Abstract
ΔNP63α, the predominant TP63 isoform expressed in diverse epithelial tissues, including the mammary gland is required for the preservation of stem cells and has been implicated in tumorigenesis and metastasis. Despite data characterizing ΔNP63α as a master regulator of stem cell activity, identification of the targets underlying these effects is incompletely understood. Recently, ΔNP63α was identified as a key regulator in the promotion of pro-inflammatory programs in squamous cell carcinoma of the head and neck. Inflammation has been implicated as a potent driver of cancer stem cell phenotypes and metastasis. In this study, we sought to identify novel targets of ΔNP63α that confer cancer stem cell and pro-metastatic properties. Data presented here identifies the gene encoding the chemokine receptor 4 (CXCR4) as a transcriptional target of ΔNP63α. Our data indicate that ΔNP63α enhances CXCR4 expression in breast cancer cells via its binding at two regions within the CXCR4 promoter. The CXCR4 antagonist AMD3100 was used to demonstrate that the pro-stem cell activity of ΔNP63α is mediated through its regulation of CXCR4. Importantly, we show that ΔNP63α promotes the chemotaxis of breast cancer cells towards the CXCR4 ligand SDF1α, a process implicated in the trafficking of breast cancer cells to sites of metastasis. This study highlights CXCR4 as a previously unidentified target of ΔNP63α which plays a significant role in mediating ΔNP63α-dependent stem cell activity and chemotaxis toward SDF1α. Our findings suggest that ΔNP63α regulation of CXCR4 may have strong implications in the regulation of cancer stem cells and metastasis.
Keywords: ΔNP63α, CXCR4, Cancer stem cells, chemotaxis, metastasis
Introduction
TP63, a member of the P53 family of transcription factors encodes multiple isoforms via a combination of differential promoter usage and alternative C-terminal splicing. TAp63 isoforms contain full-length N-terminal transactivating domains while ΔNp63 isoforms have a truncated N-terminus (1). ΔNp63α is the most abundant isoform expressed in the majority of epithelial tissues including the mammary epithelium (2). Substantial evidence implicates ΔNp63α in the maintenance of epithelial stasis, particularly through the preservation of self-renewing capacity (3, 4). Recently, we have defined a regulatory pathway by which miR203-dependent suppression of ΔNP63α promotes forfeiture of self-renewing capacity in a mammary stem cell (MaSCs) model programmed to undergo luminal differentiation (5). In the context of cancer, ΔNP63α has been identified as an oncogene, where it was found to drive epidermal stem cell proliferation and tumorigenesis (6). Likewise, in human breast tumors, ΔNp63α expression is highly enriched in the breast cancer stem cell population (7). Interestingly, a recent study determined that collective invasion in breast cancer, a process by which tumors invade surrounding normal tissue as a multicellular unit is mediated by carcinoma cells expressing basal epithelial markers including ΔNp63α and that suppression of ΔNp63α was sufficient to block collective invasion (8). These findings coupled to studies identifying ΔNp63α as a pro-survival factor and mediator of chemoresistance in basal breast cancers and head and neck squamous cell carcinomas (HNSCC), (9, 10) highlight its role in epithelial tumor initiation, progression and metastasis.
Accumulating evidence indicates that a subpopulation of cancer cells with stem cell properties play a crucial role in tumorigenesis, metastatic development and resistance to therapy. These cancer stem cells (CSCs), like normal stem cells, retain self-renewing capacity and their mitotic offspring differentiate into the various cell populations within the tumor mass (11-13). Recently we reported that ΔNp63α expression is significantly enriched in self-renewing populations of normal murine and malignant human mammary stem cells respectively (5, 14). In addition to its role in tissue and tumor stasis, ΔNp63α regulates pro-inflammatory gene expression and a malignant phenotype in HNSCC (15) by forming novel complexes with NF-κB family members. NF-κB signaling is required for cancer stem cell activity in multiple cancers including basal breast cancer (16, 17,)(18). Additionally, ΔNp63 isoforms have been identified as a marker of the basal breast cancer subtype (19), where it functions to regulate cancer cell survival and drug resistance (9). Together these studies suggest that ΔNp63α directs expression of pro-inflammatory genes via interactions with NF-κB and that this class of target genes plays a role in tumor progression and metastasis.
Accumulating evidence highlights the role of inflammation and cytokine networks in the cancer initiation and progression and more recently, in the regulation of cancer stem cell populations (20, 21). Many of the cytokines present in the tumor microenvironment and pre-metastatic niches bind to receptors present on cancer stem cells, which subsequently converge on several pathways including Notch, PI3K/AKT, Wnt, NF-κB and Jak/Stat to activate self-renewal and pro-survival pathways (21-23). Despite ΔNp63α's role in the preservation of stem cell renewal, the target genes and mechanisms by which ΔNp63α mediate such phenotypes are incompletely defined. Based upon the ability of ΔNp63α to co-regulate NF-κB target genes, we sought to identify targets of ΔNp63α implicated in inflammation and cancer stem cell activity to gain a better understanding of the mechanisms by which ΔNp63α functions. Here we report that the gene encoding the chemokine receptor 4 (CXCR4) is a transcriptional target of ΔNP63α. CXCR4 belongs to a class of receptors that bind the conserved CXC class of chemokines, which have a single nonconserved amino acid residue (X) between the first N-terminal cysteine residues (C)(24). CXCR4 is a G-protein-coupled receptor (GPCR), that, when activated by its only known ligand, CXCL12 (stromal derived factor-1) (25), initiates the activation of diverse downstream signaling pathways including MAPK, PI3K, AKT, JAK/STAT and NF-κB to promote cell survival, proliferation and migration (25-27). Under normal physiologic conditions, the CXCL12-CXCR4 network plays a vital role in the regulation of normal stem/progenitor and hematopoietic cell trafficking and homing (28, 29). However, in solid tumors, CXCR4 has been shown to be markedly overexpressed relative to matched normal tissues. In the breast, CXCR4 regulates the chemotaxis of breast cancer cells to sites of metastasis in which high levels of SDF-1α (CXCL12) have been observed (30). Additionally, CXCR4 plays a central role in metastasis and has been implicated in self-renewal and survival of cancer stem cells (31, 32). Here we demonstrate that ΔNP63α binds to conserved p63-binding motifs within the CXCR4 promoter and positively regulates CXCR4 gene expression. We demonstrate that ΔNP63α mediated expression of CXCR4 influences stem cell activity and chemotaxis in response to SDF1α. Together, this study identifies a novel and significant transcriptional target of ΔNP63α and brings to light the possible function of ΔNP63α in breast cancer stem cells to mediate metastasis.
Materials and Methods
Cell Culture
The human breast cancer cell lines MCF-7, MDA-MB-231 and the lung cancer cell line H1299 were obtained from the ATCC (Manassas, VA, USA) and grown in DMEM-medium supplemented with 10% FBS and 0.2IU/mL insulin for MCF-7 cells. Derivation and maintenance of the IMEC line has been previously described (5). The human breast cancer cell line SUM-102 was grown in Ham's/F-12 medium supplemented with 1mM HEPES, 2% FBS, 5μg/ml Insulin, 1μg/ml Hydrocortisone, 10ng/ml EGF and Penicillin/Streptomycin. No cell line authentication was done by the authors.
Plasmid and Viral Constructs and Trasfection and Infection Assays
For experiments involving stable overexpression of ΔNP63α, IMEC's were transfected with an empty control mammalian expression plasmid (pCDNA-Ctr) or one encoding human ΔNP63α (pCDNA- ΔNP63α) using lipofectamine 2000 Transfection reagent (Invitrogen). Both expression plasmids were linearized via restriction enzyme digestion. Twenty four hours post transfection, cells were selected under G418 (2μg/ml) to establish a stable cell line overexpressing ΔNP63α. For experiments involving stable overexpression of ΔNP63α in MCF-7 cells and stable overexpression of SDF1α in MCF10A cells, a retrovirus was utilized. The Platinum-A retroviral packaging cell line (Cell BioLabs Inc., San Diego, CA, USA) was transfected with the mammalian retroviral expression vector pLPC (Addgene, Cambridge, MA, USA) containing the open reading frame of ΔNP63αORF, or empty vector control (pLPC) and for SDF1α experiments, the mammalian retroviral expression vector pBABE (Addgene plasmid 12270, Addgene, Cambridge, MA, USA) containing the open reading frame of SDF- 1α, or empty vector control (pBABE) was used (33). At 24 hours post transfection, viral titers were collected and used to infect MCF-7 or MCF10A cells, which were subsequently used in mammosphere and chemotaxis experiments respectively. For all other experiments involving ΔNP63α overexpression, an adenovirus containing the open reading frame of ΔNP63α (Adv- ΔNP63α), or an empty vector control (Adv-Ctr) was used. For experiments overexpressing miR203, IMECs were transfected using Lipofectamine 2000 Transfection Reagent (Invitrogen) with 60nM of miR-control (miR-CON) or mature miR203 constructs (Applied Biosystems Inc). Cells were collected for qRT-PCR analysis 48 hours post infection.
Western Blot Analysis
Total cell protein lysates were obtained using NETN lysis buffer (100mM Tris-Cl (pH 7.8), 1mM EDTA, 100mM NaCl, and 0.1%Triton X-100) supplemented with protease and phosphatase inhibitors (Roche,Branchburg, NJ, USA). Protein lysate concentration was measured by Lowry protein assay and 10μg of protein per well was resolved on a 10% SDS-polyacrylamide gel and transferred onto a PVDF membrane. Membranes were subsequently blocked in TBST 5% dry mile for 1h at room temperature, incubated with primary antibody overnight at 4°C, washed with TBST and incubated at room temperature for 1h with horseradish peroxidase-conjugated secondary antibody and developed using enhanced chemiluminescence substrate (ECL).
Immunofluorescence
Cells were seeded onto glass chamber slides at 8000 cells/well. Cells were then fixed using CytoRich-Red 24 hours post seeding. Slides were washed three times (PBS/0.1% Tween-20) and blocked for 2 hours in 5% goat serum/0.5% Tween-20/PBS at room temperature. Samples were incubated with anti-CXCR4 primary antibody (8μg/ml) diluted in blocking buffer for 45 minutes at 37°C, washed once and subsequently incubated with anti-mouse Alexa Fluor 488 secondary antibody for 15 minutes at 37°C. Glass slides were mounted in Vectashield with 4′,6-diamino-2-phenylindole (DAPI; Vector Laboratories) and imaged via fluorescence microscopy. To control for non-specific fluorescence, a set of cells were stained with secondary antibody only. All fluorescent and phase contrast images were visualized and captured using a Nikon Eclipse TS100 inverted microscope coupled to a RT Slider Spot charge-coupled device (CCD) camera. Quantification of fluorescent intensity was performed using ImageJ software.
Antibodies
For western blotting, mouse anti-p63 (4A4) (LabVision, Kalamazoo, MI, USA; 1/1000 dilution), mouse anti-β-actin (1/1000 dilution), rabbit anti-SDF1 (1/1500 dilution) (Cell Signaling, Boston, MA, USA), rabbit anti-CXCR4 (1/500) (Abcam, Cambridge, MA, USA) antibodies were used. For immunofluorescence experiments, rabbit anti-CXCR4 (1/500) (Abcam, Cambridge, MA, USA) (Figure 1C) or mouse anti-CXCR4 (R&D Systems, Minneapolis, MN, USA; 8μg/ml dilution) (Supplemental Figure 2B) antibodies were used. Secondary antibodies used were anti-mouse and anti-rat IgG horseradish peroxidase-conjugated for immunoblot and goat anti-rabbit-alexafluor-555 or goat anti-mouse-alexafluor-488 conjugated antibodies for immunofluorescence.
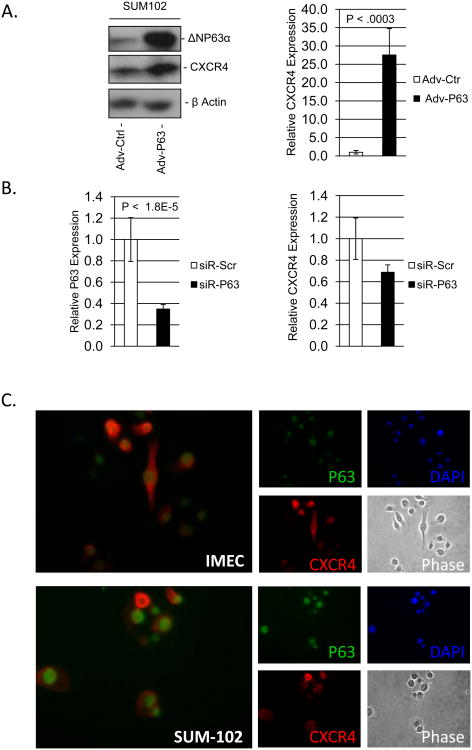
Figure 1. ΔNP63α positively regulates the expression of CXCR4 mRNA.
SUM-102 cells were infected with an empty adenovirus or an adenovirus encoding ΔNP63α. A) Left - Immunoblot detection of ΔNP63α and CXCR4 protein expression from SUM-102 protein lysates 24 hours post infection. Right - Relative mRNA expression of CXCR4 from RNA lysates 24 hours post infection. B) SUM-102 cells were transfected with a siRNA against ΔNP63α or scrambled control (20nM). Relative mRNA expression levels for Left) ΔNP63α and Right) CXCR4 was determined via qRT-PCR analysis of RNA lysates 48 hours post transfection. C) IMEC and SUM-102 cells were fixed and immunoflourescent analysis was performed to detect the expression of ΔNP63α and CXCR4 protein. 40× magnification. Data are means ± S.D.; and performed in triplicate (n=3), P-values are indicated.
Luciferase Reporter Assay
Oligonucleotides representing the two putative p63-binding elements identified in the CXCR4 promoter were concatenated and ligated uni-directionally upstream of the minimal promoter of the HSV16 thymidine kinase promoter and firefly luciferase. (ptk-Luc). Resulting reporters including parental tk-Luc were transfected into H1299 cells along with expression vectors encoding renilla luciferase and either an empty vector or ΔNp63α. Twenty-four hours after transfection, cells were harvested and analyzed for luciferase activity using the Dual-Luciferase Reporter Assay System (Promega, Madison WI) as per manufacturers' protocol.
Colony Formation Analysis
IMECs stably overexpressing ΔNP63α or empty control vector (IMEC-pCDNA- ΔNP63α or IMEC-pCDNA) were plated at colony formation density (6000 cells per 10cm tissue culture plate) and treated in the presence of vehicle control (H2O) or the CXCR4 antagonist AMD3100 (5μM). Colonies were stained using crystal violet 14 days post plating.
Mammosphere Formation Assay
The human breast cancer cell line, MCF-7 was infected with retrovirus to stably overexpress ΔNP63α or empty control (MCF7-pLPC- ΔNP63α and MCF7-pLPC) and seeded (5000 cells per plate) onto 10cm ultra-low attachment surface tissue culture plates (Corning Inc.). Cells were treated in the presence of vehicle control (H2O) or the CXCR4 antagonist AMD3100 (5μM). Cells were grown in low binding conditions for 6 days and later quantified.
RT-qPCR Analysis
RNA was isolated using the RNeasy Kit (Qiagen) as per manufacture's protocol. RNA (1ug) was reverse transcribed into cDNA using the iScript cDNA Synthesis Kit (BioRad). Quantitative PCR was carried out using the iQ SYBR Green Super mix (BioRad) and oligonucleotide primers specific to each target gene. The 2-ΔΔCT method was used for quantification of relative gene expression changes and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Chromatin Immunoprecipiation Assay
Crosslinking was performed by treating cells with media supplemented with 1% formaldehyde for 10 minutes at 37°C. Cells were washed once in PBS/BSA (5mg/ml) and then lysed at room temperature in lysis buffer (1% SDS, 10mM EDTA, 50mM Tris-HCl pH8.1 and protease inhibitor). DNA fragmentation was performed using a bioruptor. Lysates were subjected to 3×5 minute sonication periods at 30 second on/off cycles. Lysates were then spun down and aliquots of the supernatant were taken for input control. Supernatants were then diluted 1:10 in dilution buffer (1% triton, 2mM EDTA, 150mM NaCl, 20 mM Tris-HCl pH 8.1) which were subsequently added to a 1:1 mixture of A and G Dynal beads complexed with either IgG isotype control or anti-p63 (4A4) antibodies (2.5 μg) and immunoprecipitated overnight on a rotator at 4°C. The immunoprecipitation complex was washed 4×5minutes in cold RIPA buffer (50mM HEPES pH 7.6, 1mM EDTA, 0.7% Na Deoxycholate, 1% NP-40, 0.5M LiCl) followed by washing twice in cold TE (pH 7.6) buffer. The immunoprecipitation complex was then reverse cross-linked in a 0.1M NaHCO3 solution containing 1% SDS at 65°C overnight. DNA was isolated using the QIAgen PCR purification kit. Isolated DNA was analyzed using qRT-PCR.
Predicted p63 binding sites and designed PCR primers used in ChIP assay
Putative p63 binding sites on the CXCR4 gene promoter were predicted based on the p63 canonical binding motif (RRRCWWGYY). The top scored putative p63 binding sites (6 or greater complementary nucleotides per p63 binding site with minimal nucleotides between p63 dimers) were selected in the range of -2000bp to +2000bp from the transcription starting site (TSS) of the CXCR4 promoter. ChIP experiments identified significant p63 binding activity over IgG isotype controls at 2 CXCR4 promoter sites, and a known p63 binding site in the CSF2 gene. The sequences of the designed primers for these binding sites are as follows: CXCR4, p63 binding site (-547bp): forward primer: 5′- TTCGCGAATTGGTTACCG-3′; reverse primer: 5′- CAGCCCATTCAGGAGGTAAA-3′; amplicon: 206bp. CXCR4, p63 binding site (+1403bp): forward primer: 5′- CGGGTTAACTGGATCAGTGG-3′; reverse primer: 5′- AAATGAACAAACGGCACCTC-3′; amplicon: 225bp. CSF2, p63 binding site (+845bp): forward primer: 5′-GGGGTGAGAGTCACCTCCTT-3′; reverse primer: 5′-GTCATAGACCCTGCCCTGTC-3′; amplicon: 122bp.
Cell Sensitivity Assay
MDA-MB-231 cells were seeded at 1.5×10(x002C6)5 cells per well of a 6-well tissue culture plate. Upon adherence, cells were infected with an adenovirus expressing ΔNP63α or an empty adenovirus control. Cells were then treated with either vehicle (H2O) or the CXCR4 antagonist AMD3100 (10μM). Cells were fed with fresh growth medium and AMD3100 every other day for 7 days. At day 7, cells were stained with crystal violet or trypsinized and counted. Trypan blue exclusion experiments to measure cellular viability were performed by staining trypsinized cells in a 1:10 dilution of trypan blue(HyClone Cell Culture): PBS. Cells were then counted on a hemacytometer. Percent viable cells = [1.00 – (Number of blue cells ÷ Number of total cells)] × 100.
Chemotaxis Assay
For chemotaxis assays, 4×105 MCF10A-Ctrl or MCF10A-pBABE-SDF1α cells were seeded in the lower well of a boyden chamber two days prior to the addition of polycarbonate membrane (8-μM pore size). A total of 2×105 SUM102 cells that were infected with either an empty adenoviral control, or one containing the open reading frame of ΔNP63α was seeded in the upper chamber 24 hours after infection. After 20 hours of incubation at 37°C, the cells were fixed in 4% paraformaldehyde for 20 minutes, and the nonmigrated cells on the top of the membrane were removed. The migrated cells on the bottom of the chamber were stained in crystal violet and placed on glass slides. The number of stained cells was counted under a microscope at 10 different fields.
Statistical Analysis
Statistical analyses were performed using Students' t-test. A P-value < 0.05 was considered significant.
Results
ΔNP63α Positively Regulates the Expression of CXCR4 mRNA
ΔNp63α interacts with NFκB and contributes to the regulation of pro-inflammatory NFκB-driven transcriptional networks and distinct cytokines which contribute to the governance of stem and cancer stem cell activity. This suggests that ΔNP63α-mediated preservation of stem cell phenotype is carried out, through the transcriptional regulation of inflammation associated genes. We therefore analyzed the relative change in mRNA expression of several well characterized genes associated with inflammation in response to overexpression of ΔNP63α and found CXCR4 expression to be significantly induced in SUM102 cells which were selected for their intermediate level of ΔNP63α protein expression (Figure 1A – left: protein expression, right: mRNA expression, Supplemental Figure 1 –validated targets of ΔNP63α as control, Supplemental Figure 2A – endogenous ΔNP63α protein expression in mammary and breast cancer cell lines). CXCR4 was similarly induced upon ΔNP63α overexpression in immortalized mammary epithelial cells (IMEC), and the breast cancer cell lines MCF-7 and MDA-MB-231 (Supplemental Figure 2B). Additionally, immunoflourescent imaging of SUM-102 cells over-expressing ΔNP63α revealed a substantial induction in CXCR4 protein expression compared to adenoviral-control (Supplemental Figure 2C). Consistent with these results, siRNA-mediated depletion of ΔNP63α from SUM-102 cells (Figure 1B - left); decreased the expression of CXCR4 mRNA (Figure 1B - right), which was also observed in IMECs (Supplemental Figure 2D). Additionally, Immunofluorescent imaging of normal immortalized mammary epithelial cells (IMECs) and the breast cancer cell line SUM-102 revealed that both ΔNP63α and CXCR4 protein are co-expressed within the same cell (Figure 1C). Remarkably, transfection of miR203, which targets ΔNP63α in mammary epithelial cells (5), also repressed CXCR4 mRNA expression (Supplemental Figure 3), further implicating ΔNP63α in the positive regulation of CXCR4. Taken together, these results indicate that ΔNP63α positively regulates the expression of CXCR4.
ΔNP63α Binds to the CXCR4 Promoter Region
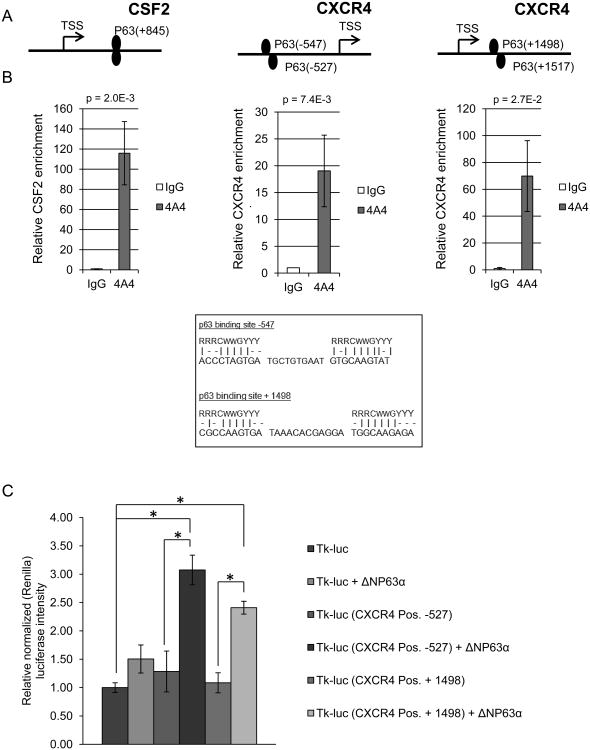
The ability of ΔNP63α to positively regulate the expression of CXCR4 mRNA (Figure 1) suggests that it may bind to its promoter region to facilitate transcription. To address this, we searched the CXCR4 promoter for consensus p63-binding elements and identified two putative p63-dimer binding sites (Figure 2A and Materials and Methods). Anti-p63 Chromatin Immune-Precipitation (ChIP) lead to a sharp enrichment of genomic fragments containing these sequences in SUM-102 cells (Figure 2A-B) and an hTERT immortalized mammary epithelial cell line (IMEC) (Supplemental Figure 4A). Consistent with this observation, ectopic expression of ΔNp63α enhanced binding of these genomic regions (Supplemental Figure 4B). Under identical conditions a known p63-binding element in the CSF2 promoter was enriched by p63-directed ChIP as a positive control and random genomic regions within the CXCR4 promoter was used as a negative control (Figure 2A&B and Supplemental Figure 4C respectively) (15). In order to determine the functional significance of the observed p63 binding within the CXCR4 promoter, the identified elements were subcloned upstream of a heterologous promoter and firefly luciferase (Figure 2C). Compared to parental luciferase control constructs (tk-luc), reporter constructs containing either of the two p63-binding sites displayed significantly greater luciferase intensity in the presence of ectopic ΔNP63α (Figure 2C). Taken together, these studies demonstrate that ΔNP63α binds to two predicted binding sites on the CXCR4 promoter and positively regulates the transcription and subsequent protein expression of CXCR4.
Figure 2. ΔNP63α binds to the CXCR4 promoter region.
A) Predicted p63 binding sites on the CSF2 (positive control) and CXCR4 gene promoter regions [base pairs from transcription start site (TSS) denoted, P63 putative binding sites – lower right)]. B) ChIP assay performed on SUM-102 cells using anti-p63 (4A4) and IgG isotype antibodies which were then subjected to real-time PCR. Values are mean relative binding activity over isotype control for endogenous p63. C) H1299 cells were transfected with a parental control luciferase plasmid or one containing the CXCR4 promoter sequences that are bound by ΔNP63α in the presence or absence of ectopic ΔNP63α. Luciferase activity was measured on a luminometer 24hr post transfection. Data are means ± S.D.; and performed in triplicate (n=3), * indicate a P-value < 0.05.
ΔNP63α-dependent regulation of stem cell activity is mediated through induction of CXCR4 expression
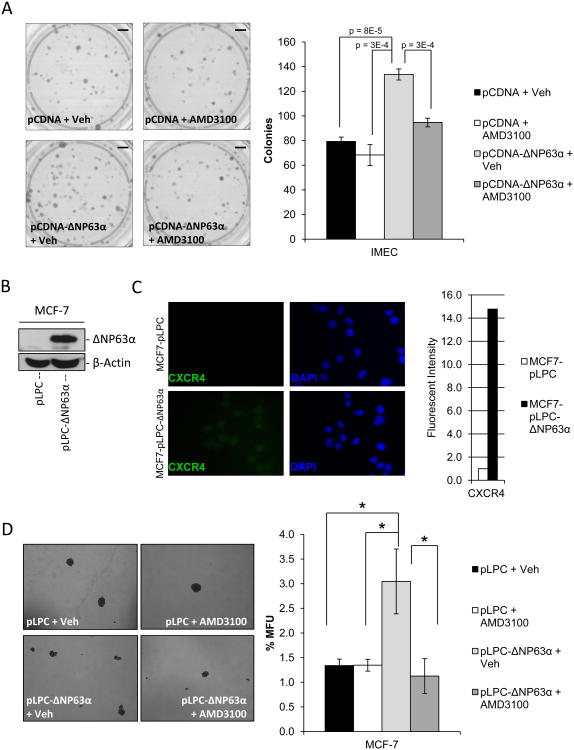
The ability of ΔNP63α to positively regulate expression of CXCR4 suggests that CXCR4 may mediate some of the activities ascribed to ΔNP63α. Several studies have begun to illuminate the influence of various cytokines over stem cell regulation and phenotype (21). CXCR4 has been implicated in normal stem cell and cancer stem cell function as well as cancer progression and metastasis (31, 32). This, combined with abundant evidence implicating ΔNP63α in the maintenance of self-renewing capabilities in stem cells suggest that this role of ΔNP63α may be mediated through its regulation of CXCR4 (3, 5). To test this, clonogenicity assays were performed on IMECs infected with control, or an adenovirus programmed to express ΔNP63α, and then treated in the presence or absence of a small molecule inhibitor against CXCR4 (AMD3100). Results revealed that ectopic ΔNP63α significantly enhanced clonogenic capacity of IMECs and this was sensitive to CXCR4 inhibition with AMD3100 (Figure 3A). Consistent with this result, ectopic expression of ΔNP63α in MCF7 cells induced expression of CXCR4 (Figure 3B & C) and sharply increased mammosphere-forming capacity, in a manner that was sensitive to AMD3100 (Figure 3D). Importantly, the ability of AMD3100 to oppose mammosphere formation required ectopic ΔNP63α (Figure 3D) (Supplemental Figure 5 – the ligand to CXCR4, SDF1α is present at detectable levels in the cell culture media used in the above experiments). Taken together these studies indicate that the ability of ΔNP63α to enhance breast cancer stem cell activity is mediated through its positive regulation of CXCR4 and its subsequent downstream activity.
Figure 3. ΔNP63α-dependent regulation of stem cell activity is mediated through induction of CXCR4 expression.
A) Clonogenicity assays were performed in SUM-102 cells that were transfected with either linearized pCDNA or pCDNA-ΔNP63α. Cells were then plated at clonogenic capacity in the presence or absence of the CXCR4 antagonist AMD3100 (5 μM). Images of colonies stained with crystal violet (left), quantification of the number of colonies formed per experimental group (right). P-values are indicated. Scale bars, 850 mm. B) Western immunoblot to detect ΔNP63α protein expression in protein lysates of MCF-7 cells stably infected with an empty retrovirus (MCF7-pLPC) or one encoding ΔNP63α (MCF7-pLPC-ΔNP63α). C) Representative immunofluorescence images of MCF7-pLPC+/- ΔNP63α cells to detect CXCR4 protein expression levels (left), quantification of fluorescent intensity of image normalized to cell number (fluorescent intensity of DAPI staining, right) 40× magnification. D) MCF7-pLPC+/- ΔNP63α were seeded on non-adherent tissue culture plates at 500 cells/mL in the presence or absence of the CXCR4 antagonist AMD3100 (5 μM). Mammosphere forming capacity was assessed 6 days post-plating. Representative 10× phase images of mammospheres (left), quantification of mammosphere forming units (MFU) (right). All data are mean ± S.D.; n=3, asterisks indicates a P<0.013.
ΔNP63α Mediates Sensitivity to CXCR4 Inhibition
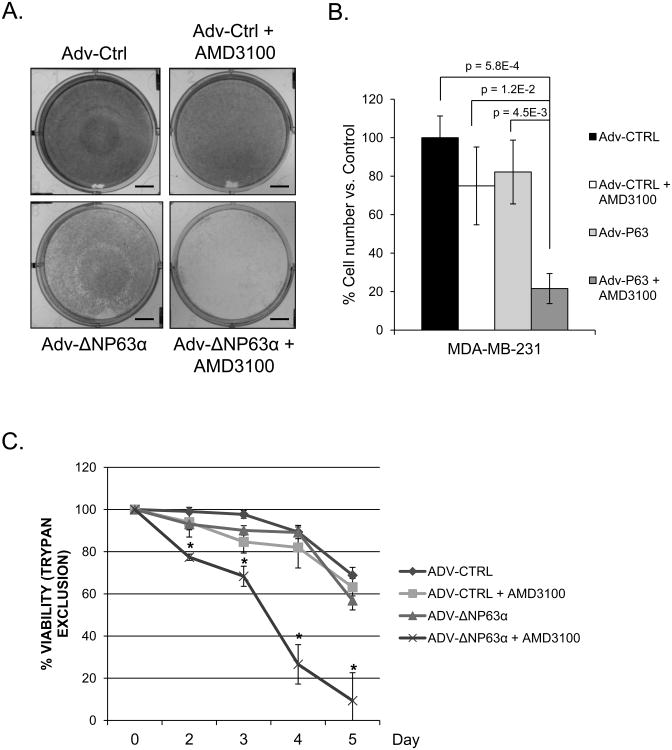
Stem cells and cancer stem cells have been shown to possess enhanced pro-survival signaling and rely heavily on, in part, AKT and JAK/STAT pathways, both of which have been demonstrated to be activated by CXCR4 signaling (25). Additionally, several studies highlight the pro-survival, anti-apoptotic activity of ΔNP63α, especially in the context of chemoresistance and tumorigenesis (9, 10, 34). These studies coupled with data indicating that ΔNP63α mediated breast cancer stem cell activity involves expression and activity of CXCR4 suggests that ΔNP63α expression may confer sensitivity to CXCR4 inhibition. To determine this, we utilized the highly aggressive breast cancer cell line MDA-MB-231, which lacks endogenous expression of ΔNP63α (Supplemental Figure 2A), and infected them with either an empty adenovirus as a control, or an adenovirus programmed to express ΔNP63α. Cells were re-fed every other day in the presence or absence of the CXCR4 inhibitor AMD3100 (5μM) for 7 days. Crystal violet staining of the cells after seven days of treatment revealed minimal to no loss in cell density (Figure 4A) or cell number (Figure 4B) between control MDA-MB-231 cells (Adv-Ctrl) treated in the presence or absence of AMD3100. However, ectopic ΔNP63α sensitized MDA-MB-231 cells to the CXCR4 inhibitor AMD3100 (Figure 4), suggesting that ΔNP63α dependent regulation of CXCR4 is critical for cellular viability. This is further supported by tyrpan blue exclusion viability experiments of MDA-MB-231 cells expressing an adenoviral empty control or one expressing ΔNP63α treated in the presence or absence of the CXCR4 inhibitor AMD3100 (Figure 4C). Results demonstrate that MDA-MB-231 cells overexpressing ΔNP63α exhibit significant reductions in cell viability in the presence of AMD3100 compared to controls (Figure 4C). Taken together with previous studies demonstrating the pro-survival effects of both ΔNP63α and CXCR4, the above data suggests that ΔNP63α regulates cellular viability in part, through its regulation of CXCR4 and also suggests that ΔNP63α may act as a marker for CXCR4 inhibition or anti-CXCR4 therapies.
Figure 4. ΔNP63α mediates sensitivity to CXCR4 inhibition.
MDA-MB-231 cells were infected with empty or an adenovirus encoding ΔNP63α and treated with vehicle or AMD3100 (5 μM) for 7 days. A) Representative images of crystal violet stained cells. Scale bars, 500 mm. B) Cell number of MDA.MB.231 cells, P-values are indicated. C) Trypan blue exclusion to measure viability of MDA-MB-231 cells infected with empty or an adenovirus encoding ΔNP63α and treated with vehicle or AMD3100 (5 μM), * indicates a P-value < 0.05. Experiments were performed in triplicate (n=3), data are means ± S.D.
ΔNP63α Positively Regulates SDF1α Dependent Chemotaxis of Breast Cancer Cells
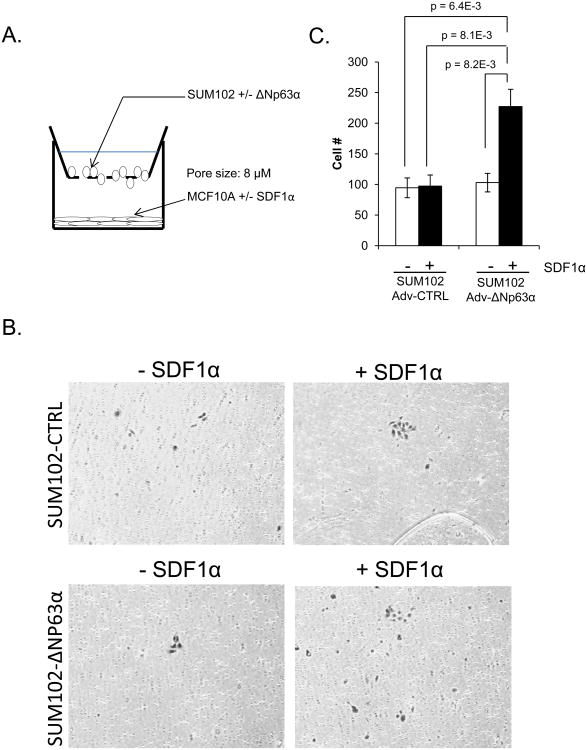
Under normal physiologic conditions, SDF1α (CXCL12) acts as a chemoattractant for immature and mature hematopoietic cells, for which the cognate receptor CXCR4 is expressed at its highest levels, thus playing a vital role in immune surveillance at distant tissues and inflammatory processes (25). More recently, it has been established that CXCR4 expression is significantly increased in various cancers, including breast cancers compared to their normal counterparts and may mediate the trafficking and invasion of these cells to distant sites of metastasis, where SDF1α is expressed. Interestingly, multiple studies have demonstrated CXCR4 as a major mediator in the development of metastasis in as many as 75% of all cancers including the breast (35). Based on the demonstration that ΔNP63α is a positive regulator of CXCR4, which contributes to the ability of ΔNP63α to maintain a breast cancer stem cell phenotype, we sought to determine if ΔNP63α can regulate the chemotaxis of breast cancer cells towards the CXCR4 ligand SDF1α. To do this, we performed chemotaxis assays in which we seeded the bottom layer with MCF10A cells infected with an empty retrovirus, or one encoding SDF1α, to act as the source of the chemoattractant (SDF1α). This was then followed by seeding the top membrane with control SUM-102 cells, or SUM-102 cells overexpressing ΔNP63α (Figure 5A). Under these conditions, only SUM-102 cells overexpressing ΔNP63α displayed significant migratory activity in the presence of SDF1α. Moreover, this ΔNp63α-mediated migration appears to be dependent on SDF1α/CXCR4 signaling, as there was no significant induction of migration in the absence of the chemokine or ΔNP63α (Figure 5B and C). Taken together, this result demonstrates the ability of ΔNP63α to promote (> 2-fold) the migratory activity of breast cancer cells via its modulation of CXCR4 and may therefore play a significant role in the development of breast cancer metastasis.
Figure 5. ΔNP63α positively regulates SDF1α dependent chemotaxis of breast cancer cells.
A) Diagram of the Boyden chamber assay. MCF10A cells (control and SDF1α overexpressing) were seeded in the bottom of the chamber and SUM102 cells (control or ΔNP63α overexpressing) were seeded onto the upper layer of the membrane. B) After 20 hours, the migrated cells were fixed and stained in crystal violet and where counted at 10 different fields. Graph represents the relative degree of migration based on the total number of cells counted. 10× magnification. C) Representative images of transmigrated cells per experimental group. Experiments were performed in triplicate (n=3), data are means ± S.D.; p-values are indicated.
Discussion
The majority of cancer mortality is the result of advanced metastatic disease (36). Cancer stem cells have been shown to be capable of migrating to metastatic sites and eventually differentiate into diverse cell types that make up the tumor bulk (37). Here, we report a novel regulatory relationship between the cancer stem cell pro-survival factor, ΔNP63α, and the pro-metastatic gene CXCR4. We demonstrate not only that CXCR4 mediates the pro-stem cell activities of ΔNP63α, but also that ΔNP63α promotes migration of breast cancer cells in a manner that is dependent upon CXCL12-CXCR4 signaling. These findings contribute to the accumulating evidence implicating ΔNP63α in cancer stem cell activity and metastasis. Epithelial-to-mesenchymal transition (EMT) has also been attributed to mediating the acquisition of a cancer stem cell and a pro-invasive and metastatic phenotype (38). Additionally, recent studies have implicated ΔNP63α in the modulation of EMT-MET transitions (39, 40). In one study in prostate cancer, p63 was shown to inhibit EMT marker expression and lung metastasis in mouse tail vein injection models via the regulation of the microRNA miR-205 in a p53-dependent context (41). However, in our studies, ΔNP63α-mediated induction of CXCR4 expression and function occurred without any changes in the protein expression of hallmark markers of EMT (Supplemental Figure 6), suggesting that the observed findings in this study are independent of ΔNP63α's regulation of EMT. Given this paradox by which p63 has been demonstrated to be capable of promoting or repressing EMT, it suggests that it may function as a regulator of EMT-MET plasticity. Under certain distinct contexts, p63 may be able to drive populations toward one state or the other. Further studies are needed to elucidate whether p63 can mediate such plasticity and under what contexts it drives one state over the other.
CXCR4 was first shown to mediate metastasis of breast cancer towards organs expressing its ligand CXCL12 and has since been implicated in the metastatic development of up to 75% of other solid tumors (25, 35). Not surprisingly, increased expression of CXCR4 in breast cancer has been found to be associated with poor overall survival, particularly due the development of metastatic disease (42). The present report demonstrates that ΔNP63α positively regulates expression of this vital pro-metastatic gene. This highlights the potential significance of ΔNP63α as a mediator of metastasis and as a potential prognostic marker of breast cancer. Additional research is necessary to identify and characterize all mechanisms by which ΔNP63α contributes to disease progression, and to what extent it is due to its regulation of CXCR4. For example, it has been demonstrated that NF-κB signaling promotes breast cancer migration and metastasis via CXCR4 (43). Additionally, ΔNP63α and NF-κB have been shown to co-regulate an overlapping subset of target genes that promote inflammation and disease progression in HNSCC (15). In addition to CXCR4, ΔNP63α can also positively regulate IL-6, IL-8 and CSF2 (Supplemental Figure 1), all of which can promote pro-inflammatory cellular responses linked to tumor progression, angiogenesis, invasion and metastasis (30). Taken together, these studies, coupled to the data presented here, illustrate the profound and potentially global influence ΔNP63α may have on breast cancer tumorigenesis and progression.
In glioblastoma stem cells, inhibition of CXCL12-CXCR4 signaling significantly reduced viability and self-renewing activity (31). Here, we identified CXCR4 as a functional mediator of the observed gains in stem cell activity in response to ΔNP63α expression. ΔNP63α-dependent enhancement of clonogenicity and mammosphere forming capacity are diminished by the CXCR4 inhibitor, AMD3100. CXCR4's role in maintaining stem cells has been reported to be via multiple pathways known to be involved in stem cell renewal, suggesting that ΔNP63α may influence these pathways via CXCR4. This highlights the complexity of ΔNP63α activity and the need to elucidate the particular contexts and networks governed. Additionally, data presented here implicate CXCR4 as a mediator of ΔNP63α-dependent viability and demonstrate that ΔNp63α expression predicts a response to AMD3100. An important feature of cancer stem cells is resistance to chemotherapy (44). Recently CXCR4 was implicated in stem cell activity in drug resistant non-small cell lung cancer cells (32). Likewise, ΔNP63α is a critical mediator of chemoresistance in HNSCC and breast cancer. These functions may overlap with and future studies should be aimed at identifying this.
CXCL12-CXCR4 signaling plays an important role in the establishment of metastasis by regulating the migration of cancer cells to distant metastatic sites and by mediating the survival and proliferation of cells at these locations (25). In a recent study from the Massague lab, investigators revealed that a subset of triple negative breast tumors with high Src activity is primed for metastasis to the bone via CXCL12 signaling (45). Given that ΔNP63α has been shown to activate the same downstream pathways as Src (46) combined with the data presented here identifying ΔNP63α as a positive regulator of CXCR4, it suggests that it may also play a role in the metastatic priming of breast cancer cells to the bone. Multiple pre-clinical studies have demonstrated that disruption of this pathway, either through inactivation of CXCL12 or CXCR4 drastically reduced the ability of breast cancer to metastasize to distant organs, specifically the most common sites of breast cancer metastasis, the bone and lungs (47, 48). However, to our knowledge, there has not been a clinical trial using inhibitors of this pathway in breast cancer. Here, we demonstrate that ΔNP63α can mediate the migration of breast cancer cells towards CXCL12, which is highly expressed in bone and lung, the most common sites of breast cancer metastasis. Again, this stresses the potential significance of ΔNP63α in mediating breast cancer metastasis. Additionally, ΔNP63α has been reported to regulate an adhesion program including beta4 integrin's, which have been implicated in the docking and survival of cancer cells at a distant tissue, thus promoting metastasis (49, 50). Given this knowledge, coupled to the findings we present here in which we demonstrate that ΔNP63α can mediate chemotaxis, it suggests that ΔNP63α may denote or identify cells of particularly high metastatic potential. Further research should aim at this goal, particularly with regards to the newly identified regulation ΔNP63α exerts over CXCR4 presented here.
Supplementary Material
Acknowledgments
Grant Support: This work was supported in part by grants to J. DiRenzo from the National Cancer Institute (NCI; 5RO1CA108539-05). A. J. DeCastro was supported by a pre-doctoral training grant from the United States Department of Defense Breast Cancer Research Program (W81XWH-11-1-0043).
Financial Support: National Cancer Institute - NCI 5RO1CA108539-05 awarded to J. DiRenzo, Department of Defense - DoD W81XWH-11-1-0043 awarded to A.J. DeCastro.
Abbreviations
- CSC
Cancer stem cell
- MaSC
Mammary stem cell
- HNSCC
Head neck squamous cell carcinoma
- CXCR4
Chemokine receptor 4
- SDF1 or CXCL12
Stromal derived factor-1
Footnotes
Conflicts of Interest: The authors declare no conflict of interests
References
- 1.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Molecular cell. 1998;2:305–16. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 2.Nylander K, Vojtesek B, Nenutil R, Lindgren B, Roos G, Zhanxiang W, et al. Differential expression of p63 isoforms in normal tissues and neoplastic cells. The Journal of pathology. 2002;198:417–27. doi: 10.1002/path.1231. [DOI] [PubMed] [Google Scholar]
- 3.Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–36. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 4.Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–8. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 5.DeCastro AJ, Dunphy KA, Hutchinson J, Balboni AL, Cherukuri P, Jerry DJ, et al. MiR203 mediates subversion of stem cell properties during mammary epithelial differentiation via repression of DeltaNP63alpha and promotes mesenchymal-to-epithelial transition. Cell death & disease. 2013;4:e514. doi: 10.1038/cddis.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keyes WM, Pecoraro M, Aranda V, Vernersson-Lindahl E, Li W, Vogel H, et al. DeltaNp63alpha is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis. Cell stem cell. 2011;8:164–76. doi: 10.1016/j.stem.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–51. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leong CO, Vidnovic N, DeYoung MP, Sgroi D, Ellisen LW. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. The Journal of clinical investigation. 2007;117:1370–80. doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Korkaya H, Wicha MS. Selective targeting of cancer stem cells: a new concept in cancer therapeutics. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2007;21:299–310. doi: 10.2165/00063030-200721050-00002. [DOI] [PubMed] [Google Scholar]
- 12.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer research. 2006;66:1883–90. doi: 10.1158/0008-5472.CAN-05-3153. discussion 95-6. [DOI] [PubMed] [Google Scholar]
- 13.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Seminars in cancer biology. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherukuri P, DeCastro AJ, Balboni AL, Downey SL, Liu JY, Hutchinson JA, et al. Phosphorylation of DeltaNp63alpha via a novel TGFbeta/ALK5 signaling mechanism mediates the anti-clonogenic effects of TGFbeta. PloS one. 2012;7:e50066. doi: 10.1371/journal.pone.0050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Lu H, Yan B, Romano RA, Bian Y, Friedman J, et al. DeltaNp63 versatilely regulates a Broad NF-kappaB gene program and promotes squamous epithelial proliferation, migration, and inflammation. Cancer research. 2011;71:3688–700. doi: 10.1158/0008-5472.CAN-10-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Y, Luo JL, Karin M. IkappaB kinase alpha kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15852–7. doi: 10.1073/pnas.0706728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast cancer research and treatment. 2008;111:419–27. doi: 10.1007/s10549-007-9798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto M, Taguchi Y, Ito-Kureha T, Semba K, Yamaguchi N, Inoue J. NF-kappaB non-cell-autonomously regulates cancer stem cell populations in the basal-like breast cancer subtype. Nature communications. 2013;4:2299. doi: 10.1038/ncomms3299. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro-Silva A, Ramalho LN, Garcia SB, Brandao DF, Chahud F, Zucoloto S. p63 correlates with both BRCA1 and cytokeratin 5 in invasive breast carcinomas: further evidence for the pathogenesis of the basal phenotype of breast cancer. Histopathology. 2005;47:458–66. doi: 10.1111/j.1365-2559.2005.02249.x. [DOI] [PubMed] [Google Scholar]
- 20.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korkaya H, Liu S, Wicha MS. Regulation of cancer stem cells by cytokine networks: attacking cancer's inflammatory roots. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:6125–9. doi: 10.1158/1078-0432.CCR-10-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer research. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. The Journal of clinical investigation. 2011;121:3804–9. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annual review of pharmacology and toxicology. 2008;48:171–97. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 25.Cojoc M, Peitzsch C, Trautmann F, Polishchuk L, Telegeev GD, Dubrovska A. Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. OncoTargets and therapy. 2013;6:1347–61. doi: 10.2147/OTT.S36109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:2927–31. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 27.Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:2074–80. doi: 10.1158/1078-0432.CCR-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maksym RB, Tarnowski M, Grymula K, Tarnowska J, Wysoczynski M, Liu R, et al. The role of stromal-derived factor-1--CXCR7 axis in development and cancer. European journal of pharmacology. 2009;625:31–40. doi: 10.1016/j.ejphar.2009.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. The Journal of experimental medicine. 1997;185:111–20. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 31.Gatti M, Pattarozzi A, Bajetto A, Wurth R, Daga A, Fiaschi P, et al. Inhibition of CXCL12/CXCR4 autocrine/paracrine loop reduces viability of human glioblastoma stem-like cells affecting self-renewal activity. Toxicology. 2013;314:209–20. doi: 10.1016/j.tox.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Jung MJ, Rho JK, Kim YM, Jung JE, Jin YB, Ko YG, et al. Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene. 2013;32:209–21. doi: 10.1038/onc.2012.37. [DOI] [PubMed] [Google Scholar]
- 33.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 34.Deyoung MP, Ellisen LW. p63 and p73 in human cancer: defining the network. Oncogene. 2007;26:5169–83. doi: 10.1038/sj.onc.1210337. [DOI] [PubMed] [Google Scholar]
- 35.Zlotnik A. New insights on the role of CXCR4 in cancer metastasis. The Journal of pathology. 2008;215:211–3. doi: 10.1002/path.2350. [DOI] [PubMed] [Google Scholar]
- 36.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F, Tiede B, Massague J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell research. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 38.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature reviews Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 39.Lindsay J, McDade SS, Pickard A, McCloskey KD, McCance DJ. Role of DeltaNp63gamma in epithelial to mesenchymal transition. The Journal of biological chemistry. 2011;286:3915–24. doi: 10.1074/jbc.M110.162511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higashikawa K, Yoneda S, Tobiume K, Taki M, Shigeishi H, Kamata N. Snail-induced down-regulation of DeltaNp63alpha acquires invasive phenotype of human squamous cell carcinoma. Cancer research. 2007;67:9207–13. doi: 10.1158/0008-5472.CAN-07-0932. [DOI] [PubMed] [Google Scholar]
- 41.Tucci P, Agostini M, Grespi F, Markert EK, Terrinoni A, Vousden KH, et al. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15312–7. doi: 10.1073/pnas.1110977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369:1742–57. doi: 10.1016/S0140-6736(07)60781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, et al. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. The Journal of biological chemistry. 2003;278:21631–8. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 44.Jordan CT, Guzman ML, Noble M. Cancer stem cells. The New England journal of medicine. 2006;355:1253–61. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XH, Jin X, Malladi S, Zou Y, Wen YH, Brogi E, et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell. 2013;154:1060–73. doi: 10.1016/j.cell.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sen T, Sen N, Brait M, Begum S, Chatterjee A, Hoque MO, et al. DeltaNp63alpha confers tumor cell resistance to cisplatin through the AKT1 transcriptional regulation. Cancer research. 2011;71:1167–76. doi: 10.1158/0008-5472.CAN-10-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richert MM, Vaidya KS, Mills CN, Wong D, Korz W, Hurst DR, et al. Inhibition of CXCR4 by CTCE-9908 inhibits breast cancer metastasis to lung and bone. Oncology reports. 2009;21:761–7. [PubMed] [Google Scholar]
- 48.Huang EH, Singh B, Cristofanilli M, Gelovani J, Wei C, Vincent L, et al. A CXCR4 antagonist CTCE-9908 inhibits primary tumor growth and metastasis of breast cancer. The Journal of surgical research. 2009;155:231–6. doi: 10.1016/j.jss.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 49.Carroll DK, Carroll JS, Leong CO, Cheng F, Brown M, Mills AA, et al. p63 regulates an adhesion programme and cell survival in epithelial cells. Nature cell biology. 2006;8:551–61. doi: 10.1038/ncb1420. [DOI] [PubMed] [Google Scholar]
- 50.Wan X, Kim SY, Guenther LM, Mendoza A, Briggs J, Yeung C, et al. Beta4 integrin promotes osteosarcoma metastasis and interacts with ezrin. Oncogene. 2009;28:3401–11. doi: 10.1038/onc.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.