version and the Conners Global Index.
On the basis of psychostimulant treatment history, we identified two ADHD subgroups: (a) medication-naïve patients never treated with psychostimulants or other psychoactive medications (ADHD-nonmedicated subgroup) and (b) patients with a history of psychostimulant treatment defined by current or past psychostimulant use (ADHD-medicated subgroup).
Image Acquisition and Processing
MR imaging data were collected with a 3.0-T unit (Tim Trio; Siemens, Erlangen, Germany) with a transmission body coil and an eight-element phased-array reception coil (total imaging time, 19 minutes 28 seconds). No anesthesia was used. For MFC estimation, asymmetric spin-echo images were acquired with segmented echo-planar imaging with the following parameters: 5550/40 (repetition time msec/echo time msec); voxel size, 1.7 × 1.7 × 1.7 mm3; number of sections, 78; number of signals acquired, four; flip angle, 90°; echo-planar imaging factor, 33; bandwidth, 1346 Hz/pixel; refocusing pulse time shifts, 0, −4, and −16 msec; and acquisition time, 6 minutes 40 seconds (16–19). For R2 estimation, T2-weighted fast spin-echo images were obtained with the following parameters (28): 6450/15, 30, 45, 60, 75; voxel size, 1.7 × 1.7 × 3.4 mm3; number of sections, 36; number of signals acquired, one; flip angle, 180°; turbo factor, 2; bandwidth, 292 Hz/pixel; and acquisition time, 3 minutes 59 seconds. For R2* estimation, T2*-weighted gradient-echo images were obtained with the following parameters: 60/7, 12.99, 16.87, 21.75, 26.63, 31.51, 36.39, 41.27, 46.15, 51.03; voxel size, 1.7 × 1.7 × 1.7 mm3; number of sections, 72; number of signals acquired, one; flip angle, 20°; bandwidth, 210 Hz/pixel; and acquisition time, 5 minutes 20 seconds. The asymmetric spin-echo, T2-weighted, and T2*-weighted images are whole brain, without gaps, with a 220 × 220-mm2 field of view and 128 × 128 matrix. Whole-brain T1-weighted magnetization-prepared rapid acquisition gradient-echo imaging was performed with the following parameters: 2200/2.26; matrix, 192 × 256 × 160; voxel size, 1 × 1 × 1 mm3; no gaps; and acquisition time, 3 minutes 29 seconds. Following routine institutional policy, all images were screened for abnormal clinical findings by board-certified neuroradiologists who were not involved in the study. Images with abnormal clinical reports or severe artifacts (ie, blurred images, loss of signal) were excluded by a reader blinded to study groups (V.A., with >6 years of experience in neuroimaging). A total of 25 images were excluded owing to an abnormal clinical MR imaging finding (n = 1), MR imaging hardware artifacts (n = 17), and severe motion artifacts (n = 7). To confirm that there was no group bias in head motion, group differences were analyzed (Appendix E1, Fig E1 [online]). MFC (16–18), R2, and R2* (18) parametric maps were generated as previously described; R2′ is defined as R2* − R2. A patent for the MFC imaging method is held by Drs Jensen and Helpern and owned by New York University.
Brain Region-of-Interest Analyses
Region-of-interest (ROI) analyses were conducted on the globus pallidus, putamen, caudate nucleus, and thalamus because these brain regions are primary targets of psychostimulants (1,4) and have the highest concentrations of dopamine and iron (11,29) (Fig 1, B). For each participant, automatic ROI segmentation of the magnetization-prepared rapid acquisition gradient-echo image was performed by using software (FreeSurfer, http://surfer.nmr.mgh.harvard.edu; R.L.D, with >3 years of experience in neuroimaging). To exclude voxels with partial volume effects, ROIs were further constrained with a consensus mask. First, ROIs and parametric maps were normalized to the MNI152 standard space (1 mm3) with the automatic registration toolbox software package (30). The consensus mask was then defined as ROI voxels with 100% (49 of 49) overlap among all participants. Anatomic accuracy of the resulting ROIs was visually verified by an author who was blinded to the study groups (V.A.). Consensus ROIs were applied to each participant’s spatially normalized parametric maps to extract ROI means. Supplementary ROI analyses were conducted on MFC restricted to voxels dominated by microscopic MFC contributions (Appendix E1 [online]).
Figure 1:
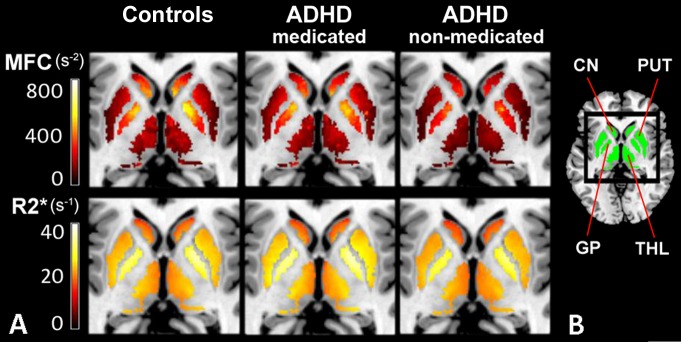
Subgroup averages of MFC and R2*. A, MFC and R2* parametric maps for 27 control subjects, 10 ADHD patients with a history of psychostimulant treatment (ADHD-medicated subgroup), and 12 medication-naïve ADHD patients (ADHD-nonmedicated subgroup). Qualitative differences between control subjects and ADHD subgroups are visible only on MFC maps. B, ROIs (green) used to mask parametric maps. CN = caudate nucleus, GP = globus pallidus, PUT = putamen, THL = thalamus.
Serum Iron Measures
Blood samples were collected after a fasting period to measure serum ferritin level, iron level, total iron binding capacity, transferrin level, and complete blood count (Appendix E1, Table E1 [online]).
Statistical Analyses
Statistical analyses were performed with software (SPSS v19.0; IBM, Armonk, NY). Data distributions were tested for normality by using the Shapiro-Wilk test. Subgroup comparisons between the control group and the ADHD subgroups were conducted by using one-way analysis of variance for normally distributed measures, the Kruskal-Wallis test for nonnormally distributed measures, and the Pearson χ2 for nominal measures. Effect sizes were reported with eta-squared (η2), which is the proportion of the total variation in brain, blood, or clinical metrics that is accounted for by group effects; η2 = 0.01, 0.06, and 0.14 correspond to small, medium, and large effects, respectively. One-way analysis of variance post hoc analyses (ie, control group vs ADHD-nonmedicated subgroup, control group vs ADHD-medicated subgroup, ADHD-nonmedicated subgroup vs ADHD-medicated subgroup) were performed with Games-Howell tests; the Cohen d provides the measure of effect size. Kruskal-Wallis post hoc analyses were conducted with two-tailed Mann-Whitney U tests; the rank-biserial correlation (rrb) reflects the effect size. A sequential Bonferroni-type false discovery rate (FDR) correction method was conducted to correct for multiple comparisons wherein an FDR-corrected P value significance threshold is calculated (31). For each metric, the FDR approach was applied over the entire set of P values from all omnibus tests (eg, MFC in the globus pallidus, putamen, caudate nucleus, and thalamus) and over the entire set of P values from all pairwise posthoc analyses (eg, MFC in ROIs with significant omnibus tests). P < .05 (FDR corrected) was indicative of a statistically significant difference.
Results
Demographics
The control group (n = 27) and ADHD group (n = 22) did not significantly differ with regard to age, sex distribution, IQ (Table E2 [online]), or parent-identified ethnicity (Appendix E1 [online]). The ADHD group was comprised of 12 medication-naïve patients (ADHD-nonmedicated subgroup, 55%) and 10 patients with a history of psychostimulant treatment (ADHD-medicated subgroup, 45%). Of the 10 patients with a history of psychostimulant treatment, medications taken at the time of the study included amphetamines (n = 3, 30%), methylphenidate with past amphetamines (n = 1, 10%), amphetamines with past atomoxetine and bupropion (n = 1, 10%), and atomoxetine with past amphetamines, escitalopram, and sertraline (n = 1, 10%). Four ADHD patients were off medication but had a history of taking methylphenidate (n = 3, 30%) or amphetamines (n = 1, 10%). The ADHD subgroups did not differ significantly with regard to age, IQ, ADHD symptom ratings, sex distribution, ADHD subtype, or comorbidity (Table 1, Appendix E1 [online]). Both ADHD subgroups differed significantly in symptom ratings from the control group but not in age, sex distribution, or IQ (Table 1).
Table 1.
Demographic Characteristics
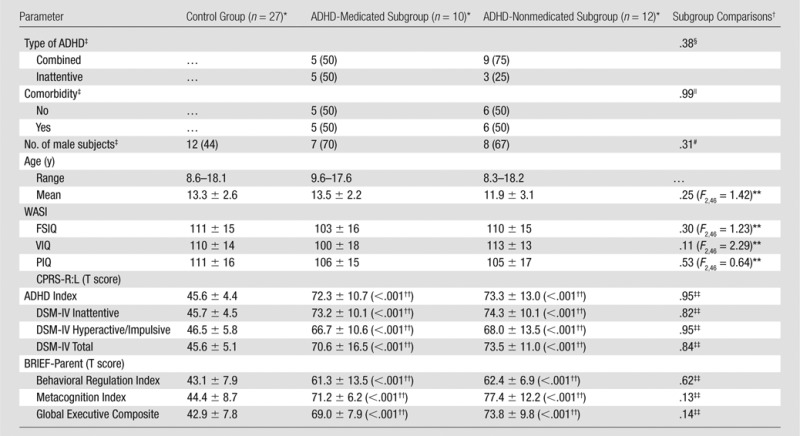
Note.—BRIEF-Parent = Behavioral Rating Inventory of Executive Function-Parent Version, CPRS-R:L = Conners Parent Rating Scale-Revised-Long Version, FSIQ = full scale IQ, PIQ = performance IQ, VIQ = verbal IQ, WASI = Wechsler Abbreviated Scale of Intelligence.
Except where indicated, data are means ± standard deviations.
Except where indicated, data are P values.
Data are numbers of subjects, with percentages in parentheses.
Fisher exact test (two sided: ADHD-medicated subgroup vs ADHD-nonmedicated subgroup).
Pearson χ2 (df = 1, n = 22) = 0.0 (two sided: ADHD-medicated subgroup vs ADHD-nonmedicated subgroup).
Exact Pearson χ2 (df = 2, n = 49) = 2.8 (two sided: control group, ADHD-medicated subgroup, ADHD-nonmedicated subgroup).
One-way analysis of variance (control group, ADHD-medicated subgroup, ADHD-nonmedicated subgroup).
Two-tailed Mann-Whitney U test P values (ADHD subgroup vs control group) following Kruskal-Wallis test, P < .001 (control group, ADHD-medicated subgroup, ADHD-nonmedicated subgroup).
Two-tailed Mann-Whitney U test (ADHD-medicated subgroup vs ADHD-nonmedicated subgroup).
Brain Iron
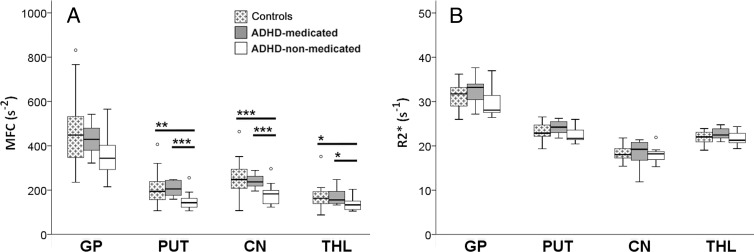
MFC data for all regions except the thalamus were normally distributed for the control and ADHD groups (Appendix E1 [online]). Analysis of variance showed a significant group main effect on MFC in bilateral putamen (P = .024, η2 = 0.15) and caudate nucleus (P = .015, η2 = 0.17). Post hoc analyses indicated that the ADHD-nonmedicated subgroup had significantly lower MFC than the control group in the putamen (P = .012, d = 1.0) and caudate nucleus (P = .008, d = 1.1). Compared with the ADHD-medicated subgroup, the ADHD-nonmedicated subgroup also had significantly lower MFC in the putamen (P = .006, d = 1.5) and caudate nucleus (P = .010, d = 1.4). Within these regions, the ADHD-medicated subgroup did not significantly differ from the control group in MFC (Figs 1, A, 2, A). All significant findings survived FDR correction (Table 2).
Figure 2:
Subgroup comparisons of MFC and R2* brain iron indexes. Box and whisker plots show subgroup variations in indexes (medians, 25th and 75th percentiles, minimum, maximum, outliers). CN = caudate nucleus, GP = globus pallidus, PUT = putamen, THL = thalamus. A, Subgroup comparisons of MFC in globus pallidus, putamen, caudate nucleus, and thalamus show a significant reduction in mean striatal (putamen and caudate nucleus) and median thalamic MFC in ADHD-nonmedicated subgroup compared with control group and ADHD-medicated subgroup. No significant differences in MFC were found between control group and ADHD-medicated subgroup. B, Subgroup comparisons of R2* indexes in all regions did not show any significant differences in means. * = P < .05, ** = P < .01, *** = P < .005 (all FDR corrected).
Table 2.
Subgroup Comparisons: MFC Indexes of Brain Iron
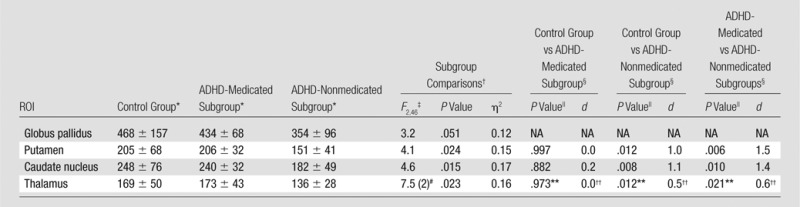
Note.—NA = not applicable.
Data are mean MFCs (in seconds−2) ± standard deviations.
Subgroup comparisons (FDR corrected, P ≤ .024).
One-way analysis of variance.
Post hoc multiple comparisons (FDR corrected, P ≤ .025).
Games-Howell test.
Kruskal-Wallis test. Data are χ2, with df in parentheses.
Two-tailed Mann-Whitney U test.
Rank-biserial correlation (rrb).
With the Kruskal-Wallis test, a significant group main effect was detected on MFC in bilateral thalamus (P = .023, η2 = 0.16). Results of post hoc analyses indicated that the MFC in the ADHD-nonmedicated subgroup (median, 134 seconds−2; range, 105–204 seconds−2) was significantly lower than that in the control group (median, 164 seconds−2; range, 88–352 seconds−2) in thalamus (U = 79.0, P = .012, rrb = 0.5). Compared with the ADHD-medicated subgroup (median, 156 seconds−2, range, 133–248 seconds−2), the ADHD-nonmedicated subgroup also had significantly lower MFC in the thalamus (U = 25.0, P = .021, rrb = 0.6). The MFC within the thalamus in the ADHD-medicated subgroup did not significantly differ from that in the control group (Figs 1, A, 2, A). All significant findings survived FDR correction (Table 2). Similar group differences in the putamen, caudate nucleus, and thalamus were detected even in MFC restricted to voxels dominated by microscopic MFC contributions (Table E3, Fig E3, A [online]).
In contrast, no significant differences were detected between the control group and the ADHD subgroups with use of R2, R2* (Figs 1, A, 2, B), and R2′ indexes (Table 3; Figs E2, E3, B, E3, C [online]). For all MR imaging data, there were no significant group differences in head motion (Table E4 [online]).
Table 3.
Subgroup Comparisons: R2, R2*, R2’ Indexes of Brain Iron
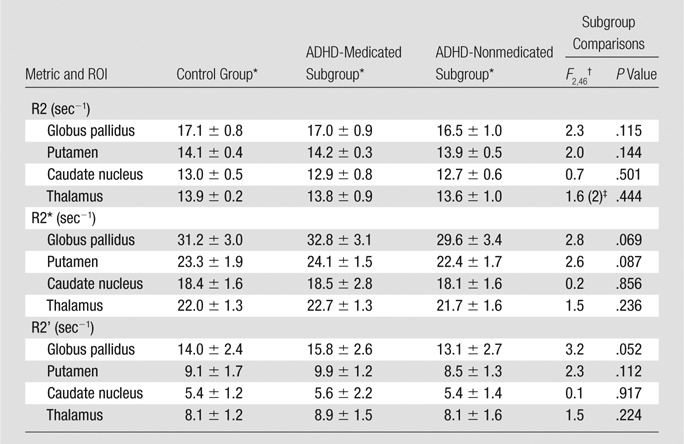
Data are means ± standard deviations.
Subgroup comparisons (control group, ADHD-medicated subgroup, and ADHD-nonmedicated subgroup) were performed with one-way analysis of variance.
Subgroup comparison (control group, ADHD-medicated subgroup, and ADHD-nonmedicated subgroup) was performed with the Kruskal-Wallis test. Data are χ2 , with df in parentheses.
Serum Iron
No significant differences were detected between the control group and the ADHD subgroups with regard to serum iron measures (even when normalized for age and sex), the number of control subjects versus ADHD patients with ferritin levels of less than normal cut-off values from the literature (8), or dietary intake of multivitamins or heme or nonheme iron (Tables E5, E6 [online]).
Discussion
In this study, we demonstrated that medication-naïve ADHD patients displayed significantly reduced MFC indexes of striatal and thalamic brain iron compared with control subjects and psychostimulant-medicated ADHD patients; these differences could not be detected with relaxation rates. Alternatively, medicated ADHD patients and control subjects did not differ significantly with regard to any brain iron index. These findings implicate reduced brain iron in ADHD pathophysiology before medication that appears to normalize with psychostimulants. If we consider that psychostimulants reduce ADHD symptoms by predominantly increasing striatal dopamine (1,4,32) and that our results parallel previous molecular imaging findings of reduced striatal dopamine biomarkers in medication-naïve ADHD patients (3,5,33,34) and greater dopamine biomarkers in those treated with psychostimulants (3), brain iron levels in ADHD may indirectly reflect the disrupted dopamine metabolic pathway targeted by psychostimulant treatment (1,4).
A fundamental relationship between brain iron and the dopaminergic system has been extensively demonstrated. Changes in brain iron and/or dopaminergic functional units are associated with alterations in motivation, attention, working memory, and motor control (11–13,35). This behavioral overlap may be due to iron’s involvement in maintaining dopamine homeostasis and may explain why high concentrations of both brain iron and dopamine are co-localized to the same basal ganglia regions (11,29). Specifically, brain iron is required for the rate-limiting step in catecholamine synthesis because it is an essential cofactor for tyrosine hydroxylase, which converts the precursor for dopamine and noradrenaline (9,10). Tyrosine hydroxylase–bound iron is also involved with a regulatory feedback mechanism that inhibits unnecessary catecholamine synthesis (9,10).
Studies have also shown that catecholamine levels are sensitive to changes in brain iron homeostasis. Rodent models of diet-induced iron deficiency have reduced brain iron levels in the striatum that coincide with reduced dopamine transporters, increased extracellular dopamine, and reduced dopamine receptors (11,13). These aberrant dopaminergic features were found only in brain regions with atypical brain iron levels and were associated with deficits in dopamine-dependent behaviors (11,13), which improved after psychostimulant treatment (6). In humans, evidence linking atypical brain iron levels with changes in the dopaminergic system has been observed in restless leg syndrome (36), a sensorimotor disorder that shows high comorbidity with ADHD (8). Along with the successful use of iron supplementation or dopaminergic drugs (36) to relieve restless leg syndrome symptoms, a reduction in brain iron (36) and dopaminergic abnormalities (36–38) have also been well documented.
Although there is still debate as to how psychostimulants modulate dopamine signaling in ADHD (32), Volkow et al (1) suggest that psychostimulants increase both tonic and phasic dopamine cell firing to amplify a weak striatal dopamine signal in ADHD. This weak dopamine signal may be due to multiple factors, including reduced dopamine synthesis and postsynaptic receptors as well as increased dopamine transporters and presynaptic autoreceptor activity. Given brain iron’s essential role in dopamine synthesis (9,10), its reduced striatal and thalamic levels in medication-naïve ADHD patients may reflect reduced dopamine availability and are consistent with the only ADHD MR imaging study of brain iron. In a study of predominantly medication-naïve children with ADHD, Cortese et al (7) associated reduced thalamic brain iron, as indexed with use of R2*, to the “hypoarousal” theory of ADHD because the thalamus is involved in cortical arousal via thalamocortical connections; similar trends were observed within the striatum.
Less is known about how the dopaminergic system adapts to long-term psychostimulant treatment; however, there is evidence suggesting that increased striatal dopamine transporters in ADHD may result from psychostimulant treatment (3). Increased dopamine transporters may reflect a compensatory response to chronic striatal dopamine transporter blockage or upregulation of dopamine (4). Findings from animal and postmortem human cocaine studies corroborate that long-term psychostimulant use results in increased dopamine transporters and greater dopamine reuptake (39). Consistent with this adaptation theory, we found that striatal and thalamic brain iron levels in psychostimulant-medicated ADHD patients were comparable to those in control subjects and significantly greater than those in medication-naive ADHD patients. If our results are replicated and striatal iron levels are found to increase in response to psychostimulant treatment, understanding the physiologic mechanisms of such putative compensation would become an important scientific priority.
The relaxation rates also displayed similar trends to the MFC indexes of brain iron but were not statistically significant. This is likely due to differences in the indexes’ sensitivity and specificity for brain iron (14,15). In contrast, similar group differences were detected even in MFC restricted to voxels dominated by microscopic MFC contributions (eg, iron-rich glial cells [17]), which verifies that the observed differences were, at least in part, intrinsic to the striatum and thalamus rather than being solely due to macroscopic gradients. Also, in agreement with the largest controlled study of serum ferritin levels in medication-naïve children with ADHD (40), none of the serum iron measures differed between control subjects and ADHD patients—even when accounting for possible confounders such as dietary iron intake. Mechanisms for iron absorption into the brain may therefore be aberrant in ADHD even when serum iron levels are normal. Finally, head motion and comorbidity unlikely biased our results as no significant differences in head motion were detected between cohorts and as comorbidity distribution was the same between ADHD subgroups.
Our study has limitations. First, we restricted our analysis to the dopaminergic system in basal ganglia regions, where substantial molecular imaging evidence has been accumulated (33) and wherein our MR imaging indexes of brain iron reflect putative brain iron levels (17,18). Second, we cannot assert that findings in the ADHD-medicated subgroup only reflect psychostimulant-specific changes because two patients also had a history of nonpsychostimulant medication. Third, given the cross-sectional study design, we could not demonstrate a causal relationship of the medication effects observed in the psychostimulant-medicated patients. Fourth, because treatment duration (ie, start and end dates) was not systematically documented, we could not assess whether medication effects on brain iron were dependent on treatment duration. Finally, because of the modest sample sizes, chance variation in the ADHD subgroups could also explain our findings; however, this is unlikely because all significant findings had large effect sizes and survived multiple comparison corrections.
In conclusion, our results implicate reduced striatal and thalamic brain iron levels in ADHD pathophysiology before medication and suggest that reduced brain iron levels may normalize with psychostimulant treatment. To our knowledge, these findings have not previously been established in the literature. When accounting for medication status, brain iron levels in ADHD parallel dopamine biomarker levels detected in molecular imaging studies (3). Although larger longitudinal studies examining pre- and postpsychostimulant treatment (accounting for comorbidity and treatment duration) are needed to validate our preliminary findings, these observations support the dopamine-deficiency theory of ADHD (1,2,5,33,34) and reinforce a fundamental brain iron-dopamine relationship (6,9,10,13,37). Thus, assessment of baseline brain iron levels with MFC imaging may provide an alternative noninvasive diagnostic biomarker for ADHD that responds to psychostimulant treatment.
Advances in Knowledge
■ Medication-naïve patients with attention deficit hyperactivity disorder (ADHD) had significantly lower striatal (putamen, caudate nucleus) and thalamic magnetic field correlation (MFC) indexes of brain iron than did typically developing control subjects (putamen, P = .012; caudate, P = .008; thalamus, P = .012) and psychostimulant-medicated ADHD patients (putamen, P = .006; caudate, P = .010; thalamus, P = .021).
■ MFC indexes in ADHD patients with a history of psychostimulant treatment were comparable to those in control subjects (P > .05).
■ No significant differences in brain iron were detected between control subjects and ADHD patients with use of R2, R2*, and R2′ (P > .05), which suggests that advanced MR imaging indexes (eg, MFC) may provide sensitive and more specific measures of brain iron than conventional water proton relaxation rates.
■ No significant differences in serum iron measures (P > .05) were detected between control subjects and ADHD patients, which suggests that mechanisms for iron absorption into the brain may be aberrant in ADHD even when serum iron levels are normal.
Implication for Patient Care
■ Noninvasive MFC detection of low brain iron in medication-naïve ADHD patients may help inform the clinical diagnosis.
PODCAST
APPENDIX
SUPPLEMENTAL FIGURES
SUPPLEMENTAL TABLES
Acknowledgments
Acknowledgments
We thank Jane Kwon, BS, for her guidance on diet questionnaires along with Rebecca Grzadzinski, BA, for both their assistance with participant recruitment. Additional thanks go to Heather Collins, PhD, for her guidance on statistical analyses.
Received January 8, 2014; revision requested February 19; revision received March 11; accepted April 3; final version accepted April 10.
Funding: This research was supported by the National Institutes of Health (grant 1RO1EB007656).
From the 2013 RSNA Annual Meeting.
J.A.H. supported by the Litwin Foundation.
Disclosures of Conflicts of Interest: V.A. No relevant conflicts of interest to disclose. J.H.J. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: author is inventor of patents owned by New York University and licensed to Siemens Heathcare. A.T. No relevant conflicts of interest to disclose. R.L.D. No relevant conflicts of interest to disclose. E.F. No relevant conflicts of interest to disclose. A.D.M. No relevant conflicts of interest to disclose. K.M.G. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: received a grant from Merck and Supernus Pharmaceuticals. Other relationships: none to disclose. F.X.C. No relevant conflicts of interest to disclose. J.A.H. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: has patents licensed to Siemens Medical.
Abbreviations:
- ADHD
- attention deficit hyperactivity disorder
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, fourth edition
- FDR
- false discovery rate
- IQ
- intelligence quotient
- MFC
- magnetic field correlation
- ROI
- region of interest
References
- 1.Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry 2005;57(11):1410–1415. [DOI] [PubMed] [Google Scholar]
- 2.Swanson JM, Kinsbourne M, Nigg J, et al. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev 2007;17(1):39–59. [DOI] [PubMed] [Google Scholar]
- 3.Fusar-Poli P, Rubia K, Rossi G, Sartori G, Balottin U. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. Am J Psychiatry 2012;169(3):264–272. [DOI] [PubMed] [Google Scholar]
- 4.Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology 2011;36(1):207–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkow ND, Wang GJ, Kollins SH, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA 2009;302(10):1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohamed WM, Unger EL, Kambhampati SK, Jones BC. Methylphenidate improves cognitive deficits produced by infantile iron deficiency in rats. Behav Brain Res 2011;216(1):146–152. [DOI] [PubMed] [Google Scholar]
- 7.Cortese S, Azoulay R, Castellanos FX, et al. Brain iron levels in attention-deficit/hyperactivity disorder: a pilot MRI study. World J Biol Psychiatry 2012;13(3):223–231. [DOI] [PubMed] [Google Scholar]
- 8.Cortese S, Angriman M, Lecendreux M, Konofal E. Iron and attention deficit/hyperactivity disorder: what is the empirical evidence so far? A systematic review of the literature. Expert Rev Neurother 2012;12(10):1227–1240. [DOI] [PubMed] [Google Scholar]
- 9.Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 2011;508(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsey AJ, Hillas PJ, Fitzpatrick PF. Characterization of the active site iron in tyrosine hydroxylase: redox states of the iron. J Biol Chem 1996;271(40):24395–24400. [DOI] [PubMed] [Google Scholar]
- 11.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr 2003;23:41–58. [DOI] [PubMed] [Google Scholar]
- 12.McCann JC, Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr 2007;85(4):931–945. [DOI] [PubMed] [Google Scholar]
- 13.Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr 2011;141(4):740S–746S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dusek P, Dezortova M, Wuerfel J. Imaging of iron. Int Rev Neurobiol 2013;110:195–239. [DOI] [PubMed] [Google Scholar]
- 15.Haacke EM, Cheng NY, House MJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 2005;23(1):1–25. [DOI] [PubMed] [Google Scholar]
- 16.Jensen JH, Chandra R, Ramani A, et al. Magnetic field correlation imaging. Magn Reson Med 2006;55(6):1350–1361. [DOI] [PubMed] [Google Scholar]
- 17.Jensen JH, Szulc K, Hu C, et al. Magnetic field correlation as a measure of iron-generated magnetic field inhomogeneities in the brain. Magn Reson Med 2009;61(2):481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adisetiyo V, Jensen JH, Ramani A, et al. In vivo assessment of age-related brain iron differences by magnetic field correlation imaging. J Magn Reson Imaging 2012;36(2):322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raz E, Jensen JH, Ge Y, et al. Brain iron quantification in mild traumatic brain injury: a magnetic field correlation study. AJNR Am J Neuroradiol 2011;32(10):1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumas EM, Versluis MJ, van den Bogaard SJ, et al. Elevated brain iron is independent from atrophy in Huntington’s disease. Neuroimage 2012;61(3):558–564. [DOI] [PubMed] [Google Scholar]
- 21.Ge Y, Jensen JH, Lu H, et al. Quantitative assessment of iron accumulation in the deep gray matter of multiple sclerosis by magnetic field correlation imaging. AJNR Am J Neuroradiol 2007;28(9):1639–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler abbreviated scale of intelligence (WASI-II). San Antonio, Tex: Psychological Corporation, 1999. [Google Scholar]
- 23.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Arlington, Va: American Psychiatric Association, 2000. [Google Scholar]
- 25.Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Diagnostic interview: Kiddie-Sads-present and lifetime version (K-SADS-PL)—screen interview. University of Pittsburgh Department of Psychiatry Web site. http://psychiatry.pitt.edu/node/8233. Published October 1996. Accessed June 2006. [Google Scholar]
- 26.Conners CK. Conners rating scales–revised: technical manual. North Tonawanda, NY: Multi-Health Systems, 1997. [Google Scholar]
- 27.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol 2000;6(3):235–238. [DOI] [PubMed] [Google Scholar]
- 28.Kim D, Jensen JH, Wu EX, Sheth SS, Brittenham GM. Breathhold multiecho fast spin-echo pulse sequence for accurate R2 measurement in the heart and liver. Magn Reson Med 2009;62(2):300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem 1958;3(1):41–51. [DOI] [PubMed] [Google Scholar]
- 30.Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods 2005;142(1):67–76. [DOI] [PubMed] [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57(1):289–300. [Google Scholar]
- 32.Engert V, Pruessner JC. Dopaminergic and noradrenergic contributions to functionality in ADHD: the role of methylphenidate. Curr Neuropharmacol 2008;6(4):322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry 2011;69(12):e145–e157. [DOI] [PubMed] [Google Scholar]
- 34.Del Campo N, Müller U, Sahakian BJ. Neural and behavioral endophenotypes in ADHD. Curr Top Behav Neurosci 2012;11:65–91. [DOI] [PubMed] [Google Scholar]
- 35.Egerton A, Mehta MA, Montgomery AJ, et al. The dopaminergic basis of human behaviors: a review of molecular imaging studies. Neurosci Biobehav Rev 2009;33(7):1109–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen RP, Earley CJ. The role of iron in restless legs syndrome. Mov Disord 2007;22(Suppl 18):S440–S448. [DOI] [PubMed] [Google Scholar]
- 37.Rizzo G, Tonon C, Manners D, Testa C, Lodi R. Imaging brain functional and metabolic changes in restless legs syndrome. Curr Neurol Neurosci Rep 2013;13(9):372–378. [DOI] [PubMed] [Google Scholar]
- 38.Connor JR, Wang XS, Allen RP, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain 2009;132(Pt 9):2403–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci 2010;1187:316–340. [DOI] [PubMed] [Google Scholar]
- 40.Donfrancesco R, Parisi P, Vanacore N, Martines F, Sargentini V, Cortese S. Iron and ADHD: time to move beyond serum ferritin levels. J Atten Disord 2013;17(4):347–357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.