Abstract
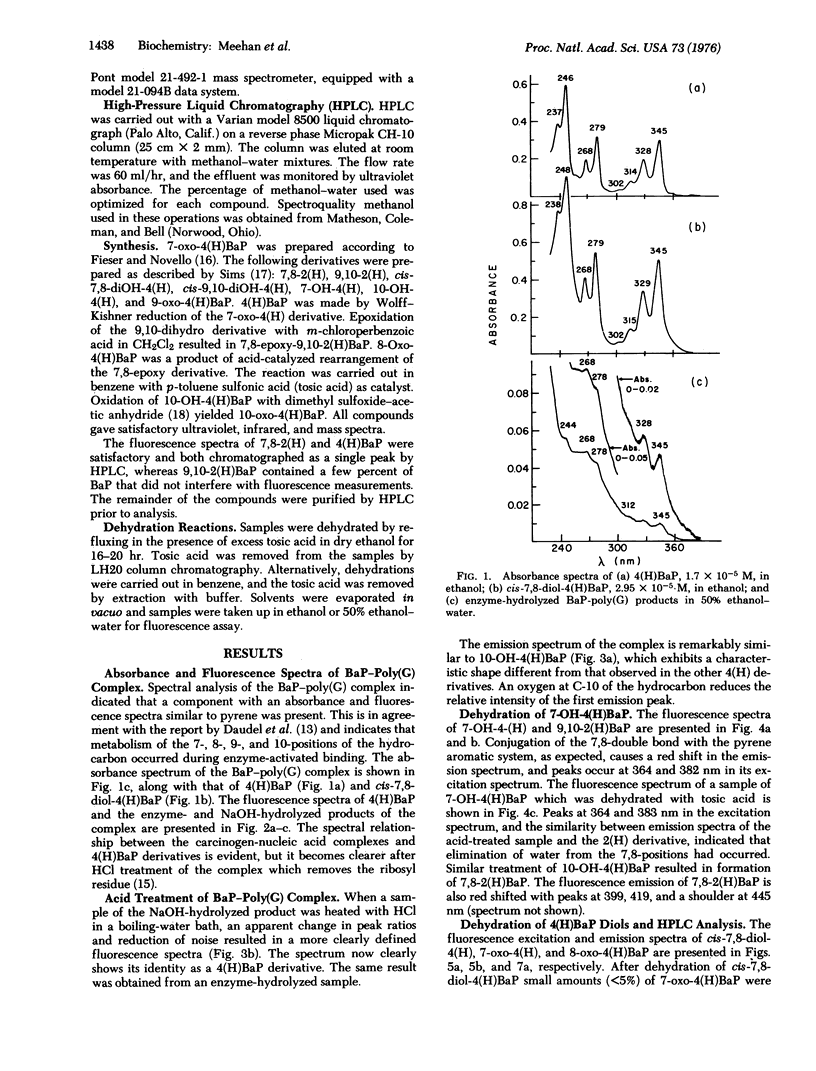
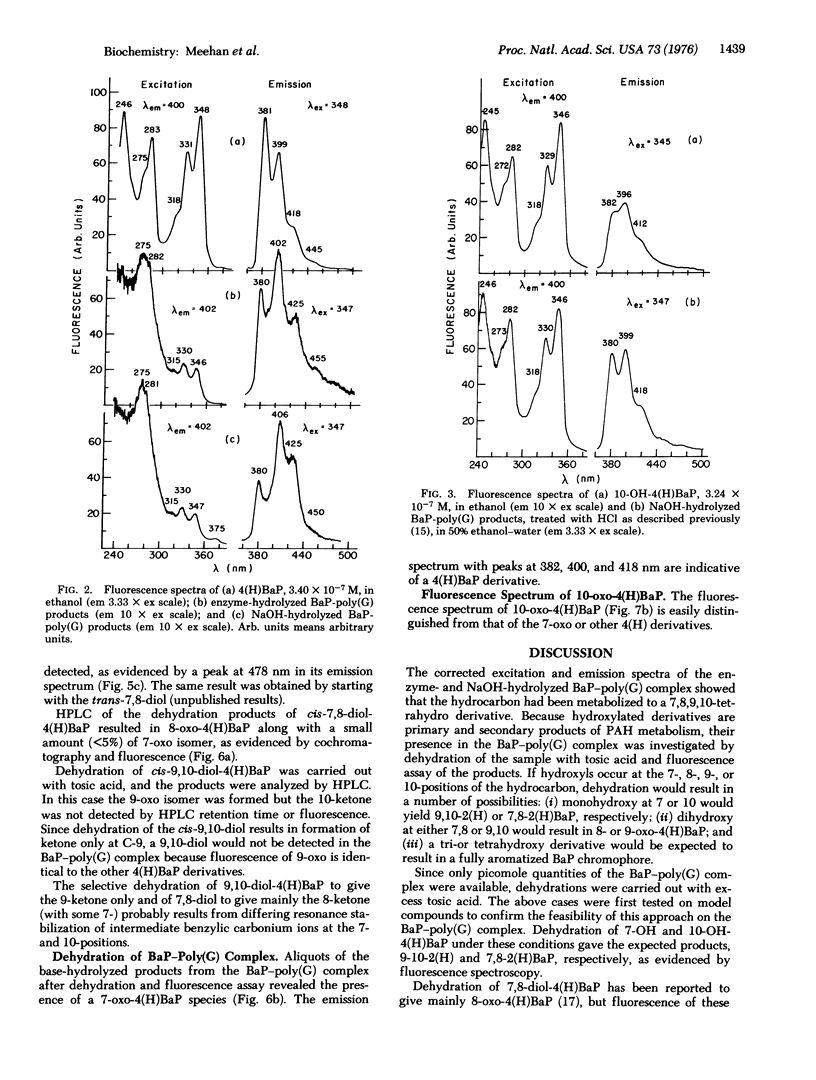
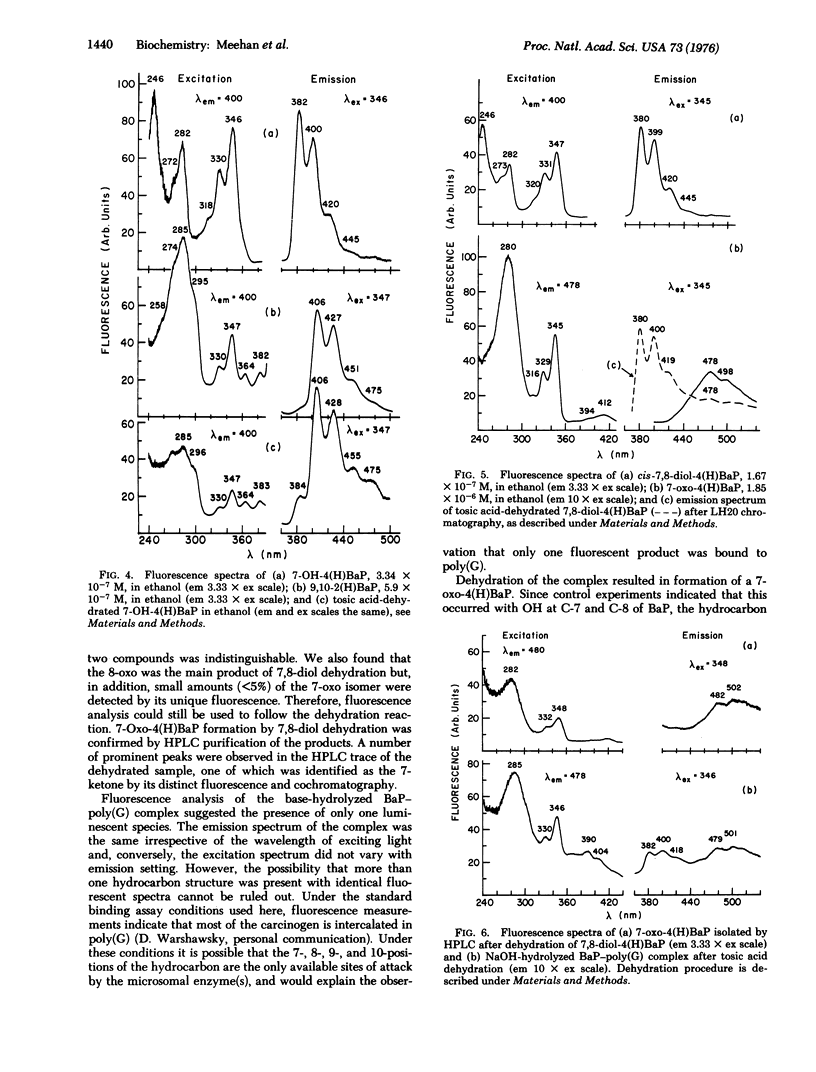
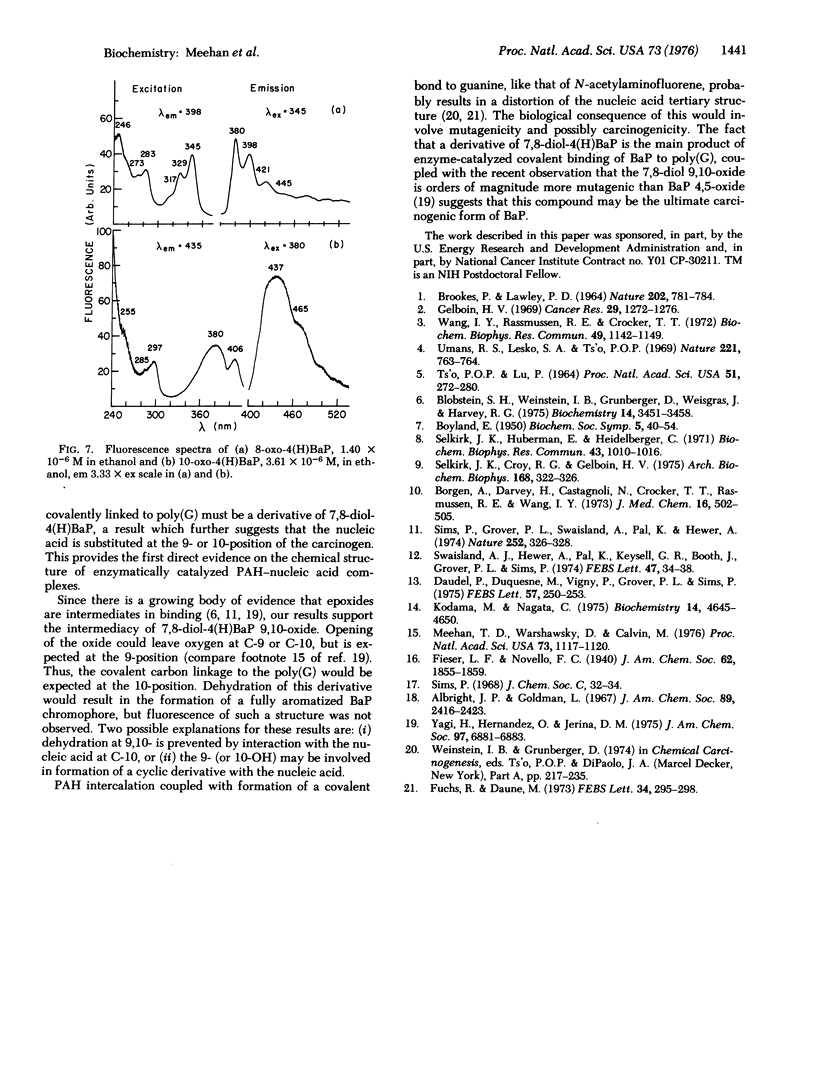
The carcinogen, benzo[a]pyrene, was covalently attached to poly (G) by liver microsomes from rats pretreated with 3-methylcholanthrene. The complex was hydrolyzed with enzymes or base and products were isolated by Sephadex chromatography. Absorbance and fluorescence spectra of the products fit that of red-shifted pyrene aromatic system and suggest that metabolism has occurred at the 7-, 8-, 9-, and 10-positions of the hydrocarbon. Benzanthracene or chrysene fluorescence were not observed in these preparations. Benzo[a]pyrene derivatives were synthesized and purified by high-pressure liquid chromatography. Dehydration of 7,8-dihydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene resulted in the formation of small amounts of 7-oxo-7,8,9,10-tetrahydrobenzoa[a]pyrene. A 7-keto species was also observed after similar treatment of the hydrocarbon-poly(G) hydrolysis products. Evidence of dehydration at the 9,10-positions was not observed. The hydrocarbon covalently bound to poly(G) is, therefore, a derivative of 7,8-dihydroxy-7,8,9,10-tetrahydrobenzol[a]pyrene with nucleic acid substitution at C-10 or 9.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKES P., LAWLEY P. D. EVIDENCE FOR THE BINDING OF POLYNUCLEAR AROMATIC HYDROCARBONS TO THE NUCLEIC ACIDS OF MOUSE SKIN: RELATION BETWEEN CARCINOGENIC POWER OF HYDROCARBONS AND THEIR BINDING TO DEOXYRIBONUCLEIC ACID. Nature. 1964 May 23;202:781–784. doi: 10.1038/202781a0. [DOI] [PubMed] [Google Scholar]
- Blobstein S. H., Weinstein I. B., Grunberger D., Weisgras J., Harvey R. G. Products obtained after in vitro reaction of 7,12-dimethylbenz[alpha]anthracene 5,6-oxide with nucleic acids. Biochemistry. 1975 Jul 29;14(15):3451–3458. doi: 10.1021/bi00686a025. [DOI] [PubMed] [Google Scholar]
- Borgen A., Darvey H., Castagnoli N., Crocker T. T., Rasmussen R. E., Wang I. Y. Metabolic conversion of benzo(a)pyrene by Syrian hamster liver microsomes and binding of metabolites to deoxyribonucleic acid. J Med Chem. 1973 May;16(5):502–506. doi: 10.1021/jm00263a020. [DOI] [PubMed] [Google Scholar]
- Daudel P., Duquesne M., Vigny P., Grover P. L., Sims P. Fluorescence spectral evidence that benzo(a)pyrene-DNA products in mouse skin arise from diol-epoxides. FEBS Lett. 1975 Oct 1;57(3):250–253. doi: 10.1016/0014-5793(75)80310-3. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Daune M. Physical basis of chemical carcinogenesis by N-2-fluorenylacetamide derivatives and analogs. FEBS Lett. 1973 Aug 15;34(2):295–298. doi: 10.1016/0014-5793(73)80815-4. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V. A microsome-dependent binding of benzo[a]pyrene to DNA. Cancer Res. 1969 Jun;29(6):1272–1276. [PubMed] [Google Scholar]
- Kodama M., Nagata C. A fluorometric study of DNA-bound benzo[a]pyrene. Biochemistry. 1975 Oct 21;14(21):4645–4650. doi: 10.1021/bi00692a013. [DOI] [PubMed] [Google Scholar]
- Meehan T., Warshawsky D., Calvin M. Specific positions involved in enzyme catalyzed covalent binding of benzo[a]pyrene to poly(G). Proc Natl Acad Sci U S A. 1976 Apr;73(4):1117–1120. doi: 10.1073/pnas.73.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkirk J. K., Croy R. G., Gelboin H. V. Isolation by high pressure liquid chromatography and characterization of benzo(a)pyrene-4'5-epoxide as a metobolite of benzoapyrene. Arch Biochem Biophys. 1975 May;168(1):322–326. doi: 10.1016/0003-9861(75)90256-8. [DOI] [PubMed] [Google Scholar]
- Selkirk J. K., Huberman E., Heidelberger C. An epoxide is an intermediate in the microsomal metabolism of the chemical carcinogen, dibenz(a,h)anthracene. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1010–1016. doi: 10.1016/0006-291x(71)90562-6. [DOI] [PubMed] [Google Scholar]
- Sims P., Grover P. L., Swaisland A., Pal K., Hewer A. Metabolic activation of benzo(a)pyrene proceeds by a diol-epoxide. Nature. 1974 Nov 22;252(5481):326–328. doi: 10.1038/252326a0. [DOI] [PubMed] [Google Scholar]
- Sims P. The synthesis of 8- and 9-hydroxybenzo[a]pyrene and the role of the products in benzo[a]pyrene metabolism. J Chem Soc Perkin 1. 1968;1:32–34. doi: 10.1039/j39680000032. [DOI] [PubMed] [Google Scholar]
- Swaisland A. J., Hewer A., Pal K., Keysell G. R., Booth J., Grover P. L., Sims P. Polycyclic hydrocarbon epoxides: the involvement of 8,9-dihydro-8,9-dihydroxybenz (a) anthracene 10,11-oxide in reactions with the DNA of benz (a) anthracene-treated hamster embryo cells. FEBS Lett. 1974 Oct 1;47(1):34–38. doi: 10.1016/0014-5793(74)80420-5. [DOI] [PubMed] [Google Scholar]
- TSO P. O., LU P. INTERACTION OF NUCLEIC ACIDS, II. CHEMICAL LINKAGE OF THE CARCINOGEN 3,4-BENZPYRENE TO DNA INDUCED BY PHOTORADIATION. Proc Natl Acad Sci U S A. 1964 Feb;51:272–280. doi: 10.1073/pnas.51.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umans R. S., Lesko S. A., Jr, Ts'o P. O. Chemical linkage of carcinogenic 3,4-benzpyrene to DNA in aqueous solution induced by peroxide and iodine. Nature. 1969 Feb 22;221(5182):763–764. doi: 10.1038/221763a0. [DOI] [PubMed] [Google Scholar]
- Wang I. Y., Rasmussen R. E., Crocker T. T. Isolation and characterization of an active DNA-binding metabolite of benzo(a)pyrene from hamster liver microsomal incubation systems. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1142–1149. doi: 10.1016/0006-291x(72)90332-4. [DOI] [PubMed] [Google Scholar]
- Yagi H., Hernandez O., Jerina D. M. Letter: Synthesis of (+/-)-7 beta,8alpha-dihydroxy-9 beta,10beta-epoxy-7,8,-9,10-tetrahydrobenzo(a)pyrene, a potential metabolite of the carcinogen benzo(a)pyrene with stereochemistry related to the antileukemic triptolides. J Am Chem Soc. 1975 Nov 12;97(23):6881–6883. doi: 10.1021/ja00856a057. [DOI] [PubMed] [Google Scholar]


