Abstract
STUDY QUESTION
How does the placenta protect the fetus from immune rejection by the mother?
SUMMARY ANSWER
The placenta can produce IgG that is glycosylated at one of its Fab arms (asymmetric IgG; aIgG) which can interact with other antibodies and certain leukocytes to affect local immune reactions at the junction between the two genetically distinct entities.
WHAT IS KNOWN ALREADY
The placenta can protect the semi-allogenic fetus from immune rejection by the immune potent mother. aIgG in serum is increased during pregnancy and returns to the normal range after giving birth. aIgG can react to antigens to form immune complexes which do not cause a subsequent immune effector reaction, including fixing complements, inducing cytotoxicity and phagocytosis, and therefore has been called ‘blocking antibody’.
STUDY DESIGN, SIZE, DURATION
Eighty-eight human placentas, four trophoblast cell lines (TEV-1, JAR, JEG and BeWo), primary culture of human placental trophoblasts and a gene knock-out mouse model were investigated in this study.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The general approach included the techniques of cell culture, immunohistochemistry, in situ hybridization, immuno-electron microscopy, western blot, quantitative PCR, protein isolation, glycosylation analysis, enzyme digestion, gene sequencing, mass spectrophotometry, laser-guided microdissection, enzyme-linked immunosorbent assay, pulse chase assay, double and multiple staining to analyze protein and DNA and RNA analysis at the cellular and molecular levels.
MAIN RESULTS AND THE ROLE OF CHANCE
Three major discoveries were made: (i) placental trophoblasts and endothelial cells are capable of producing IgG, a significant portion of which is aberrantly glycosylated at one of its Fab arms to form aIgG; (ii) the asymmetrically glycosylated IgG produced by trophoblasts and endothelial cells can react to immunoglobulin molecules of human, rat, mouse, goat and rabbit at the Fc portion; (iii) asymmetrically glycosylated IgG can react to certain leukocytes in the membrane and cytoplasm, while symmetric IgG from the placenta does not have this property.
LIMITATIONS, REASONS FOR CAUTION
Most of the experiments were performed in vitro. The proposed mechanism calls for verification in normal and abnormal pregnancy.
WIDER IMPLICATIONS OF THE FINDINGS
This study identified a number of new phenomena suggesting that aIgG produced by the placenta would be able to react to detrimental antibodies and leukocytes and interfere with their immune reactions against the placenta and the fetus. This opens a new dimension for further studies on pregnancy physiology and immunology. Should the mechanism proposed here be confirmed, it will have a direct impact on our understanding of the physiology and pathology of human reproduction and offer new possibilities for the treatment of many diseases including spontaneous abortion, infertility and pre-eclampsia. It also sheds light on the mechanism of immune evasion in general including that of cancer.
STUDY FUNDING/COMPETING INTEREST(S)
Funded by the Li KaShing Foundation and the National Natural Science Foundation to Jiang Gu, grant no. 30971150 and 81030033. There is no competing interest.
Trial registration number: N/A.
Keywords: asymmetric immunoglobulin G, placenta, immune evasion, leukocyte, fetus
Introduction
The placenta is an organ that protects the fetus from being rejected by the mother as the fetus is a semi-allogenic entity capable of eliciting a maternal immune response (Schroder and De la Chapelle, 1972; Pitcher-Wilmott et al., 1980; Reed et al., 1991; Roopenian et al., 2003). An effective mechanism of immune evasion is known to take place at the junction between the pregnant mother and the fetus, but this mechanism has not been fully explained. IgG is known to be present in the placenta as the human fetus acquires humoral immunity through transfer of maternal IgG across the placental barrier (Franklin and Kunkel, 1958; Faulk et al., 1980; Roopenian and Akilesh, 2007). However, without an immune evasion mechanism, maternal anti-fetus antibodies or leukocytes would cause damage to the placenta and the fetus. It has been proposed that the placenta functions as an ‘antibody sink’ that can filter out these detrimental anti-paternal antibodies, thus preventing them from crossing the placental barrier (Gitlin and Biasucci, 1969, Simister and Story, 1997, Simister, 1998), but no concrete evidence has been forthcoming to support this hypothesis.
Recently, it has been found that several cell types other than activated B lymphocytes and plasma cells can produce IgG. These cells include neoplastic cells (Qiu et al., 2003; Chen and Gu, 2007; Chen et al., 2010), cerebral neurons (Huang et al., 2008; Niu et al., 2011), human ocular epithelial and ganglion cells (Niu et al., 2010), spermatogenic cells (Huang et al., 2009), mammary gland epithelial cells (Franklin and Kunkel, 1958; Zhang et al., 2010) and human umbilical cord endothelial cells (Zhao et al., 2011). Previously, such non-lymphocytic IgG was found to promote cancer cell growth and metastasis and inhibit cell apoptosis (Niu et al., 2012; Ma et al., 2013; Jiang et al., 2014; Lei et al., 2014). However, the specificity and reactivity of such non-lymphocytic antibody have never been established. We speculate that such IgG may play a role in interacting with the immune system and therefore provide the long sought after mechanism of immune evasion.
Although most of the IgG in the fetal circulation appears to be of maternal origin, small amounts of IgG with a haplotype different from that of the mother have been detected as early as 17 weeks of gestation (Johnson et al., 1977). In addition, previous studies have demonstrated that part of the IgG present in the placenta is of fetal origin and this fetal IgG can be detected as early as 20 weeks of gestation (Babbage et al., 2006). In this study, we investigated the possible production of IgG by human placenta and the possible function that such locally produced IgG may play during pregnancy. We obtained extensive evidence to show that the placenta is capable of synthesizing IgG, which can react to both the hormonal and the cellular immunity and may play a central role in immune evasion.
Materials and Methods
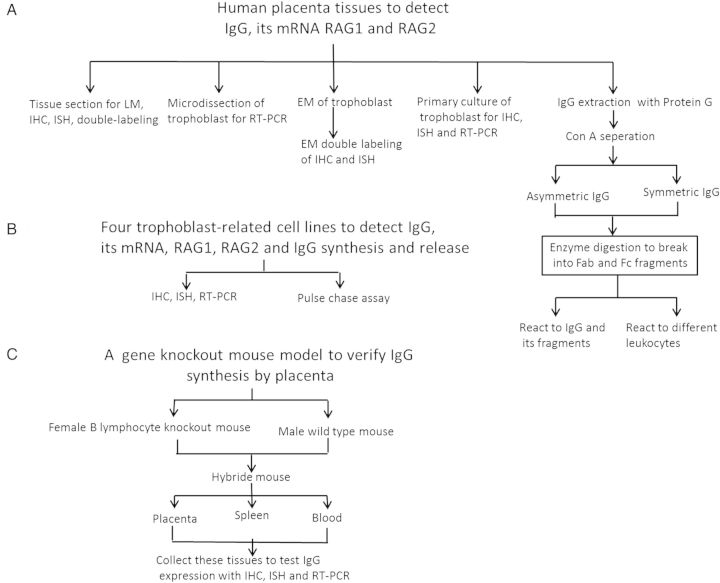
The design of the experiments is shown in Fig. 1.
Figure 1.
A diagram shows the experimental design of the study. (A) Human fresh placenta tissues were processed for morphologic examination [immunohistochemistry (IHC), in situ hybridization (ISH) and double labeling], microdissection for PCR, ultrastructural investigation, primary culture and IgG extraction. The extracted IgG was further separated into asymmetric and symmetric IgGs and subjected to electrophoresis and immunohistochemistry for their reactivities. (B) Established cell lines of trophoblast-derived cells to investigate IgG gene expression and protein synthesis. (C) A gene knock out mouse model that does not possess B lymphocytes, therefore does not produce classic IgG. Such a female mouse was mated with a wild-type male mouse to create a hybrid pregnant mother mouse. The placenta as well as the spleen and the blood of this mouse were examined and showed that even though the mother did not produce IgG, the placenta still contained IgG protein and mRNA indicating that the IgG was indeed produced by the placenta itself rather than transported from the mother.
Materials
A total of 88 human placentas were used in this study, among which 53 were full term, 11 were mid-term and 24 were first-term placentas. The following cell lines were used: human first-trimester extravillous trophoblast cell line (TEV-1) (Pattillo et al., 1971), and the human choriocarcinoma cell lines JAR, JEG and BeWo [American Type Culture Collection (ATCC), VA, USA]. A primary trophoblast cell culture was established as described previously (Bright and Ockleford, 1995, Zhao et al., 2011). A gene knock-out mouse model (Mu-MT mouse) (Kitamura et al., 1991) with no B lymphocyte development was also used. The female gene knock-out mice were mated with wild-type male mice and the pregnant mother mice had placenta but no B lymphocyte therefore no IgG detected in the serum.
Methods
Immunohistochemistry, immuno-electron microscopy, in situ hybridization, electron microscopic in situ hybridization and double labeling
Immunohistochemistry was performed on human placentas following standard procedures with primary antibodies, as described in Supplementary data, Table S1. Immuno-electron microscopy was also performed with antibodies to Igγ and Igκ labeled with colloidal gold. Immunofluorescence was performed on trophoblast cell lines with primary antibodies to IgG. In situ hybridization (ISH) at both the light and the electron microscopic levels was performed on human placentas, the cell lines and the primary trophoblast culture, according to a previously published protocol (Chen et al., 2010) with a cRNA probe against human immunoglobulin heavy constant gamma 1 (IGHG1). The probe was produced as described previously (Chen et al., 2010). ISH/IHC double labeling at both the light and the electron microscopic levels with antibodies to Igγ or placental alkaline phosphatase (PLAP) on the primary trophoblast culture and with antibody to IgG and probe to IgG mRNA was performed. In this protocol, ISH was performed first on one side of the grid and immunostaining was performed second on the other side of the grid holding the ultrathin electron microscopic sections.
A gene knock-out mouse model with no IgG production from B lymphocytes
To further establish that the Ig detected in the trophoblasts were locally produced but not absorbed from the mother, we used a mouse model (Mu-MT mouse) that had a gene knock-out resulting in no B lymphocyte development, and therefore, no Ig production from the immune system (Kitamura et al., 1991). Mu-MT mouse was mated with a normal mouse. We examined the distribution of IgG and mRNA in the placenta of pregnant Ig-deficient mice using the same set of techniques as for the human placentas.
Laser-assisted microdissection, RT–PCR and single cell RT–PCR without prior RNA purification
Laser-assisted microdissection (LMD) and single cell isolation of trophoblasts were performed on frozen samples from human placentas and cultured cells as described previously(Clement-Ziza et al., 2008; Chen et al., 2010). RNA extraction and RT–PCR were performed as described in the Supplementary data with the primers described in Supplementary data, Table S2.
Primary culture of placental trophoblasts, IgG immunostaining and ISH
Eight first trimester placentas were used for primary trophoblast cell culture. Cells were grown on slides and fixed with 4% paraformaldehyde for 20 min. IHC and ISH, separately or simultaneously as double labeling, were performed as described above.
VH sequencing
Following cloning into a pGEM-T vector, we next sequenced three randomly chosen variable region of the IgG heavy chain (VH) encoding clones and compared the VH sequences with sequences published in BLAST of the National Center for Biotechnology Information (NCBI) (BLAST number: 2.2.20) (Dieterich et al., 2006).
Pulse chase assay to detect in vitro synthesis of IgG by trophoblasts
Pulse chase assay was performed both with radioisotope (35S) labeled and non-radioisotope (biotin) labeled proteins in BeWo and TEV-1 cell lines to verify incorporation of labeled amino acids into newly synthesized IgG in culture as described previously (Wang et al., 2003; Dieterich et al., 2010). We substituted one of the amino acids in the culture medium, l-methionine, with alkyne-bearing l-homopropargylglycine (HPG). The alkine groups of proteins extracted from trophoblast cells were in vitro labeled with azide coupled biotin. A skin fibroblast cell line and addition of a protein translational elongation inhibitor, cycloheximide (Sigma, St Louis, MO, USA) served as negative controls.
Isolation of IgG from human placental and rat spleen lysates
Total IgG was purified from placental and spleen lysates using Protein G agarose after extensive washing to remove traces of blood following the manufacturer's instructions (Invitrogen, USA).
Separation of glycosylated IgG from non-glycosylated IgG
The separation of glycosylated IgG from non-glycoslated IgG was performed using Concanavalin A (Con A) affinity chromatography according to the manufacturer's instruction (GE Healthcare, Sweden) (Gercel-Taylor et al., 2001; Canellada et al., 2002).
Preparation of IgG Fab and Fc fragments
Fab and Fc segments were prepared from placental IgG and maternal serum IgG using papain digestion following the manufacturer's instructions (Pierce® Fab Preparation Kit, Pierce Biotechnology, Rockford, IL, USA).
The labeling of IgG, Fc and Fab with biotin
The process of labeling IgG, Fc fragment and Fab fragment with biotin was performed following the instructions of the manufacturer of AnaTag™ Biotin Protein Labeling Kit (AnaSpec Corporate, San Jose, CA, USA).
Reaction of Con A-reactive IgG to other IgG molecules
The reaction of Con A extracted IgG to other IgG was demonstrated with standard sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and western blot. Briefly, mouse, rat, rabbit, goat and human IgG were subjected to SDS–PAGE and transferred to immobilon polyvinyl transfer membrane, followed by incubation with biotin-labeled aIgG and sIgG IgG overnight at 4°C, incubated with horse-radish peroxidase (HRP)-labeled streptavidin (ZhongShan Golden Bridge Biotechnology Cooperation, Beijing, China) for 1 h at room temperature and then visualized.
Separation of different leukocyte types
Human lymphocytes, NK cells, monocytes and neutrophil granulocytes were isolated from normal adult blood following the instructions of the isolation kits (Haoyang Biotechnology Cooperation, Shanghai, China).
Stain-decolorize-stain method for human leukocytes
The stain-decolorize-stain method was performed to show cell types reactive to Con A-reactive IgG on blood leukocyte according to a newly established procedure (Li et al., 2014). Briefly, different types of white blood cells were fixed onto slides and IHC was performed with different cell marker antibodies (CD3, CD19, CD20, CD57, CD68). After visualizing and taken photos, the slides were decolorized with 80% alcohol for half an hour at room temperature and then heated in a microwave oven for 10 min to remove the bound antibodies and the labeling color. The biotin-labeled glycosylated and non-glycosylated IgGs were used as the primary antibodies and the slides then were incubated with HRP-labeled streptavidin. After visualization of labeling with the AEC kit, photos were taken of the same fields as for the first immunostaining. The specificity of this technique has been reported previously (Li et al., 2014).
Removal of glycans from the glycosylated IgG with deglycosylation enzymes
PNG-F was used to remove the N-link glycan from the Con A-reactive IgG according to the manufacturer's instructions (Invitrogen). Endoglycosidases F3 was also used to remove glycan from the aIgG, which would not damage the reactivity of the IgG molecule (Sigma).
Results
Abundant IgG mRNA and IgG synthesizing enzymes are present in placental trophoblast cells, endothelial cells and cultured trophoblast cells
We first confirmed that abundant IgG molecules are present in the human placenta. Immunohistochemistry with different monoclonal and polyclonal antibodies to the heavy chain (Igγ), and κ and λ light chains (Igκ and Igλ) of IgG (Supplementary data, Table S1) detected strong immunoreactivity of all forms of IgG in trophoblasts and endothelial cells in all three trimesters of the placenta (Fig. 2A–C and Supplementary data, Fig. S1).
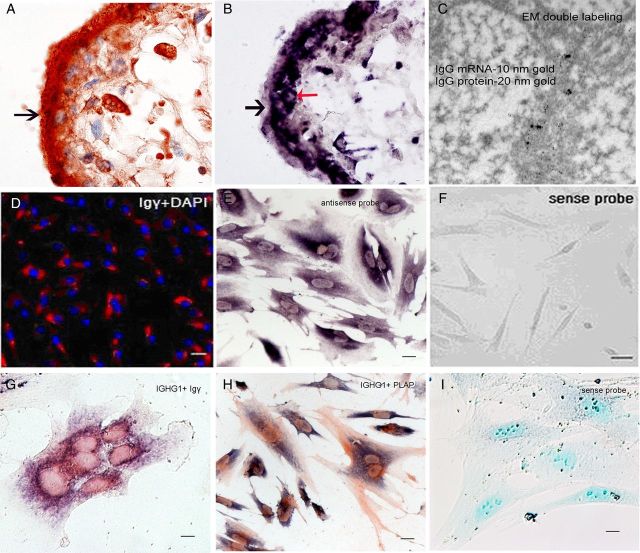
Figure 2.
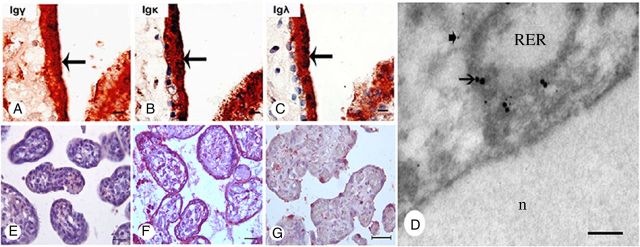
IgG immunoreactivity present in the trophoblasts of the human placenta. (A–C) Immunoreactivity of IgG γ, IgG κ and IgG λ detected in the trophoblasts (arrows). (D) Double electron microscopy ISH and immunostaining to demonstrate co-localization of IgG immunoreactivity and IgG mRNA on the membrane of RER of trophoblast with 15 nm (arrows) and 5 nm (arrow-heads) colloidal gold particles. (E) Symmetric IgG did not react to any cell of the placenta. (F) aIgG isolated from the placenta reacted positively to the trophoblasts and endothelial cells of the placenta. (G) Positive signal disappeared with pre-absorbed IgG. The nuclei were stained blue with hematoxylin. A–C, scale bar = 10 μm; D, scale bar = 100 nm; E–G, scale bar = 10 μm.
ISH on placental tissue sections with a cRNA probe against the constant region of the IgG1 heavy chain (IGHG1) showed abundant IGHG1 mRNA transcripts present in the cytoplasm of syncytiotrophoblasts, cytotrophoblasts and vascular endothelial cells in all trimesters (Fig. 3B and Supplementary data, Fig. S2I–L). Double labeling of IgG protein and its mRNA with electron microscope (EM) immuno-gold labeling and EM ISH in the trophoblasts demonstrated IgG immunoreactivity and IgG mRNA on the membrane of rough endoplasmic reticulum (RER) of the cytoplasm (Figs 2D and 3C). RT–PCR with primers for IGHG1, Igκ and Igλ detected their transcripts in RNA extracted from placental tissues of all three trimesters (Fig. 4A).
Figure 3.
Evidence of IgG synthesis by placental trophoblasts. (A) Positive Igγ IHC staining (reddish brown) in trophoblasts (arrows) of a mid-term placenta. (B) IGHG1 mRNA (purple black) detected in the cytoplasm of trophoblasts (black arrow: syncytiotrophoblast, red arrow: cytotrophoblast) with ISH. (C) Electron microscopic double labeling showing colocalization of IgG protein antigen with immunostaining (15 nm gold labeling) and mRNA with ISH(5 nm gold labeling) at the membrane of RER in a cultured syncytiocytoblast (n, nuclear). (D) Positive immunofluorescence staining of Igγ (red signal) in the cytoplasm of trophoblasts of TEV-1 cell line with nuclear counterstaining in blue. (E) Positive ISH signal (black–blue) of IGHG1 mRNA present in the cytoplasm of cultured human syncytiotrophoblasts. (F) Negative control: sense probe on the same cell line. (G and H) Double labeling showing colocalization of IGHG1 mRNA (blue) and Igγ (brown) (G), as well as IGHG1 mRNA (blue) and PLAP (brown) (H) in the cytoplasm of primary cultured human trophoblasts. (I) Negative control: sense probe on the same primary culture of human trophoblasts. The nuclei were counterstained light blue. Scale bars, 10 μm (A, B, G, I), 40 nm (C), 30 μm (D–F), 20 μm (H).
Figure 4.
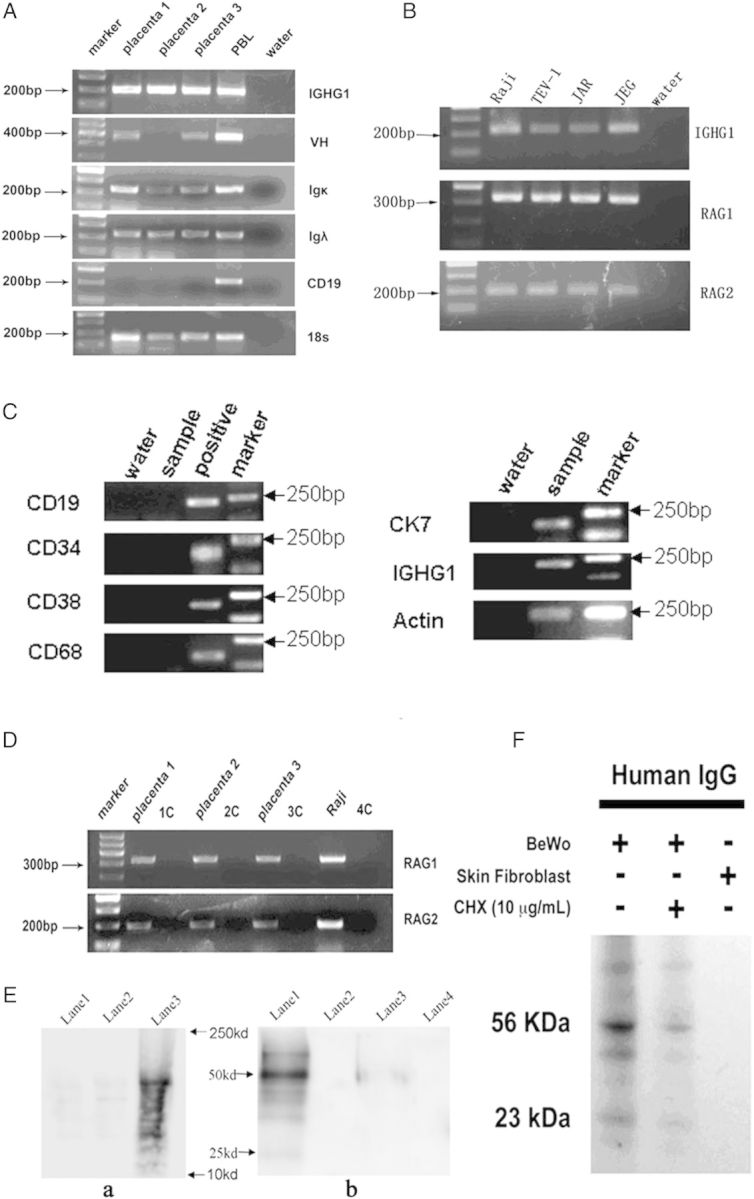
RT–PCR, single cell RT–PCR and western blot. (A) mRNA transcripts of IGHG1, Igκ and Igλ were detected in placentas from all three trimesters. PBL (peripheral blood lymphocyte): positive control. 18s: internal control. The absence of CD19 transcripts excluded B lymphocyte contamination. (B) RT–PCR on trophoblast cell lines. Amplification of IGHG1, recombination activating gene (RAG)1 and RAG2 were detected. (C) Single cell RT–PCR. IGHG1 and cytokeratin (CK) successfully amplified from placenta samples. Positive controls: liver samples for CD34, CD38 and CD68; Raji cell line for CD19. (D) Both RAG 1 and RAG 2 were detectable in placentas from all three trimesters. 1C–4C: RNA without reverse transcriptase as template used as negative controls. Raji cell line: Positive control. Placenta 1, 2 and 3 represent first, second and third trimester placentas, respectively. (E) Pulse chase assay using alkyne-bearing L-HPG followed by tagging with azide coupled biotin in TEV-1 cell line. a, Alkyne-bearing l-HPG tagged with azide coupled biotin is incorporated into newly synthesized total protein in TEV-1 cell line as detected by western blot with streptavidin HRP. Lane 1: negative control (TEV-1 cultured with DMEM/F12). Lane 2: negative control (TEV-1 +HPG + anisomycin). Lane 3: experimental group showing HPG was incorporated into newly synthesized total protein. b, Alkyne-bearing HPG tagged with azide coupled biotin is incorporated into newly synthesized IgG in TEV-1, Lane 1: HPG. Lane 2: final wash HPG solution. Lane 3: negative control (TEV-1 cell line cultured in DMEM/F12). Lane 4: final wash as a negative control. (F) 35S-metabolic labeling for IgG synthesis in cultured human BeWo cells with IgG immunoprecipitated from extracted proteins detected by autophotograph. Cycloheximide (CHX) treatment inhibiting protein translation elongation decreased radio-labeled IgG. Skin fibroblasts treated identically did not show any immunoglobulin synthesis. Lane 1: BeWo cell line, Lane 2: CHX treatment, Lane 3: skin fibroblast.
In addition, we performed LMD to capture trophoblasts only prior to RT–PCR (Supplementary data, Fig. S3G and H). To exclude placental multipotent hematopoietic progenitors, and possible contamination by B cells, plasma cells and macrophages, we used primers for CD34, Ck7, CD 19/20, CD38 and CD68 (Supplementary data, Table S2) to ensure isolation of trophoblasts only (Fig. 4C). Collectively, our findings demonstrate that trophoblasts contain IgG mRNA, and are capable of synthesizing IgG.
Specific Igγ immunoreactivity was detected in the cytoplasm of established placental trophoblast cell lines (TEV-1, JAR, JEG and BeWo) and primary cultures of human trophoblasts maintained in immunoglobulin-free medium (Fig. 3D). In addition, ISH detected IGHG1 mRNA in the cytoplasm of the cultured primary trophoblasts (Fig. 3G and H) and the four cell lines (Fig. 3E). Double labeling combining IHC with antibody to Ck7 or PLAP, and ISH showed that the multi-nucleated IGHG1-positive cells in the primary culture were PLAP-positive syncytiotrophoblasts (Fig. 3G and H). IGHG1 mRNA transcripts were also amplified from mRNA extracted from trophoblast cell lines and the primary trophoblast culture (Fig. 4B).
Upon sequencing, two sequences showed high homology (89.3 and 86.7%) to IGHV4-61*08, while one was different and showed high homology to IGHV4-59*01 (87.3%) (Supplementary data, Fig. S4).
VH gene transcripts (Fig. 4A) and expression of RAG 1 and RAG 2 genes were identified in placentas from all three trimesters (Fig. 4D) and in cultured cell lines (Fig. 4B) with RT–PCR. In addition, IHC with antibodies to RAG enzymes showed extensive staining in the cytoplasm of trophoblasts and endothelial cells in all three trimesters (Supplementary data, Fig. S5).
IgG synthesis in placental trophoblasts in gene knock-out mice with no B lineage cells
In the placenta of the pregnant gene knockout mouse model (no IgG produced by the mother), Ig immunoreactivity, Ig mRNA and Ig synthesizing enzymes were detected in the trophoblasts by RT–PCR (Supplementary data, Fig. S6), immunohistochemistry, ISH (Supplementary data, Fig. S7), sequencing and western blot (Supplementary data, Fig. S8). Positive signals of IgG immunoreactivity and IgG mRNA ISH were detected in the cytoplasm of trophoblasts in the labyrinth (Supplementary data, Fig. S7Ca) and spongiotrophoblast layers (Supplementary data Fig. S7Ba).
Slides incubated with sense probes were served as negative controls (Supplementary data, Fig. S7Db). Sections of the spleen of normal ICR mouse incubated with antisense probes were served as positive controls (Supplementary data, Fig. S7Da). The protein of IgG was located with immunohistochemistry in the same cells that contained Ig mRNA (Supplementary data, Fig. S7Ab and Ac). Tissue sections of the spleen of normal ICR mouse incubated with antibodies against IgG were served as positive controls (Supplementary data, Fig. S7Dc). Antibodies against hemagglutinin to substitute the primary antibodies were used as a negative control. Western blot was also used to identify IgG protein in placentas of the mutant mouse and normal ICR mouse. The positive signals were detected at MW 170 000 (Supplementary data, Fig. S8). The spleen of normal ICR mouse was served as a positive control for western blot. The lysis buffer was served as a negative control (Supplementary data, Fig. S8). All controls gave appropriate results, supporting that in the mutant mice, placental trophoblasts can still produce IgG, while the spleen of the mother mice cannot.
Pulse chase labeling technique demonstrating IgG synthesis by human trophoblasts
We performed a pulse chase experiment to verify incorporation of labeled amino acid into newly synthesized IgG. Using protein G-coupled magnetic beads and western blot analysis, we detected newly synthesized IgG with incorporated biotin-labeled amino acid in the trophoblast lysates, thereby firmly establishing that trophoblasts are capable of synthesizing IgG (Fig. 4E). The pulse chase experiment in the human placental trophoblast culture and cultured trophoblast cell lines, including a human choriocarcinoma cell line (BeWo), gave identical results (Fig. 4F). In addition, we detected IgG immunoreactivity in the supernatant from primary cultures by western blot analysis. The result indicates that IgG was not only synthesized in but also secreted by the trophoblasts. The control experiments showed no IgG synthesis.
About 30% of IgG isolated from the placenta is glycosylated at one of the Fab arms
We isolated IgG from human placenta with protein G column after extensive washing to remove trace of blood. We further separated glycosylated IgG and non-glycosylated IgG with a Con A coupled sepharose 4B column which binds to α-d-mannosyl and α-d-glucosyl groups (Sumner et al., 1938; Petryniak and Goldstein, 1986) linked to IgG. The proportion of glycosylated IgG in total IgG isolated from the placenta was ∼30%. Silver staining of the electrophoresis preparation showed the Con A-reactive IgG and the non-Con A-reactive IgG both had their heavy chain at 50 kDa but were different in that the former showed a double band while the latter showed a single band (Fig. 5A). As it is impossible to obtain spleen tissue sample from pregnant healthy women, we measured IgG and glycosylated IgG in normal spleen obtained from human autopsy and normal adult rats and found little Con A-reactive IgG, although normal IgG can be detected in abundance (Supplementary data, Fig. S5F). This indicates that the glycosylated IgG extracted from the placenta was unlikely to be produced by B lymphocytes in the spleen but locally by the placenta itself.
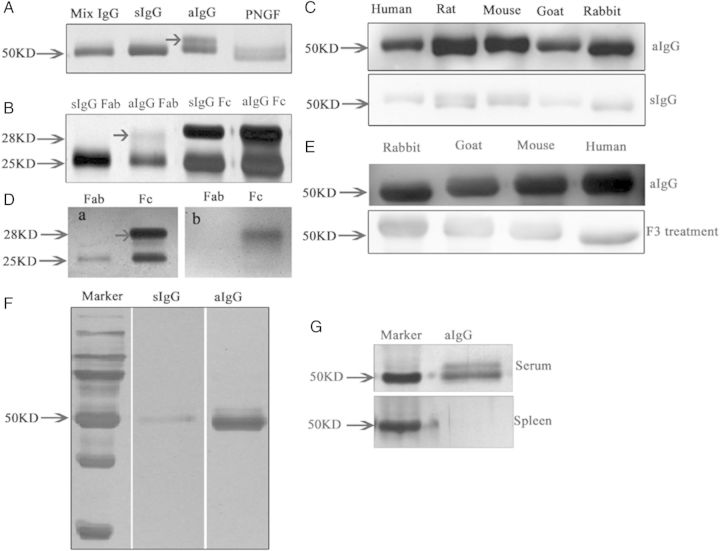
Figure 5.
Electrophoresis of asymmetrically glycosylated IgG (aIgG) and symmetrically glycosylated IgG (sIgG) extracted from placenta. (A) Silver stain of IgG extracted from the human placenta showing aIgG had a double band (arrow) at around 50 kDa. When the aIgG was treated with deglycosylation enzyme PNGF, the double band became a single band. (B) Sliver stain showing that the aberrant glycan is located in one of the Fab arms. (C) Western blot of aIgG and sIgG showing that aIgG reacted to IgGs from human, rat, mouse, goat and rabbit, while sIgG only reacted very weakly to IgG molecules of different species. (D) a, silver stain of Fab and Fc (arrow) fragment; b, western blot of aIgG showing that aIgG reacted to the Fc fragment of the other IgGs but not to the Fab fragment. (E) When the aIgG was digested with the enzyme to remove the glycan, the reactivity to IgG molecules was significantly weakened. (F) In a separate experiment where the aIgG and sIgG were coated onto magnetic beads and reacted to human IgG, the aIgG trapped significantly more IgG than the sIgG indicating that aIgG was indeed capable of reacting to other IgG molecules. (G) Silver stain showing that aIgG can be detected in the serum but not in the spleen of normal adult rat.
We further found with electrophoresis that the Con A-reactive glycosylated IgG had glycan molecules attached to one of the Fab fragments making the IgG molecule asymmetric (Fig. 5B). We used papain to digest the IgG whole molecule into Fab and Fc fragments and then separated them with electrophoresis. We again observed that the glycosylated IgG Fab fragment but not the Fc fragment has two bands with the same size and intensity, indicating that the glycan is located in only one of the Fab fragment arms. Therefore, we called the Con A-reactive IgG asymmetric IgG (aIgG), the non-Con A-reactive IgG symmetric IgG (sIgG).
aIgG but not sIgG reacted to the Fc portion of IgG from many sources
We then labeled the two kinds of IgG with biotin and used them separately as the primary antibodies in western blot and immunohistochemistry. IgG from different species including human, rat, mouse, goat and rabbit reacted positively to aIgG but not to the sIgG (Fig. 5C). The trophoblasts, endothelial cells and Haufbour cells (macrophages) on the human placental tissue section showed immunoreactivity with the aIgG (Fig. 2F) but not with the sIgG (Fig. 2E). The positive IHC staining was significantly decreased and eventually abolished in the pre-absorption tests in which human IgG was pre-incubated with the sIgG at increasing concentrations prior to applying to the preparations (Fig. 2F and G). This experiment established that the Fab glycosylated IgG (aIgG) was capable of reacting to the IgG molecule in western blot and IHC.
In addition, we found the aIgG isolated from one individual reacted to the IgG from other individuals, and also to those of other species.
To further confirm the ability of aIgG to bind to other IgGs, we coated two agarose preparations with Con A-reactive IgG and Con A non-reactive IgG separately and then let the same amount of IgG from different sources to react to the columns. We then measured the unbound IgG after centrifugation. We found that much less IgG remained after they reacted to the aIgG column than to the sIgG column which was almost unchanged. In addition, we eluted the bound IgG from the two columns and measured their concentrations and found that the aIgG column trapped a lot more IgG than the sIgG column (Fig. 5F).
We also used the biotin-labeled Fc or Fab fragment of the aIgG to react to the whole IgG molecule, the Fc fragment or the Fab fragment of IgG in western blot and in the pre-absorption tests. The Fab fragment of the aIgG reacted to the Fc fragment of other IgGs (Fig. 5D). IHC gave identical results. Only the biotin-labeled Fab fragment of aIgG reacted to trophoblasts and endothelial cells, while the biotin-labeled Fc fragment did not (Supplementary data, Fig. S9).
In addition, we found that both aIgG and sIgG extracted from human placenta reacted to an identical range of protein antigens extracted from Hela cells except that aIgG reacted to IgG, while sIgG did not (Supplementary data, Fig. S10).
aIgG, but not sIgG, reacted to the membrane and cytoplasm of monocytes and neutrophils
Using human white blood cell preparations from normal adults aIgG but not sIgG, reacted to the cell membrane and cytoplasm of monocytes and neutrophils in the blood (Fig. 6). On series sections with the techniques of double labeling and the stain-decolorize-stain method, the cell types reacting to aIgG were monocytes and neutrophils in blood (Fig. 6A–D). NK cells and B and T lymphocytes were negative (Fig. 6E–J). The different cell types in blood were identified by specific monoclonal antibodies (Fig. 6A, C, E, G and I). When we used human sIgG with increasing concentrations to pre-incubate the aIgG, the positive reactions in the leukocytes decreased significantly (Fig. 6L).
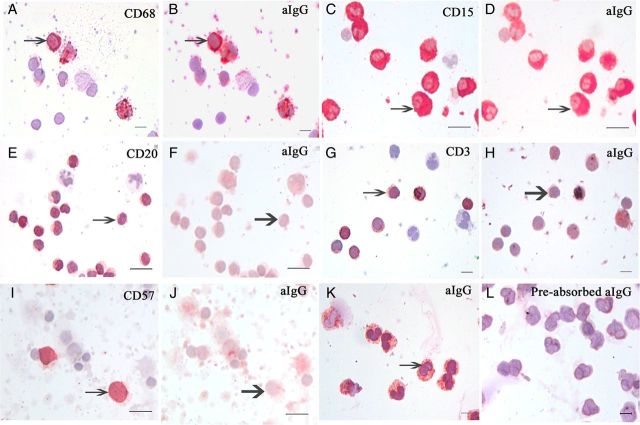
Figure 6.
Reaction of placenta-extracted aIgG with different leukocyte types demonstrated with the stain-decolorize-stain method and the pre-absorption test. (A and B) aIgG reacted to CD68-positive monocytes (arrows point to the same monocyte). (C and D) aIgG reacted to CD15-positive neutrophils (arrows point to the same neutrophils). (E and F) CD20-positive B lymphocytes were negative for aIgG (thick arrows point to the same B-cell). (G and H) CD3-positive T lymphocytes were negative for aIgG (thick arrows point to the same T-cell). (I and J) CD57-positive natural killer (NK) cells were negative for aIgG (thick arrows point to the same NK cell). (K and L) aIgG-positive neutrophils (K) were abolished when the antibody was pre-incubated with IgG molecule in a pre-absorption test on a different slide of the same neutrophil preparation (L). The reactions occurred with leukocytes from the same and different individuals. Symmetric IgG did not react to any leukocyte (not shown). Scale bar, 10 μm.
When the Fab glycosylated IgG was deglycosylated, the above reactivities disappeared
We treated the Fab glycosylated IgG with the enzymes PNGase and endoglycosidases F1, F2 and F3 in a native protein deglycosylation kit to remove the oligosaccharides from the IgG (Kim and Leahy, 2013). The later enzymes are capable of deglycoslylation of N-linked oligosaccharides from glycoproteins under native conditions without denaturing the protein. Following deglycosylation with PNGase, the two bands at 50kDa, usually seen in Fab glycosylated IgG in the western blot, became one (Fig. 5A). More importantly, after deglycosylation, the IgG lost its reactivity to other IgGs or to leukocytes in both western blot and immunohistochemistry and behaved like sIgG (Fig. 5E). This result indicates that glycosylation of IgG Fab is essential for it to bind to other IgG molecules and to leukocytes.
Discussion
In this study, we observed three novel phenomena. The first is that human placental trophoblasts and endothelial cells are capable of producing IgG, a significant portion of which is aberrantly glycosylated at one of its Fab arms to form aIgG. The second is that the asymmetrically glycosylated IgG produced by trophoblasts and endothelial cells can react to IgG molecules from human, rat, mouse, goat and rabbit at the Fc portion. Third, asymmetrically glycosylated IgG can react to certain leukocytes on the membrane and in the cytoplasm, while sIgG from the placenta does not have such properties.
The first aim of this study is to demonstrate that, in addition to transporting IgG from mother to fetus, the placenta is capable of synthesizing and secreting IgG. The evidence we obtained from IgG mRNA, relevant synthesizing enzymes, detection of IgG and its mRNA in cultured trophoblasts, pulse chase experiments and the gene-knockout mouse model unequivocally established that placental trophoblasts and endothelial cells can synthesize IgG.
In agreement with our findings, Margni et al. reported that aIgG isolated with Con A represented ∼30% of total IgG extracted from the placenta (Zenclussen et al., 2001). However, these authors believed that the aIgG was produced from the spleen or placental B cells, and interleukin-6 released by the trophoblasts was the mediator to induce hybridoma cells to produce aIgG (Canellada et al., 2002; Shields et al., 2002). In this study, we found that the aIgG was most likely synthesized by the placenta itself. The spleen is unlikely to be responsible for its production as in the normal spleen of human and rat, little aIgG could be detected. In the gene knockout mice that had no B lymphocytes, no IgG could be produced by the mother, but the placental trophoblast cells still contained IgG. In this study, multiple lines of evidence point to the conclusion that the origin of IgG extracted from the human placenta is likely, at least in part, to be the placenta itself.
The findings of aIgG, but not sIgG (normal IgG), reacting to other IgG molecules and to leukocytes are intriguing. This is likely a fundamental physiological event essential for the survival of life as it appears to operate across species, a sign of functional conservation during evolution. As the reactions can be abolished by pre-incubation with the human IgG molecule, the reaction is specifically directed against IgG. The specificity of this reaction was further confirmed with two additional straight-forward experiments in which no biotin labeling or western blot was involved. As for the nature of antigenic epitopes in leukocytes reacting to aIgG, it is possible that it reacted to immunoglobulin molecules or molecules of the immunoglobulin superfamily in these cells. Using different fragments of aIgG and sIgG as well as the whole IgG molecule in the western blot, immunohistochemistry and the pre-absorption tests, we established that the glycans are likely attached to the heavy chain of the Fab fragment and the reactions between aIgG and other IgGs took place between the Fab of aIgG and the Fc of the target immunoglobulin. More interestingly, when we removed the glycans from the aIgG with enzymes, its ability to react to other IgG and to leukocytes disappeared. This further established that the aIgG was indeed capable of reacting to the IgG molecule and leukocytes and glycosylation of the Fab fragment is necessary for this reaction. IgG is a glycoprotein with glycans attached to different portions of the molecule that would affect the reactivity of the antibody, a field that biomedical scientists have just begin to appreciate (Shields et al., 2002; Satoh et al., 2006). Recently, there has been a surge of interest in studying the patterns of glycosylation of immunoglobulin and their effects on Ig reactivity. Apart from a single covalently attached bi-antennary N-glycan at the highly conserved asparagine 297 residue in the CH2 domains of the heavy chain of Fc region (Wright and Morrison, 1997; Raju, 2008), IgG molecules are known to bear complex bi-antennary oligosaccharides attached to the variable regions of the light chain, heavy chain or both (Jefferis, 2007). Decreased galactosylation of IgG glycans was found to be associated with rheumatoid arthritis (Ercan et al., 2010). Studies of IgG glycosylation revealed a number of important functional consequences of structural alterations in IgG glycans. The addition of sialic acids dramatically changed the physiological role of IgGs by converting them from pro-inflammatory to anti-inflammatory (Pattillo et al., 1971; Petryniak and Goldstein, 1986; Kaneko et al., 2006; Abramovich et al., 2012). Another structural change to IgG glycans, the addition of fucose to the glycan core, interferes with binding of IgG to Fc_RIIIa receptor on leukocytes and dampens its ability to destroy target cells through antibody dependent cell-mediated cytotoxicity (Shields et al., 2002; Satoh et al., 2006).
In this study, we found that Fab glycosylation of IgG extracted from the placenta with Con A rendered the IgG antibody highly reactive to other IgG molecules and to certain leukocytes, thus giving it great significance in interacting with the humoral and cellular immunity. There is extensive evidence to show that IgG isolated with Con A would not trigger complement fixation, phagocytosis or T-cell toxicity (Gentile et al., 1998; Margni and Malan Borel, 1998; Gutierrez et al., 2005). With a local relatively high concentration of aIgG, any antibody bound to the placenta surface paved with trophoblasts and endothelial cells would be bound by these aIgG antibodies and thereby terminate any possible immune effector reaction that would otherwise occur at the antibody binding sites. Should there be any monocytes/macrophages getting close to, or penetrating, the placenta, the locally produced aIgG would bind to them and interfere or block their activities. This would be the most effective and efficient way for the placenta to protect the fetus at the junction of the two genetically different entities without altering the balance of the immune equilibrium of the pregnant mother.
Results obtained from our study indicate a possible novel immune evasion mechanism employing locally produced immunoglobulin that actively interacts with potentially damaging antibodies and leukocytes at the barrier separating the mother and the fetus. This protective mechanism appears to be an active exertion by the placenta rather than a passive escape and is likely to act on both innate and adaptive immune responses. Intriguing as it is, many questions about this delicate new mechanism remain unanswered and await further investigation. Nevertheless, our observations unveiled new basic facts about human pregnancy and a new mechanism of immune evasion in reproductive physiology and pathology is evident, with profound biological and clinical implications.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
J.G.: initiated the project, designed the experiments, supervised the protocols, analyzed the results, wrote the manuscript and provided the funding. Y.L.: designed and performed some experiments, collected and analyzed data, searched literature, wrote, revised and submitted the manuscript. Y.H., J.L. and Y.Z.: performed some experiments, collected and analyzed data. T.H., J.Z., J.W. and X.D.: performed some experiments. Z.C.: performed tissue sample preparation and technique support. C.K.: performed literature search and analysis, manuscript organization and writing and some experimental design. R.D., M.Y. and S.D.: performed some experiments. M.C. and L.L.: provided tissues samples. G.H.: technique support. Y.W.: administration and technical support. Q.L., C.L. and M.S.: performed tissue collection, fixation and immunohistochemistry. C.Y.: performed radioactive-labeled pulse chase assay. Z.Z.: participated in result analysis and pulse chase experimental design.
Funding
This work was supported by grants from the National Nature Science Foundation of China (No. 30971150 and 81030033 to J.G.) and The Li KaShing Foundation.
Conflict of interest
There is no competing interest by any of the authors.
Supplementary Material
Acknowledgements
We would like to thank Professor Helen Durkin of State University of New York, Health Science Center at Brooklyn, NY, USA, for her constructive comments and insightful advises on this study, Dr Lini Lu, Dr Jidong Chen, Ms Linhong Luo, Ms Xiuling Lin, Ms Jingchun Cai, Ms Meiru Yang of the First Affiliated Hospital, and Dr Lian Ma of the Second Affiliated Hospital, Shantou University Medical College for providing the fresh placental tissue and blood samples, Professor George Tsai of Hong Kong University for providing the trophoblast cell lines. We also thank Jianrong Tian for her help to collect the tissue samples, Professor Jen-Fu Chiu and Professor Steve Lin for their valuable advices.
References
- Abramovich D, Irusta G, Bas D, Cataldi NI, Parborell F, Tesone M. Angiopoietins/TIE2 system and VEGF are involved in ovarian function in a DHEA rat model of polycystic ovary syndrome. Endocrinology. 2012;153:3446–3456. doi: 10.1210/en.2012-1105. [DOI] [PubMed] [Google Scholar]
- Babbage G, Ottensmeier CH, Blaydes J, Stevenson FK, Sahota SS. Immunoglobulin heavy chain locus events and expression of activation-induced cytidine deaminase in epithelial breast cancer cell lines. Cancer Res. 2006;66:3996–4000. doi: 10.1158/0008-5472.CAN-05-3704. [DOI] [PubMed] [Google Scholar]
- Bright NA, Ockleford CD. Cytotrophoblast cells: a barrier to maternofetal transmission of passive immunity. J Histochem Cytochem. 1995;43:933–944. doi: 10.1177/43.9.7642966. [DOI] [PubMed] [Google Scholar]
- Canellada A, Gentile T, Dokmetjian J, Margni RA. Occurrence, properties, and function of asymmetric IgG molecules isolated from non-immune sera. Immunol Invest. 2002;31:107–120. doi: 10.1081/imm-120004802. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gu J. Immunoglobulin G expression in carcinomas and cancer cell lines. FASEB J. 2007;21:2931–2938. doi: 10.1096/fj.07-8073com. [DOI] [PubMed] [Google Scholar]
- Chen Z, Huang X, Ye J, Pan P, Cao Q, Yang B, Li Z, Su M, Huang C, Gu J. Immunoglobulin G is present in a wide variety of soft tissue tumors and correlates well with proliferation markers and tumor grades. Cancer. 2010;116:1953–1963. doi: 10.1002/cncr.24892. [DOI] [PubMed] [Google Scholar]
- Clement-Ziza M, Munnich A, Lyonnet S, Jaubert F, Besmond C. Stabilization of RNA during laser capture microdissection by performing experiments under argon atmosphere or using ethanol as a solvent in staining solutions. RNA. 2008;14:2698–2704. doi: 10.1261/rna.1261708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT) Proc Natl Acad Sci USA. 2006;103:9482–9487. doi: 10.1073/pnas.0601637103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich DC, Hodas JJ, Gouzer G, Shadrin IY, Ngo JT, Triller A, Tirrell DA, Schuman EM. In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nat Neurosci. 2010;13:897–905. doi: 10.1038/nn.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan A, Cui J, Chatterton DEW, Deane KD, Hazen MM, Brintnell W, O'Donnell CI, Derber LA, Weinblatt ME, Shadick NA, et al. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2010;62:2239–2248. doi: 10.1002/art.27533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk WP, Jarret R, Keane M, Johnson PM, Boackle RJ. Immunological studies of human placentae: complement components in immature and mature chorionic villi. Clin Exp Immunol. 1980;40:299–305. [PMC free article] [PubMed] [Google Scholar]
- Franklin EC, Kunkel HG. Comparative levels of high molecular weight (19S) gamma globulin in maternal and umbilical cord sera. J Lab Clin Med. 1958;52:724–727. [PubMed] [Google Scholar]
- Gentile T, Llambias P, Dokmetjian J, Margni RA. Effect of pregnancy and placental factors on the quality of humoral immune response. Immunol Lett. 1998;62:151–157. doi: 10.1016/s0165-2478(98)00041-8. [DOI] [PubMed] [Google Scholar]
- Gercel-Taylor C, Bazzett LB, Taylor DD. Presence of aberrant tumor-reactive immunoglobulins in the circulation of patients with ovarian cancer. Gynecol Oncol. 2001;81:71–76. doi: 10.1006/gyno.2000.6102. [DOI] [PubMed] [Google Scholar]
- Gitlin D, Biasucci A. Development of gamma G, gamma A, gamma M, beta IC-beta IA, C 1 esterase inhibitor, ceruloplasmin, transferrin, hemopexin, haptoglobin, fibrinogen, plasminogen, alpha 1-antitrypsin, orosomucoid, beta-lipoprotein, alpha 2-macroglobulin, and prealbumin in the human conceptus. J Clin Invest. 1969;48:1433–1446. doi: 10.1172/JCI106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez G, Gentile T, Miranda S, Margni RA. Asymmetric antibodies: a protective arm in pregnancy. Chem Immunol Allergy. 2005;89:158–168. doi: 10.1159/000087964. [DOI] [PubMed] [Google Scholar]
- Huang J, Sun X, Mao Y, Zhu X, Zhang P, Zhang L, Du J, Qiu X. Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse brain neurons. Int J Biochem Cell Biol. 2008;40:1604–1615. doi: 10.1016/j.biocel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang L, Ma T, Zhang P, Qiu X. Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse testis and epididymis. J Histochem Cytochem. 2009;57:339–349. doi: 10.1369/jhc.2008.951434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther. 2007;7:1401–1413. doi: 10.1517/14712598.7.9.1401. [DOI] [PubMed] [Google Scholar]
- Jiang C, Huang T, Wang Y, Huang G, Wan X, Gu J. Immunoglobulin G expression in lung cancer and its effects on metastasis. PLosOne. 2014;9:e97359. doi: 10.1371/journal.pone.0097359. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Johnson PM, Natvig JB, Ystehede UA, Faulk WP. Immunological studies of human placentae: the distribution and character of immunoglobulins in chorionic villi. Clin Exp Immunol. 1977;30:145–153. [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F, Ravetch EV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- Kim MS, Leahy D. Enzymatic deglycosylation of glycoproteins. Methods Enzymol. 2013;533:259–263. doi: 10.1016/B978-0-12-420067-8.00019-2. [DOI] [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Lei Y, Huang T, Su M, Luo J, Korteweg C, Li J, Chen Z, Qiu Y, Liu X, Yan M, et al. Expression and distribution of immunoglobulin G in normal liver, hepatocarcinoma and post partial-hepatectomy liver. Lab Invest. 2014;94:1283–1295. doi: 10.1038/labinvest.2014.114. [DOI] [PubMed] [Google Scholar]
- Li J, Zhou Y, Gu J. Stain-decolorize-stain (SDS): a new technique for multiple staining. Histochem Cell Biol. 2014;141:251–262. doi: 10.1007/s00418-013-1177-7. [DOI] [PubMed] [Google Scholar]
- Ma C, Wang Y, Zhang G, Chen Z, Qiu Y, Li J, Luo J, Huang B, Jiang C, Huang G, et al. IgG expression and its potential role in primary and metastatic breast cancers. Curr Mol Med. 2013;13:429–437. [PubMed] [Google Scholar]
- Margni RA, Malan Borel I. Paradoxical behavior of asymmetric IgG antibodies. Immunol Rev. 1998;163:77–87. doi: 10.1111/j.1600-065x.1998.tb01189.x. [DOI] [PubMed] [Google Scholar]
- Niu N, Zhang J, Sun Y, Wang S, Korteweg C, Gao W, Gu J. Expression and distribution of immunoglobulin G and its receptors in an immune privileged site: the eye. Cell Mol Life Sci. 2010;68:2481–2492. doi: 10.1007/s00018-010-0572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu N, Zhang J, Guo Y, Zhao Y, Korteweg C, Gu J. Expression and distribution of immunoglobulin G and its receptors in the human nervous system. Int J Biochem Cell Biol. 2011;43:556–563. doi: 10.1016/j.biocel.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Niu N, Zhang J, Huang T, Sun Y, Chen Z, Yi W, Korteweg C, Wang J, Gu J. IgG expression in human colorectal cancer and its relationship to cancer cell behaviors. PLoS One. 2012;7:e4736. doi: 10.1371/journal.pone.0047362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattillo RA, Hussa RO, Garancis JC. Glycogen metabolism in human hormone-producing trophoblastic cells in continuous culture. I. Regulation of glycogen metabolism by glucose. In Vitro. 1971;7:59–67. doi: 10.1007/BF02628263. [DOI] [PubMed] [Google Scholar]
- Petryniak J, Goldstein IJ. Immunochemical studies on the interaction between synthetic glycoconjugates and alpha-L-fucosyl binding lectins. Biochemistry. 1986;25:2829–2838. doi: 10.1021/bi00358a014. [DOI] [PubMed] [Google Scholar]
- Pitcher-Wilmott RW, Hindocha P, Wood CB. The placental transfer of IgG subclasses in human pregnancy. Clin Exp Immunol. 1980;41:303–308. [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao P, Li G, Lv P, Li Z, Sun X, et al. Human epithelial cancers secrete immunoglobulin g with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 2003;63:6488–6495. [PubMed] [Google Scholar]
- Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471–478. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Reed E, Beer AE, Hutcherson H, King DW, Suciu-Foca N. The alloantibody response of pregnant women and its suppression by soluble HLA antigens and anti-idiotypic antibodies. J Reprod Immunol. 1991;20:115–128. doi: 10.1016/0165-0378(91)90028-o. [DOI] [PubMed] [Google Scholar]
- Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- Satoh M, Iida S, Shitara K. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin Biol Ther. 2006;6:1161–1173. doi: 10.1517/14712598.6.11.1161. [DOI] [PubMed] [Google Scholar]
- Schroder J, De la Chapelle A. Fetal lymphocytes in the maternal blood. Blood. 1972;39:153–162. [PubMed] [Google Scholar]
- Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- Simister NE. Human placental Fc receptors and the trapping of immune complexes. Vaccine. 1998;16:1451–1455. doi: 10.1016/s0264-410x(98)00107-8. [DOI] [PubMed] [Google Scholar]
- Simister NE, Story CM. Human placental Fc receptors and the transmission of antibodies from mother to fetus. J Reprod Immunol. 1997;37:1–23. doi: 10.1016/s0165-0378(97)00068-5. [DOI] [PubMed] [Google Scholar]
- Sumner JB, Gralen N, Eriksson-Quensel IB. The molecular weights of urease, canavalin, concanavalin a and concanavalin B. Science. 1938;87:395–396. doi: 10.1126/science.87.2261.395. [DOI] [PubMed] [Google Scholar]
- Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J Am Chem Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- Wright A, Morrison SL. Effect of glycosylation on antibody function: implications for genetic engineering. Trends Biotechnol. 1997;15:26–32. doi: 10.1016/S0167-7799(96)10062-7. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Gentile T, Kortebani G, Mazzolli A, Margni R. Asymmetric antibodies and pregnancy. Am J Reprod Immunol. 2001;45:289–294. doi: 10.1111/j.8755-8920.2001.450504.x. [DOI] [PubMed] [Google Scholar]
- Zhang S, Mao Y, Huang J, Ma T, Zhang L, Zhu X, Zheng J, Wu L, Yin CC, Qiu X. Immunoglobulin gene locus events in epithelial cells of lactating mouse mammary glands. Cell Mol Life Sci. 2010;67:985–994. doi: 10.1007/s00018-009-0231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Liu Y, Chen Z, Korteweg C, Gu J. Immunoglobulin g (IgG) expression in human umbilical cord endothelial cells. J Histochem Cytochem. 2011;59:474–488. doi: 10.1369/0022155411400871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.