Abstract
Evaluation of cannabinoid stability in authentic oral fluid (OF) is critical, as most OF stability studies employed fortified or synthetic OF. Participants (n=16) smoked a 6.8% delta-9-tetrahydrocannabinol (THC) cigarette, and baseline concentrations of THC, 11-nor-9-carboxy-THC (THCCOOH), cannabidiol (CBD), and cannabinol (CBN) were determined within 24h in 16 separate pooled samples (collected 1h before to 10.5 or 13h after smoking). OF was collected with the StatSure Saliva Sampler™ and Oral-Eze® devices. Oral-Eze samples were re-analyzed after room temperature (RT) storage for 1 week, and for both devices after 4°C for 1 and 4 weeks, and –20°C for 4 and 24 weeks. Concentrations ±20% from initial concentrations were considered stable. With the StatSure device, all cannabinoids were within 80-120% median %baseline for all storage conditions. Individual THC, CBD, CBN and THCCOOH pool concentrations were stable in 100%, 100%, 80-94% and >85%, respectively, across storage conditions. With the Oral-Eze device, at RT or refrigerated storage (for 1 and 4 weeks), THC, CBD and THCCOOH were stable in 94-100%, 78-89% and 93-100% of samples, respectively, while CBN concentrations were 53–79% stable. However, after 24 weeks at -20°C, stability decreased, especially for CBD, with a median of 56% stability. Overall, the collection devices’ elution/stabilizing buffers provided good stability for OF cannabinoids, with the exception of the more labile CBN. To ensure OF cannabinoid concentration accuracy, these data suggest analysis within 4 weeks at 4°C storage for Oral-Eze collection and within 4 weeks at 4°C or 24 weeks at -20°C for StatSure collection.
Keywords: oral fluid, cannabis, cannabinoids, stability, THC
Introduction
More individuals use cannabis than any other illicit drug worldwide [1]. Oral fluid (OF) is advantageous over other biological matrixes (e.g. blood, urine, plasma) for drug testing in workplace, drug treatment, forensic, and driving under influence of drugs (DUID) testing programs for several reasons: sample collection is simple and noninvasive; infection risk is reduced compared to blood; OF concentrations may reflect recent drug use better than urine; special collection facilities and same-sex collectors are not required; and specimen adulteration is more difficult [2; 3; 4]. OF testing often requires specialized collection devices and specific legislation for screening and confirmatory cut-off concentrations. The U.S. Substance Abuse and Mental Health Services Administration (SAMHSA) and the European initiative, Driving Under the Influence of Drugs, Alcohol, and Medicines (DRUID), [5; 6] proposed specific OF cannabinoids cut-off concentrations for screening and confirmation. Currently, THC is the only confirmation analyte monitored in the SAMHSA (2 ng/mL) and DRUID (1 ng/mL) proposals. Understanding analyte stability during specimen storage is critical to ensure accurate result interpretation for clinical and forensic purposes.
The main psychoactive cannabis constituent, delta-9-tetrahydrocannabinol (THC), is sensitive to several factors during storage: air oxidation [7]; degradation when exposed to light [7; 8], acids [9], high temperatures [10]; and adsorption to materials such as glass, plastic, and precipitant material [11; 12]. OF collection devices with elution/stabilization buffers are preferred over expectorated samples due to increased analyte stability during storage and improved analytical precision [13] [14]; however, most stability studies [15; 16; 17; 18] focused on fortified authentic or synthetic oral fluid. Moore et al. [16; 17] showed that THC, cannabidiol (CBD), cannabinol (CBN) and 11-nor-9-carboxy-THC (THCCOOH) concentrations were stable in fortified synthetic OF collected with the Quantisal device when refrigerated for 10 days; instability occurred when cannabinoids were stored at room temperature (RT) for the same period. Only one study evaluated cannabinoid stability from authentic OF collected with the Quantisal device and by expectoration [14]. THC, THCCOOH, CBN, and CBD concentrations in OF collected with the Quantisal device were stable for at least 4 weeks at 4°C, while significant degradation of THCCOOH, CBD, and CBN was observed after 24 weeks at -20°C. In expectorated authentic OF, cannabinoids concentrations were less stable than specimens collected with Quantisal under all storage conditions, demonstrating that cannabinoid stability varies by collection method and storage conditions.
There is a strong need to determine cannabinoid stability in authentic OF collected with commercial OF collection devices after cannabis smoking, as stability in fortified authentic or synthetic OF may not be the same. In this study, after controlled smoked cannabis administration, cannabinoid stability in authentic OF collected with StatSure Saliva Sampler™ and the Oral-Eze® collection devices were characterized after storage at RT, 4°C, and -20°C for 1-24 weeks. We provide stability data for THC, THCCOOH, CBN, and CBD, due to the importance all these cannabinoids have in improving interpretation of OF results.
Materials and Methods
Participants
Frequent and occasional cannabis smokers were recruited from the community by print, radio, internet and television advertisements. Subjects were required to be 18-45 years old and physically and psychologically healthy based on comprehensive medical and psychological evaluation. Self-reported cannabis smoking at least four times per week (frequent cannabis smokers) or less than twice per week (occasional cannabis smokers) in the 3 months prior to study entry, and for frequent smokers, a positive urine cannabinoid screen (Iscreen™ >50 μg/L, Alere, Waltham, MA) was required for inclusion. Exclusion criteria included clinically significant illness or adverse event associated with cannabis intoxication, more than 450 mL blood donation within the previous 30 days, participation in drug abuse treatment within the previous 60 days, or interest in treatment at any time, and pregnant or nursing women.
All subjects provided written informed consent to participate in this National Institute on Drug Abuse Institutional Review Board-approved study and were remunerated for their participation. Participants resided on a secure clinical research unit the nights before and after drug administration.
Oral Fluid Stability Sample Collection
OF was collected with the StatSure Saliva Sampler™ (StatSure Diagnostic Systems, Inc., Brookline, MA) and Oral-Eze® (Quest Diagnostics, Madison, NJ) devices upon admission to the clinical unit (approximately -19 h) and -1.0, 0.5, 1, 2, 3, 4, 4.5, 5, 6, 8, 10.5, 13.5, 21, 24, 26, 28, and 30 h post dose. Cannabis cigarettes were obtained through the NIDA Chemistry and Physiological Systems Research Branch. Participants smoked one (mean±SD) 6.8±0.2% THC (54mg), 0.25±0.08% CBD (2mg), and 0.21±0.02% CBN (1.6mg) cannabis cigarette ad libitum within 10min. OF was collected first with the StatSure and then with the Oral-Eze device. Both collection devices contain an indicator that turns blue when 1mL OF is collected. Specimens were processed according to manufacturers’ recommendations. StatSure collection pads were placed into tubes containing 1mL elution/stabilization buffer (yielding a 1:2 v/v OF dilution) and stored at 4°C. Oral-Eze pads were stored in 2mL buffer (1:3 v/v OF dilution) at RT. OF/buffer mixtures were transferred to 3.6 mL Nunc cryotubes (Thomas Scientific, Swedesboro, NJ) 12h later. These OF samples were originally used for pharmacokinetic analyses [19; 20]. Aliquots from each time point sample except those at 4.5 h (from -1 to 10.5 h for StatSure and from -1 to 13h for Oral-Eze) from each participant were combined to create individual StatSure and Oral-Eze stability pools with enough volume for analysis at multiple storage conditions. After vortexing, each pool was divided into 5 and 6 aliquots for StatSure and Oral-Eze specimens, respectively, and one aliquot was assayed immediately to establish baseline concentrations. Two of four StatSure aliquots were stored at 4°C and analyzed after 7 days and 1 month. The other StatSure aliquots were stored at -20°C and analyzed after 4 and 24±2 weeks. Oral-Eze aliquots were refrigerated for analysis after 7 days and 1 month, and frozen for analysis after 4 and 24 ± 2 weeks. One additional Oral-Eze aliquot remained at room temperature for 7 days prior to analysis (Figure 1A).
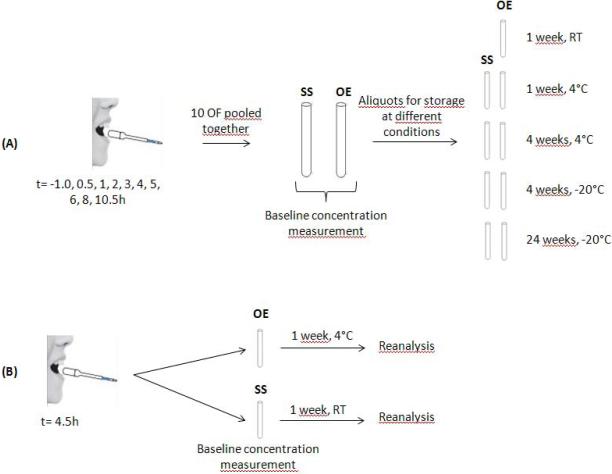
Figure 1.
Stability evaluation design. (A) For each participant, one collection with each device at each time point (t= -1.0, 0.5, 1, 2, 3, 4, 5, 6, 8, 10.5h ) was performed resulting in 10 different OF for each device. After individual analyses for pharmacokinetics purposes, the remaining OF from each of 10 OF were pooled in one tube for each device (StatSure=SS and Oral-Eze=OE) which was analyzed within 24h for baseline concentrations. After baseline quantification, different aliquots were stored under different conditions for different durations for stability analyses. (B) To evaluate manufacturers’ recommended storage conditions, the 4.5h time point was collected with each device and analyzed within 24h for baseline concentration. After analysis, the tubes were kept for 1 week at 4° (Oral-Eze) or room temperature (StatSure), analyzed and compared to baseline for stability purpose.
In order to compare manufacturers’ recommended storage temperatures and to evaluate potential changes during different shipping conditions, specimens collected 4.5 h post dose were not included in the stability pool and were analyzed only within the initial 24h (baseline concentration) and again after 7 days storage at RT for StatSure and 4°C for Oral-Eze samples (Figure 1B). This allowed us to evaluate the robustness of these collection devices in samples that were not processed according to the recommendations of the manufacturer.
Oral Fluid Cannabinoid Analysis
OF THC, CBD, CBN, 11-Hydroxy-THC (11-OH-THC), and THCCOOH were quantified by a fully validated 2-dimensional gas chromatography-mass spectrometry (2D-GC-MS) method with minor modifications [21]. The electron ionization chromatographic system included a DB- 1MS (Agilent Technologies) as the primary and ZB-50 (Phenomenex) as the secondary column, and an oven temperature program utilized in our plasma method [22]. Minor sample preparation modifications also were required to process Oral-Eze and StatSure specimens. During solid phase extraction, 0.4 mL methanol (StatSure) or hexane (Oral-Eze) was added to the column prior to the elution solvent for THC, CBD and CBN in order to reduce baseline interferences and improve chromatography. StatSure calibrators and quality controls were prepared in 0.25 mL blank OF and 0.25 mL StatSure buffer; linear ranges were 0.5–50 μg/L for THC, CBD, CBN and 11-OH-THC; and 15–500 ng/L for THCCOOH; intra- and inter-assay imprecision were ≤ 7.7%, and analytical bias was within 97.1-107%. To account for specimen dilution, Oral-Eze calibrators and quality controls were prepared in 0.25 mL blank OF and 0.50 mL Oral-Eze buffer; linear ranges were 0.5–50 µg/L for THC and 11-OH-THC, 1.0–50 μg/L for CBD and CBN, and 15–500 ng/L for THCCOOH; while intra- and inter-assay imprecision were ≤ 7.6%, and analytical bias was 88.2-110%.
Data analysis
Baseline cannabinoid concentrations were established within 24h of collection. Concentration changes are reported as %baseline and calculated as (stored sample concentration / baseline concentration × 100%). Concentration changes within ±20% of baseline were considered stable. Specimens for which %baseline could not be determined due to concentrations < limit of quantification (LOQ) at baseline, insufficient OF volume or chromatographic interferences were excluded from calculations. Samples with initial concentrations <20% above the LOQ and then falling below LOQ were excluded from stability comparisons, while analyte concentrations falling below LOQ were considered unstable only if initial baseline concentrations were >20% above the LOQ.
Results
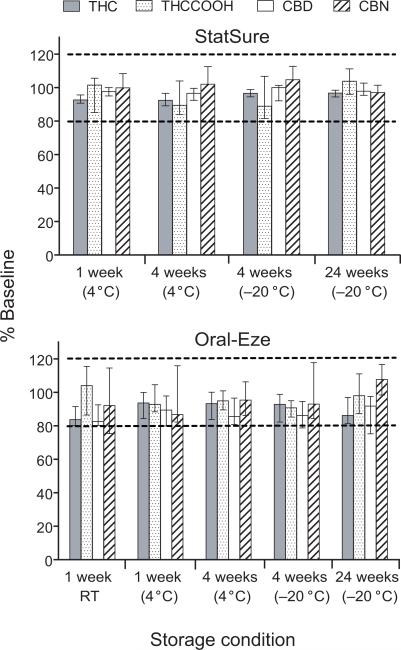
THC, CBD, CBN, 11-OH-THC and THCCOOH concentrations were quantified in 16 pools (one for each of 16 participants) to investigate inter-individual differences in stability from authentic OF collected with StatSure and Oral-Eze devices. All specimens collected with StatSure (n=80) and Oral-Eze (n=96) devices were analyzed; 11-OH-THC was not detected in any specimen; therefore, no stability data were available. Cannabinoid concentration changes from baseline at each storage condition are displayed in Figure 2 and Table 1. Percent differences from baseline concentrations of specimens collected 4.5 h post dose and stored for one week at RT (StatSure) and one week at 4°C (Oral-Eze) are shown in Table 2.
Figure 2.
Median delta-9-tetrahydrocannabinol (THC), cannabidiol (CBD), cannabinol (CBN) and 11-nor-9-carboxy-THC (THCCOOH) %baseline concentration after different storage conditions for the StatSure (A) and Oral-Eze device pools (B). Each bar represents median data from 16 individual sample pools (pooling 1h before to 10.5h [StatSure] or 13 h [Oral-Eze] after smoking a 6.8% delta-9-tetrahydrocannabinol cigarette) collected from 16 adult cannabis smokers. Error bars indicate interquartile ranges. RT is room temperature.
Table 1.
Delta-9-tetrahydrocannabinol (THC), cannabidiol (CBD), cannabinol (CBN), and 11-nor-9-carboxy-THC (THCCOOH) concentration changes from baseline for oral fluid specimens collected with the StatSure and Oral-Eze devices after being stored at room temperature (RT) for 1 week (Oral-Eze only), 4°C for 1 and 4 weeks, and –20°C for 4 and 24 weeks (both devices).
| Analyte | Baseline concentration range 1 | Storage condition | # Samples with analytes >LOQ2 | # Stable samples (%stable) | %Baseline, range |
|---|---|---|---|---|---|
| A. StatSure collection device | |||||
| THC | 6.8 – 509 μg/L | 1 week, 4°C | 16 | 16 (100) | 85.6–111 |
| 4 weeks, 4°C | 16 | 16 (100) | 82.3–111 | ||
| 4 weeks, –20°C | 16 | 16 (100) | 89.7–117 | ||
| 24 weeks, –20°C | 16 | 16 (100) | 84.6–110 | ||
| CBD | < LOQ –15.3 μg/L | 1 week, 4°C | 11 | 11 (100) | 94.3–109 |
| 4 weeks, 4°C | 10 | 10 (100) | 89.4–108 | ||
| 4 weeks, –20°C | 9 | 9 (100) | 88.4–108 | ||
| 24 weeks, –20°C | 9 | 9 (100) | 89.1–108 | ||
| CBN | 0.6 – 35.8 μg/L | 1 week, 4°C | 16 | 15 (94) | 80.4–167 |
| 4 weeks, 4°C | 15 | 13 (87) | 87.2–166 | ||
| 4 weeks, –20°C | 15 | 12 (80) | 91.9–1653 | ||
| 24 weeks, –20°C | 15 | 13 (87) | 90.3–183 | ||
| THCCOOH | < LOQ –150 ng/L | 1 week, 4°C | 12 | 12 (100) | 80.4–115 |
| 4 weeks, 4°C | 13 | 11 (85) | 68.4–142 | ||
| 4 weeks, –20°C | 13 | 11 (85) | 66.7–148 | ||
| 24 weeks, –20°C | 13 | 13 (100) | 88.3–118 | ||
| B. Oral-Eze collection device | |||||
| THC | 6 – 376 μg/L | 1 week, RT | 16 | 15 (94) | 70.7–108 |
| 1 week, 4°C | 16 | 16 (100) | 80.0–119 | ||
| 4 weeks, 4°C | 16 | 16 (100) | 80.1–119 | ||
| 4 weeks, –20°C | 16 | 15 (94) | 46.4–113 | ||
| 24 weeks, –20°C | 16 | 13 (81) | 60.5–99.8 | ||
| CBD | < LOQ – 15.1 μg/L | 1 week, RT | 8 | 7 (88) | 75.1–99.4 |
| 1 week, 4°C | 9 | 7 (78) | 66.1–111 | ||
| 4 weeks, 4°C | 9 | 8 (89) | 80.0–102 3 | ||
| 4 weeks, –20°C | 9 | 7 (78) | 40.8–99.8 | ||
| 24 weeks, –20°C | 9 | 5 (56) | 31.9–98.7 | ||
| CBN | <LOQ – 16.8 μg/L | 1 week, RT | 15 | 8 (53) | 70.2–155 |
| 1 week, 4°C | 15 | 9 (60) | 57.3–163 | ||
| 4 weeks, 4°C | 14 | 11 (79) | 20.1–147 | ||
| 4 weeks, –20°C | 13 | 9 (69) | 66.4–146 | ||
| 24 weeks, –20°C | 14 | 12 (86) | 83.2–129 | ||
| THCCOOH | < LOQ – 327 ng/L | 1 week, RT | 14 | 13 (93) | 70.1–120 |
| 1 week, 4°C | 13 | 13 (100) | 81.4–119 | ||
| 4 weeks, 4°C | 13 | 13 (100) | 82.4–118 | ||
| 4 weeks, –20°C | 13 | 12 (92) | 60.8–104 | ||
| 24 weeks, –20°C | 14 | 11 (79) | 75.1–139 | ||
No significant differences (p>0.05, Mann-Whitney test for median comparison) were observed between baseline concentrations after StatSure or Oral-Eze OF collections
LOQ: Limit of Quantification
1 CBD and 1 CBN sample concentration were quantified at >20% of LOQ at baseline but <LOQ after storage. No %baseline was calculated, but the sample was considered unstable.
Table 2.
Delta-9-tetrahydrocannabinol (THC), cannabidiol (CBD), cannabinol (CBN), and 11-nor-9-carboxy-THC (THCCOOH) concentration changes from baseline for oral fluid specimens collected 4.5 h after cannabis smoking with the StatSure (A) and Oral-Eze (B) devices and stored for 1 week at 4°C (Oral-Eze) and room temperature (RT) (StatSure).
| Analyte | Baseline concentration range 1 | Storage condition | # Samples with determined %baseline | # Stable samples (%stable) | %Baseline range |
|---|---|---|---|---|---|
| A. StatSure collection device | |||||
| THC | 4.4 – 81.4 μg/L | 1 week, RT | 16 | 16 (100) | 80.7–107 |
| CBD | < LOQ – 3.2 μg/L | 7 | 6 (86) | 50.8–108 | |
| CBN | < LOQ – 3.9 μg/L | 12 | 12 (100) | 83.3–114 | |
| THCCOOH | < LOQ – 165 ng/L | 13 | 12 (92) | 60.0–119 | |
| B. Oral-Eze collection device | |||||
| THC | 2.5 – 49.6 μg/L | 1 week, 4°C | 16 | 16 (100) | 80.7–119 |
| CBD | < LOQ – 2.2 μg/L | 1 | 0 | 162 | |
| CBN | < LOQ – 3.5 μg/L | 6 | 4 (67) | 82.0–145 | |
| THCCOOH | < LOQ – 166 ng/L | 12 | 11 (92) | 77.4–119 | |
No significant differences (p>0.05, Mann-Whitney test for median comparison) were observed between baseline concentrations after StatSure or Oral-Eze OF collections
THC Stability
In pooled OF collected with the StatSure device, all 16 participants’ samples were stable under all conditions tested. Median %baseline concentrations (range) after 1 week at 4°C were 92.6% (85.6-111%), after 4 weeks 92.4% (82.3-111%). Median %baseline concentrations after 4 weeks at –20°C were 96.6% (89.7-117%), and after 24 weeks 96.7% (84.6-110%). In OF collected with the StatSure device 4.5 h after smoking following 1 week storage at RT, all participants’ samples (n=16) were stable, with median %baseline concentrations of 90.1% (80.7-107%).
In pooled OF collected with the Oral-Eze device, all 16 participants’ samples were stable at 4°C for 1 and 4 weeks, with median %baseline concentrations of 93.7% (80.0-119%) and 93.3% (80.1-119%), respectively. However, during frozen storage (–20°C), THC in one sample decreased to 46.4% of baseline after 4 weeks, and 3 of 16 samples’ THC concentrations decreased to 60.5-75.1% of baseline after 24 weeks. THC was stable in all but one pool after RT storage for 1 week. Median %baseline concentrations at RT were 83.8%, including OF from the one participant where THC concentration decreased to 70.7% of baseline. All individual OF specimens collected with the Oral-Eze device 4.5 h after smoking and stored for 1 week at 4°C also were stable with median %baseline concentrations of 97.3% (80.7–119%).
THCCOOH Stability
In StatSure pooled OF, all THCCOOH concentrations (n=12) were stable at 4°C for 1 week, but 3 weeks later, only 11 of 13 were stable, with one pool increasing (141.9% baseline) and one decreasing (68.4% baseline) concentration. These same 2 samples were the only unstable samples after 4 weeks at -20°C. After 24 weeks at -20°C, 100% of samples (n=13) were stable, with median %baseline concentrations of 104% (88.3-118%). In OF collected 4.5 h after smoking with the StatSure device and stored for 1 week at RT, 12 of 13 participants’ samples had stable THCCOOH concentrations, with one sample's THCCOOH decreasing to 60.0% of baseline.
In pooled OF collected with the Oral-Eze device, all samples (n=13) were stable when refrigerated for one and 4 weeks. After 4 weeks at -20°C, concentrations from one sample decreased to 60.8% baseline. This sample was also unstable for THC under the same storage condition (4 weeks at -20°C). After 24 weeks at -20°C, this sample's THCCOOH concentration remained unstable (75.1% baseline), with 2 additional samples’ concentrations increasing to 127 and 139%. In OF collected 4.5 h after smoking with the Oral-Eze device, 11 of 12 participants’ samples were stable after 1 week at 4°C with median %baseline concentrations of 91.4%, including OF from one participant where THCCOOH concentrations decreased to 77.4% of baseline.
CBD Stability
In pooled OF samples collected with the StatSure device, 100% were stable under all tested conditions, with median %baseline concentrations of 97.6, 96.5, 100 and 97.8% after 1 and 4 weeks storage at 4°C (n= 11 and 10) and 4 and 24 weeks at -20°C (n=9 and 9), respectively. In OF collected with StatSure device 4.5 h after smoking, 6 of 7 samples were stable after one week storage at RT. Oral fluid from one participant decreased to 50.8% of baseline.
In pooled OF samples collected with the Oral-Eze device, 7 participants’ samples were stable after 1 week at RT with median %baseline of 84.1%, with only 1 sample decreased to 75.1%. Similar results were obtained at lower temperatures for storage times up to 4 weeks; 78, 89 and 78% of participants’ CBD concentrations were stable at 4°C for 1 and 4 weeks (n=9) and at -20°C for 4 weeks (n=9), respectively. Longer storage (24 weeks) at -20°C increased CBD instability; 44% of participants’ samples were unstable with %baseline between 31.9 and 77.1%. In OF collected with the Oral-Eze device 4.5 h after smoking, only 1 participant had a CBD concentration above the LOQ and after 1 week at 4°C, however, the CBD concentration was 162% of baseline.
CBN Stability
In pooled OF collected with the Statsure device, 15 of 16 samples were stable for 1 week at RT, with one increasing to 167% of baseline. After 4 weeks, 87 and 80% of samples were stable at 4°C (n=15) and -20°C (n=15), respectively. Statsure OF from the same 2 participants were unstable at both temperatures exhibiting %baseline increases of up to 166%. Similarly, 13 of 15 samples were stable after 24 weeks at -20°C (n=15) with median %baseline of 95.9% (90.3 – 106%). As expected, the 2 unstable samples increasing to 133 and 183% baseline were from the same participants. In OF collected 4.5 h after smoking with the StatSure device, 100% of participants’ samples (n=12) were stable, with %baseline concentrations ranging between 83.3 and 111%.
In pooled OF collected with the Oral-Eze device, only 53% of samples (n=15) were stable after 1 week at RT; concentrations in 2 unstable samples increased up to 155% baseline, while concentrations from 5 other samples decreased to 70.2 and 78.4% of baseline. For the same storage duration, decreasing temperature to 4°C did not increase CBN stability. Longer storage at the same temperature (4°C or -20°C) sometimes appeared to increase stability. Indeed, at 4°C, %stable samples increased from 60% for 1 week (n=15) to 79% for 4 weeks (n=14) storage; at -20°C, %stable samples increased from 64% for 1 week (n=14) to 86% for 4 weeks (n=14). In OF collected 4.5 h after smoking with the Oral-Eze device, 4 of 6 participants’ samples were stable with median %baseline 93.5% (82-109%), with 2 increasing to 137 and 145%.
Discussion
We present cannabinoid stability in authentic OF specimens collected with the StatSure and Oral-Eze OF collection devices after controlled smoking of a 6.8% THC cigarette. This study included the sequential collection of authentic OF (StatSure Saliva Sampler followed by Oral-Eze) to evaluate cannabinoid stability. Evaluation of stability was performed on pooled participant samples and the order of collection did not affect these calculations. No significant differences were observed between baseline concentrations collected with either device (p>0.05), and stability was calculated as change from baseline. The strengths of this study include the variety of storage conditions evaluated and baseline specimen analysis within 24h of collection after controlled cannabis smoking, allowing precise determination of authentic OF concentrations. In addition, individually prepared pools for all 16 participants allowed evaluation of inter-subject variability. However, there were limitations to using authentic specimens, as some analyte baseline concentrations quantified <LOQ and could not be included in stability calculations. Forty percent of all specimens had CBD <LOQ at baseline.
In pooled OF collected with the Statsure device, all analytes were stable (median %baseline between 80-120%) and were, most of the time, not affected by temperature or duration of storage. THC and CBD were the most stable analytes with concentrations at all stability conditions 82.3–117% of baseline (100% stable). THCCOOH, an important analyte that may help discriminate passive environmental contamination from active cannabis smoking [23] was also highly stable under all storage conditions. THCCOOH concentrations were always stable with the exception of 2 of 13 samples stored for 4 weeks at 4°C. The same 2 samples also were unstable when stored for 4 weeks at -20°C. The few unstable samples for CBN were always the consequence of an increase compared to the baseline concentration. In our previous stability study with authentic OF collected by expectoration or with the Quantisal device, a similar phenomenon was observed with CBN concentrations [24]. This may be explained by the conversion during storage of THC to CBN [8; 25; 26; 27]. These results indicated that OF collected with the StatSure device for cannabinoid quantification should be stored for 4 weeks at 4°C or 24 weeks at -20°C before analysis without significantly affecting THC, CBD and THCCOOH concentrations. However, the best storage condition for CBN was 1 week at 4°C (94% of specimens stable.)
In OF collected with the Oral-Eze device, changes in temperature and storage conditions adversely affected analyte stability. At RT all analytes except CBN (only 53% stable) had at least 88% of samples stable for 1 week. As previously discussed, CBN instability may be due to concentration increases from baseline, as also observed with other collection devices. Decreasing temperature from RT to 4°C resulted in slightly better stability for all analytes, including CBN. CBN stability increased from 53% after 1 week of storage at RT to 79% after 4 weeks of storage at 4°C. All THC and THCCOOH samples were stable at 4°C for 1 and 4 weeks. After 4 weeks, all analytes were more stable at 4°C than -20°C. This trend of decreasing stability was amplified after 24 weeks of storage at -20°C for all analytes except CBN. In order to accurately determine THC, CBN, CBD and THCCOOH concentrations in Oral-Eze collected OF specimens, we suggest storage at 4°C and analysis within 4 weeks.
As expected, baseline concentration ranges were similar for each device, and overall, there were few stability differences between StatSure and Oral-Eze collections. THC and CBD were the most stable analytes collected by each device, and CBN the least. With the StatSure collection device all THC (n=64) and CBD (n=39) stability samples were stable. In comparison, for pooled samples collected with the Oral-Eze device, 75 of 80 (93.8%) and 34 of 44 (77.3%) were stable for THC and CBD, respectively. Similar results were observed for THCCOOH, as 47 of 51 (92.2%) and 62 of 67 (92.5%) samples were stable when collected with the StatSure or Oral-Eze device, respectively. For similar storage conditions, OF StatSure specimens were slightly more stable than Oral-Eze OF specimens, suggesting that cannabinoids were better preserved in the StatSure buffer. Our previous study evaluated cannabinoid stability in authentic expectorated OF and in authentic OF collected with the Quantisal device [24]. Specimens collected with a device showed improved stability over expectorated OF specimens. In that previous study, less than 50% of expectorated OF specimens remained stable 4 weeks after collection with storage at 4°C or -20°C. Stability of cannabinoids in OF collected with the Quantisal device was similar to the stability observed in this study with Oral-Eze, in that specimens were generally less stable when stored for longer periods of time at lower temperatures (-20°C). The differences in stability between devices are most likely due to the proprietary buffer composition and OF/buffer volume ratio that is unique for each manufacturer. Although each device collects 1 mL OF, the total OF/buffer mixture volume varied for each device. The buffer volumes for StatSure (1 mL), Oral-Eze (2 mL), and Quantisal (3 mL) help to stabilize drugs but yield dramatically different dilutions for each device. The most diluted OF (Quantisal) had the lowest cannabinoid stability, whereas the least diluted OF (StatSure) exhibited the greatest stability. However, less device buffer results in lower total sample volume; which may be problematic in workplace, clinical and forensic drug testing settings where screening and multiple drug confirmation assays on the same specimen are commonplace. Other studies evaluated THC stability in fortified OF [18; 28; 29,30]. Wille et al. [18] documented THC stability in fortified OF specimens collected with the StatSure device. Specimens stored for 8 weeks at 4°C and –20°C displayed similar stability with our results in authentic OF. However, others observed a loss of THC in fortified OF specimens collected with the Intercept device and stored between 2 and 4 weeks at all conditions (4°C, 21°C or –20°C); or in fortified neat OF stored for 24h at RT (>50% loss) and stored for 24h or 2 weeks at 4°C and – 18°C (15-35% loss) [28; 29]. Sergi et al, evaluated short (14 days) and long term (2 months) stability in fortified OF at -20°C for THC, CBD, CBN and THCCOOH. All analytes were within intermediate reproducibility of the method [30]. These results demonstrate the variability between authentic and fortified OF, and how OF collection method and device buffer composition can affect cannabinoid stability and test result interpretation. It is essential that a thorough evaluation of cannabinoid stability be performed for all commercially available OF collection devices.
Using the 4.5h specimen, our evaluation of manufacturers’ recommended storage conditions (Table 2) demonstrated that OF collected from either device may be shipped at RT or refrigerated to analytical laboratories, as recommended by the collection device manufacturer. Acceptable stability results were obtained for StatSure specimens containing CBN and CBD. However, Oral-Eze stability results should be qualified as 10/16 (CBN) and 15/16 (CBD) participants’ OF samples had concentrations <LOQ 4.5 h after dosing and were not included in stability calculations.
In summary, these OF cannabinoid stability results obtained after controlled smoked cannabis administration and collected with StatSure and Oral-Eze devices, suggest performing analyses within 4 weeks of storage at 4°C for Oral-Eze and within 4 weeks at 4°C or 24 weeks at -20°C for StatSure. These data contribute to the OF cannabinoid scientific database and permit the development of evidence-based OF drug testing policy and legislation, and improve interpretation of authentic OF cannabinoid results.
Acknowledgements
The authors acknowledge the contribution of the Chemistry and Drug Metabolism Section staff, and the clinical staff of the National Institute on Drug Abuse, Intramural Research Program and the Behavioral Pharmacology Research Unit and Clinical Research Unit, Johns Hopkins Bayview Medical Center. This research was supported by the Intramural Research Program, National Institute on Drug Abuse, NIH. MMB received grants from CAPES and FAPESP (Brazil) and NAD received a fellowship from the “Fondation Baxter et Alma Ricard” (Canada).
References
- 1.United Nations Office on Drugs and Crime (UNODC) World Drug Report 2011. Vienna: 2011. p. 272. [Google Scholar]
- 2.Bosker WM, Huestis MA. Oral Fluid Testing for Drugs of Abuse. Clin. Chem. 2009;55:1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cone EJ, Huestis MA. Interpretation of oral fluid tests for drugs of abuse. Ann. N.Y. Acad. Sci. 2007;1098:51–103. doi: 10.1196/annals.1384.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee D, Huestis MA. Current knowledge on cannabinoids in oral fluid. Drug Test. Anal. 2013 doi: 10.1002/dta.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration (SAMHSA) Proposed Revisions to the Mandatory Guidelines for Federal Workplace Drug Testing Programs. 2004;69:19673–19732. [Google Scholar]
- 6.Verstraete A, Knoche A, Jantos R, Skopp G, Gjerde H, Vindenes V, Mørland J, Langel K, Lillsunde P. Per se limits - Methods of defining cut-off values for zero tolerance, 6th Framework Programme, Deliverable 1.4.2, Driving under the Influence of Drugs, Alcohol and Medicines. 2011.
- 7.Fairbairn JW, Liebmann JA, Rowan MG. The stability of cannabis and its preparations on storage. J. Pharm. Pharmacol. 1976;28:1–7. doi: 10.1111/j.2042-7158.1976.tb04014.x. [DOI] [PubMed] [Google Scholar]
- 8.Turner CE, Hadley KW, Fetterman PS, Doorenbos NJ, Quimby MW, Waller C. Constituents of Cannabis sativa L. IV: Stability of cannabinoids in stored plant material. J. Pharm. Sci. 1973;62:1601–1605. doi: 10.1002/jps.2600621005. [DOI] [PubMed] [Google Scholar]
- 9.Garrett ER, Gouyette AJ, Roseboom H. Stability of tetrahydrocannabinols II. J. Pharm. Sci. 1978;67:27–32. doi: 10.1002/jps.2600670108. [DOI] [PubMed] [Google Scholar]
- 10.ElSohly MA. Chemical Constituents of Cannabis. In: Grotenhermen F, Russo E, editors. Cannabis and Cannabinoids: Pharmacology, Toxicology, and Therapeutic Potential. The Haworth Integrative Healing Press; New York: 2002. pp. 27–36. [Google Scholar]
- 11.Garrett ER, Hunt CA. Physiochemical properties, solubility, and protein binding of delta-9-tetrahydrocannabinol. J. Pharm. Sci. 1974;63:1056–1064. doi: 10.1002/jps.2600630705. [DOI] [PubMed] [Google Scholar]
- 12.Molnar A, Lewis J, Fu S. Recovery of spiked Delta9-tetrahydrocannabinol in oral fluid from polypropylene containers. Forensic Sci. Int. 2013;227:69–73. doi: 10.1016/j.forsciint.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Houwing S, Smink BE, Legrand SA, Mathijssen RP, Verstraete AG, Brookhuis KA. Repeatability of oral fluid collection methods for THC measurement. Forensic Sci. Int. 2012;223:266–72. doi: 10.1016/j.forsciint.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Lee D, Milman G, Schwope DM, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid stability in authentic oral fluid after controlled cannabis smoking. Clin. Chem. 2012;58:1101–9. doi: 10.1373/clinchem.2012.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langel K, Engblom C, Pehrsson A, Gunnar T, Ariniemi K, Lillsunde P. Drug testing in oral fluid-evaluation of sample collection devices. J. Anal. Toxicol. 2008;32:393–401. doi: 10.1093/jat/32.6.393. [DOI] [PubMed] [Google Scholar]
- 16.Moore C, Vincent M, Rana S, Coulter C, Agrawal A, Soares J. Stability of [Delta]9-tetrahydrocannabinol (THC) in oral fluid using the Quantisal(TM) collection device. Forensic Sci. Int. 2006;164:126–130. doi: 10.1016/j.forsciint.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Moore C, Rana S, Coulter C. Simultaneous identification of 2-carboxy-tetrahydrocannabinol, tetrahydrocannabinol, cannabinol and cannabidiol in oral fluid. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2007;852:459–64. doi: 10.1016/j.jchromb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Wille SM, Di Fazio V, Ramirez-Fernandez Mdel M, Kummer N, Samyn N. Driving under the influence of cannabis: pitfalls, validation, and quality control of a UPLC-MS/MS method for the quantification of tetrahydrocannabinol in oral fluid collected with StatSure, Quantisal, or Certus collector. Ther. Drug. Monit. 2013;35:101–11. doi: 10.1097/FTD.0b013e318278dbe4. [DOI] [PubMed] [Google Scholar]
- 19.Newmeyer MN, Desrosiers NA, Lee D, Mendu DR, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid disposition in oral fluid after controlled cannabis smoking in frequent and occasional smokers. Drug Test. Anal. 2014 doi: 10.1002/dta.1632. doi: 10.1002/dta.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anizan S, Milman G, Desrosiers N, Barnes AJ, Gorelick DA, Huestis MA. Oral fluid cannabinoid concentrations following controlled smoked cannabis in chronic frequent and occasional smokers. Anal. Bioanal. Chem. 2013;405:8451–8461. doi: 10.1007/s00216-013-7291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milman G, Barnes AJ, Lowe RH, Huestis MA. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J. Chromatogr. A. 2010;1217:1513–1521. doi: 10.1016/j.chroma.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowe RH, Karschner EL, Schwilke EW, Barnes AJ, Huestis MA. Simultaneous quantification of delta-9-tetrahydrocannabinol (THC), 11-hydroxy-delta-9-tetrahydrocannabinol (11-OH-THC), and 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH) in human plasma using two-dimensional gas chromatography, cryofocusing, and electron impact-mass spectrometry. J. Chromatogr. A. 2007;1163:318–327. doi: 10.1016/j.chroma.2007.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore C, Coulter C, Uges D, Tuyay J, van der Linde S, van Leeuwen A, Garnier M, Orbita J., Jr Cannabinoids in oral fluid following passive exposure to marijuana smoke. Forensic Sci. Int. 2011;212:227–30. doi: 10.1016/j.forsciint.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Lee D, Milman G, Schwope DM, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid Stability in Authentic Oral Fluid after Controlled Cannabis Smoking. Clin. Chem. 2012;58:1101–1109. doi: 10.1373/clinchem.2012.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross SA, MA E. CBN and D9-THC concentration ratio as an indicator of the age of stored marijuana samples. United Nations Office on Drugs and Crime. Bull. Narc. 1997;1:1–8. [Google Scholar]
- 26.Turner CE, Elsohly MA. Constituents of cannabis sativa L. XVI. A possible decomposition pathway of Δ9-tetrahydrocannabinol to cannabinol. J. Heterocycl. Chem. 1979;16:1667–1668. [Google Scholar]
- 27.Mechoulam R. Chapter 1. Cannabinoid Chemistry. In: Mechoulam R, editor. Marijuana. Academic Press; New York: 1973. pp. 1–87. [Google Scholar]
- 28.Crouch DJ. Oral fluid collection: the neglected variable in oral fluid testing. Forensic Sci. Int. 2005;150:165–73. doi: 10.1016/j.forsciint.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Molnar A, Lewis J, Doble P, Hansen G, Prolov T, Fu S. A rapid and sensitive method for the identification of delta-9-tetrahydrocannabinol in oral fluid by liquid chromatography-tandem mass spectrometry. Forensic Sci. Int. 2011;215:92–96. doi: 10.1016/j.forsciint.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 30.Sergi M, Montesano C, Odoardi S, Mainero RL, Fabrizi G, Compagnone D, Curini R. Micro extraction by packed sorbent coupled to liquid chromatography tandem mass spectrometry for the rapid and sensitive determination of cannabinoids in oral fluids. J. Chromatogr. A. 2013;1301:139–146. doi: 10.1016/j.chroma.2013.05.072. [DOI] [PubMed] [Google Scholar]