Abstract
Inflammation-related changes in the concentrations of kynurenine-pathway metabolites occur in depression secondary to medical conditions but have not been well characterized in primary bipolar disorder (BD), with contradictory results potentially attributable to the presence or absence of psychosis and/or medication effects. In contrast, reductions in hippocampal and amygdalar volume that theoretically reflect dendritic atrophy occurring in the context of a neurotoxic process are commonly reported in unmedicated BD patients. Here we tested whether the concentrations of putatively neuroprotective (kynurenic acid, KynA) and neurotoxic (3-hydroxy-kynurenine, 3HK and quinolinic acid, QA) kynurenine-pathway metabolites were altered in primary BD and whether these metabolites were associated with hippocampal and amygdalar volume. Twenty-five moderately-to-severely depressed unmedicated subjects and 38 moderately-to-severely depressed medicated subjects who met DSM-IV-TR criteria for BD, as well as 48 healthy controls (HCs) completed a structural MRI scan and provided a blood sample for kynurenine metabolite analysis, performed using high performance liquid chromatography with tandem mass spectrometry. Gray matter volumes were measured with the automated segmentation software, FreeSurfer. A putative neuroprotective index, KynA/QA, was significantly lower in the BD subjects relative to the HCs, a finding that was unrelated to current treatment with medication or a prior history of psychosis. Further, another putative neuroprotective index, KynA/3HK was positively associated with hippocampal volume in the BD group after controlling for age, sex, body mass index (BMI), and intracranial volume (ICV). Kyn/3HK was significantly associated with total amygdalar volume in the BD group, but after controlling for age, sex, BMI, but not ICV, this association was reduced to a trend. In addition, Kyn/3HK was positively associated with amygdalar volume in the HCs although the association was no longer significant after accounting for the effects of age, sex, and BMI. The results raise the possibility that BD-associated abnormalities in kynurenine metabolism may impact the structure of the hippocampus and amygdala, highlighting a pathway through which inflammation may exert neuropathological effects in the context of depression.
Keywords: Bipolar disorder, inflammation, kynurenine, quinolinic acid, magnetic resonance imaging, hippocampus
Introduction
The phenomenological overlap between the behavioral changes observed in humans and animals suffering from infection (e.g. anhedonia, fatigue, sleep disturbances, and the desire for social isolation, a.k.a. sickness behavior) and certain symptoms of idiopathic major depression led to the hypothesis that the peripheral immune system communicates with the central nervous system (CNS) to modulate emotions and behavior, and that immunological dysregulation may be an important component of primary mood disorders (Dantzer et al., 2008). Immune mediators may affect neuronal functions directly or indirectly via alterations in the balance between potentially neuroprotective and neurotoxic kynurenine metabolites as a result of the activation of the kynurenine metabolism pathway (figure S1) (Dantzer et al., 2011; Miller et al., 2009; Schwarcz et al., 2012).
One of these kynurenine metabolites, quinolinic acid (QA), is a known neurotoxin that is produced in the brain by microglia and infiltrating macrophages QA activates N-methyl-D-aspartate (NMDA) receptors and additionally exerts neurotoxic effects via lipid peroxidation, and disruption of the blood-brain barrier (Schwarcz et al., 2012; Stone et al., 2012). Further, QA has the ability to initiate an inflammatory response or augment existing disease-associated inflammation by enhancing the production of pro-inflammatory proteins (Stone et al., 2013). Elevated concentrations of QA have been reported in both the serum and the cerebrospinal fluid (CSF) of patients with neurodegenerative and inflammatory disorders such as Alzheimer’s disease and (AD) and systemic lupus erythematosus patients with neuropsychiatric symptoms (Ting et al., 2009; Vogelgesang et al., 1996).
Another kynurenine metabolite produced by macrophages and microglia that may have neurotoxic properties is 3HK, a free radical generator. In vitro work has demonstrated that the administration of pharmacological doses of 3-hydroxy-kynurenine (3HK) may kill hippocampal neurons (Okuda et al., 1996) and cortical neurons (Chiarugi et al., 2001), perhaps explaining why levels of 3HK have been reported to be elevated in the serum of AD patients (Schwarz et al., 2013) and in the brains of Parkinson’s disease (PD) (Ogawa et al., 1992) patients postmortem.
In contrast, kynurenic acid (KynA) is a pleiotropic astrocyte-derived metabolite that at least in vitro, acts as an endogenous competitive antagonist of all ionotrophic excitatory amino acid receptors including the NMDA receptor (Birch et al., 1988). In addition, KynA is an α7 nicotinic receptor antagonist, an orphan G-protein-coupled receptor (GPR35) agonist, an aryl hydrocarbon receptor (AHR) agonist, and an enhancer of nerve growth factor (NGF) expression, potentially regulating the inflammatory response together with glutamatergic, cholinergic, and dopaminergic neurotransmission (Stone et al., 2013). Inducing an elevation in peripheral KynA by blocking the enzyme, kynurenine 3-monooxygenase (KMO), in mouse models of AD and Huntington’s disease (HD) suppresses microglial activation, limits neuronal and synaptic loss, improves memory, alleviates anxiety, and extends lifespan possibly by decreasing QA concentrations and/or glutamate-induced excitotoxicity (Zwilling et al., 2011). Similarly, pharmacological inhibition of KMO is neuroprotective in animal models of cerebral ischemia (Moroni et al., 2003), a genetic reduction in KynA production increases vulnerability to excitotoxic insults (Sapko et al., 2006), while in humans, reduced concentrations of KynA have been reported in the CSF of MS patients (Rejdak et al., 2002). These data have led to a heuristic model that regards 3HK and QA as potentially neurotoxic and KynA as potentially neuroprotective (Amaral et al., 2013; Stone et al., 2012).
Because the various kynurenine metabolites impact the brain differently, their concentrations may be better expressed as ratios of neuroprotective to neurotoxic metabolites or neurotoxic to neuroprotective metabolites rather than as absolute values. Our previous work provided some support for this model in the context of primary mood disorders. In a morphometric MRI study, we reported a positive correlation between the KynA/QA ratio, a putative neuroprotective index (Johansson et al., 2013; Kocki et al., 2012), and total gray matter (GM) volumes of the hippocampus and amygdala in unmedicated patients with MDD but not in healthy controls (Savitz et al., 2014).
Histopathological studies of rodents and humans (Cobb et al., 2013; Duric et al., 2013) indicate that reductions in GM volume of brain regions such as the hippocampus and amygdala, which are widely reported in MRI studies of mood disorders (Phillips and Swartz, 2014; Savitz and Drevets, 2009), are reflective of dendritic atrophy (Cobb et al., 2013; McEwen, 1999; Stockmeier et al., 2004) occurring in the context of excitotoxicity, oxidative stress, decreased neurogenesis, and impaired neurotrophic function (Petrik et al., 2012).
Because of the reductions in the GM volume of cortico-limbic structures in BD (Phillips and Swartz, 2014; Savitz and Drevets, 2009), coupled with the reports of increased microglia function and/or impaired astrocytic function in the prefrontal cortices, hippocampi and/or amygdalae of depressed populations at postmortem (Haarman et al., 2014; Steiner et al., 2011; Webster et al., 2005) one might expect to find a decrease in putative neuroprotective kynurenine metabolites (i.e. KynA) and/or an increase in putative neurotoxic metabolites (i.e. 3HK and QA) in BD. Unfortunately, very few studies have measured KynA, 3HK, and QA in the same samples.
With respect to the putative neurotoxic metabolites, (Steiner et al., 2011) reported an increased density of QA-positive microglial cells in the subgenual anterior cingulate cortex (sgACC) and the mid-anterior cingulate cortex in postmortem samples of subjects with MDD and BD versus control samples. Regarding KynA, (Myint et al., 2007) reported a significant decrease in tryptophan and KynA in the BD group versus the controls. In contrast, KynA concentrations in the CSF of medicated, euthymic males with type I BD (BD1) and type 2 BD (BD 2) were reported to be elevated versus healthy controls (Olsson et al., 2010), and the elevation in KynA was associated with a history of psychotic episodes (Olsson et al., 2012). Consistent with these data, (Lavebratt et al., 2014) found a reduction in the postmortem expression of the enzyme, KMO in the dorsolateral prefrontal cortices of medicated BD1 patients with a history of psychosis, putatively altering the metabolism of kynurenine such that an increased concentration of KynA was present in these patients.
In one ex vivo study of skin fibroblasts from predominantly depressed and lithium-treated BD subjects and neuroleptic-treated schizophrenic subjects that did measure both putative neurotoxic and neuroprotective metabolites, (Johansson et al., 2013) reported basal elevations in both 3HK and KynA in the patient groups versus the healthy control group. Exposure to various pro-inflammatory cytokines further increased the levels of 3HK, but not KynA, in the schizophrenia and BD groups, raising the possibility that 3HK is disproportionately elevated in schizophrenia and BD (Johansson et al., 2013). These data are consistent with our recent report of a nominal decrease in the ratio of KynA/QA in depressed, unmedicated subjects with MDD versus healthy controls; this despite the absence of significant group differences in the concentrations of KynA, alone or QA, alone (Savitz et al., 2014).
Based on the literature discussed above, we hypothesized that the putative neuroprotective indices KynA/3HK and KynA/QA would be decreased in patients with BD and would positively correlate with the GM volume of the hippocampus and the amygdala in patients with BD. Nevertheless, as discussed above, the literature is complicated by the fact that elevations in KynA have been reported in BD patients with a history of psychosis. Thus conceivably there may be a different relationship between GM volumes and kynurenine-pathway metabolites in BD patients with and without a history of psychosis. Further, interpretation of the extant literature is complicated by the potentially confounding influence of the medications used to treat BD since certain of these medications have themselves been shown to affect GM volume and inflammation (Nassar and Azab, 2014; Savitz and Drevets, 2009). Here we extend the extant literature by measuring a range of kynurenine-pathway metabolites in serum samples from both unmedicated and medicated BD patients, evaluating the effect of a history of psychosis on kynurenine metabolite concentrations, and testing for correlations between hippocampal and amygdalar volumes and the peripheral concentrations of putatively neuroactive kynurenine metabolites.
Methods
Subjects
Subjects provided written informed consent after receiving a full explanation of the study procedures and risks, as approved by the local IRB.
All BD subjects (n=63) and healthy controls (n=48) were interviewed with the Structured Clinical Interview for the DSM-IV-TR. In addition, unstructured psychiatric interviews with board-certified psychiatrists were obtained on all participants with BD.
The majority of the BD subjects (both medicated and unmedicated) had a Hamilton Depression Rating Scale (HAM-D, 24-item) score in the moderately-to-severely depressed range (table 1). Anhedonia symptoms were measured with the Snaith-Hamilton Pleasure Scale (SHAPS) and manic symptoms with the Young Mania Rating Scale (YMRS). The unmedicated BD group did not receive any psychotropic medications for at least 3 weeks (8 for fluoxetine) prior to the blood-draw and MRI scanning (mean length of time off medication: 39.6±30.0 months; 6 participants treatment-naïve). The medicated BD subjects were receiving treatment with the following medications at the time of the study: adderall, n=2, aripiprazole, n=8; benzodiazepines, n=13; bupropion, n=5; carbazepine, n=1; divalproex sodium, n=4; gabapentin, n=3; lamotrigine, n=18; lithium, n=2; methylphenidate, n=1; olanzapine, n=2; quetiapine, n=2; risperidone, n=1; SSRI/SNRI, n=16; trazodone, n=4; zolpidem, n=3. Exclusion criteria were as follows: serious suicidal ideation or behavior; medical conditions or concomitant medications likely to influence CNS or immunological function including cardiovascular, respiratory, endocrine and neurological diseases; a history of drug or alcohol abuse within 6 months or a history of drug or alcohol dependence within 1 year (DSM-IV-TR criteria), and general MRI exclusion criteria such as magnetic implants or claustrophobia.
Table 1.
Demographic and Clinical Information for the BD and the HC Groups (mean ± SE)
| Unmed BD |
Med BD | All BD | All BD P+ | All BD P− | HC | Comparison | |
|---|---|---|---|---|---|---|---|
| N | 25 | 38 | 63 | 18 | 45 | 48 | - |
| BD1/BD2 | 9/16 | 24/14 | 33/30 | 14/4 | 17/28 | - | BD1>BD2 in med BD vs unmed BD and in BD P+ vs BD P− |
| Sex (% F) | 84 | 79 | 81 | 89 | 78 | 60 | F>M in All BD vs HC |
| Age | 36.2 ± 2.1 | 40.5 ± 1.9 | 38.8 ± 1.4 | 40.7 ± 2.8 | 38.0 ± 1.7 | 32.6 ± 1.5 | All BD>HC |
| BMI | 28.5 ± 1.1 | 29.1 ± 1.0 | 28.9 ± 0.7 | 28.1 ± 1.6 | 29.2 ± 0.8 | 28.0 ± 0.8 | NS |
| HAM-D (24-item) | 22.5 ± 1.6 | 24.2 ± 1.9 | 23.5 ± 1.3 | 23.8 ± 3.0 | 23.4 ± 1.4 | 0.9 ± 0.3 | All BD>HC |
| SHAPS | 30.9 ± 1.5 | 27.3 ± 1.3 | 28.7 ± 1.0 | 29.7 ± 1.8 | 28.3 ± 1.2 | 18.6 ± 0.8 | All BD>HC |
| YMRS | 5.6 ± 1.1 | 7.7 ± 1.3 | 6.9 ± 0.9 | 4.7 ± 1.6 | 7.7 ± 1.0 | 0.1 ± 0.1 | All BD>HC |
| ICV (mL) | 1374 ± 45 | 1387 ± 29 | 1382 ± 25 | 1301 ± 44 | 1414 ± 29 | 1375 ± 31 | BD P+ < BD P− |
| Total Hippocampus (mL) | 7.80 ± 0.14 | 7.75 ± 0.14 | 7.77 ± 0.14 | 7.52 ± 0.21 | 7.87 ± 0.12 | 8.15 ± 0.10 | All BD<HC |
| Total Amygdala (mL) | 2.83 ± 0.07 | 2.90 ± 0.07 | 2.88 ± 0.05 | 2.77 ± 0.09 | 2.92 ± 0.06 | 3.10 ± 0.07 | All BD<HC |
| hs_CRP (µg/mL) | 5.8 ± 2.3 | 4.4 ± 1.3 | 4.9 ± 1.1 | 5.2 ± 2.2 | 4.7 ± 1.3 | 3.1 ± 0.8 | NS |
| TRP (µM) | 55.6 ± 2.0 | 59.1 ± 1.6 | 57.7 ± 1.3 | 56.0 ± 2.3 | 58.4 ± 1.5 | 64.4 ± 2.9 | All BD<HCa |
| KYN (nM) | 1.77 ± 0.10 | 1.98 ± 0.09 | 1.90 ± 0.07 | 1.93 ± 0.10 | 1.89 ± 0.08 | 1.99 ± 0.07 | NS |
| KynA (nM) | 34.0 ± 2.6 | 39.1 ± 2.9 | 37.1 ± 2.0 | 36.9 ± 3.0 | 37.1 ± 2.6 | 40.3 ± 2.4 | NS |
| 3HK (nM) | 30.9 ± 2.3 | 31.6 ± 1.7 | 31.3 ± 1.4 | 33.1 ± 3.1 | 30.6 ± 1.4 | 34.3 ± 2.4 | NS |
| QA (nM) | 314 ± 22 | 327 ± 17 | 322 ± 13 | 335 ± 25 | 317 ± 16 | 311 ± 14 | NS |
| KYN/TRP | 0.03 ± 0.002 | 0.03 ± 0.002 | 0.03 ± 0.001 | 0.03 ± 0.002 | 0.03 ± 0.001 | 0.03 ± 0.001 | NS |
| KynA/3HK | 1.15 ± 0.08 | 1.24 ± 0.06 | 1.20 ± 0.048 | 1.19 ± 0.10 | 1.21 ± 0.05 | 1.26 ± 0.06 | NS |
| KynA/QA | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.14 ± 0.01 | All BD<HCb |
Note: With the exception of a chi-squared test for testing sex differences as well as differences in the ratio of BD1 versus BD2, group comparisons were made using independent sample t-tests. MRI data were available for 56 individuals with BD and 46 healthy controls. CRP data were available for 50 individuals with BD and 35 healthy controls.
Abbreviations: a = no longer significant after controlling for age and sex; b = significant after controlling for age and sex; Unmed BD=Unmedicated Bipolar Disorder; Med BD=Medicated Bipolar Disorder; All BD P+ = Unmedicated and medicated bipolar subjects with a positive history of psychosis; All BD P− = Unmedicated and medicated bipolar subjects without a history of psychosis; HC=Healthy Control; NS=nonsignificant; BMI=Body Mass Index; HAM-D=Hamilton Depression Rating Scale; SHAPS=Snaith-Hamilton Pleasure Scale; YMRS=Young Mania Rating Scale; ICV=intracranial volume; hs-CRP = high-sensitivity C-reactive protein; TRP=Tryptophan; KYN=Kynurenine; 3HK=3-hydroxykynurenine; KynA=Kynurenic Acid; QA=Quinolinic Acid.
The healthy control individuals met the same exclusion criteria except that they had no personal or family (first-degree relatives) history of psychiatric illness assessed using the Structured Clinical Interview for the DSM-IV-TR and the Family Interview for Genetic Studies (FIGS).
MRI
The scans were acquired on a 3 Tesla GE Discovery MR750 MRI scanner (GE Medical Systems) with a receive-only 32 elements surface coil brain array (Nova Medical) using a magnetization-prepared, rapid gradient echo (MP-RAGE) pulse sequence with sensitivity encoding (SENSE) optimized for tissue contrast resolution: (TR=5msec, TE=2.01 msec, FOV 240×192 mm; voxel size=0.94 × 0.94 × 0.90 mm; prep=725 msec, delay=1400 msec, flip=8° SENSE acceleration R=2). The automated segmentation program, FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) was used to obtain unbiased GM volumes of the hippocampus, amygdala and whole brain. The T1-weighted MPRAGE images were processed using the default analysis settings of FreeSurfer. Representative examples of the hippocampal and amygdala masks from a single subject are shown in supplementary figures, S2 and S3. Visual inspection of the FreeSurfer-based segmentations was performed to confirm accurate segmentation of the hippocampus and the amygdala. MRI data were either not available or the FreeSurfer segmentations were judged to be inaccurate based on a manual check for 7 individuals with BD and 2 healthy controls leaving a total of 56 BD subjects and 46 healthy controls with valid MRI data. An example of an error in the segmentation of the hippocampus is provided in figure S4.
Kynurenine Pathway Metabolites
A blood sample was obtained from each subject within three days of completing the MRI scan. Subjects fasted overnight and the blood sample was drawn by venipuncture between 8am and 11am. Serum samples were collected with BD Vacutainer serum tubes, processed according to the standard BD Vacutainer protocol, and stored at −80° C.
Concentrations of tryptophan (TRP), kynurenine (KYN), kynurenic acid (KynA), 3-hydroxykynurenine (3HK), and quinolinic acid (QA) were measured blind to diagnosis by Brains Online, LLC (www.brainsonline.org/home). The serum metabolite concentrations were determined by high performance liquid chromatography (HPLC) with tandem mass spectrometry (MS/MS) detection using their standard protocols (Appendix A). High-sensitivity C-reactive protein (hs-CRP) was measured in a clinical laboratory using the Kamiya Biomedical K-Assay (Appendix A).
Statistical Analysis
All the statistical tests conducted were two-tailed. Deviations from normality were tested using the Kolmogorov-Smirnov test and non-normally distributed variables were log normalized. Differences in kynurenine pathway metabolites between the BD subjects (combined medicated and unmedicated samples) and the healthy controls were evaluated with ANOVA. Since age and sex differed between the BD subjects and healthy controls (table 1), age and sex were used as covariates. For the primary variables of interest (KynA/3HK and KynA/QA) a statistical threshold of p<0.05 was used for determining statistical significance whereas for the individual metabolites and the kynurenine to tryptophan ratio (KYN/TRP) we used a Bonferroni correction for multiple testing (p<0.008). Similarly, differences in total hippocampal volume and amygdalar volume between the BD subjects and healthy controls were evaluated using ANOVA with age, sex and intracranial volume (ICV) as covariates. We used a statistical threshold of p<0.05 since there is extant evidence that hippocampal and amygdalar volume is decreased in unmedicated patients with BD.
Linear regression was used in order to evaluate the effects of medication status (yes/no), a history of psychosis (yes/no; BD P+/BD P-), and a history of suicide attempts (yes/no) on the concentration of kynurenine metabolites and GM volume within the BD group. Statistical thresholds were as above.
Pearson’s (r) or Spearman’s (rs) tests were used to measure the correlations between the kynurenine metabolites and the GM volumes as well as between the kynurenine metabolites and the severity of depression and the degree of anhedonia within the BD group. For those kynurenine metabolites that were statistically correlated with hippocampal and/or amygdalar volume and/or the clinical rating scales, we subsequently conducted a linear regression analysis to control for the potential confounds, age, sex, BMI, and ICV (model 3). However, in order to reduce the possibility of Type II error (false negatives) we also show the unadjusted regression models (model 1) and the regression models controlling for age, sex, BMI, but not ICV (model 2). A statistical threshold of p<0.05 was used for the two primary variables of interest (KynA/3HK and KynA/QA) whereas for the individual metabolites and KYN/TRP a statistical threshold of p<0.008 was used to determine significance.
Results
Deviations from normality
With the exception of kynurenine (p=0.141) and KynA/3HK (p=0.200) the statistical distribution of all other kynurenine pathway metabolites violated the assumption of normality and thus were log10 normalized prior to the use of parametric statistics.
The relationship between inflammation and the kynurenine metabolites
A general marker of inflammation, high-sensitivity CRP (hs-CRP), was significantly correlated with QA (rs=0.24, p=0.025), KynA/3HK (r=−0.28, p=0.011), and KynA/QA (rs=−0.28, p=0.008) in the total sample (table A8).
Diagnostic group differences in kynurenine pathway metabolites
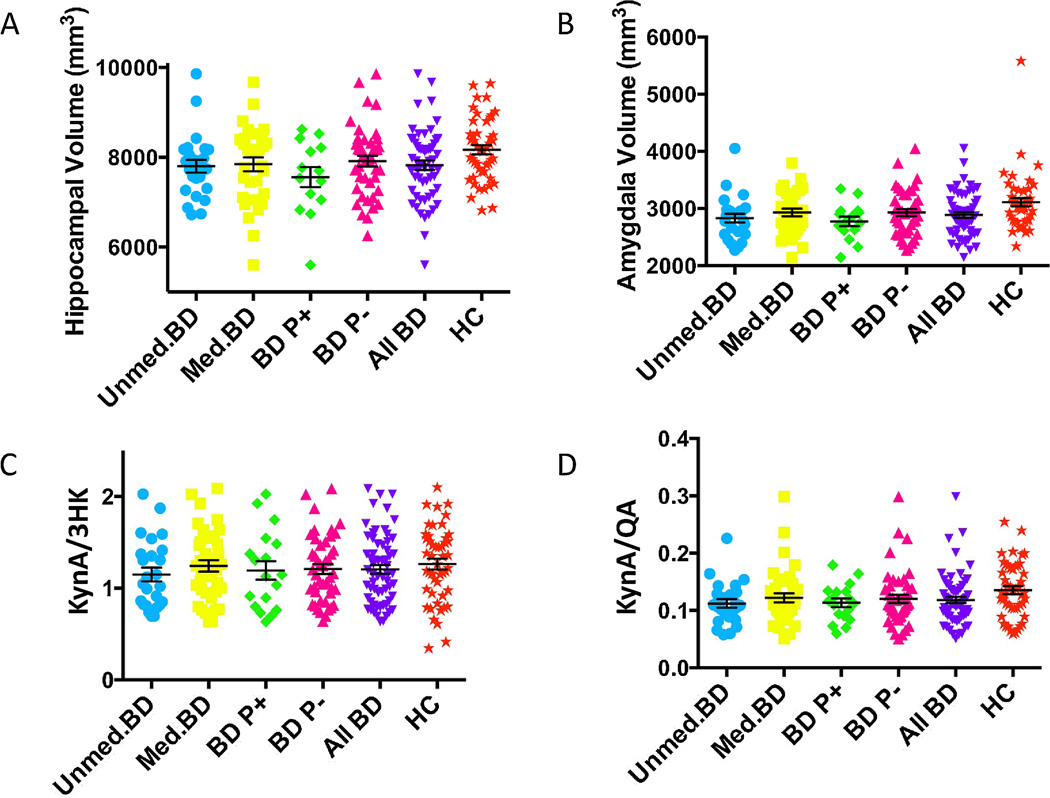
After controlling for age and sex no statistically significant differences in the concentration of CRP was found between the BD subjects (“All BD” in table 1) and the healthy controls. Similarly, no statistically significant differences in the concentration of any of the individual kynurenine metabolites were found between the BD and healthy control groups. However, the ratio KynA/QA (but not KynA/3HK) was significantly lower in the BD subjects compared with the healthy controls (F3, 107=4.2, p=0.043, figure 1D) after controlling for age and sex. There were no significant effects of medication status, history of psychosis, or past history of suicide attempts on the concentrations of CRP or the kynurenine metabolic pathway metabolites (all p-values>0.1).
Figure 1.
Scatterplots of group differences in (A) hippocampal volume, (B) amygdalar volume, (C) KynA/3HK, and (D) KynA/QA across the BD and healthy control groups. The unmedicated BD group is shown in blue circles, the medicated BD group is displayed with yellow squares, the BD subjects with a history of psychosis (BD P+) are shown in green diamonds, the BD subjects without a history of psychosis (BD P−) are shown in pink triangles, the combined unmedicated and medicated BD group is shown in inverted purples triangles, and the healthy controls are illustrated with orange stars. The mean and standard error of the mean is displayed for each group in black. The healthy control group had significantly larger hippocampal and amygdalar volumes than all five of the BD groups, but the results were no longer significant after regressing out the effects of age, sex, and intracranial volume. The unmedicated BD group had reduced levels of KynA/3HK compared with the healthy controls but the results were no longer significant after regressing out the effects of age and sex. The unmedicated BD (F=4.4, p=0.039) and the combined BD (F=4.2, p=0.043) group had reduced levels of KynA/QA compared with the healthy control group after controlling for age and sex.
Diagnostic group differences in GM volume of the hippocampus and amygdala
The BD subjects had smaller hippocampal volumes (t=2.2, d.f.=100, p=0.034) and amygdalar volumes (t=2.4, d.f. =100, p=0.017) than healthy controls (table 1, figures 1A and B). However, after controlling for age, sex, and ICV, the differences in hippocampal volume (F4, 97=1.9, p=0.168) and amygdalar volume (F4, 97=2.1, p=0.154) between the BD subjects and the healthy controls were no longer significant. Further, a history of psychotic symptoms, suicide behavior, and medication status did not significantly impact hippocampal or amygdalar volume (all p-values>0.2).
Associations between the kynurenine metabolites and hippocampal and amygdalar volumes in the combined BD group
Table 2 shows that KynA/3HK was positively correlated with both hippocampal and amygdalar volume whereas KYN was positively correlated with amygdalar volume. We therefore conducted additional regression analyses to further explore these correlations.
Table 2.
Pearson's (r) and Spearman's (rs) correlation coefficients between the kynurenine pathway metabolites and brain volumes within the BD (A), and HC (B) groups.
| (A). BD | ICV | Total Hipp | Total Amyg |
| TRP (rs) | 0.27 | 0.20 | 0.24 |
| KYN (r) | 0.15 | 0.23 | 0.34* |
| KYN/TRP (rs) | 0.06 | 0.04 | 0.11 |
| KA (rs) | 0.10 | 0.24 | 0.21 |
| 3HK (rs) | −0.03 | −0.08 | 0.002 |
| QA (rs) | −0.01 | 0.04 | 0.12 |
| KynA/3HK (r) | 0.11 | 0.35** | 0.32* |
| KynA/QA (rs) | 0.03 | 0.25 | 0.12 |
| CRP (rs) | 0.07 | −0.05 | 0.06 |
| (B). HC | ICV | Total Hipp | Total Amyg |
| TRP (rs) | 0.19 | 0.20 | 0.28 |
| KYN (r) | −0.25 | 0.09 | 0.05 |
| KYN/TRP (rs) | −0.31* | −0.10 | −0.11 |
| KynA (rs) | −0.08 | −0.03 | 0.13 |
| 3HK (rs) | −0.24 | −0.25 | −0.30* |
| QA (rs) | −0.27 | −0.12 | −0.05 |
| KynA/3HK (r) | 0.10 | 0.11 | 0.34* |
| KynA/QA (rs) | 0.28 | 0.13 | 0.23 |
| CRP (rs) | −0.50** | −0.11 | −0.34* |
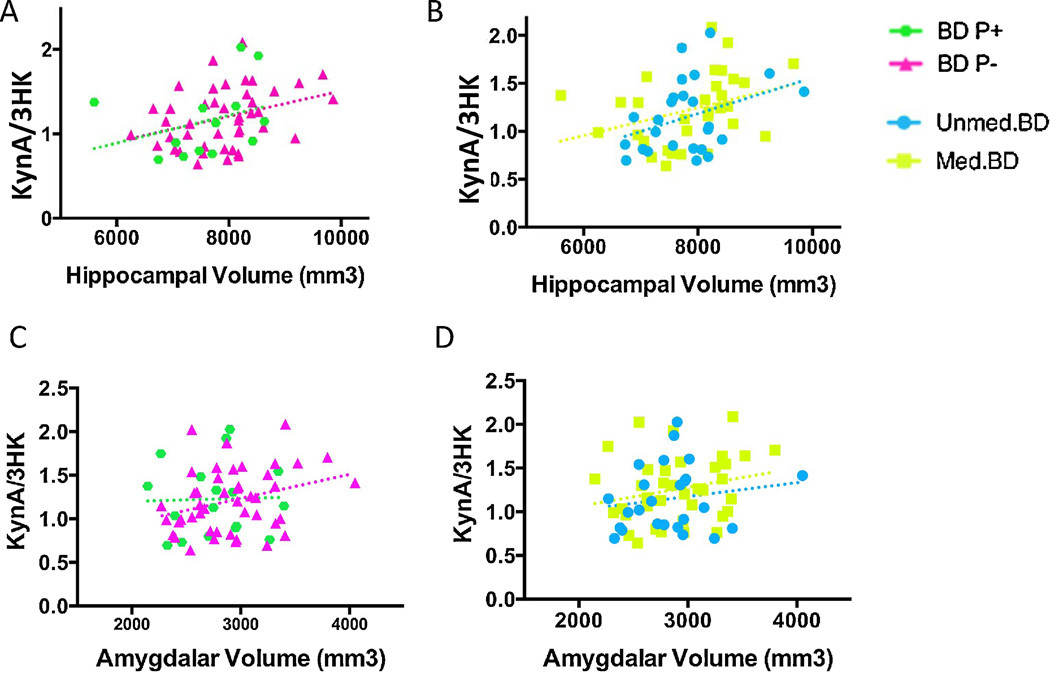
In the unadjusted regression models, KynA/3HK was positively associated with both hippocampal volume (β-weight = 0.35, t=2.8, p=0.008) and amygdalar volume (β-weight = 0.32, t=2.5, p=0.015) in the combined BD sample (table 3, figure 2). After controlling for age, sex, and BMI (model 2), the association between KynA/3HK and hippocampal volume remained statistically significant (β-weight = 0.30, t=2.4, p=0.022) with the association between KynA/3HK and amygdalar volume trending significant (β-weight = 0.25, t=2.0, p=0.051). Similarly, after controlling for age, sex, BMI, and ICV (model 3), KynA/3HK remained significantly associated with hippocampal volume (β-weight = 0.25, t=2.0, p=0.048), but not amygdalar volume (β-weight = 0.18, t=1.6, p=0.124).
Table 3.
Regression models showing the relationship between the kynurenine pathway metabolites and brain volumes within the BD (A), and HC (B) groups.
| Association | Model 1 (unadjusted) | Model 2 (sex, age, and BMI) |
Model 3 (sex, age, BMI, ICV) |
|---|---|---|---|
| A. BD Group | β-weight, t-score, p-value | β-weight, t-score, p-value | β-weight, t-score, p-value |
| KYN & Amyg | 0.34, 2.7, 0.010 | 0.19, 1.2, 0.254 | 0.20, 1.4, 0.174 |
| KynA/3HK & Hipp | 0.35, 2.8, 0.008** | 0.30, 2.4, 0.022* | 0.25, 2.0, 0.048* |
| KynA/3HK & Amyg | 0.32, 2.5, 0.015* | 0.25, 2.0, 0.051 | 0.18, 1.6, 0.124 |
| B. HC Group | β-weight, t-score, p-value | β-weight, t-score, p-value | β-weight, t-score, p-value |
| KYN/TRP & ICV | 0.35, 2.4, 0.022 | 0.34, 1.7, 0.088 | NA |
| CRP & ICV | 0.44, 2.7, 0.011 | 0.30, 1.6, 0.131 | NA |
| 3HK & Amyg | 0.15, 1.0, 0.317 | 0.04, 0.25, 0.805 | 0.03, 0.21, 0.836 |
| KynA/3HK & Amyg | 0.34, 2.4, 0.023* | 0.20, 1.3, 0.185 | 0.21, 1.4, 0.161 |
| CRP & Amyg | 0.29, 1.7, 0.093 | 0.26, 1.4, 0.173 | 0.18, 0.9, 0.387 |
p<0.05, corrected,
p<0.01, corrected
Figure 2.
Scatterplots showing the correlation between KynA/3HK (y-axis) and GM volume of the hippocampus (x-axis) in the the BD subjects with and without a history of psychosis (A) and the unmedicated and medicated BD groups (B). Scatterplots showing the correlation between KynA/3HK (y-axis) and GM volume of the amygdala (x-axis) in the the BD subjects with and without a history of psychosis (C) and the unmedicated and medicated BD groups (D). The BD patients with a past history of psychosis (BD P+) are displayed in green diamonds, and the BD subjects without a history of psychosis are displayed in pink triangles. The unmedicated BD subjects are shown with blue circles. The medicated BD group is represented by yellow squares. After controlling for the effects of age, sex, BMI, and intracranial volume, KynA/3HK was significantly associated with GM volume of the hippocampus in the combined BD group (β-weight = 0.25, t=2.0, p=0.048). After controlling for age, sex, BMI, but not intracranial volume, KynA/3HK was nominally associated with GM volume of the amygdala in the combined BD group (β-weight = 0.25, t=2.0, p=0.051).
KYN was significantly associated with amygdalar volume in the unadjusted regression model (β-weight = 0.34, t=2.7, p=0.010) but not regression models 2 or 3 (table 3). Nevertheless, the positive association between KYN and amygdalar volume in the unadjusted model did not remain significant after correction for multiple testing (significance threshold p<0.008).
Associations between the kynurenine metabolites and hippocampal and amygdalar volumes in the HC group
Table 2 shows the significant correlations between the kynurenine pathway metabolites and GM volumes in the healthy control group. Total amygdalar volumes were positively correlated with KynA/3HK but negatively correlated with 3HK and CRP. In addition, KYN/TRP and CRP were significantly negatively correlated with ICV.
The unadjusted regression analysis (model 1, table 3) showed that KynA/3HK was significantly predictive of amygdala volume (β-weight = 0.34, t=2.4, p=0.023). However, this result was no longer significant for model 2 (β-weight = 0.20, t=1.3, p=0.185) or model 3 (β-weight = 0.21, t=1.4, p=0.161). Regression models 1, 2, and 3 all showed a non-significant association between 3HK and amygdala volume and between CRP and amygdala volume (all p-values > 0.09, table 3). On the other hand, there was a nominally significant association between CRP and ICV in regression model 1 (β-weight = 0.44, t=2.7, p=0.011, statistical threshold of p=0.008) but not regression model 2. Similarly, KYN/TRP was nominally associated with ICV in model 1 (β-weight = 0.35, t=2.4, p=0.022, statistical threshold of p=0.008) but not model 2 (table 3).
Associations between the kynurenine metabolites and the clinical rating scales in the BD group
Within the BD subjects, there were no statistically significant correlations between the SHAPS and the kynurenine metabolites. 3HK was positively correlated with the HAM-D scores (rs=0.27, p=0.043) and the association remained nominally significant after controlling for age, sex, and BMI (β-weight=0.28, t=2.1, p=0.039; statistical threshold p=0.008).
Discussion
Here we found a reduction in the putative neuroprotective index, KynA/QA, but no difference in any of the individual kynurenine metabolites, in the BD group versus the healthy controls. This result confirms our hypothesis that the relative balance in activation of the KynA-pathway versus the QA-pathway is disturbed in BD, and extends our previously published finding of a reduction in KynA/QA in unmedicated patients with MDD (Savitz et al., 2014). We hypothesize that a key abnormality in mood disorders is the ratio of KynA to QA-pathway metabolites because these metabolites affect brain function and structure differently. That is, KynA and QA exert opposite effects on the NMDA receptor. Thus it may be more informative to consider their levels relative to each other than their absolute levels. Consistent with our data/hypothesis, an ex vivo study suggested that 3HK is disproportionately elevated relative to KynA in the skin fibroblasts of BD samples after stimulation of the cells with pro-inflammatory cytokines (Johansson et al., 2013). Further, a postmortem study reported an increased density of QA-positive cells in the sgACC in a depressed sample which included individuals with BD (Steiner et al., 2011), and (Myint et al., 2007) found a decrease in KynA in BD subjects relative to healthy controls.
In contrast to the results of two other studies that reported elevations in KynA in psychotic disorders, including BD (Lavebratt et al., 2014; Olsson et al., 2010), we found no indication of increased KynA in BD subjects with a history of psychosis (table 1). The reason for the discrepancy in results is unclear but could potentially be related to mood state or treatment with lithium. For instance, in the (Olsson et al., 2010) study, the BD subjects were euthymic at the time of testing and most were receiving treatment with lithium. Since KynA is putatively neuroprotective, conceivably levels of KynA will increase as the patients remit, although this hypothesis needs to be tested in longitudinal studies. Secondly, lithium has been demonstrated to possess immune-modulating properties (Nassar and Azab, 2014) and exerts therapeutic effects across a range of CNS disorders (Leeds et al., 2014), raising the possibility that lithium-treated groups of BD patients display different kynurenine metabolite profiles to the medicated BD patients studied here (see further discussion below). Thirdly, we measured kynurenine metabolites in serum rather than in CSF, and it is thus theoretically possible that our data are not reflective of central kynurenine metabolite concentrations. Kynurenine and 3HK are however, able to cross the blood brain barrier into the brain parenchyma where they may be metabolized into KynA and QA by astrocytes and microglial cells, respectively (Schwarcz et al., 2012). This may explain why (Raison et al., 2010) found that INF-α-treated hepatitis C patients showed both plasma and CSF increases in kynurenine and QA that correlated with depressive symptoms (although this was an extreme case of immune-induced changes in kynurenine metabolites and their association with clinical features of depression). Further, (Zwilling et al., 2011) demonstrated using mouse models of AD and HD that peripheral administration of an experimental drug that directly inhibits KMO, elevated brain levels of KynA and decreased glutamate release and excitotoxicity in the brain parenchyma despite the fact that neither the prodrug nor its metabolite were capable of crossing the blood brain barrier. A recent study provides additional evidence that the peripheral immune system plays a critical role in the stress-associated induction of depressive behavior (Hodes et al., In press). Stress susceptible mice were found to produce higher levels of interleukin-6 (IL-6) compared with resilient mice. The authors subsequently generated bone marrow chimeras transplanted with hematopoietic progenitor cells from the stress susceptible mice or IL-6 knockout mice. The stress susceptible chimeras exhibited increased depression-like behavior, whereas the knockouts were resistant to the stress, this despite the fact that the resident microglia population was left intact in the chimeras (Hodes et al., In press).
One unexpected finding was that the medicated BD group did not differ significantly from the unmedicated BD group in terms of kynurenine metabolite concentrations or GM volumes of the hippocampus and amygdala after covarying for age, sex, and ICV. We and others have demonstrated that lithium (and potentially valproic acid) is associated with an increase in GM volume to normative levels in multiple brain regions, including the hippocampus and amygdala (Savitz and Drevets, 2009; Savitz et al., 2010). In our current sample of 38 BD patients, only 2 were treated with lithium and 4 were treated with divalproex, suggesting that atypical antipsychotics, lamotrigine, and SSRIs, the most commonly prescribed medications in our current sample, may not exert the equivalent hypertrophic effects. Interestingly, administration of valproic acid, which we previously demonstrated to be associated with larger amygdala volumes in BD patients (Savitz et al., 2010), reportedly increased the levels of KynA in the rat brain (Maciejak et al., 2013). Similarly, (Kocki et al., 2012) reported that 24–48 hours of exposure to selective serotonin reuptake inhibitors or tricyclic antidepressant medications stimulated the de novo synthesis of KynA and decreased 3HK production in astroglial cultures, thus resulting in an increase in the KynA to 3HK ratio. Another study reported that patients with schizophrenia had lower plasma concentrations of KynA and higher plasma concentrations of 3HK than HCs, an effect that was ameliorated by 6 weeks of treatment with antipsychotic treatment such that a significant increase in the KynA/3HK ratio was observed post-treatment (Myint et al., 2011). One possible explanation for the absence of medication effects in our study is that despite treatment, the medicated patients remained on average moderately-to-severely depressed, raising the possibility that a proportion of the patients were treatment resistant. A number of studies have reported that resistance to treatment with SSRIs/SNRIs in the context of MDD is associated with increased inflammation (Uher et al., 2014) and a SNP in the IDO gene was found to be associated with response to treatment with citalopram in the Sequenced Treatment Alternatives to Relieve Depression study (STAR*D) cohort (Cutler et al., 2012). Nevertheless, since we did not formally assess treatment resistance, our hypothesis remains speculative.
A potentially important finding is the significant positive association between KynA/3HK and hippocampal volume (and nominally significant association with amygdalar volume) in the BD group. Previously we reported a positive association between KynA/QA and hippocampal and amygdalar volume in an independently-typed group of depressed patients with MDD but not in the independently-typed healthy controls. These data are potentially consistent with a heuristic model that regards KynA as neuroprotective and 3HK and QA as neurotoxic at high enough concentrations. Preclinical and in vitro data support the possibility that hippocampal structure may be impacted by neuroactive kynurenine metabolites. For instance, in a rat model of pneumococcal meningitis, acute infection caused 3HK to accumulate in the hippocampus and the concentration of 3HK was positively correlated with the extent of apoptosis in this region (Bellac et al., 2006). Conversely, neurons exposed to extracellular glutamate showed a reduction in dendritic growth that was prevented by administration of KynA (Monnerie et al., 2003). (Zunszain et al., 2012) demonstrated that the suppressive effects of IL-1 on human hippocampal progenitor cells in vitro can be reversed by treatment with a KMO inhibitor. Nevertheless, the relationship between hippocampal neurogenesis and hippocampal GM volume is complex has yet to be fully clarified in primates (Wu et al., 2014).
In the HC group, we observed a number of suggestive associations between GM volumes and metabolites of the kynurenine pathway or CRP that did not remain significant after correction for multiple testing. Perhaps most importantly, KynA/3HK was positively associated with amygdalar volume prior to controlling for sex, age, and BMI. One possible explanation for this result is the presence of BMI-driven variation in inflammation in the healthy controls: the mean BMI of the HC group was 28.0 (range 19.9 – 43.9) with a standard deviation of 5.8. The mean CRP concentration of the HC group was 3.1 with a standard deviation of 4.7 (range 0.2 – 26.8).
A limitation of our study is that it is cross-sectional such that the data reported here are correlative in nature and unlike some of the animal studies cited above, do not provide information concerning cause and effect relationships. Further, the sample sizes of certain subgroups such as the BD P+ group (n=18) and the suicide attempter group (n=14) were relatively modest and given the fact that the study was performed in a heterogeneous population of patients, the statistical power needed to detect differences among the diagnostic groups may not have been adequate.
Another limitation is that we were only able to measure circulating levels of kynurenine-pathway metabolites, and it therefore remains unclear whether the hippocampal and amygdalar concentrations of KynA, 3HK, and QA in the brains’ of the volunteers were sufficient to exert neurotoxic or neuroprotective effects. However, (Stone et al., 2012) have argued that the levels of kynurenine metabolites in the plasma/serum are substantially diluted relative to the extracellular spaces that are in close proximity to activated macrophages in the periphery, and microglia and astrocytes in the CNS.
In conclusion, our results raise the possibility that BD-associated abnormalities in kynurenine metabolism may negatively impact the hippocampus and possibly the amygdala, structures that have been shown to be both functionally impaired and reduced in volume in unmedicated patients with BD (Savitz and Drevets, 2009). These changes in kynurenine metabolism are partly driven by inflammation and as such our data strengthen the inflammation model of depression by providing some of the first evidence that neuroactive kynurenine metabolites may impact limbic structures such as the hippocampus and amygdala, in vivo.
Supplementary Material
Main branches of the kynurenine pathway. Each box represents a metabolite resulting from the oxidation of tryptophan. Putative neurotoxic metabolites are colored red while KynA, which is putatively neuroprotective, is colored green. The black italicized text shows the enzymes that catalyze each step in the metabolic pathway. The blue and red stars indicate that the metabolite is able to cross the blood-brain barrier.
Representative example of the segmentation of the hippocampus by FreeSurfer shown in the sagittal (A and B) and coronal planes (C and D). The FreeSurfer mask is shown in red in B and D with the corresponding unsegmented image to the reader’s left in A and C.
TLV=temporal horn of the lateral ventricle.
Representative example of the segmentation of the amygdala by FreeSurfer shown in the sagittal (A and B) and coronal planes (C and D). The FreeSurfer mask is shown in red in B and D with the corresponding unsegmented image to the reader’s left in A and C. The amygdala can be clearly differentiated from the hippocampus by the alveus (white matter band) in the coronal slices.
LV=lateral ventricle.
Representative example of an error in the segmentation of the left hippocampus made by FreeSurfer. The erroneous inclusion of the left fusiform gyrus, illustrated by the yellow arrowhead can be clearly seen in the axial (A) and coronal (B) planes. The corresponding unsegmented images are displayed for purposes of comparison (C and D).
Highlights.
We measured serum concentrations of kynurenine metabolites in bipolar disorder (BD)
We also measured gray matter volume of the hippocampus and amygdala in BD
The ratio of kynurenic acid (KynA) to quinolinic acid (QA) was reduced in BD
The ratio of KynA/3-hydroxykynurenine was correlated with hippocampal volume in BD
Abnormalities in kynurenine metabolism may impact the structure of the hippocampus
Acknowledgements
The authors acknowledge Marieke van der Hart, Ph.D., at Brains Online for excellence in kynurenine metabolite analysis.
The authors also thank all the research participants and wish to acknowledge the contributions of Brenda Davis, Debbie Neal, Chibing Tan, and Ashlee Taylor from the laboratory of TKT at the University of Oklahoma Integrative Immunology Center towards the transport, processing and handling of all blood samples.
Role of Funding Source
This study was funded by a grant from the National Institute of Mental Health to JS (K01MH096077). JS, WCD, TAV, BNF, BEW, PFSB, and JB received support from The William K. Warren Foundation. TKT received support from the Oklahoma Tobacco Research Foundation.
The funders played no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Jonathan Savitz, Ph.D. has received research funding from Janssen Pharmaceuticals for an independent study and a lecture honorarium from University of Kansas-Wichita. Dr. Dantzer has received consulting fees from Ironwood Pharma, Cambridge, MA, and an honorarium from Pfizer, France. Wayne Drevets, M.D. is an employee of Janssen Pharmaceuticals of Johnson & Johnson, Inc., Titusville, NJ, USA, and received within the past 3 years lecture honoraria or consulting fees from Johns-Hopkins University, The University of Michigan, University of Illinois at Chicago, University of Kansas-Wichita, Washington University School of Medicine, St. Louis, the Taiwanese Society of Biological Psychiatry and Neuropsychopharmacology, Janssen Pharmaceuticals, Inc. and Myriad/ Rules Based Medicine, Inc.
APPENDIX A
BIOANALYTICAL METHODS FOR KYNURENINE METABOLIC PATHWAY MEASUREMENTS
Concentrations of Quinolinic acid (QA), 3-Hydroxy-kynurenine (3HK), Tryptophan (Trp), Kynurenine (Kyn), and Kynurenic Acid (KynA) in serum were determined by HPLC with tandem mass spectrometry (MS/MS) detection.
A.1 Analysis of Quinolinic Acid
Serum samples (20 µl) were mixed with 20 µl internal standard (2.5*10−6 M QA-d3) in acetonitrile and thoroughly mixed. After 5 min samples were centrifuged at 13000 rpm for 5 min at 4 °C. 10 µl supernatant was transferred into a separate vial and mixed with 90 µl ultrapure water already containing 0.2 % tri-fluoric acid (TFA). 30 µl of this mixture was automatically injected into the LC system by an automated sample injector (SIL-10AD, Shimadzu, Japan). Chromatographic separation was performed on a reversed phase Synergi max-RP 3.0 * 100 mm, particle size: 2.5 µm (Phenomenex, USA) held at a temperature of 35° C. Components were separated using a linear gradient of ACN/0.2% TFA in UP/0.2% TFA (flow rate 0.3 ml/min) following this scheme:
Table A1.
LC gradient time scheme
| Time (min) | % ACN |
|---|---|
| 0 | 0 |
| 5 | 24 |
| 5.1 | 30 |
| 5.9 | 30 |
| 6 | 0 |
The flow of the LC was diverted to the waste for 3 minutes, after which it was switched to the MS for detection of QA. MS analyses was performed using a API 4000 MS/MS system consisting of a API 3000 MS/MS detector and a Turbo Ion Spray interface (both from Applied Biosystems, USA). The acquisitions were performed in positive ionization mode, with ion spray voltage set at 5 kV with a probe temperature of 550 °C. The instrument was operated in multiple-reaction-monitoring (MRM). The collision gas (nitrogen) pressure was held at 10 psig. Data were calibrated using calibration curve prepared in serum matrix and quantified using the Analysttm data system (Applied Biosystem, version 1.4.2).
Table A2.
MRM for QA and its deuterated internal standard
| Analyte | Q1 | Q3 |
|---|---|---|
| QA | 168.1 | 78.2 |
| QA-d3 | 171.2 | 81.25 |
A.2 Analysis of 3HK
Serum (10 µl) were mixed with 40 µl internal standard (2*10−7 M d5-KYNA) in acetonitrile and thoroughly mixed. After 5 min samples were centrifuged at 13000 rpm for 5 min at 4 °C. 10 µl supernatant was transferred into a separate vial and mixed with 100 µl ultrapure water already containing 0.1 % formic acid (FA) and 0.01% Ascorbic acid. 25 µl of the mixture was injected into the HPLC-MS/MS system by an automated sample injector (SIL-10AD, Shimadzu, Japan). Chromatographic separation was performed on reversed phase column, BDS Hypersil C18, 2.1×150 mm Particle size 3µm (Thermo Scientific, USA), held at a temperature of 25° C with a total flow rate of 0.20 ml/min over the column. Components were separated using a linear gradient of ACN in UP, and contained 0.1 % FA. The gradient followed this scheme:
Table A3.
LC gradient time scheme acetylcholine
| Time (min) | % ACN |
|---|---|
| 0 | 0 |
| 3.5 | 27 |
| 6.9 | 95 |
| 7.4 | 83 |
| 7.8 | 0 |
The flow of the LC was directed to the MS from 4.5 to 8.2 minutes of the run for detection of 3HK. MS analyses was performed using an API 4000 MS/MS system consisting of an API 4000 MS/MS detector and a Turbo Ion Spray interface (both from AB Sciex, USA). The acquisitions were performed in positive ionization mode, with ion spray voltage set at 5 kV with a probe temperature of 600° C. The instrument was operated in multiple-reaction-monitoring (MRM) mode Data were calibrated using calibration curve prepared in serum matrix and quantified using the Analysttm data system (Applied Biosystem, version 1.4.2).
Table A4.
MRM for the respective analytes
| Analyte | Q1 | Q3 |
|---|---|---|
| 3HK | 225.3 | 207.95 |
| d5-KynA | 195.05 | 149.0 |
A.3. Analysis of Kynurenine, Kynurenic Acid and Tryptophan
Serum (10 µl) were mixed with 100 µl internal standard (2*10−7 M d5-KYNA & 3*10−4 M d2-KYN) in acetonitrile and thoroughly mixed. After 5 min samples were centrifuged at 13000 rpm for 5 min at 4 °C. 10 µl supernatant was transferred into a separate vial and mixed with 100 µl ultrapure water already containing 0.1 % formic acid (FA) and 0.01% Ascorbic acid. 20 µl of the mixture was injected into the HPLC-MS/MS system by an automated sample injector (SIL-10AD, Shimadzu, Japan). Chromatographic separation was performed on reversed phase column, XBridge C18, 2.1×100mm particle size 3.5µm (Waters, USA), held at a temperature of 30° C with a total flow rate of 0.25 ml/min over the column. Components were separated using a linear gradient of ACN in UP, and contained 0.1 % FA. The gradient was performed according to the following scheme:
Table A5.
LC gradient time scheme.
| Time (min) | % ACN |
|---|---|
| 0 | 0 |
| 4.5 | 15 |
| 8.0 | 85 |
| 8.5 | 85 |
| 9.0 | 0 |
The flow of the LC was directed to the MS from 4.0 to 8.1 minutes of the run for detection of Kyn, KynA and Trp (post column make up flow 0.1 mL/min (acetonitril/0.1% FA). MS analyses was performed using an API 4000 MS/MS system consisting of an API 4000 MS/MS detector and a Turbo Ion Spray interface (both from AB Sciex, USA). The acquisitions were performed in positive ionization mode, with ion spray voltage set at 5 kV with a probe temperature of 600° C. The instrument was operated in multiple-reaction-monitoring (MRM) mode. Data were calibrated using calibration curve prepared in serum matrix and quantified using the Analysttm data system (Applied Biosystem, version 1.4.2).
Table A6.
MRM for the analytes and their internal standard
| Analyte | Q1 | Q3 |
|---|---|---|
| Kyn | 209.15 | 146.1 |
| d2-Kyn | 211.15 | 148.1 |
| Trp | 205.2 | 170.1 |
| KynA | 190.05 | 144.0 |
| d5-KynA | 195.05 | 149.0 |
Table A7.
Lowest level of quantification and the intra-assay percentage of coefficient of variation for the analytes measured by high performance liquid chromatography with tandem mass spectrometry.
| Analyte | LLOQ | % CV |
|---|---|---|
| TRP | 10 µM | 5.70 |
| KYN | 0.75 µM | 5.83 |
| KynA | 12.5 nM | 5.36 |
| 3HK | 10 nM | 4.50 |
| QA | 50 nM | 3.70 |
Note: The LLOQ and % CV data are derived from a group of subjects with MDD in addition to the BD subjects and healthy controls included in this study.
A.4 HIGH SENSITIVITY C-REACTIVE PROTEIN (CRP) ASSAY
The Kamiya Biomedical K-Assay for C-Reactive Protein (CRP) was used for the quantitative determination of CRP in serum by immunoturbidimetric assay (Abbott c8000 System). The degree of light scattering is measured by reading turbidity at 570 nm. The sample CRP concentration is determined versus dilutions of a CRP standard of known concentration. The analytical measurement range for the assay is 0.2 mg/L to 480.0 mg/L.
Table A8.
Pearson's (r) and Spearman's (rs) correlation coefficients between the kynurenine pathway metabolites and CRP in the total sample.
| CRP | |
|---|---|
| TRP | rs=−0.17, p=0.130 |
| KYN | r=0.02, p=0.826 |
| KYN/TRP | rs=0.13, p=0.224 |
| KynA | r=−0.20, p=0.071 |
| 3HK | rs=0.19, p=0.076 |
| QA | rs=0.24, p=0.025 |
| KynA/3HK | r=−0.28, p=0.011 |
| KynA/QA | rs=−0.28, p=0.008 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The other authors have no disclosures.
References
- Amaral M, Levy C, Heyes DJ, Lafite P, Outeiro TF, Giorgini F, Leys D, Scrutton NS. Structural basis of kynurenine 3-monooxygenase inhibition. Nature. 2013;496:382–385. doi: 10.1038/nature12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellac CL, Coimbra RS, Christen S, Leib SL. Pneumococcal meningitis causes accumulation of neurotoxic kynurenine metabolites in brain regions prone to injury. Neurobiology of disease. 2006;24:395–402. doi: 10.1016/j.nbd.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Birch PJ, Grossman CJ, Hayes AG. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988;154:85–87. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Meli E, Moroni F. Similarities and differences in the neuronal death processes activated by 3OH-kynurenine and quinolinic acid. Journal of neurochemistry. 2001;77:1310–1318. doi: 10.1046/j.1471-4159.2001.00335.x. [DOI] [PubMed] [Google Scholar]
- Cobb JA, Simpson J, Mahajan GJ, Overholser JC, Jurjus GJ, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA. Hippocampal volume and total cell numbers in major depressive disorder. Journal of psychiatric research. 2013;47:299–306. doi: 10.1016/j.jpsychires.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler JA, Rush AJ, McMahon FJ, Laje G. Common genetic variation in the indoleamine-2,3-dioxygenase genes and antidepressant treatment outcome in major depressive disorder. Journal of psychopharmacology. 2012;26:360–367. doi: 10.1177/0269881111434622. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, Jurjus GJ, Dieter L, Duman RS. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16:69–82. doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarman BC, Riemersma-Van der Lek RF, de Groot JC, Ruhe HG, Klein HC, Zandstra TE, Burger H, Schoevers RA, de Vries EF, Drexhage HA, Nolen WA, Doorduin J. Neuroinflammation in bipolar disorder - A [(11)C]-(R)-PK11195 positron emission tomography study. Brain Behav Immun. 2014;40:219–225. doi: 10.1016/j.bbi.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Stelzhammer V, Wong EHF, Rothermundt M, Bahn S, Merad M, Russo SJ. Leukocyte-derived Interleukin 6 predicts individual differences in susceptibility to social defeat stress. Science. In press. [Google Scholar]
- Johansson AS, Owe-Larsson B, Asp L, Kocki T, Adler M, Hetta J, Gardner R, Lundkvist GB, Urbanska EM, Karlsson H. Activation of kynurenine pathway in ex vivo fibroblasts from patients with bipolar disorder or schizophrenia: cytokine challenge increases production of 3-hydroxykynurenine. Journal of psychiatric research. 2013;47:1815–1823. doi: 10.1016/j.jpsychires.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Kocki T, Wnuk S, Kloc R, Kocki J, Owe-Larsson B, Urbanska EM. New insight into the antidepressants action: modulation of kynurenine pathway by increasing the kynurenic acid/3-hydroxykynurenine ratio. Journal of neural transmission. 2012;119:235–243. doi: 10.1007/s00702-011-0668-8. [DOI] [PubMed] [Google Scholar]
- Lavebratt C, Olsson S, Backlund L, Frisen L, Sellgren C, Priebe L, Nikamo P, Traskman-Bendz L, Cichon S, Vawter MP, Osby U, Engberg G, Landen M, Erhardt S, Schalling M. The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Molecular psychiatry. 2014;19:334–341. doi: 10.1038/mp.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds PR, Yu F, Wang Z, Chiu CT, Zhang Y, Leng Y, Linares GR, Chuang DM. A New Avenue for Lithium: Intervention in Traumatic Brain Injury. ACS Chem Neurosci. 2014 doi: 10.1021/cn500040g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejak P, Szyndler J, Turzynska D, Sobolewska A, Kolosowska K, Lehner M, Plaznik A. The kynurenine pathway: a missing piece in the puzzle of valproate action? Neuroscience. 2013;234:135–145. doi: 10.1016/j.neuroscience.2012.12.052. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnerie H, Shashidhara S, Le Roux PD. Effect of excess extracellular glutamate on dendrite growth from cerebral cortical neurons at 3 days in vitro: Involvement of NMDA receptors. J Neurosci Res. 2003;74:688–700. doi: 10.1002/jnr.10797. [DOI] [PubMed] [Google Scholar]
- Moroni F, Carpenedo R, Cozzi A, Meli E, Chiarugi A, Pellegrini-Giampietro DE. Studies on the neuroprotective action of kynurenine mono-oxygenase inhibitors in post-ischemic brain damage. Advances in experimental medicine and biology. 2003;527:127–136. doi: 10.1007/978-1-4615-0135-0_15. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Park SH, Scharpe S, Steinbusch HW, Leonard BE. Tryptophan breakdown pathway in bipolar mania. J Affect Disord. 2007;102:65–72. doi: 10.1016/j.jad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Myint AM, Schwarz MJ, Verkerk R, Mueller HH, Zach J, Scharpe S, Steinbusch HW, Leonard BE, Kim YK. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naive and medication-free schizophrenic patients. Brain, behavior, and immunity. 2011;25:1576–1581. doi: 10.1016/j.bbi.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Nassar A, Azab AN. Effects of Lithium on Inflammation. ACS Chem Neurosci. 2014 doi: 10.1021/cn500038f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Matson WR, Beal MF, Myers RH, Bird ED, Milbury P, Saso S. Kynurenine pathway abnormalities in Parkinson's disease. Neurology. 1992;42:1702–1706. doi: 10.1212/wnl.42.9.1702. [DOI] [PubMed] [Google Scholar]
- Okuda S, Nishiyama N, Saito H, Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12553–12558. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson SK, Samuelsson M, Saetre P, Lindstrom L, Jonsson EG, Nordin C, Engberg G, Erhardt S, Landen M. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J Psychiatry Neurosci. 2010;35:195–199. doi: 10.1503/jpn.090180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson SK, Sellgren C, Engberg G, Landen M, Erhardt S. Cerebrospinal fluid kynurenic acid is associated with manic and psychotic features in patients with bipolar I disorder. Bipolar disorders. 2012;14:719–726. doi: 10.1111/bdi.12009. [DOI] [PubMed] [Google Scholar]
- Petrik D, Lagace DC, Eisch AJ. The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology. 2012;62:21–34. doi: 10.1016/j.neuropharm.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA. A Critical Appraisal of Neuroimaging Studies of Bipolar Disorder: Toward a New Conceptualization of Underlying Neural Circuitry and a Road Map for Future Research. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejdak K, Bartosik-Psujek H, Dobosz B, Kocki T, Grieb P, Giovannoni G, Turski WA, Stelmasiak Z. Decreased level of kynurenic acid in cerebrospinal fluid of relapsing-onset multiple sclerosis patients. Neuroscience letters. 2002;331:63–65. doi: 10.1016/s0304-3940(02)00710-3. [DOI] [PubMed] [Google Scholar]
- Sapko MT, Guidetti P, Yu P, Tagle DA, Pellicciari R, Schwarcz R. Endogenous kynurenate controls the vulnerability of striatal neurons to quinolinate: Implications for Huntington's disease. Experimental neurology. 2006;197:31–40. doi: 10.1016/j.expneurol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neuroscience and biobehavioral reviews. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS, Bodurka J, Teague TK, Dantzer R. Putative Neuroprotective and Neurotoxic Kynurenine Pathway Metabolites are Associated with Hippocampal and Amygdalar Volumes in Subjects with Major Depressive Disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Nugent AC, Bogers W, Liu A, Sills R, Luckenbaugh DA, Bain EE, Price JL, Zarate C, Manji HK, Cannon DM, Marrett S, Charney DS, Drevets WC. Amygdala volume in depressed patients with bipolar disorder assessed using high resolution 3T MRI: the impact of medication. Neuroimage. 2010;49:2966–2976. doi: 10.1016/j.neuroimage.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nature reviews. Neuroscience. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz MJ, Guillemin GJ, Teipel SJ, Buerger K, Hampel H. Increased 3-hydroxykynurenine serum concentrations differentiate Alzheimer's disease patients from controls. European archives of psychiatry and clinical neuroscience. 2013;263:345–352. doi: 10.1007/s00406-012-0384-x. [DOI] [PubMed] [Google Scholar]
- Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, Mawrin C, Brisch R, Bielau H, Meyer zu Schwabedissen L, Bogerts B, Myint AM. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? Journal of neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW, Forrest CM, Darlington LG. Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. Febs J. 2012;279:1386–1397. doi: 10.1111/j.1742-4658.2012.08487.x. [DOI] [PubMed] [Google Scholar]
- Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends in pharmacological sciences. 2013;34:136–143. doi: 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Ting KK, Brew BJ, Guillemin GJ. Effect of quinolinic acid on human astrocytes morphology and functions: implications in Alzheimer's disease. Journal of neuroinflammation. 2009;6:36. doi: 10.1186/1742-2094-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A, McGuffin P. An Inflammatory Biomarker as a Differential Predictor of Outcome of Depression Treatment With Escitalopram and Nortriptyline. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.14010094. [DOI] [PubMed] [Google Scholar]
- Vogelgesang SA, Heyes MP, West SG, Salazar AM, Sfikakis PP, Lipnick RN, Klipple GL, Tsokos GC. Quinolinic acid in patients with systemic lupus erythematosus and neuropsychiatric manifestations. J Rheumatol. 1996;23:850–855. [PubMed] [Google Scholar]
- Webster MJ, O'Grady J, Kleinman JE, Weickert CS. Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience. 2005;133:453–461. doi: 10.1016/j.neuroscience.2005.02.037. [DOI] [PubMed] [Google Scholar]
- Wu MV, Shamy JL, Bedi G, Choi CW, Wall MM, Arango V, Boldrini M, Foltin RW, Hen R. Impact of social status and antidepressant treatment on neurogenesis in the baboon hippocampus. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:1861–1871. doi: 10.1038/npp.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, Thuret S, Price J, Pariante CM. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, Lee J, Truong J, Andrews-Zwilling Y, Hsieh EW, Louie JY, Wu T, Scearce-Levie K, Patrick C, Adame A, Giorgini F, Moussaoui S, Laue G, Rassoulpour A, Flik G, Huang Y, Muchowski JM, Masliah E, Schwarcz R, Muchowski PJ. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145:863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Main branches of the kynurenine pathway. Each box represents a metabolite resulting from the oxidation of tryptophan. Putative neurotoxic metabolites are colored red while KynA, which is putatively neuroprotective, is colored green. The black italicized text shows the enzymes that catalyze each step in the metabolic pathway. The blue and red stars indicate that the metabolite is able to cross the blood-brain barrier.
Representative example of the segmentation of the hippocampus by FreeSurfer shown in the sagittal (A and B) and coronal planes (C and D). The FreeSurfer mask is shown in red in B and D with the corresponding unsegmented image to the reader’s left in A and C.
TLV=temporal horn of the lateral ventricle.
Representative example of the segmentation of the amygdala by FreeSurfer shown in the sagittal (A and B) and coronal planes (C and D). The FreeSurfer mask is shown in red in B and D with the corresponding unsegmented image to the reader’s left in A and C. The amygdala can be clearly differentiated from the hippocampus by the alveus (white matter band) in the coronal slices.
LV=lateral ventricle.
Representative example of an error in the segmentation of the left hippocampus made by FreeSurfer. The erroneous inclusion of the left fusiform gyrus, illustrated by the yellow arrowhead can be clearly seen in the axial (A) and coronal (B) planes. The corresponding unsegmented images are displayed for purposes of comparison (C and D).