Abstract
Vibrio cholerae is the causative agent of the severe diarrheal disease cholera. The production of the virulence factors that are required for human disease is controlled by a complex network of transcriptional and posttranscriptional regulators. ToxT is the transcription regulator that directly controls the production of the two major virulence factors, toxin-coregulated pilus (TCP) and cholera toxin (CT). The solved crystal structure of ToxT revealed an unstructured region in the N-terminal domain between residues 100 and 110. This region and the surrounding amino acids have been previously implicated in ToxT proteolysis, resistance to inhibition by negative effectors, and ToxT dimerization. To better characterize this region, site-directed mutagenesis was performed to assess the effects on ToxT proteolysis and bile sensitivity. This analysis identified specific mutations within this unstructured region that prevent ToxT proteolysis and other mutations that reduce inhibition by bile and unsaturated fatty acids. In addition, we found that mutations that affect the sensitivity of ToxT to bile also affect the sensitivity of ToxT to its positive effector, bicarbonate. These results suggest that a small unstructured region in the ToxT N-terminal domain is involved in multiple aspects of virulence gene regulation and response to human host signals.
INTRODUCTION
Vibrio cholerae is the etiological agent of the severe diarrheal disease cholera. Cholera disease is characterized by extreme water loss and dehydration due to diarrhea and if left untreated can result in death. The bacteria are usually ingested through contaminated food or water and colonize the upper small intestine (1). When the V. cholerae bacterium is in the optimal environment within the intestine, it begins producing the major virulence factors responsible for causing disease, cholera toxin (CT) and toxin-coregulated pilus (TCP) (2–4). CT is an ADP-ribosylating toxin composed of five binding B subunits and one enzymatic A subunit (5). After binding the GM1 ganglioside via the B subunits, the A subunit is translocated into the intestinal epithelial cell, where it modifies Gsα1, leading to aberrant secretion of chloride, water, and other electrolytes (6). TCP is a type IV bundle-forming pilus that is responsible for bacterium-bacterium interactions that result in microcolony formation during intestinal colonization (4, 7, 8).
TCP and CT are produced via a virulence regulatory cascade known as the ToxR regulon. The expression of CT and TCP is directly activated by the major virulence transcription regulator, ToxT (9, 10). ToxT binds “toxbox” motifs in the promoters of ctxAB and tcpA, as well as in the promoters of other accessory virulence factors, such as acfA, acfD, tagA, aldA, and tcpI, and small regulatory RNAs tarA and tarB, resulting in the expression of these genes under appropriate conditions (9, 11–17). ToxT is a 276-amino-acid protein that is part of the AraC/XylS family of transcription regulators (18). ToxT consists of two domains, the N-terminal domain (NTD; amino acids 1 to 160) and the C-terminal domain (CTD; amino acids 170 to 276), which are separated by a short linker (amino acids 161 to 169) (19). The CTD comprises the DNA-binding domain, which consists of two helix-turn-helix motifs and shares homology with AraC (20). The NTD shares no significant sequence similarity with any other protein, but shares secondary structural similarity with AraC (19) despite having only 14% identity at the amino acid level.
The transcription of toxT is initially activated by both TcpP/H and ToxR/S (10, 21–23). After ToxT protein is present, it can produce more of itself independently of TcpP/H and ToxR/S by binding to the promoter of tcpA and activating the transcription of a long, polycistronic mRNA containing toxT (14, 22). Proteolysis of ToxT is required to break this autoregulatory loop and completely shut off virulence gene expression prior to escape from the host (24). A region of the ToxT NTD between amino acids 100 and 109 was found to be required for the proteolysis of ToxT (24). The region of amino acids 101 to 110 was not resolved in the ToxT crystal structure, indicating the absence of a fixed structure, at least in ToxT crystals (19).
The activation of ToxT-dependent promoters is further regulated by effector molecules that act on ToxT. ToxT activity is inhibited by bile and, to a greater extent, the unsaturated fatty acid (UFA) components of bile, including oleic, linoleic, and arachidonic acids (25, 26). The ToxT crystal structure contained a buried 16-carbon fatty acid, palmitoleic acid, that was shown to decrease binding of ToxT to the tcpA promoter when added exogenously (19). Another inhibitor of ToxT, virstatin, decreases ToxT activation of ctxAB and tcpA by inhibiting ToxT dimerization (27, 28). On the other hand, ToxT activity is enhanced by bicarbonate, which is abundant within the upper small intestine where V. cholerae colonizes (29). Given their high concentrations in the upper small intestine, bile and bicarbonate are likely to be in vivo effectors used by V. cholerae to determine the optimal location for colonization.
The unstructured region between amino acids 101 and 110, together with the surrounding amino acids, has also been implicated in responding to bile, unsaturated fatty acids (UFAs), and virstatin (28, 30, 31). Due to its importance for ToxT proteolysis and sensing of ToxT inhibitory substances, we performed site-directed mutagenesis on this unstructured region and the surrounding amino acids to identify specific amino acid changes that alter ToxT function. Mutational analysis of amino acids 100 to 109 confirmed that this region is important for control of ToxT proteolysis. We have further identified specific mutations that prevent the ToxT effectors bile, UFAs, virstatin, and bicarbonate from affecting ToxT activity. Our results suggest that the unstructured region in the ToxT NTD plays a central role in the control of V. cholerae virulence by affecting ToxT activity at multiple levels.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli JM101 was used for cloning. All strains were maintained at −70°C in LB containing 20% glycerol. For determination of responses to negative effectors, overnight cultures of classical O395 V. cholerae were diluted 1:40 into LB, pH 6.5, in the presence or absence of 0.05% sodium choleate (Sigma-Aldrich) as a substitute for crude bile, 32 μM linoleic acid (Acros Organics), or 50 μM virstatin (Santa Cruz Biotechnology). Cultures were grown with shaking for 3 h at 30°C and analyzed. Virulence-inducing conditions using bicarbonate were as previously described (29). Briefly, classical O395 strains were grown overnight in LB and subcultured 1:100 into AKI medium (1.5% peptone, 0.3% yeast extract, 0.5% NaCl) in the presence or absence of freshly prepared 0.3% sodium bicarbonate (36 mM). Cultures were grown statically for 4 h at 37°C and analyzed. Induction of protein expression from pMAL-c2e derivatives under all of these conditions was done using a final concentration of 25 μM isopropyl-β-d-thiogalactopyranoside (IPTG). Proteolysis experiments were conducted as previously described (24). Overnight cultures were subcultured 1:40 into LB, pH 8.5, and grown at 37°C for 4 h. pBAD33 derivatives were induced using a final concentration of 0.2% arabinose. To assess proteolysis with additional effectors, pBAD33-ToxT was induced with or without the addition of the following final concentrations of effectors: linoleic acid (32 μM, 160 μM, or 320 μM), palmitic acid (380 μM) (Sigma), virstatin (100 μM or 500 μM), or sodium bicarbonate (36 mM). To evaluate ToxT degradation after a shift to repressing conditions, bacteria were cultured under virulence-inducing conditions (LB, pH 6.5, 30°C, with shaking) for 3 h with the addition of 0.2% arabinose. Then, cells were harvested by centrifugation and resuspended under virulence-repressing conditions (LB, pH 8.5, 37°C, with shaking). Linoleic acid was added to appropriate cultures to a concentration of 320 μM after the shift.
Plasmid and strain construction.
Construction of site-directed ToxT mutants was done using splicing by overlap extension PCR (32). For cloning C-terminally His-tagged toxT into pBAD33, outside primers BP22 and BP195 (24) were paired with inside primers containing desired mutations using V. cholerae O395 toxT as a template. PCR products were inserted into pBAD33 using restriction enzymes XbaI and PstI. For cloning MBP-ToxT, outside primers BP171 and BP172 (24) were paired with inside primers and PCR products were inserted into the pMAL-c2e vector using restriction enzymes KpnI and PstI. A list of primers used in this study is available in the supplemental material (see Table S1). Plasmids pBAD33 and pMAL-c2e containing WT or mutated toxT were electroporated into a previously constructed V. cholerae classical biotype strain O395 ΔtoxT mutant with a chromosomal tcpA::lacZ fusion (29).
Western blot analysis of ToxT.
Western blot analysis was performed as previously described (29). Briefly, subcultures were normalized by optical density at 600 nm, harvested by centrifugation, and resuspended in 10 μl water and 10 μl 2× protein buffer (123 mM Tris-HCl, 4% sodium dodecyl sulfate, 1.4 M 2-mercaptoethanol, 20% glycerol, 0.2% bromophenol blue). Samples were boiled for 5 min and subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gel was blotted on nitrocellulose and probed with a 1:5,000 dilution of mouse monoclonal anti-His tag antibody (Millipore). Secondary goat anti-mouse IgG conjugated to alkaline phosphatase (AP) was used at a dilution of 1:5,000 (Southern Biotech). After blots were washed, they were developed using immuno-BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium liquid substrate (MP Biomedicals). Densitometry of Western blots was performed using ImageJ software (NIH). The percentage of ToxT degradation was quantified by comparing the degradation band to total ToxT.
β-Galactosidase assays.
Cells were grown under the appropriate conditions, and β-galactosidase activity was measured and expressed in Miller units as previously described (24, 29, 33, 34). The fold change in tcpA::lacZ activation in response to effector molecules was calculated for WT and mutant ToxT. The fold change was calculated for each biological replicate, and the average and standard error were calculated. The fold change in activation of tcpA::lacZ in response to an effector by mutant ToxT was compared to the fold change in response to the effector by wild-type (WT) ToxT, and statistical significance was calculated using Student's t test.
Protein purification and EMSAs.
Protein purification and electrophoretic mobility shift assays (EMSAs) were performed as previously described (35). Maltose-binding protein (MBP) fusions were purified from E. coli BL21(DE3) with plasmid pMAL-c2e containing either MBP-ToxT WT or MBP-ToxT N106F (ToxT with a change of asparagine to phenylalanine at position 106). DNA probes for EMSA were produced by PCR of plasmid pTL61t containing PtcpA. Binding reactions were performed in a 30-μl total volume containing 10 μg/ml salmon sperm DNA, 10 mM Tris-acetate (pH 7.4), 1 mM potassium EDTA (pH 7.0), 100 mM KCl, 1 mM dithiothreitol (DTT), 0.3 mg/ml bovine serum albumin (BSA), and 10% glycerol, along with titrations of MBP-ToxT WT or N106F. Binding reactions were performed in the presence or absence of 36 mM sodium bicarbonate or 32 μM linoleic acid for 30 min at 37°C, and the mixtures were run through a 6% polyacrylamide gel at 4°C. Binding reaction mixtures for comparison to linoleic acid reaction mixtures contained 3.33% dimethyl sulfoxide (DMSO).
Binding curve analysis.
Autoradiographs were analyzed using ImageJ software (NIH) as previously described (37). Briefly, the percentage of labeled DNA bound by MBP-ToxT WT or N106F was determined for each lane, and GraphPad Prism 5 software was used for curve fitting to the equation % bound = Bmax × [protein]h/(Kdh + [protein]h), where Bmax is the amount of bound DNA where the curve plateaus, which we set to a constraint of 100% (this constraint represents all free DNA being bound by ToxT), and h is the Hill coefficient. The dissociation constant (Kd) for each condition was calculated, and the significance of differences in Kd between conditions was determined.
RESULTS
Identification of residues essential for ToxT proteolysis.
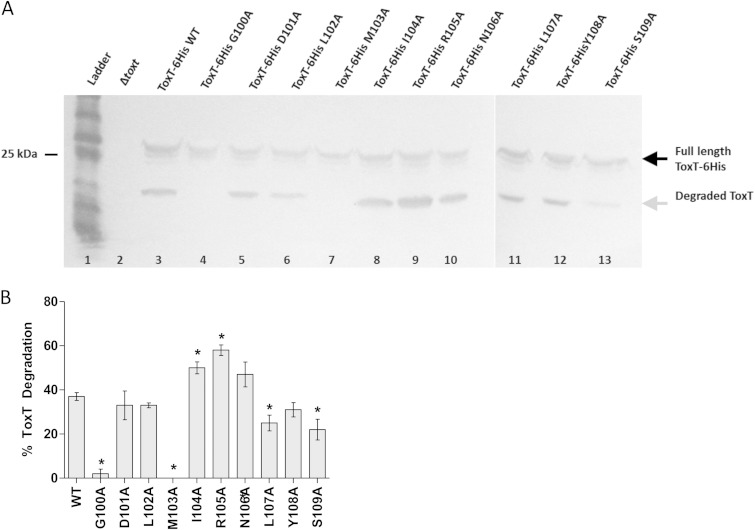
The crystal structure of ToxT revealed a small, unstructured region in the NTD of the protein (19). This region, as well as the surrounding amino acids, has been implicated in ToxT proteolysis, resistance to negative effectors of ToxT activity, and dimerization (24, 27, 28, 30, 31, 36). To further characterize this region in regard to these roles, we performed site-directed alanine mutagenesis of ToxT amino acids 100 to 109, shown in Fig. 1. To assess ToxT proteolysis, we grew V. cholerae strains expressing plasmid-borne WT or mutated ToxT proteins, which carried a polyhistidine tag at the C terminus (ToxT-6His) for detection, under repressing conditions (LB, pH 8.5, 37°C, with shaking) for 4 h. The previously described degradation intermediate of ToxT (24) was present in cell extracts from strains carrying each of the mutations in the unstructured region between amino acids 100 and 109 except for the G100A and M103A mutant strains (Fig. 2A). Densitometry was performed using ImageJ software (Fig. 2B) and revealed that the L107A and S109A mutants also had decreased levels of degradation compared to the results for the WT. Mutations I104A and R105A resulted in increased degradation of ToxT, possibly due to decreased ToxT stability. The identification of mutations in this region affecting ToxT proteolysis both confirms previous work demonstrating the importance of amino acids 100 to 109 and pinpoints amino acids 100 and 103 as crucial for ToxT proteolysis.
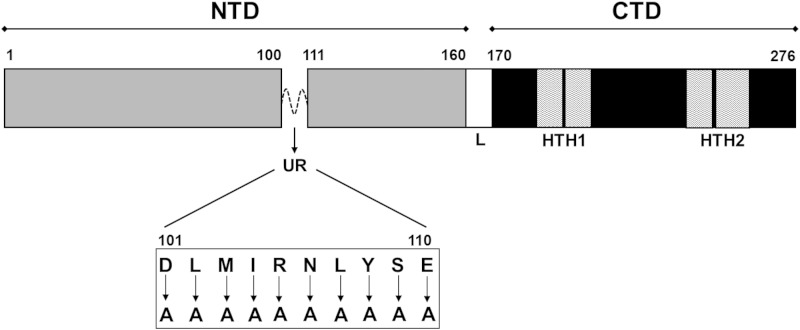
FIG 1.
Linear representation of ToxT domain orientation and mutagenesis sites. Amino acids located in the unstructured region (UR) of ToxT between amino acids 100 and 110 were mutated to alanine for initial analysis of the region. The N-terminal domain (NTD) is located between amino acids 1 and 160, the linker (L) between amino acids 161 and 169, the C-terminal domain (CTD) between amino acids 170 and 276, and helix-turn-helix domains 1 and 2 (HTH1 and HTH2) within the CTD.
FIG 2.
Effects of mutations in unstructured region of ToxT on proteolysis. (A) V. cholerae was grown under virulence-repressing conditions (LB, pH 8.5, 37°C, shaking) for 4 h, producing a degradation product of ToxT (gray arrow). Full-length ToxT-6His expressed from pBAD33 migrates to ∼32 kDa (black arrow). Cells with ToxT G100A and M103A mutations lacked the degradation product (lanes 4 and 7). Cells with I104A and R105A mutations had increased degradation of ToxT (lanes 8 and 9). Blots were probed with mouse monoclonal anti-His tag antibody, and samples were normalized by optical density at 600 nm (OD600). The gap between gels indicates two separate gels. Experiments were performed a minimum of three times, and representative data are shown. (B) Quantification of band intensities was performed using ImageJ software. Graph represents percentages of ToxT intermediate compared to total ToxT protein. Statistical significance of the percentage of ToxT mutant degradation compared to the percentage of wild-type (WT) degradation was determined by Student's t test (*, P < 0.05). Error bars represent ± standard errors of the means (SEM).
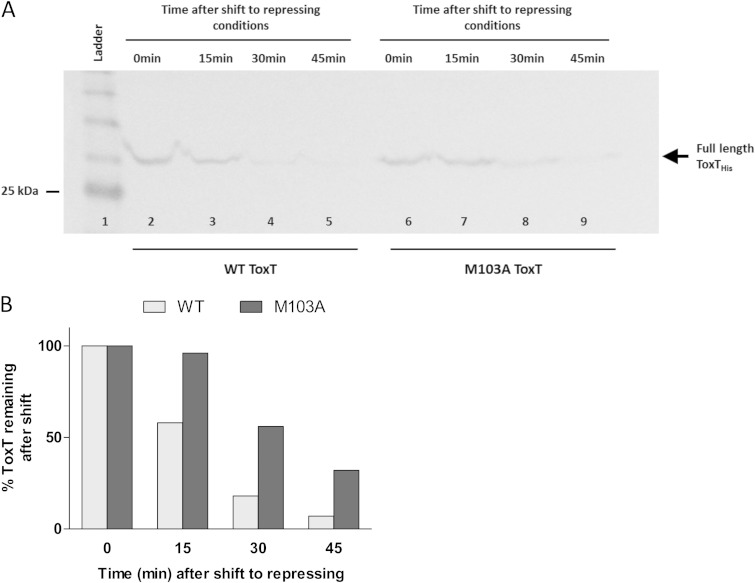
To distinguish whether the lack of degradation intermediates for the G100A and M103A mutants represented decreased ToxT degradation or increased degradation of the intermediates, we performed culture condition shift experiments. The shift experiment methods of Abuaita and Withey were amended slightly for use with mutant ToxT strains (24). The degradation of full-length WT ToxT and ToxT M103A was analyzed by Western blotting with anti-His tag antibody (Fig. 3A). WT ToxT was 82% degraded by 30 min after being shifted to virulence-repressing conditions. ToxT M103A was also degraded; however, 30% of the starting ToxT remained after 45 min. The ToxT intermediate degradation product is not visible in the culture shift experiments, as ToxT is no longer being actively induced after the shift to repressing conditions, so accumulation of the product does not occur. The degradation of ToxT G100A was also evaluated after a shift to repressing conditions and was similar to that of WT ToxT (data not shown). A likely explanation for the lack of intermediate degradation product seen with the G100A mutant (Fig. 2A) is enhanced proteolytic degradation of the intermediate. Together, these findings reveal that amino acid 103 is important for normal proteolysis and that the loss of the intermediate degradation product under virulence-repressing conditions is not only due to a decrease in the stability of the intermediate.
FIG 3.
Full-length WT and M103A ToxT degradation after shift to virulence-repressing conditions. (A) V. cholerae was grown under virulence-inducing conditions (LB, pH 6.5, 30°C, shaking) for 3 h while inducing WT ToxT or ToxT M103A from pBAD33. Samples were resuspended under virulence-repressing growth conditions (LB, pH 8.5, 37°C, shaking). Samples were normalized by OD600, and equivalent culture volumes were taken at each time point to monitor degradation of full-length ToxT. Full-length ToxT migrates to ∼32 kDa (arrow). WT ToxT degradation over time is shown in lanes 2 to 5, and ToxT M103A degradation over time is shown in lanes 6 to 9. The blot shown is representative of at least 3 separate experiments. (B) Quantification of band intensities from Western blot in panel A was performed using ImageJ software. Graph represents the percentages of ToxT remaining after shift to virulence-repressing conditions compared to the amounts at time zero. WT ToxT was 82% degraded by 30 min after shift to virulence-repressing growth conditions, while ToxT M103A persisted to a greater extent.
Identification of ToxT residues involved in responding to natural negative effectors bile and linoleic acid.
The ToxT unstructured region contains several residues that are required for normal proteolysis. Previously published evidence has also shown that residues in this region are required for the normal response of ToxT to negative effectors, such as bile and UFAs (31, 36). Bile and UFAs decrease the ability of ToxT to activate the transcription of virulence genes (25, 26). Two ToxT mutants, M103A and N106A, have previously been described as insensitive to bile and UFAs (31). We tested strains carrying these ToxT mutations, as well as strains carrying other alanine substitutions in the unstructured region, to determine whether their response to these negative ToxT effectors was altered. Overexpression of ToxT eliminates the effects of bile and linoleic acid on ToxT activity. Therefore, we chose to use the IPTG-inducible expression vector pMAL-c2e in place of arabinose-inducible pBAD33 due to the smaller amount of ToxT produced from the vector and, also, to facilitate protein purification for downstream applications. Previous work that used MBP-ToxT fusions showed no significant difference in activity compared to that of untagged ToxT (25). As a further control for any effect the MBP tag may have had on ToxT activity, we also performed experiments with untagged ToxT mutants of interest and found that they responded to effector molecules similarly to MBP fusions using the pMAL-c2e vector (see Fig. S2 in the supplemental material). The MBP-ToxT mutants were induced from pMAL-c2e in a ΔtoxT derivative of the classical biotype V. cholerae strain O395 containing a chromosomal tcpA::lacZ transcriptional fusion. The induction of tcpA transcription in the presence and absence of negative effectors was measured by β-galactosidase reporter assay and used as a metric for ToxT activity. The mean fold change for each mutant after the addition of effectors was determined and will be referred to as ToxT's response to effectors.
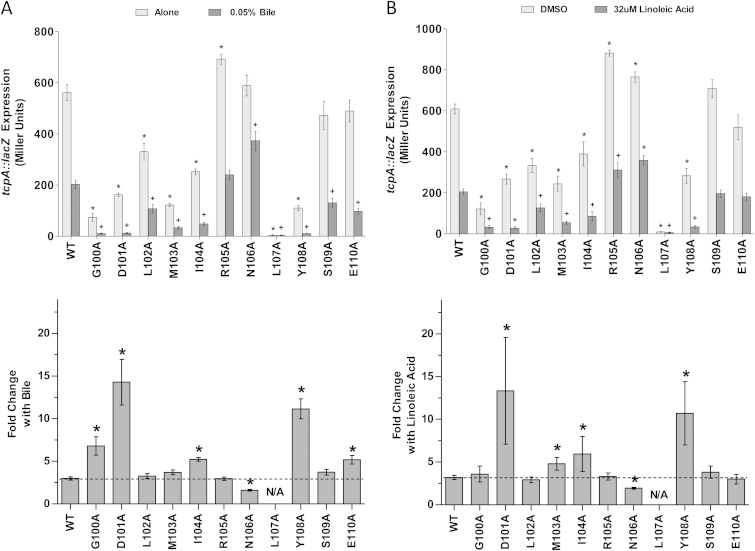
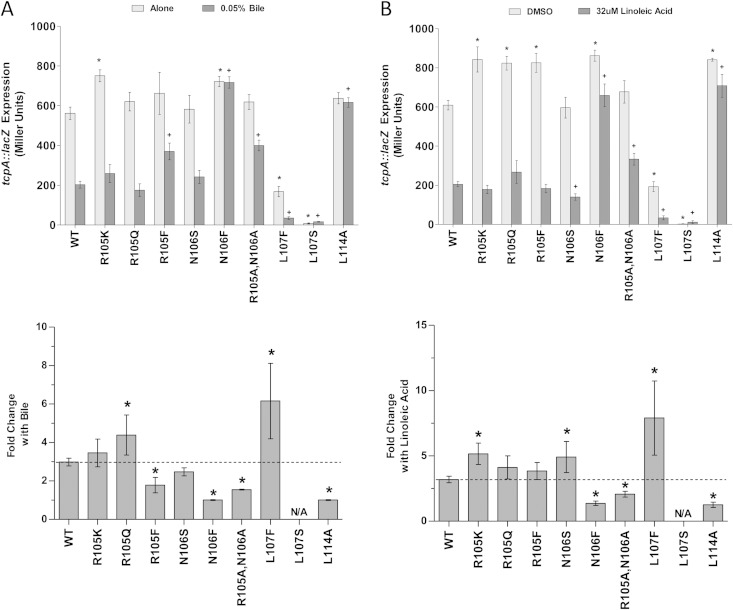
The results in Fig. 4A show the activities and responses of the ToxT mutants to 0.05% sodium choleate, a crude derivative of bile. The lowest concentration of sodium choleate that still decreased ToxT activity in titration experiments was 0.05% (data not shown). Alanine mutations to amino acids 100 to 104, 107, and 108 caused reductions in the overall activity of ToxT in the presence and absence of bile compared to the activity of WT MBP-ToxT. These results agree with previous reports that mutations at these residues reduce the activation of acfA::phoA (30). The L107A mutation completely eliminated tcpA::lacZ expression. Prior reports have suggested that this mutation inhibits ToxT dimerization and, therefore, inhibits ToxT activity (30, 31). Additionally, ToxT R105A had higher overall activity than the WT, as has been previously reported (30). The fold decrease in activation of tcpA::lacZ upon the addition of bile was calculated for each mutant (Fig. 4A). This analysis revealed ToxT mutations (G100A, D101A, I104A, Y108A, and E110A) that caused greater sensitivity to the negative effect of bile. The M103A mutation did not affect the sensitivity of ToxT to bile compared to that of the WT, which was unexpected, as this mutant reportedly had decreased sensitivity to bile when measuring CT and TCP production (31). However, a mutation at amino acid 106, which had previously been shown to decrease sensitivity to bile when measuring CT and TCP production (31), decreased sensitivity to bile in our work also.
FIG 4.
Effects of MBP-ToxT alanine mutagenesis on response to negative effectors bile and linoleic acid. V. cholerae O395 ΔtoxT mutant with plasmid-borne WT or mutant MBP-ToxT was grown under virulence-inducing conditions (LB, pH 6.5, 30°C, shaking) with or without the addition of negative effectors for 3 h. (Top) Light gray bars, V. cholerae grown without bile (A) or with dimethyl sulfoxide (DMSO) alone (B); dark gray bars, V. cholerae grown with 0.05% bile (A) or 32 μM linoleic acid (B) dissolved in DMSO. β-Galactosidase activity produced from chromosomal tcpA::lacZ in classical strain O395 ΔtoxT was measured. Statistical significance of ToxT mutant activation of tcpA::lacZ under each condition compared to WT ToxT activation of tcpA::lacZ under the same condition was calculated using Student's t test. Statistical significance of activation by a ToxT mutant without effector or with is denoted by an asterisk or plus sign, respectively (* or +, P < 0.05). (Bottom) Mean fold decrease in activation of tcpA::lacZ upon the addition of 0.05% bile (A) or 32 μM linoleic acid (B) for each ToxT mutant. Statistical significance of fold change in activity of a MBP-ToxT mutant compared to the activity of WT MBP-ToxT was determined by Student's t test (*, P < 0.05); the dashed line represents fold change of WT ToxT with the addition of effector. ToxT mutants with results above the line had increased sensitivity to effector, while results below the line represent decreased sensitivity to effector. Error bars represent ±SEM.
The ability of these ToxT mutants to activate tcpA::lacZ in the presence or absence of 32 μM linoleic acid was also assessed (Fig. 4B). As was observed in the bile experiments, mutation of residues 100 to 104, 107, and 108 caused decreased overall transcriptional activity with and without added effector. Also, the D101A, I104A, and Y108A mutants had increased sensitivity to linoleic acid, as we had observed with bile. Previous work suggested that the M103A ToxT mutant has diminished sensitivity to another UFA, palmitoleic acid (31). However, we observed increased sensitivity of the M103A mutant to the negative effector linoleic acid. The N106A mutant, which was previously reported to cause a reduced response to palmitoleic acid in terms of CT and TCP production (31), also exhibited decreased activation of tcpA::lacZ in the presence of linoleic acid, verifying the importance of this residue for the response to negative effectors.
Identification of ToxT residues involved in responding to a positive effector, bicarbonate.
We have previously shown that bicarbonate induces virulence gene expression by enhancing ToxT activity (29). It is possible that the response of ToxT to this positive effector is mediated by the same region of the protein that mediates the response to negative effectors. To determine whether the unstructured region is involved in the response of ToxT to bicarbonate, as well as to bile and UFAs as shown above, we analyzed these ToxT mutants in the absence or presence of the positive effector.
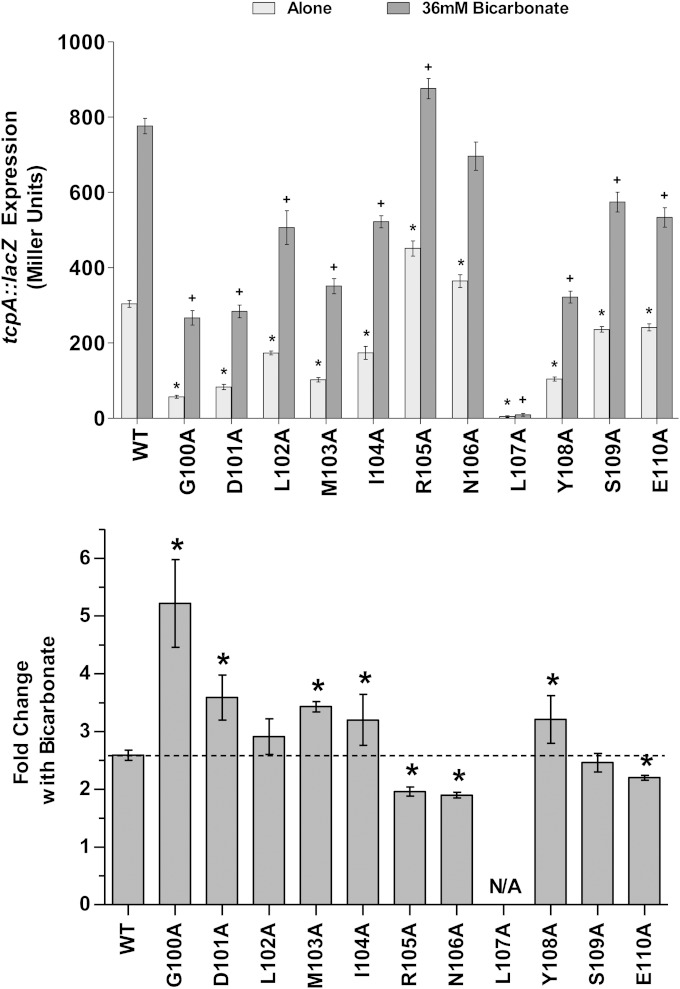
V. cholerae was grown in the presence and absence of 36 mM bicarbonate while expressing WT or mutant MBP-ToxT from a plasmid. β-Galactosidase assays were used to determine the activation levels of chromosomal tcpA::lacZ (Fig. 5). ToxT alanine substitutions at amino acids 100 to 104, 107, and 108 caused decreased overall ToxT activity, as we observed in the bile/UFA experiments described above. Additionally, mutation of S109 and E110 caused slight decreases in overall activity compared to that of the WT. The L107A mutant was completely inactive, and bicarbonate had no activating effect on this variant of ToxT. Bicarbonate increased the activity of WT MBP-ToxT 2.6-fold in these experiments (Fig. 5). ToxT G100A, D101A, M103A, I104A, and Y108A mutants all had an increased response to the activating effect of bicarbonate. Similarly, ToxT G100A, D101A, I104A, and Y108A mutants had increased sensitivity to bile or linoleic acid. The R105A, N106A, and E110A ToxT mutants had significantly reduced fold increases in tcpA::lacZ expression upon the addition of bicarbonate. The N106A mutant is notable because it also showed decreased sensitivity to the negative effectors bile and linoleic acid. These results suggest that the same ToxT residues within the unstructured region from amino acid 100 to 109 may be involved in the response to both positive and negative effectors.
FIG 5.
Effects of alanine mutagenesis of unstructured region amino acids on ToxT response to positive effector bicarbonate. V. cholerae O395 ΔtoxT mutant with plasmid-borne WT or mutant MBP-ToxT was grown under virulence-inducing conditions with or without bicarbonate (AKI medium, 37°C, static culture) for 4 h. (Top) Cells were grown without (Alone) or with 36 mM bicarbonate (0.3%). β-Galactosidase activity produced from chromosomal tcpA::lacZ in classical strain O395 ΔtoxT was measured. Statistical significance of ToxT mutant activation of tcpA::lacZ under each condition compared to WT ToxT activation of tcpA::lacZ under the same condition was calculated using Student's t test. Statistical significance of activation by a ToxT mutant without effector or with is denoted by an asterisk or plus sign, respectively (* or +, P < 0.05). (Bottom) Mean fold increase in activation of tcpA::lacZ upon the addition of bicarbonate is shown for each ToxT mutant. Statistical significance of fold change for a MBP-ToxT mutant compared to fold change for WT MBP-ToxT was determined by Student's t test (*, P < 0.05); the dashed line represents fold change of WT ToxT with the addition of effector. ToxT mutants with results above the line had increased sensitivity to effector, while results below the line represent decreased sensitivity to effector. Error bars represent ± SEM.
Alternate substitutions in the ToxT unstructured region affect responses to positive and negative effectors.
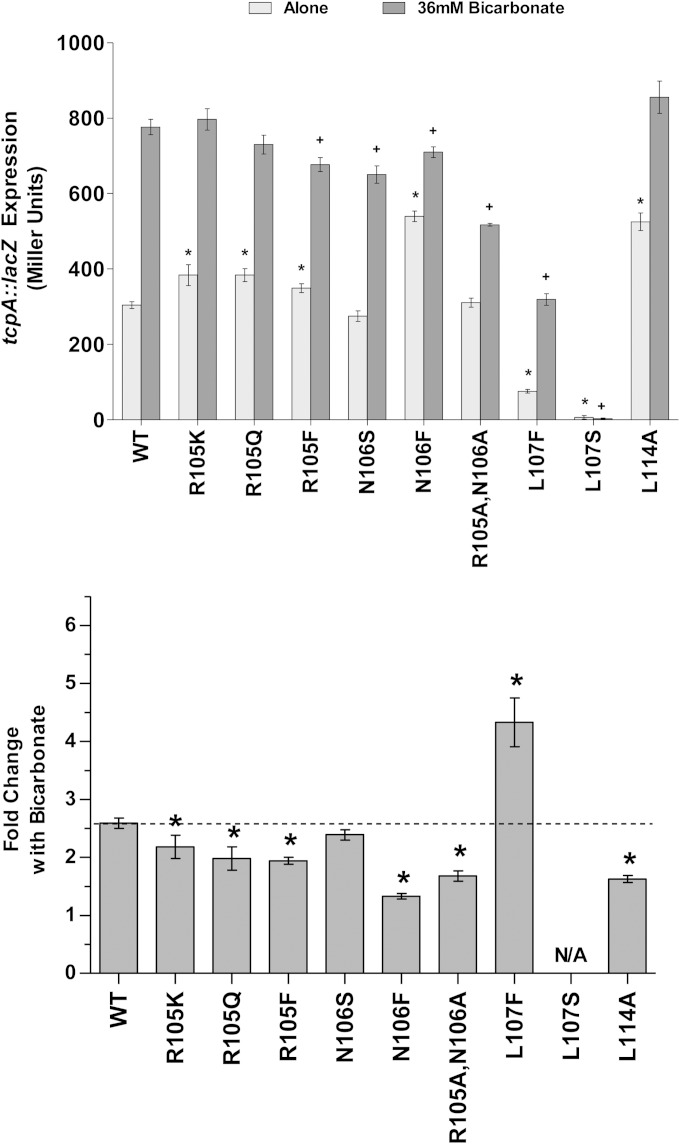
The results described above show that alanine mutagenesis within the unstructured region of ToxT alters the protein's sensitivity to bile, unsaturated fatty acids, and bicarbonate. Because amino acids in the unstructured region showed reduced sensitivity to both bile/UFA and bicarbonate when mutated to alanine, we generated alternate substitutions of amino acids 105 and 106 to further investigate the role of these residues in ToxT function. We also made mutations to amino acid 107, because the L107A mutant was completely inactive and a previous report showed that an L107F mutant had a decreased response to bile (36). Finally, we made an alanine substitution to amino acid L114, which has been shown to cause insensitivity to the inhibitory effects of bile, UFAs, and virstatin and is located in close proximity to the unstructured region from amino acid 100 to 109 (28, 31). The ToxT R105K, R105Q, R105F, N106S, N106F, L107F, L107S, and L114A mutants and an R105A/N106A double mutant were tested using the β-galactosidase assay described above under inhibiting conditions with bile or linoleic acid (Fig. 6A and B), as well as inducing conditions with bicarbonate (Fig. 7). The fold change in activity with the addition of effectors was calculated for each mutant and compared to the fold change for the response of WT ToxT.
FIG 6.
Alternate substitutions for amino acids in the unstructured region of ToxT affect responses to negative effectors. V. cholerae O395 ΔtoxT mutant with plasmid-borne WT or mutant MBP-ToxT was grown in the absence and presence of negative effectors. (Top) β-Galactosidase produced in the absence and presence of 0.05% bile (A) or with dimethyl sulfoxide (DMSO) or 32 μM linoleic acid dissolved in DMSO (B). β-Galactosidase activity produced from chromosomal tcpA::lacZ in classical strain O395 ΔtoxT was measured. Statistical significance of ToxT mutant activation of tcpA::lacZ under each condition compared to WT ToxT activation of tcpA::lacZ under the same condition was calculated using Student's t test. Statistical significance of activation by a ToxT mutant without effector or with is denoted by an asterisk or plus sign, respectively (* or +, P < 0.05). (Bottom) Mean fold decrease in activation of tcpA::lacZ upon the addition of effector is shown for each ToxT mutant. Statistical significance of fold change for an MBP-ToxT mutant compared to fold change for WT MBP-ToxT was determined by Student's t test (*, P < 0.05); the dashed line represents fold change for WT ToxT with the addition of effector. ToxT mutants with results above the line had increased sensitivity to effector, while results below the line represent decreased sensitivity to effector. Error bars represent ± SEM.
FIG 7.
Alternate substitutions for amino acids in the ToxT unstructured region affect responses to bicarbonate. V. cholerae O395 ΔtoxT mutant with plasmid-borne WT or mutant MBP-ToxT was grown in the absence or presence of 36 mM bicarbonate (0.3%). (Top) β-Galactosidase activity from chromosomal tcpA::lacZ in classical strain O395 ΔtoxT produced in the absence or presence of bicarbonate was measured. Statistical significance of ToxT mutant activation of tcpA::lacZ under each condition compared to WT ToxT activation of tcpA::lacZ under the same condition was calculated using Student's t test. Statistical significance of activation by a ToxT mutant without or with effector is denoted by an asterisk or a plus sign, respectively (* or +, P < 0.05). (Bottom) Mean fold increase in activation of tcpA::lacZ upon the addition of bicarbonate is shown for each ToxT mutant. Statistical significance of fold change for an MBP-ToxT mutant compared to fold change for WT MBP-ToxT was determined by Student's t test (*, P < 0.05); the dashed line represents fold change for WT ToxT with the addition of effector. ToxT mutants with results above the line had increased sensitivity to effector, while results below the line represent decreased sensitivity to effector. Error bars represent ± SEM.
Mutants with alternate substitutions for amino acid R105 generally exhibited higher than normal ToxT activity in the absence of effector molecules, similar to what was observed after mutation to alanine. ToxT R105Q had an increased response to the negative effector bile, while ToxT R105F exhibited a decreased response to bile (Fig. 6A). On the other hand, ToxT R105K exhibited increased sensitivity to linoleic acid (Fig. 6B). The increased sensitivity to negative effectors of the R105K and R105Q mutants may be due to a conformational change in the protein that increases the accessibility of the negative effector binding sites. ToxT L107F, which has previously been reported as having decreased responses to bile and UFAs (36), had increased responses to both negative effectors compared to the responses of the WT ToxT. ToxT L107S was similar to the L107A mutant in having inactivity. ToxT N106S had a response to bile similar to that of the WT, while the R105A/N106A double mutant had decreased sensitivity to bile, presumably due to the N106A mutation (Fig. 6A). The N106S mutation caused an increase in the response to linoleic acid, but the double R105A/N106A mutant displayed reduced sensitivity to linoleic acid (Fig. 6B). L114A ToxT was insensitive to bile and UFAs, as previously described (31). Another mutant, N106F ToxT, revealed complete insensitivity to the negative effectors bile and linoleic acid. This is a novel mutation that exhibits greatly decreased responses to negative effectors.
In addition to showing reduced responsiveness to the negative effectors, ToxT mutants with N106F, R105A/N106A, and L114A mutations exhibited decreased sensitivity to the positive effector bicarbonate (Fig. 7). All mutations to amino acid 105 resulted in decreased responses of ToxT to bicarbonate. Of all the mutations in this region, the N106F mutation caused the greatest decrease in the response to bicarbonate, followed by L114A and the double mutation R105A/N106A.
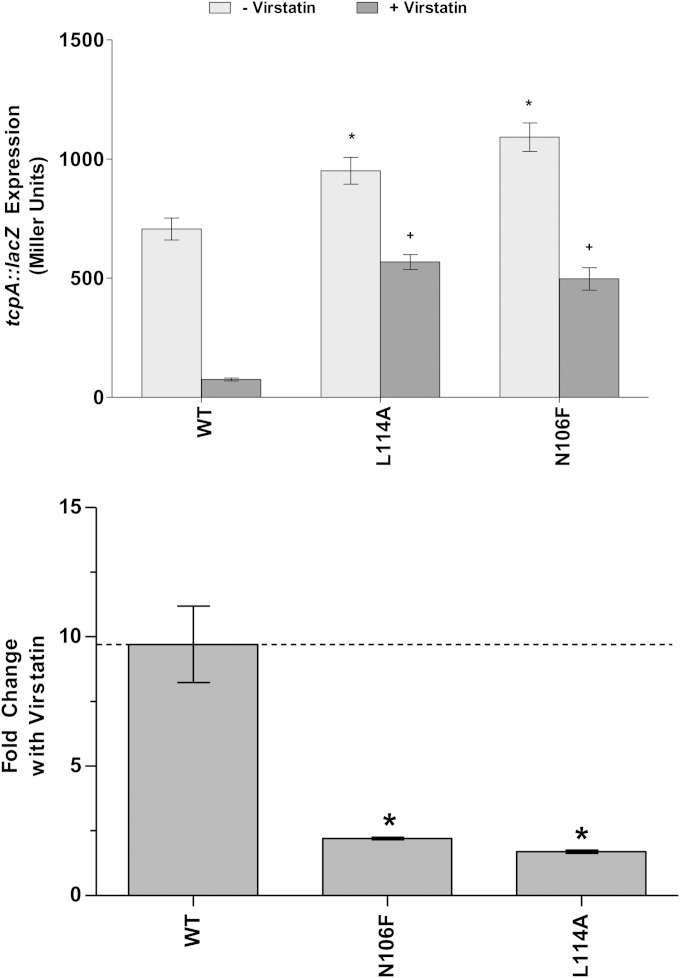
ToxT N106F has decreased sensitivity to virstatin.
In addition to bile and UFAs, mutations to amino acid residue L114 have been found to cause resistance to a synthetic inhibitory molecule, virstatin (27, 28, 31). We have shown by the results described above that the ToxT L114A mutant also has decreased sensitivity to bicarbonate (Fig. 7). The N106F mutant that we discovered to play a role in resistance to bile, UFAs, and bicarbonate behaves similarly to L114A under the same conditions. In order to determine whether the N106F mutant is also insensitive to virstatin, we grew the bacteria containing either the WT or N106F ToxT under virulence-inducing conditions in the presence and absence of 50 μM virstatin. In the presence of virstatin, there was a 9.7-fold reduction in tcpA::lacZ activation when the expression of WT MBP-ToxT was induced (Fig. 8). The effect of virstatin on ToxT activity was decreased in the resistant L114A mutant, consistent with the results of previous work (27, 28, 31). The effect of virstatin was also greatly decreased in the N106F mutant, as mentioned above.
FIG 8.
MBP-ToxT N106F mutant is insensitive to the negative effector virstatin. (Top) V. cholerae O395 ΔtoxT mutant with plasmid-borne WT or mutant MBP-ToxT in dimethyl sulfoxide (DMSO) alone or 50 μM virstatin dissolved in DMSO. β-Galactosidase activity produced from chromosomal tcpA::lacZ in classical strain O395 ΔtoxT was measured. Statistical significance of ToxT mutant activation of tcpA::lacZ under each condition compared to WT ToxT activation of tcpA::lacZ under the same condition was calculated using Student's t test. Statistical significance of activation by a ToxT mutant without or with effector is denoted by an asterisk or plus sign, respectively (* or +, P < 0.05). (Bottom) Mean fold decrease in activation of tcpA::lacZ upon the addition of virstatin is shown for each ToxT mutant. Statistical significance of fold change for an MBP-ToxT mutant compared to fold change for WT MBP-ToxT was determined by Student's t test (*, P < 0.05); the dashed line represents fold change for WT ToxT with the addition of effector. ToxT mutants with results above the line had increased sensitivity to effector, while results below the line represent decreased sensitivity to effector. Error bars represent ± SEM.
The DNA binding affinity of ToxT N106F is less affected than that of wild-type ToxT by linoleic acid and bicarbonate.
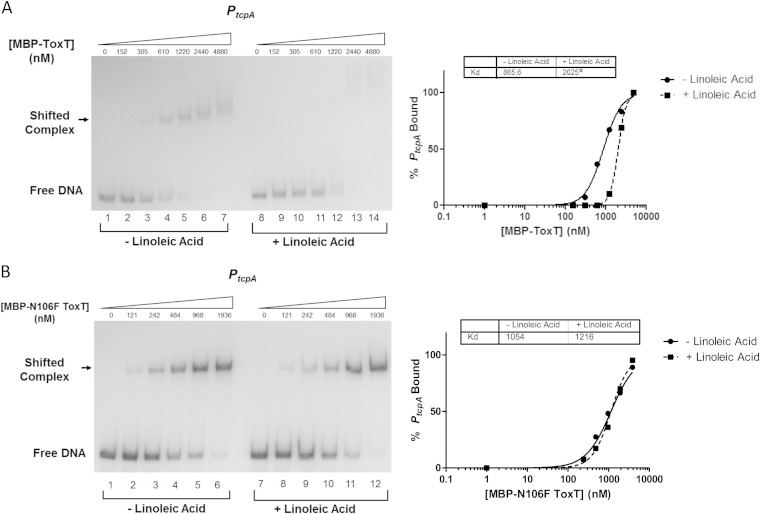
Previous work has suggested that palmitoleic acid decreases the ability of ToxT to bind to PtcpA (19). Additionally, we have shown that bicarbonate increases the binding affinity of ToxT to virulence gene promoters regardless of toxbox orientation (37). To assess the ability of linoleic acid and bicarbonate to alter the binding affinity of ToxT N106F to PtcpA, we used electrophoretic mobility shift assays (EMSAs) to determine the equilibrium binding affinity of WT MBP-ToxT and the N106F derivative for the major virulence gene promoter, PtcpA, in the absence and presence of effector.
A representative autoradiograph showing the titrations of WT MBP-ToxT binding in the absence (lanes 1 to 6) and presence of 32 μM linoleic acid (lanes 7 to 12) is shown in Fig. 9. The Kd of WT ToxT for PtcpA was increased with linoleic acid, corresponding to a decrease in binding affinity. The MBP-ToxT N106F mutant was also assessed for changes in Kd in the presence of linoleic acid. The EMSA and resulting binding curve for reaction mixtures containing linoleic acid revealed no significant change in Kd compared to those for the DMSO control (Fig. 9B).
FIG 9.
MBP-ToxT N106F has no change in binding affinity to PtcpA after the addition of linoleic acid. MBP-ToxT WT and N106F binding to PtcpA was analyzed using EMSA. Autoradiographs of EMSAs presented are representative of three or more independent experiments. (Left) Binding reactions between MBP-ToxT WT (A) or MBP-ToxT N106F (B) and PtcpA in lanes 1 to 7 took place with the addition of DMSO. Lanes 8 to 14 were incubated in the presence of 32 μM linoleic acid. Reaction mixtures in lanes 1 and 8 contained PtcpA DNA in the absence of MBP-ToxT. Reaction mixtures in subsequent lanes contained titrations of MBP-ToxT with the concentrations indicated above the lanes. (Right) Binding curves for the autoradiographs at the left. Densitometry of autoradiographs was performed with ImageJ software. Circles represent percentages of PtcpA bound by MBP-ToxT in the absence of linoleic acid, and the solid line corresponds to the binding curve for MBP-ToxT to PtcpA, determined by the following equation using GraphPad Prism 5 software: % bound = Bmax × [protein]h/(Kdh + [protein]h), with the Bmax constraint set to 100. The squares and dashed line represent percentages of PtcpA bound by MBP-ToxT and the binding curve, respectively, in the presence of linoleic acid. The Kd for each condition is inset, and a significant difference between the best-fit values of each data set is denoted by an asterisk (P < 0.00025).
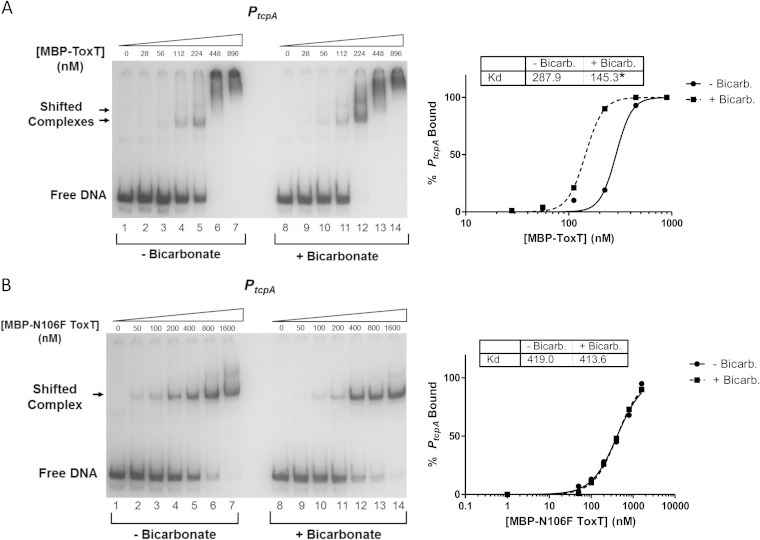
Similarly, we examined the binding affinities of WT MBP-ToxT and N106F MBP-ToxT for PtcpA in the absence and presence of 36 mM bicarbonate (Fig. 10). Binding reaction mixtures with no added bicarbonate are shown in lanes 1 to 7, while binding reaction mixtures with bicarbonate are shown in lanes 8 to 14. The binding curves revealed that bicarbonate decreased the Kd for WT MBP-ToxT (Fig. 10A), while there was no statistical difference in the binding affinity of ToxT N106F to PtcpA when bicarbonate was added (Fig. 10B). The lack of change in the binding affinity of ToxT N106F with the addition of these ToxT effector molecules correlates with the results of the transcriptional reporter assays described above (Fig. 6B and 7), providing a direct link between changes in DNA binding and transcription activation.
FIG 10.
MBP-ToxT N106F has no change in binding affinity to PtcpA after the addition of bicarbonate. MBP-ToxT WT and N106F binding to PtcpA was analyzed using EMSA as previously described (37). Autoradiographs of EMSAs presented are representative of three or more independent experiments. Panel A is taken from our previous study of the effects of bicarbonate on ToxT binding (37). (Left) Binding reactions between MBP-ToxT WT (A) or MBP-ToxT N106F (B) and PtcpA in lanes 1 to 7 took place in the absence of NaHCO3. Reaction mixtures in lanes 8 to 14 were incubated in the presence of 36 mM NaHCO3. Reaction mixtures in lanes 1 and 8 contained PtcpA DNA in the absence of MBP-ToxT. Reaction mixtures in subsequent lanes contained titrations of MBP-ToxT with the concentrations indicated above the lanes. (Right) Binding curves for the autoradiographs at the left. Densitometry of autoradiographs was performed with ImageJ software. Circles represent percentages of PtcpA bound by MBP-ToxT in the absence of bicarbonate, and the solid line corresponds to the binding curve for MBP-ToxT to PtcpA, determined by the following equation using GraphPad Prism 5 software: % bound = Bmax × [protein]h/(Kdh + [protein]h), with the Bmax constraint set to 100. The squares and dashed line represent percentages of PtcpA bound by MBP-ToxT and the binding curve, respectively, in the presence of bicarbonate. The Kd for each condition is inset, and a significant difference between the best-fit values of each data set is denoted by an asterisk (P < 0.00025).
Addition of unsaturated fatty acids prevents ToxT proteolysis.
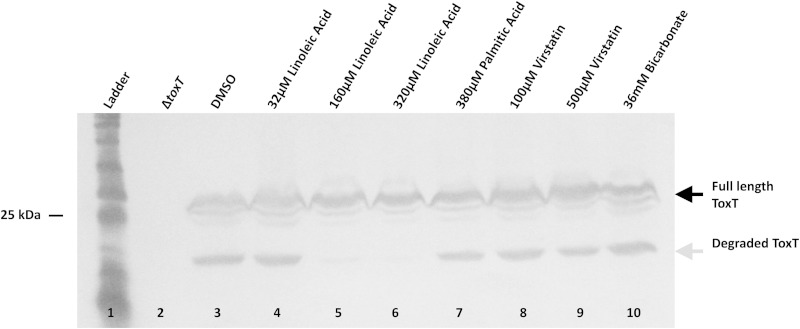
The work described above strongly suggests that the short unstructured region of ToxT between amino acids 101 and 110 and the surrounding amino acids are involved both in the response to positive and negative effectors and in ToxT proteolysis. Since such a small segment of ToxT was implicated in these different functions, we next investigated the possibility that the addition of effector molecules inhibits proteolysis by altering this region of ToxT and/or potentially blocking access to it by protease(s). We induced the production of WT ToxT under virulence-repressing conditions (LB, pH 8.5, 37°C, with shaking) that normally result in proteolysis of ToxT and allow the detection of proteolytic intermediates (24). We added various concentrations of effector molecules known to modulate ToxT activity positively or negatively and found that proteolysis of ToxT was eliminated in a dose-dependent manner by the UFA linoleic acid (Fig. 11). In contrast, a saturated fatty acid, palmitic acid, which does not inhibit ToxT activity (26), also does not inhibit ToxT proteolysis. The addition of effectors virstatin and bicarbonate did not significantly affect proteolysis.
FIG 11.
Linoleic acid inhibits ToxT proteolysis. (A) WT ToxT was produced from pBAD-toxT (29) under virulence-repressing conditions (LB, pH 8.5, 37°C, shaking) for 4 h, leading to production of the proteolytic intermediate (gray arrow). Full-length WT ToxT (black arrow) is produced under each condition. Control DMSO does not affect proteolysis (lane 3). The addition of increasing concentrations of linoleic acid blocked the formation of the ToxT degradation product (lanes 5 and 6). Palmitic acid, virstatin, and bicarbonate did not affect ToxT proteolysis (lanes 7 to 10). Blots were probed with mouse monoclonal anti-His tag antibody.
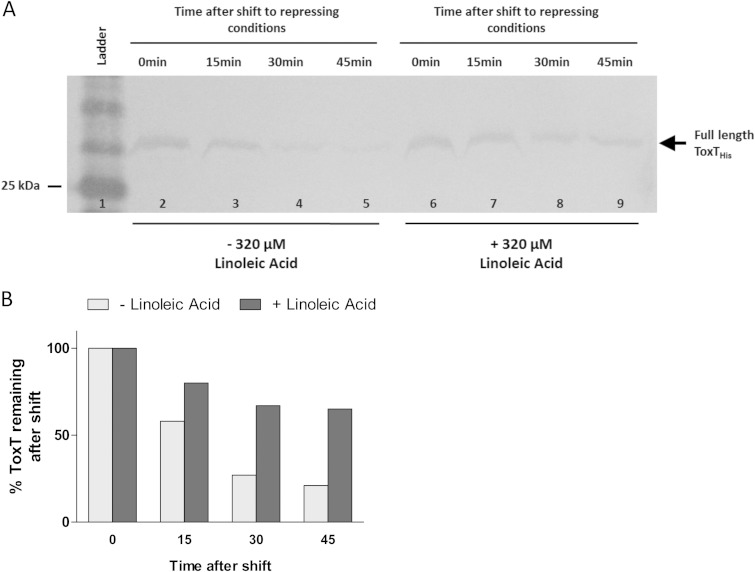
The elimination of proteolysis by linoleic acid could be due to a conformational change in ToxT upon binding of the effector, a steric interruption resulting from the large size of the effector, or inhibition of the protease responsible for degradation of ToxT. An additional possibility is that linoleic acid increases the degradation rate of the ToxT intermediate, which would resemble a defect in proteolysis. To assess this possibility, we used the assay discussed above (Fig. 3A), in which ToxT is induced under virulence-inducing conditions and then the cells are shifted to virulence-repressing conditions. Again, WT ToxT was 75% degraded after 30 min in the absence of linoleic acid (Fig. 12A). However, when linoleic acid was present after the shift, full-length ToxT persisted to a greater extent over time, as shown by densitometry (Fig. 12B). A portion of full-length ToxT was degraded when linoleic acid was present, but that could be attributed to the on/off rate of linoleic acid, allowing occasional access by the protease.
FIG 12.
Full-length WT ToxT degradation is inhibited by the addition of linoleic acid after shift to virulence-repressing conditions. (A) V. cholerae was grown under virulence-inducing conditions (LB, pH 6.5, 30°C, shaking) for 3 h to induce the production of WT ToxT from pBAD33. Samples were resuspended under virulence-repressing growth conditions (LB, pH 8.5, 37°C, shaking) with the addition of DMSO or 320 μM linoleic acid. Equivalent culture volumes were taken at each time point to monitor the degradation of full-length ToxT. Full-length ToxT migrates to ∼32 kDa (arrow). WT ToxT degradation over time in the absence of linoleic acid is shown in lanes 2 to 5, and WT ToxT degradation over time with the addition of linoleic acid is shown in lanes 6 to 9. (B) Quantification of band intensities from blot shown in panel A was performed using ImageJ software. Graph represents percentages of ToxT remaining after shift to virulence-repressing conditions compared to the amounts at time zero. WT ToxT was 75% degraded by 30 min after shift to virulence-repressing growth conditions, while ToxT in the presence of linoleic acid persisted to a greater extent. Blot shown is representative of at least 3 separate experiments.
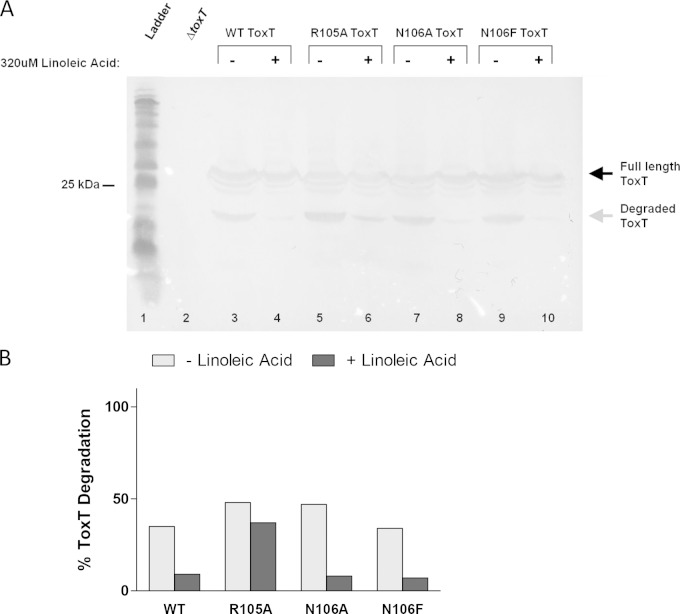
To determine whether linoleic acid interferes sterically with proteolysis in the unstructured region due to binding at this site, we analyzed the proteolysis of effector-insensitive ToxT mutants (R105A, N106A, and N106F) in the absence and presence of linoleic acid (Fig. 13A). Proteolysis of WT ToxT was almost fully inhibited by the addition of 320 μM linoleic acid, as shown earlier by the results presented in Fig. 11. ToxT R105A exhibited a lower degree of protection from proteolysis in the presence of linoleic acid, confirmed by densitometry (Fig. 13B). These data suggest that linoleic acid is not inhibiting the activity of the protease itself, because there would be similar detection of the degradation intermediate of ToxT R105A as with WT ToxT with linoleic acid present if the protease were inactivated. The effector-insensitive N106A and N106F mutants showed complete inhibition of proteolysis, similar to the results for WT ToxT. Therefore, it is unlikely that linoleic acid is binding to these amino acids and sterically interfering with the protease. This is not surprising due to the previous finding that a palmitoleic acid molecule was found in the interface between the NTD and CTD in the crystal structure of ToxT (19). As a result of our findings, we hypothesize that the closed conformation of ToxT upon binding UFA, introduced in Lowden et al., precludes access of the ToxT protease to the unstructured region and, thus, inhibits degradation (19).
FIG 13.
Linoleic acid inhibits proteolysis of effector-insensitive ToxT mutants. (A) Degradation of WT ToxT and R105A, N106A, and N106F mutants with DMSO (−) and with 320 μM linoleic acid (+) is shown. Linoleic acid inhibits proteolysis with each of the mutations (lanes 4, 6, 8, and 10). The R105A mutant still showed a small band of degraded ToxT in the presence of linoleic acid (lane 6). Samples were normalized by OD600. Experiments were performed a minimum of three times, and a representative blot is shown. (B) Quantification of band intensities from the blot shown in panel A was performed by densitometry using ImageJ software. Graph represents percentages of ToxT intermediate compared to total ToxT protein.
DISCUSSION
ToxT is the major transcription activator that induces CT and TCP production and, thus, is responsible for initiating cholera disease. It has previously been shown that amino acids in the region of positions 101 to 110 of ToxT are important for the response to negative effector molecules and dimerization (27, 28, 30, 31, 36). Also, this region was required for ToxT proteolysis (24). Interestingly, this region was not visible in the solved ToxT structure (19). We found in the work presented here that ToxT variants with G100A and M103A mutations abrogated normal proteolysis of ToxT, M103A being more important for protection from degradation. We further discovered that mutants with N106A, R105A/N106A, N106F, and L114A substitutions showed decreased responses to bile, linoleic acid, and bicarbonate. The N106F mutant also showed a reduced response to the negative effector virstatin, as did the previously reported L114A mutant (28). Most mutations to amino acid 105 had no effect on reducing the response to bile and linoleic acid but caused reduced sensitivity to bicarbonate. Additionally, the effector-insensitive ToxT N106F demonstrated no change in binding affinity to PtcpA in response to UFA and bicarbonate.
ToxT proteolysis occurs as a mechanism to break the autoregulatory loop that is initiated after ToxT protein production has begun. We found that ToxT G100A and M103A mutants had reduced ToxT proteolysis; however, further analysis indicated that the G100A mutation likely caused enhanced degradation of the proteolytic intermediate rather than reducing the degradation of full-length ToxT. The culture shift experiments showed that the rates of degradation for WT ToxT and the M103A mutant are nearly identical. However, the degradation of the M103A mutant is delayed by 15 min compared to that of the WT (Fig. 3A and B). This could be due to alteration of a protease recognition site, resulting in initially low degradation since a low concentration of protease cannot degrade M103A ToxT as efficiently as WT ToxT. However, as the cultures grow for longer times under the conditions that induce protease activity, a higher level of protease activity can be reached, and subsequently, the mutated form of ToxT is degraded. Possible mechanisms for resistance of the M103A mutant to proteolysis are alteration of the cleavage site, inaccessibility of the protease to the cleavage site due to a structural change, or an increase in protein stability. Previous work indicated that the protease cleavage site is located between amino acids 100 and 109, so any of these mechanisms could be consistent with the known data (24). The M103A mutation, which renders ToxT resistant to proteolysis, was previously reported to result in 300% more activation than WT ToxT in ctxA::lacZ assays but 44% of the WT level in acfA::phoA fusion assays (30). In our study, the M103A mutation caused decreased activation of tcpA::lacZ. Due to the observed decrease in transcription activation by this mutant, it is probable that the M103A mutation either alters a protease cleavage site or makes the site inaccessible to a protease, rather than increasing protein stability. Similarly, the addition of linoleic acid inhibited proteolysis, presumably due to a conformational change upon binding, resulting in an inactive state of ToxT. The inactive state of ToxT with bound linoleic acid may resemble the inactive conformation of ToxT M103A, preventing access to the unstructured region by the protease. However, mutations to amino acids 101, 102, 104, and 108 that also displayed decreased overall ToxT activity did not alter the proteolysis of ToxT. Therefore, we hypothesize that M103 is part of a protease recognition site.
ToxT L107A and S109A mutants also exhibited reduced degradation compared to that of the WT. There was still a proteolytic intermediate present; however, the replacement of leucine with alanine is very conservative and may not have changed the side chain enough to eliminate proteolysis completely. When tested, the L107F mutation caused no change in ToxT proteolysis, so residue L107 most likely is not directly involved in proteolysis (data not shown). Two other mutations in the unstructured region, I104A and R105A, increased ToxT degradation intermediate formation. This could be due to a decrease in ToxT stability, an increase in access by the protease, or enhanced stability of the intermediate. ToxT I104A exhibited a lower level of degradation of the full-length protein than WT ToxT, implying that full-length ToxT I104A has increased stability (see Fig. S3 in the supplemental material). ToxT R105A had increased degradation compared to that of WT ToxT in the culture shift assay, and we therefore conclude that it is indeed degraded to a higher level than the WT (see Fig. S3). ToxT R105A has overall enhanced activity compared to that of the WT (30). This form of ToxT, despite its higher activity, may have been evolutionarily selected against due to its increased proteolytic degradation.
The protease responsible for ToxT cleavage remains undiscovered, but locating a candidate cleavage site could aid in protease identification. Our work shows the involvement of multiple amino acids, primarily M103, in the proteolytic cleavage of ToxT, and the current thought is that multiple ATP-dependent proteases may be involved (24). The discovery that multiple amino acids are involved in the proteolytic degradation of ToxT supports the possibility of cleavage by multiple proteases. ATP-dependent proteases, such as Clp, Lon, and HsIV, recognize stretches of hydrophobic amino acids that are normally buried within the protein structure (38). Mutation of methionine to alanine at amino acid 103 could change the degree of hydrophobicity of this region of the protein, resulting in altered proteolytic cleavage.
Our findings revealed that the addition of linoleic acid blocked the proteolysis of ToxT. However, the UFA-insensitive ToxT N106F did not reverse the effect of linoleic acid on ToxT proteolysis. Therefore, we conclude that amino acid N106 is not part of a binding site for UFAs, in agreement with results from reference 19. The protective effect of linoleic acid on proteolysis is likely due to a ToxT conformational change upon binding of the effector molecule, effectively blocking access to the unstructured region by the protease. Interestingly, the degradation of ToxT was unchanged when bicarbonate was present in the proteolysis culture conditions. These findings, together with results from transcription reporter assays, led to the development of a model for modulation of ToxT activity under different culture conditions (Fig. 14). This model proposes that when ToxT is produced under virulence-inducing conditions, it fluctuates between active and inactive conformations in the absence of effector molecules. This fluctuation leads to midlevel virulence promoter activity. In the presence of negative effector molecules, ToxT becomes locked into an inactive conformation, resulting in low virulence gene promoter activity. In this conformation, it is proposed that the NTD and CTD are pulled together by the effector molecule (19), and we add that this conformation impedes access to the unstructured region by the ToxT protease. Alternatively, in the presence of the positive effector molecule bicarbonate, ToxT is locked into an active conformation, causing the highest virulence gene promoter activity. We propose that the active conformation of ToxT has an exposed unstructured region that leads to a high degree of proteolysis.
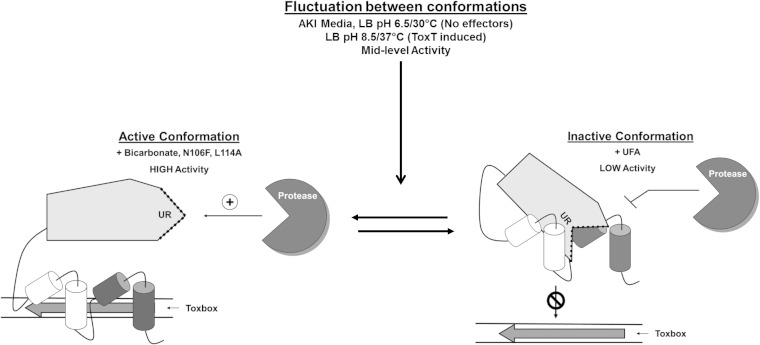
FIG 14.
Model for effector control of ToxT activity. (Left) High-level transcriptional activation by ToxT with the addition of positive effector bicarbonate. ToxT undergoes conformational change with the addition of bicarbonate that allows increased DNA binding affinity to toxbox and leaves the unstructured region (UR) accessible by protease. The active conformation resembles those of ToxT N106F and L114A mutants. (Right) Low-level transcriptional activation by ToxT with the addition of unsaturated fatty acids (UFA). Upon binding UFA, ToxT takes on an inactive conformation that results in low toxbox binding affinity and decreased proteolysis due to unexposed unstructured region. (Center) Midlevel transcriptional activation by ToxT in the absence of effector molecules. ToxT fluctuates between inactive and active conformations, leading to potential ToxT proteolysis. The culture conditions used were AKI without added bicarbonate, virulence-inducing growth conditions (LB, pH 6.5, 30°C, shaking) without negative effectors, or virulence-repressing growth conditions (LB, pH 8.5, 37°C, shaking) for ToxT induction in trans.
It has been proposed that the unstructured region of ToxT could be functionally related to the N-terminal arm of related family members AraC and RegA (19). The N-terminal arms of these proteins are involved in effector control of transcription activity (39, 40). In particular, when the N-terminal arm of RegA is mutated, it leads to a constitutively active form of RegA, comparable to RegA in the presence of bicarbonate (39). It was proposed that the N-terminal arm normally blocks DNA binding by RegA and mutation of the arm or the addition of bicarbonate relieves the inhibition and allows DNA binding (39). ToxT does not contain such an N-terminal arm. However, effector-insensitive ToxT mutants, the N106F and L114A mutants, exhibit a similar phenomenon, wherein virulence gene promoter activity is increased in the absence of effector compared to the virulence gene promoter activity of the WT ToxT (Fig. 6 and 7). These mutants, regardless of effector presence, have activities that resemble the high-level activity of ToxT in the presence of bicarbonate. Therefore, we hypothesize that the unstructured region of ToxT may play a role similar to that of the N-terminal arm of RegA, acting to impair DNA binding in the absence of bicarbonate.
The negative effectors of ToxT activity, bile, UFAs, and virstatin, have been implicated in reducing dimerization of ToxT monomers, leading to reduced overall activity (27, 31). The inactive conformation that we propose could be due to decreased interaction between ToxT monomers through the unstructured region, which has been previously proposed (19). Bicarbonate has not been implicated in dimerization, although increasing interaction between ToxT monomers has been suggested as a possible mechanism for bicarbonate-dependent enhancement of ToxT activity (29). Bicarbonate has been shown to increase ToxT's DNA binding affinity, even at the aldA promoter, which has a single toxbox, suggesting that the mechanism is a structural change not related to dimerization (37).
The unstructured region of ToxT is a likely candidate for effector molecule binding due to its importance in the responses to negative and positive effectors and in solvent accessibility (19) for on/off binding and its disorder in the crystal structure. Unstructured regions in crystal structures are often disordered due to the lack of a stabilizing molecule (41), which could also be the case with ToxT. It is unlikely that UFAs bind the unstructured region of ToxT, as mutations in the region do not reverse the proteolytic blockade by linoleic acid. Conversely, we hypothesize that the positive effector bicarbonate could bind in this region. First, it is possible that bicarbonate binds to ToxT in the unstructured region, as a bulky phenylalanine substitution at N106 results in an active conformation of ToxT, similar to that caused by bicarbonate. Furthermore, the X-ray structure of E. coli aminopeptidase A (PepA) revealed a bicarbonate anion bound to an arginine side chain (42). Our work here shows the importance of an arginine (R105) in the ToxT response to bicarbonate, raising the possibility that bicarbonate is binding to this residue.
We previously proposed a model wherein ToxT becomes activated as the bicarbonate concentration increases when V. cholerae enters the mucus layer of the small intestine (29). This model proposed that ToxT is in an inactive form in the lumen of the intestine due to a lower local concentration of bicarbonate. The model was modified in reference 43 to propose that bile/UFAs in the lumen of the intestine inactivate ToxT and are replaced by bicarbonate as the bacterium moves closer to the mucus/epithelial layer. Using this model of ToxT-dependent virulence in the host, it is plausible that the inactive state of ToxT with UFA bound can be converted to the active state in the presence of enough bicarbonate, even though the effectors may not be bound in the same region.
The discovery that UFAs block ToxT proteolysis may support a mechanism for inhibiting the premature degradation of ToxT. After ToxT is produced and V. cholerae is in the lumen of the intestine, ToxT must remain intact but inactive so that CT and TCP are not produced prematurely in infection. UFAs in bile provide a mechanism for ToxT inactivity in the lumen and also protect ToxT from proteolysis. As the bacteria encounter higher concentrations of bicarbonate and lower concentrations of UFA closer to the epithelial surface, bicarbonate binds and converts ToxT to its active conformation. If bicarbonate also blocked proteolysis, V. cholerae could not undergo the mucosal escape response and be released from the intestine, as there would be no mechanism for breaking the ToxT positive autoregulatory loop that causes continued production of TCP and CT.
In summary, we determined that the responses of ToxT to both negative and positive effectors present in the intestine are mediated by an unstructured region in the NTD. Amino acids in this region were of high importance for the proteolysis of ToxT and led to the finding that the negative effector linoleic acid blocks the degradation of ToxT. This unstructured region plays a role in the response of ToxT to environmental signals and has implications for the temporal and spatial regulation of virulence factor production in the host.
Supplementary Material
ACKNOWLEDGMENTS
We thank the members of the Withey and Neely laboratories for helpful discussions.
This work was supported by P.H.S. grants K22AI071011 and R56AI093622 (to J.H.W.) and by Wayne State University funds.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02068-14.
REFERENCES
- 1.Holmgren J, Svennerholm AM. 1977. Mechanisms of disease and immunity in cholera: a review. J Infect Dis 136:S105–S112. doi: 10.1093/infdis/136.Supplement.S105. [DOI] [PubMed] [Google Scholar]
- 2.Miller VL, Mekalanos JJ. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol 170:2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. 1986. Identification of a pilus colonization factor that is coordinately regulated with cholera toxin. Ann Sclavo Collana Monogr 3:51–61. [PubMed] [Google Scholar]
- 4.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A 84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill DM. 1976. The arrangement of subunits in cholera toxin. Biochemistry 15:1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- 6.Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233. doi: 10.1016/S0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 7.Kirn TJ, Lafferty MJ, Sandoe CM, Taylor RK. 2000. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol Microbiol 35:896–910. doi: 10.1046/j.1365-2958.2000.01764.x. [DOI] [PubMed] [Google Scholar]
- 8.Thelin KH, Taylor RK. 1996. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun 64:2853–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulbert RR, Taylor RK. 2002. Mechanism of ToxT-dependent transcriptional activation at the Vibrio cholerae tcpA promoter. J Bacteriol 184:5533–5544. doi: 10.1128/JB.184.20.5533-5544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiRita VJ, Parsot C, Jander G, Mekalanos JJ. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci U S A 88:5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Withey JH, Dirita VJ. 2005. Vibrio cholerae ToxT independently activates the divergently transcribed aldA and tagA genes. J Bacteriol 187:7890–7900. doi: 10.1128/JB.187.23.7890-7900.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Withey JH, DiRita VJ. 2005. Activation of both acfA and acfD transcription by Vibrio cholerae ToxT requires binding to two centrally located DNA sites in an inverted repeat conformation. Mol Microbiol 56:1062–1077. doi: 10.1111/j.1365-2958.2005.04589.x. [DOI] [PubMed] [Google Scholar]
- 13.Withey JH, DiRita VJ. 2006. The toxbox: specific DNA sequence requirements for activation of Vibrio cholerae virulence genes by ToxT. Mol Microbiol 59:1779–1789. doi: 10.1111/j.1365-2958.2006.05053.x. [DOI] [PubMed] [Google Scholar]
- 14.Yu RR, DiRita VJ. 1999. Analysis of an autoregulatory loop controlling ToxT, cholera toxin, and toxin-coregulated pilus production in Vibrio cholerae. J Bacteriol 181:2584–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu RR, DiRita VJ. 2002. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol Microbiol 43:119–134. doi: 10.1046/j.1365-2958.2002.02721.x. [DOI] [PubMed] [Google Scholar]
- 16.Richard AL, Withey JH, Beyhan S, Yildiz F, DiRita VJ. 2010. The Vibrio cholerae virulence regulatory cascade controls glucose uptake through activation of TarA, a small regulatory RNA. Mol Microbiol 78:1171–1181. doi: 10.1111/j.1365-2958.2010.07397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley ES, Bodi K, Ismail AM, Camilli A. 2011. A genome-wide approach to discovery of small RNAs involved in regulation of virulence in Vibrio cholerae. PLoS Pathog 7:e1002126. doi: 10.1371/journal.ppat.1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins DE, Nazareno E, DiRita VJ. 1992. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J Bacteriol 174:6974–6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowden MJ, Skorupski K, Pellegrini M, Chiorazzo MG, Taylor RK, Kull FJ. 2010. Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes. Proc Natl Acad Sci U S A 107:2860–2865. doi: 10.1073/pnas.0915021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin RG, Rosner JL. 2001. The AraC transcriptional activators. Curr Opin Microbiol 4:132–137. doi: 10.1016/S1369-5274(00)00178-8. [DOI] [PubMed] [Google Scholar]
- 21.Hase CC, Mekalanos JJ. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A 95:730–734. doi: 10.1073/pnas.95.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins DE, DiRita VJ. 1994. Transcriptional control of toxT, a regulatory gene in the ToxR regulon of Vibrio cholerae. Mol Microbiol 14:17–29. doi: 10.1111/j.1365-2958.1994.tb01263.x. [DOI] [PubMed] [Google Scholar]
- 23.Krukonis ES, Yu RR, Dirita VJ. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol Microbiol 38:67–84. doi: 10.1046/j.1365-2958.2000.02111.x. [DOI] [PubMed] [Google Scholar]
- 24.Abuaita BH, Withey JH. 2011. Termination of Vibrio cholerae virulence gene expression is mediated by proteolysis of the major virulence activator, ToxT. Mol Microbiol 81:1640–1653. doi: 10.1111/j.1365-2958.2011.07798.x. [DOI] [PubMed] [Google Scholar]
- 25.Schuhmacher DA, Klose KE. 1999. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J Bacteriol 181:1508–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatterjee A, Dutta PK, Chowdhury R. 2007. Effect of fatty acids and cholesterol present in bile on expression of virulence factors and motility of Vibrio cholerae. Infect Immun 75:1946–1953. doi: 10.1128/IAI.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shakhnovich EA, Hung DT, Pierson E, Lee K, Mekalanos JJ. 2007. Virstatin inhibits dimerization of the transcriptional activator ToxT. Proc Natl Acad Sci U S A 104:2372–2377. doi: 10.1073/pnas.0611643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. 2005. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science 310:670–674. doi: 10.1126/science.1116739. [DOI] [PubMed] [Google Scholar]
- 29.Abuaita BH, Withey JH. 2009. Bicarbonate induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect Immun 77:4111–4120. doi: 10.1128/IAI.00409-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Childers BM, Weber GG, Prouty MG, Castaneda MM, Peng F, Klose KE. 2007. Identification of residues critical for the function of the Vibrio cholerae virulence regulator ToxT by scanning alanine mutagenesis. J Mol Biol 367:1413–1430. doi: 10.1016/j.jmb.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 31.Childers BM, Cao X, Weber GG, Demeler B, Hart PJ, Klose KE. 2011. N-terminal residues of the Vibrio cholerae virulence regulatory protein ToxT involved in dimerization and modulation by fatty acids. J Biol Chem 286:28644–28655. doi: 10.1074/jbc.M111.258780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 33.Bellair M, Withey JH. 2008. Flexibility of Vibrio cholerae ToxT in transcription activation of genes having altered promoter spacing. J Bacteriol 190:7925–7931. doi: 10.1128/JB.00512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 35.Dittmer JB, Withey JH. 2012. Identification and characterization of the functional toxboxes in the Vibrio cholerae cholera toxin promoter. J Bacteriol 194:5255–5263. doi: 10.1128/JB.00952-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prouty MG, Osorio CR, Klose KE. 2005. Characterization of functional domains of the Vibrio cholerae virulence regulator ToxT. Mol Microbiol 58:1143–1156. doi: 10.1111/j.1365-2958.2005.04897.x. [DOI] [PubMed] [Google Scholar]
- 37.Thomson JJ, Withey JH. 2014. Bicarbonate increases binding affinity of Vibrio cholerae ToxT to virulence gene promoters. J Bacteriol 196:3872–3880. doi: 10.1128/JB.01824-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sauer RT, Baker TA. 2011. AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem 80:587–612. doi: 10.1146/annurev-biochem-060408-172623. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Dogovski C, Hocking D, Tauschek M, Perugini M, Robins-Browne RM. 2009. Bicarbonate-mediated stimulation of RegA, the global virulence regulator from Citrobacter rodentium. J Mol Biol 394:591–599. doi: 10.1016/j.jmb.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 40.Soisson SM, MacDougall-Shackleton B, Schleif R, Wolberger C. 1997. Structural basis for ligand-regulated oligomerization of AraC. Science 276:421–425. doi: 10.1126/science.276.5311.421. [DOI] [PubMed] [Google Scholar]
- 41.Dyson HJ, Wright PE. 2005. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol 6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 42.Strater N, Sun L, Kantrowitz ER, Lipscomb WN. 1999. A bicarbonate ion as a general base in the mechanism of peptide hydrolysis by dizinc leucine aminopeptidase. Proc Natl Acad Sci U S A 96:11151–11155. doi: 10.1073/pnas.96.20.11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J, Tauschek M, Robins-Browne RM. 2011. Control of bacterial virulence by AraC-like regulators that respond to chemical signals. Trends Microbiol 19:128–135. doi: 10.1016/j.tim.2010.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.