Abstract
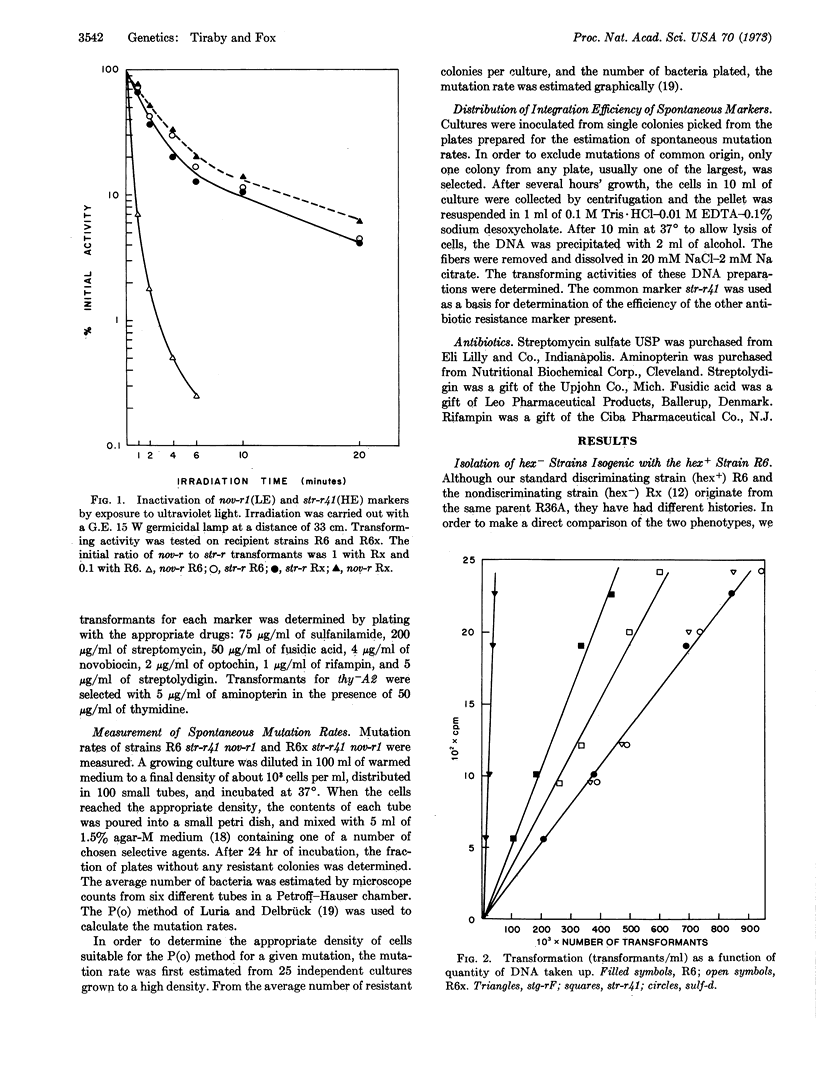
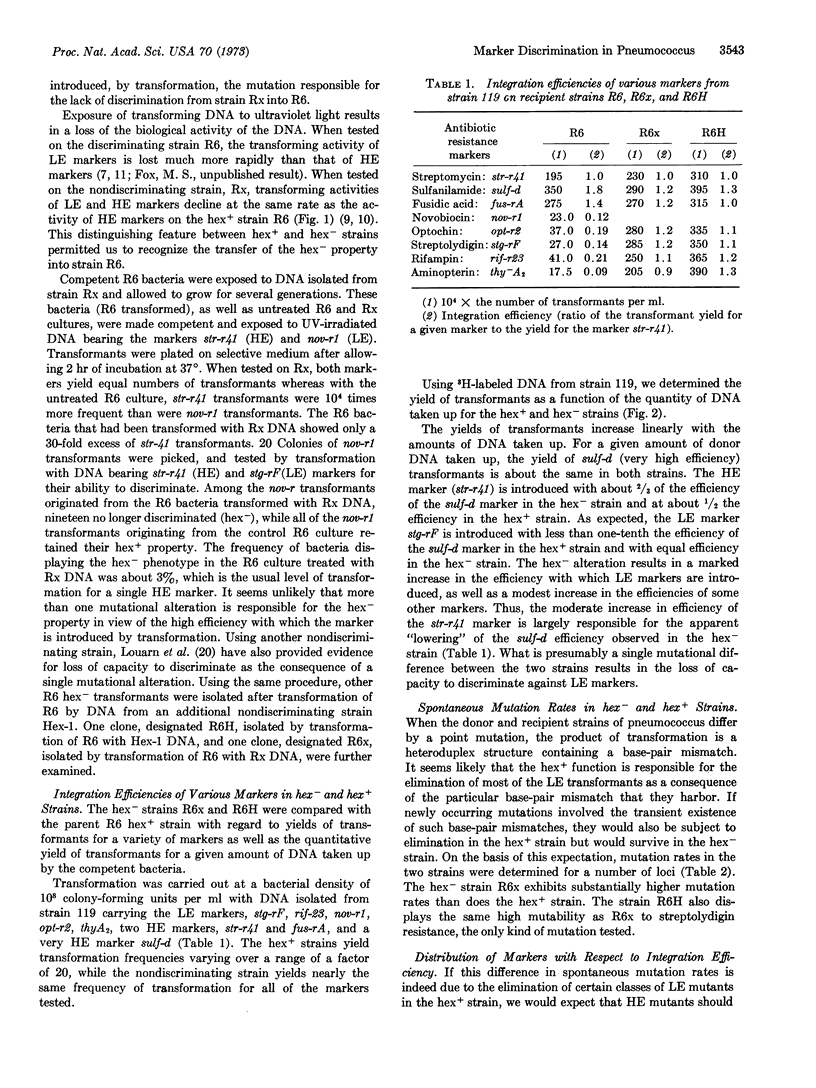
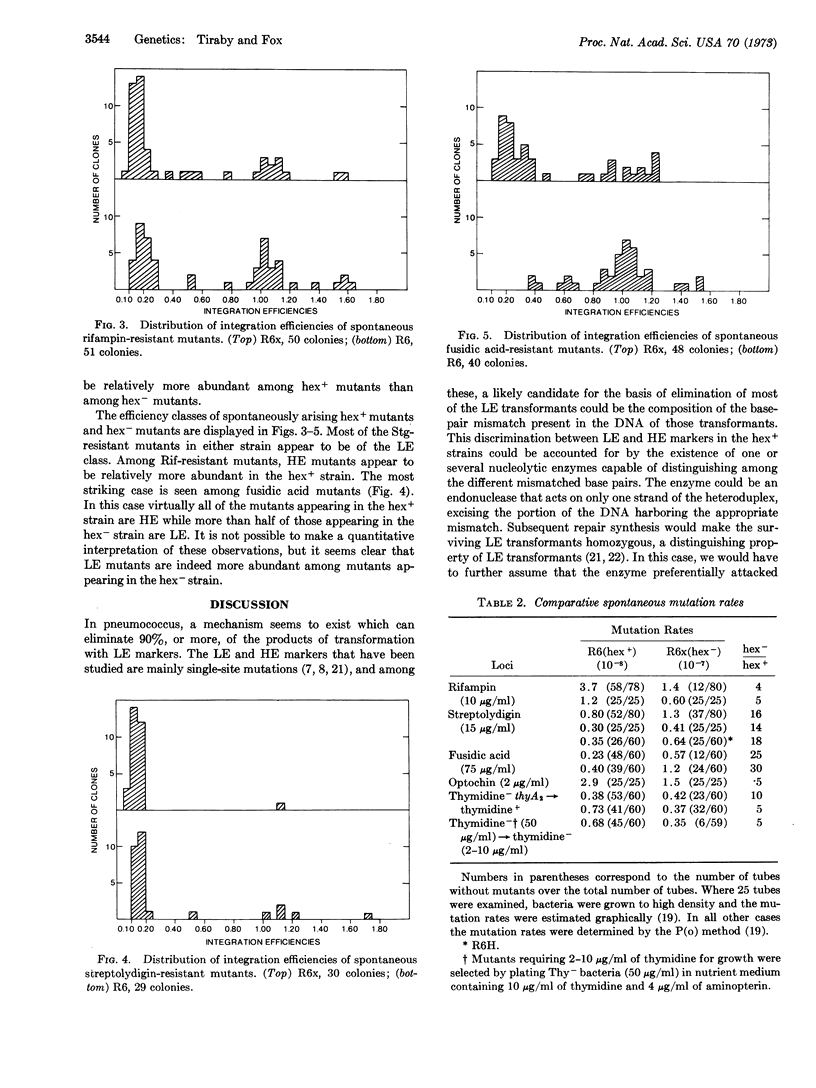
Earlier investigations of pneumococcal transformation revealed a function (hex+) responsible for severely reducing the transformation yield of certain markers. A mutational alteration (hex-) responsible for the loss of this function has been transferred into a hex+ strain to permit a comparison of the hex+ and hex- phenotypes in an isogenic background.
The loss of the hex+ function results both in a loss of the capacity to eliminate the low efficiency markers in transformation and a substantial increase in the spontaneous mutation rate. These properties of the hex- strain could result from the loss of a capacity to eliminate certain classes of mismatched base pairs that occur as intermediates in both transformation and mutagenesis.
Keywords: bacterial transformation, spontaneous mutation, mismatched base pairs
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cato A., Jr, Guild W. R. Transformation and DNA size. I. Activity of fragments of defined size and a fit to a random double cross-over model. J Mol Biol. 1968 Oct 14;37(1):157–178. doi: 10.1016/0022-2836(68)90080-6. [DOI] [PubMed] [Google Scholar]
- Ephrussi-Taylor H., Gray T. C. Genetic studies of recombining DNA in pneumococcal transformation. J Gen Physiol. 1966 Jul;49(6):211–231. doi: 10.1085/jgp.49.6.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi-Taylor H., Sicard A. M., Kamen R. Genetic Recombination in DNA-Induced Transformation of Pneumococcus. I. the Problem of Relative Efficiency of Transforming Factors. Genetics. 1965 Mar;51(3):455–475. doi: 10.1093/genetics/51.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S. Deoxyribonucleic acid incorporation by transformed bacteria. Biochim Biophys Acta. 1957 Oct;26(1):83–85. doi: 10.1016/0006-3002(57)90056-2. [DOI] [PubMed] [Google Scholar]
- Friedman L. R., Ravin A. W. Genetic and biochemical properties of thymidine-dependent mutants of pneumococcus. J Bacteriol. 1972 Jan;109(1):459–461. doi: 10.1128/jb.109.1.459-461.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini F., Fox M. S. Effects of DNA repair in transformation-heterozygotes of pneumococcus. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1116–1123. doi: 10.1073/pnas.59.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney T., Jr, Fox M. S. Physical and genetic hybrids formed in bacterial transformation. J Mol Biol. 1968 Feb 28;32(1):83–100. doi: 10.1016/0022-2836(68)90147-2. [DOI] [PubMed] [Google Scholar]
- HOTCHKISS R. D., EVANS A. H. Analysis of the complex sulfonamide resistance locus of pneumococcus. Cold Spring Harb Symp Quant Biol. 1958;23:85–97. doi: 10.1101/sqb.1958.023.01.012. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S., TOLMACH L. J. Genetic transformation. I. Cellular incorporation of DNA accompanying transformation in Pneumococcus. Biochim Biophys Acta. 1957 Oct;26(1):68–82. doi: 10.1016/0006-3002(57)90055-0. [DOI] [PubMed] [Google Scholar]
- Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966 Jan;53(1):207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVIN A. W. Reciprocal capsular transformations of pneumococci. J Bacteriol. 1959 Mar;77(3):296–309. doi: 10.1128/jb.77.3.296-309.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHEIM M. B., RAVIN A. W. SITES OF BREAKAGE IN THE DNA MOLECULE AS DETERMINED BY RECOMBINATION ANALYSIS OF STREPTOMYCIN-RESISTANCE MUTATIONS IN PNEUMOCOCCUS. Proc Natl Acad Sci U S A. 1964 Jul;52:30–38. doi: 10.1073/pnas.52.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard A. M., Ephrussi-Taylor H. Recombinaison génétique dans la transformation chez le pneumocoque. Etude des réversions au locus amiA. C R Acad Sci Hebd Seances Acad Sci D. 1966 May 23;262(21):2305–2308. [PubMed] [Google Scholar]


