Abstract
Importance
Several management strategies may improve outcomes in patients with Staphylococcus aureus bacteremia (SAB). The strength of evidence supporting these management strategies, however, varies widely.
Objective
To perform a systematic review of the evidence for two unresolved questions involving management strategies for SAB: 1) is transesophageal echocardiography (TEE) necessary in all cases of SAB; and 2) what is the optimal antibiotic therapy for methicillin resistant Staphylococcus aureus (MRSA) bacteremia?
Evidence acquisition
A PubMed search from inception through May 2014 was performed to find studies that addressed the role of TEE in SAB. A second search of PubMed, EMBASE, and The Cochrane Library from 1/1/1990 to 5/28/2014 was performed to find studies that addressed antibiotic treatment of MRSA bacteremia. Studies that reported outcomes of systemic antibiotic therapy for MRSA bacteremia were included. All searches were augmented by review of bibliographic references from included studies. The quality of evidence was assessed using the GRADE system by consensus of independent evaluations by at least two authors.
Results
In 9 studies with a total of 3513 patients, use of TEE was associated with higher rates of diagnosis of endocarditis (14–25%) when compared with TTE (2–14%). Five studies proposed criteria to identify patients in whom TEE might safely be avoided. Only one high-quality trial of antibiotic therapy for MRSA bacteremia was identified from the 83 studies considered.
Conclusions and relevance
Most contemporary management strategies for SAB are based upon low quality evidence. TEE is indicated in most patients with SAB. It may be possible to identify a subset of SAB patients for whom TEE can be safely avoided. Vancomycin and daptomycin are the first-line antibiotic choices for MRSA bacteremia. Well-designed studies to address the management of SAB are desperately needed.
Introduction
Case fatality rates for Staphylococcus aureus bacteremia (SAB) have improved only modestly in recent decades. The emergence of methicillin resistance in S. aureus complicates therapy and is an independent risk factor for mortality in SAB.1,2 Several management strategies for SAB are accepted as standard of care,3–5 including a) performing a thorough history and physical examination; b) obtaining follow-up blood cultures to document resolution of bacteremia; and c) draining abscesses and removing infected prosthetic material.
Other SAB management strategies are unresolved. Despite almost 20 years of deliberation and at least three major treatment guidelines,3–5 the ideal role of transesophageal echocardiography (TEE) in the management of SAB remains unresolved. The optimal antibiotic therapy of MRSA bacteremia is also unresolved, particularly for infections due to MRSA isolates with high, but still susceptible minimum inhibitory concentrations to vancomycin.6 Thus, this manuscript systematically reviews the evidence informing two key questions:
Do all patients with SAB require transesophageal echocardiography (TEE)?
What is the optimal antibiotic therapy for MRSA bacteremia?
Methods
Do all patients with SAB require transesophageal echocardiography (TEE)?
To evaluate the evidence surrounding this question, PubMed was queried from inception to May 2014 for the following terms: Staphylococcus aureus or MRSA, echocardiography, and bacteremia. Queries were limited to English language studies that involved adults only. References of included studies were also searched. The abstracts of studies being considered for inclusion were reviewed independently by two authors (TH, CA). To be included, studies had to specifically address the role of TEE in SAB. Reviews, editorials, case reports, studies reporting data that were included in part within previous publications, and studies not reporting echocardiography results by organism were excluded. Those remaining were selected for full text review.
What is the optimal antibiotic therapy for MRSA bacteremia?
To evaluate the final question, searches were performed on PubMed, EMBASE, and The Cochrane Library from January 1990 to May 2014 for the following terms: Staphylococcus aureus or MRSA; bacteremia or bloodstream infection; antibiotic or antimicrobial; vancomycin, daptomycin, linezolid, teicoplanin, trimethoprim-sulfamethoxazole (TMP/SMX), clindamycin, quinupristin-dalfopristin, tigecycline, ceftaroline, telavancin, dalbavancin, oritavancin, or tedizolid. In addition, www.ClinicalTrials.gov was queried for bacteremia and methicillin-resistant Staphylococcus aureus or MRSA. Search results were limited to English-language studies in adults. Finally, the references of included studies were also reviewed. Studies that reported outcomes of systemic antibiotic therapy for MRSA bacteremia were included for review. Case reports involving fewer than 5 patients with MRSA bacteremia, review articles, editorials, guidelines, and studies reporting duplicate data or subgroup analyses of earlier published studies were excluded.
Grading and review of studies
The level of evidence of reviewed studies was assigned a score of high, moderate, low, or very low based upon the GRADE system.7 Studies were graded by consensus of independent reviews by two authors (TH, CA). Studies for which the two original scores disagreed underwent a resolution review by a third author (VGF). For the antibiotic therapy question, studies in which only a subset of included patients had MRSA bacteremia were graded upon the quality of evidence for MRSA bacteremia specifically.
Results of Evidence Review
Do all patients with SAB require transesophageal echocardiography (TEE)?
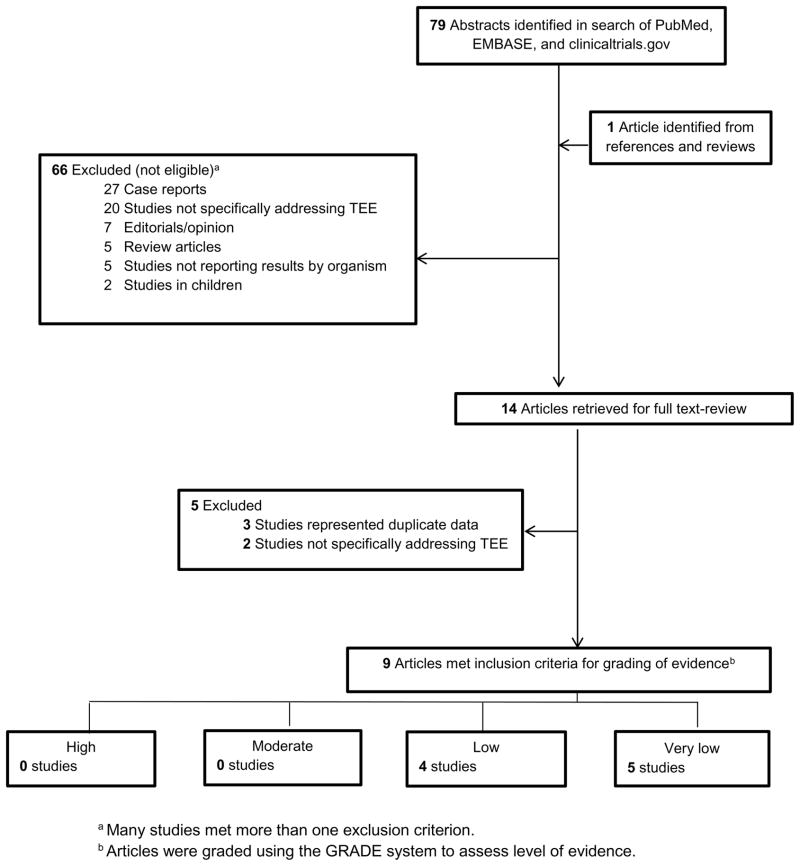
Of the 79 publications identified by search definitions, 14 met inclusion criteria. Five were subsequently excluded based on the full-text review (Figure 1), yielding 9 studies that underwent quality assessment review. The independent quality assessments agreed in all 9 cases. All studies were observational (Table 1). Sample sizes of included studies ranged from 98 to 877 patients. TEE was performed in 12–63% of these patients. Endocarditis was defined in all studies via either the original8 or modified9 Duke criteria. Among the 4 studies10–13 that evaluated endocarditis rates by both transthoracic (TTE) and TEE, detection of infective endocarditis (IE) was higher with TEE (14–25%) than with TTE (2–14%).
Figure 1.
Identification of Manuscripts for Systematic Review of the Role of Transesophageal Echocardiogram for Patients with S. aureus Bacteremia.
Table 1.
Role of Transesophageal Echocardiography in S. aureus Bacteremia
| Author, year | GRADE category | Study Design | Study population | Total # SAB cases | # IE cases | # with TEE (%) | IE # and rate by TTE | IE # and rate by TEE | Key Outcomes | Risk stratification |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies which suggest that TEE should be required for all SAB cases | ||||||||||
| Fowler, 199710 | Low | Prospective cohort | Adults with SAB (age 56 ± 15) who underwent both TTE and TEE PV=5, CD=4 |
176 | 26 | 103 (58%) | 7 (7%) | 26 (25%) | TEE(+) in 19 (19%) of TTE(−)patients | Clinical findings and TTE results did not predict TEE results |
| Sullenberger, 200511 | Very low | Retrospective cohort | Adults with SAB (age 56.5 ± 19.1) who underwent TEE PV=1, CD=0 |
176 | 11 | 64 (36%) | 1 (2%) | 9 (14%) | 0/9 TEE (+)patients had (+)TTE 1 TTE(+)/TEE(−)patient |
Clinical findings and TTE results did not predict TEE results |
| Incani, 201314 | Low | Prospective cohort | Adults with SAB (age 68, IQR 53– 76) who underwent TEE PV=9, CD=7 |
230 | 41 | 144 (63%) | N/A | 41 (29%) | 19 (46%) IE cases not suspected clinically | Clinical findings did not predict TEE results |
| Holden, 201415 | Very low | Prospective cohort | Adults with SAB (age 62 [19–100]) PV=0, CD=4 |
98 | 13 | 58 (59%) | 3 (14%) | 9 (16%) | 6/13 (46%) IE cases had no risk factors | Clinical findings did not predict TEE findings |
| Studies which suggest that TEE may be unnecessary in some SAB cases | ||||||||||
| Van Hal, 200512 | Very low | Retrospective cohort | Adults without cardiac prostheses with SAB (age: non-IE 61.4 [22–92], IE 56.3 [28–84]) who underwent both TTE and TEE PV=0, CD=0 |
808 | 22 | 125 (15%) | 18 (14%) | 20 (16%) | 2 IE cases had both (−)TTE and (−)TEE 2/125 patients had (−)TTE, (+)TEE |
Proposed low-risk group:
|
| Kaasch, 201116 | Low | Prospective cohorts (2 separate cohorts) | Hospitalized patients (age: INSTINCT cohort 67 [21–91], SABG cohort 65 [15–95]) with nosocomial SAB PV=43, CD=92 |
736 | 53 | 175 (24%) | N/A | N/A | Low-risk criteria patients: only 1/208 had IE 52/53 patients with IE fulfilled at least one “high risk criteria” |
Proposed low-risk group:
|
| Rasmussen, 201117 | Low | Prospective cohort | Adults with SAB (age: IE 65 ± 16, non-IE 64 ± 16) who underwent echocardiography PV=20, CD=14 |
244 | 53 | 152 (62%) | N/A | N/A | 47 (87%) IE cases: predicted by “high risk” criteria 6 IE cases missed by “high-risk” criteria: 4: (+)TTE/ (+)TEE 2: (−)TTE/ (+)TEE |
Proposed low-risk group:
|
| Joseph, 201318 | Very low | Retrospective cohort | Hospitalized patients (age: IE 50.7 ± 3.6, non-IE 61.1 ± 1.1) with SAB PV=20, CD=14 |
668 | 31 | 82 (12%) | N/A | N/A | Highest rate IE in:
|
Proposed low-risk group:
|
| Khatib, 201313 | Very low | Retrospective cohort | Adults (age N/A) with SAB CD=104a |
877 | 64 | 177 (20%) | 25 (8%) | 42 (24%) | “Low-risk” group: only 1 patient with (+) TEE | Proposed low-risk group:
|
Abbreviations: SAB, Staphylococcus aureus bacteremia; IE, infective endocarditis; TTE, transthoracic echocardiogram; TEE; transesophageal echocardiogram; IQR, interquartile range; PV, prosthetic valve; CD, cardiac device
Included prosthetic valves, pacemakers, defibrillators
Two studies10,11 reported that clinical findings and TTE results were poorly predictive of subsequent TEE findings. In Fowler et al, TEE detected endocarditis in 19% of patients with a negative TTE.10 Among 230 Australian patients with SAB, 144 of whom underwent TEE, 46% of IE cases were not suspected on clinical grounds, and 15% of patients without clinical evidence of IE were reclassified by TEE.14 Most recently, 13 of 98 SAB patients (16%) were found to have IE in a “mandatory TEE” approach.15
Five studies12,13,16–18 to date have proposed that TEE might safely be avoided in SAB patients without any of several specific IE risk factors. Identified low risk factors include: a) absence of a permanent intracardiac device (e.g. prosthetic valve, pacemaker, cardioverter defibrillator),12,13,16–18 short duration of bacteremia, 13,16 no hemodialysis dependence,16 nosocomial acquisition of infection,17 absence of secondary foci of infection,13,16 and no evidence of embolic or immunologic phenomena. 12,17
Recommendation
Obtain an echocardiogram for all patients with SAB. TEE is preferred for most patients. TTE may be adequate for patients without identified risk factors for IE. (weak recommendation based on low quality evidence).
What is the optimal antibiotic therapy for MRSA bacteremia?
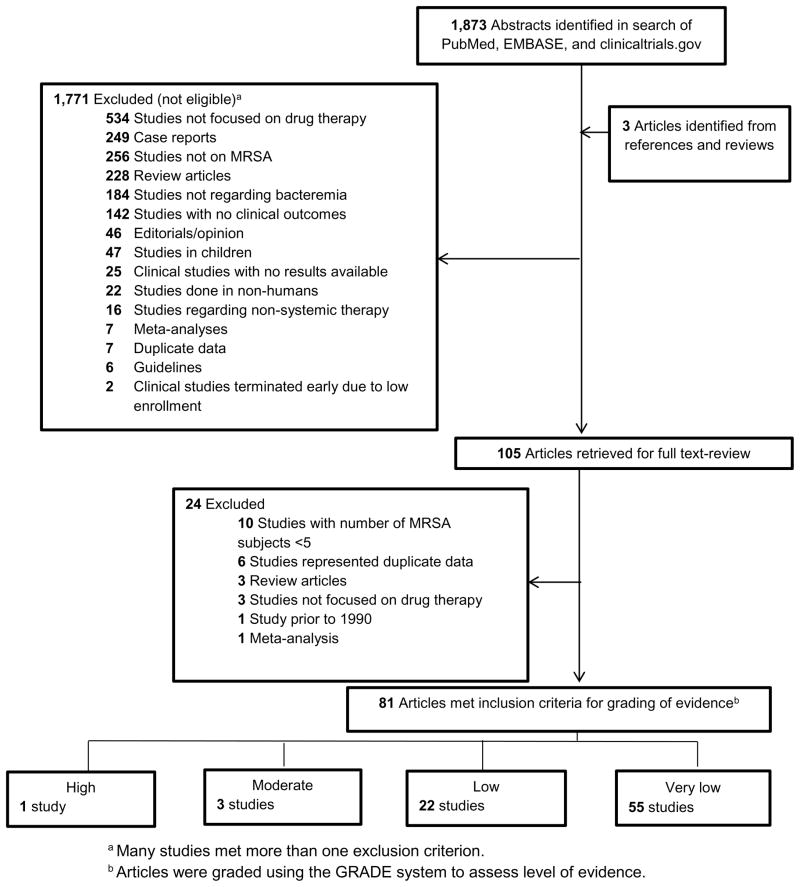
Of the 1876 publications identified by search definitions, 105 met inclusion criteria. Of these, 24 were subsequently excluded based on the full-text review (Figure 2), yielding 81 studies that underwent quality assessment review. The sample size of included studies ranged from 6 to 337. The independent quality assessments agreed in 68 of 81 cases (84%). All 13 discrepancies in assessment varied by one level of evidence, and 11 of the 13 differed between scores of very low and low. These 13 studies underwent resolution review to determine their final evidence grade.
Figure 2.
Identification of Manuscripts for Systematic Review of Antibiotic Therapy for Patients with MRSA Bacteremia.
Overall, data quality was poor. Only one met GRADE criteria for high quality evidence.19 Three were categorized as moderate,20–22 22 as low,23–44 and 55 as very low. Studies with a grade of high, moderate or low are summarized in Table 2. Study outcomes were variable and included mortality, clinical success (variably defined), microbiological success, duration of SAB, and recurrence.
Table 2.
Studies of Antibiotic Therapy in MRSA Bacteremia.
| Author, year |
GRADE category |
Population | Study design | Number of patients |
Treatment | Primary Endpoints | Results |
|---|---|---|---|---|---|---|---|
| VANCOMYCIN DOSING STUDIES | |||||||
| Kullar, 201123 | Low | Adults with MRSAB (age: vancomycin success 53 [45–64]; vancomycin failure 54 [46–61]) who received vancomycin >3 days as initial therapy | Retrospective cohort | 320 | Vancomycin, (attention to dosing regimens) | Treatment failure (30-day mortality, persistent infection or bacteremia ≥7 days) | 168/320 (52.5%) failure rate Independent predictors of failure: Vancomycin trough <15mg/L and MIC>1 |
| Moore, 201124 | Low | Adults with MRSAB (age 57 ± 17) treated with vancomycin | Retrospective cohort | 200 | Vancomycin | Predictors of clinical failure (30-day mortality, persistent bacteremia ≥7 days while on therapy, bacteremia recurrence within 30 days) | Predictors of failure:
|
| Hall, 201225 | Low | Adults with MRSAB (age: survivors 53 [42–63]; non-survivors 65 [56–77]) | Retrospective cohort | 337 | Vancomycin, (dosing of ≥15mg/kg vs <15mg/kg) | In-hospital mortality | Dosing not significantly associated with mortality |
| Forstner, 201326 | Low | Adults with MRSAB (age 64.5 [18–96]) | Retrospective cohort | 124 | Vancomycin (N=63), teicoplanin (N=28), linezolid (N=7), tigecycline (N=2), other (N=24) | 28-day mortality, persistent bacteremia ≥7 days, treatment failure | Vancomycin trough levels 15–20mg/L associated with lower odds of persistent bacteremia and treatment failure |
| DAPTOMYCIN | |||||||
| Fowler, 200619 | High | Adults with SAB (age: daptomycin 50.5 [21–87]; standard therapy 55 [25–91]) | Open-label RCT | 246 | Daptomycin 6mg/kg/day vs. initial low-dose gentamicin plus either an anti-staphylococcal penicillin or vancomycin | Treatment success 42 days after the end of therapy | Daptomycin non-inferior to vancomycin for SAB and right-sided endocarditis |
| Kullar, 201143 | Low | Adults with complicated Gram-positive infections (age 55 [45–65]) who received high-dose daptomycin | Retrospective cohort | 250 (126 MRSAB) | Daptomycin, (median dose 8.9mg/kg/day) | Clinical response (cure, improvement, or failure) Adverse events |
Cure rate for bacteremic patients was 175/218 (80.3%) 3/250 (1.2%) experienced adverse event attributed to high dose daptomycin |
| Moore, 201242 | Low | Adults with MRSAB (age: vancomycin 52 ± 14; daptomycin 51 ± 14) and vancomycin MIC 1.5 or 2 | Retrospective case-control | 177 | Daptomycin (N=59) vs. vancomycin (N=118) | Clinical failure (composite of 60-day mortality, persistent bacteremia ≥7 days, or recurrence within 30 days) | Non-significant trend toward lower failure rate with daptomycin (10/59 [17%] vs. 37/118 [31%], p=0.084) |
| Falcone, 201227 | Low | All staphylococcal invasive infections (age: daptomycin 67.2; vancomycin 66.7) | Retrospective case-control | 106 (57 bacteremia, 35 MRSAB) | Daptomycin (N=23), glycopeptide (N=34) | Duration of antibiotic therapy, length of stay, attributable mortality |
|
| Murray, 201328 | Low | Adults with MRSAB and vancomycin MIC>1 (age: daptomycin 57 [51–65]; vancomycin 56 [51–64]) | Retrospective matched cohort | 170 | Daptomycin or vancomycin | Clinical failure (composite of all-cause 30-day mortality or persistent bacteremia ≥7 days) | Higher risk of failure with vancomycin (OR 4.5, 95% CI 2.1–9.8) |
| Cheng, 201329 | Low | Adults with MRSAB (age N/A) and vancomycin MIC≥1.5 | Retrospective case-control | 78 | Daptomycin 8–10mg/kg or vancomycin | 14-day and 30-day clinical outcome (cure or improvement vs failure or death) | Daptomycin associated with favorable outcome (OR 0.27, 95% CI 0.084–0.857) |
| Carugati, 201330 | Low | Adults with Gram-positive left-sided endocarditis (age: daptomycin 62.5 [54–72.5]; standard therapy 60.5 [44–73]) | Prospective cohort | 178 (86 SAB, 25 MRSAB) | Daptomycin (N=29) vs. standard of care (N=149) | In-hospital mortality | Daptomycin (mean dose 9.2mg/kg/day) not associated with mortality (RR 0.8, 95% CI 0.4–1.3, p=0.35) |
| Weston, 2014 | Low | Adults with MRSAB (mean age 61) | Retrospective matched cohort | 150 | Daptomycin (N=50) vs. vancomycin (N=100) | Treatment failure (composite of in-hospital mortality, 30-day recurrence, or persistent bacteremia ≥ 5 days) | Daptomycin use not associated with treatment failure in patients with preserved (OR 0.45, 95% CI 0.11–1.79) or with impaired renal function (OR 0.46, 95% CI 0.11–1.94) |
| TEICOPLANIN | |||||||
| Menichetti, 199431 | Low | Adults with febrile neutropenia (age: teicoplanin 44 (14–78); vancomycin 42 (14–72)) | Open-label RCT | 635 (527 evaluable, 102 Gram-positive bacteremia, 12 MRSAB) | Vancomycin vs. teicoplanin, (each in combination with amikacin/ceftazi dime) | Success (resolution of signs of infection, eradication of organism) | No difference in rates of treatment success: both overall and among those with Gram-positive bacteremia |
| Yoon, 201432 | Low | Adults with healthcare-associated MRSAB (age 66 [51–73]) | Prospective cohort | 190 | Vancomycin (N=134) vs. teicoplanin (N=56) | Clinical failure (composite of MRSAB-attributed mortality, bacteremia duration ≥7 days, and/or fever duration ≥7 days) | Choice of antibiotic not associated with clinical failure |
| LINEZOLID | |||||||
| Birmingham, 200333 | Low | Patients with signs and symptoms of a serious infection (age: adults 55.8 [18–93]; children 8.7 [0.1–17]) | Open-label compassionate-use cohort | 796 (378 bacteremia, 14 evaluable MRSAB) | Linezolid | Clinical and microbiological outcome (cure, failure, or indeterminate) |
|
| Shorr, 200534 | Low | Adults with nosocomial pneumonia, cSSTI, or general MRSA infections who also had bacteremia (age: linezolid 63.5 ± 17.1; vancomycin 59.3 ± 18.9) | Retrospective pooled analysis of subgroups with bacteremia in 5 RCTs | 3228 in parent studies (144 SAB, 64 MRSAB) | Vancomycin 1g q12h vs. linezolid | Clinical cure, microbiologic success, survival | No significant differences in clinical cure, microbiologic success, or survival |
| Gomez, 200735 | Low | Patients with MRSAB (mean age: 60 [14–95]) | Prospective cohort | 100 | Vancomycin (N=49), teicoplanin (N=20), linezolid (N=17), other (N=14) | Influence of empiric antibiotic choice on mortality | Empiric therapy with linezolid: lower mortality than glycopeptides in bivariate analysis |
| Wilcox, 200936 | Low | Adults with suspected catheter-related infection (age: linezolid 53.7 ± 18.1; vancomycin 53.8 ± 17.6) | Open-label RCT | 739 (49 MRSAB in evaluable population) | Linezolid or vancomycin (or β-lactam for methicillin-susceptible pathogens) | Microbiologic outcome at test-of-cure |
|
| Park, 201237 | Low | Adults with persistent MRSAB (age: linezolid 63.7 ± 11.6; glycopeptide 62.4 ± 14.2) | Prospective cohort | 90 | Linezolid-based salvage therapy (with or without carbapenem) vs. continued glycopeptide | Early microbiologic response, duration of bacteremia, salvage success |
|
| TRIMETHOPRIM/SULFAMETHOXAZOLE | |||||||
| Markowitz, 199221 | Moderate | Adult IVDU with suspected SAB, without left-sided IE (age: TMP/SMX 32.6 [31.1–34.1]; vancoymcin 32.5 [30.7–34.3]) | Double-blind RCT | 228 (65 SAB, 38 MRSAB) | TMP/SMX 320/1600 q12h vs. vancomycin 1g q12h | Cure rate in those with S. aureus infection (not limited to bacteremia) |
|
| Goldberg, 201038 | Low | Adults with MRSAB (age: TMP/SMX 74.7 ± 15.9; vancomycin 75.8 ± 13.7) | Retrospective matched cohort | 114 | TMP/SMX (N=38) vs. vancomycin (N=76) | 30-day mortality, persistent bacteremia >14 days, relapse, adverse events | No significant differences in any of the outcomes |
| COMBINATION THERAPY | |||||||
| Levine, 199120 | Moderate | Adults with MRSA endocarditis (age 32 [23–61]) | Open-label RCT | 42 | Vancomycin 1g q12h vs. vancomycin 1g q12h + rifampin 600mg daily | Duration of bacteremia |
|
| Lemonovich, 201139 | Low | Adults with persistent SAB and/or S. aureus endocarditis (median age: aminoglycoside 58 [50–70]; no aminoglycoside 57 [53–71]) | Retrospective cohort | 87 (48 MRSAB) | Aminoglycoside use (N=49) vs. no aminoglycoside (N=38) | Incidence of recurrent SAB within 6 months, duration of bacteremia, 6-month mortality, incidence of complications of bacteremia, incidence of renal failure |
|
| Dilworth, 201440 | Low | Adults with MRSAB with vancomycin MIC≤2 (age: combination 51.6 ± 15; vancomycin 50.5 ± 16.8) | Retrospective cohort | 80 | Vancomycin vs. vancomycin/β-lactam combination therapy | Microbiological eradication (negative blood cultures and no relapse within 30 days of completing therapy) | Microbiological eradication more likely with combination therapy (AOR 11.24, 95% CI 1.72–144.34, p=0.01) |
| DALBAVANCIN | |||||||
| Raad, 200522 | Moderate | Adults with Gram(+) catheter-related bloodstream infection (age: dalbavancin 54 [20–78]; vancomycin 58 [19–85]) | Open-label RCT | 75 (14 MRSAB) | Dalbavancin vs. vancomycin | Overall efficacy at test-of-cure visit in microITT population | Overall success higher with dalbavancin (21/23 [87%] vs 14/28 [50%], p<0.05), in all study patients (not limited to MRSAB only) |
| TREATMENT DURATION | |||||||
| Chong, 201341 | Low | Adults with uncomplicated SAB (age: 60 [49.5–68]) | Prospective cohort | 111 (53 MRSAB) | Treatment duration <14 days vs. ≥14 days | Relapse, crude mortality, and 12-week treatment failure | Higher relapse (3/38 [7.9%] vs. 0/73 [0%], p=0.036) and non-significant trend toward increased treatment failure with short-course therapy |
Abbreviations: SAB; Staphylococcus aureus bacteremia; MSSA, methicillin susceptible Staphylococcus aureus bacteremia; MRSA, methicillin resistant Staphylococcus aureus; MRSAB, methicillin resistant Staphylococcus aureus bacteremia; MIC, minimum inhibitory concentration; RCT, randomized controlled trial; cSSTI, complicated skin and soft tissue infection; TMP/SMX, trimethoprim/sulfamethoxazole; microITT, microbiological intention-to-treat
Evidence for Vancomycin
Vancomycin was the comparator in most MRSA bacteremia antibiotic studies. In the sole high quality randomized control trial, vancomycin was part of standard therapy compared to daptomycin for patients with SAB with or without endocarditis.19 In that open-label clinical trial, for which the primary outcome was treatment success in the modified intention-to-treat population 42 days after completion of therapy, daptomycin was non-inferior (44.2% [53/120] vs 41.7% [48/115], absolute difference 2.4%, 95% CI −10.2 to 15.1%) to low-dose, short course gentamicin plus either an antistaphylcoccal penicillin (for MSSA bacteremia) or vancomycin (for MRSA bacteremia or penicillin-allergic patients). Vancomycin was also compared in open-label randomized trials to teicoplanin,31 TMP/SMX,21 linezolid,34,36 and dalbavancin.22 None of these antibiotics performed significantly better than vancomycin.
Evidence for Daptomycin
As noted above, daptomycin at a dose of 6mg/kg/day was not inferior to standard therapy for SAB and right-sided endocarditis.19 In the pre-defined subgroup of patients with MRSA bacteremia, the success rate was 20/45 (44.4%) among daptomycin recipients vs. 14/44 (31.8%) in patients who were randomized to receive standard therapy; this difference was not statistically significant (absolute difference 12.6%, 95% CI −7.4% to 32.6%, p=0.28). This study led to FDA approval of daptomycin for SAB and right-sided endocarditis. Presently, vancomycin and daptomycin are the only two agents approved for MRSA bacteremia.
Subsequent investigators have employed cohort 28,30 or case-control 27,29,42 studies to test the hypothesis that daptomycin, either at 28 or above 29,30 the FDA-approved dose of 6mg/kg daily, was associated with better clinical outcomes than vancomycin in patients with bacteremia due to MRSA with high vancomycin minimum inhibitory concentrations (MIC) values. In infections caused by MRSA with vancomycin MIC >1mg/dl, outcomes generally appeared better with daptomycin. In a prospective cohort study of patients with left-sided endocarditis, use of high-dose daptomycin (mean dose 9.2mg/kg/day) was not significantly associated with in-hospital mortality when compared to standard of care (daptomycin: 1/7 [14.3%] vs. standard of care: 8/18 [44.4%], p=0.35).30 Among a retrospective cohort of 250 patients with complicated Gram-positive infections, 126 of which were MRSA bacteremia, daptomycin at a mean dose of 8.9mg/kg/day was associated with a very low adverse event rate.43 Generalizability of these results was limited by suboptimal trial design and non-random receipt of antibiotics and Infectious Diseases consultation.
Evidence for Linezolid
Observations from a compassionate-use program suggested that linezolid might have utility for bacteremia.33 Shorr et al compiled data on patients with bacteremia from five earlier randomized trials comparing linezolid to vancomycin. Of 3228 enrolled patients in the original studies, 53 had MRSA bacteremia and were evaluable. Among these 53 patients, rates of clinical cure were not significantly different (14/25 [56%]) linezolid recipients vs.13/28 (46%) vancomycin recipients; OR 1.47, 95% CI 0.50–4.34).34 Linezolid was also compared to vancomycin in an open-label phase 3 non-inferiority study involving patients with suspected catheter-related bacteremia.36 Linezolid was non-inferior among patients with Gram-positive infections. However, patients in the linezolid group had a higher chance of death than did those in the comparator group. This led to an FDA “black box” warning advising against the empiric use of linezolid in catheter-related bacteremia, if Gram-negative infection is known or suspected.45 Linezolid was evaluated as a salvage agent for MRSA bacteremia persisting ≥7 days despite glycopeptide (e.g. vancomycin or teicoplanin) therapy. Outcomes including microbiologic response, treatment success, and mortality were not significantly different among those switched to linezolid versus those continued on glycopeptide therapy.37
Evidence for TMP/SMX
TMP/SMX was compared to vancomycin in a randomized trial of intravenous drug users with suspected SAB.21 Of 228 enrolled patients, 65 had SAB, of which 38 were due to MRSA. Overall, vancomycin was superior to TMP/SMX (98% vs. 86% cure rate; p=0.014). All failures in both groups were in patients with MSSA. More recently, 38 retrospectively-identified patients treated with TMP/SMX for MRSA bacteremia were compared to 76 matched controls who received vancomycin for the same diagnosis. Thirty-day mortality, relapse or persistent bacteremia, and rates of renal failure were not significantly different between groups.38
Evidence for Combination therapy
Results of combination therapy for MRSA bacteremia have been largely disappointing. In a small randomized trial, addition of rifampin to vancomycin for treatment of MRSA IE was not associated with improved duration of bacteremia or rates of treatment failure, as compared to patients randomized to vancomycin alone.20 In a classic randomized trial of patients with MSSA IE, addition of gentamicin to nafcillin did not alter morbidity or mortality.46 This concept was revisited in a retrospective evaluation of 87 patients with persistent SAB or IE, 48 of whom had MRSA infection. Those treated with an aminoglycoside had lower incidence of recurrence within 6 months, though there was no significant association with other outcomes.39 In an analysis of the safety data from the Fowler et al daptomycin trial, 19 Cosgrove et al noted that 27/122 (22%) of patients who received initial low-dose gentamicin therapy experienced a clinically significant decrease in renal function, compared to 8/100 (8%) of those who did not receive gentamicin.47 There are case reports of the successful use of fluoroquinolone/rifampin combination therapy for right-sided MRSA IE.48,49 Last, there have been small case series of successful treatment of MRSA bacteremia with the addition of β-lactam antibiotics to linezolid37,50 and daptomycin.51
Evidence for other antibiotics
Several other antibiotics have either preliminary or limited data in treatment of MRSA bacteremia. Moderate quality data from a single randomized trial suggested that dalbavancin is a potential alternative to vancomycin for catheter-related Gram-positive bacteremia. However, only 14 patients in the trial had MRSA bacteremia. 22
Very low quality data from an emergency-use program suggested that quinupristin-dalfopristin could be a treatment option for MRSA infections, including bacteremia.52 Use of this antibiotic, however, has been limited by its unfavorable adverse event profile.
Telavancin is a lipoglycopeptide antibiotic approved for MRSA soft tissue infection 53 and hospital acquired pneumonia.54 In subgroup analysis of the 73 patients with bacteremic pneumonia, 33 of whom had MRSA bacteremia, telavancin therapy, when compared to vancomycin, was not associated with a difference in cure rate.55 Additionally, telavancin was compared to standard therapy for the treatment of uncomplicated SAB in a small “proof-of-concept” randomized trial. All 9 clinically evaluable patients with MRSA, of whom 5 received telavancin, were cured.56
In a retrospective evaluation of patients treated with ceftaroline, clinical success was reported in 101/129 (78.3%) of patients with SAB (of which 92.5% had MRSA).57
Pooled results of patients with secondary bacteremia from 8 trials with tigecycline have been reported; however, only 10 patients had MRSA bacteremia.58 A subsequent analysis by the FDA of patients in 10 trials demonstrated increased risk of death with tigecycline.59 This led to an FDA “black box” warning recommending that tigecycline be reserved only for situations in which alternative treatments are not suitable.
Evidence for duration of therapy
In addition to selecting an antibiotic to treat MRSA bacteremia, clinicians must also decide how long to treat. Historically, SAB was treated with 4–6 weeks of intravenous antibiotics.60 Over the past 3 decades, clinicians have repeatedly tried to identify a subgroup of patient with SAB who could safely be treated with shorter durations of therapy. A prerequisite for short course therapy is the ability to prospectively differentiate patients with uncomplicated SAB, who might be cured with a short treatment course, from complicated SAB, for whom longer treatment is necessary. MRSA treatment guidelines define uncomplicated SAB as infection in which: a) endocarditis has been excluded; b) no implanted prostheses are present; c) follow-up blood cultures drawn 2–4 days after the initial set are sterile; d) the patient defervesces within 72 hours of initiation of effective antibiotic therapy; and e) no evidence of metastatic infection is present on exam.3 Generally, only a minority of all cases of MRSA bacteremia meet these guideline-based criteria for uncomplicated infection. In these cases, the recommended treatment duration is at least ≤14 days of intravenous (IV) antibiotics from time of first negative blood culture. There is limited evidence supporting this recommendation. One prospective cohort study reported unacceptably high relapse rates in patients meeting the guideline definition of uncomplicated bacteremia who were treated for less than 2 weeks.41 A 1993 meta-analysis of older studies evaluated the effectiveness of 14 days of antibiotic therapy for intravascular catheter-associated SAB.61 This study estimated a 6.1% late complication rate (such as relapse and metastatic infections) for short-course therapy, albeit with low statistical precision, and concluded that until a means exists to identify patients who may safely receive short course therapy, more than 2 weeks of IV antibiotics should be administered. In an attempt to provide a means of identifying patients with catheter-associated SAB who might safely receive 14 days of IV therapy, Rosen et al used outcomes data from a prospective cohort of patients with uncomplicated SAB to show that a TEE-guided approach to identify patients in whom short courses of antibiotics are adequate was cost-effective.62 A multicenter randomized trial of treatment duration in staphylococcal bacteremia is currently recruiting participants.63
Recommendation
Use vancomycin or daptomycin as first-line therapy for MRSA bacteremia. Treat for at least 14 days from the first negative blood culture. (weak recommendation based on low quality evidence).
Discussion
Do all patients with SAB require transesophageal echocardiography (TEE)?
S. aureus IE is common, often clinically indistinguishable from SAB, and lethal if inadequately treated.64,65 Thus, it is important to consider the possibility of underlying IE, which will impact management and prognosis, in all patients found to have S. aureus in their blood. TEE is significantly better than either TTE or physical examination in identifying findings of IE in patients with SAB. For example, three prospective cohort studies evaluating TEE in patients with SAB conducted on different continents all identified IE in ~ one-quarter of patients.10,14,17 While this prevalence is almost certainly enriched by a heightened clinical suspicion for IE in patients whose clinicians refer them for TEE, 66 it is clear that TEE will diagnose IE in a subset of SAB patients with a non-diagnostic TTE.
Several points argue against a universal recommendation for TEE in all cases of SAB. First, TEE has associated cost and risks. Major complications such as esophageal perforation occur in approximately 1 in 5000 TEEs performed.67 Second, there is virtually no evidence to suggest that the improved detection of small valvular vegetations or oscillating targets by TEE actually improves clinical outcome in patients with SAB. Although one small, single center study reported that patients with smaller vegetations discovered by TEE only (after negative TTE) were less likely than those with positive TTE to suffer an embolic event or die of their infection, 68 this finding was not externally validated.65 Third, several studies now suggest that it is possible to identify a subset of patients with SAB at low risk of IE in whom TEE is not essential. 12,13,16–18 This low-risk subset could be conservatively defined as patients meeting all of the following criteria: a) a negative TTE,12,18 b) nosocomial acquisition of bacteremia,17,18 c) sterile follow-up blood cultures,13,16 d) absence of permanent intracardiac device,12,13,16–18 e) absence of hemodialysis dependence,16 and f) no clinical signs of endocarditis or secondary foci of infection.12,13,16,17 Alternatively, patients whose SAB has resolved and who are already scheduled to receive extended courses of antibiotics for other forms of complicated S. aureus infection may not always require TEE. Fourth, imaging technology in echocardiography is not static. Indeed, improvements in TTE image quality have already narrowed the diagnostic gap between the two modalities, especially for the evaluation of native valves.69 Collectively, these results suggest that all patients with SAB should undergo echocardiography.3,70 Although TEE is preferred when feasible, there may be identifiable low-risk patients in whom TEE is not required. A trial comparing a strategy of universal versus targeted TEE, with attention to clinical outcomes, would be a pivotal contribution to the literature.
What is the optimal antibiotic therapy for MRSA bacteremia?
Vancomycin and daptomycin are the only FDA-approved agents for the treatment of MRSA bacteremia in the United States. Approval for vancomycin is based largely on historical precedent. Recently, concerns have emerged regarding clinical isolates of MRSA exhibiting rising MIC to vancomycin.6 These concerns were underscored by the observation that patients with MRSA bacteremia due to isolates with higher (but still susceptible) vancomycin MIC had significantly higher all-cause mortality than those infected with lower vancomycin MIC isolates.71 The cause of this association is unknown, and the subject has been recently reviewed.72 One response to the perceived threat of rising vancomycin MICs in clinical MRSA isolates is to target higher vancomycin trough levels of 15–20mg/L for serious infections, an approach that was recommended in consensus guidelines.73 The relationship of these higher vancomycin trough levels to the outcome of patients with MRSA bacteremia, however, is also unresolved.72
Daptomycin is FDA-approved for SAB and right-sided IE based upon a pivotal randomized trial. 19 Observational data from patients with MRSA bacteremia due to high-vancomycin MIC isolates suggest the possibility that daptomycin might be preferred in this setting.28,29,42 Randomized trials are required to confirm this possibility. Increasingly, clinicians employ daptomycin at doses exceeding the FDA-approved dose of 6mg/kg IV once daily for MRSA bacteremia;43 the quality of evidence for this practice is low.
Teicoplanin represents another potential alternative to vancomycin but is unavailable in the US.31,32 The addition of gentamicin and/or rifampin to vancomycin for the treatment of MRSA bacteremia and native valve endocarditis appears to offer no meaningful benefit and to confer significant risk for harm. Addition of a β-lactam antibiotic to vancomycin or daptomycin for the treatment of MRSA bacteremia is interesting but unproven. Options for salvage therapy, based on low-quality data, include linezolid, TMP/SMX, dalbavancin, ceftaroline, quinupristin/dalfopristin, and telavancin. Tigecycline should be avoided. No data in MRSA bacteremia are yet available for other recently approved (e.g., tedizolid) or late-stage investigational compounds (e.g., oritavancin, ceftobiprole).
All cases of MRSA bacteremia should be treated with IV antibiotics for a minimum of 14 days from the time of blood culture clearance. For those patients not meeting the stringent definition of uncomplicated bacteremia, 4–6 weeks of therapy is recommended. Readers are additionally directed to current guidelines for more detailed discussion of treatment issues.3–5
Evidence for other Components of SAB management
The use of anti-staphylococcal β-lactam antibiotics whenever possible to treat methicillin-susceptible S. aureus (MSSA) infections is widely accepted as standard of care. The level of evidence for this practice is poor, consisting of several observational studies suggesting higher treatment failure rates in MSSA-infected patients treated with vancomycin.74–80 For example, one prospective cohort of 298 patients with MSSA bacteremia reported that the rate of microbiologic failure was lower (0/18 [0%] vs. 13/70 [19%], OR 6.5, 95% CI 1.0–53) among patients with MSSA bacteremia who were treated with nafcillin instead of vancomycin.76 Several other prospective75 and retrospective78–80 cohort studies documented lower overall78 and infection-related79,80 mortality rates among MSSA-infected patients who were treated with β-lactam antibiotics. The importance of using β-lactam antibiotics when possible is a crucial message, as patients often receive vancomycin or other alternative antibiotics due to a reported penicillin allergy. However, most patients with a self-reported penicillin allergy do not have a true allergy by skin testing and would tolerate β-lactam therapy.81 While clinical data is scarce, skin testing appeared cost-effective in a decision analysis for treatment of MSSA IE, even assuming equal efficacy of vancomycin and β-lactam therapy.82
At least 15 studies have now evaluated the role of Infectious Diseases consultation (IDC) for SAB (Table 3). All found clinical benefit; 11 reported improved mortality among patients who received IDC.83–93 IDC was also associated with increased adherence to standards of care, including selection of a β-lactam for MSSA infections,84–88,94,95 longer duration of therapy for complicated infection,83–88,92,94,95 removal of intravenous catheters,84,94 obtaining follow-up blood cultures to assess for clearance of bacteremia,84,85,87,88,92–94 obtaining echocardiography,83,85,86,88,93,96 draining abscesses,84 and removing infected prosthetic material. 84,95 Thus, while the evidence for routine IDC in patients with SAB is limited to observational studies, it supports the conclusion that IDC should be considered for every case of SAB at institutions where such expertise is available.
Table 3.
Impact of Infectious Diseases Consultation on Outcomes in S. aureus Bacteremia
| Author, year | Study Design | SAB cases | Patients with IDC (%) | Outcome Measures | Key Results | Adherence to Standards of Care |
|---|---|---|---|---|---|---|
| Fowler, 199897 | Prospective cohort | 244 | 244/244 (100%) | 12-week outcome according to whether or not IDC recommendations were followed | Following IDC advice associated with higher cure rate (89/112 [79.5%] vs. 85/132 [64.4%], p=0.01) and lower relapse rate (7/112 [6.3%] vs 24/132 [18.2%], p<0.01). Rates of death were not significantly different | N/A |
| Kaech, 200698 | Retrospective cohort | 308 | 253/308 (82%) | In-hospital complications and all-cause mortality | IDC associated with better outcome in univariate, but not multivariate, analysis | N/A |
| Jenkins, 200894 | Retrospective cohort | 234 | 161/234 (69%) overall, increase from 71/134 (53%) to 90/100 (90%) after policy of routine IDC enacted | 12-week outcome, comparing groups before (N=134) and after (N=100) a policy of mandatory IDC for SAB was enacted, and adherence to 4 pre-identified standards of care | Rates of failure and death were not significantly different between groups. | Higher adherence to standards of care with mandatory IDC (74/100 [74%] vs.53/134 [40%], p<0.001): removal of IV catheters, obtaining follow-up blood cultures, using β-lactam for MSSA, appropriate duration of therapy for complicated infection |
| Rieg, 200983 | Retrospective cohort/prospective cohort | 521 | 350/521 (67%) overall, increase from 33% to 81% after policy of routine IDC enacted | In-hospital mortality based on patient characteristics and whether IDC was performed | IDC associated with lower in-hospital mortality (66/350 [19%] vs. 47/171 [28%], OR 0.6, CI 0.4–1.0, p=0.045) and 90-day mortality (28% vs. 43%, OR 0.5, CI 0.3–0.9, p=0.02). | Patients with IDC more likely to have echocardiography, bone scan, and ≥14 days parenteral therapy |
| Lahey, 200984 | Retrospective cohort | 240 | 122/240 (51%) | Clinical management and in-hospital mortality | IDC associated with lower mortality (13.9% vs. 23.7%, OR 0.45, CI 0.22–0.93, p=0.03). | Patients with IDC more likely to have follow-up blood cultures, appropriate antibiotic selection, longer duration of therapy, interventions to drain abscesses and remove prosthetic material |
| Nagao, 201085 | Retrospective cohort | 346 | N/A | Comparison of outcomes between initial (N=194) and latter (N=152) halves of observation period after mandatory IDC policy enacted | Mortality rate decreased (25.8% to 16.4%, p=0.04) during the study period | Adherence to standards of care improved: follow-up blood cultures, obtaining echocardiogram, appropriate duration of therapy and antibiotic choice |
| Honda, 201086 | Prospective cohort | 341 | 111/341 (33%) | 28-day and 365-day all-cause mortality | IDC associated with 56% reduction in 28-day mortality (adjusted HR, 0.44; 95% CI, 0.22–0.89), but not 365-daymortality (HR 0.88, CI 0.56–1.38 p=0.579). | Patients with IDC more likely to have appropriate antibiotic choice and duration of therapy, TEE performed |
| Choi, 201187 | Retrospective cohort | 100 | 42/100 (42%) | Unfavorable outcome (30-day mortality or relapse) | IDC associated with lower rate of unfavorable outcome (31.0% vs. 53.4%, p=0.025). | Patients with IDC more likely to have selection of optimal antimicrobials, appropriate duration of therapy, and follow-up blood cultures performed |
| Robinson, 201288 | Retrospective cohort | 599 | 162/599 (27%) | 7-day, 30-day, and 1-year mortality | In univariate analysis, 7-day (3.1% vs 16.5%, p<0.001), 30-day (8.0% vs. 27%, p<0.001), and 1-year mortality (22.2% vs 44.9%, p<0.001) all lower with IDC. | Patients with IDC more likely to receive appropriate empiric antibiotics, have TEE performed, have follow-up blood cultures, and had longer duration of therapy |
| Pragman, 201295 | Retrospective cohort | 233 | 179/233 (77%) | Conformance to standards of care (use of β-lactam for MSSA, at least 28 days’ treatment for complicated infection, removal of infected prosthetic device) | IDC associated with lower risk of 12-week relapse but not with mortality | IDC associated with increased conformance to standards of care (adjusted OR 5.9, 95% CI 2.5–13.8). |
| Pastagia, 201289 | Retrospective cohort | 699 | (461/699) 66% | Predictors of 90-day all-cause mortality in MRSA bacteremia | IDC associated with lower adjusted risk ratio (0.69, 95% CI 0.57–0.86) and adjusted risk difference (−0.11, 95% CI −0.16 to − 0.04) of 90-day mortality | N/A |
| Isobe, 2012 90 | Retrospective cohort | 115 | 28/115 (24%) | 30-day mortality | IDC associated with lower 30-day mortality (6/28 [21.4%] vs. 40/87 [46.0%], p=0.02) | N/A |
| Forsblom, 201391 | Retrospective cohort | 342 | 245/342 (72%) bedside IDC, 62/342 (18%) telephone IDC | Primary: 28-day and 90-day mortality. Secondary: number of deep infection foci, time to defervescence, inadequate antibiotic therapy, duration of hospitalization, 90-day relapse | Bedside IDC, compared to telephone IDC, associated with lower 28-day (12/245 [5%] vs 10/62 [16%], OR 0.27; 95% CI 0.11–0.65), and 90-day mortality (23/245 [9%] vs 18/62 [29%], OR 0.25; 95% CI 0.13–0.51). | Patients with bedside IDC more likely to have deep focus of infection identified, compared to telephone IDC |
| Fries, 201492 | Retrospective cohort | 177 | 142/177 (80%) | Crude and attributable mortality | IDC associated with lower crude mortality (10/142 [7%] vs. 7/35 [20%], p=0.02) and attributable mortality (9/142 [6%] vs. 4/35 [11%], p=0.3). | Patients with IDC more likely to have follow-up blood cultures, focal source removed, echocardiography, and had longer duration of therapy |
| Tissot, 201493 | Retrospective cohort | 148, all MRSA | 124/148 (80%) overall, increase from 58% to 91% after policy of routine IDC enacted | Primary: 7-day, 30-day, and in-hospital mortality. Secondary: appropriateness of antibiotic therapy, performance of follow-up cultures and echocardiography, eradication of removable foci | IDC associated with lower 7-day (6/124 [5%] vs. 7/32 [22%], OR 0.18, 95% CI 0.06–0.59, p<0.01), 30-day, 23/114 [20%] vs. 12/30 [40%], OR 0.38, 95% CI 0.16–0.90, p=0.03) and in-hospital mortality (36/124 [29%] vs. 17/32 [53%], HR 0.38, 95% CI 0.20–0.74, p<0.01). | IDC associated with longer duration of therapy. |
Abbreviations: SAB; Staphylococcus aureus bacteremia; MSSA, methicillin susceptible Staphylococcus aureus bacteremia; MRSA, methicillin resistant Staphylococcus aureus; IDC, infectious diseases consultation; IVDU, intravenous drug use; IE, infective endocarditis; TEE, transesophageal echocardiogram; ICU, intensive care unit
Conclusion
The evidence for most management strategies in SAB is poor. The overall evidence guiding the use of TEE in patients with SAB is weak. It may be possible to prospectively identify a low-risk group of patients for whom TTE is adequate. Vancomycin and daptomycin remain the first-line therapies for MRSA bacteremia. Treatment should extend for at least 14 days from the first negative blood culture, and it should be longer in those with complicated disease. High quality trials comparing treatment strategies, antibiotics, and treatment durations are needed to optimize the management of this common, serious infection.
Key Take-Home Messages.
All patients with S. aureus bacteremia should be evaluated with echocardiography. Transesophageal echocardiography is preferred, but it may be possible to identify a low-risk group of patients for whom transthoracic echocardiography is adequate. This low-risk group could include all of the following: a) nosocomial acquisition of bacteremia; b) sterile follow-up blood cultures; c) no permanent intracardiac device; d) no hemodialysis dependence; and e) no clinical signs of endocarditis or secondary foci of infection.
Vancomycin and daptomycin are first-line antibiotic options for MRSA bacteremia.
For patients meeting a stringent definition of uncomplicated MRSA bacteremia, at least 14 days of antibiotic therapy from the first negative culture may be adequate. For all others, a longer course (e.g. 4–6 weeks) is recommended.
Acknowledgments
Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number UM1-AI104681. Dr. Fowler was supported by NIH K24-AI093969. The sponsor had no role in data generation, data analysis, interpretation of results, or manuscript preparation.
Footnotes
Additional contributions: We are grateful to Megan van Noord, MSIS, Duke University Medical Center Librarian, for her assistance in conducting the antibiotic therapy searches. She was not compensated for this work.
Disclosures
VGF served as Chair of V710 Scientific Advisory Committee (Merck), has received grant support or has grants pending from Cerexa, Pfizer, Advanced Liquid Logic, MedImmune, and Cubist, has been a paid consultant for Merck, Astellas, Affinium, Theravance, Cubist, Cerexa, Durata, Pfizer, NovaDigm, Novartis, Medicines Company, Biosynexus, MedImmune, and Inimex, Bayer, and has received honoraria from Merck, Astellas, Cubist, Pfizer, Theravance, and Novartis.
TH has been a paid consultant for The Medicines Company.
References
- 1.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36(1):53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 2.Kaasch AJ, Barlow G, Edgeworth JD, et al. Staphylococcus aureus bloodstream infection: A pooled analysis of five prospective, observational studies. J Infect. 2014;68(3):242–251. doi: 10.1016/j.jinf.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 4.Gould FK, Brindle R, Chadwick PR, et al. Guidelines (2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother. 2009;63(5):849–861. doi: 10.1093/jac/dkp065. [DOI] [PubMed] [Google Scholar]
- 5.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deresinski S. Counterpoint: Vancomycin and Staphylococcus aureus--an antibiotic enters obsolescence. Clin Infect Dis. 2007;44(12):1543–1548. doi: 10.1086/518452. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200–209. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 9.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 10.Fowler VG, Jr, Li J, Corey GR, et al. Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol. 1997;30(4):1072–1078. doi: 10.1016/s0735-1097(97)00250-7. [DOI] [PubMed] [Google Scholar]
- 11.Sullenberger AL, Avedissian LS, Kent SM. Importance of transesophageal echocardiography in the evaluation of Staphylococcus aureus bacteremia. J Heart Valve Dis. 2005;14(1):23–28. [PubMed] [Google Scholar]
- 12.Van Hal SJ, Mathur G, Kelly J, Aronis C, Cranney GB, Jones PD. The role of transthoracic echocardiography in excluding left sided infective endocarditis in Staphylococcus aureus bacteraemia. J Infect. 2005;51(3):218–221. doi: 10.1016/j.jinf.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Khatib R, Sharma M. Echocardiography is dispensable in uncomplicated Staphylococcus aureus bacteremia. Medicine (Baltimore) 2013;92(3):182–188. doi: 10.1097/MD.0b013e318294a710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Incani A, Hair C, Purnell P, et al. Staphylococcus aureus bacteraemia: evaluation of the role of transoesophageal echocardiography in identifying clinically unsuspected endocarditis. Eur J Clin Microbiol Infect Dis. 2013;32(8):1003–1008. doi: 10.1007/s10096-013-1838-4. [DOI] [PubMed] [Google Scholar]
- 15.Holden E, Bashir A, Das I, et al. Staphylococcus aureus bacteraemia in a UK tertiary referral centre: a ‘transoesophageal echocardiogram for all’ policy. J Antimicrob Chemother. 2014 doi: 10.1093/jac/dku082. [DOI] [PubMed] [Google Scholar]
- 16.Kaasch AJ, Fowler VG, Jr, Rieg S, et al. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis. 2011;53(1):1–9. doi: 10.1093/cid/cir320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen RV, Host U, Arpi M, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr. 2011;12(6):414–420. doi: 10.1093/ejechocard/jer023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph JP, Meddows TR, Webster DP, et al. Prioritizing echocardiography in Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2013;68(2):444–449. doi: 10.1093/jac/dks408. [DOI] [PubMed] [Google Scholar]
- 19.Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 20.Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674–680. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 21.Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390–398. doi: 10.7326/0003-4819-117-5-390. [DOI] [PubMed] [Google Scholar]
- 22.Raad I, Darouiche R, Vazquez J, et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis. 2005;40(3):374–380. doi: 10.1086/427283. [DOI] [PubMed] [Google Scholar]
- 23.Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8):975–981. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 24.Moore CL, Lu M, Cheema F, et al. Prediction of failure in vancomycin-treated methicillin-resistant Staphylococcus aureus bloodstream infection: a clinically useful risk stratification tool. Antimicrob Agents Chemother. 2011;55(10):4581–4588. doi: 10.1128/AAC.00115-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall RG, 2nd, Giuliano CA, Haase KK, et al. Empiric guideline-recommended weight-based vancomycin dosing and mortality in methicillin-resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Infect Dis. 2012;12:104. doi: 10.1186/1471-2334-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forstner C, Dungl C, Tobudic S, Mitteregger D, Lagler H, Burgmann H. Predictors of clinical and microbiological treatment failure in patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: a retrospective cohort study in a region with low MRSA prevalence. Clin Microbiol Infect. 2013;19(7):E291–297. doi: 10.1111/1469-0691.12169. [DOI] [PubMed] [Google Scholar]
- 27.Falcone M, Russo A, Pompeo ME, et al. Retrospective case-control analysis of patients with staphylococcal infections receiving daptomycin or glycopeptide therapy. Int J Antimicrob Agents. 2012;39(1):64–68. doi: 10.1016/j.ijantimicag.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Murray KP, Zhao JJ, Davis SL, et al. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration >1 mg/L: a matched cohort study. Clin Infect Dis. 2013;56(11):1562–1569. doi: 10.1093/cid/cit112. [DOI] [PubMed] [Google Scholar]
- 29.Cheng CW, Hsu PC, Yang CC, et al. Influence of early daptomycin therapy on treatment outcome of meticillin-resistant Staphylococcus aureus bacteraemia with high vancomycin minimum inhibitory concentrations. Int J Antimicrob Agents. 2013;41(3):293–294. doi: 10.1016/j.ijantimicag.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Carugati M, Bayer AS, Miro JM, et al. High-dose daptomycin therapy for left-sided infective endocarditis: a prospective study from the international collaboration on endocarditis. Antimicrob Agents Chemother. 2013;57(12):6213–6222. doi: 10.1128/AAC.01563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menichetti F, Martino P, Bucaneve G, et al. Effects of teicoplanin and those of vancomycin in initial empirical antibiotic regimen for febrile, neutropenic patients with hematologic malignancies. Gimema Infection Program. Antimicrob Agents Chemother. 1994;38(9):2041–2046. doi: 10.1128/aac.38.9.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon YK, Park DW, Sohn JW, et al. Multicenter prospective observational study of the comparative efficacy and safety of vancomycin versus teicoplanin in patients with health care-associated methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2014;58(1):317–324. doi: 10.1128/AAC.00520-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birmingham MC, Rayner CR, Meagher AK, Flavin SM, Batts DH, Schentag JJ. Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience from a compassionate-use program. Clin Infect Dis. 2003;36(2):159–168. doi: 10.1086/345744. [DOI] [PubMed] [Google Scholar]
- 34.Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923–929. doi: 10.1093/jac/dki355. [DOI] [PubMed] [Google Scholar]
- 35.Gomez J, Garcia-Vazquez E, Banos R, et al. Predictors of mortality in patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: the role of empiric antibiotic therapy. Eur J Clin Microbiol Infect Dis. 2007;26(4):239–245. doi: 10.1007/s10096-007-0272-x. [DOI] [PubMed] [Google Scholar]
- 36.Wilcox MH, Tack KJ, Bouza E, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis. 2009;48(2):203–212. doi: 10.1086/595686. [DOI] [PubMed] [Google Scholar]
- 37.Park HJ, Kim SH, Kim MJ, et al. Efficacy of linezolid-based salvage therapy compared with glycopeptide-based therapy in patients with persistent methicillin-resistant Staphylococcus aureus bacteremia. J Infect. 2012;65(6):505–512. doi: 10.1016/j.jinf.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg E, Paul M, Talker O, et al. Co-trimoxazole versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus bacteraemia: a retrospective cohort study. J Antimicrob Chemother. 2010;65(8):1779–1783. doi: 10.1093/jac/dkq179. [DOI] [PubMed] [Google Scholar]
- 39.Lemonovich TL, Haynes K, Lautenbach E, Amorosa VK. Combination therapy with an aminoglycoside for Staphylococcus aureus endocarditis and/or persistent bacteremia is associated with a decreased rate of recurrent bacteremia: a cohort study. Infection. 2011;39(6):549–554. doi: 10.1007/s15010-011-0189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dilworth TJ, Ibrahim O, Hall P, Sliwinski J, Walraven C, Mercier RC. beta-Lactams enhance vancomycin activity against methicillin-resistant Staphylococcus aureus bacteremia compared to vancomycin alone. Antimicrob Agents Chemother. 2014;58(1):102–109. doi: 10.1128/AAC.01204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong YP, Moon SM, Bang KM, et al. Treatment duration for uncomplicated Staphylococcus aureus bacteremia to prevent relapse: analysis of a prospective observational cohort study. Antimicrob Agents Chemother. 2013;57(3):1150–1156. doi: 10.1128/AAC.01021-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore CL, Osaki-Kiyan P, Haque NZ, Perri MB, Donabedian S, Zervos MJ. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: a case-control study. Clin Infect Dis. 2012;54(1):51–58. doi: 10.1093/cid/cir764. [DOI] [PubMed] [Google Scholar]
- 43.Kullar R, Davis SL, Levine DP, et al. High-dose daptomycin for treatment of complicated gram-positive infections: a large, multicenter, retrospective study. Pharmacotherapy. 2011;31(6):527–536. doi: 10.1592/phco.31.6.527. [DOI] [PubMed] [Google Scholar]
- 44.Weston A, Golan Y, Holcroft C, Snydman DR. The Efficacy of Daptomycin Versus Vancomycin for Methicillin-Resistant Staphylococcus aureus Bloodstream Infection in Patients With Impaired Renal Function. Clin Infect Dis. 2014;58(11):1533–1539. doi: 10.1093/cid/ciu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.FDA. [Accessed March 2, 2014];Information for Healthcare Professionals: Linezolid (marketed as Zyvox) 2007 http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/drugsafetyinformationforheathcareprofessionals/ucm085249.htm.
- 46.Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: A prospective study. Ann Intern Med. 1982;97(4):496–503. doi: 10.7326/0003-4819-97-4-496. [DOI] [PubMed] [Google Scholar]
- 47.Cosgrove SE, Vigliani GA, Fowler VG, Jr, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis. 2009;48(6):713–721. doi: 10.1086/597031. [DOI] [PubMed] [Google Scholar]
- 48.Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071–1073. doi: 10.1016/s0140-6736(89)91083-0. [DOI] [PubMed] [Google Scholar]
- 49.Heldman AW, Hartert TV, Ray SC, et al. Oral antibiotic treatment of right-sided staphylococcal endocarditis in injection drug users: prospective randomized comparison with parenteral therapy. Am J Med. 1996;101(1):68–76. doi: 10.1016/s0002-9343(96)00070-8. [DOI] [PubMed] [Google Scholar]
- 50.Jang HC, Kim SH, Kim KH, et al. Salvage treatment for persistent methicillin-resistant Staphylococcus aureus bacteremia: efficacy of linezolid with or without carbapenem. Clin Infect Dis. 2009;49(3):395–401. doi: 10.1086/600295. [DOI] [PubMed] [Google Scholar]
- 51.Dhand A, Bayer AS, Pogliano J, et al. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis. 2011;53(2):158–163. doi: 10.1093/cid/cir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drew RH, Perfect JR, Srinath L, Kurkimilis E, Dowzicky M, Talbot GH. Treatment of methicillin-resistant Staphylococcus aureus infections with quinupristin-dalfopristin in patients intolerant of or failing prior therapy. For the Synercid Emergency-Use Study Group. J Antimicrob Chemother. 2000;46(5):775–784. doi: 10.1093/jac/46.5.775. [DOI] [PubMed] [Google Scholar]
- 53.Stryjewski ME, O’Riordan WD, Lau WK, et al. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin Infect Dis. 2005;40(11):1601–1607. doi: 10.1086/429914. [DOI] [PubMed] [Google Scholar]
- 54.Rubinstein E, Lalani T, Corey GR, et al. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis. 2011;52(1):31–40. doi: 10.1093/cid/ciq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stryjewski ME, Barriere SL, Rubinstein E, et al. Telavancin versus vancomycin for bacteraemic hospital-acquired pneumonia. Int J Antimicrob Agents. 2013;42(4):367–369. doi: 10.1016/j.ijantimicag.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Stryjewski ME, Lentnek A, O’Riordan W, et al. A randomized Phase 2 trial of telavancin versus standard therapy in patients with uncomplicated Staphylococcus aureus bacteremia: the ASSURE study. BMC Infect Dis. 2014;14(1):289. doi: 10.1186/1471-2334-14-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Casapao AM, Davis SL, Barr VO, et al. Large retrospective evaluation of the effectiveness and safety of ceftaroline fosamil therapy. Antimicrob Agents Chemother. 2014;58(5):2541–2546. doi: 10.1128/AAC.02371-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gardiner D, Dukart G, Cooper A, Babinchak T. Safety and efficacy of intravenous tigecycline in subjects with secondary bacteremia: pooled results from 8 phase III clinical trials. Clin Infect Dis. 2010;50(2):229–238. doi: 10.1086/648720. [DOI] [PubMed] [Google Scholar]
- 59.FDA. [Accessed March 2, 2014];FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new Boxed Warning. http://www.fda.gov/drugs/drugsafety/ucm369580.htm.
- 60.Nolan CM, Beaty HN. Staphylococcus aureus bacteremia. Current clinical patterns. Am J Med. 1976;60(4):495–500. doi: 10.1016/0002-9343(76)90715-4. [DOI] [PubMed] [Google Scholar]
- 61.Jernigan JA, Farr BM. Short-course therapy of catheter-related Staphylococcus aureus bacteremia: a meta-analysis. Ann Intern Med. 1993;119(4):304–311. doi: 10.7326/0003-4819-119-4-199308150-00010. [DOI] [PubMed] [Google Scholar]
- 62.Rosen AB, Fowler VG, Jr, Corey GR, et al. Cost-effectiveness of transesophageal echocardiography to determine the duration of therapy for intravascular catheter-associated Staphylococcus aureus bacteremia. Ann Intern Med. 1999;130(10):810–820. doi: 10.7326/0003-4819-130-10-199905180-00004. [DOI] [PubMed] [Google Scholar]
- 63. [Accessed March 17, 2014];Treatment Algorithm to Reduce the Use of Vancomycin in Adults With Blood Stream Infection (Bacteremia) http://clinicaltrials.gov/ct2/show/NCT01191840?term=staphylococcal+bacteremia&rank=12.
- 64.Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation. 2005;112(1):69–75. doi: 10.1161/CIRCULATIONAHA.104.493155. [DOI] [PubMed] [Google Scholar]
- 65.Fowler VG, Jr, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293(24):3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 66.Soriano A, Mensa J. Is transesophageal echocardiography dispensable in hospital-acquired Staphylococcus aureus bacteremia? Clin Infect Dis. 2011;53(1):10–12. doi: 10.1093/cid/cir305. [DOI] [PubMed] [Google Scholar]
- 67.Khandheria BK, Seward JB, Tajik AJ. Transesophageal echocardiography. Mayo Clin Proc. 1994;69(9):856–863. doi: 10.1016/s0025-6196(12)61788-1. [DOI] [PubMed] [Google Scholar]
- 68.Fowler VG, Jr, Sanders LL, Kong LK, et al. Infective endocarditis due to Staphylococcus aureus: 59 prospectively identified cases with follow-up. Clin Infect Dis. 1999;28(1):106–114. doi: 10.1086/515076. [DOI] [PubMed] [Google Scholar]
- 69.Casella F, Rana B, Casazza G, et al. The potential impact of contemporary transthoracic echocardiography on the management of patients with native valve endocarditis: a comparison with transesophageal echocardiography. Echocardiography. 2009;26(8):900–906. doi: 10.1111/j.1540-8175.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- 70.Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30(19):2369–2413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 71.van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012;54(6):755–771. doi: 10.1093/cid/cir935. [DOI] [PubMed] [Google Scholar]
- 72.van Hal SJ, Fowler VG., Jr Is it time to replace vancomycin in the treatment of methicillin-resistant Staphylococcus aureus infections? Clin Infect Dis. 2013;56(12):1779–1788. doi: 10.1093/cid/cit178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 74.McConeghy KW, Bleasdale SC, Rodvold KA. The empirical combination of vancomycin and a beta-lactam for Staphylococcal bacteremia. Clin Infect Dis. 2013;57(12):1760–1765. doi: 10.1093/cid/cit560. [DOI] [PubMed] [Google Scholar]
- 75.Stryjewski ME, Szczech LA, Benjamin DK, Jr, et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44(2):190–196. doi: 10.1086/510386. [DOI] [PubMed] [Google Scholar]
- 76.Chang FY, Peacock JE, Jr, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003;82(5):333–339. doi: 10.1097/01.md.0000091184.93122.09. [DOI] [PubMed] [Google Scholar]
- 77.Khatib R, Saeed S, Sharma M, Riederer K, Fakih MG, Johnson LB. Impact of initial antibiotic choice and delayed appropriate treatment on the outcome of Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis. 2006;25(3):181–185. doi: 10.1007/s10096-006-0096-0. [DOI] [PubMed] [Google Scholar]
- 78.Schweizer ML, Furuno JP, Harris AD, et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279. doi: 10.1186/1471-2334-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lodise TP, Jr, McKinnon PS, Levine DP, Rybak MJ. Impact of empirical-therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(10):3731–3733. doi: 10.1128/AAC.00101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim SH, Kim KH, Kim HB, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52(1):192–197. doi: 10.1128/AAC.00700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(3 Suppl 3):S1–148. doi: 10.1016/s1081-1206(10)60305-5. [DOI] [PubMed] [Google Scholar]
- 82.Dodek P, Phillips P. Questionable history of immediate-type hypersensitivity to penicillin in Staphylococcal endocarditis: treatment based on skin-test results vers-us empirical alternative treatment--A decision analysis. Clin Infect Dis. 1999;29(5):1251–1256. doi: 10.1086/313435. [DOI] [PubMed] [Google Scholar]
- 83.Rieg S, Peyerl-Hoffmann G, de With K, et al. Mortality of S. aureus bacteremia and infectious diseases specialist consultation--a study of 521 patients in Germany. J Infect. 2009;59(4):232–239. doi: 10.1016/j.jinf.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 84.Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore) 2009;88(5):263–267. doi: 10.1097/MD.0b013e3181b8fccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagao M, Iinuma Y, Saito T, et al. Close cooperation between infectious disease physicians and attending physicians can result in better management and outcome for patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2010;16(12):1783–1788. doi: 10.1111/j.1469-0691.2010.03156.x. [DOI] [PubMed] [Google Scholar]
- 86.Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med. 2010;123(7):631–637. doi: 10.1016/j.amjmed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi SH, Cho SY, Park JH, Chung JW. Impact of infectious-disease specialist consultations on outcomes of Staphylococcus aureus bacteremia in a hospital with a low volume of patients with S. aureus bacteremia. J Infect. 2011;62(2):181–185. doi: 10.1016/j.jinf.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 88.Robinson JO, Pozzi-Langhi S, Phillips M, et al. Formal infectious diseases consultation is associated with decreased mortality in Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis. 2012;31(9):2421–2428. doi: 10.1007/s10096-012-1585-y. [DOI] [PubMed] [Google Scholar]
- 89.Pastagia M, Kleinman LC, Lacerda de la Cruz EG, Jenkins SG. Predicting risk for death from MRSA bacteremia. Emerg Infect Dis. 2012;18(7):1072–1080. doi: 10.3201/eid1807.101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Isobe M, Uejima E, Seki M, et al. Methicillin-resistant Staphylococcus aureus bacteremia at a university hospital in Japan. J Infect Chemother. 2012;18(6):841–847. doi: 10.1007/s10156-012-0423-6. [DOI] [PubMed] [Google Scholar]
- 91.Forsblom E, Ruotsalainen E, Ollgren J, Jarvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527–535. doi: 10.1093/cid/cis889. [DOI] [PubMed] [Google Scholar]
- 92.Fries BL, Licitra C, Crespo A, et al. Infectious diseases consultation and the management of Staphylococcus aureus bacteremia. Clin Infect Dis. 2014;58(4):598–599. doi: 10.1093/cid/cit730. [DOI] [PubMed] [Google Scholar]
- 93.Tissot F, Calandra T, Prod’hom G, et al. Mandatory infectious diseases consultation for MRSA bacteremia is associated with reduced mortality. J Infect. 2014 doi: 10.1016/j.jinf.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 94.Jenkins TC, Price CS, Sabel AL, Mehler PS, Burman WJ. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(7):1000–1008. doi: 10.1086/529190. [DOI] [PubMed] [Google Scholar]
- 95.Pragman AA, Kuskowski MA, Abraham JM, Filice GA. Infectious Disease Consultation for Staphylococcus aureus Bacteremia Improves Patient Management and Outcomes. Infect Dis Clin Pract (Baltim Md) 2012;20(4):261–267. doi: 10.1097/IPC.0b013e318255d67c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fowler VG, Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163(17):2066–2072. doi: 10.1001/archinte.163.17.2066. [DOI] [PubMed] [Google Scholar]
- 97.Fowler VG, Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27(3):478–486. doi: 10.1086/514686. [DOI] [PubMed] [Google Scholar]
- 98.Kaech C, Elzi L, Sendi P, et al. Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre. Clin Microbiol Infect. 2006;12(4):345–352. doi: 10.1111/j.1469-0691.2005.01359.x. [DOI] [PubMed] [Google Scholar]