Abstract
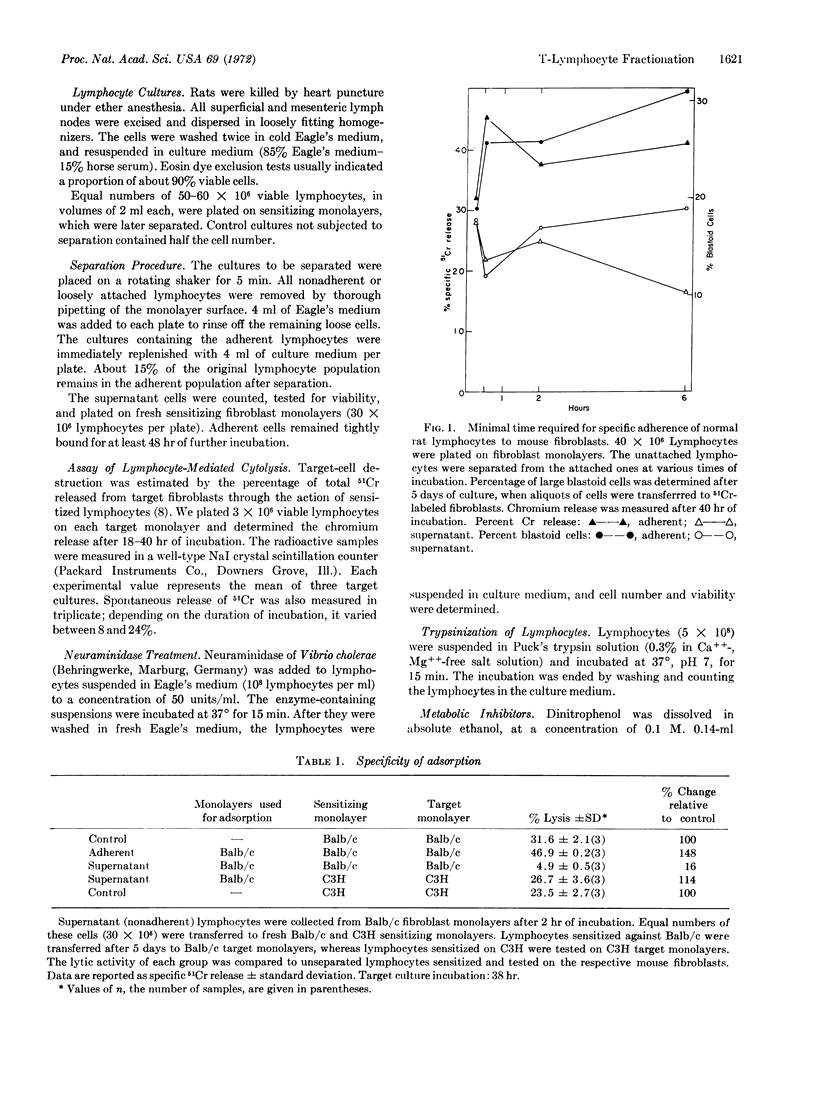
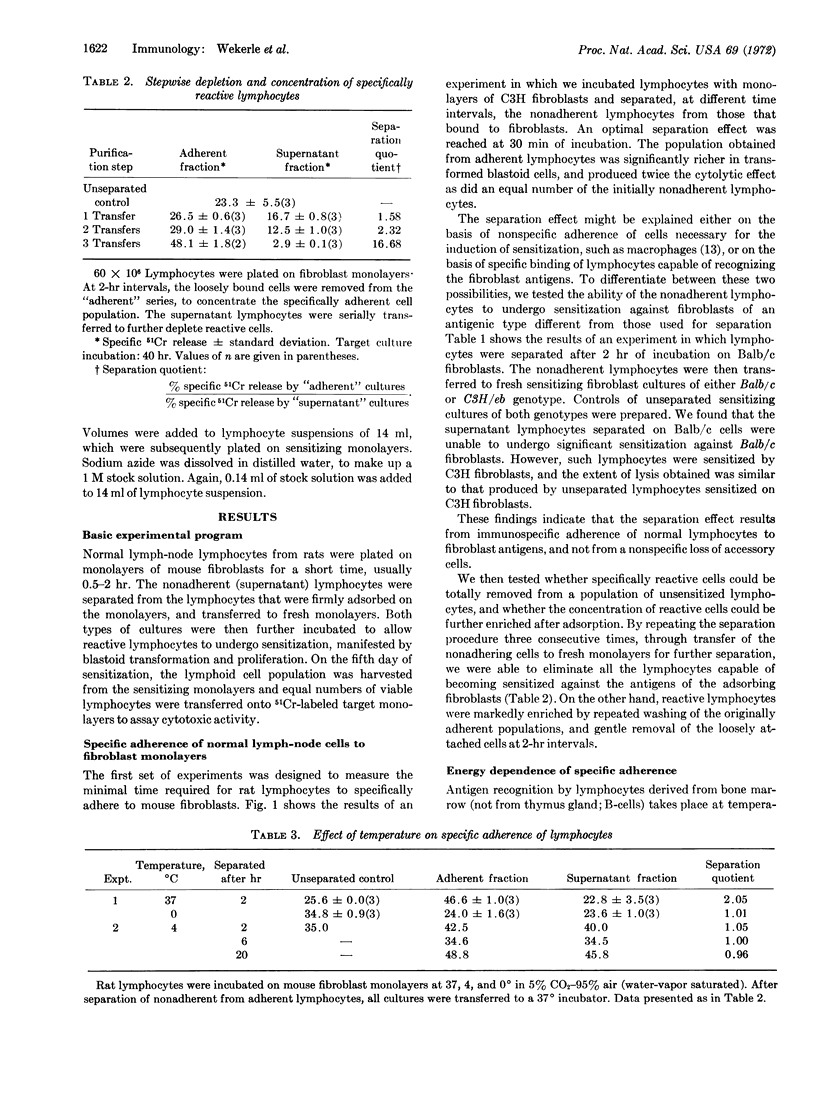
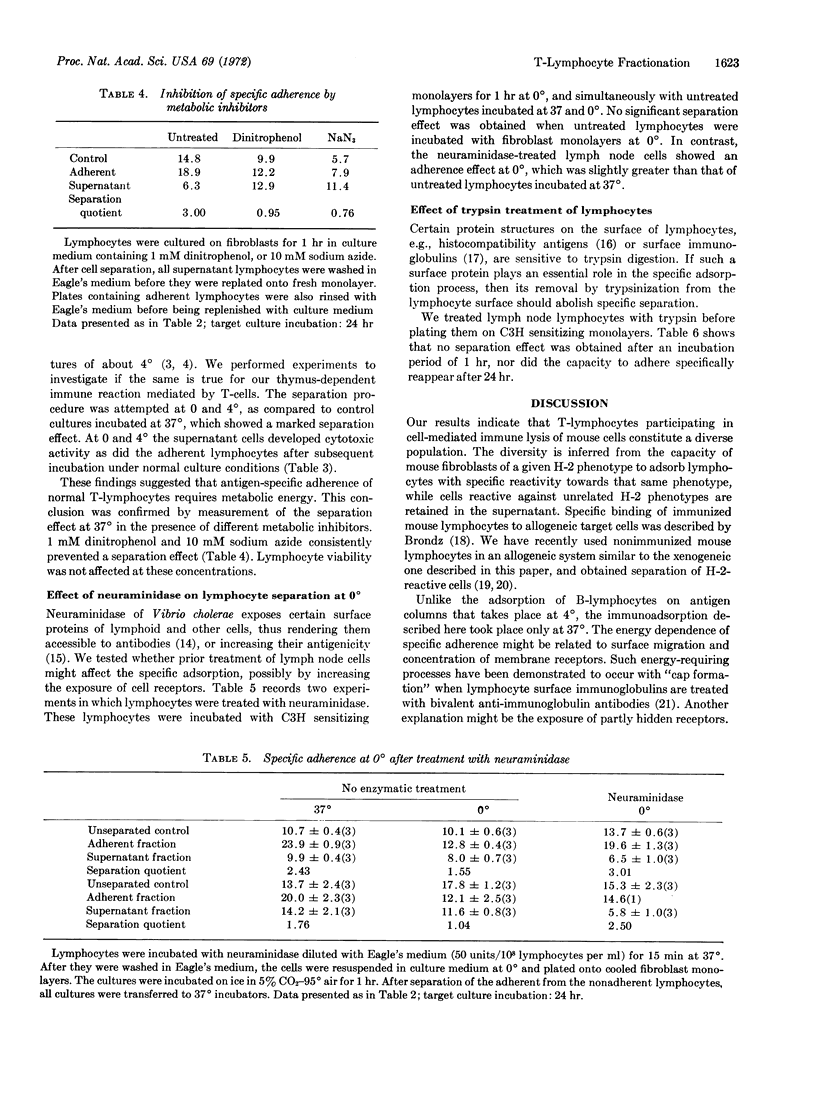
Monolayers of mouse fibroblasts were used as cellular immunoadsorbents to separate rat lymphocytes that recognize specific mouse histocompatibility antigens. Normal lymphocytes were incubated on fibroblasts of strain C3H/eb, and nonadherent cells were separated from adherent cells, then transferred for sensitization onto fresh monolayers of C3H. When tested on 51Cr-labeled target monolayers the nonadherent cells manifested significantly lower cytotoxicity than the adherent cells. However, the nonadherent cells could be sensitized against mouse fibroblasts of an unrelated H-2 phenotype (strain Balb/c). The immune specificity of the adherence was further demonstrated by a stepwise adsorption, which resulted in complete loss of the capacity to acquire specific lytic activity towards C3H antigens. Lymphocytes recognizing strain-specific antigens of the mouse were separated at 37° (not at 0° or 4°); separation was inhibited after treatment with dinitrophenol, sodium azide, or trypsin. Prior treatment with neuraminidase rendered the lymphocytes suceptible to separation at 0°.
Keywords: mouse fibroblasts, cell receptors, cell culture
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Byrt P. Specific inactivation of antigen-reactive cells with 125I-labelled antigen. Nature. 1969 Jun 28;222(5200):1291–1292. doi: 10.1038/2221291a0. [DOI] [PubMed] [Google Scholar]
- Berke G., Ax W., Ginsburg H., Feldman M. Graft reaction in tissue culture. II. Quantification of the lytic action on mouse fibroblasts by rat lymphocytes sensitized on mouse embryo monolayers. Immunology. 1969 May;16(5):643–657. [PMC free article] [PubMed] [Google Scholar]
- Brondz B. D., Snegiröva A. E. Interaction of immune lymphocytes with the mixtures of target cells possessing selected specificities of the H-2 immunizing allele. Immunology. 1971 Apr;20(4):457–468. [PMC free article] [PubMed] [Google Scholar]
- Clark W. R., Berke G., Feldman M., Sarid S. Macromolecular synthesis during the sensitization of rat lymphcytes on mouse fibroblasts in vitro. Immunochemistry. 1971 Jun;8(6):487–498. doi: 10.1016/0019-2791(71)90400-9. [DOI] [PubMed] [Google Scholar]
- Cohen I. R., Feldman M. The lysis of fibroblasts by lymphocytes sensitized in vitro: specific antigen activates a nonspecific effect. Cell Immunol. 1970 Nov;1(5):521–535. doi: 10.1016/0008-8749(70)90039-0. [DOI] [PubMed] [Google Scholar]
- Currie G. A., Bagshawe K. D. The role of sialic acid in antigenic expression: further studies of the Landschütz ascites tumour. Br J Cancer. 1968 Dec;22(4):843–853. doi: 10.1038/bjc.1968.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Sachs L. Destruction of mouse and rat embryo cells in tissue culture by lymph node cells from unsensitized rats. J Cell Physiol. 1965 Oct;66(2):199–219. doi: 10.1002/jcp.1030660207. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Torrigiani G., Roitt I. M. Inhibition of human mixed lymphocyte reaction by antibodies to immunoglobulin light chain determinants. Clin Exp Immunol. 1971 Sep;9(3):313–328. [PMC free article] [PubMed] [Google Scholar]
- Kunin S., Shearer G. M., Segal S., Globerson A., Feldman M. A bicellular mechanism in the immune response to chemically defined antigens. 3. Interaction of thymus and bone marrow-derived cells. Cell Immunol. 1971 Jun;2(3):229–238. doi: 10.1016/0008-8749(71)90042-6. [DOI] [PubMed] [Google Scholar]
- Lesley J. F., Kettman J. R., Dutton R. W. Immunoglobulins on the surface of thymus-derived cells engaged in the initiation of a humoral immune response. J Exp Med. 1971 Sep 1;134(3 Pt 1):618–629. doi: 10.1084/jem.134.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonai P., Clark W. R., Feldman M. Participation of theta-bearing cell in an in vitro assay of transplantation immunity. Nature. 1971 Feb 19;229(5286):566–567. doi: 10.1038/229566a0. [DOI] [PubMed] [Google Scholar]
- Lonai P., Feldman M. Cooperation of lymphoid cells in an in vitro graft reaction system. The role of the thymus cell. Transplantation. 1970 Nov;10(5):372–381. doi: 10.1097/00007890-197011000-00003. [DOI] [PubMed] [Google Scholar]
- Lonai P., Feldman M. Studies on the effect of macrophages in an in vitro graft reaction system. Immunology. 1971 Nov;21(5):861–867. [PMC free article] [PubMed] [Google Scholar]
- Lonai P., Wekerle H., Feldman M. Fractionation of specific antigen-reactive cells in an in vitro system of cell-mediated immunity. Nat New Biol. 1972 Feb 23;235(60):235–236. doi: 10.1038/newbio235235a0. [DOI] [PubMed] [Google Scholar]
- Mason S., Warner N. L. The immunoglobulin nature of the antigen recognition site on cells mediating transplantation immunity and delayed hypersentivity. J Immunol. 1970 Mar;104(3):762–765. [PubMed] [Google Scholar]
- Naor D., Sulitzneau D. Binding of radioiodinated bovine serum albumin to mouse spleen cells. Nature. 1967 May 13;214(5089):687–688. doi: 10.1038/214687a0. [DOI] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. F. The dispositions of proteins in the plasma membranes of animal cells: analytical approaches using controlled peptidolysis and protein labels. Biochim Biophys Acta. 1972 Feb 14;265(1):61–83. doi: 10.1016/0304-4157(72)90019-6. [DOI] [PubMed] [Google Scholar]
- Walters C. S., Wigzell H. Demonstration of heavy and light chain antigenic determinants on the cell-bound receptor for antigen. Similarities between membrane-attached and humoral antibodies produced by the same cell. J Exp Med. 1970 Dec 1;132(6):1233–1249. doi: 10.1084/jem.132.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Mäkelä O. Separation of normal and immune lymphoid cells by antigen-coated coated columns. Antigen-binding characteristics of membrane antibodies as analyzed by hapten-protein antigens. J Exp Med. 1970 Jul 1;132(1):110–126. doi: 10.1084/jem.132.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]


