Abstract
Endothelial cells (ECs) are central regulators of hemostasis, inflammation, and other vascular processes. ECs have been used to cover vascular graft materials in an attempt to improve the biological integration of the grafts with the surrounding tissue. Although EC seeded grafts demonstrated improved patency, the invasive nature of EC harvest has limited the clinical translation of this technique. Endothelial outgrowth cells (EOCs) can be derived from circulating endothelial progenitor cells, which are noninvasively isolated from a peripheral blood draw. Although EOCs have been presumed to regulate hemostasis and inflammation similarly to arterial ECs, there has been limited research that directly compares EOCs to arterial ECs, particularly using pairs of donor-matched cells. This study provides a multifaceted characterization of hemostasis regulation by baboon EOCs and carotid ECs, both in the presence and absence of an inflammatory stimulus, tumor necrosis factor α (TNFα). The expression of genes involved in thrombosis and inflammation was highly similar between ECs and EOCs at a basal state and following TNFα stimulation. ECs and EOCs activated similar levels of protein C and Factor X (FX) at a basal state. Following TNFα treatment, EOCs had less of an increase in tissue factor activity than ECs. Cell-seeded expanded polytetrafluoroethylene vascular grafts demonstrated no significant differences between ECs and EOCs in platelet accumulation or fibrinogen incorporation in a baboon femoral arteriovenous shunt loop. This work demonstrates that EOCs regulate thrombus formation and respond to an inflammatory stimulus similar to ECs, and supports utilizing EOCs as a source for an autologous endothelium in tissue engineering applications.
Introduction
Blood coagulation is a highly regulated process designed to enable rapid blood clot formation at the sites of vascular injury (hemostasis), while limiting excessive blood clot formation, which can result in vessel occlusion (thrombosis) or the release of a clot into circulation (embolism), where the clot may occlude distant vessels. Endothelial cells (ECs) line the vasculature and inhibit thrombus formation through the expression of both surface-bound molecules such as thrombomodulin and cluster of differentiation 39 (CD39), as well as secreted factors such as nitric oxide (NO) and tissue factor pathway inhibitor (TFPI). Conversely, at sites of vascular injury, ECs initiate thrombus formation to prevent vascular leakage and hemorrhage. The process of hemostasis is promoted by endothelial expression of tissue factor, platelet endothelial cell adhesion molecule (PECAM), and downregulation of normal antithrombotic activity. In healthy vasculature, ECs are responsible for clot dissolution (fibrinolysis) to prevent excessive thrombus formation and to permit reendothelialization of the injured vessel wall. In addition to regulating thrombus formation, ECs regulate inflammation by controlling leukocyte adhesion and transmigration, as well as vascular smooth muscle cell proliferation and vascular tone.1–5 Leukocyte adhesion and transmigration are facilitated by endothelial expression of adhesion molecules, predominantly intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin, whereas smooth muscle cell contractility is regulated by NO generated by endothelial nitric oxide synthase (eNOS). Thus, ECs serve as an active interface between the blood and surrounding tissue to regulate many vascular physiological processes.
Unlike the native vascular endothelium, synthetic vascular grafts developed thus far have sought to remain inert to avoid activation of coagulation. One example of such a clinically utilized inert material is expanded polytetrafluoroethylene (ePTFE). However, at small diameters (<6 mm) these grafts frequently become occluded due to thrombus formation and intimal hyperplasia, both of which a healthy endothelium inhibits.6 Tissue engineering strategies to develop biological vascular replacements or cover synthetic graft materials have used ECs to improve the bioactivity of the engineered vessels.7–11 Despite the promising results of endothelialized vascular grafts in clinical trials, the limited availability of autologous ECs and the donor site morbidity resulting from EC harvest have limited the clinical potential of these grafts.8,12 ECs have also been incorporated into tissue engineering strategies seeking to engineer thick or metabolically active tissues.13–15 In these applications, the ECs are incorporated to form a capillary-like network to transport nutrients through the construct.
In contrast to ECs, endothelial progenitor cells can be isolated noninvasively from peripheral blood.16 Although a variety of cell types can be derived from endothelial progenitor cells, a population termed endothelial outgrowth cells (EOCs; also known as late outgrowth, blood outgrowth endothelial, or endothelial colony-forming cells) most closely resemble ECs in their expression of EC surface antigens, activation of pro- and anticoagulant factors, and influence on smooth muscle cell proliferation and vascular tone.17–21 Thus, EOCs are an attractive cell source for engineering an autologous endothelium that can properly recapitulate EC interactions with the blood and surrounding vascular tissue.
Although prior work has demonstrated that EOCs modulate their expression of pro- and anticoagulant factors in response to stimuli, including fluid shear17,22,23 and calcium,24 a direct comparison of the ability of ECs and EOCs to regulate thrombus formation in a model using flowing blood has not been performed. Thrombus formation is a dynamic process with the continuous deposition and removal of circulating cells and coagulation factors as well as the continuous activation of zymogens to both pro- and anticoagulant proteases. Characterizing thrombus formation with flowing blood is necessary to incorporate the transport dynamics of circulating coagulation factors and shear-dependent cell adhesion.25,26 To characterize the ability of EOCs to regulate vascular physiological processes, we have compared donor-matched EOCs and carotid ECs in their expression of thrombosis-regulating surface antigens and gene expression, activation of coagulation factors, and regulation of thrombus formation in an ex vivo shunt model of graft thrombosis. In addition, we performed these analyses before and after stimulation with the inflammatory cytokine tumor necrosis factor α (TNFα) to identify differences in the cells' inflammatory response. Although ECs and EOCs exhibited distinct profiles of gene expression and coagulation factor activation, ECs and EOCs were similar in their ability to regulate thrombus formation on a synthetic vascular graft. This work supports the use of EOCs as an autologous endothelium for various tissue engineering applications.
Materials and Methods
EC isolation
ECs were isolated from explanted baboon carotid arteries as previously described.27 Briefly, explanted arteries were clamped shut at one end of the vessel and collagenase type II (Worthington Biochemical; 600 U/mL) was dripped into the lumen. Blood vessels were treated with collagenase for 5 min and manually compressed to detach ECs from the vessel wall. The cells were then deposited into well plates coated with 50 μg/mL collagen I. ECs were purified by positive selection at the first passage using the CD31 Dynabeads® (Life Technologies) according to the manufacturer's protocol.
EOC isolation
EOCs were isolated from the peripheral blood of baboons as previously described.19 Briefly, peripheral blood was diluted 1:1 in the Hank's Balanced Salt Solution (HBSS) and gently layered over Histopaque-1077 (Sigma-Aldrich). The mononuclear cell layer was collected and diluted 1:1 with HBSS. After washing the cells three times with HBSS, the cells were plated into a 12-well plate coated with 50 μg/mL fibronectin at a density of 10–20 M cells/well. Individual colonies were isolated and expanded in culture flasks coated with 50 μg/mL collagen I. All isolated colonies were sorted using the CD31 Dynabeads® according to the manufacturer's protocol to positively select for EOCs.
Cell culture and TNFα treatment
Following isolation, cells were cultured in EGM-2 (Lonza) supplemented with 10% fetal bovine serum (FBS; HyClone). Media was exchanged every 2–3 days. Analyses were performed on cells at passages 4–5. For all experiments, ECs and EOCs from multiple donors were used, with both ECs and EOCs being derived from the same donor. To establish a dose-response effect of TNFα treatment, 1–100 U/mL of TNFα (R&D Systems) in EGM-2 was applied for 4 h before analysis and ICAM-1 expression was measured by flow cytometry (below). For all other experiments, ECs and EOCs were treated with 100 U/mL TNFα for 4 h.
Flow cytometry
ECs and EOCs were dissociated from culture flasks with TrypLE (Life Technologies) and resuspended in a phosphate buffered saline (PBS) with 1% FBS at a concentration of 10 M cells/mL. Nonspecific antibody binding was blocked using 5% mouse serum for 20 min on ice. A master mixture of fluorescent antibodies was prepared with each antibody being used at the concentration recommended by the manufacturer. The antibodies used were Brilliant Violet 421–anti-CD34 (Biolegend), FITC–anti-ICAM-1, PE–anti-CD141 (thrombomodulin; BD Pharmingen), PE/Cy7–anti-CD146 (BD Pharmingen), AlexaFluor647–anti-CD309 (VEGFR2; BD Pharmingen), APC/Cy7–anti-CD31 (PECAM; Biolegend), and PerCP–anti-CD45 (BD Pharmingen). The multicolor antibody mixture was added to each sample tube and incubated for 30 min on ice in the dark. The cells were then washed twice in PBS with 1% FBS and transferred into wells of a 96-well plate. Fluorescent measurements were acquired using a MACSQuant analyzer (Miltenyi Biotec). FlowJo vX was used to perform automated compensation and plot histograms of fluorescence intensity. Fluorescent-labeled cells were compared with unstained controls or fluorescent minus one controls for channels in which accessory fluorophores caused the background to be evidently higher than unstained samples after compensation.
Reverse transcription and quantitative polymerase chain reaction
Cells from three different donors were grown in culture flasks, dissociated with TrypLE and pelleted. Cell pellets were resuspended in Buffer RLT (Qiagen) with 1% β-mercaptoethanol. RNA isolation was performed using the RNeasy Mini kit (Qiagen) according to the manufacturer's protocol. Samples were treated with DNAse I (Fermentas) and RNA was reverse transcribed using the SuperScript III Reverse Transcriptase (Life Technologies) with random primers according to the manufacturer's instructions. Gene expression was quantified with the Platinum® SYBR® Green quantitative polymerase chain reaction (qPCR) SuperMix-UDG using custom primers (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/tea) with ROX reference dye and the Applied Biosystems 7500 Fast Real-Time PCR system. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as a housekeeping gene for calculating dCT values with dCT equal to the threshold cycle of GAPDH subtracted from the threshold cycle of the gene of interest. Each sample was performed in duplicate, and the mean Ct was used to calculate dCt values. Data are presented as the mean 2(−dCt) plus standard deviation.
Coagulation factor activation
Cells which were either cultured in well plates or seeded on ePTFE graft sections were used to characterize coagulation factor activation. When cultured in well plates, cells were seeded at a density of 100,000 cells/cm2 and cultured overnight, producing a confluent monolayer of cells. To measure tissue factor pathway-dependent FX activation, a solution of FVIIa (20 nM; Enzyme Research Laboratories) and FX (200 nM, Enzyme Research Laboratories) in HBSS with Ca2+ and Mg2+ supplemented with 0.1% bovine serum albumin was added to each sample and incubated for 1 h at 37°C. Ethylenediaminetetraacetic acid (15 mM) was used to quench the reaction, Spectrozyme FXa (American Diagnostica) was added for a final concentration of 1 mM, and the absorbance at 405 nm was measured every 20 s for 20 min. To measure protein C activation, thrombin (5 nM; Haemotologic Technologies) and protein C (100 nM, Haemotologic Technologies) in PBS with Ca2+ and Mg2+ were added to sample wells and incubated for 1 h at 37°C. Hirudin (500 nM) was used to inhibit thrombin protease activity, the chromogenic substrate S-2366 (Chromogenix; 1 mM) was added to each sample, and the absorbance at 405 nm was measured every 20 s for 20 min. For both FX and protein C activation assays, the maximum slope of absorbance increase over 10 points was used to calculate the concentration by comparing to a standard curve of FXa or activated protein C (APC). Each biological sample group had three replicates, and each of these sample replicates was split into duplicates for FXa and APC quantification.
Animal care
Male baboons (Papio anubis) used in this study were cared for and housed at the Oregon National Primate Research Center at Oregon Health & Science University. Experiments (IS00002496) were approved by the Oregon Health & Science University West Campus Institutional Animal Care and Use Committee according to the guidelines of the NIH “Guide for the Care and Use of Laboratory Animals” prepared by the Committee on Care & Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (International Standard Book, Number 0-309-05377-3, 1996).
Ex vivo arteriovenous shunt
Four millimeter internal diameter ePTFE grafts were seeded with cells. ePTFE grafts 5 cm in length were adjoined to silicone tubing and wetted with 95% ethanol, washed with water, and perfused with 0.02 N acetic acid. Collagen I (MP Biomedicals) in 0.02 N acetic acid (4 mg/mL) was then perfused through the graft material from the distal side to coat the graft lumen and permit cell adhesion, and the distal silicone tubing was washed with 0.02 N acetic acid to remove the residual collagen. The grafts were seeded with cells by perfusing the grafts with a cell suspension of 3×105 cells/mL. Three cell perfusions were performed 40 min apart, and the grafts were rotated 120° between perfusions to produce a uniform cell monolayer. The seeded grafts were cultured overnight in a custom biochamber to enable cell adhesion and spreading on the graft material. Following overnight incubation, the cell-seeded grafts were connected to a baboon arteriovenous femoral shunt (Supplementary Fig. S1) in the absence of antiplatelet or anticoagulant therapies.25 Autologous platelets and allogeneic fibrinogen were radiolabeled with 111In and 125I, respectively and infused into the baboon before the shunt study. Blood flow through the graft was held constant at 100 mL/min using a clamp downstream of the graft. The shunt loop was located above a gamma camera to enable the real-time measurement of platelet accumulation using 5 min exposure times over the course of the 60 min shunt study. Following the shunt study, the grafts were fixed in 3.7% paraformaldehyde and placed at 4°C until the 111In had decayed >10 half-lives, at which point the grafts were sectioned and fibrinogen incorporation was measured using a WIZARD automatic gamma camera (PerkinElmer). Platelet accumulation and fibrinogen incorporation were measured on the central 2 cm of the graft to eliminate the potentially confounding effect of increased platelet accumulation at the ePTFE-silicone junctions.
Statistical analyses
Statistical analyses were performed using the SPSS 12.0 or JMP 11.1.1. To determine significant differences between experimental groups for all studies, except ICAM-1 flow cytometry, a two-way ANOVA was performed with the treatment factor containing four levels (ECs, EOCs, TNFα-treated ECs, and TNFα-treated EOCs) and donor as a blocking factor. For analyzing ICAM-1 flow cytometry, a two-way ANOVA was performed with the treatment factor consisting of the TNFα concentrations (no TNFα, 1, 10, 100 U/mL) for each of the two cell types for a total of eight levels and with donor as a blocking factor. A Tukey post hoc analysis was used to determine homogenous subsets of experimental groups, and differences between groups were considered significant if p<0.05. All data are presented as the mean±standard deviation.
Results
Morphology and surface marker expression
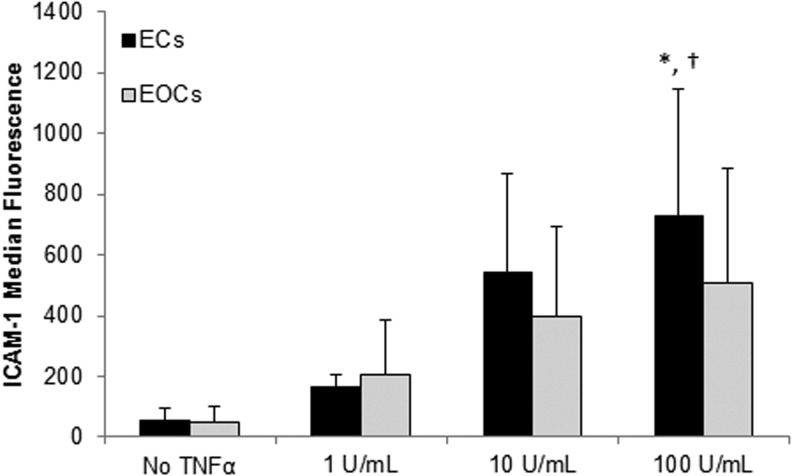
As previously reported, ECs and EOCs share a similar cobblestone morphology and form a confluent monolayer in culture (Fig. 1). Flow cytometry characterization of ECs and EOCs with and without TNFα stimulation demonstrated a similar expression of endothelial surface markers, and the absence of leukocyte marker CD45 (Fig. 2, data not shown in the absence of TNFα). Of note, ICAM-1 expression by both ECs and EOCs was dose-dependent (Fig. 3). Our results are consistent with the previous work demonstrating ECs treated with 100 U/mL TNFα for 4 h exhibit a significant upregulation of ICAM-1. The magnitude of the response to TNFα varied considerably between donors; as a result, EOCs treated with 100 U/mL TNFα did not show statistically significant increase in ICAM-1 expression compared with untreated EOCs (p=0.190 by two-way ANOVA). However, each individual animal demonstrated the expected dose-dependent increase in ICAM-1 expression (Supplementary Fig. S2). Similar upregulation of ICAM-1 between ECs and EOCs indicates a similar response to inflammatory stimuli.
FIG. 1.
Morphology of endothelial and endothelial outgrowth cells. Representative brightfield images of baboon carotid endothelial cells (A) and endothelial outgrowth cells (B), both of which exhibit similar cobblestone morphology. Scale bar=200 μm.
FIG. 2.
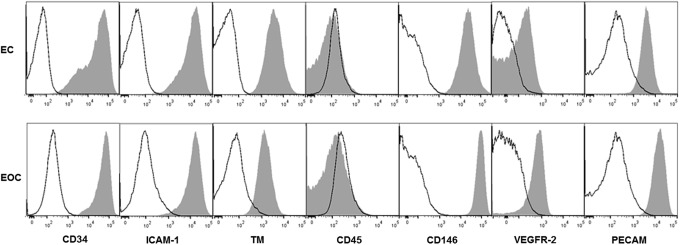
Representative flow cytometry histograms of ECs and EOCs treated with TNFα. Shaded histograms indicate samples stained with the multicolor antibody mixture, whereas the open histograms are the corresponding unstained or fluorescent-minus-one controls. EOCs exhibit the typical EC surface markers and are negative for the leukocyte marker CD45. ECs, endothelial cells; EOCs, endothelial outgrowth cells; TM, thrombomodulin; TNFα, tumor necrosis factor α.
FIG. 3.
Median fluorescence of ICAM-1 by ECs and EOCs measured through flow cytometry. Both ECs and EOCs showed a dose-dependent upregulation of ICAM-1 expression induced by TNFα treatment. Data are charted as the mean±standard deviation. *p<0.05 versus untreated ECs; †p<0.05 versus untreated EOCs based on the post hoc analysis, n=3. ICAM-1, intercellular adhesion molecule 1.
Thrombus formation and inflammation: gene expression
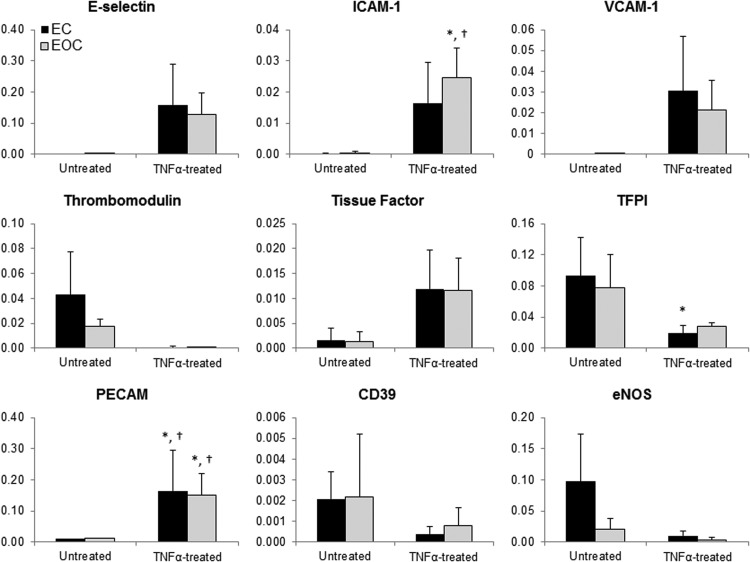
Quantitative PCR was performed to identify differences in gene expression between cell types and in response to TNFα (Fig. 4). Expression of the gene targets varied considerably between donors, although trends indicate similar TNFα-induced responses between ECs and EOCs. At a basal state, ECs and EOCs had very similar expression of all genes, with the exception of trending lower eNOS expression by EOCs. TNFα-treatment resulted in proinflammatory and prothrombotic trends in both ECs and EOCs. Following TNFα treatment, both ECs and EOCs trended toward increases in genes encoding for ICAM-1, VCAM-1, PECAM, and E-selectin, all of which are adhesion molecules that facilitate inflammation. In addition, in response to TNFα treatment, ECs downregulated the antithrombotic effector TFPI, and both ECs and EOCs trended toward decreased thrombomodulin expression as well as increased expression of the prothrombotic effector tissue factor. The genes encoding eNOS and CD39 also trended toward downregulation with TNFα treatment, indicating a potential reduced inhibition of platelet activation. In summary, despite the variability between individual donors, ECs and EOCs similarly modulated inflammation- and thrombosis-related gene expression in response to TNFα.
FIG. 4.
Gene expression of ECs and EOCs in the presence and absence of TNFα treatment. Gene expression of ECs and EOCs was measured with quantitative polymerase chain reaction. Data are expressed as the mean 2−(dCt) value using GAPDH as the housekeeping gene, error bars represent standard deviation.*p<0.05 versus untreated ECs; †p<0.05 versus untreated EOCs based on the post hoc analysis, n=3. TFPI, tissue factor pathway inhibitor.
Coagulation factor activation
Protein C activation and FX activation by ECs and EOCs were quantified to determine how EOCs regulate specific anti- and pro-thrombotic pathways in response to TNFα. In the absence of TNFα, ECs and EOCs exhibited very low levels of FX activation (Fig. 5A). Following TNFα stimulation, both ECs and EOCs significantly increased FX activation, indicating an increase in tissue factor activity. Furthermore, TNFα-treated ECs had a significantly greater increase in FX activation than EOCs (two-way ANOVA, p<0.01), indicating that EOCs may have a dampened prothrombotic response following inflammatory cytokine stimulation. There was no significant difference in the average protein C activation between ECs and EOCs cultured in well plates (Fig. 5B), although there was considerable inter-donor variability (Supplementary Fig. S3). TNFα treatment did not significantly change the average protein C activation by either cell type. ECs and EOCs demonstrated similar activation of FX and protein C when seeded on vascular grafts (Fig. 6) as when seeded in 96-well plates, with the exception that on the vascular grafts ECs had heightened FX activation in the absence of TNFα.
FIG. 5.
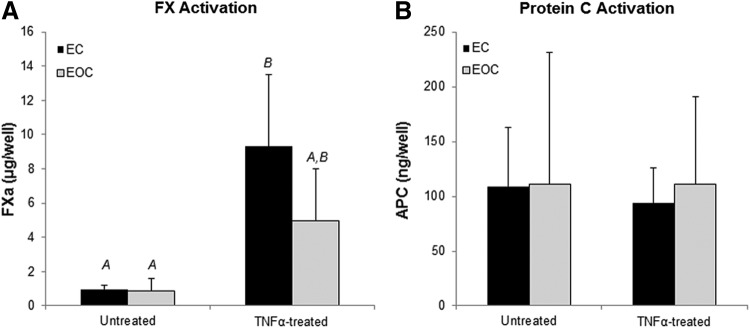
Coagulation factor activation by untreated and TNFα-treated ECs and EOCs. ECs and EOCs were assessed for FX activation (A) and protein C activation (B) at a basal state and following TNFα treatment. Letters denote groups belonging to homogenous subsets, whereas groups not sharing a letter are considered significantly different based on the post hoc analysis, n=8. APC, activated protein C.
FIG. 6.
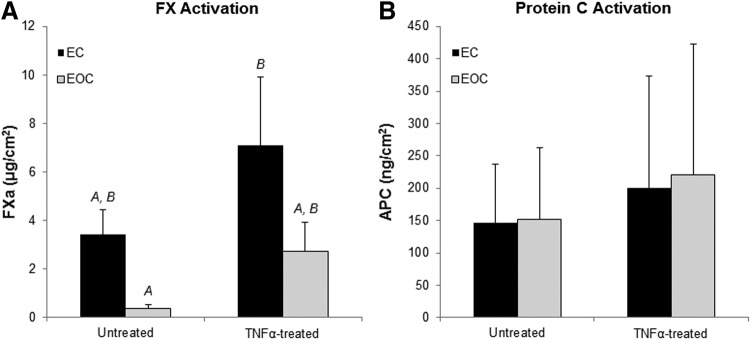
Coagulation factor activation on cell-seeded vascular grafts in the presence and absence of TNFα treatment. Sections of EC- and EOC-seeded ePTFE vascular grafts were assessed for FX activation (A) and protein C activation (B) at a basal state and following TNFα treatment. Letters denote groups belonging to homogenous subsets based on the post hoc analysis, whereas groups not sharing a letter are considered significantly different based on the post hoc analysis, n=3. ePTFE, expanded polytetrafluoroethylene.
Platelet accumulation on cell-seeded vascular grafts
Following TNFα treatment, EC-seeded vascular grafts had similar platelet accumulation over the course of 1 h as EOC-seeded grafts in the arteriovenous shunt model (Fig. 7a). Interestingly, TNFα treatment did not significantly increase platelet accumulation on cell-seeded vascular grafts compared to untreated donor matched cell-seeded grafts (Fig. 7a). This indicates that platelet accumulation in this model is not significantly altered by changes in cell phenotype induced by TNFα treatment. Similarly, there was no significant difference in fibrinogen incorporation between ECs and EOCs treated with TNFα or untreated (Fig. 7b).
FIG. 7.
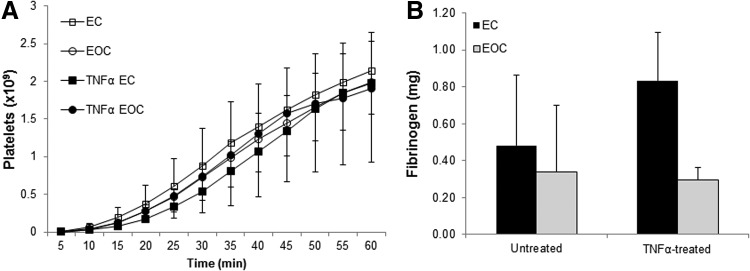
Thrombus formation on cell-seeded vascular grafts in a baboon arteriovenous shunt. Platelet accumulation (A) and fibrinogen incorporation (B) were measured on cell-seeded vascular grafts with or without TNFα treatment connected to a baboon arteriovenous shunt loop. Both platelet accumulation and fibrinogen incorporation were measured on the central 2 cm of the graft to eliminate the potentially confounding effect of increased platelet accumulation at the ePTFE-silicone junction. Data are presented as the mean±standard deviation, n=3.
Discussion
The vascular endothelium serves as an active interface between the blood and surrounding vascular tissue. Although harvesting ECs to serve as an autologous endothelium in vascular tissue engineering applications has reduced graft complications, the process of harvesting vascular ECs is invasive. EOCs may provide an alternative source for engineering an autologous endothelium; however, there has been limited work in characterizing the capacity of EOCs to regulate thrombus formation and respond to inflammatory stimuli. This work contrasted EOCs with donor-matched, carotid ECs to characterize the capacity of EOCs to functionally regulate vascular physiological processes. Considering that the inability of acellular, synthetic vascular grafts to regulate processes such as intimal proliferation and thrombus formation has precluded the success of small diameter synthetic grafts, characterizing the performance of EOCs in these processes has great implications for novel strategies to reduce graft thrombosis and enable vascular tissue engineering applications.
This study compared ECs and EOCs from the same animal donor, and utilized multiple donors in each analysis. As expected, there was considerable inter-donor variability in cellular function. Inter-donor differences are particularly apparent in the gene expression and flow cytometry analysis (see Supplementary Fig. S2 for flow cytometry data of individual donors). This variability may have hindered the identification of statistically significant differences between cell types considering the low number of donors (n=3 for qPCR and flow cytometry analyses). Increasing the number of animals would likely cause the results to become statistically significant; however, a power analysis of the data indicated that due to the large variability between baboons, 24 donors would be required for sufficient statistical power to distinguish between treatment groups, and obtaining that quantity of baboon donors is not technically feasible. Tissue engineering applications using EOCs as an autologous endothelium would likely also exhibit this inter-donor variability. Therefore, rather than using cells from only one donor, we used multiple donors to identify general population trends and characterize how EOCs typically compare with ECs. The variability in EOC phenotype is likely intrinsic to the individual and not the result of a pathological state, as prior work has indicated that EOCs derived from patients with coronary artery disease exhibit few differences from EOCs derived from healthy patients.28 The inter-donor variability of EOCs may be beneficial, as EOCs may be better tailored to an individual's unique hemostatic and inflammatory state as compared to the standardized properties of synthetic vascular grafts. More research on inter-donor EOC variability may identify relationships between EOC function and individual variances in blood composition or other health metrics.
In both ECs and EOCs, the inflammatory cytokine TNFα caused a prothrombotic shift in cell phenotype. Genes encoding antithrombotic agents such as TFPI, CD39, thrombomodulin, and eNOS all trended lower in TNFα stimulated cells than in unstimulated cells, whereas prothrombotic tissue factor expression increased. In general, the pre- and posttreatment expression of these genes was similar between ECs and EOCs, although eNOS was trending toward lower expression in EOCs than in ECs, which agrees with the previous studies.19,20,22,24,29 The functional impact of lower eNOS gene expression by EOCs is uncertain, although it is suggested that nitric oxide production is not significantly lower in EOCs than in ECs.24,30 Prior work on ECs response to TNFα demonstrated that TNFα causes a modest reduction in thrombomodulin cofactor activity.31 Although thrombomodulin activity averaged across individuals was not reduced by TNFα treatment, individual donors exhibited consistent decreases in thrombomodulin activity (Supplementary Fig. S3). The average individual reduction in thrombomodulin activity was 9.4% in ECs and 10.2% in EOCs, which closely resembles prior findings.31 A major limitation of this study is that all in vitro analyses utilized ECs and EOCs that had been cultured in static conditions. Future work could investigate differences between donor-matched EOCs and arterial ECs following fluid shear stress, as preconditioning EOCs and umbilical vein ECs with fluid shear stress has been shown to attenuate the cells' inflammatory response to TNFα.17
Increased tissue factor activity, resulting from both increased tissue factor expression and reduced TFPI secretion, by the vascular endothelium and the resulting increase in local FX activation is generally considered to be a major contributor to platelet accumulation and hemostasis in vivo.32 Perhaps the most surprising result in this work was that increased tissue factor activity resulting from TNFα treatment did not significantly alter platelet accumulation. This finding was demonstrated by the lack of difference in platelet accumulation between TNFα-treated and untreated grafts, as well as a lack of difference between EC- and EOC-seeded grafts treated with TNFα. In the latter case, ECs were shown to have increased tissue factor-mediated FX activation following TNFα treatment, but did not have a corresponding increase in platelet accumulation. Although unexpected, this result demonstrates how the complex interaction of pro- and antithrombotic pathways, as well as fibrinolytic activity, can dominate single procoagulant factors such as endothelial tissue factor expression. In this shunt model, tissue factor from other cell types, such as activated leukocytes or platelets, may be greater than EC- or EOC-expressed tissue factor, thereby overpowering any differences between these cell types. Alternatively, the contact activation pathway initiating with Factor XII activation could drive the initial generation of FXa. The initiation of fluid flow through the grafts may have caused the cells to release NO, a process which occurs in <2 min after the onset of flow,33 and may have overpowered differences in cell phenotype due to cell type and treatment. Regardless of the mechanism, once a small amount of thrombin has been generated, FXa generation in vivo is predominantly accomplished through the tissue factor-independent intrinsic pathway due to thrombin's positive feedback on intrinsic coagulation factors.34
The baboon arteriovenous shunt model used in this work allows for a characterization of thrombus formation on cell-seeded vascular grafts with flowing whole blood in the absence of anticoagulants. This system enables flow conditions that are hemodynamically similar to the in vivo arterial environment and does not limit the activity of key coagulation factors in the highly-regulated process of hemostasis. As blood coagulation and platelet accumulation are regulated by multiple interdependent pathways, the absence of anticoagulants is notable as it maintains the function of all regulatory pathways and feedback mechanisms. This work demonstrates how the complex regulation of coagulation, and the flow-dependent transport dynamics of coagulation factors, which are not accounted for in many in vitro analyses of thrombogenicity, can have a significant impact on cell thrombogenicity. Specifically, although TNFα treatment altered the cellular thrombotic phenotype, including increased FX activation, platelet accumulation on cell-seeded vascular grafts was not affected. This result should be cautionary to drawing conclusions regarding cell thrombogenicity based on the activity of individual procoagulant pathways or the expression of thrombosis-related genes.
This work highlights the utility of characterizing thrombus formation in models that include flowing whole blood to properly simulate the in vivo regulatory pathways and transport dynamics. The similarity of EOCs and ECs in gene expression, coagulation factor activation, and regulation of platelet accumulation, at both a basal state and following TNFα treatment, supports the use of EOCs as an autologous endothelium in cardiovascular applications.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the technical support provided by Jennifer Greisel and the OHSU Flow Cytometry Shared Resource. Generous thanks are also given to Deirdre Anderson for her assistance with performing statistical analyses. This work was supported by the National Science Foundation Graduate Research Fellowship DGE-0925180, as well as funding from NIH grants R01HL 095474 and R01HL103728. Operation of the Oregon National Primate Research Center was supported by NIH grant OD011092.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Davies P.F.Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6,16, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu J.-J., and Chien S.Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev 91,327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aird W.C.Spatial and temporal dynamics of the endothelium. J Thromb Haemost 3,1392, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Zarbock A., and Ley K.Neutrophil adhesion and activation under flow. Microcirc N Y N 199416,31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller W.A.Mechanisms of transendothelial migration of leukocytes. Circ Res 105,223, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S., and Henry J.J.D.Nonthrombogenic Approaches to Cardiovascular Bioengineering. Annu Rev Biomed Eng 13,451, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Deutsch M., Meinhart J., Fischlein T., Preiss P., and Zilla P.Clinical autologous in vitro endothelialization of infrainguinal ePTFE grafts in 100 patients: A 9-year experience. Surgery 126,847, 1999 [PubMed] [Google Scholar]
- 8.Deutsch M., Meinhart J., Zilla P., Howanietz N., Gorlitzer M., Froeschl A., et al. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J Vasc Surg 49,352, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Zilla P., Deutsch M., Meinhart J., Puschmann R., Eberl T., Minar E., et al. Clinical in vitro endothelialization of femoropopliteal bypass grafts: an actuarial follow-up over three years. J Vasc Surg 19,540, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H., Kabuto M., Ide H., Hosotani K., and Kubota T.An artificial blood vessel with an endothelial-cell monolayer. J Neurosurg 77,397, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Pasic M., Muller-Glauser W., Von Segesser L.K., Lachat M., Mihaljevic T., and Turina M.I.Superior late patency of small-diameter dacron grafts seeded with omental microvascular cells: An experimental study. Ann Thorac Surg 58,677, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Tiwari A., Salacinski H.J., Hamilton G., and Seifalian A.M.Tissue engineering of vascular bypass grafts: role of endothelial cell extraction. Eur J Vasc Endovasc Surg 21,193, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Levenberg S., Rouwkema J., Macdonald M., Garfein E.S., Kohane D.S., Darland D.C., et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol 23,879, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Baptista P.M., Siddiqui M.M., Lozier G., Rodriguez S.R., Atala A., and Soker S.The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 53,604, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Stevens K.R., Kreutziger K.L., Dupras S.K., Korte F.S., Regnier M., Muskheli V., et al. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A 106,16568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asahara T., Murohara T., Sullivan A., Silver M., Van Der Zee R., Li T., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275,964, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Ahmann K.A., Johnson S.L., Hebbel R.P., and Tranquillo R.T.Shear stress responses of adult blood outgrowth endothelial cells seeded on bioartificial tissue. Tissue Eng Part A 17,2511, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen J.B., Khan S., Lapidos K.A., and Ameer G.A.Toward engineering a human neoendothelium with circulating progenitor cells. Stem Cells 28,318, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Hinds M.T., Ma M., Tran N., Ensley A.E., Kladakis S.M., Vartanian K.B., et al. Potential of baboon endothelial progenitor cells for tissue engineered vascular grafts. J Biomed Mater Res A 86A,804, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Shirota T., He H., Yasui H., and Matsuda T.Human endothelial progenitor cell-seeded hybrid graft: Proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng 9,127, 2003 [DOI] [PubMed] [Google Scholar]
- 21.He H., Shirota T., Yasui H., and Matsuda T.Canine endothelial progenitor cell-lined hybrid vascular graft with nonthrombogenic potential. J Thorac Cardiovasc Surg 126,455, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Ensley A.E., Nerem R.M., Anderson D.E.J., Hanson S.R., and Hinds M.T.Fluid shear stress alters the hemostatic properties of endothelial outgrowth cells. Tissue Eng Part A 18,127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lund T., Hermansen S.E., Andreasen T.V., Olsen J.O., Østerud B., Myrmel T., et al. Shear stress regulates inflammatory and thrombogenic gene transcripts in cultured human endothelial progenitor cells. Thromb Haemost 104,582, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Gulati R., Jevremovic D., Peterson T.E., Chatterjee S., Shah V., Vile R.G., et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res 93,1023, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Hanson S.R., Kotze H.F., Savage B., and Harker L.A.Platelet interactions with dacron vascular grafts. A model of acute thrombosis in baboons. Arteriosclerosis 5,595, 1985 [DOI] [PubMed] [Google Scholar]
- 26.McGuigan A.P., and Sefton M.V.The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials 28,2547, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vartanian K.B., Kirkpatrick S.J., Hanson S.R., and Hinds M.T.Endothelial cell cytoskeletal alignment independent of fluid shear stress on micropatterned surfaces. Biochem Biophys Res Commun 371,787, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Stroncek J.D., Grant B.S., Brown M.A., Povsic T.J., Truskey G.A., and Reichert W.M.Comparison of endothelial cell phenotypic markers of late-outgrowth endothelial progenitor cells isolated from patients with coronary artery disease and healthy volunteers. Tissue Eng Part A 15,3473, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuccuini W., Poitevin S., Poitevin G., Dignat-George F., Cornillet-Lefebvre P., Sabatier F., et al. Tissue factor up-regulation in proinflammatory conditions confers thrombin generation capacity to endothelial colony-forming cells without influencing non-coagulant properties in vitro. J Thromb Haemost 8,2042, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Brown M.A., Wallace C.S., Angelos M., and Truskey G.A.Characterization of umbilical cord blood–derived late outgrowth endothelial progenitor cells exposed to laminar shear stress. Tissue Eng Part A 15,3575, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nan B., Lin P., Lumsden A.B., Yao Q., and Chen C.Effects of TNF-α and curcumin on the expression of thrombomodulin and endothelial protein C receptor in human endothelial cells. Thromb Res 115,417, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Steffel J., Lüscher T.F., and Tanner F.C.Tissue factor in cardiovascular diseases: Molecular mechanisms and clinical implications. Circulation 113,722, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Andrews A.M., Jaron D., Buerk D.G., Kirby P.L., and Barbee K.A.Direct, real-time measurement of shear stress-induced nitric oxide produced from endothelial cells in vitro. Nitric Oxide 23,335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mann K.G., Orfeo T., Butenas S., Undas A., and Brummel-Ziedins K.Blood coagulation dynamics in haemostasis. Hamostaseologie 29,7, 2009 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.