Abstract
The nuclear receptor NR2E1 (also known as TLX or tailless) controls the self-renewal of neural stem cells (NSCs) and has been implied as an oncogene which initiates brain tumours including glioblastomas. Despite NR2E1 regulating targets like p21CIP1 or PTEN we still lack a full explanation for its role in NSC self-renewal and tumorigenesis. We know that Polycomb repressive complexes (PRC) also control stem cell self-renewal and tumorigenesis, but so far, no formal connection has been established between NR2E1 and PRCs. In a screen for transcription factors regulating the expression of the Polycomb protein CBX7, we identified NR2E1 as one of its more prominent regulators. NR2E1 binds at the CBX7 promoter, inducing its expression. Notably CBX7 represses NR2E1 as part of a regulatory loop. Ectopic NR2E1 expression inhibits cellular senescence, extending cellular lifespan in fibroblasts via CBX7-mediated regulation of p16INK4a and direct repression of p21CIP1. In addition NR2E1 expression also counteracts oncogene-induced senescence (OIS). The importance of NR2E1 to restrain senescence is highlighted through the process of knocking down its expression, which causes premature senescence in human fibroblasts and epithelial cells. We also confirmed that NR2E1 regulates CBX7 and restrains senescence in NSCs. Finally, we observed that the expression of NR2E1 directly correlates with that of CBX7 in human glioblastoma multiforme. Overall we identified control of senescence and regulation of Polycomb action as two possible mechanisms that can join those so far invoked to explain the role of NR2E1 in control of NSC self-renewal and cancer.
Keywords: NR2E1, senescence, CBX7, p21CIP1, p16INK4a, Polycomb
INTRODUCTION
The orphan nuclear receptor NR2E1 (also known as tailless or TLX) is critical for maintaining the undifferentiated characteristics and proliferative potential of neural stem cells (NSCs)19, 36, 43. NR2E1 is expressed in the vertebrate forebrain24 and at elevated levels in a number of brain cancers, such as astrocytomas, ependymomas and gliomas suggesting a role in tumorigenesis20, 22, 29, 30, 32, 40, 47.
By analogy to tailless in Drosophila, NR2E1 has generally been viewed as a transcriptional repressor that is able to recruit co-repressors such as histone deacetylase complexes39 and the histone demethylase LSD141 to consensus binding sites in target gene promoters. Prominent examples include the Pten tumour suppressor and Cdkn1a, which encodes the p21CIP1 cyclin-dependent kinase inhibitor39, 42. De-repression of Cdkn1a could in part explain the proliferative impairment of NSCs observed following conditional knockout of Nr2e1/Tlx in the mouse. However, crossing the Nr2e1−/− mice into a Cdkn1a−/− background does not fully rescue the phenotype47. NR2E1 also participates in a feedback loop with the brain specific microRNA, miR-946. Thus, miR-9 down-regulates NR2E1 via a target sequence in the 3′-UTR, whereas NR2E1 represses the expression of miR-9-1 by binding to consensus site(s) in the adjacent DNA.
A broader view of NR2E1 function has emerged from expression profiling of the Nr2e1 knockout cells, which revealed equivalent numbers of up- and down-regulated genes43. It was subsequently confirmed that NR2E1 activates the Wnt7a gene via two consensus binding sites in the promoter, and that Wnt/β-catenin signalling can partially rescue the defect in NSC proliferation caused by NR2E1 knockdown33. However, the possibility that NR2E1 regulates additional targets relevant to its function in NSC self-renewal and cancer clearly remains open.
The Polycomb group (PcG) protein CBX7 is also implicated in the maintenance of stem cell characteristics and cancer. CBX7 is one of five mammalian orthologues of Drosophila Polycomb (Pc) and participates in Polycomb repressive complex 1 (PRC1) along with members of the Posterior sex combs (Psc), Polyhomeotic (Ph) and Sex combs extra (Sce) families37. CBX7 is the predominant Pc orthologue in ES cells and upon differentiation its levels decline and are replaced by CBX4 and CBX825, 28. This down-regulation of CBX7 is in part orchestrated by micro-RNAs from the miR-125 and miR-181 families and by feedback loops with PRC complexes6, 25, 28.
In contrast to ES cells, human diploid fibroblasts (HDFs) express multiple PRC1 components, including CBX4, CBX6, CBX7 and CBX831. CBX7 was first identified in a screen for the bypass of replicative senescence, the state of profound cell cycle arrest that occurs when cells reach replicative exhaustion or are exposed to stress caused by oncogene activation or DNA damaging agents12. Although traditionally studied in cultured human fibroblasts (HFs), senescence is relevant in several physiological contexts including development, ageing and premalignant lesions in vivo8, 9, 17, 27, 38. In particular, senescence limits tumour progression and escape from senescence is one of the hallmarks of cancer15. The role of CBX7 and PRC1 complexes in senescence is largely explained by repression of the CDKN2A tumour suppressor locus, and its primary product, the CDK inhibitor p16INK4a 13. Although this regulation contributes to explain, at least in part, the oncogenic properties of CBX7 in prostate cancer or follicular lymphomas5, 35, the role of CBX7 in cancer is context-dependent as it can behave as a tumor suppressor in lung and pancreatic cancer10, 16.
Here, we identify NR2E1 in a screen for regulators of CBX7 expression and show that NR2E1 can downregulate p16INK4a via effects on CBX7. As a consequence, NR2E1 expression inhibits senescence. In addition to maintain p16INK4a repressed, NR2E1 also directly repressed p21CIP1 with the downregulation of both CDK inhibitors contributing to the ability of NR2E1 to control senescence. Besides identifying a novel pathway regulating CBX7 expression, our work suggests that modulation of Polycomb function and control of senescence are additional mechanisms by which NR2E1 might regulate NSC self-renewal and cancer.
RESULTS
A reporter-based screen for regulators of CBX7 transcription
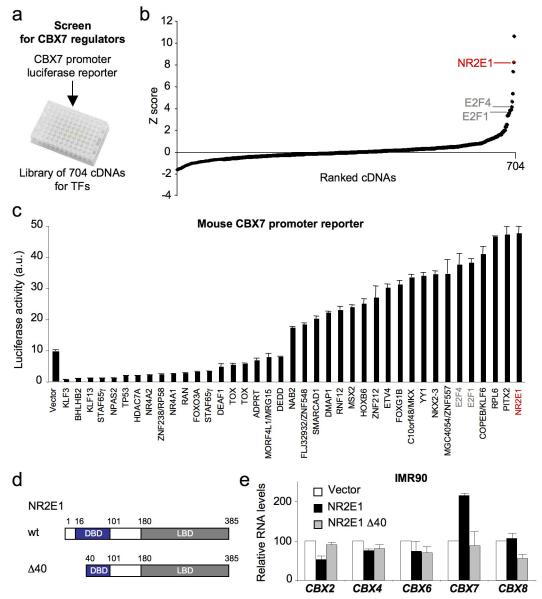
To identify novel factors controlling CBX7 expression, we screened a library of 704 cDNAs encoding known transcription regulators for their ability to regulate a reporter in which a region of the mouse Cbx7 promoter was cloned upstream of the luciferase gene (Fig. 1a, b). Among the top Cbx7 activators we identified several members of the E2F-family, the homeobox protein PITX2, and the nuclear receptor NR2E1. Re-testing of candidates with either the mouse Cbx7 promoter (Fig 1c) or an equivalent reporter based on the human CBX7 promoter (Fig. S1a) confirmed these observations.
Figure 1. A screen for transcription factors regulating CBX7 expression identifies NR2E1/TLX.
(a) Scheme depicting the screen. A mouse CBX7 promoter luciferase reporter was co-transfected in HEK293T cells with a library of cDNAs of transcription factors to screen for cDNAs regulating CBX7 transcription. (b) Results of the screen. (c) Re-testing of candidates for CBX7 regulation identifies E2F family proteins and NR2E1 as activators of CBX7 transcription. (d) Cartoon showing the structure of NR2E1 wt and a DNA binding domain mutant (NR2E1 Δ40). DBD: DNA-binding domain; LBD: ligand binding domain. (e) NR2E1 induces CBX7 but not other CBX family genes. qRT-PCR for CBX family members in IMR90 cells infected with expression vectors for NR2E1 wt or NR2E1 Δ40 or with a control vector.
As E2F-family members are already known to regulate the expression of PcG genes, such as EZH2, SUZ12 and BMI126, we chose to focus on NR2E1, an orphan nuclear receptor without any previously known connections to PcG regulation14. In common with other nuclear receptors, NR2E1 has an amino terminal DNA binding domain and a ligand-binding domain in the carboxy terminal region (Fig. 1d). Whereas wild-type NR2E1 activates the CBX7 promoter, this effect is not seen with a mutated form of NR2E1 (NR2E1Δ40) that lacks 40 residues from the amino-terminal DNA binding domain (Fig. S1b, c). We recently showed that the expression of the five Pc orthologues in mammalian cells is differentially regulated28. In agreement with these observations, NR2E1 wt, but not NR2E1Δ40, induced CBX7 in IMR90 primary HFs without affecting the levels of other Pc orthologues (Fig. 1e).
NR2E1 binds directly to the CBX7 promoter
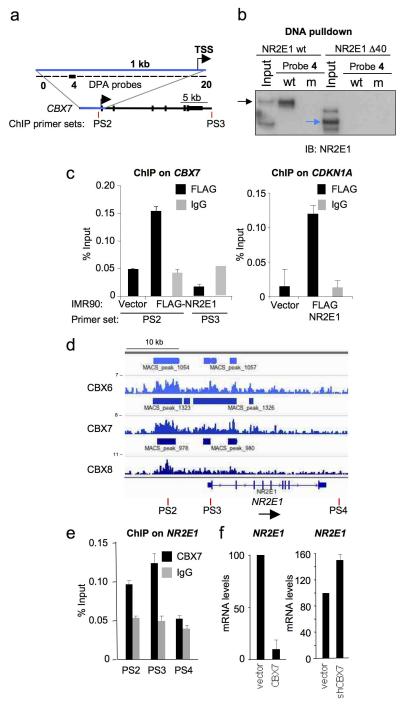
A search for NR2E1 consensus binding sites in the human CBX7 locus revealed multiple candidates, including a site in the promoter region used for the screening. To better define this, we performed a DNA pulldown assay (DNA) using 21 probe sets spanning a region of 1 kb upstream the CBX7 TSS (Fig 2a). Probe 4 resulted in a specific pulldown of NR2E1 (Sup Fig S2a). We observed that a consensus NR2E1 binding sequence was present in that probe, and its mutation resulted in abrogation of NR2E1 binding (Fig 2b). However, we did not observe a significant reduction in NR2E1 activation of a mouse CBX7 reporter lacking the equivalent region (Sup Fig S2b). This suggests the presence of alternative NR2E1 binding sites or indirect mechanisms by which NR2E1 regulated CBX7. To confirm that NR2E1 was binding at the CBX7 locus on native chromatin, we performed chromatin immunoprecipitation (ChIP). Human fibroblasts were infected with a retrovirus encoding a FLAG-tagged version of NR2E1 and FLAG immunoprecipitates were interrogated using two PCR primer sets that sample the 5′ and 3′ ends of CBX7 (Fig. 2c). Clear enrichment, relative to the IgG control, was observed with the primer set 2 (PS2) from the promoter region but not with the primer set 3 (PS3), which served as a specificity control (Fig. 2d). In addition, we also observed NR2E1 binding to the CDKN1A promoter (Fig. 2d), consistent with previous reports showing direct repression of CDKN1A by NR2E139.
Figure 2. A regulatory feedback loop between NR2E1 and CBX7.
(a) Scheme of the CBX7 gene showing the location of primers sets used for ChIP and probes used for DPA. (b) DNA pulldown analysis. DNA pulldown assay confirmed NR2E1 binding to a DNA motif upstream of CBX7 transcription start site. Lysates of HEK293T cells overexpressing NR2E1 wt or the DNA binding domain mutant NRE1 Δ40 were incubated with probe 4, harbouring a wt or mutated NR2E1 DNA binding motif. NR2E1 binding was assessed by immunoblot (IB). Black arrow, NR2E1 wt; blue arrow, NRE1 Δ40. (c) NR2E1 is a direct transcriptional regulator of CBX7. ChIP with FLAG antibody or control IgG were performed on CBX7 (left) or CDKN1A (right, as positive control) in IMR90 cells infected with FLAG-NR2E1 overexpressing vector or with an empty vector. (d) CBX6, CBX7 and CBX8 associate with NR2E1 promoter. ChIP-seq data in Hs68 HFs showing CBX6, CBX7 and CBX8 binding profiles on NR2E1. The location of primer sets used for ChIP on NR2E1 is also depicted. (e) ChIP in IMR90 cells confirming that CBX7 binds to NR2E1 promoter. (f) CBX7 regulate NR2E1 expression, as shown by qRT-PCR upon CBX7 overexpression (left) or knockdown (right) in IMR90 cells.
Evidence for a feedback loop between CBX7 and NR2E1
We recently compared the genome-wide binding profiles of several CBX proteins in HFs31. Strikingly, we found that CBX6, CBX7 and CBX8 co-localize at multiple sites in the genome and, as others have reported, NR2E1 was among the potential PRC1 target genes6, 25 (Fig. 2d). In common with many other loci, the binding profile at NR2E1 has a complicated architecture, with several peaks located upstream, across and downstream of the predicted TSS. To confirm the ChIP-seq data, we prepared a series of PCR primer sets that can discriminate between regions of high and low/no binding in the NR2E1 gene and used these for conventional ChIP assays (Fig. 2d). In line with our previous findings, we could show that CBX7 bind at the NR2E1 locus (Fig. 2e).
The presence of PRC1 proteins at the locus implies that NR2E1 expression could be regulated by CBX7. Consistent with this idea, overexpression of CBX7 resulted in down-regulation of NR2E1 and conversely knockdown of CBX7 up-regulated NR2E1 (Fig. 2f). Taken together, the data imply that NR2E1 and CBX7 expression levels are intimately linked by regulatory feedback mechanisms.
NR2E1 expression inhibits senescence
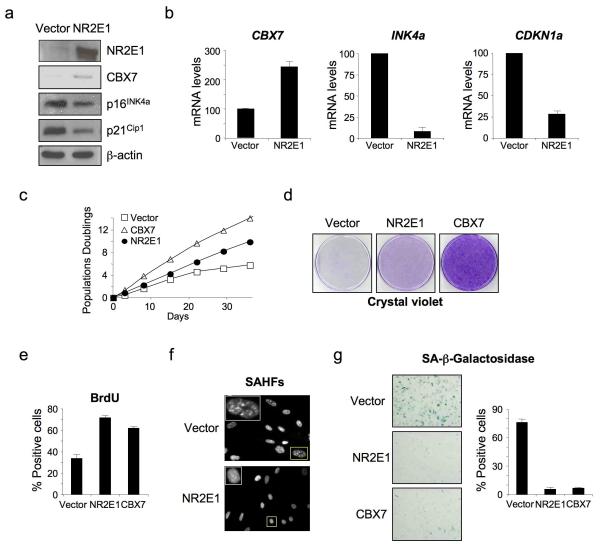
To investigate whether NR2E1 has a role in controlling senescence, we first expressed NR2E1 in late passage IMR90 cells. Upon NR2E1 expression we observed up-regulation of CBX7 accompanied by down-regulation of p16INK4a (Fig. 3a); a more pronounced effect was observed at the RNA level (Fig. 3b). Importantly, the expression of NR2E1 also reduced the levels of p21CIP1 protein and RNA (Fig 3a, b) as previously reported that NR2E1 can do in other contexts39, and in agreement with our data showing binding of NR2E1 to the CDKN1A promoter (Fig 2b).
Figure 3. Expression of NR2E1 inhibits senescence.
(a, b) NR2E1 overexpression in IMR90 results in an increase in CBX7 and decrease in p16INK4a and p21CIP1a levels, as shown by immunoblot (a) and qRT-PCR (b). (c, d) NR2E1 overexpression extends the lifespan of IMR90 cells. IMR90 cells were infected with vectors expressing CBX7 or NR2E1 or a control vector and growth curves were performed (c). Crystal violet staining is also shown (d). (e) BrdU incorporation is increased in NR2E1 overexpressing cells. BrdU incorporation was assessed at passage 24 in IMR90 cells infected with the indicated vectors. (f) NR2E1 overexpression results in prevention of SAHF formation. IMR90 cells infected with NR2E1 expression vector or control vector were fixed and stained with DAPI at passage 24. Representative images are shown. (g) SA-β-galactosidase activity is decreased in cells overexpressing NR2E1. IMR90 cells infected with the indicated vectors were stained for SA-β-galactosidase at passage 24. Representative pictures (left) and the percentage of SA-β-Gal positive cells (right) are shown.
IMR90 cells were then passaged until the empty vector controls reached senescence. NR2E1 expression resulted in an appreciable extension of replicative lifespan, as judged by cumulative population doublings and colony formation assays (Fig 3c and 3d). Consistent with NR2E1 counteracting senescence, a higher percentage of the NR2E1-expressing cells incorporated BrdU (Fig 3e) and a smaller percentage stained positively for SA-β-Gal activity, or showed evidence of senescence-associated heterochromatin foci (SAHFs) (Fig 3f and 3g). The effects of NR2E1 on senescence were dependent on DNA binding and were not observed with the NR2E1Δ40 mutant (Fig S3a-c). In addition NR2E1 expression also affected replicative senescence in a different strain of fibroblasts, WI-38 (Fig S3d).
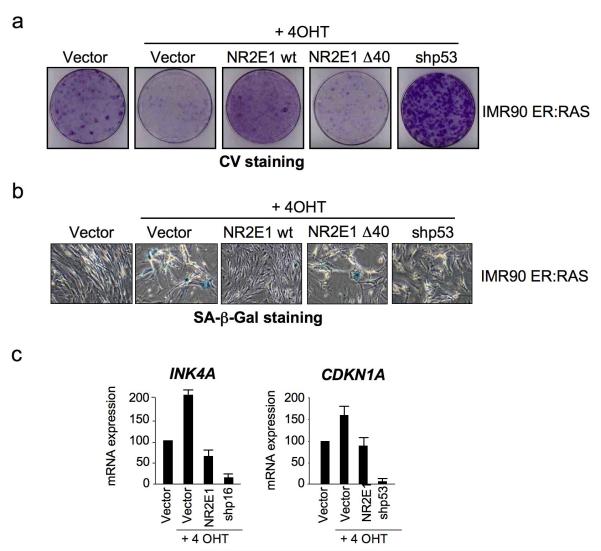
Next we investigated a possible role of NR2E1 in oncogene-induced senescence (OIS). To this end, we overexpressed NR2E1 wt or a mutant version in IMR90 ER:RAS cells, a model of OIS. IMR90 ER:RAS express a chimeric protein that upon 4-hydroxy-tamoxifen (4OHT) addition becomes activated, triggering senescence4. The expression of NR2E1 partially prevented the growth arrest and senescence observed upon RAS induction (Fig 4a, b). Expression of NR2E1 also blunted the induction of p16INK4a and p21CIP1 during OIS (Fig 4c). All together the above results suggest that one of NR2E1 functions is to suppress senescence.
Figure 4. NR2E1 expression controls oncogene-induced senescence (OIS).
(a-b) NR2E1 expression prevents OIS. (a) IMR90 ER:RAS cells were infected with the indicated vectors. ER:RAS was induced by 200 nM 4OHT. 12 days after, cell growth was evaluated by crystal violet staining (a) and senescence induction by SA-β-Gal staining (b). (c) NR2E1 expression prevents the induction of INK4a and CDKN1a during OIS. IMR90 ER:RAS cells were infected with the indicated vectors, the expression of INK4a and CDKN1a was monitored by qRT-PCR.
Loss of NR2E1 expression induces premature senescence
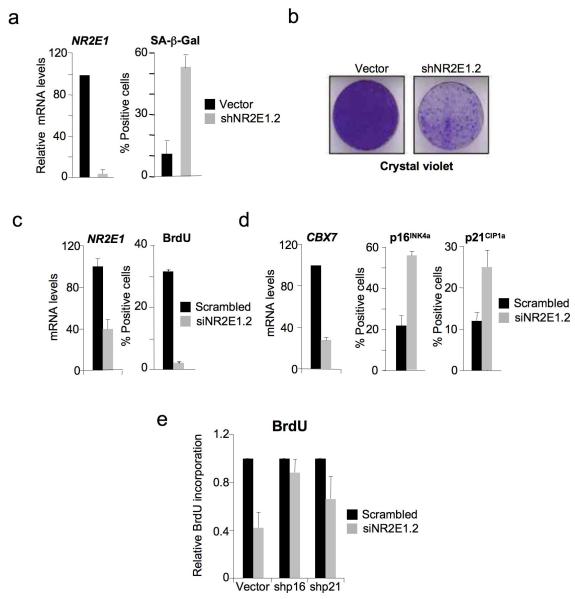
To extend these observations, we investigated whether depletion of NR2E1 would be sufficient to induce premature senescence. To this end, we used recombinant retroviruses to deliver short hairpin RNAs (shRNAs) that target NR2E1 (Fig. S4a). IMR90 infection with shNR2E1.2, which was capable of reducing NR2E1 expression by over 90%, led to decreased cell proliferation and premature senescence (Fig. 5a, b and S3b). These observations were confirmed using shNR2E1.3, another shRNA targeting NR2E1 (Fig. S4b). Similar results were obtained following transfection of IMR90 cells with an NR2E1-specific siRNA. Although the knock-down was less efficient, there was a significant decrease in the proportion of BrdU-positive cells (Fig. 5c). As anticipated, NR2E1 knockdown resulted in down-regulation of CBX7 levels and upregulation of p16INK4a and p21CIP1 (Fig. 5d). Further extending the generality and significance of our results, knockdown of NR2E1 in primary human prostate epithelial cells also causes a senescence-like response accompanied by upregulation of p16INK4a and p21CIP1 (Fig. S4c, d).
Figure 5. NR2E1 depletion causes premature senescence.
(a, b) NR2E1 knockdown by shRNA induces cellular senescence in IMR90. Knockdown efficiency of NR2E1 shRNA (a, left) is shown. The effect of NR2E1 knockdown on the percentage of SA-β-galactosidase positive cells (a, right) and cell growth (b) is shown. (c) NR2E1 knockdown by siRNA inhibits cell proliferation in IMR90, as shown by BrdU incorporation in IMR90 transfected with a scrambled siRNA or a siRNA targeting NR2E1 (right). The efficiency of NR2E1 knockdown was assessed by qRT-PCR (left). (d) NR2E1 knockdown results in a decrease in CBX7 level and an increase in p16INK4a and p21CIP1 levels in IMR90, as shown by CBX7 qRT-PCR (left), p16INK4a IF (middle) and p21CIP1 IF (right). (e) The effect of NR2E1 on senescence is dependent on p16INK4a and p21CIP1a. BrdU incorporation was assessed in IMR90 cells infected with an empty vector (vector) or vectors knocking down p16INK4a (shp16) or p21CIP1a (shp21) and then transfected with a scrambled siRNA or a siRNA targeting NR2E1.
To investigate the contributions of NR2E1 regulation of p16INK4a and p21CIP1 to senescence, we used previously validated shRNAs3 to knock down the expression of these genes (Fig. S4e) and asked what effects they had on the premature senescence caused by depletion of NR2E1. In accordance with our previous data, the proliferation block was partially dependent on both p16INK4a and p21CIP1 (Fig. 5e).
NR2E1 controls CBX7 and prevents senescence in neural stem cells
NR2E1 has been primarily implicated in the regulation and function of neural stem cells (NSC) and initiation of brain tumours20. We therefore sought to investigate whether the mechanisms that we have uncovered in primary fibroblasts also operates in neural cells. We used two different siRNAs to knock down Nr2e1 expression in postnatal mouse neural stem cells (NSCs) (Fig. S5a). Although the knockdown was partial, both siRNAs resulted in reduced expression of Cbx7 and the upregulation of both Ink4a and Cdkn1a (Fig. 6a). The cells also underwent a senescence-like arrest as judged by increased numbers of SA-β–Gal positive cells (Fig. 6b, c). Similar results were obtained using lentiviral vectors to express different shRNAs targeting mouse Nr2e1 (Fig. S5a, b), suggesting that NR2E1 can also regulate CBX7 and senescence in NSCs.
Figure 6. NR2E1 and CBX7 levels are linked in gliomas and neural stem cells.
(a) Nr2e1 knockdown by siRNA in mouse NSC downregulates Cbx7 and upregulates Ink4a and Cdkn1a. NSC were transfected with two different siRNA targeting NR2E1 or a scrambled sequence and qRT-PCR were performed 2 days later. All differences in expression of Nr2e1, Cbx7, Ink4a and Cdkn1a between the scrambled and siNR2e1.4 and scrambled and siNR2e1.5 are p<0.05 as calculated using paired t-test. (b, c) Nr2e1 knockdown by siRNA in NSC causes senescence. NSC were subjected to SA-β-Gal staining. Quantification (b) and representative pictures (c) are shown. (d) The expression of CBX7 and NR2E1 correlate in GBM, as shown by qRT-PCR in samples from GBM patients. a.u, arbitrary units.
Finally, we took advantage of a collection of samples from glioblastoma multiforme (GBM) patients and asked whether there was a link between the expression levels of NR2E1 and CBX7, as judged by qRT-PCR. Interestingly, we observed a direct correlation, suggesting that enhanced levels of NR2E1 associated with GBM formation result in a concomitant increase in CBX7 expression (Fig. 6d). The above results suggest that control of CBX7 and senescence could contribute to explain the role of NR2E1 in NSC self-renewal and its role in gliomagenesis.
DISCUSSION
There has been a great deal of interest in the mechanisms by which PRC complexes regulate gene expression and how they are recruited to target loci, but relatively little is known about the transcriptional regulation of the PcG genes themselves. In particular CBX7 is a PcG important to control senescence12, stem cell self-renewal28 and cancer5, 23, 35, 44. Recently others and we described that CBX7 expression is subjected to repression by PRCs and is controlled by miR-125 and miR-18125, 28, but no much else is known about its regulation. Here, we identify the orphan nuclear receptor NR2E1 as a novel regulator of CBX7 expression.
There are intriguing parallels between CBX7 and NR2E1 as both are implicated in the maintenance of stem-cell characteristics and both were historically viewed as transcriptional repressors. However, there is increasing evidence that they can have both positive and negative influences on gene expression, albeit in different ways. NR2E1 is a DNA binding transcription factor that recruits histone modifying enzymes to regulate transcription of target genes whereas CBX7 operates within a multicomponent complex that associates with and modifies histone tails, rather than interacting directly with DNA. Whereas NR2E1 operates predominantly in neural lineages, CBX7 is more widely expressed. The role of CBX7 in the regulation of INK4a has been well documented in human fibroblasts, the classical model for cellular senescence12. Although the endogenous levels of NR2E1 in HFs are low, ectopically expressed NR2E1 associates with the CBX7 locus and activates its expression. Conversely, CBX7 binds to the NR2E1 locus as a component of PRC1 complexes, and repressed it as part of a regulatory feedback loop.
Our work shows how high levels of NR2E1 restrain senescence by keeping p16INK4a and p21CIP1 expression downregulated. Therefore, besides regulating p16INK4a, NR2E1 also impacts on p21/p53 as suggested from previous work showing that p21CIP1 is a direct NR2E1 target39, confirmed by us in the context of senescence. In addition, the engagement of p16INK4a potentially explains why p21CIP1 knockout does not fully rescue the proliferation of Nr2e1−/− cells47.
As most of the previous literature on NR2E1 relates to its role in neural cells and brain-derived tumours20, we investigated whether the regulation of CBX7 and senescence by NR2E1 that we have uncovered here also operates in these systems. Analysis of the gene expression data from human gliomas provided tentative evidence for a correlation between NR2E1 and CBX7. This is interesting, as a previous work has suggested that Cbx7 behave as a tumor suppressor in gliomas11. However, in that study the effect of Cbx7 was analysed on Cdkn2a−/− mice, which don’t express p16Ink4a or p19Arf, key effectors of senescence. More importantly, knockdown of Nr2e1 in NSCs recapitulated our observations in HFs, suggesting that the ability of NR2E1 to prevent senescence is relevant for its role in self-renewal of NSCs as well as in cancer. Preliminary results with NSCs derived from Cdkn2a−/− mice, suggest that similar to that observed in HFs, the Ink4a/Arf locus contributes to mediate senescence caused by NR2E1 loss (data not shown).
In summary, we show that NR2E1 regulates CBX7 expression. As well as adding to our understanding of the transcriptional control of PcG genes, the data suggest that CBX7 can mediate some of the effects of NR2E1 in NSC and cancer. By controlling senescence via CBX7-dependent and independent mechanisms, NR2E1 adds additional functions to its already long repertoire that could explain its function in controlling NSC self-renewal and its role in gliomagenesis.
MATERIALS & METHODS
Cell culture and retroviral infection
HEK293T, IMR90 and WI-38 cells were obtained from the ATCC. Leiden cells were described previously7. Cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen) with 10% fetal bovine serum (PAA) and 1% antibiotic-antimycotic solution (Invitrogen). Primary human prostate epithelial cells (HPrEC) were obtained from Lonza and maintained in prostate epithelial cell growth medium (PrEGM, Lonza). Methods used for retrovirus production and infection have been previously described1.
NSC Isolation and culture of neural stem cells (NSC)
For isolation of NSCs, the SVZ was dissected from P6-P10 mouse brains as described previously18. Briefly, following isolation, the SVZ was dissociated in HBSS medium (Invitrogen) containing 0.05% trypsin (Invitrogen) and 60U/ml Dnase I (Sigma), washed and plated on poly-l-lysine (PLL)-coated plates in SVZ explant medium [DMEM/F12 (Invitrogen), 3%FBS, 20ng/ml EGF (Peprotech)]. The next day NSCs were purified by fractionation and the medium was replaced with SVZ culture medium [DMEM/F12 (Invitrogen), 0.25% FBS (invitrogen), N2 (Invitrogen), 20ng/ml EGF (Peprotech), 10ng/ml bFGF (Peprotech) and 35 μg/ml bovine pituitary extract] modified from34. NSC were grown to confluency and subcultured in adhesion under the same conditions for up to 8-10 passages. All experiments were performed on freshly isolated NSCs.
Transcription factor library and plasmids
The Transcription Factor library was obtained from Origene. The library contains 704 full-length human cDNAs cloned in the pCMV6-XL5 vector. NR2E1 was cloned into pMIV retroviral vector. NR2E1 Δ40 mutant and FLAG-NR2E1 were generated by PCR. Human and mouse CBX7 promoters were cloned into a pGL3-basic luciferase reporter vector by PCR using a BAC or FOSMID plasmid as template. Plasmids expressing CBX7 have been described previously28.
Reverse transfection and luciferase assay
For the luciferase screen, reverse transfection using Polyethylenimine (PEI) (Polysciences, 23966) was performed in HEK293T cells to individually transfect 704 clones from the transcription factor library in a 96-well plate format. A 20:1 ratio of promoter Luciferase reporter to Renilla construct was used. pGL3-basic was used as control vector. Firefly and Renilla luciferase activities were evaluated using the Dual-Luciferase Reporter Assay system (Promega) 48 h after transfection. Each luciferase value was expressed as the number of median-adjusted standard deviation (z-score value) and a threshold was established to Z-score value <−2.
BrdU assay and crystal violet staining
BrdU labelling and crystal violet staining were performed as previously described1.
Senescence-associated β-Galactosidase staining
IMR90 fibroblasts or NSC were seeded in 6-well plates. Two days later, the cells were fixed with 0.5% glutaraldehyde (w/v) for 15 min and then washed twice with 1 mM MgCl2 in PBS (pH 6.0). X-Gal staining solution (1 mg mL−1 X-Gal, 5 mM K3[Fe(CN)6] and 5 mM K4[Fe(CN)6] in 1 mM MgCl2/PBS (pH 6.0)) was added to the cells for 2-24 h, after which the cells were washed with water and stored at 4 °C, in the dark. The cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 30 min. Bright field and DAPI images were taken and the percentage of SA-β-Gal-positive cells was determined upon counting at least 100 cells per condition.
Antibodies, immunofluorescence and immunoblotting
Immunofluorescence (IF) was performed using an automated high throughput microscope (InCell Analyzer 2000, GE). Image processing and quantification were performed using InCell Investigator software (GE). Immunoblot (IB) was performed following standard procedures. Primary antibodies used in this study are summarized in Sup Table 1.
Quantitative RT-PCR analysis
Total RNA was extracted using miRCURY RNA isolation kit (Exiqon). cDNAs were generated using SuperScript II reverse transcriptase (Invitrogen). PCR reactions were performed in a Real-Time PCR Detection System (BioRad) using Power SYBR Green Master Mix or TaqMan Universal PCR Master Mix (Applied Biosystems). Expression was normalized to ribosomal protein S14 (RPS14) or TATA box binding protein (TBP) expression. Primers and Taqman probes used are listed in Sup Table 2.
RNA interference
IMR90 cells, HPrEC and NSC were transfected with 20-30 nM siRNA (Qiagen), miR or anti-miR (Ambion) in 96-well or 6-well plates. A 3.5% solution of HiPerFect transfection reagent (QIAGEN) was prepared in serum-free medium and then mixed with the siRNA. The mix was incubated for 30 min at room temperature and then added to the cells. The cell culture medium was replaced the following day and cells were either fixed for immunofluorescence (IF) or harvested for RNA extraction 48-120 h later. The Cy™ 3-labelled siGLO™ cyclophilin B siRNA (Dharmacon) was used to monitor transfection efficiency. Sequences specifying shRNAs against human NR2E1 were cloned in pRetroSuper as previously described12. Lentiviral pLKO-based shRNA targeting mouse NR2E1 (pLKO-shNR2E1.4 and pLKO-shNR2E1.4) were obtained from Sigma (TRCN0000026019 and TRCN0000026030). The sequences of siRNA and shRNAs used are listed in Sup Table 3. Knockdown of p16INK4a, and CBX7 was achieved using validated shRNA constructs2, 28.
Chromatin immunoprecipitation (ChIP)
ChIP experiment were performed as described previously21. Immunoprecipitation of crosslinked chromatin was conducted with antibodies listed above. After immunoprecipitation, DNA was extracted using the QIAquick PCR purification kit (Qiagen) and an aliquot amplified by real time qPCR using primers described in Sup Table 4. To confirm target enrichment each PCR product was evaluated first by standard end point PCR.
ChIP-seq and bioinformatics analysis
Parallel ChIP were performed using approximately 5 μg of antibody with 500 μg chromatin. The recovered material was pooled and concentrated to a minimum of 0.2 μg/μl. Input DNA was used as control for the ChIP-seq analysis. Library preparation and Solexa genome-wide sequencing were performed as recommended by the manufacturer. The alignments were performed using novoalign (version 2.07.14; http://novocraft.com) allowing for a single mismatch per read. Duplicates were removed using the Picard MarkDuplicates program (picard-tools package version 1.48; http://picard.sourceforge.net) and peak calling was performed using MACS (version 1.4.0rc2;45).
DNA pulldown assay (DPA)
Lysates from HEK293T cells overexpressing wild type or mutant NR2E1 were prepared in HKMG buffer (10 mM Hepes pH 7.9, 100 mM KCl, 5mM MgCl2, 10% glycerol, 0.5% NP40, 1mM DTT and protease inhibitors (Complete EDTA-free, Roche) and pre-cleared with pre-equilibrated streptavidin-coupled Dynabeads (Invitrogen). Pairs of complementary oligonucleotides (44 bp-long, with the sense oligonucleotide biotinylated at the 5′ end, Sigma, sequences described in Sup Table 5) were annealed and incubated overnight at 4°C with the cell lysates. DNA-bound proteins were collected by incubation for 1h at 4°C with streptavidin-coupled Dynabeads (Invitrogen), washed four times in HKMG buffer and separated by SDS-PAGE. NR2E1 was detected by IB.
Glioblastoma samples
Samples from 28 patients with newly diagnosed, untreated, histologically proven GBM (glioblastoma multiforme) according to the World Health Organization classification, were used. Samples were collected at the Clínica Universidad de Navarra (CUN; Pamplona, Spain). The study protocol was approved by the Institutional Review Board, and all the participants signed the informed consent form approved by the respective Institutional Review Boards or Ethical Committees. NR2E1 and CBX7 transcript levels were measured by RT-PCR using an ABI 7700 sequence detection system (Applied Biosystems). The expression levels relative to GAPDH were calculated and normalized relative to expression in normal brain RNA.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to G. Dharmalingam and S. Khadayate for help with bioinformatics and to I. Fariñas and J.M. Morente for advise and help with NSCs. Core support from MRC and grants from MRCT, CRUK and the AICR funded the research in J. Gil’s laboratory. N Martin was funded by EMBO and Marie Curie fellowships. J. Gil is also supported by the EMBO Young Investigator Programme.
REFERENCES
- 1.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 2.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barradas M, Anderton E, Acosta JC, Li S, Banito A, Rodriguez-Niedenfuhr M, et al. Histone demethylase JMJD3 contributes to epigenetic control of INK4a/ARF by oncogenic RAS. Genes Dev. 2009;23:1177–1182. doi: 10.1101/gad.511109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernard D, Martinez-Leal JF, Rizzo S, Martinez D, Hudson D, Visakorpi T, et al. CBX7 controls the growth of normal and tumor-derived prostate cells by repressing the Ink4a/Arf locus. Oncogene. 2005 doi: 10.1038/sj.onc.1208735. [DOI] [PubMed] [Google Scholar]
- 6.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20:1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brookes S, Rowe J, Ruas M, Llanos S, Clark PA, Lomax M, et al. INK4a-deficient human diploid fibroblasts are resistant to RAS-induced senescence. Embo J. 2002;21:2936–2945. doi: 10.1093/emboj/cdf289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 9.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Forzati F, Federico A, Pallante P, Fedele M, Fusco A. Tumor suppressor activity of CBX7 in lung carcinogenesis. Cell Cycle. 2012;11:1888–1891. doi: 10.4161/cc.20022. [DOI] [PubMed] [Google Scholar]
- 11.Gargiulo G, Cesaroni M, Serresi M, de Vries N, Hulsman D, Bruggeman SW, et al. In vivo RNAi screen for BMI1 targets identifies TGF-beta/BMP-ER stress pathways as key regulators of neural- and malignant glioma-stem cell homeostasis. Cancer Cell. 2013;23:660–676. doi: 10.1016/j.ccr.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Gil J, Bernard D, Martinez D, Beach D. Polycomb CBX7 has a unifying role in cellular lifespan. Nat Cell Biol. 2004;6:67–72. doi: 10.1038/ncb1077. [DOI] [PubMed] [Google Scholar]
- 13.Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol. 2006;7:667–677. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 14.Gui H, Li ML, Tsai CC. A tale of tailless. Developmental neuroscience. 2011;33:1–13. doi: 10.1159/000321585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Karamitopoulou E, Pallante P, Zlobec I, Tornillo L, Carafa V, Schaffner T, et al. Loss of the CBX7 protein expression correlates with a more aggressive phenotype in pancreatic cancer. Eur J Cancer. 2010;46:1438–1444. doi: 10.1016/j.ejca.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 17.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu HK, Belz T, Bock D, Takacs A, Wu H, Lichter P, et al. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 2008;22:2473–2478. doi: 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu HK, Wang Y, Belz T, Bock D, Takacs A, Radlwimmer B, et al. The nuclear receptor tailless induces long-term neural stem cell expansion and brain tumor initiation. Genes Dev. 2010;24:683–695. doi: 10.1101/gad.560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maertens GN, El Messaoudi-Aubert S, Racek T, Stock JK, Nicholls J, Rodriguez-Niedenfuhr M, et al. Several distinct polycomb complexes regulate and co-localize on the INK4a tumor suppressor locus. PLoS One. 2009;4:e6380. doi: 10.1371/journal.pone.0006380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modena P, Lualdi E, Facchinetti F, Veltman J, Reid JF, Minardi S, et al. Identification of tumor-specific molecular signatures in intracranial ependymoma and association with clinical characteristics. J Clin Oncol. 2006;24:5223–5233. doi: 10.1200/JCO.2006.06.3701. [DOI] [PubMed] [Google Scholar]
- 23.Mohammad HP, Cai Y, McGarvey KM, Easwaran H, Van Neste L, Ohm JE, et al. Polycomb CBX7 promotes initiation of heritable repression of genes frequently silenced with cancer-specific DNA hypermethylation. Cancer Res. 2009;69:6322–6330. doi: 10.1158/0008-5472.CAN-09-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monaghan AP, Grau E, Bock D, Schutz G. The mouse homolog of the orphan nuclear receptor tailless is expressed in the developing forebrain. Development. 1995;121:839–853. doi: 10.1242/dev.121.3.839. [DOI] [PubMed] [Google Scholar]
- 25.Morey L, Pascual G, Cozzuto L, Roma G, Wutz A, Benitah SA, et al. Nonoverlapping functions of the Polycomb group Cbx family of proteins in embryonic stem cells. Cell Stem Cell. 2012;10:47–62. doi: 10.1016/j.stem.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Muller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, et al. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 2001;15:267–285. doi: 10.1101/gad.864201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, et al. Programmed Cell Senescence during Mammalian Embryonic Development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 28.O’Loghlen A, Munoz-Cabello AM, Gaspar-Maia A, Wu HA, Banito A, Kunowska N, et al. MicroRNA Regulation of Cbx7 Mediates a Switch of Polycomb Orthologs during ESC Differentiation. Cell Stem Cell. 2012;10:33–46. doi: 10.1016/j.stem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park HJ, Kim JK, Jeon HM, Oh SY, Kim SH, Nam DH, et al. The neural stem cell fate determinant TLX promotes tumorigenesis and genesis of cells resembling glioma stem cells. Molecules and cells. 2010;30:403–408. doi: 10.1007/s10059-010-0122-z. [DOI] [PubMed] [Google Scholar]
- 30.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pemberton H, Anderton E, Patel H, Brookes S, Chandler H, Palermo R, et al. Genome-wide co-localization of Polycomb orthologs and their effects on gene expression in human fibroblasts. Genome Biol. 2014;15:R23. doi: 10.1186/gb-2014-15-2-r23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Qu Q, Sun G, Li W, Yang S, Ye P, Zhao C, et al. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol. 2010;12:31–40. doi: 10.1038/ncb2001. sup pp 31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheffler B, Walton NM, Lin DD, Goetz AK, Enikolopov G, Roper SN, et al. Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci U S A. 2005;102:9353–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott CL, Gil J, Hernando E, Teruya-Feldstein J, Narita M, Martinez D, et al. Role of the chromobox protein CBX7 in lymphomagenesis. Proc Natl Acad Sci U S A. 2007;104:5389–5394. doi: 10.1073/pnas.0608721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, et al. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 37.Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- 38.Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, et al. Senescence Is a Developmental Mechanism that Contributes to Embryonic Growth and Patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 39.Sun G, Yu RT, Evans RM, Shi Y. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc Natl Acad Sci U S A. 2007;104:15282–15287. doi: 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Yokoyama A, Takezawa S, Schule R, Kitagawa H, Kato S. Transrepressive function of TLX requires the histone demethylase LSD1. Mol Cell Biol. 2008;28:3995–4003. doi: 10.1128/MCB.02030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang CL, Zou Y, Yu RT, Gage FH, Evans RM. Nuclear receptor TLX prevents retinal dystrophy and recruits the corepressor atrophin1. Genes Dev. 2006;20:1308–1320. doi: 10.1101/gad.1413606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 44.Zhang XW, Sheng YP, Li Q, Qin W, Lu YW, Cheng YF, et al. BMI1 and Mel-18 oppositely regulate carcinogenesis and progression of gastric cancer. Mol Cancer. 2010;9:40. doi: 10.1186/1476-4598-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16:365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou Y, Niu W, Qin S, Downes M, Burns DK, Zhang CL. The nuclear receptor TLX is required for gliomagenesis within the adult neurogenic niche. Mol Cell Biol. 2012;32:4811–4820. doi: 10.1128/MCB.01122-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.