Abstract
Humans and their predecessors evolved in environments where they were challenged intermittently with: 1) food scarcity; 2) the need for aerobic fitness to catch/kill prey and avoid or repel attackers; and 3) exposure to biological toxins present in foodstuffs. Accordingly, cells and organ systems acquired and retained molecular signaling and metabolic pathways through which the environmental challenges enhanced the functionality and resilience of the cells and organisms. Within the past 60 years there has been a precipitous diminution of such challenges in modern societies because of the development of technologies that provide a continuous supply of energy-dense processed foods and that largely eliminate the need for physical exertion. As a consequence of the modern ‘couch potato’ lifestyle, signaling pathways that mediate beneficial effects of environmental challenges on health and disease resistance are disengaged, thereby rendering people vulnerable to obesity, diabetes, cardiovascular disease, cancers and neurodegenerative disorders. Reversal of the epidemic of diseases caused by unchallenging lifestyles will require a society-wide effort to re-introduce intermittent fasting, exercise and consumption of plants containing hormetic phytochemicals into daily and weekly routines.
Keywords: brain function, exercise, hormesis, intermittent fasting
EVOLUTIONARY PERSPECTIVE: THE CHALLENGE OF COMPETITION FOR LIMITED FOOD SOURCES
In order to survive and reproduce, our ancestor hominids spent most of their waking hours working to find food, either by ‘grazing’ on plants or by hunting animals. In many instances, the food supply was very limited and so there was a survival advantage for those who could tolerate and adapt to periods of food deprivation. One such adaptation is the metabolic shift from the use of glycogen stores in liver and muscle cells, to the mobilization of fatty acids in adipose cells and their conversion to ketones, an alternative cellular energy substrate (Longo and Mattson, 2014). Another interesting adaptation suggested by studies of animal models, is that cognitive function and stress resistance improve in response to intermittent fasting (Wan et al., 2003; Ahmet et al., 2005; Mattson, 2012a; Marosi and Mattson, 2014). Similarly, endurance running, such as is required to chase and kill a deer (Zimmer, 2004), not only strengthens the muscles and heart, but also improves brain function (Ahlskog et al., 2011; Mattson, 2012b; Voss et al., 2013) (Figure 1). As with lower species of mammals, humans evolved the ability to consume a range of plant species, many of which contain chemicals that exert noxious effects on cells. Such natural pesticides/deterrents in plants typically have a bitter taste and, as described below and elsewhere (Mattson and Cheng, 2006; Hooper et al., 2010), these phytochemicals activate adaptive cellular stress response pathways that not only protect cells against the phytochemicals but also against injury and disease. The remainder of this article will present examples of signaling and metabolic pathways by which laboratory animals and human subjects respond to the challenges of food deprivation/ fasting, running, and ingestion of phytochemical ‘toxins’. What follows is not intended to be a comprehensive review article, but instead provides brief overviews in which relevant review articles are cited, combined with specific examples from research in the author’s laboratory.
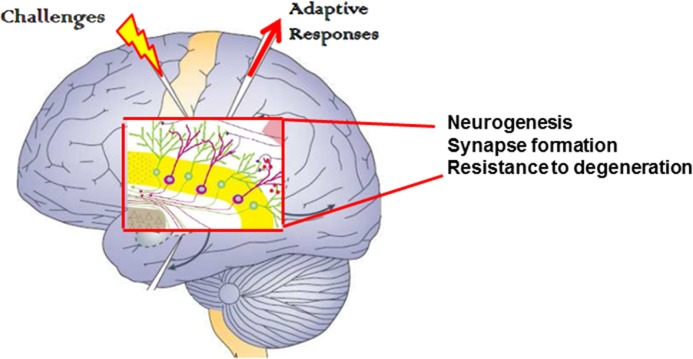
FIGURE 1.
Illustration of the simple fundamental concept that optimal health is promoted by intermittent challenges (mild stressors). Cells in the organ, in this case the brain, respond adaptively by enhancing their ability to function and resist disease.
CHALLENGE 1: INTERMITTENT FOOD RESTRICTION/FASTING
It is not possible to precisely simulate in a laboratory the environment in which wild animals and our hominid ancestors lived. However, it is clearly evident that the diets (meal frequency and composition) and activity level of lab animals are abnormal. In effect, lab animals live a ‘couch potato’ lifestyle. They eat as much as they like whenever they like, they eat only processed food, and they get little exercise in their ‘living rooms’ (Martin et al., 2010). Even when consuming only the usual processed food diet, a simple change in the eating pattern can greatly improve the health and increase the lifespan of mice and rats. To simulate a more natural environment, animals can be fed intermittently. Animals that eat only every other day (alternate day fasting) live up to 30% longer than those that eat every day (Goodrick et al., 1983), and exhibit resistance to diabetes (Anson et al., 2003; Belkacemi et al., 2010), cancers (Berrigan et al., 2002; Lee et al., 2012) and neurodegenerative disease (Duan et al., 2003; Halagappa et al., 2007; Arumugam et al., 2010).
In contrast to daily calorie restriction which typically results in a reduction in lean mass and fat mass, animals and human subjects on intermittent fasting (IF) lose fat while retaining lean mass (Anson et al., 2003; Harvie et al., 2010). During the fasting period, fatty acids are released from adipose cells and enter the liver where they are converted to ketone bodies (β-hydroxybutyrate and acetoacetate) which provide a robust energy source by muscle cells and neurons (Johnson et al., 2007; Maalouf et al., 2009). Ketone bodies have beneficial effects on cells that can counteract disease processes. For example, ketones protect neurons against dysfunction and degeneration in experimental models of epilepsy (McNally and Hartman, 2012) and Alzheimer’s disease (Kashiwaya et al., 2013). In addition to being used as an energy source, ketone bodies can modulate signaling pathways and gene expression by, for example, inhibiting certain histone deacetylases (Newman and Verdin, 2014). Fasting can enhance the ability of cells to remove molecular ‘garbage’ (damaged proteins and organelles), a process called autophagy, and can potentiate the effects of endurance exercise on autophagy (Jamart et al., 2013).
A major mechanism by which fasting can protect against tissue injury and disease is by activating adaptive cellular stress responses by hormesis-based processes (Figure 2). For example, IF induces the expression of protein chaperones (e.g., heat-shock protein 70 and glucose-regulated protein 78) and stimulates autophagy in many cell types (Duan and Mattson, 1999; Yu and Mattson, 1999; Alirezaei et al., 2010; Arumugam et al., 2010; Lee and Notterpek, 2013). The latter actions of IF enhance the ability of cells to eliminate damaged proteins and organelles such as mitochondria. Indeed, autophagy is required for the maintenance of muscle mass (Masiero et al., 2009), consistent with the preservation of muscle mass during IF (Harvie et al., 2010). IF decreases levels of circulating leptin and increases levels of adiponectin, changes associated with improved energy metabolism and cardioprotection (Wan et al., 2010; Hui et al., 2012). IF can also increase neurotrophic factor signaling in brain cells, which may contribute to its abilities to enhance hippocampal neurogenesis (Lee et al., 2002) and protect neurons against oxidative and metabolic stress in animal models of Parkinson’s disease (Duan and Mattson, 1999), Huntington’s disease (Duan et al., 2003), Alzheimer’s disease (Halagappa et al., 2007) and stroke (Yu and Mattson, 1999; Arumugam et al., 2010).
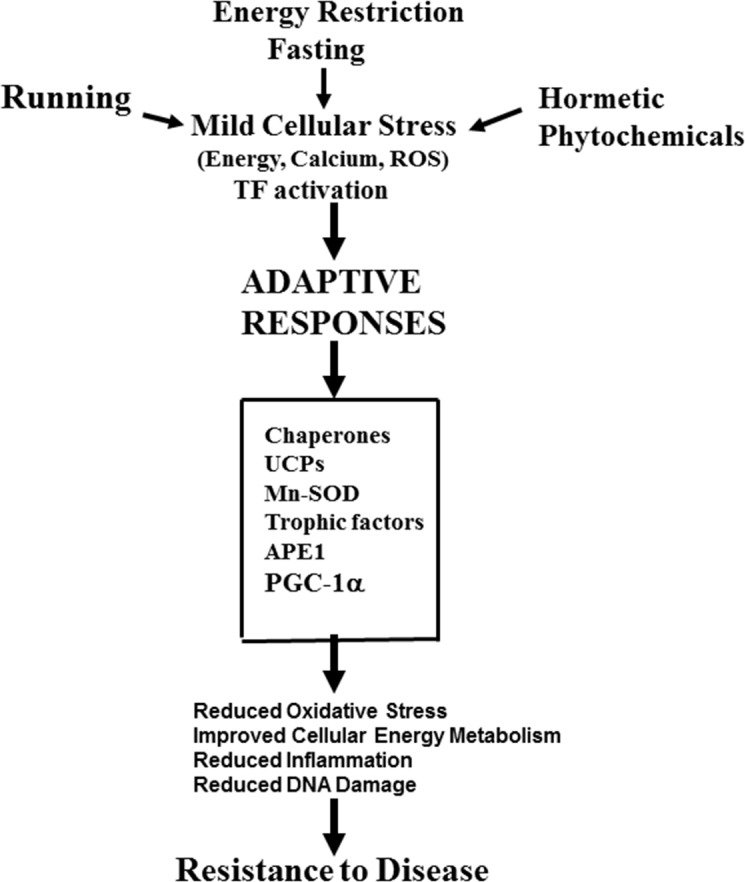
FIGURE 2.
Mechanisms by which the intermittent challenges of running, intermittent fasting and ingestion of hormetic phytochemicals may protect cells against injury and disease. This example focuses on the brain. See text and the following references for further information: Mattson and Cheng, 2006; Stranahan and Mattson, 2012; Mattson, 2012a; Longo and Mattson, 2014.
There is considerable evidence from the historical record, self-reports, interviews and case studies that IF improves health and can counteract disease processes. Numerous books have been written on the topic including one more than a century ago by Sinclair (1911) and very recently by Mosley and Spencer (2013) and Harvie and Howell (2013). In a case series study, patients with different types of cancer experienced fewer side effects of chemotherapy when fasting (Safdie et al., 2009). In a case study of a patient with glioblastoma multiforme, a rapidly progressing and almost always fatal brain cancer, fasting and a ketogenic diet resulted in an apparent complete arrest of tumor growth (Zuccoli et al., 2010). Importantly, recent controlled trials in human subjects support the evidence in animals that IF can promote optimal health and counteract disease processes. In a study of asthma patients, an alternate day energy restriction/fasting diet resulted in improvement of symptoms, reduced airway resistance, and reduced inflammation and oxidative stress during a 2-month diet period (Johnson et al., 2007). A similar alternate day fasting diet resulted in improvements in measures of cardiovascular risk in obese subjects (Varady et al., 2009). In two studies, women at risk for breast cancer based on family history and their being obese were maintained for 6 months on either a daily calorie restriction diet (25% reduction in calories) or a diet on which they ate regular size meals 5 days per week and on the other two days ate only 500–600 calories (‘5:2 diet’). Women in both groups lost a similar amount of weight, but those on the 5:2 diet lost more abdominal fat and exhibited a greater improvement of insulin sensitivity compared to those on daily calorie restriction (Harvie et al., 2011; Harvie et al., 2013).
CHALLENGE 2: RUNNING
As a result of several key structural and physiological adaptations, humans are extraordinary endurance runners. During evolution humans acquired: 1) an upright bipedal posture with changes in the musculoskeletal system that increased running efficiency; 2) reduced body hair, a large skin surface area and abundant sweat glands to enhance body cooling; and 3) a brain with a remarkable capacity to establish a pace conducive to the expected running distance (Bramble and Lieberman, 2004; Mattson, 2012b). There is a vast literature concerning the physiological and psychological effects of running that includes human studies that compare non-runners to runners, elite runners to ‘joggers’, and changes in individuals prior to and during a running program. There is also a large literature from randomized controlled trials of wheel or treadmill running in rats and mice. Of course, running is not the only type of exercise that improves health. However, because most of the data regarding the cellular and molecular mechanisms by which exercise affects various organ systems is from studies of rodents, which do not ride bicycles or workout on elliptical or rowing machines, the focus of the following discussion is on running.
Running is a challenge, imposing a heavy metabolic load on skeletal muscles and the cardiovascular system, and milder stress on other tissues. Cells throughout the body and brain respond adaptively to running, bolstering their ability to withstand injury and disease. Highly reproducible robust improvements in metabolic and cardiovascular profiles occur in response to regular endurance running in human subjects. These include increased insulin sensitivity, reduced levels of triglycerides and cholesterol, reduced blood pressure and resting heart rate, increased heart rate variability, and reduced levels of pro-inflammatory cytokines (for a recent review see Rowe et al., 2014). Endurance exercise increases the number of mitochondria in skeletal muscle, liver and brain cells (Little et al., 2011), which presumably increases their energy production capacity. In muscle cells, exercise-induced mitochondrial biogenesis is triggered by calcium influx, reactive oxygen species and activation of AMP-activated protein kinase (AMPK) which induce the expression of the transcriptional regulator peroxisome proliferator receptor gamma coactivator 1α (PGC-1α). PGC-1α induces the expression of genes encoding proteins involved in mitochondrial growth and division (Diaz and Moraes, 2008; Wenz, 2013). Exercise also stimulates autophagy and expression of the anti-apoptotic protein Bcl2 which, interestingly, are changes tightly linked to improved glucose metabolism (He et al., 2012).
Running has profound beneficial effects on the brain in animal models. It induces the expression of brain-derived neurotrophic factor (BDNF) which activates receptors in neurons and neural stem cells. BDNF plays critical roles in learning and memory (Gomez-Pinilla and Hillman, 2013), and may mediate the improved cognitive function that is observed in runners compared to non-runner rats and mice (Marosi and Mattson, 2014). The mechanisms by which running ‘strengthens’ synapses involves binding of BDNF to a receptor called TrkB resulting in the activation of the kinases Akt and ERK (extracellular signal regulated kinase), and the downstream transcription factors cyclic AMP response element binding protein (CREB) and NF-kB (Kuipers and Bramham, 2006; Gavalda et al., 2009; Chen et al., 2012). The hippocampus, a brain region critical for learning and memory, contains stem cells that can divide and can differentiate into new neurons or glial cells. Exercise increases the proliferation of stem cells and the formation of new neurons that can integrate into the existing neuronal circuits by forming synapses with other neurons (Vivar et al., 2013). BDNF promotes the differentiation of new neurons from stem cells and enhances their survival and formation of synapses with other neurons (Cheng et al., 2003; Cheng et al., 2012). Numerous studies have shown that BDNF can protect neurons against degeneration and death in experimental models relevant to the pathogenesis of Alzheimer’s, Parkinson’s and Huntington’s diseases (Duan et al., 2003; Zuccato and Cattaneo, 2009; Intlekofer and Cotman, 2013). Exercise and BDNF signaling bolster the stress resistance of neurons in multiple ways including by enhancing DNA repair (Yang et al., 2014), stimulating mitochondrial biogenesis (Cheng et al., 2012), inducing the expression of ‘anti-apoptotic’ proteins such as Bcl-2 and Bcl-xL (Allsopp et al., 1995; Chao et al., 2011), and upregulating expression of antioxidant enzymes (Mattson et al., 1995; Marosi et al., 2012). Even in aged mice, running can upregulate the expression of genes involved in synaptic plasticity and cellular bioenergetics, while downregulating genes associated with oxidative stress (Stranahan et al., 2010).
In addition to its beneficial effects on the musculoskeletal, cardiovascular and nervous systems, intermittent exercise, including running, can modify the activation states of immune cells in ways that enhance health. A well-established example is in patients with chronic inflammatory disorders such as arthritis and multiple sclerosis; exercise improves their mobility, lessens their pain and improves their mood (Motl and Pilutti, 2012; Tierney et al., 2012). Data from studies of rats suggest that running wheel exercise can enhance innate immune function by modulating the expression of cytokines and chemokines in white adipose cells (Speaker et al., 2013). The function of the adaptive immune system can also be enhanced by endurance running, as indicated by increased levels of natural killer cells (Nieman et al., 1995). More direct evidence that exercise can improve immune function comes from studies in which the primary antibody and T cell responses to foreign antigens were measured in sedentary and physically active subjects. In one study, young and old men who either exercised regularly or were sedentary were first immunized with keyhole-limpet hemocyanin (KLH) intramuscularly, and then three weeks later were given an intradermal injection of KLH to induce a hypersensitivity response (Smith et al., 2004). Older subjects exhibited reduced anti-KLH immunoglobulin responses. However, the immunoglobulin responses, and delayed hypersensitivity response, of older subjects who exercised were greater than sedentary old subjects. In a related study previously sedentary subjects were divided into an aerobic exercise group and a stretching control group, and 8 months later the subjects were challenged with KLH (Grant et al., 2008). The authors found that subjects in the aerobic exercise group exhibited significantly greater IgG1 and IgM antibody responses compared to the subjects in the control group. Collectively, the data suggest that regular exercise can bolster immune responses in ways that may protect against infectious agents.
It should be noted, however, that as with any factor that improves health by inducing adaptive stress responses, excessive exercise can be detrimental to health. This is most obvious from the perspective of overuse injuries to the musculoskeletal system, but is also well-established for the immune system (Haaland et al., 2008). For example, marathon and ultra-distance running increases susceptibility to upper respiratory tract infections (Nieman et al., 2003). This biphasic response of the immune system to exercise provides another good example of hormesis (Radak et al., 2008).
Finally, it is well-established that chronic uncontrollable stress, such as psychosocial stress and sleep deprivation, can increase the risk of many different disorders including cardiovascular disease, gastrointestinal disorders, anxiety disorders and depression (Charney and Manji, 2004; Everson-Rose and Lewis ., 2005). Studies of animal models have demonstrated robust beneficial effects of exercise in counteracting pathogenic processes underlying the adverse effects of chronic stress on the brain and immune system (Fleshner, 2005; Greenwood and Fleshner, 2008). For example, voluntary wheel running prevented the anxiety/fear-like behaviors, and associated increases in activation of serotonergic neurons in the raphe nucleus caused by uncontrollable tail shock in sedentary rats (Greenwood et al., 2003). Rats that ran on running wheels for six weeks exhibited resistance to the reduction in social exploration that otherwise occurs in non-runners; and this resistance to chronic stress persisted for more than 2 weeks after removal of running wheels from the cages (Greenwood et al., 2012). With regards to suppression of immune function by chronic uncontrollable stress, it was shown that running prevented the reduction in immune response to KLH otherwise caused by uncontrollable tail shock in rats (Moraska and Fleshner 2001). Thus, intermittent challenges such as aerobic exercise can increase the resistance of many different organ systems to chronic stress.
CHALLENGE 3: NOXIOUS DIETARY PHYTOCHEMICALS
There is no doubt that some of the chemicals present in the vegetables, fruits and other plant materials that we consume function as toxins in those plants; they are natural pesticides/biopesticides (Koul, 2005; Mattson and Cheng, 2006). Because herbivores and omnivores evolved consuming diets that contain such chemicals, they have developed two general mechanisms to prevent those phytochemicals from damaging them. One mechanism involves rapid metabolism/detoxification of the phytochemicals by cytochrome p450 and ‘phase 2’ enzymes, and urinary excretion (Shapiro et al., 2006; Wu et al., 2013). The second mechanism involves activation of adaptive cellular stress response pathways by the phytochemicals. The stress response pathways include those resulting in the activation of the transcription factors nuclear regulatory factor 2 (Nrf2), hypoxia inducible factor 1α (HIF-1α) and nuclear factor κB (NF-κB) and peroxisome proliferator activated receptors (PPARs) (Mattson and Meffert, 2006; Copple et al., 2010; Majmundar et al., 2010; Yessoufou and Wahli, 2010). Activation of the latter pathways results in increased production of a range of proteins including: antioxidant enzymes such as NAD(P)H quinone oxidoreductase (NQO1), superoxide dismutase 2, heme oxygenase 1 (HO-1) and glutathione peroxidase; erythropoietin and adiponectin; and the cell survival proteins Bcl-2 and Bcl-xL (Sen, 2006; Joshi and Johnson, 2012; Ong and Hausenloy, 2012). In addition, activation of PPARs can suppress inflammation by inhibiting the production of proinflammatory cytokines and cell adhesion molecules (Gervois and Mansouri, 2012).
There are several hormetic phytochemicals that have been intensively studied to elucidate the mechanisms by which they improve health and protect against disease. Sulforaphane (from broccoli and other cruciferous vegetables), curcumin (the curry spice from turmeric root), resveratrol (from red grapes and certain types of nuts) and epicatechins (present in high amounts in green tea and dark chocolate) are four prominent examples (for review see Calabrese et al., 2010; Bhardwaj and Khanna, 2013; Kensler et al., 2013; Nehlig et al., 2013; Witkin and Li, 2013). Sulforaphane activates Nrf2 resulting in the up-regulation of NQO1, HO-1 and phase 2 enzymes. This adaptive stress response can protect cells against dysfunction and degeneration in experimental models; for example, sulforaphane protects neurons and cardiac myocytes in models of stroke and myocardial infarction, respectively (Zhao et al., 2006; Piao et al., 2010). Sulforaphane is also beneficial in animal models of Parkinson’s disease (Morroni et al., 2013). Other noxious phytochemicals have been identified that activate Nrf2 and are neuroprotective, with plumbagin being one example (Son et al., 2010). Similarly, the phytochemical Nrf2 activator naphthazarin reduced damage to dopaminergic neurons and improved functional outcome in a mouse model of Parkinson’s disease (Choi et al., 2012). Curcumin can protect synapses and neurons against dysfunction and degeneration in experimental models relevant to the pathogenesis of Alzheimer’s disease (Zhang et al., 2010; Ahmed et al., 2011), Parkinson’s disease (Liu et al., 2011), retinal degeneration (Mandal et al., 2009) and neuroinflammation (Kawamoto et al., 2013). The beneficial effects of curcumin in these models involves activation of Nrf2 and differential modulation of NF-κB activity in neurons and glial cells (Kang et al., 2004; Kawamoto et al., 2013). A remarkable array of disease processes may be ameliorated by curcumin acting to reduce inflammation and induce adaptive cellular stress responses including diabetes, cardiovascular disease, arthritis irritable bowel disease and gastric inflammation (for review see Gupta et al., 2013).
The flavonoids resveratrol and epicatechin are also emerging as phytochemicals that can improve health by hormesis-based mechanisms. Resveratrol can activate several cellular stress response pathways including those involving AMPK, Akt and the histone deacetylase Sirt1 (Mattson, 2008; Sun et al., 2010; Turan et al., 2012). Recent findings indicate that resveratrol inhibits a cyclic AMP phosphodiesterase resulting in an increase in intracellular cyclic AMP and calcium release from the endoplasmic reticulum and that, as a result, NAD+ levels increase and Sirt1 is activated (Park et al., 2012). Selective inhibitors of the same phosphodiesterase with the drug rolipram mimics metabolic benefits of resveratrol by protecting mice against diet-induced obesity and diabetes (Park et al., 2012). Epicatchins have been found to have broad neuroprotective actions against oxidative and metabolic stress in experimental models relevant to the pathogenesis of stroke, Alzheimer’s and Parkinson’s diseases, and HIV dementia (Sutherland et al., 2006; Lin et al., 2009; Schroeder et al., 2009; Nath et al., 2012). Studies of cultured neural cells suggest that the underlying mechanisms involve activation of the PI3 kinase – Akt pathways and stimulation of BDNF production (Jang et al., 2010; Nath et al., 2012). Epicatechins may also reduce the risk of cardiovascular disease by lowering levels of pro-atherosclerotic lipids and reducing blood pressure (Moore et al., 2009; Ellam and Williamson, 2013).
One interesting aspect of the health-promoting actions of some phytochemicals is that, while they can protect normal cells against various types of stress, they can also inhibit the growth of, and even kill, tumor cells. Sulforaphane and curcumin have been widely reported to have anti-cancer actions. In multiple types of cancers sulforaphane can cause cell cycle arrest and cell death, and may also suppress tumor growth by inhibiting angiogenesis resulting in reduced blood supply to the tumor (for review see Juge et al., 2007). Cultured colon cancer cells can be killed by sulforaphane by a mechanism involving production of the pro-apoptotic protein Bax (Gamet-Payrastre, 2000). In prostate cancer cells sulforaphane can cause glutathione depletion which triggers mitochondrial membrane depolarization, release of cytochrome c and activation of caspases (Singh et al., 2005). The reason that, at a given concentration, sulforaphane can kill cancer cells but not normal cells is poorly understood but may involve inhibition of histone deacetylases in the cancer cells (Clarke et al., 2011) and differential effects on mitogen-activated protein kinases in normal and cancer cells (Zeng et al., 2011). The anti-cancer effects of curcumin may involve mechanisms that overlap with those of sulforaphane. Curcumin can trigger apoptosis of cancer cells by inhibiting anti-apoptotic pathways and activating pro-apoptotic pathways (for review see Reuter et al., 2008).
CAN SOCIETY EMBRACE THE REALITIES OF THE HORMESIS-BASED REQUIREMENTS FOR OPTIMAL HEALTH AND DISEASE PREVENTION?
Intermittent fasting, regular exercise and consumption of dietary phytochemicals can have a major positive impact on health by bolstering adaptive cellular stress response pathways that protect against and counteract a range of major diseases. Why then are these pillars of health crumbling in many industrialized countries? Unfortunately, the United States has been the major source of the epidemics of obesity, diabetes and associated chronic diseases (cardiovascular disease, stroke, cancers, neurodegenerative disorders and others). Compared to all other countries, the US spends more money on health care and yet has poorer outcomes (US Burden of Disease Collaborators, 2013). Most of the poor outcomes are the result of “anhormesis”, a lack of hormesis resulting from unchallenging diets and lifestyles. Among the US States, and within large cities, there is a strong association of diet and lifestyle (and socioeconomic status) with the incidence of obesity, diabetes, cardiovascular disease and stroke. Technological advances have greatly reduced the need for exercise in the workplace, as well as the need to walk or ride a bicycle to work. Thus, in addition to having continuous access to high energy density processed foods, the US population is largely sedentary. The southeastern states (e.g., Louisiana, Mississippi and Georgia) and inner cities have the highest disease burdens (Liao et al., 2009; Barker et al., 2011). Why should those with less income be more likely to be obese and diabetic? If they ate less food, their health would improve and they would have more money to spend on other things. While this is a complex issue, it is certainly the case that the processed food industry has played a major role in supplying our populace with relatively low cost high energy density, phytochemical poor foods. Moreover, most such foods are addictive in that they are formulated to be highly palatable and often contain high amounts of simple carbohydrates (e.g., fructose) that stimulate appetite. Contributing to the pervasiveness of poor quality addictive foods has been large-scale agriculture which produces large amounts of corn, wheat and soybeans which are used to make corn syrup, refined flour and hydrogenated oils that have proven to be detrimental to health (Fields, 2004).
Another factor that has likely contributed to low numbers of people who are on restricted energy/intermittent fasting diets that include large amounts of fruits and vegetables is, ironically, the pharmaceutical and health care industries. During the past century there were several major advances in medicine that reduced early deaths; these included antibiotics, vaccinations, and life-extending treatments for cardiovascular disease and some cancers. Many among the US populace have come to believe that if they develop a disease there will be a drug they can take to reverse the disease process. Unfortunately for them and the overall health of our nation, this is not true. In fact, deaths from cancers, cardiovascular disease and complications of diabetes remain high. Moreover, while lives of many in their 50s and 60s can be prolonged by aggressive drug treatment or surgery, those who survive are likely to develop Alzheimer’s disease, Parkinson’s disease or suffer a stroke, three devastating conditions for which there are no disease-modifying treatments (Figure 3).
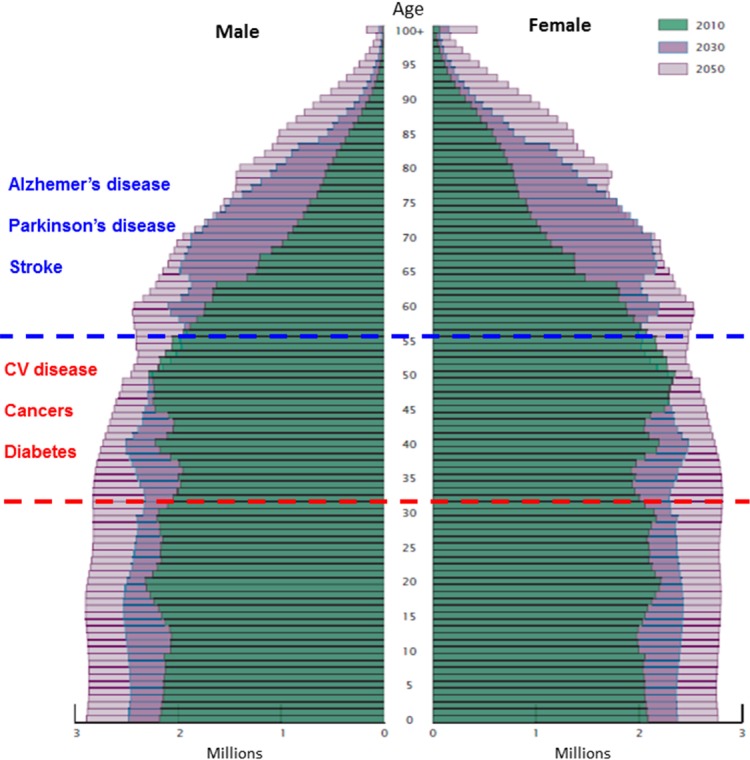
FIGURE 3.
Age and sex structure of the U. S. population in the years 2010, 2030 and 2050. Because of advances in the early diagnosis and treatment of diabetes, cardiovascular disease and some cancers, many individuals who would have previously died when they were 40–60 years old are living into their 70s and 80s. However, those who live into their 70s and 80s are at high risk for stroke, Alzheimer’s and Parkinson’s disease. Unfortunately, there are no effective treatments for stroke, Alzheimer’s and Parkinson’s diseases. Source: Vincent and Velkoff, 2010.
While largely unintentional, medical education and practice have changed during the past 50 years in ways that have led to a hastening of drug and surgical interventions in individuals who would benefit more from prescriptions (and rigorous counseling) for dietary modifications and exercise. Medical school curricula place little or no emphasis on diet and exercise; instead, the focus is on specific diseases and the drugs or surgical treatments for those diseases. Specialization of physicians has become so focused that the underlying causes of many diseases go largely unaddressed. Cardiologists, oncologists, gastroenterologists, pulmonologists and even orthopedists ‘fix’ (albeit often only transiently incompletely) diseases fostered by sedentary gluttonous lifestyles. The need for early intervention with prescriptions for dietary restriction/intermittent fasting and exercise is urgent. In this modern information age it should be and, indeed is, possible for health care providers to give patients choices for intermittent fasting and exercise routines from which they can choose those that best fit their daily and weekly routines. For example, a patient who is overweight and insulin resistant should be told that unless they follow the doctor’s prescription they will develop diabetes and will be likely to die from a heart attack or stroke at an early age. The patient would then give the patient a choice of two weekly intermittent fasting-based diets: 1) two days each week they eat only one meal and the other five days they eat normally; or 2) Five days each week they do not eat between the hours of 6 PM and 10 AM. Whichever diet the patient chooses, someone in the physician’s office staff would keep in close contact with the patient via text messaging and/or social media to monitor the patient’s progress and to be a ‘cheerleader’ to help the patient adjust to their new eating pattern. Based upon recent findings (Harvie et al., 2011, 2013), a high percentage of patients will be able to adapt to the healthier eating pattern and may incorporate it into their lifestyle long-term as they experience better overall health.
Finally, parents should be better educated as to healthy diet and exercise patterns, and why hormesis/adaptive responses are responsible for the beneficial effects of exercise, intermittent fasting, and consumption of fruits and vegetables. Importantly, myths surrounding diet and exercise should be dispelled. It was not too long ago that it was a common belief that someone with heart disease should not exercise because ‘it puts too much stress on the heart’. However, that myth was ‘busted’ by highly reproducible evidence that exercise increases the survival of patients with heart disease. One example of an ongoing myth that is refuted by scientific studies is the notion that eating numerous small meals is healthier than skipping meals. In fact, short fasts of 16–24 hours activate adaptive stress responses that protect against disease, whereas eating three or more meals per day prevents activation of the adaptive responses. Another myth is that ingestion of antioxidant vitamins can substitute for the consumption of fruits and vegetables. Recent placebo-controlled trials of vitamins in human subjects refute the latter myth and, in fact, emerging findings suggest that the chemicals in fruits and vegetables that are good for health actually act by inducing adaptive stress responses (see preceding section). Children’s behaviors, including eating patterns and exercise, are learned and reinforced by their parents. Until parents are well informed regarding what they and their children should do to optimize their health, and until they understand the consequences of their not doing so, it will be difficult to implement hormesis-based lifestyles on a large-scale basis.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, NIH.
REFERENCES
- Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T, Gilani AH, Hosseinmardi N, Semnanian S, Enam SA, Fathollahi Y. Curcuminoids rescue long-term potentiation impaired by amyloid peptide in rat hippocampal slices. Synapse. 2011;65:572–582. doi: 10.1002/syn.20876. [DOI] [PubMed] [Google Scholar]
- Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6:702–710. doi: 10.4161/auto.6.6.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp TE, Kiselev S, Wyatt S, Davies AM. Role of Bcl-2 in the brain-derived neurotrophic factor survival response. Eur J Neurosci. 1995;7:1266–1272. doi: 10.1111/j.1460-9568.1995.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker LE, Kirtland KA, Gregg EW, Geiss LS, Thompson TJ. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. Am J Prev Med. 2011;40:434–439. doi: 10.1016/j.amepre.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Bhardwaj P, Khanna D. Green tea catechins: defensive role in cardiovascular disorders. Chin J Nat Med. 2013;11:345–353. doi: 10.1016/S1875-5364(13)60051-5. [DOI] [PubMed] [Google Scholar]
- Belkacemi L, Selselet-Attou G, Louchami K, Sener A, Malaisse WJ. Intermittent fasting modulation of the diabetic syndrome in sand rats. II. In vivo investigations. Int J Mol Med. 2010;26:759–765. doi: 10.3892/ijmm_00000523. [DOI] [PubMed] [Google Scholar]
- Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23:817–822. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Mattson MP, Calabrese V. Resveratrol commonly displays hormesis: occurrence and biomedical significance. Hum Exp Toxicol. 2010;29:980–1015. doi: 10.1177/0960327110383625. [DOI] [PubMed] [Google Scholar]
- Chao CC, Ma YL, Lee EH. Brain-derived neurotrophic factor enhances Bcl-xL expression through protein kinase casein kinase 2-activated and nuclear factor kappa B-mediated pathway in rat hippocampus. Brain Pathol. 2011;21:150–162. doi: 10.1111/j.1750-3639.2010.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004;2004(225):re5. doi: 10.1126/stke.2252004re5. 2004 Mar 16. [DOI] [PubMed] [Google Scholar]
- Chen DY, Bambah-Mukku D, Pollonini G, Alberini CM. Glucocorticoid receptors recruit the CaMKIIα-BDNF-CREB pathways to mediate memory consolidation. Nat Neurosci. 2012;15:1707–1714. doi: 10.1038/nn.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Wang S, Cai J, Rao MS, Mattson MP. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol. 2003;258:319–333. doi: 10.1016/s0012-1606(03)00120-9. [DOI] [PubMed] [Google Scholar]
- Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Son TG, Park HR, Jang YJ, Oh SB, Jin B, Lee J. Naphthazarin has a protective effect on the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson’s disease model. J Neurosci Res. 2012;90:1842–1849. doi: 10.1002/jnr.23061. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Hsu A, Yu Z, Dashwood RH, Ho E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol Nutr Food Res. 2011;55:999–1009. doi: 10.1002/mnfr.201000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copple IM, Goldring CE, Kitteringham NR, Park BK. The keap1-nrf2 cellular defense pathway: mechanisms of regulation and role in protection against drug-induced toxicity. Handb Exp Pharmacol. 2010;196:233–266. doi: 10.1007/978-3-642-00663-0_9. [DOI] [PubMed] [Google Scholar]
- Diaz F, Moraes CT. Mitochondrial biogenesis and turnover. Cell Calcium. 2008;44:24–35. doi: 10.1016/j.ceca.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellam S, Williamson G. Cocoa and human health. Annu Rev Nutr. 2013;33:105–128. doi: 10.1146/annurev-nutr-071811-150642. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Fields S. The fat of the land: do agricultural subsidies foster poor health? Environ Health Perspect. 2004;112:A820–823. doi: 10.1289/ehp.112-a820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M. Physical activity and stress resistance: sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc Sport Sci Rev. 2005;33:120–126. doi: 10.1097/00003677-200507000-00004. [DOI] [PubMed] [Google Scholar]
- Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Tercé F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- Gavaldà N, Gutierrez H, Davies AM. Developmental switch in NF-kappaB signalling required for neurite growth. Development. 2009;136:3405–3412. doi: 10.1242/dev.035295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervois P, Mansouri RM. PPARα as a therapeutic target in inflammation-associated diseases. Expert Opin Ther Targets. 2012;16:1113–1125. doi: 10.1517/14728222.2012.715633. [DOI] [PubMed] [Google Scholar]
- Gomez–Pinilla F, Hillman C. The influence of exercise on cognitive abilities. Compr Physiol. 2013;3:403–428. doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Differential effects of intermittent feeding and voluntary exercise on body weight and lifespan in adult rats. J Gerontol. 1983;38:36–45. doi: 10.1093/geronj/38.1.36. [DOI] [PubMed] [Google Scholar]
- Grant RW, Mariani RA, Vieira VJ, Fleshner M, Smith TP, Keylock KT, Lowder TW, McAuley E, Hu L, Chapman-Novakofski K, Woods JA. Cardiovascular exercise intervention improves the primary antibody response to keyhole limpet hemocyanin (KLH) in previously sedentary older adults. Brain Behav Immun. 2008;22:923–932. doi: 10.1016/j.bbi.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. 2008;10:81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Loughridge AB, Sadaoui N, Christianson JP, Fleshner M. The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behav Brain Res. 2012;233:314–321. doi: 10.1016/j.bbr.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland DA, Sabljic TF, Baribeau DA, Mukovozov IM, Hart LE. Is regular exercise a friend or foe of the aging immune system? A systematic review. Clin J Sport Med. 2008;18:539–548. doi: 10.1097/JSM.0b013e3181865eec. [DOI] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, Cutler RG, Evans G, Whiteside S, Maudsley S, Camandola S, Wang R, Carlson OD, Egan JM, Mattson MP, Howell A. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110:1534–1547. doi: 10.1017/S0007114513000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie M, Howell T. Genesis Breast Cancer Prevention. Random House, Inc.; New York, New York: 2013. The 2 Day Diet; p. 360. [Google Scholar]
- He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL, Tytell M, Vígh L. Xenohormesis: health benefits from an eon of plant stress response evolution. Cell Stress Chaperones. 2010;15:761–770. doi: 10.1007/s12192-010-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012;165:574–590. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Jamart C, Naslain D, Gilson H, Francaux M. Higher activation of autophagy in skeletal muscle of mice during endurance exercise in the fasted state. Am J Physiol Endocrinol Metab. 2013;305:E964–974. doi: 10.1152/ajpendo.00270.2013. [DOI] [PubMed] [Google Scholar]
- Jang S, Jeong HS, Park JS, Kim YS, Jin CY, Seol MB, Kim BC, Lee MC. Neuroprotective effects of (−)-epigallocatechin-3-gallate against quinolinic acid-induced excitotoxicity via PI3K pathway and NO inhibition. Brain Res. 2010;1313:25–33. doi: 10.1016/j.brainres.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G, Johnson JA. The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat CNS Drug Discov. 2012;7:218–229. doi: 10.2174/157488912803252023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Kong PJ, Yuh YJ, Lim SY, Yim SV, Chun W, Kim SS. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappab bindings in BV2 microglial cells. J Pharmacol Sci. 2004;94:325–328. doi: 10.1254/jphs.94.325. [DOI] [PubMed] [Google Scholar]
- Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34:1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto EM, Scavone C, Mattson MP, Camandola S. Curcumin requires tumor necrosis factor α signaling to alleviate cognitive impairment elicited by lipopolysaccharide. Neurosignals. 2013;21:75–88. doi: 10.1159/000336074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD, Chen JG, Chen TY, Fahey JW, Talalay P. Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Top Curr Chem. 2013;329:163–177. doi: 10.1007/128_2012_339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul O. Insect Antifeedants. CRC Press; New York, NY: 2005. p. 1005. [Google Scholar]
- Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr Opin Drug Discov Devel. 2006;9:580–586. [PubMed] [Google Scholar]
- Lee S, Notterpek L. Dietary restriction supports peripheral nerve health by enhancing endogenous protein quality control mechanisms. Exp Gerontol. 2013;48:1085–1090. doi: 10.1016/j.exger.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, Emionite L, de Cabo R, Longo VD. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4(124):124ra27. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Greenlund KJ, Croft JB, Keenan NL, Giles WH. Factors explaining excess stroke prevalence in the US Stroke Belt. Stroke. 2009;40:3336–3341. doi: 10.1161/STROKEAHA.109.561688. [DOI] [PubMed] [Google Scholar]
- Lin CL, Chen TF, Chiu MJ, Way TD, Lin JK. Epigallocatechin gallate (EGCG) suppresses beta-amyloid-induced neurotoxicity through inhibiting c-Abl/FE65 nuclear translocation and GSK3 beta activation. Neurobiol Aging. 2009;30:81–92. doi: 10.1016/j.neurobiolaging.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Little JP, Safdar A, Benton CR, Wright DC. Skeletal muscle and beyond: the role of exercise as a mediator of systemic mitochondrial biogenesis. Appl Physiol Nutr Metab. 2011;36:598–607. doi: 10.1139/h11-076. [DOI] [PubMed] [Google Scholar]
- Liu Z, Yu Y, Li X, Ross CA, Smith WW. Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for Parkinsonism. Pharmacol Res. 2011;63:439–444. doi: 10.1016/j.phrs.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal MN, Patlolla JM, Zheng L, Agbaga MP, Tran JT, Wicker L, Kasus-Jacobi A, Elliott MH, Rao CV, Anderson RE. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Med. 2009;46:672–679. doi: 10.1016/j.freeradbiomed.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K, Bori Z, Hart N, Sárga L, Koltai E, Radák Z, Nyakas C. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience. 2012;226:21–28. doi: 10.1016/j.neuroscience.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008;7:43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012a;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Evolutionary aspects of human exercise—born to run purposefully. Ageing Res Rev. 2012b;11:347–352. doi: 10.1016/j.arr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally MA, Hartman AL. Ketone bodies in epilepsy. J Neurochem. 2012;121:28–35. doi: 10.1111/j.1471-4159.2012.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RJ, Jackson KG, Minihane AM. Green tea (Camellia sinensis) catechins and vascular function. Br J Nutr. 2009;102:1790–1802. doi: 10.1017/S0007114509991218. [DOI] [PubMed] [Google Scholar]
- Moraska A, Fleshner M. Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression. Am J Physiol Regul Integr Comp Physiol. 2001;281:R484–489. doi: 10.1152/ajpregu.2001.281.2.R484. [DOI] [PubMed] [Google Scholar]
- Morroni F, Tarozzi A, Sita G, Bolondi C, Zolezzi Moraga JM, Cantelli-Forti G, Hrelia P. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson’s disease. Neurotoxicology. 2013;36:63–71. doi: 10.1016/j.neuro.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Mosley M, Spencer M. The Fast Diet. Atria Books; New York, New York: 2013. p. 208. [Google Scholar]
- Motl RW, Pilutti LA. The benefits of exercise training in multiple sclerosis. Nat Rev Neurol. 2012;8:487–497. doi: 10.1038/nrneurol.2012.136. [DOI] [PubMed] [Google Scholar]
- Nath S, Bachani M, Harshavardhana D, Steiner JP. Catechins protect neurons against mitochondrial toxins and HIV proteins via activation of the BDNF pathway. J Neurovirol. 2012;18:445–455. doi: 10.1007/s13365-012-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br J Clin Pharmacol. 2013;75:716–727. doi: 10.1111/j.1365-2125.2012.04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25:42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman DC, Buckley KS, Henson DA, Warren BJ, Suttles J, Ahle JC, Simandle S, Fagoaga OR, Nehlsen-Cannarella SL. Immune function in marathon runners versus sedentary controls. Med Sci Sports Exerc. 1995;27:986–992. doi: 10.1249/00005768-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Dumke CI, Henson DA, McAnulty SR, McAnulty LS, Lind RH, Morrow JD. Immune and oxidative changes during and following the Western States Endurance Run. Int J Sports Med. 2003;24:541–547. doi: 10.1055/s-2003-42018. [DOI] [PubMed] [Google Scholar]
- Ong SG, Hausenloy DJ. Hypoxia-inducible factor as a therapeutic target for cardioprotection. Pharmacol Ther. 2012;136:69–81. doi: 10.1016/j.pharmthera.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao CS, Gao S, Lee GH, Kim do S, Park BH, Chae SW, Chae HJ, Kim SH. Sulforaphane protects ischemic injury of hearts through antioxidant pathway and mitochondrial K(ATP) channels. Pharmacol Res. 2010;61:342–348. doi: 10.1016/j.phrs.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Reuter S, Eifes S, Dicato M, Aggarwal BB, Diederich M. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem Pharmacol. 2008;76:1340–1351. doi: 10.1016/j.bcp.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Rowe GC, Safdar A, Arany Z. Running forward: new frontiers in endurance exercise biology. Circulation. 2014;129:798–810. doi: 10.1161/CIRCULATIONAHA.113.001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD. Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 2009;1:988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder EK, Kelsey NA, Doyle J, Breed E, Bouchard RJ, Loucks FA, Harbison RA, Linseman DA. Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antioxid Redox Signal. 2009;11:469–480. doi: 10.1089/ars.2008.2215. [DOI] [PubMed] [Google Scholar]
- Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- Sen R. Control of B lymphocyte apoptosis by the transcription factor NF-kappaB. Immunity. 2006;25:871–883. doi: 10.1016/j.immuni.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Sinclair U. The Fasting Cure. Mitchell Kennerley; New York: 1911. p. 153. [Google Scholar]
- Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- Smith TP, Kennedy SL, Fleshner M. Influence of age and physical activity on the primary in vivo antibody and T cell-mediated responses in men. J Appl Physiol. 2004;97:491–498. doi: 10.1152/japplphysiol.01404.2003. [DOI] [PubMed] [Google Scholar]
- Son TG, Camandola S, Arumugam TV, Cutler RG, Telljohann RS, Mughal MR, Moore TA, Luo W, Yu QS, Johnson DA, Johnson JA, Greig NH, Mattson MP. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem. 2010;112:1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speaker KJ, Cox SS, Paton MM, Serebrakian A, Maslanik T, Greenwood BN, Fleshner M. Six weeks of voluntary wheel running modulates inflammatory protein (MCP-1, IL-6, and IL-10) and DAMP (Hsp72) responses to acute stress in white adipose tissue of lean rats. Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2013.10.028. 2013 Nov 15. pii: S0889-1591(13)00527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain ageing. Nat Rev Neurosci. 2012;13:209–216. doi: 10.1038/nrn3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging. 2010;31:1937–1949. doi: 10.1016/j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland BA, Rahman RM, Appleton I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J Nutr Biochem. 2006;17:291–306. doi: 10.1016/j.jnutbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Tierney M, Fraser A, Kennedy N. Physical activity in rheumatoid arthritis: a systematic review. J Phys Act Health. 2012;9:1036–1048. doi: 10.1123/jpah.9.7.1036. [DOI] [PubMed] [Google Scholar]
- Turan B, Tuncay E, Vassort G. Resveratrol and diabetic cardiac function: focus on recent in vitro and in vivo studies. J Bioenerg Biomembr. 2012;44:281–296. doi: 10.1007/s10863-012-9429-0. [DOI] [PubMed] [Google Scholar]
- US Burden of Disease Collaborators The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent GK, Velkoff VA. The next four decades: the older population in the United States 2010 to 2050. U. S. Census Bureau; 2010. p. 1138. P25. [Google Scholar]
- Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90:1138–1143. doi: 10.3945/ajcn.2009.28380. [DOI] [PubMed] [Google Scholar]
- Vivar C, Potter MC, van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci. 2013;15:189–210. doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133:1921–1929. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, Mattson MP. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem. 2010;21:413–417. doi: 10.1016/j.jnutbio.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz T. Regulation of mitochondrial biogenesis and PGC-1α under cellular stress. Mitochondrion. 2013;13:134–142. doi: 10.1016/j.mito.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Li X. Curcumin, an active constiuent of the ancient medicinal herb Curcuma longa L.: some uses and the establishment and biological basis of medical efficacy. CNS Neurol Disord Drug Targets. 2013;12:487–497. doi: 10.2174/1871527311312040007. [DOI] [PubMed] [Google Scholar]
- Wu TY, Khor TO, Lee JH, Cheung KL, Shu L, Chen C, Kong AN. Pharmacogenetics, pharmacogenomics and epigenetics of Nrf2-regulated xenobiotic-metabolizing enzymes and transporters by dietary phytochemical and cancer chemoprevention. Curr Drug Metab. 2013;14:688–694. doi: 10.2174/1389200211314060005. [DOI] [PubMed] [Google Scholar]
- Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolecular Med. 2014;16:161–174. doi: 10.1007/s12017-013-8270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yessoufou A, Wahli W. Multifaceted roles of peroxisome proliferator-activated receptors (PPARs) at the cellular and whole organism levels. Swiss Med Wkly. 2010;140:w13071. doi: 10.4414/smw.2010.13071. 2010 Sep 15. [DOI] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- Zeng H, Trujillo ON, Moyer MP, Botnen JH. Prolonged sulforaphane treatment activates survival signaling in nontumorigenic NCM460 colon cells but apoptotic signaling in tumorigenic HCT116 colon cells. Nutr Cancer. 2011;63:248–255. doi: 10.1080/01635581.2011.523500. [DOI] [PubMed] [Google Scholar]
- Zhang C, Browne A, Child D, Tanzi RE. Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J Biol Chem. 2010;285:28472–28480. doi: 10.1074/jbc.M110.133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Zimmer C. Human evolution. Faster than a hyena? Running may make humans special. Science. 2004;306:1283. doi: 10.1126/science.306.5700.1283. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, Seyfried TN. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr Metab (Lond) 2010;7:33. doi: 10.1186/1743-7075-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]