Summary
Homing endonuclease genes (HEGs) exist naturally in many single‐celled organisms and can show extremely strong genetic drive allowing them to spread through populations into which they are introduced. They are being investigated as tools to manipulate the populations of important vectors of human disease, in particular the mosquitoes that transmit malaria. Before HEGs can be deployed, it is important to study their spatial spread in order to design efficient release strategies.
A spatially explicit model is developed to study the spread of a HEG through a landscape whose structure is defined by the distribution of mosquito breeding and feeding sites. The model is motivated by the biology of the major vectors of malaria in Africa. The conditions for spread, fixation and loss of two major types of HEG are explored in different landscapes.
In landscapes where mosquito resources are abundant, the conditions for spread are well approximated by a mean‐field model. Where a HEG imposes a genetic load, it can cause population extinction, though spatial models more often predict population suppression.
In certain types of landscapes where mosquito resources are rare, an introduced HEG may be prevented from moving between local mosquito populations and so a simple release strategy is unlikely to be effective, yet if the HEG succeeds in spreading population extinction is a feasible outcome. Increasing the number of release sites at the expense of releasing fewer mosquitoes per site reduces the probability that a HEG will fail.
Synthesis and applications. The model presented asks for the first time how the spatial structure of mosquito populations will influence the effectiveness of a technology that is being rapidly developed for vector control. If homing endonuclease genes (HEGs) are to be used in this way, we have qualified the importance of accounting for landscape characteristics in both the execution and the expectation of their application. The next stage is to use the model to study the spread of HEGs through real landscapes where releases may take place, something that will be facilitated by the results of the present study.
Keywords: Anopheles, epidemiology, malaria, spatial modelling, spatial spread, vector‐borne disease
Short abstract
The model presented asks for the first time how the spatial structure of mosquito populations will influence the effectiveness of a technology that is being rapidly developed for vector control. If homing endonuclease genes (HEGs) are to be used in this way, we have qualified the importance of accounting for landscape characteristics in both the execution and the expectation of their application. The next stage is to use the model to study the spread of HEGs through real landscapes where releases may take place, something that will be facilitated by the results of the present study.
Introduction
Vector‐borne pathogens are responsible for many of the most significant infectious diseases afflicting mankind today, and of these the most important is malaria (Gilles, Warrell & Bruce‐Chwatt 1993). Approximately 200 million cases of malaria occur each year, and estimates of annual mortality range from three‐quarters of a million to 1·2 million people (WHO 2010; Murray et al. 2011). The major current strategies used to combat malaria involve drug therapy (typically based on artemisinin and its derivatives, Klayman 1985) and mosquito control by spraying insecticide indoors and through the use of insecticide‐treated bed nets (Lengeler 2004; Pluess et al. 2010). Such tactics can be highly effective where well‐functioning public health infrastructure exists, but are difficult to implement in many least‐developed countries (Cohen & Dupas 2010). There is also worrying evidence of the spread of resistance in both the pathogen and the vector (White 2010; Abilio et al. 2011; Chanda et al. 2011). In consequence, there is a global effort to develop new strategies to control malaria and other vector‐borne diseases (Greenwood et al. 2008). One very promising approach is vector population suppression using constructs spread by genetic drive (Sinkins & Gould 2006). As this technology begins to move into a pre‐implementation assessment phase, a critical question is how the genetic elements will spread through landscapes with different spatial distributions of humans and vectors. Spatially explicit population modelling provides valuable tools to address this question.
Homing endonuclease genes (HEGs) are a type of autonomously spreading genetic element found naturally in some single‐celled eukaryotes (Burt & Trivers 2006). The gene is inserted on the chromosome in the middle of a unique DNA sequence that in its uninterrupted form is also the sequence recognized and cut by the endonuclease which the HEG encodes. In a heterozygote (where the HEG is present on only one of the two homologous chromosomes), the endonuclease makes a double‐strand break at the uninterrupted recognition site which is normally repaired using the other chromosome as a template (Stoddard 2005; Taylor & Stoddard 2012). The action of the endonuclease, referred to as ‘homing’, thus converts the heterozygote to a homozygote and all gametes will carry the HEG. This is an exceptionally strong drive mechanism, and in many circumstances the HEG will quickly increase in frequency and becomes fixed in the population.
There are a number of ways that HEGs might be used to help combat vector‐borne diseases (Burt 2003; Deredec, Burt & Godfray 2008). First, the HEG may be appropriately engineered and inserted inside a gene of functional significance. When homing occurs and a homozygote is created, this gene function is lost. If a gene could be identified that was essential for pathogen transmission, but whose loss had minor consequences for vector fitness, then HEG spread could interrupt disease spread. Alternatively, a gene essential for vector survival or reproduction could be targeted so that the spread of the HEG would impose a genetic load leading to population suppression or extinction. A different approach to employing HEG is called X‐shredding. If a HEG is inserted on the Y‐chromosome but with the endonuclease's recognition site on the X, then if the protein is active at spermatogenesis, a heterogametic male would produce only viable Y gametes and the modified Y‐chromosome would be at a selective advantage compared to its wild‐type counterpart. The spread of the HEG‐carrying Y would cause the population sex ratio to become male‐biased leading to population suppression and, if strong‐enough, extinction. Recent progress has demonstrated in the laboratory both classical HEG action and X‐shredding in Anopheles gambiae, the most important vector of malaria in Africa (Windbichler et al. 2007, 2011; Windbichler, Papathanos & Crisanti 2008; Klein et al. 2012).
Investigation of HEGs in the laboratory has been accompanied by population genetic and population dynamic modelling, both to explore different homing scenarios and to guide the choice of different strategies and constructs for detailed development (Deredec, Burt & Godfray 2008; Deredec, Godfray & Burt 2011). For example, population genetic models showed the potential for HEGs to increase to high frequencies even when they exert a heavy fitness load on their host, and indicated that maximum suppression would occur if genes essential for reproduction (especially if limited to females) rather than viability were targeted (Deredec, Burt & Godfray 2008). Models incorporating both population genetics and population dynamics suggested that manipulations involving 2–3 HEGs targeting female fecundity, or an X‐shredding strategy resulting in male‐biased sex ratios of the order 75–95%, could both eliminate A. gambiae populations given our best estimates of their intrinsic population growth rates (Deredec, Godfray & Burt 2011).
All the modelling to date has assumed spatially homogeneous (mean‐field) populations, but in assessing how HEG control might be implemented in the field, it will be important to consider HEG spread and efficiency in heterogeneous landscapes. The aim of the work described here was to develop the appropriate modelling framework and explore which aspects of landscape structure have the greatest effect on spatial spread and optimum release strategy. Simple landscape structures are explored as a prelude to studying spread in real landscapes described by geographic data from malaria‐endemic regions in Africa.
Materials and methods
The models are motivated by the biology of Anopheles gambiae in Africa though could easily be adapted to many other insect vector species. Mosquitoes lay eggs in small water bodies where there is evidence that the larvae experience density‐dependent competition for food (Gimnig et al. 2002; White et al. 2011; Muriu et al. 2013). Like nearly all models of A. gambiae population dynamics, we assume that population regulation occurs at this stage. Emerging adult mosquitoes search for mates and then for hosts in order to blood feed. After digesting the blood meal, the female mosquito searches for oviposition sites. The sequence of host feeding and oviposition is called the gonotrophic cycle and can be completed several times. At least two gonotrophic cycles are required for disease transmission to occur.
Mosquitoes in the model are characterized as either juvenile (J) or adult with the latter divided into males (M), unmated females (U), mated females searching for hosts (H) and ovipositing females (O).
In studying X‐shredding strategies, males are characterized by whether or not their Y‐chromosome carries the HEG, and mated females by whether the sperm stored in their spermatheca are derived from a wild‐type or HEG‐bearing male. The probability that a randomly chosen gamete from a HEG‐bearing male carries the Y‐chromosome is ½(e + 1), where e (0 ≤ e ≤ 1) is the cleavage rate, a measure of how reliably the HEG cleaves the Y‐chromosome.
In models of ‘classical’ HEG spread, the individuals are indexed by whether they carry 0, 1 or 2 copies of the gene, and mated females also by the genotype of their mate. HEG heterozygotes are converted to HEG homozygotes prior to gametogenesis with probability e (0 ≤ e ≤ 1). In the simulations we run, we assume that the effects of the HEG are fully recessive with female homozygote fitness zero.
Non‐Spatial Model
For comparison with other models and to obtain analytical insight into the more complex spatial model, we developed a non‐spatial or ‘mean‐field’ model. The model (fully described in the Appendix S1 in Supporting Information) is phrased as a series of ordinary differential equations describing the rates of change in the numbers of mosquitoes in the different stage categories described above. All mosquitoes were assumed to experience density‐independent mortality at constant stage‐specific rates, while larval mosquitoes suffered additional density‐dependent mortality described by a linear function of juvenile density. Juveniles were assumed to recruit to the adult stage at a constant rate, and the probability of searching females successfully finding hosts and oviposition sites was assumed to be constant. The probability of mating is a linear function of male density. Table S1 (Supporting Information) lists the parameters in the model and the default values we used.
Spatial Model
We used a stochastic individual‐based model to study mosquito dynamics in different landscapes. Mosquitoes are treated as individuals and are indexed by their stage category (described above) and their spatial location in two dimensions. Demographic stochasticity is incorporated by supposing that transition events occur sporadically, with the time intervals separating events described by exponentially distributed random variables with rates corresponding to the equivalent parameters of the non‐spatial model. The rate at which an unmated female encounters a male is a linear function of the number of males within a certain radius, and the rate at which a female successfully locates a feeding site is a linear function of the number of houses within a detection radius. Searching for oviposition sites is described in a similar manner. Female egg load after host feeding is Poisson distributed and a Poisson distribution conditional on sufficient eggs being available also describes the number of eggs deposited per breeding site. The density of other mosquitoes in the same breeding site influences larval survival. Note that in contrast to the mean‐field model, the two processes influenced by vector density (mate location and larval survival) now operate locally.
We assume that the landscape is completely described by the distribution of larval breeding sites (small water bodies) and adult mosquito feeding sites (houses), which are treated as point locations. In general, the landscape can be specified by the mean densities of breeding and feeding sites, their spatial variances and covariances, and functions describing the creation and loss of the two types of sites. Here, we assume that houses are permanent features of the landscape while breeding sites arise stochastically at location‐specific rates that may be influenced by local house densities giving rise to negative, zero or positive covariance. Breeding sites disappear at fixed rates (with the death of any larvae they contain).
Mosquito dispersal is a complex process affected by many visual and other cues. To construct a tractable model, we make some simple assumptions about movement but which we believe still capture the essence of host and breeding site location. We assume that mosquitoes have a basic tendency to move that is reduced in the vicinity of a feeding or oviposition site leading to area‐restricted searching. The basic tendency, the strength of the reduction in movement near the object of search and the maximum dispersal jump together characterize mosquito movement and can be parameterized separately for host and breeding site location (see the Supporting Information for a more formal description).
Mosquito Suppression
Control of mosquitoes using HEG constructs was studied by modelling the release of male mosquitoes into an environment where it was assumed that the wild‐type population was at an equilibrium or quasi‐equilibrium. Male‐only introductions were assumed so that only non‐biting individuals were introduced into the environment. The models developed here can be used to study different release strategies though we initially assume that mosquitoes are released at a single randomly chosen site. Population simulations were run for the equivalent of 1000 days pre‐intervention and a further 2500 days after HEG introduction. At the end of the simulation, we recorded whether HEG establishment or mosquito extinction occurred, as well as HEG frequency and mosquito population density.
Results
Landscapes were generated that differed in their densities of mosquito feeding and breeding sites and in the covariance between them (Fig. 1). On some landscapes, if the densities of breeding or feeding sites (or both) are too low, the mosquito population cannot persist, even in the absence of any intervention (Fig. 2). The effects of low density are amplified or mitigated if the distributions are negatively or positively correlated, respectively. Population persistence is facilitated when adults do not have to travel long distances between breeding/oviposition and feeding sites and hence persistence is promoted by both higher resource density and greater covariance between the resource categories.
Figure 1.
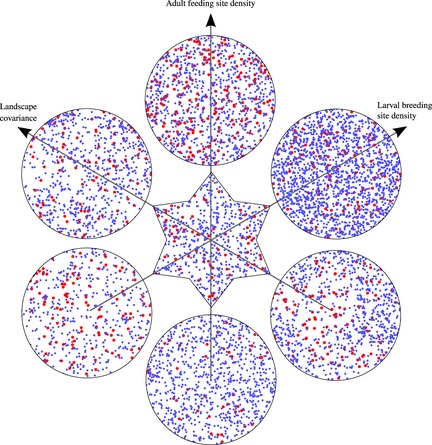
Examples of the generated landscapes in which spatial spread of the HEG was studied. Red discs represent houses, and blue discs larval breeding sites. House densities increase from south to north, and breeding site densities from south‐west to north‐east. The covariance in the distribution of houses is zero in the centre, negative to the south‐east and positive to the north‐west. Parameter values: house density km−1, {4, 8, 16}; breeding site density km−1, {32, 64,128}; covariance, {−0·25, 0, 0·25}.
Figure 2.
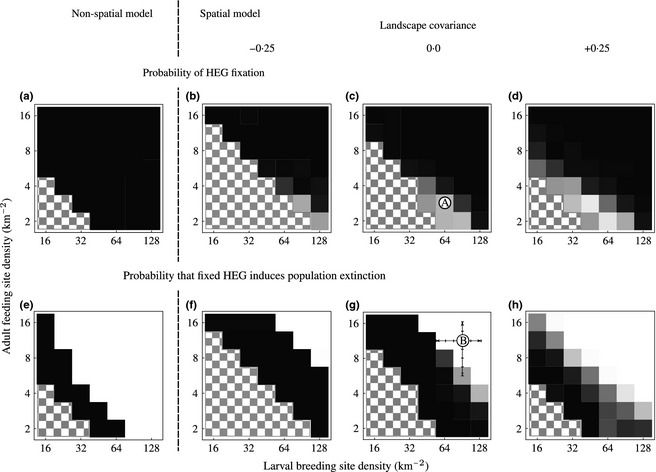
The effects of landscape structure on the probability of the fixation of an X‐shredder HEG (top row) and, if fixation occurs, the probability of mosquito population extinction (bottom row). Results from the non‐spatial model are shown to the far left (a, e), while the three plots to the right are from the spatially explicit model assuming (in order b, c, d and f, g, h) negative, zero and positive covariance between the mosquito breeding and feeding sites. Landscapes in which the mosquito population cannot persist even in the absence of a HEG are shown throughout by checkerboard shading. Probabilities of fixation or extinction from 1 to 0 are indicated by shading from black to white. The dynamic behaviours of the system at A (in plot c) and at, and at points in the vicinity of, B (in plot g) are discussed later in the paper. Results from the non‐spatial model equivalent to those from the spatial model were obtained by varying the feeding and oviposition rate parameters. Simulations of the spatial model were run for 1000 days prior to release and 2500 afterwards, and releases consisted of 200 transgenic males liberated at one location. For full details of parameters and their default values, see Table S1 (Supporting Information).
Spread of an X‐shredder
Consider first the non‐spatial model (Fig. 2a,e). Where a wild‐type population is feasible, we found that for the parameter values we assumed HEG establishment always occurred and that there were two broad classes of outcome. First, if either feeding site density or larval site density is low, so that the mosquito intrinsic population growth rate is relatively small and the environment can only sustain a sparse wild‐type population density, then HEG establishment leads to population extinction. Secondly, if both breeding and feeding sites are more common, then HEG establishment results in population suppression rather than extinction.
Turning to the spatial model (Fig. 2b–d,f–h), the same general pattern is found. However, now spread of the HEG to fixation is not certain, and where feeding site and breeding site densities are low (though still high enough to ensure that mosquito populations are viable in the absence of HEGs), the HEG normally goes extinct locally followed by resurgence of the wild type (Fig. 2b–d). When fixation does occur then as in the non‐spatial model (Fig. 2e), the mosquito population goes extinct provided that resource densities are relatively low. The area of parameter space in which HEG fixation leads to mosquito extinction is greater in the spatial model, yet extinction becomes less likely as the covariance between the two resource types moves from negative to positive (Fig. 2f–h).
To explore the various outcomes that can occur for certain landscape structures, consider the point marked A in Fig. 2c. The nearest equivalent non‐spatial model predicts HEG establishment and population suppression for these parameter values. In the spatial model, a wider set of outcomes are possible (Fig. 3). Fixation occurred in 32% of the simulations but now, unlike the situation with the non‐spatial model, the result is population extinction rather than suppression, probably because the wild‐type intrinsic population growth rate is lower in the spatial model (due to stochastic problems finding mates and non‐zero density dependence at low density). Where fixation did occur, we observed substantial variation in the speed with which it happened (compare Figs 3a,b). In 52% of simulations, the HEG went extinct followed by the recovery of the wild‐type mosquito population (Fig. 3c). Inspection of these runs showed that typically the HEG became temporarily localized in a subregion of the environment where it extirpated the mosquito leading to its own loss, but allowing recolonization of wild‐type mosquitoes from other unaffected areas. Finally, the random algorithm that determined landscape structure sometimes (16%) produced environments that from the mosquito's point of view were disconnected and where the HEG could eliminate vectors only in the subpopulations where it was introduced (Fig. 3d). Spatial animations of these four outcomes can be viewed in Appendix S2 (Supporting Information).
Figure 3.

Examples of different runs of the simulation in the landscape at point A in Fig. 2c; densities of wild‐type (grey) and HEG‐bearing adult males (black). (a) Heg induces population extinction; (b) Heg induces delayed extinction; (c) Drawn‐out contest, wildtype prevails; (d) Heg reduces population to isolated region.
The elimination of the mosquito population fails when the driving HEG chromosome eliminates local populations (and so itself) before it has time to disperse to all nearby populations. The risk of failure thus depends critically on both the pattern of mosquito dispersal and the rapidity with which elimination occurs. Changes in any of the parameters describing mosquito dispersal which increase the spatial extent of vector movement make HEG fixation and mosquito extinction more likely. Thus, as shown in Fig. 4, an increase in the basic propensity to disperse, a decrease in the strength of the reduction in movement near the object of search and an increase in the maximum dispersal jump all tend to make it more likely that introduction of the HEG will lead to the extinction of the mosquito.
Figure 4.
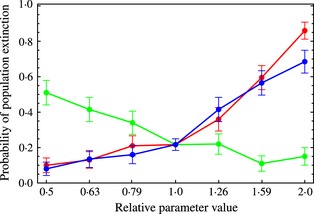
Factors that increase the extent of mosquito dispersal heighten the probability an introduced HEG becomes established and causes population extinction. The plot shows mean probability of extinction (with 95% confidence limits based on 200 runs) when the parameters describing the basic propensity to disperse (blue), the strength of the reduction in movement near the object of search (green) and the maximum dispersal jump (red) are changed around the values used to draw Fig. 2 and in a landscape corresponding to point A in Fig. 2c.
The increased likelihood of HEG extinction and wild‐type recovery when feeding sites are relatively rare and breeding sites are relatively common occurs because of the assumptions we made about landscape structure, motivated by the biology of Anopheles gambiae. Adult feeding sites are relatively rare in the landscape compared to breeding sites, and when this pattern is accentuated, the mosquito population structure becomes a metapopulation: an ensemble of subpopulations connected by dispersal. Further reductions in larval breeding site densities undermine the viability of individual subpopulations and hence the overall metapopulation. Further reductions in adult feeding site densities decrease the connectivity of the metapopulation and make local extinction and the loss of the HEG more likely.
The cleavage rate also influences the fate of the HEG and the consequences of HEG spread for the mosquito population. Figure 5 plots the probability of the loss of the HEG‐bearing Y‐chromosome against cleavage rate. When the cleavage rate is zero, the Y‐chromosome is selectively neutral and its fate is determined purely by neutral stochastic dynamics. For the landscape and release strategy assumed here, the HEG was lost in 80% of simulations but was still present at the end of the runs in 20% of cases. Increasing the cleavage rate increases the probability the HEG establishes to a maximum, after which it declines again. Since cleavage confers a selective advantage, a HEG with a low degree of cleavage is protected against loss through drift alone and, because the impact on local population dynamics is small, has a high probability of successful establishment. At higher cleavage rates, there is a greater chance that the spread of the HEG will result in the extinction of the host population, but also a greater risk that the HEG will be lost through local extinction while wild‐type refuges remain.
Figure 5.

Cleavage rate influences the probability of both HEG establishment (with or without subsequent extinction, grey) and the extinction of the mosquito population (black). Means and standard errors based on 200 simulation runs in a landscape corresponding to point A in Fig. 2c.
To study the factors that influence the extent of population suppression considers the point marked B in Fig. 2g. Here, fixation always occurs but leading to population suppression rather than extinction. At B, fixation results in a 92% drop in the density of adult female mosquitoes from c. 2300 to 190 km−2. This point is marked B on Fig. 6. We then varied adult feeding and breeding site densities in the vicinity of point B across the ranges indicated by the orthogonal lines centred at B in Fig. 2. Increases in the densities of either factor result in higher numbers of mosquitoes at equilibrium prior to intervention, as indicated by the grey and black lines on Fig. 6. It also leads to reduced levels of population suppression after the HEG has become fixed.
Figure 6.

The effect of landscape structure on mosquito population suppression. In the landscapes studied here, HEG fixation leads to mosquito population suppression rather than extinction. Point B in this plot is the equivalent to point B in Fig. 2g now plotted in a space with axes pre‐intervention population density and the proportional reduction in density after the HEG has become fixed. The grey and black lines show the result of changing either breeding site or feeding site density along the orthogonal range of values around B indicated in Fig. 2g. Increasing either leads to higher pre‐intervention population densities and lower population reductions. Error bars show one standard error based on 10 runs.
Classical‐action HEGs
We now explore the dynamics of a classical HEG that homes at a rate of 0·6 and where female (but not male) homozygotes are completely infertile. The non‐spatial model predicts that a HEG will spread through any viable wild‐type mosquito population (Fig. 7a). Unlike the X‐shredder, a classical HEG will not become fixed following its spread. Where feeding or breeding site densities are relatively low leading to small mosquito populations, the HEG can exert a load sufficient to drive the vector population to extinction. Where mosquito population densities are higher, the HEG forms a stable polymorphism with its wild‐type counterpart, whereby the mosquito survives but their numbers are suppressed (Fig. 7e).
Figure 7.

The effects of landscape structure on the probability of the establishment of a classical HEG (top row) and the probability of mosquito population extinction (bottom row). Note that unlike X‐shredder HEGs, a classical HEG never becomes fixed with the parameters we assume and a stable polymorphism occurs. See legend to Fig. 2 for further details.
The spatially explicit model shows the same general pattern but, as we found with the X‐shredder strategy, there were regions of parameter space where HEG establishment did not invariably occur (Fig. 7b–d). This was most likely to be observed in landscapes with high breeding site density and low adult feeding site density and in landscapes with a positive correlation between these two resource types. In these landscapes, the host population has a fragmented spatial structure and the HEG can drive local populations and itself to extinction while leaving wild‐type refugia elsewhere. As with the X‐shredder HEG, we find that increasing the extent of mosquito dispersal reduces the risk of HEG extinction (Fig. S1, Supporting Information). Homing rate has a less critical role in determining the probability of population extinction, although homing must be above a minimum and below a maximum rate for the HEG to be effectual (Fig. S2, Supporting Information). In landscapes where the probability of HEG establishment is high, we find, as with the X‐shredder, that the highest load is exerted by a classical‐action HEG when the initial pre‐intervention population size is relatively low (Fig. S3, Supporting Information).
Release Strategies
Spatially explicit models of HEG spread may also be valuable in exploring different release strategies. We illustrate this here though such studies prior to an actual release would almost certainly make use of spatial data from the proposed release site.
We consider the release of an X‐shredder HEG into a landscape structure the same as point A in Fig. 2c (and with all parameters except release strategy the same as those used above). In this region, establishment is not certain and quite frequently the HEG eliminates a local population of mosquitoes before going extinct itself, followed by recovery of the wild type, though successful extirpation of the mosquito can also occur. Increasing the number of males released from a single site initially increases the probability of mosquito population extinction, but this plateaus quite quickly (Fig. 8a). In contrast, distributing HEG‐carrying males amongst many sites, even with no increase in the total number of insects released, has a marked effect in increasing the likelihood of mosquito extinction (Fig. 8b). Very similar results were obtained for the release of a classic HEG (Fig. S4, Supporting Information).
Figure 8.

The effects of different release strategies on the probability that an X‐shredder HEG becomes established and causes mosquito population extinction. The landscape structure and other parameters are as at point A in Fig. 2c. In (a), varying numbers of male HEG‐carrying mosquitoes are released at a single site, while in (b) 200 males are distributed at a different number of sites from one release of 200 mosquitoes to 200 releases of a single mosquito. Means and 95% confidence limits from 100 simulation runs are shown.
Discussion
Genetic control is a potentially important strategy to target vectors of major human diseases. Some control strategies rely on the mass release of particular vector genotypes that do not persist in the environment, a control mechanism similar to the sterile insect technique (e.g. Thomas et al. 2000). Other strategies involve the release of insects with genes that spread through the vector population, imposing a genetic load or modifying the vector in a way that reduces transmission (Sinkins & Gould 2006). Such strategies require a gene drive mechanism to be bred or engineered into the insect vector.
Homing endonuclease genes (HEGs) offer particular promise as a drive mechanism because of their capacity to increase in frequency from arbitrary low values. This compares with other potential strategies that rely on constructs that increase in frequency only when a threshold frequency is exceeded. The ability to increase from low numbers also facilitates their spatial spread. The spatial spread of a HEG is described by a Fisherian process (Fisher 1937), while the spread of a threshold drive mechanism is described by a Bartonian process (Barton 1979; Turelli & Hoffmann 1991). Bartonian processes, unlike Fisherian spread, have a much greater propensity to become arrested in regions of low population density.
Population genetic and population dynamic models of HEG spread have shown that these genes can spread rapidly through populations even when at the same time they cause substantial reductions in population fitness (Burt 2003; Deredec, Burt & Godfray 2008; Deredec, Godfray & Burt 2011). HEG spread has now been confirmed in laboratory populations of mosquitoes (Windbichler et al. 2011), but actual deployment of HEGs will occur in environments that are inevitably spatially heterogeneous and spatially explicit models will be essential to guide development and implementation. The models developed here are informed by the biology of Anopheles gambiae, the most important mosquito vector of malaria in Africa. They explore movement of a HEG through an environment characterized by the distribution and covariance of the two resource types used by mosquitoes: larval breeding and adult feeding sites. We hope that understanding spread in such a simplified spatial setting will help the analysis of spread in more complex environments parameterized by data from potential release sites.
The majority of the model runs we report here explore the fate of HEGs released at a single randomly chosen site in the environment, a particularly stringent test for assessing the ability of a construct to spread. Our main results are as follows:
For many parameter values, the probability of HEG establishment given by the spatially explicit model is similar to that predicted by the nearest equivalent non‐spatial model.
The HEG interventions we model impose a genetic load, and again for most parameter values, spatial and non‐spatial models predict similar patterns of population suppression or extinction.
With our assumptions about mosquito biology, in environments where mosquito resources are sparsely distributed, an introduced HEG can drive a local population to extinction and itself become lost while wild‐type refugia still exist. The probability of this occurring increases both with the rarity of adult feeding sites and with the covariance between adult feeding and breeding sites, since both changes act to increase the autonomy of the local populations. After HEG extinction occurs, the wild‐type vectors can recolonize the environment restoring the status quo ante.
Increasing the number of release sites, even at the expense of releasing fewer mosquitoes per site, can greatly increase the probability of successful population suppression or extinction in environments where local HEG extinction may occur.
Reducing the density of mosquito resource sites leads to a more disconnected population structure. Our results suggest that there is a threshold above which the HEG spreads easily through the environment but below which spread may only be local. HEG spread may thus have features in common with a percolation process where landscape parameters determine a threshold for global spread. Percolation theory has been applied to population dynamic processes in ecology (e.g. With & Crist 1995) where it has been shown that understanding the spatial structure of the environment at the scales appropriate to the organism is critical in determining the percolation threshold. For mosquito vectors of human diseases, the distribution of human feeding sites and potential breeding sites can be determined relatively easily from ground surveys and increasingly by remote observation and image analysis (e.g. Machault et al. 2011). Much more challenging is estimating the parameters describing mosquito dispersal, in particular the role of occasional long‐distance movement. Information on the geographic spread of genes under strong recent selection (e.g. insecticide resistance genes) might give useful information here (Hemingway & Ranson 2000; Abilio et al. 2011).
The HEG interventions modelled here are designed to impose a load on their host leading to population suppression and extinction. Their spread is thus reminiscent of a virulent disease that can cause local extinction of its host so hindering its transmission (Bull 1994). Again, percolation theory can be applied, and depending on the biology of the disease, thresholds in density or network connectedness can be identified below which the spread of the pathogen will be restricted (Newman 2002; Sander et al. 2002). In some landscapes, relatively non‐virulent pathogens can spread, while more virulent ones fail. This suggests that there may be some environments where HEGs that impose a lower load on their hosts may overall be more efficacious than those with a high fitness cost.
The modelling described here is a first step in exploring spatial models of HEG release, and the obvious next step is to incorporate actual rather than artificial landscapes. It is clearly not possible to model very large geographic areas at the level of resource granularity attempted here. One approach to study broader spread might be to use detailed models such as ours to study the rate of spread of HEGs in different environments and then to incorporate these estimates in a less‐detailed larger‐scale model. Incorporating seasonal variation in resource density, especially larval breeding site densities that are correlated with rainfall, will also be required to produce a more realistic model of spread. Finally, better understanding of mosquito biology, in particular local and long dispersal, the use of nectar as an energy resource, movement of males, density‐dependent processes and behaviour during dry seasons, would all improve the ability of the model to predict the outcome of HEG release.
In this paper, we have concentrated on the release of mosquitoes in an environment where no other control mechanisms are in operation. It is straightforward to extend the model to more complicated situations where larval or adult insecticide control is also being carried out. We have also only modelled simple strategies with the release of constructs containing single HEGs. To reduce the probability of resistance evolving, more complicated interventions may be used which can be incorporated in the current framework. Similarly, the spread of a resistant mutant that has arisen by chance or has been deliberately released (a ‘recall’ strategy) can be modelled (Burt 2003).
The release of genetically manipulated individuals of species that bite humans must be done with great care and subject to extensive regulatory review. But were the strategy to succeed, it offers great benefits, especially for people in least‐developed countries where conventional health services are absent. It also provides a further means of attacking malaria and other diseases, which may become important if insecticide and drug resistance continue to spread. The models developed here show that many of the insights from simple non‐spatial models also apply in the spatial case, though they also indicate circumstances where spread may be arrested. The risk of such outcomes can be reduced by multiple releases, though can never be wholly eliminated. Post‐release monitoring will thus be required, with the possibility of further releases. Models of the type developed here can help predict in which environments such eventualities are most likely to occur, and where follow‐up resources will need to be most concentrated.
Supporting information
Appendix S1. Full model description.
Appendix S2. Animations of the simulation runs plotted in Fig. 3.
Fig. S1. The effect of dispersal parameters on the probability a classical HEG induces population extinction.
Fig. S2. The effect of homing rate on the outcome of a classical HEG introduction.
Fig. S3. The effect of landscape structure on mosquito population suppression following the spread of a classical HEG.
Fig. S4. The effects of different release strategies on the probability that a classical HEG becomes established and causes mosquito population extinction.
Table S1. Model parameters and their default values.
Acknowledgements
This work is funded by a grant from the Foundation for the National Institutes of Health through the Vector‐Based Control of Transmission: Discovery Research program of the Grand Challenges in Global Health initiative. HCJG is part of the Research and Policy for Infectious Disease Dynamics program of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center. The authors would like to acknowledge the use of the Oxford Supercomputing Centre (OSC) and the HPC services of Imperial College London (http://www.imperial.ac.uk/ict/services/teachingandresearchservices/highperformancecomputing) in carrying out this work.
References
- Abilio, A.P. , Kleinschmidt, I. , Rehman, A.M. , Cuamba, N. , Ramdeen, V. , Mthembu, D.S. et al (2011) The emergence of insecticide resistance in central Mozambique and potential threat to the successful indoor residual spraying malaria control programme. Malaria Journal, 10, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton, N.H. (1979) The dynamics of hybrid zones. Heredity, 43, 341–359. [Google Scholar]
- Bull, J.J. (1994) Virulence. Evolution, 48, 1423–1437. [DOI] [PubMed] [Google Scholar]
- Burt, A. (2003) Site‐specific selfish genes as tools for the control and genetic engineering of natural populations. Proceedings of the Royal Society of London Series B‐Biological Sciences, 270, 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt, A. & Trivers, R. (2006) Genes in Conflict. Belknap Press, Cambridge, MA. [Google Scholar]
- Chanda, E. , Hemingway, J. , Kleinschmidt, I. , Rehman, A.M. , Ramdeen, V. , Phiri, F.N. et al (2011) Insecticide resistance and the future of malaria control in Zambia. PLoS ONE, 6, e24336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. & Dupas, P. (2010) Free distribution or cost sharing? Evidence from a randomized malaria prevention experiment. Quarterly Journal of Economics, 125, 1–45. [Google Scholar]
- Deredec, A. , Burt, A. & Godfray, H.C.J. (2008) The population genetics of using homing endonuclease genes in vector and pest management. Genetics, 179, 2013–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deredec, A. , Godfray, H.C.J. & Burt, A. (2011) Requirements for effective malaria control with homing endonuclease genes. Proceedings of the National Academy of Sciences of the United States of America, 108, E874–E880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R.A. (1937) The wave of advance of advantageous genes. Annals of Eugenics, 7, 355–369. [Google Scholar]
- Gilles, H.M. , Warrell, D.A. & Bruce‐Chwatt, L.J. (1993) Bruce‐Chwatt's Essential Malariology. Edward Arnold, London. [Google Scholar]
- Gimnig, J.E. , Ombok, M. , Otieno, S. , Kaufman, M.G. , Vulule, J.M. & Walker, E.D. (2002) Density‐dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. Journal of Medical Entomology, 39, 162–172. [DOI] [PubMed] [Google Scholar]
- Greenwood, B.M. , Fidock, D.A. , Kyle, D.E. , Kappe, S.H.I. , Alonso, P.L. , Collins, F.H. & Duffy, P.E. (2008) Malaria: progress, perils, and prospects for eradication. Journal of Clinical Investigation, 118, 1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway, J. & Ranson, H. (2000) Insecticide resistance in insect vectors of human disease. Annual Review of Entomology, 45, 371–391. [DOI] [PubMed] [Google Scholar]
- Klayman, D.L. (1985) Qinghaosu (artemisinin) ‐ an antimalarial drug from China. Science, 228, 1049–1055. [DOI] [PubMed] [Google Scholar]
- Klein, T.A. , Windbichler, N. , Deredec, A. , Burt, A. & Benedict, M.Q. (2012) Infertility resulting from transgenic I‐PpoI male Anopheles gambiae in large cage trials. Pathogens and Global Health, 106, 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler, C. (2004) Insecticide‐treated bednets and curtains for preventing malaria. Cochrane Database of Systematic Reviews, 2, 1–52. [DOI] [PubMed] [Google Scholar]
- Machault, V. , Vignolles, C. , Borchi, F. , Vounatsou, P. , Pages, F. , Briolant, S. , Lacaux, J.P. & Rogier, C. (2011) The use of remotely sensed environmental data in the study of malaria. Geospatial Health, 5, 151–168. [DOI] [PubMed] [Google Scholar]
- Muriu, S.M. , Coulson, T. , Mbogo, C.M. & Godfray, H.C.J. (2013) Larval density dependence in Anopheles gambiae s.s., the major African vector of malaria. Journal of Animal Ecology, 39, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, C.J.L. , Rosenfeld, L.C. , Lim, S.S. , Andrews, K.G. , Foreman, K.J. , Haring, D. et al (2011) Global malaria mortality between 1980 and 2010: a systematic analysis. The Lancet, 379, 413–431. [DOI] [PubMed] [Google Scholar]
- Newman, M.E.J. (2002) Spread of epidemic disease on networks. Physical Review E, 66, 016128. [DOI] [PubMed] [Google Scholar]
- Pluess, B. , Tanser, F.C. , Lengeler, C. & Sharp, B.L. (2010) Indoor residual spraying for preventing malaria. Cochrane Database of Systematic Reviews, 4, 1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander, L.M. , Warren, C.P. , Sokolov, I.M. , Simon, C. & Koopman, J. (2002) Percolation on heterogeneous networks as a model for epidemics. Mathematical Biosciences, 180, 293–305. [DOI] [PubMed] [Google Scholar]
- Sinkins, S.P. & Gould, F. (2006) Gene drive systems for insect disease vectors. Nature Reviews Genetics, 7, 427–435. [DOI] [PubMed] [Google Scholar]
- Stoddard, B.L. (2005) Homing endonuclease structure and function. Quarterly Reviews of Biophysics, 38, 49–95. [DOI] [PubMed] [Google Scholar]
- Taylor, G.K. & Stoddard, B.L. (2012) Structural, functional and evolutionary relationships between homing endonucleases and proteins from their host organisms. Nucleic Acids Research, 40, 5189–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, D.D. , Donnelly, C.A. , Wood, R.J. & Alphey, L.S. (2000) Insect population control using a dominant, repressible, lethal genetic system. Science, 287, 2474–2476. [DOI] [PubMed] [Google Scholar]
- Turelli, M. & Hoffmann, A.A. (1991) Rapid spread of an inherited incompatibility factor in California Drosophila . Nature, 353, 440–442. [DOI] [PubMed] [Google Scholar]
- White, N.J. (2010) Artemisinin resistance‐the clock is ticking. Lancet, 376, 2051–2052. [DOI] [PubMed] [Google Scholar]
- White, M.T. , Griffin, J.T. , Churcher, T.S. , Ferguson, N.M. , Basanez, M.G. & Ghani, A.C. (2011) Modelling the impact of vector control interventions on Anopheles gambiae population dynamics. Parasites & Vectors, 4, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2010) World Malaria Report. WHO, Geneva. [Google Scholar]
- Windbichler, N. , Papathanos, P.A. & Crisanti, A. (2008) Targeting the X chromosome during spermatogenesis induces Y chromosome transmission Ratio distortion and early dominant embryo lethality in Anopheles gambiae . Plos Genetics, 4, e1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windbichler, N. , Papathanos, P.A. , Catteruccia, F. , Ranson, H. , Burt, A. & Crisanti, A. (2007) Homing endonuclease mediated gene targeting in Anopheles gambiae cells and embryos. Nucleic Acids Research, 35, 5922–5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windbichler, N. , Menichelli, M. , Papathanos, P.A. , Thyme, S.B. , Li, H. , Ulge, U.Y. et al (2011) A synthetic homing endonuclease‐based gene drive system in the human malaria mosquito. Nature, 473, 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- With, K.A. & Crist, T.O. (1995) Critical thresholds in species responses to landscape structure. Ecology, 76, 2446–2459. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Full model description.
Appendix S2. Animations of the simulation runs plotted in Fig. 3.
Fig. S1. The effect of dispersal parameters on the probability a classical HEG induces population extinction.
Fig. S2. The effect of homing rate on the outcome of a classical HEG introduction.
Fig. S3. The effect of landscape structure on mosquito population suppression following the spread of a classical HEG.
Fig. S4. The effects of different release strategies on the probability that a classical HEG becomes established and causes mosquito population extinction.
Table S1. Model parameters and their default values.


