Abstract
The human settlement of the Pacific in general, and the origin of the Polynesians in particular, have been topics of debate for over two centuries. Polynesian origins are most immediately traced to people who arrived in the Fiji, Tonga, and Samoa region ≈3,000 B.P. and are clearly associated with the Lapita Cultural Complex. Although this scenario of the immediate origins of the Polynesians is generally accepted, the debate on the ultimate origin of the Polynesians and the Lapita cultural complex continues. Our previous research has shown that analyses of mtDNA variation in the Pacific rat (Rattus exulans), often transported as a food item in the colonizing canoes, are valuable for tracing prehistoric human migration within Polynesia. Here we present mtDNA phylogenies based on ≈240 base pairs of the d-loop from both archaeological and modern samples collected from Island Southeast Asia and the Pacific. We identify three major haplogroups, two of which occur in the Pacific. Comparing our results with Lapita models of Oceanic settlement, we are able to reject two often cited but simplistic models, finding support instead for multifaceted models incorporating a more complex view of the Lapita intrusion. This study is unique and valuable in that R. exulans is the only organism associated with the Lapita dispersal for which there are sufficient ancient and extant populations available for genetic analysis. By tracking population changes through time, we can understand more fully the settlement process and population interactions in both Near and Remote Oceania.
Keywords: Oceania, Lapita, prehistory, ancient DNA, phylogeography
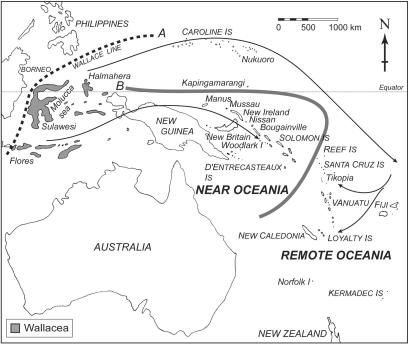
The history of human settlement in the island Pacific can generally be divided into two major phases: the settlement of Near Oceania, which commenced ≈40,000 B.P., and that of Remote Oceania, which began only ≈3,100 B.P. (1) (see Fig. 1). The initial settlement of Near Oceania involved the peopling of greater New Guinea followed by colonization of the Bismarck archipelago by 33,000 B.P. and the Solomon Islands by 29,000 B.P., if not before. Many modern-day descendants of these people speak what is often termed “Papuan” languages, which includes a remarkably diverse group of languages quite distinct from the more recently introduced Austronesian languages. The introduction of the Oceanic subgroup of Austronesian languages is associated by many with the appearance of the Lapita cultural complex into previously uninhabited coastal sites and on small off-shore islands in Near Oceania at ≈3,500-3,300 B.P. (3). The Lapita cultural complex is currently identified not only by the distinctive “Lapita” pottery and other artifacts, but also by the introduction of a number of plant and animal species, apparently including the Pacific rat, Rattus exulans.
Fig. 1.
Map of the Pacific showing the Wallace Line (A) and the line delineating Near and Remote Oceania (B). Arrows mark the proposed routes of dispersal of R. exulans according to Tate (2). Wallacea is shown in gray.
Within a few hundred years of the initial appearance of the Lapita cultural complex in Near Oceania, the voyaging barrier beyond it into Remote Oceania was breached, and Lapita settlements appear from the Reef/Santa Cruz group east of the Solomon chain through as far as Fiji, Samoa, and Tonga, on the western edge of the Polynesian Triangle, defined by the apices of Hawaii, Easter Island, and New Zealand. It is generally believed that these phenotypically Oceanic “Lapita people” from Vanuatu, New Caledonia, and Fiji are the ancestors of the Polynesians (1, 4). After a pause of ≈500-1,000 years, Polynesians then settled the rest of the Polynesian Triangle (1).
In a recent review of the role of Lapita in Pacific prehistory, Green (4) outlines current models for Lapita in both Near and Remote Oceania. For Lapita origins in Near Oceania, Green identifies four sets of models: set A, or the “Express Train to Polynesia” (ETP), focuses on a rapid dispersal from Southeast Asia (Taiwan) ultimately to Polynesia, with little or no contact with indigenous populations in between; set B, or the “Bismarck Archipelago Indigenous Inhabitants” (BAII), the other extreme perspective, argues that there is no need to consider any major migration into Near Oceania to account for the appearance of the Lapita cultural complex, rather that it can be explained as an indigenous development; set C, the “Slow Boat to the Bismarcks” (SBB) focuses on interactions within a “voyaging corridor stretching from eastern Indonesia to the Bismarck and Solomon Islands” from 6,000 to 3,500 B.P., followed by rapid expansion out into Remote Oceania ≈3,100 B.P.; and set D, the “Voyaging Corridor Triple I” (VC Triple-I), seen by some (5) as a “compromise solution” builds on the SBB model but allows for various components of the Lapita cultural complex to be the result of intrusion of new components along with the integration of materials from indigenous inhabitants in Near Oceania and innovation or development of new, unique components. For Lapita settlement of Remote Oceania, Green presents set E, which describes a rapid and rather unstable process he identifies as a Mobile Founding Migrant category of models. Using mtDNA phylogenies of the commensal Pacific rat, we can test these models of Lapita origins for both Near and Remote Oceania.
The Pacific rat, R. exulans, is the third most widely dispersed rat species, with a distribution that ranges from mainland Southeast Asia, throughout Island Southeast Asia and across the Pacific as far as Easter Island. It is believed to originate in island or peninsular southeast Asia (2, 6) and was not in Near Oceania before the Holocene (7). Rattus exulans skeletal remains first appear in Remote Oceania in Lapita settlements, generally in the earliest layers, and are present in all archaeological sites associated with both Lapita and with the later Polynesian settlement. The ubiquitous distribution and the fact that skeletal remains occur in large numbers in archaeological middens suggests that R. exulans was intentionally introduced, possibly as a food item. These rats do not swim so cannot self disperse (8). Therefore a phylogeographic analysis of R. exulans populations should provide evidence of the origins of the canoes that transported the animals, and thus shed light on the origins of both the Polynesians and the Lapita peoples.
In addition to R. exulans, people associated with the Lapita horizon also introduced dogs (Canis familiaris), pigs (Sus sp.), and jungle fowl (Gallus gallus) to the islands they settled. Unlike R. exulans, these animals were the same species carried by Europeans as they moved into the Pacific. Thus, just as with humans, there has been substantial admixture between Pacific and European populations over the intervening 300 plus years. R. exulans, on the other hand, is a distinct species from the rats introduced by Europeans (Rattus rattus and Rattus norvegicus), and so does not interbreed with them. Unlike human remains in the Pacific, R. exulans remains are numerous in archaeological sites and generally available for analyses. Therefore, R. exulans is particularly valuable in that the analysis of both ancient and modern samples provides a diachronic approach to population studies. This allows the rare opportunity to identify changes through time and to see how well modern populations represent past populations.
Previous research focused on mtDNA variation within East Polynesian populations of R. exulans (9) and showed that this rat was an excellent proxy for tracing the movement of prehistoric Polynesian peoples. Our analyses identified interaction spheres and specific population origins within Polynesia. Here, to address the more contentious debate of Polynesian and Lapita origins, we have combined these and additional Polynesian data with mtDNA sequences from the Western Pacific and Island Southeast Asian R. exulans populations, both ancient and modern. This analysis of R. exulans through time and space allows us the unique opportunity to test the various theories proposed for the human settlement of the Pacific with a degree of control not previously possible.
Materials and Methods
Overall, we analyzed sequence data from a total of 131 samples in this study. A collection of 64 R. exulans bone samples were acquired from the American Museum of Natural History for analysis. We obtained sequence data for 33 of the 64 samples. Most of these specimens were collected between 1921 and 1963. Three from Halmahera were collected in 1993. We also obtained mtDNA sequence from 87 bone samples from R. exulans recovered from archaeological excavations and natural cave sites throughout the Pacific. Fresh tissue samples were obtained from the north coast of Papua New Guinea and Thailand. These and six of our previously studied samples from Polynesia (9) were reamplified and sequenced with the same primers used for all other samples in this study. Sample information is available in Table 1, which is published as supporting information on the PNAS web site.
DNA was extracted from bones by using a modified silica/guanidinium thiocyanate method (10). Fresh tissues were extracted as described (9). We amplified and sequenced ≈240 base pairs of the hypervariable mitochondrial control region; see Supporting Text, which is published as supporting information on the PNAS web site, for specific methods.
All extraction and pre-PCR processing of bone material was conducted in a separate, dedicated ancient DNA laboratory with all precautions taken to avoid and identify any potential contamination (11). In addition, a randomly chosen subsample of specimens representing each identified haplogroup was replicated in an independent laboratory at Massey University (Palmerston North, New Zealand). From each of these specimens, a second bone was removed from an articulated cranial sample, and sent for DNA extraction, PCR amplification, and direct sequencing. All resulting sequences were identical to those obtained originally. Ancient DNA sequences were also compared to those obtained from fresh tissue in each of the major geographic regions (Southeast Asia, New Guinea, and Polynesia), to confirm their “phylogenetic sense” (11).
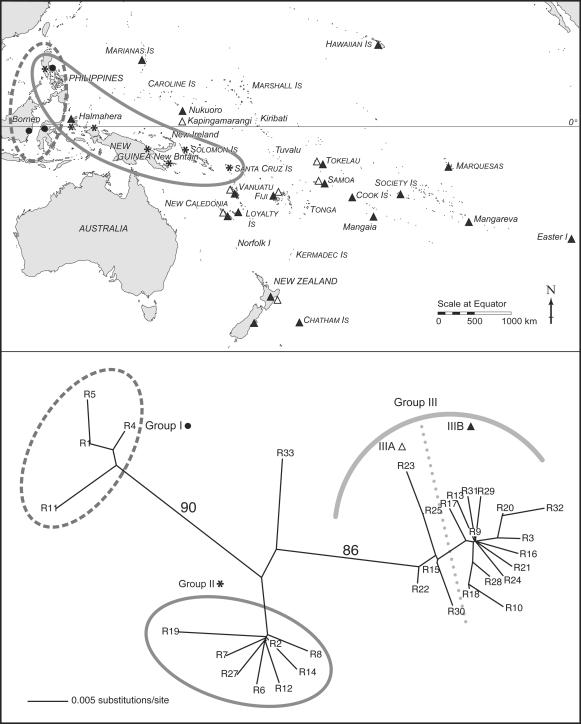
A total of 32 distinct haplotypes with 27 variable sites were identified. We constructed an unrooted neighbor-joining (NJ) tree (12) by using the distance matrix calculated by the Kimura two-parameter model of evolution (13) as implemented in the paup* software package (v 4.0b10; ref. 14). A bootstrap analysis using 1,000 pseudoreplicates was performed on the resulting tree. Trees were also estimated by using maximum likelihood with the general time reversible model of evolution. Eleven equally likely trees were found and had nearly identical topologies to that of the NJ tree. A strict consensus parsimony tree also identified the same major haplogroups. Our unrooted NJ tree is shown in Fig. 2.
Fig. 2.
An unrooted NJ tree and map of the Pacific showing location of samples and associated haplogroups. Bootstrap values for main branches are shown. Ellipses and symbols identify regions associated with particular haplogroups: filled circle, haplogroup I; asterisk, haplogroup II; open triangle, haplogroup IIIa; filled triangle, haplogroup IIIb. Haplotype R33 represents the three Thai samples.
Results and Discussion
Three distinct haplogroups, groups I, II, and III are identified in our analyses, and bootstrap values support the general structure of the phylogenetic tree shown in Fig. 2. Perhaps the most remarkable aspect of the tree is the clear geographic patterning. Haplogroup I consists solely of Southeast Asian samples from the Philippines, Borneo, and Sulawesi, suggesting an interaction sphere within Southeast Asia that has no relationship to the Oceanic settlement. Haplogroup II includes Southeast Asian and Near Oceanic samples, and indicates an eastern route of dispersal from the Philippines into Wallacea and then out into Oceania. Haplogroup III represents Remote Oceanic populations, with the exception of samples from Halmahera, in Wallacea, which appear in both haplogroups II and III.
To test the significance of the geographic structure identified on our NJ tree, specifically the separation of Near and Remote Oceanic samples, we compared it to both randomly generated trees and randomly allocated sample locations on the observed tree by using macclade V.4. Our results showed that the geographic pattern of haplotype locations is highly nonrandom (see Figs. 3-5 and Supporting Text, which are published as supporting information on the PNAS web site). Therefore, the clear geographic distinction, particularly between the Oceanic populations (groups II and III), allows us to reject two of Green's models for Lapita origins.
The BAII model predicts a reticulate pattern of variation without strong tree-like structure. This model would also require that any lineage found in Remote Oceania would originate in Near Oceania, particularly within the Bismarck Archipelago. Given the clear phylogenetic signal, combined with the fact that we find no haplogroup III lineages in Near Oceania, we can confidently reject the BAII model.
A strong tree-like pattern is predicted by the ETP model. However, given this model, we would expect to see a single central node which includes Taiwan samples, with Oceanic samples radiating out from that node. In Wodzicki and Taylor's map for the distribution of R. exulans (15), the species is not recorded in Taiwan. Recently, however, R. exulans has been reported in both Taiwan and the Ryukyu Islands (16), although the authors suggest that it is a recent “invader” there. mtDNA analyses of the Taiwanese R. exulans (17) identified very little variation (<0.5%) in the 35 samples sequenced, which is consistent with a recent introduction. To date, no archaeological evidence of R. exulans in Taiwan exists. In addition, the proposed speed of dispersal associated with the ETP model would not result in the two very distinct and rather distant groups identified in Oceanic populations. Unless the antiquity of R. exulans in Taiwan is demonstrated, we must reject the ETP model.
This leaves us with two models, the SBB and the VC Triple-I, which are difficult to distinguish based on any one data set alone, because the VC Triple-I model argues for differing processes for the various aspects of the Lapita Cultural Complex. However, by looking more closely at the structure within the haplogroups II and III, we can perhaps identify what is happening with at least one component of that complex: R. exulans.
The node at the center of haplogroup II (haplotype 2) is a DNA sequence found in samples from the Philippines, New Guinea mainland, the D'Entrecasteaux group, Woodlark Island, and Bougainville in the Solomon Islands. All other haplotypes in group II differ from that of this central node by one mutation, with the exception of the archaeological sample from the late completely post-Lapita RF3 site in the Reef/Santa Cruz group, bordering Near and Remote Oceania, which differs by two mutations. The distribution of haplotype 2 is particularly interesting, suggesting an interaction sphere/spheres encompassing this region, from the Philippines and Southern Indonesia through the Solomon Islands. This is consistent with archaeological evidence of obsidian trade (18, 19), animal translocations (7) between ISEA and Near Oceania, and post-Lapita interactions including the Reef/Santa Cruz group (20, 21). Thus, it fits with the first part of the SBB model for Lapita origins. However, the relationship between haplogroups II and III is not consistent with the second part of the SBB model, which posits a rapid dispersal from Near to Remote Oceania, which would result in the inclusion of Near Oceanic samples in haplogroup III.
Haplogroup III is clearly distinct from haplogroup II and consists of all samples from Remote Oceania, including all of those from Polynesia, the Northern Marianas, and the Polynesian outliers of Kapingamarangi and Nukuoro, in the south of the main Caroline group. These outliers are believed to be settled as the result of a back migration from Polynesia (1).
Unlike haplogroup II, in which samples all radiate from the central node of halplotype 2, haplogroup III appears to be more complex. The majority of samples belong to and/or radiate from the central node, haplotype 9, which represents not only the consensus sequence from our previous study of extant Polynesian R. exulans (n = 132), but also is found in most archaeological samples from East and West Polynesia, New Caledonia, Vanuatu, the Northern Marianas, and Nukuoro. We refer to this as subgroup IIIB. Group IIIB appears to be derived from subgroup IIIA, which includes samples from the more western locations in Remote Oceania of Vanuatu, New Caledonia, Fiji, Samoa, Tokelau, and Kapingamarangi. Interestingly, an archaeological sample from the Washpool site at the south of the North Island of New Zealand is also in subgroup IIIA (haplotype 22). With the exception of this New Zealand sample, the IIIA distribution is remarkably similar to that of Lapita in Remote Oceania, with haplotype 15 being found in Vanuatu, New Caledonia, Samoa, and Fiji. Tokelau and Kapingamarangi, also part of IIIA, were settled much later than the Lapita period, but probably in a Polynesian expansion from Samoa ≈1,000 B.P. (1).
Therefore, based on our rat data, we are able to reject the ETP, BAII, and SBB models for the settlement of Near Oceania. Our data do suggest a voyaging corridor model, but it appears that the rats in haplogroup III are an intrusive element as described by the VC Triple-I model for Lapita.
When we turn our attention to testing the model of settlement for Remote Oceania, we find that 82 of the 94 Remote Oceanic samples analyzed belong to haplogroup IIIB. Of these, 70 belong to haplotype 9. This overall pattern of limited variation within the haplogroup (all other samples in IIIB differ by only one or two base pairs) is thoroughly consistent with Green's Mobile Founding Migrant model, and with the Lapita origin of Polynesian populations. But the question remains: What is the source of these mobile founding migrants?
Origins of Remote Oceanic (Haplogroup III) Lineages. The only western samples in haplogroup III are from Halmahera, in Wallacea, making this, so far, the most likely point of origin for Remote Oceanic R. exulans populations. Halmahera rats are found in both haplogroup II and haplogroup III, and in fact represent the most variable population in our sample (see Table 2, which is published as supporting information on the PNAS web site). The implications of this result are significant, most particularly in the absence of haplogroup III lineages in Near Oceania.
Perhaps the simplest explanation would be that group III rats were transported directly from Halmahera to Remote Oceania, sailing past Near Oceania with no interaction. However, this would contradict all archaeological evidence linking Lapita in Near and Remote Oceania (1, 22). Although our data show no connection between Near and Remote Oceanic R. exulans populations, there is a second rat species, Rattus praetor, that is found in both Near and some Remote Oceanic Lapita sites. R. praetor is a New Guinea native rat not found in Island Southeast Asia or Wallacea (with the possible exception of Salawate and Gebe, just northwest of the birds head region of western New Guinea; ref. 7). R. praetor was first identified in Remote Oceania on Tikopia in archaeological layers dated to 2,300 B.P. (23). It appears that R. praetor was also present with R. exulans as early as 3,000 B.P. in the RF 2 Site in Reef/Santa Cruz (23). Roberts (6) previously suggested an “R. exulans only” boundary east of Tikopia. However, more recent reports indicate that R. praetor was also introduced prehistorically to both Vanuatu and Fiji (24), where it appears in early layers in natural cave deposits along with R. exulans remains. Therefore, if we have clear evidence that boats were transporting R. praetor between Near and Remote Oceania, why do we not see Near Oceanic R. exulans in Remote Oceania?
Two R. exulans Introductions in Near Oceania? The clear distinction between haplogroups II and III suggests that there were at least two R. exulans populations introduced into Oceania. Given that it is impossible to identify mtDNA lineages morphologically, it is highly unlikely that, from a variable source population, only one particular lineage could be selectively introduced to islands in one region, with the second lineage reserved for islands in another. Thus, a much more likely scenario is that these haplogroups represent two major introductions, possibly from the same source: Halmahera, or the general Wallacea region.
With two R. exulans introductions, the distinction between groups II and III could be the result of some kind of competitive exclusion, resulting in unsuccessful establishment of the later introduced lineage. If this is the case, then we must assume that the lineages present in any given location represent the initial founding population (with the possibility of lineage extinction over time). Given this scenario, locations that have multiple lineages would have had them introduced at the same time, and the source populations must also have been polymorphic. In this case, rat phylogenies are perhaps even more valuable in providing evidence of only the initial introductions.
If we apply this explanation to our observed data, specifically regarding possible competitive exclusion of haplogroup III in Near Oceania, we might be seeing the result of an initial pre-Lapita introduction of R. exulans (haplogroup II) from Wallacea to Near Oceania. Later, perhaps with Lapita peoples, rats of haplogroup III were carried from Wallacea, through Near Oceania, where they were not successfully introduced to occupied islands because of competition from the wide range of rats, including the earlier introduced R. exulans. However, when the boats reach islands previously uninhabited and where there were no other rodents, haplogroup III could successfully establish.
Lapita populations typically settled the previously uninhabited coastal and off-shore island regions in Near Oceania through the Solomon Islands and from there moved out to Remote Oceania. But our R. exulans samples from Near Oceania are from large islands (e.g., New Guinea, New Britain, Bougainville), which were not Lapita targets. If the Near Oceanic lineages of R. exulans (group II) were introduced and established on the large islands earlier than 3,500 B.P. and Lapita peoples then introduced the Remote Oceanic lineage (group III), it should be detectable on the smaller islets, for example, those off Mussau in the Bismarcks, or Anir, off New Ireland. This is a major goal for future research.
R. exulans is associated with all Lapita sites and was clearly introduced by Lapita peoples in Remote Oceania. The current archaeological evidence regarding the date of its initial introduction to Near Oceania, however, is ambiguous. Cave sites in the Bismarck Archipelago, which have provided faunal remains from the late Pleistocene, appear to be abandoned in the mid-Holocene (19). R. exulans and R. praetor remains were only found in level 2 and above in Baluf, New Ireland, which dates to 3,120 B.P. (7). However, at Panakiwuk, also on New Ireland, where R. praetor was found as early as 13,000 B.P., three R. exulans bones were also recovered from levels dated to between 8,000 and 13,000 B.P. It has been suggested (25) that the R. exulans bones were not in situ and are present in these layers as a result of disturbance, although there is no other evidence for this. Given that people were transporting a range of other animals within and between Near Oceania and Island Southeast Asia during the early Holocene and before, it is possible that R. exulans was also introduced before the arrival of the Lapita peoples. Only precise dating and sequencing of rodent remains from sites with faunal material from 10,000-3,000 B.P. in Near Oceania will resolve this question.
Alternate Routes of Dispersal? If we sample the small Lapita targets in Near Oceania, and do not find group III rats on those islands, we have to look at the possibility of an alternative route of dispersal to Remote Oceania. Two distinct routes of introduction of R. exulans into the Pacific were suggested by Tate (2) based on morphological variation in the species. Tate proposed one introduction from Island Southeast Asia into Near Oceania, and another from Island Southeast Asia, through Micronesia into the Fiji, Samoa, and Tonga region, and from there both west into Vanuatu and New Caledonia and east into east Polynesia (see arrows on Fig. 1). Geomorphological evidence (26) allows us to limit the timing of any migration route through the low island and atoll chains of Micronesia to the first millennium AD at the earliest, when lower sea levels first exposed these islands. However, full exposure of the palaeo-reef flats occurred even later, so most of them were only habitable within the last 1,000 years or so (26).
The Micronesian archaeological evidence, although limited, suggests that R. exulans was a late (not before 1,000-800 B.P.) introduction to the Northern Marianas and elsewhere where it is found in Micronesia (27, 28). Rattus tanezumi, the Asian house rat, is also found throughout Micronesia east of Enewetak in the Marshalls, and predates R. exulans where they are both found archaeologically. R. tanezumi is not recorded in Near Oceania, but is present on several islands in the Molucca group and further north in Island Southeast Asia. Musser (29) claims that it is also present in Fiji, but we have yet to confirm this with any modern or archaeological samples. This evidence suggests that at least one other rat species, R. tanezumi, was transported prehistorically from Island Southeast Asia through Micronesia, and possibly into Fiji. It is therefore possible that R. exulans was also taken along this route at a later time, although the presence of R. exulans in Remote Oceania from 3,100 B.P. would require this to be a secondary introduction. Further archaeological excavations in Micronesia with dating and DNA analyses of rat remains throughout the Pacific, particularly from the earliest Lapita layers, will help shed light on the chronology of rat introductions. We have established a DNA database for other Pacific rodents that will help in species identification of rodent remains in the archaeological record.
Comparison with Human Data. Our result highlighting the significance of Island Southeast Asia, in particular Halmahera, or the Wallacea region, for Lapita origins is consistent with genetic analyses of human population origins in the Pacific. Analyses of maternally inherited mtDNA variation have identified and traced the distribution of a number of population-specific markers in the Pacific, most importantly the Asian-derived 9-bp deletion coupled with three point mutations, which is often referred to as the “Polynesian motif” (30-32). Although the 9-bp deletion and Polynesian motif is the predominant mitochondrial lineage in both Polynesian and Micronesian populations, other “non-Asian” lineages are found in both regions, and those are traced to Near Oceania. Richards et al. (33) suggest that this motif originated in eastern Indonesia (Wallacea), where its immediate precursor, the 9-bp deletion with two of the point mutations, occurs.
More recently, researchers have begun looking at the paternally inherited Y-chromosome diversity in Pacific populations (34, 35). The Y chromosome data suggest a “Melanesian” or Eastern Indonesian origin for the predominant lineage found in Micronesia and Polynesia, identified as lineage 10.2 (36, 37). However, they have also found a number of Island Southeast Asian derived lineages, identified as haplogroup 26 and more specifically lineage 26.4, which are distributed throughout both Near and Remote Oceania. These results, compared with the mtDNA evidence, have been interpreted as possibly representing differential settlement or patterns of gene flow between males and females in the founding populations of Remote Oceania, specifically related to matrilineality (38). The conclusions of researchers regarding the origins of both Central Micronesian and Polynesian mtDNA and Y chromosomes are similar. They argue that these populations share common origins in both Island Southeast Asia and “Melanesia” (by which they mean either the Bismarck Archipelago or adjacent North Coastal New Guinea), yet are the result of distinct and separate settlement histories that include a significant amount of postsettlement gene flow (37, 39). Analyses of biparental genetic markers show similar results (40). Our rat results indicate the importance of targeting the Wallacea region specifically, rather than other general Island Southeast Asian populations, for further human DNA analyses. However, we also suggest that, given the high degree of admixture in the region over thousands of years, ancient lineages are most likely to be rare in modern populations, and therefore might not appear in modern sampling.
Intentionality and Multiple Introductions. Since our first application of the “rat as proxy” model for human mobility, we have regularly faced questions regarding the intentionality of the introduction of rats and the possibility of recent historic transport of the species (41, 42). The only evidence of an historic introduction of R. exulans is Taiwan (17), although given the degree of interaction around the Pacific, if rats were moving as stowaways historically, we would expect to see much more mixing of populations and reintroduction of haplogroup III to Near Oceania and visa versa. So, as our R. exulans data accumulate, we have increasing evidence for intentional introductions only and/or competitive exclusion of secondary introductions.
Conclusions
The results of our analyses of mtDNA variation in Pacific populations of R. exulans allow us to clearly reject two of the model sets described by Green (4) for Lapita origins. Specifically, the somewhat simplistic ETP set of models, although perhaps appropriate for describing the spread of the Austronesian languages (43), are inadequate for biological data in general and for our R. exulans data in particular. Similarly, the various BAII models, which argue for no clear phylogenetic signal, are also rejected by the results of our analyses. In contrast, our data do support the voyaging corridor aspect of both the SBB and the VC Triple-I models, but at this point, with no R. exulans evidence linking Near and Remote Oceania, we would have to suggest that haplogroup III R. exulans was an intrusive component of Lapita, and we therefore find strongest support for Green's VC Triple-I model. Versions of this model, which do not try to condense all types of data (linguistic, archaeological, and biological) into a single event, although difficult to test, are much more realistic.
Given the more recent settlement of Remote Oceania, it is easier to track population movements and tease apart the postsettlement interactions in this region (44). Green's Mobile Founding Migrant models for the settlement of Remote Oceania that suggest rapid dispersal are consistent with the distribution and variation identified in our haplogroup III. They lend this model set robust support and enable one to clearly trace the origins of Polynesian R. exulans back to the Lapita introductions in Remote Oceania.
This research has again shown the value of using commensal animals to trace human migration patterns. To further address this topic, we must continue to sample R. exulans populations throughout Southeast Asia and the Pacific, and we must sequence complete R. exulans mitochondrial genomes, investigate nuclear DNA markers, and track mtDNA variation in the other rat species transported into Remote Oceania, specifically R. praetor and R. tanezumi. Similarly, analyses of the distribution and genetic variation in the other commensal animals, the pig, dog, and chicken, should be initiated. Integrating these results with those from other fields such as archaeology, comparative linguistics, and molecular biology of human populations will be the only way we can fully understand the complex prehistory of this region. We argue that each of these data sets need to be analyzed independently and then synthesized within a model or models that allow for such complexity. Simplistic models that constrain the history of language, biology, and culture with a single explanation are clearly inappropriate for understanding the human settlement of the Pacific.
Supplementary Material
Acknowledgments
We thank The American Museum of Natural History, the Archaeozoology Laboratory of The Museum of New Zealand, The Otago Museum, The Canterbury Museum, The South Australia Museum, Melinda Allen, Atholl Anderson, Stuart Bedford, Simon Best, Eric Brothers, Dave Burley, Janet Davidson, Louise Furey, Roger Green, Richard Holdaway, Roz Hunter-Anderson, Thegn Ladefoged, Foss Leach, Koji Lum, Jack Grant-Mackie, Guy Musser, Doug Sutton, Richard Walter, Marshall Weisler, and Joan Wozniak for access to samples. For replication and verification of our results, we thank Peter Ritchie and David Lambert (Massey University, Auckland, New Zealand). We thank Ann Horsburgh, Jane Allen, and Sonia Townsend for laboratory assistance, and Seline McNamee for illustrations. We are most grateful to Roger Green, Howard Ross, Allen Rodrigo, Don Love, and two anonymous reviewers for helpful comments and advice. Funding for this research was provided by the New Zealand Foundation for Research, Science and Technology and the Centres of Research Excellence Fund.
Abbreviations: ETP, Express Train to Polynesia; BAII, Bismark Archipelago Indigenous Inhabitants; SBB, Slow Boat to the Bismarcks; VC Triple-I, Voyaging Corridor Triple-I; NJ, neighbor joining.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY604202-AY604233).
References
- 1.Kirch, P. V. (2000) On The Road of The Winds: An Archaeological History of the Pacific Islands Before European Contact (Univ. of California Press, Berkeley).
- 2.Tate, G. H. H. (1935) Bull. Am. Mus. Nat. Hist. 68, 145-178. [Google Scholar]
- 3.Kirch, P. V. (2001) in Lapita and its Transformations in Near Oceania, ed. Kirch, P. V. (Archaeological Research Facility Contribution No. 59, Berkeley), Vol. 1, pp. 196-222. [Google Scholar]
- 4.Green, R. C. (2003) in Pacific Archaeology: Assessments and Anniversary of the First Lapita Excavation (July 1952), ed. Sand, C. (Le Cahiers de l'Archeologie en Nouvelle-Caledonie, Noumea, New Caledonia), Vol. 15, pp. 95-120. [Google Scholar]
- 5.Terrell, J. E. & Welch, R. L. (1997) Antiquity 71, 548-572. [Google Scholar]
- 6.Roberts, R. M. (1991) Pacific Sci. 45, 123-130. [Google Scholar]
- 7.Flannery, T. (1995) Mammals of the South-West Pacific and Moluccan Islands (Cornell Univ. Press, Ithaca, NY).
- 8.Matisoo-Smith, E. (1994) J. Polynesian Soc. 103, 75-87. [Google Scholar]
- 9.Matisoo-Smith, E., Roberts, R. M., Irwin, G. J., Allen, J. S., Penny, D. & Lambert, D. M. (1998) Proc. Natl. Acad. Sci. USA 95, 15145-15150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matisoo-Smith, E., Allen, J. S., Ladefoged, T. N., Roberts, R. M. & Lambert, D. M. (1997) Electrophoresis 18, 1534-1537. [DOI] [PubMed] [Google Scholar]
- 11.Handt, O., Höss, M., Krings, M. & Pääbo, S. (1994) Experentia 50, 524-529. [DOI] [PubMed] [Google Scholar]
- 12.Saitou, N. & Nei, M. (1987) Mol. Biol. Evol. 4, 406-425. [DOI] [PubMed] [Google Scholar]
- 13.Kimura, M. (1980) J. Mol. Evol. 16, 111-120. [DOI] [PubMed] [Google Scholar]
- 14.Swofford, D. L. (2001) paup*: Phylogentic Analysis Using Parsimony (* and Other Methods) (Sinauer, Sunderland, MA).
- 15.Wodzicki, K. & Taylor, R. H. (1984) Acta Zool. Fenn. 172, 99-101. [Google Scholar]
- 16.Motukawa, M., Lu, K.-H., Harada, M. & Lin, L.-K. (2001) Zool. Stud. 40, 299-304. [Google Scholar]
- 17.Wu, H. Y., Wu, I. H., Chu, J. H. & Lin, Y. S. (2001) Plant Prot. Bull. 43, 205-214. [Google Scholar]
- 18.Bellwood, P. S. & Koon, P. (1989) Antiquity 63, 613-621. [Google Scholar]
- 19.Kirch, P. V. (1997) The Lapita Peoples: Ancestors of the Oceanic World (Blackwell Scientific, Oxford).
- 20.Green, R. C. (1997) N. Z. J. Archaeol. 17, 5-27. [Google Scholar]
- 21.Friedlaender, J. S., Gentz, F., Green, K. & Merriwether, A. (2002) Hum. Biol. 74, 453-471. [DOI] [PubMed] [Google Scholar]
- 22.Spriggs, M. J. T. (1997) The Island Melanesians (Blackwell, Cambridge, MA).
- 23.Flannery, T. F., Kirch, P. V., Specht, J. & Spriggs, M. (1988) Archaeol. Oceania 23, 89-94. [Google Scholar]
- 24.White, P. J., Clark, G. & Bedford, S. (2000) Pacific Sci. 54, 105-117. [Google Scholar]
- 25.Marshall, B. & Allen, J. (1988) in Report on the Lapita Homeland Project, eds. Allen, J. & Gosden, C. (Occasional Papers in Prehistory, Canberra, Australia), pp. 59-90.
- 26.Dickinson, W. R. (2003) J. Coastal Res. 19, 489-502. [Google Scholar]
- 27.Steadman, D. (1999) Micronesica 31, 319-345. [Google Scholar]
- 28.Wickler, S., in Colonisation, Migration and Marginal Areas: A Zooarchaeological Approach, eds. Mondini, M., Munoz, S. & Wickler, S. (Oxbow, Oxford), in press.
- 29.Musser, G. & Carleton, M. (1993) in Mammal Species of the World: A Taxonomic & Geographic Reference, eds. Wilson, D. E. & Reeder, D. M. (Smithsonian Inst. Press, Washington, DC), pp. 501-755.
- 30.Lum, J. K. & Cann, R. L. (1994) Hum. Biol. 66, 567-590. [PubMed] [Google Scholar]
- 31.Melton, T., Peterson, R., Redd, A. J., Saha, N., Sofro, A. S. M., Martinson, J. & Stoneking, M. (1995) Am. J. Hum. Genet. 57, 403-414. [PMC free article] [PubMed] [Google Scholar]
- 32.Sykes, B. C., Leiboff, A., Low-Beer, J., Tetzner, S. & Richards, M. (1995) Am. J. Hum Genet. 57, 1463-1475. [PMC free article] [PubMed] [Google Scholar]
- 33.Richards, M., Oppenheimer, S. J. & Sykes, B. (1998) Am. J. Hum. Genet. 63, 1234-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurles, M. E., Irven, C., Nicholson, J., Taylor, P. G., Santos, F. R., Loughlin, J., Jobling, M. A. & Sykes, B. C. (1998) Am. J. Hum. Genet. 63, 1793-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su, B., Jin, L., Underhill, P., Martinson, J., Saha, N., McGarvey, S. T., Shriver, M. D., Chu, J., Oefner, P., Chakraborty, R. & Deka, R. (2000) Proc. Natl. Acad. Sci. USA 97, 8225-8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kayser, M., Brauer, S., Weiss, G., Underhill, P. A., Roewer, L., Schiefenhövel, W. & Stoneking, M. (2000) Curr. Biol. 10, 1237-1246. [DOI] [PubMed] [Google Scholar]
- 37.Hurles, M. E., Nicholson, J., Bosch, E., Renfrew, C., Sykes, B. C. & Jobling, M. A. (2002) Genetics 160, 289-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hage, P. & Marck, J. (2003) Curr. Anthropol. 44, S121-S127. [Google Scholar]
- 39.Lum, J. K. & Cann, R. L. (2000) Am J. Phys. Anthropol. 113, 151-168. [DOI] [PubMed] [Google Scholar]
- 40.Lum, J. K., Jorde, L. B. & Schiefenhövel, W. (2002) Hum. Biol. 74, 413-430. [DOI] [PubMed] [Google Scholar]
- 41.Anderson, A. J. (1996) Rapa Nui J. 10, 31-36. [Google Scholar]
- 42.Langdon, R. (1995) Rapa Nui J. 9, 77-80. [Google Scholar]
- 43.Gray, R. D. & Jordan, F. M. (2000) Nature 405, 1052-1055. [DOI] [PubMed] [Google Scholar]
- 44.Hurles, M., Matisoo-Smith, E., Gray, R. D. & Penny, D. (2003) Trends Ecol. Evol. 18, 531-540. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.