Abstract
Human immunodeficiency virus-1 (HIV) infection can cause characteristic neural defects such as progressive motor dysfunction, striatal pathology, and gliosis. Recent evidence suggests that HIV-induced pathogenesis is exacerbated by heroin abuse and that the synergistic neurotoxicity is a direct effect of heroin on the CNS, an alarming observation considering the high incidence of HIV infection with injection drug abuse. Although HIV infection results in neurodegeneration, neurons themselves are not directly infected. Instead, HIV affects microglia and astroglia, which subsequently contributes to the neurodegenerative changes. Opioid receptors are widely expressed by macroglia and macroglial precursors, and the activation of µ-opioid receptors can modulate programmed cell death, as well as the response of neural cells to cytotoxic insults. For this reason, we questioned whether opioid drugs might modify the vulnerability of macroglia and macroglial precursors to HIV-1 Tat protein. To address this problem, the effects of morphine and/or HIV Tat1–72 on the viability of macroglia and macroglial precursors were assessed in mixed-glial cultures derived from mouse striatum. Our findings indicate that sustained exposure to morphine and Tat1–72 viral protein induces the preferential death of glial precursors and immature oligodendroglia. Moreover, the increased cell death is mediated by µ-opioid receptors and accompanied by the activation of caspase-3. Our results imply that opiates can enhance the cytotoxicity of HIV-1 Tat through direct actions on glial precursors and/or astroglia, suggesting novel cellular targets for HIV-opiate interactions.
Keywords: Human immunodeficiency virus, opioid receptors, striatum, drug abuse, morphine, glial precursors
Introduction
Drug abuse contributes to the spread of the human immunodeficiency virus (HIV) pandemic (Petito et al., 1999). Besides needle sharing among injection drug users, or the exchange of sex for drugs, as modes for viral spread, the drugs themselves may intrinsically affect the pathogenesis of HIV (Nath et al., 2000; Gurwell et al., 2001; Turchan et al., 2001; Nath et al., 2002). Heroin, morphine, and other opiate drugs of abuse promote HIV infection and the progression to AIDS (Arora 1990; Peterson et al., 1991; Rouveix 1992; Peterson et al., 1993), and appear to accelerate the frequency and severity of HIV encephalitis (HIVE) (Bell et al., 1996; Bell et al., 1998). The enhanced pathogenesis is likely caused by both indirect (e.g., immune suppression) and direct neurotoxic mechanisms (Donahoe & Falek., 1988; Arora 1990; Peterson et al., 1991; Rouveix 1992; Peterson et al., 1993).
Drugs derived from the opium poppy (referred to as “opiates”) mimic endogenous opiate peptides (referred to as “opioids”) through preferential actions at µ-opioid receptors. Opioids modify immune function including HIV propagation in lymphocytes and monocytes/macrophages (Carr & Serou., 1995; Nair et al., 1997; Stoll-Keller et al., 1997; Peterson et al., 1998; Houghtling et al., 2000; Gekker et al., 2001; Suzuki et al., 2001; Suzuki et al., 2002a; Suzuki et al., 2002b; Rogers & Peterson., 2003). Morphine increases transforming growth factor-α production in human and murine microglia (Chao et al., 1994). Despite the prevalence of HIV infection among injection drug users and the importance of the opioid system in the pathogenesis of HIV, it is uncertain how opiate abuse augments the neuropathology of HIV (Nath et al., 2000; Nath et al., 2002).
Many brain regions that are preferentially disrupted by HIV are also enriched in µ-opioid receptors. The basal ganglia are vulnerable to HIV infection and are important targets for opiate abuse (Mansour et al., 1988; Masliah et al., 1992a; Glass et al., 1993; Mansour et al., 1994; Berger & Nath., 1997; Nestler & Aghajanian., 1997; Kreek & Koob., 1998; Nath et al., 2000; Bansal et al., 2000; Koob 2000). Morphine potentiates the toxic effects of HIV-derived proteins (Gurwell et al., 2001; Nath et al., 2002),which are intrinsically neurotoxic (Epstein & Gendelman., 1993; Masliah et al., 1996; Scorziello et al., 1998; Rappaport et al., 1999; Wesselingh & Thompson., 2001; Kaul et al., 2001; Nath 2002; Garden et al., 2002; Haughey & Mattson., 2002). However, because subpopulations of striatal glia (and glia from other brain regions) express µ-opioid receptors (Eriksson et al., 1991; Stiene-Martin et al., 1998; Gurwell et al., 2001), and opioids can modulate programmed cell death normally or following exposure to an apoptotic insult (Meriney et al., 1985; Singhal et al., 2002), we assessed opiates might modify the pathogenesis of HIV by affecting macroglial survival directly. Our results show that morphine acts synergistically with HIV-1 Tat1–72 to increase the rate of death to glial precursors and their progeny, which may contribute to the accelerated neurodegenerative changes seen with HIV and opiate abuse.
Materials and methods
Cell culture medium consisted of Dulbecco’s Modified Eagle Medium (DMEM without phenol red) supplemented with antibiotic-antimycotic (100x), L-glutamine, and Dulbecco’s phosphate buffered saline (Gibco Life Technologies; Gaithersburg, MD, USA). Morphine sulfate, β-funaltrexamine hydrochloride (β-FNA), naloxone hydrochloride, glucose, dimethyl sulfoxide, MgCl2, and HEPES were purchased from RBI/Sigma-Aldrich (St. Louis, MO, USA). Trypsin and DNAse were obtained from Worthington Biochemical Corporation (Lakewood, NJ, USA).
Cell Culture
Mixed glia were isolated from newborn or 1-day-old ICR mice (Charles River) as previously described (Stiene-Martin et al., 1998). All experiments conformed to the local Institutional Animal Care and Use Committee (IACUC) and national (PHS) guidelines on the care and ethical use of animals. All experiments were conducted to minimize the number of mice used and their suffering. Briefly, mice were anesthetized by gas anesthesia and euthanized by decapitation as previously described (Stiene-Martin et al., 1998). Striata were aseptically isolated and cells pooled from 2–3 striata. Growth medium favoring mixed glia consisted of DMEM supplemented with glucose (27 mM), Na2HCO3 (6 mM), and 10% (v/v) Fetal Bovine Serum (FBS; JRH Biosciences; Lenexa, KS, USA). Cells were plated at 100,000 cells/well in poly-L-lysine-coated 24-well plates (Costar, Corning Life Sciences; Acton, MA, USA). Mixed glial cultures contained differentiated astrocytes and oligodendrocytes, as well as undifferentiated glial precursors. In the present study, we define “glial precursors” as nestin+/A2B5+/GD3+ and did not attempt to further distinguish among glial restricted precursor (GRP) types [e.g., GRP1 versus GRP2; see (Liu et al., 2002)], or attempt to distinguish among GRP1 and oligodendrocyte-type 2 astrocyte (O-2A) progenitors, which some investigators consider to be similar (Liu et al., 2002). Less than 0.4% microglia are present in striatal cultures (Stiene-Martin et al., 1998; Gurwell et al., 2001) (Hauser, unpublished). Cells were maintained for 6–10 days in vitro at 35–36°C in 5% CO2/ 95% air at high humidity.
Tat
The tat gene encoding the first 72 amino acids was amplified from HIVBRU obtained from Dr. Richard Gaynor through the AIDS repository at the NIH and inserted into an E. coli (PinPoint Xa-2) vector (Promega, Madison, WI, USA). Recombinant active Tat1–72 was prepared as described previously (Ma & Nath., 1997) with minor modifications (Gurwell et al., 2001). Inactive Tat (TatΔ31–61) was generated from a deletion mutant of the active Tat plasmid, which lacked the sequence encoding the neurotoxic epitope (amino acids 31–61) of Tat1–72 (Nath et al., 1996). Tat1–72 proteins expressed from this construct are naturally biotinylated and can be purified on a column of soft release avidin resin, cleaved from the fusion protein-using factor Xa, eluted from the column followed by desalting on a PD10 column.
Opioid Treatments
Cells were treated with medium alone (vehicle-treated controls), morphine sulfate (500 nM) (Sigma) with or without naloxone (1.5 µM) (a µ-, δ-, and κ-opioid receptor antagonist) or β-FNA (1.5 µM) (a selective µ-opioid receptor antagonists). Opioid drugs and Tat were prepared in basal cell culture medium before use; concentrations and treatment intervals varied as noted.
Viability
Mixed glial cultures were continuously exposed to opioids and/or HIV-1BRU Tat1–72 (or TatΔ31–61) for 24 or 96 h, while vehicle-treated cultures served as vehicle-treated controls. Following treatments, cultures were rinsed and incubated in Dulbecco’s phosphate buffered saline (DPBS) containing ethidium homodimer (3.5 µM) and calcein-AM (4 µM) for 30 min at 35°C in 5% CO2/95% air using the Live/Dead assay kit (Molecular Probes, Eugene, OR, USA) as previously described (Hauser et al., 2000). The proportion of dead/dying flat, polyhedral (type 1) astroglia, as well as undifferentiated glial precursors and their progeny, were sampled and determined as previously described (Hauser et al., 2000). As noted before, the identity of glial precursors was confirmed by nestin+/A2B5+/GD3+ immunoreactivity. However, because the expression of a cell type specific marker might be attenuated or lost in dying cells, studies using viability assays and morphologic criteria (not relying on immunocytochemical markers criteria) were also performed. Approximately 400–600 glia were counted per experimental group. Independent determinations were made from 4–5 experiments and reported as the mean ± SEM.
To detect active caspase-3, phospho-specific rabbit anti-human/mouse caspase-3 antisera was obtained from R & D Systems, Inc (Minneapolis, MN, USA) and used at a concentration of 1:400. Anti-phosphospecific caspase-3 antibodies were detected using goat anti-rabbit conjugated to Cy3 (Jackson ImmunoResearch, West Grove, PA, USA; 1:300 dilution). Caspase-3 becomes activated in the cytoplasm before translocating into the nucleus during apoptosis. Because caspase-3 activation precedes apoptotic cell death, caspase-3 was assayed at 24 h following opioid and/or Tat exposure. Some cell cultures were pretreated with opioid antagonists (β-FNA and naloxone) for 15 min prior to morphine treatment. The proportion of undifferentiated glia displaying active caspase-3 positive in their nuclei versus non-labeled cells was determined using a Nikon Diaphot (Melville, NY, USA) fluorescent microscope with 100x–fluorecent objective.
To determine the phenotypic identity of the dying cells, cultures were incubated with 0.5 µg/ml ethidium monoazide (EMA; Molecular Probes) in DPBS at 36°C. EMA was photoaffinity linked (exposure at a 20 cm distance from a 60 W fluorescent lamp for 30 min) to the DNA of dying cells and combined with immunocytochemical detection of phenotypic determinants as described below.
Immunocytochemistry
Immature neural cells were detected using anti-nestin monoclonal IgG1 [1:1 dilution; Developmental Studies Hybridoma Band (DSHB), University of Iowa, Iowa City, USA], and mouse anti-GD3 monoclonal IgG3 (1:50; National Cancer Institute BRB Preclinical Repository, Bethesda, MD, USA) (Goldman & Reynolds., 1996) antibodies. Mouse anti-A2B5 IgM (1:200, Chemicon) was used to detect oligodendrocyte-type 2 astrocyte bipotential (O-2A) progenitors (Raff et al., 1983; Raff et al., 1984; Gard & Pfeiffer., 1990). As mentioned, some investigators consider O-2A progenitors to be similar to oligodendrocyte precursors (Dietrich et al., 2002) or GRP 1 cells (Liu et al., 2002). Neurons were detected using anti-polysialyated neural cell adhesion molecule (E-NCAM) IgM (1:1; DSHB), mouse anti-neuronal nuclear (NeuN) monoclonal IgG1 (1:100; Chemicon, Temecula, CA, USA), or rabbit anti-protein gene product (PGP) 9.5 antisera (1:1800 dilution; Chemicon). PGP 9.5 is a neuronal ubiquitin carboxyl-terminal hydrolase (Wilkinson et al., 1989), which may additionally label some non-neuronal neural crest derivatives and endocrine cells (Thompson et al., 1983). Astrocytes were detected using mouse anti-glial fibrillary acidic protein (GFAP) monoclonal antibodies (1:300, Boehringer Mannheim, Indianapolis, IN, USA) or rabbit anti-GFAP antisera (1:600 dilution; Chemicon). Oligodendrocytes were identified using anti-O4 rat IgM monoclonal antibodies [1:2 dilution from ascites fluid; gift from Dr. M. Schachner (Sommer & Schachner., 1981; Bansal et al., 1989)]. Immunodetection of µ-opioid receptors (µOR) was performed using rabbit anti-µOR1 (MOR1) (1:1000) affinity purified, polyclonal antisera (PharMingen; San Diego, CA, USA). In some instances, cells were counterstained with Hoechst 33342 dye (Sigma).
Primary antibodies were detected using appropriate species and/or immunoglobulin-specific secondary antibodies conjugated to fluorescent tags. Secondary anti-rabbit antibodies conjugated to CyDyes™ (Cy3 or Cy5, 1:250 dilution; Amersham Biosciences, Pittsburgh, PA, USA), or Alexa (Alexa 488 or 350; Molecular Probes) fluorochromes. Viability was measured in distinct cell types by combining cell-type-selective immunocytochemical markers with a functional assay for viability (EMA photoaffinity labeling).
Statistical Analysis
Effects of opioids and/or Tat on glial viability were assessed using ANOVA and followed by Duncan’s post hoc test when significant ANOVA treatment effects were noted (Statistica 6.0, StatSoft, Tulsa, OK, USA). Concentration-dependent differences in Tat toxicity ± morphine were compared using Student’s t test. Data are reported as the mean ± SEM of 4-6 experiments. Treatment effects were considered significant if P < 0.05.
Results
Effects of opiates & Tat1–72 on glial cell viability
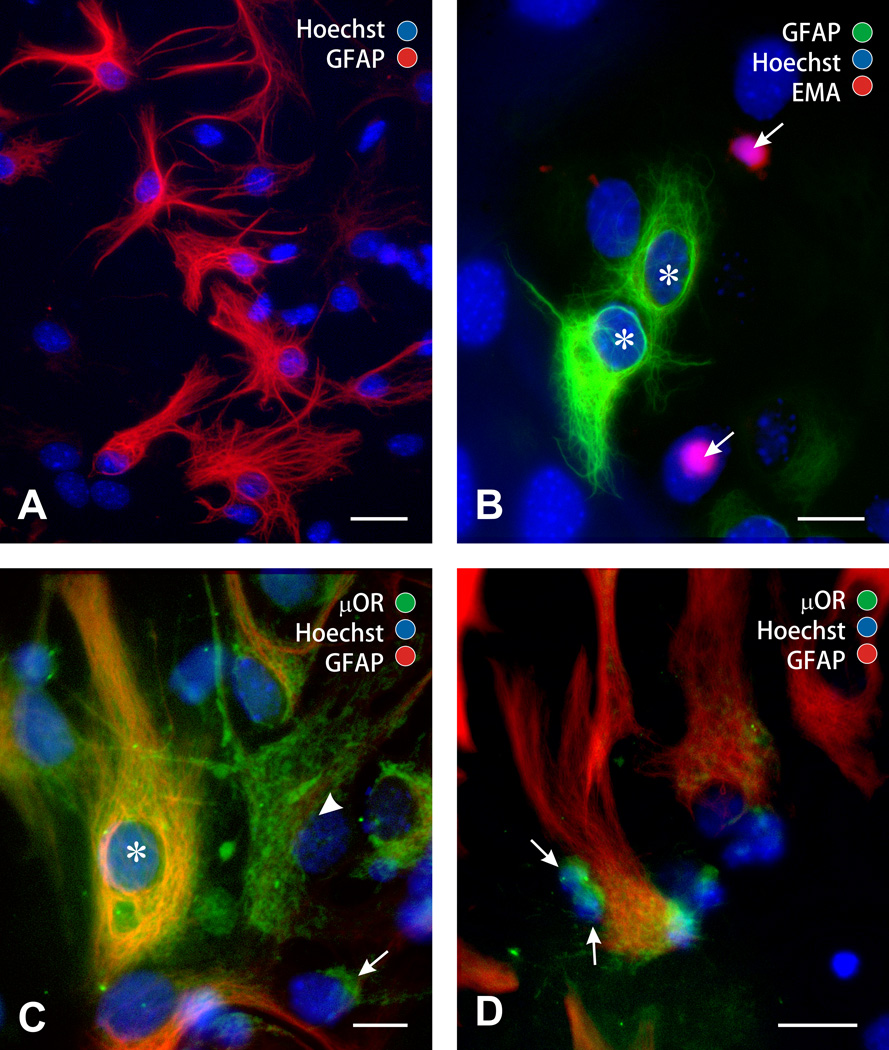
Flat, polyhedral (type 1) astroglia were identified by distinct morphologic and immunologic characteristics (GFAP+/A2B5−). Type 1 astrocytes were readily discerned from, immature neural precursors, which are round and undifferentiated, smaller diameter, and morphologically distinct (arrows; Fig. 1A,B) (Table 1). µ-Opioid receptors are expressed by a subpopulation of type 1 astrocytes in the striatum (Stiene-Martin et al., 1998; Stiene-Martin et al., 2001), as well as nestin+/GFAP− less differentiated glial precursors. Most of the small, undifferentiated neural precursors (91.0 ± 1.3%) expressed µ receptor immunoreactivity (Fig. 1C,D).
Figure 1.
Phenotypic characterization of neural cell populations in mixed-glial cultures. Mixed- glial cultures contained large numbers of flat, polyhedral (type 1) astrocytes that were glial fibrillary acidic protein (GFAP+) immunoreactive (A); scale bar = 25 µm. Besides type 1 astrocytes (asterisks), mixed glial cultures also contained large numbers of undifferentiated glia/glial precursors (arrows) (B). The undifferentiated variants were round, small-diameter, and morphologically distinct lacking mature cytoplasmic processes (arrows) from type 1 astrocytes (asterisks); scale bar 15 = µm (B). µ-Opioid receptors (µOR) are expressed by subpopulations of type 1 astrocytes (µOR+/GFAP+; asterisk), as well as less (arrow) and partially (arrowhead) differentiated flat, polyhedral cells presumed to be type 1 astrocyte precursors (µOR+/GFAP−) (C). Small, undifferentiated glial precursors almost uniformly expressed µ-opioid receptors (arrows) (D); scale bar = 20 µm.
Table 1.
Characterization of the cell types present within striatal mixed glial cultures.
| Cell Type | Percentage of the Total Cells (%) |
|---|---|
| Astrocytes | |
| Type 1 (GFAP+ / A2B5−; large diameter, flat polyhedral) |
61.0 ± 3.1 |
| Type 2 (GFAP+ / A2B5+; small diameter, process-bearing) |
0.4 ± 0.1 |
|
Glial precursors/Immature oligodendroglia* |
38.7 ± 3.0 |
| (non-GFAP) (A2B5+ / Nestin+ / GD3+) |
|
| Neurons | ND |
| (NeuN+ / E-NCAM+) (GFAP−) |
|
Glial precursor/immature oligodendroglia expressed immature markers (A2B5, nestin, and GD3) and were morphologically undifferentiated (small diameter, round, and lacking cytoplasmic processes). Mean ±SEM from 4 experiments.
ND = Not Detected; NeuN+ or E-NCAM+ cells were not detected
Astroglia
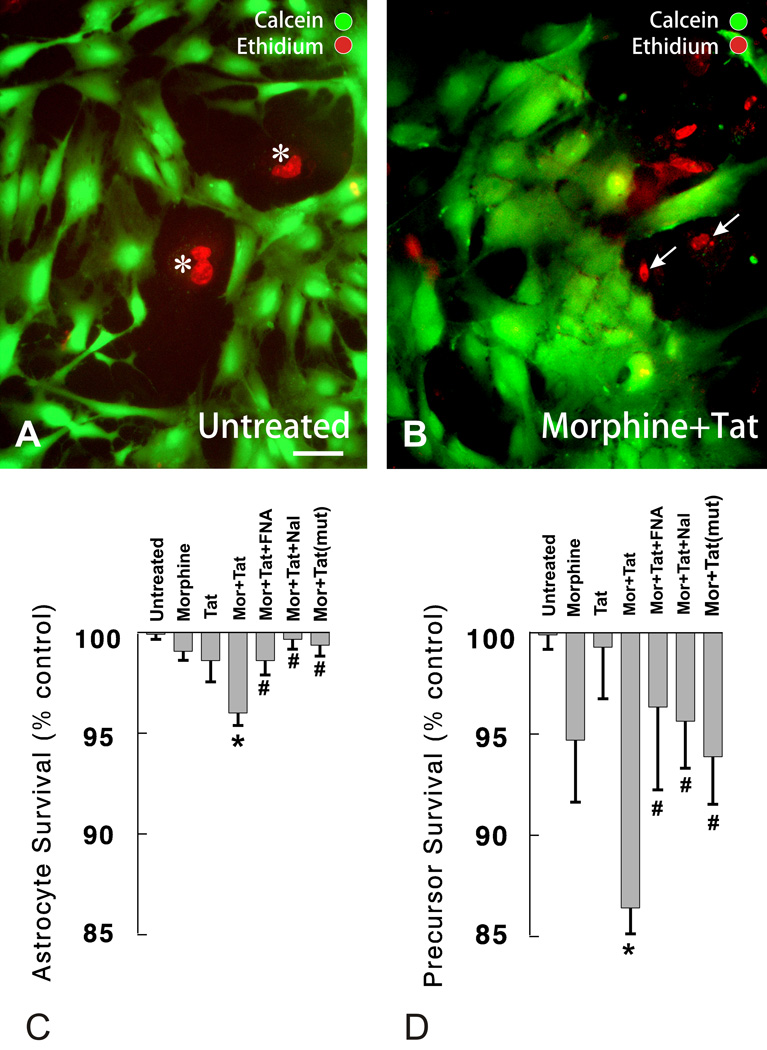
Fluorescently labeled living (calcein+) and dying (ethidium homodimer+) cells were characterized based on morphologic criteria as (i) type 1 astrocytes—flat and polyhedral with a single, large ovoid nuclei containing multiple nucleoli or as (ii) small, round, process-bearing (or without processes), and undifferentiated cells (Fig. 2). Further characterization of the glial subpopulations using EMA and cell-type-specific antigenic markers confirmed that the calcein/ethidium homodimer labeled cells were GFAP-immunoreactive type 1 astrocytes (Table 2).
Figure 2.
Effects of morphine and/or Tat1–72 on cell survival at 96 h was assessed in mixed-glial cultures by fluorescently labeling living (calcein+) and dying (ethidium homodimer+) cells. Cells were characterized morphologically as flat and polyhedral with single, large ovoid nuclei containing multiple nucleoli [morphologically identical to (type 1) astrocytes] (A) or as small, round or process-bearing, and undifferentiated cells. Combined morphine (Mor) (500 nM) and Tat1–72 (Tat) (100 nM) exposure, increased the proportion of dying type 1 astrocytes (asterisks in A; C), as well as the proportion of dying small-diameter, undifferentiated glia/glial precursors (arrows in B; D). Combined morphine and Tat1–72 toxicity was significantly attenuated by coadministering naloxone (Nal; 1.5 µM) or β-funaltrexamine (β-FNA; 1.5 µM) (C, D), or by substituting TatΔ31–61 [Tat (mut)] instead of Tat1–72 (Tat) (C,D); *P < 0.05 vs. vehicle-treated cultures, or cultures treated with morphine or Tat1–72 alone; #P < 0.05 vs. morphine + Tat1–72-treated cultures; A, B; same magnification; scale bar = 15 µm.
Table 2.
The cytotoxic effects of concurrent morphine and Tat1–72 exposure varied among different glial types. Combined morphine and Tat1–72 treatment increased the proportion of ethidium monoazide-labeled (EMA+) dying glial precursors and flat, polyhedral (type 1) astrocytes.
| Cell Type | Vehicle-treated controls (% dying)b |
Combined morphine-Tat1–72 exposed (% dying)b |
|---|---|---|
|
Undifferentiated Neural Cells (non-GFAP) (small, morphologically undifferentiated) |
11.6 ± 1.9 | 24.0 ± 3.5* |
|
Immature Oligodendrocytes (O4+) |
14.9 ± 1.4 | 20.7 ± 4.7 |
|
Astrocytes (GFAP+) |
||
| Type 1 (flat, polyhedral) | 1.4 ± 0.6 | 6.2 ± 0.8* |
| Type 2 (process-bearing) | 6.3 ± 3.2 | 8.8 ± 6.8 |
P < 0.05 versus vehicle-treated controls (Student’s t test).
Values are the number of dying cells as a percentage of the total cells of a particular phenotype in striatal mixed glial cultures. For example, % dying oligodendrocytes = [(EMA+ oligodendrocytes/total oligodendrocytes) x 100] and are presented as the mean ± SEM from 4 experiments.
At 24 h following exposure, neither morphine (500 nM) nor Tat1–72 (100 nM) alone or in combination induced significant increases in astroglial death compared to vehicle-treated controls (data not shown), confirming previous findings (Gurwell et al., 2001).
Unlike at 24 h, significant increases in type 1 astrocyte death were apparent in a small proportion of astrocytes following combined morphine (500 nM) and Tat1–72 (100 nM) exposure at 96 h (Fig. 2A–C ). Morphine and Tat1–72 in combination showed significantly greater toxicity compared to morphine (P < 0.01) or Tat alone (P < 0.025). Neither morphine nor Tat alone was toxic compared to vehicle-treated controls. The interactive toxicity was significantly attenuated by naloxone (1.5 µM) (P < 0.0025, β-FNA (1.5 µM) (P < 0.025), or when morphine was coadministered with a non-toxic deletion mutant of Tat (TatΔ31–61) (P < 0.005). Although there were significant increases in the death of type 1 astrocytes, it is noteworthy that the dying cells only represent a small proportion of the total astrocyte population (Fig. 2). Only 1.2 ± 0.3% of cells with type 1 astroglial morphology normally die in vehicle-treated cultures. Therefore, a vast majority of type 1 astrocytes (∼95%) survived irrespective of morphine-Tat1–72 treatment and displayed no cytological evidence of impending death such as plasmalemmal blebbing, nonuniform calcein-AM distribution, and cytoplasmic vacuoles. Although morphine alone failed to increase astroglial death, morphine appeared to induce cellular hypertrophy as previously described (Stiene-Martin et al., 1991; Hauser et al., 1996). Moreover, similar types of changes were observed in astrocytes with combined morphine and Tat1–72 exposure, although cellular hypertrophy was not measured directly.
Glial precursors/Immature glia
At 24 h, few small, undifferentiated glia were observed in mixed-glial cultures derived from the striatum. At this time, few dying cells were evident using viability assays in vehicle-control or experimental treatment groups.
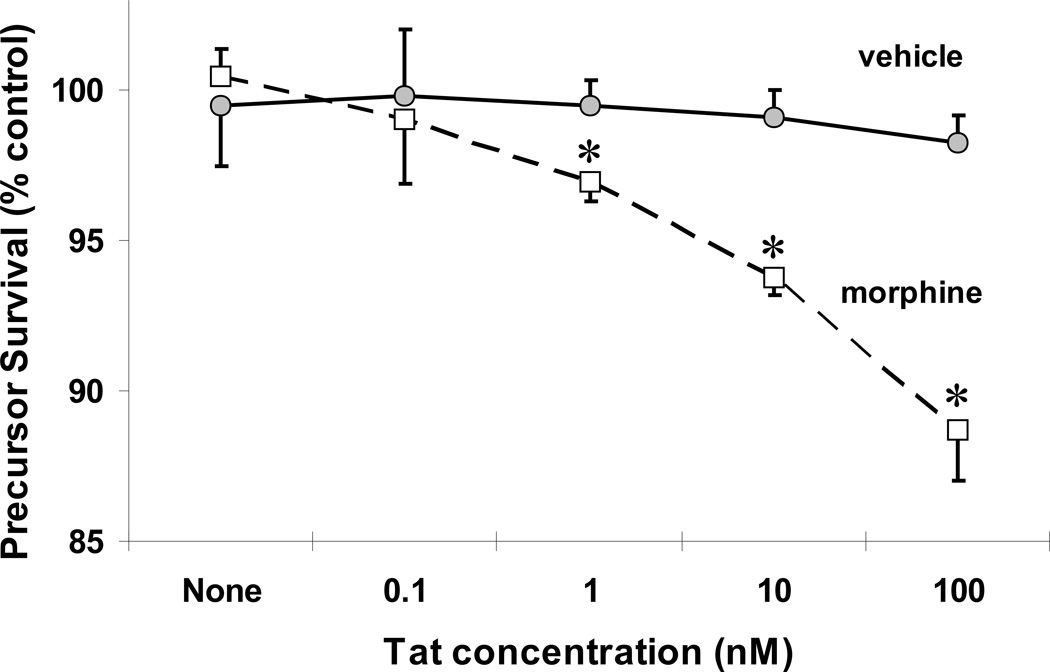
By contrast, at 96 h, greater numbers of undifferentiated glia were present suggesting the rapid growth and proliferation of glial precursors during the first few days in vitro. Using the ethidium homodimer and calcein viability assay, significant increases in the death of small, undifferentiated glia were readily seen following combined morphine (500 nM) and Tat1–72 (100 nM) exposure (P < 0.001 vs. vehicle-treated controls), that were not apparent with morphine or Tat treatment alone (Fig. 2D). To further explore the interactive toxicity between morphine and Tat, mixed glial were exposed to a saturating concentration of morphine (500 nM), and increasing concentrations of Tat (Fig. 3). In the presence of morphine, Tat caused significant, concentration-dependent losses in glial precursors at 1 nM (P < 0.025), 10 nM (P < 0.005) or 100 nM (P < 0.001) concentrations, but not at a 0.1 nM concentration (Fig. 3). Cytotoxicity was not evident with 10 nM or 100 nM Tat alone (Fig. 3). To better identify the type(s) of cells that were dying following combined morphine and Tat1–72 exposure, cells were photoaffinity labeled with EMA and assessed immunocytochemically. The findings showed that many of the cells displaying significant increases in EMA+-labeling were dying glial precursors based on morphologic (small diameter, undifferentiated cells) and immunologic (e.g., the cells did not express GFAP) criteria (see Table 2).
Figure 3.
Effect of Tat1–72 concentration on the survival of glial precursors in the presence and absence of morphine. Mixed glia were exposed to increasing concentrations of Tat with or without morphine (500 nM) and the viability of undifferentiated glia was assessed in calcein+ and ethidium homodimer+-labeled cells. In the presence of morphine, Tat caused significant, concentration-dependent losses in glial precursors at 1 nM, 10 nM or 100 nM concentrations (*P < 0.025 vs. treatment without morphine), but not at 0.1 nM. Cytotoxicity was not evident with when cells were exposed to Tat alone at 0.1 to 100 nM concentrations.
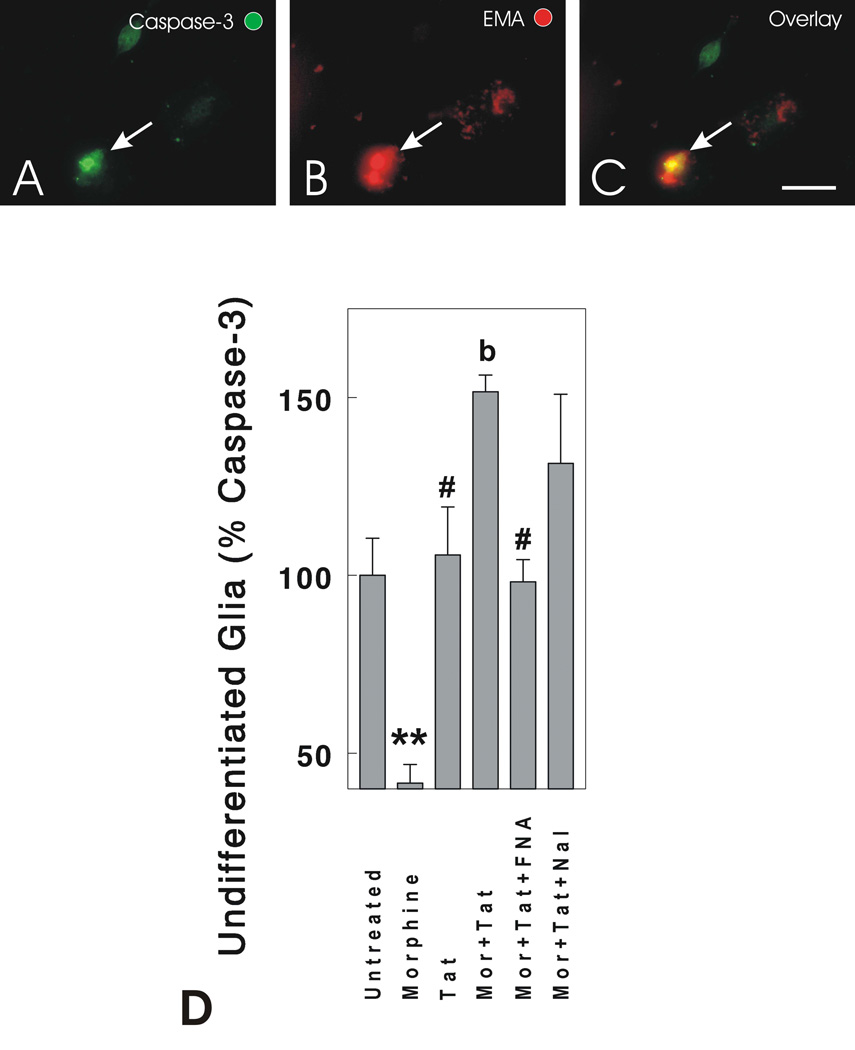
Although large numbers of dying undifferentiated glia were not seen at 24 h using cell viability assays (ethidium homodimer or EMA), significant increases in caspase-3 activation were noted at 24 h in combined morphine- (500 nM) and Tat1–72- (100 nM) treated cultures compared to cultures treated with vehicle (P < 0.01), morphine (P < 0.001), or Tat1–72 (P < 0.05) alone (Fig. 4). In the vehicle-treated controls, 15.4 ± 1.6 of the undifferentiated glia were caspase-3 immunopositive. Interestingly, morphine exposure alone caused significant reductions in caspase-3 activation compared to vehicle-treated controls suggesting that morphine is anti-apoptotic (P < 0.005) (Fig. 4). In a few cases, cells expressing activated caspase-3 were EMA+ (Fig. 4A–C); however, most caspase-3-positive cells did not yet display EMA suggesting that caspase-3 activation precedes cell death by a significant amount of time, which has been observed in striatal neurons (Singh et al., 2003).
Figure 4.
Effects of opioids and/or Tat1–72 on caspase-3 activation in undifferentiated glia at 24 h following exposure. Phospho-specific antibodies were used to detect activated caspase-3 in the nuclei of dying cells using fluorescence microscopy (A,C). Caspase-3 activation preceded cell death, although a subset of ethidium monoazide positive cells (EMA) also possessed activated caspase-3 (arrow shows the same dying cell in A-C). Morphine (Mor) caused a significant reduction in the proportion of undifferentiated glia possessing active caspase-3, while significant increases in caspase-3 activation and translocation into cell nuclei were seen when morphine and Tat were combined (D). The combined effects of morphine + Tat were blocked by 15 min preexposure to the non-competitive, selective µ-opioid receptor antagonist β-FNA, but not by the competitive µ, δ, and κ-opioid receptor antagonist naloxone (Nal) (D); **P < 0.01 vs. other treatment groups; #P < 0.05 vs. morphine + Tat1–72-treated cultures; bP < 0.05 vs. other treatment groups—except Mor+Tat+Nal; scale bar = 10 µm).
Further examination indicated that a majority of the undifferentiated cells exhibited an antigenic profile indicative of GRPs or O-2A cells, which included immunoreactivity for nestin (55.7 ± 2.9%), GD3 (81.7 ± 3.3%), and A2B5 (65.1 ± 1.8%), but failed to display immunoreactivity for neuronal (NeuN or E-NCAM), oligodendroglial (O4), or astroglial (GFAP) phenotypic markers (Fig. 5). A subset of undifferentiated cells (1.2 ± 0.3%) expressed PGP 9.5, which labels neurons, as well as some non-neuronal neural crest-derivatives and endocrine cells (Thompson et al., 1983; Wilkinson et al., 1989). However, because NeuN and E-NCAM immunoreactivity were not evident, and the PGP 9.5-labeled cells did not have a neuronal morphology, suggested that PGP 9.5 was not labeling neurons. A smaller proportion of the dying cells displayed O4 immunoreactivity, but also expressed immature markers (e.g., nestin, A2B5, and GD3), suggesting they were immature oligodendria (Fig. 5). Although there was a trend toward increased death of O4-immunoreactive oligodendrocytes following combined morphine and Tat1–72 exposure compared to vehicle-treated controls, the effect was not significant (Table 2). The proportion of ethidium homodimer+, undifferentiated glia (12.4 ± 1.6%; Fig. 2D) or EMA+/GFAP− undifferentiated glia (11.6 ± 1.9; Table 2) in vehicle-treated control cultures was similar. Some cell death in vehicle-treated control cultures was not surprising since some glial precursors die during normal development (Raff et al., 1993).
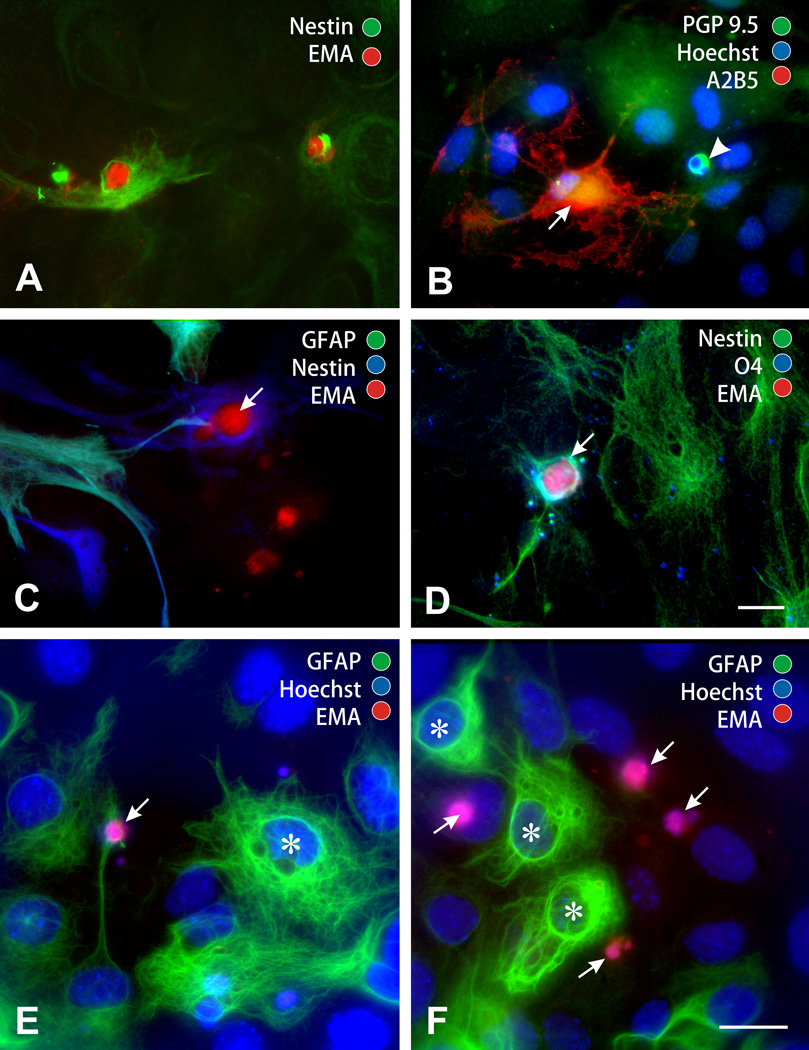
Figure 5.
Identification of dying cells in morphine and HIV-1 Tat1–72 exposed mixed glial cultures at 96 h. Dead cells were photoaffinity labeled with ethidium monoazide (EMA+) in combination with cell-type selective immunocytochemical markers (A–F). Most dying cells were undifferentiated or partially differentiated glia (e.g., arrows in F), which expressed immature (e.g., nestin) but not mature phenotypic markers (e.g., GFAP or O4). Dying cells included: immature neural cells (nestin+/EMA+) (A); immature glia (A2B5+/PGP 9.5−/EMA+) with oligodendroglial morphology (arrow) (B); small numbers of immature neural cells were morphologically similar to type 1 astrocytes but lacked GFAP (arrow) (C); immature oligodendroglia (nestin+/O4+/EMA+) (arrow) (D); astroglia (GFAP+) with process-bearing (type 2) morphology (GFAP+/EMA+; arrow) (E) (asterisk; a type 1 astrocyte for comparison) (E); and astroglia (GFAP+) with flat, polyhedral (type 1) morphology (asterisks) (F). Neurons (arrowhead) were absent or rarely present (B). The scale bar in D = 10 µm (A-D, same magnification); the scale bar in f = 10 µm (E-F, same magnification).
Each glial subtype responded differently to combined morphine and Tat1–72 exposure (Table 2; Fig. 6). This includes glial precursors, as well as immature type 1 astrocytes (Knapp et al., 1998) Khurdayan and Hauser, unpublished). By contrast, increased rates of oligodendrocyte and type 2 astrocyte deaths were not seen in the present study (Table 2). It was anticipated that type 2 astrocytes would not be responsive to morphine or morphine plus Tat1–72, because they do not express opioid receptors or respond functionally to opioids (Hauser & Stiene-Martin., 1991) (Hauser, unpublished observations). By contrast, immature oligodendrocytes express µ-opioid receptors and activating opioid receptor can affect oligodendrocyte cell death (Knapp et al., 2001). However, despite expressing µ-opioid receptors, morphine did not alter Tat1–72 toxicity in O4+/nestin− oligodendrocytes, although as noted, concurrent morphine and Tat1–72 is cytotoxic to oligodendrocyte precursors.
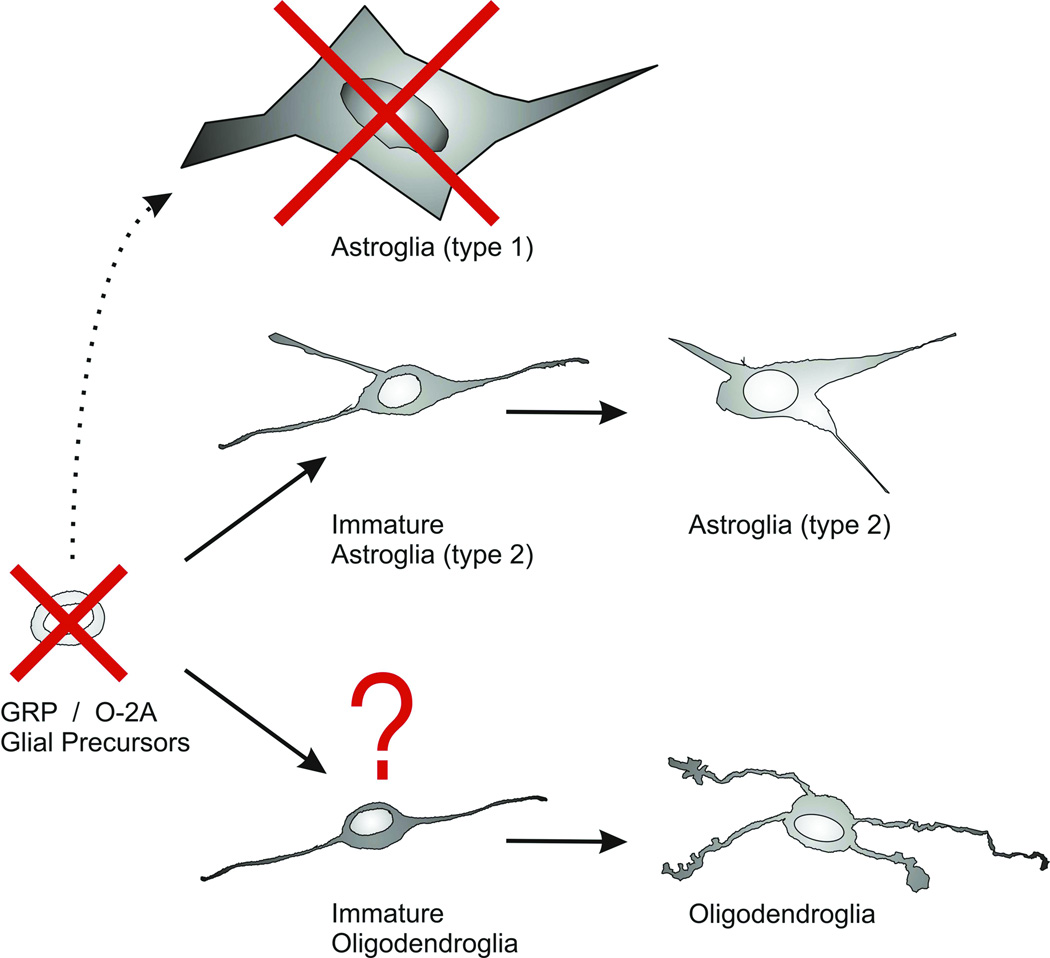
Figure 6.
Summary of the immature glial types that are preferentially vulnerable to combined morphine plus Tat1–72 exposure. Glial precursors [glial restricted precursors (GRPs) or oligodendrocyte-type 2 astrocyte (O-2A) progenitors] are preferentially destroyed by combined morphine and Tat exposure (red X). Type 1 astrocytes (dashed arrow) also showed significant losses (red X), while the viability of oligodendroglia and type 2 astrocytes was unaffected by combined morphine-Tat1–72 exposure. It is noteworthy that most GRPs in our cultures will develop into oligodendroglia and there was a trend toward a reduction in the number of immature oligodendrocytes (red ?).
Discussion
Our findings indicate that opiates modify the effects of Tat1–72 in astroglia and glial precursors, and suggest that the interaction is mediated through opioid receptors. Moreover, the ability to attenuate morphine’s effects significantly using the selective antagonist, β-FNA, suggests that opiate-HIV Tat1–72 glial interactions are mediated by µ-opioid receptors in particular. Although additional study is needed to confirm the role of µ-opioid receptor types, morphine is a preferential µ receptor agonist and µ receptors are widely expressed by astrocytes, oligodendrocytes, and glial precursors (Stiene-Martin et al., 1998; Knapp et al., 1998; Stiene-Martin et al., 2001; Persson et al., 2003a; Persson et al., 2003b) (Khurdayan, Buch, and Hauser, unpublished). An interesting observation was that the cytotoxic effects of morphine and Tat1–72 differed depending on the particular type of glial cell involved. Although our initial goal was to characterize the effects of opioids and Tat1–72 on type 1 astrocytes, as the studies progressed, it became apparent that in combination morphine and Tat1–72 were toxic to glial precursors/immature glia. For this reason, viability was assessed within several glial types.
HIV is a multisystem disease that acts through multiple toxic events and signaling cascades. HIVE is characterized by astrocytosis, the presence of multinucleate giant cells and viral products, and neuronal degeneration (neuronal death and/or dendritic pruning) (Navia et al., 1986; Masliah et al., 1992a; Masliah et al., 1992b; Nath 1999; Petito et al., 1999). Astroglial apoptosis is evident in the CNS of individuals with HIVE (Petito & Roberts., 1995; Thompson et al., 2001), and the severity of HIV dementia correlates positively with astrocytic cell death (Thompson et al., 2001).
Tat is a transactivating, nonstructural viral regulatory protein encoded by two exons (Atwood et al., 1993). Several alternatively processed forms of Tat are released by infected lymphoid and glial cells including Tat formed from the first (Tat1–72) and second (Tat1–86, Tat1–101) exons (Malim & Cullen., 1991; Tardieu et al., 1992; Atwood et al., 1993; Ensoli et al., 1993; Ma & Nath., 1997; Chang et al., 1997). All forms of Tat are present extracellularly in the CNS of infected individuals (Hudson et al., 2000) and all are neurotoxic (Sabatier et al., 1991; Nath & Geiger., 1998; Jones et al., 1998; Nath 1999; Nath 2002). Potential molecular targets of Tat include CXCR4 (Marechal et al., 1999; Xiao et al., 2000; Ghezzi et al., 2000), αv integrin subunit-containing receptors (Etienne-Manneville & Hall., 2001; Milner et al., 2001), vascular endothelial growth factor-1 receptor (VEGF-1 receptor or flt-1) (Krum & Rosenstein., 1998), and low density lipoprotein receptors (Liu et al., 2000).
Statistically significant increases in astroglial toxicity were apparent at 96 h, although the total proportion of dying astrocytes was relatively small (about 5%) compared to those astrocytes not displaying cytopathology. In combination, morphine and Tat1–72 can synergistically alter intracellular Ca2+ homeostasis, reactive oxygen species, and cytokine production in astrocytes at 1–4 h following exposure (Gurwell, El-Hage and Hauser, unpublished observations). Whether this contributes to moderate increases in astrocyte death at 96 h is uncertain and is being investigated; however, the disruptions in astroglial function are probably unrelated to astrocyte cell death. The reasons for this include: (i) an inexplicably long delay (at least 72 h) between dysfunction and death; (ii) the proportion of dying astrocytes is relatively low (∼5%) compared to viable cells; and (iii) astrocytes can adapt to disruptions in ion homeostasis caused by opioids (Hauser et al., 1998).
Synergistic death of glial precursors
Few studies have characterized mixed glial cultures derived from the neonatal striatum. Because the subventricular zone comprises a considerable proportion of the striatum of newborn or P1 mice, it was anticipated that a disproportionately large number of cells in our cultures would include glia precursors. Interestingly, our results show that about 40% of the cells in the striatal cultures are undifferentiated glia/glial precursors (Table 1), which may exceed the proportion of immature glia in neonatal cortical or whole forebrain cultures.
Glial cell death was accompanied by chromatin condensation and nuclear shrinkage (pyknosis), and/or cellular fragmentation characteristic of apoptosis. Tat has been shown to activate apoptotic cascades involving caspase-3 and/or Par-4 in primary neurons in culture (Kruman et al., 1998; Kruman et al., 1999; Singh et al., 2004). The present findings suggest that morphine and Tat1–72 in combination kill glial precursors through a caspase-3-dependent apoptotic mechanism similar to neurons, although additional studies are needed to confirm this. Morphine alone significantly reduced the proportion of active-caspase-3-immunoreactive immature glia, although morphine exposure did not result in enhanced survival as assessed by EMA at 96 h. The reason why morphine reduced the proportion of active caspase-3 positive immature glia is uncertain. Morphine can prevent apoptosis (Meriney et al., 1985) and this may involve the activation of PI-3-kinase and Akt (Polakiewicz et al., 1998; Persson et al., 2003a), which has been proposed as a pathway mediating neuroprotection downstream of the µ opioid receptor (Polakiewicz et al., 1998). Studies in progress are addressing how morphine modulates apoptosis.
By co-localizing cell type-specific markers in EMA-photoaffinity-labeled cells, we found a majority of the dying cells were glial precursors having characteristics of O-2A glial progenitors [A2B5+, GFAP− and O4− (Raff et al., 1983; Fulton et al., 1992)] or GRP1s [A2B5+/nestin+/GD3+ (Liu et al., 2002)], while some immature oligodendrocytes (A2B5+/O4+) and immature astrocytes (nestin+/GFAP+) were also preferentially lost. Since our cell culture conditions encourage glial precursors to develop into oligodendroglia and many precursors would have become oligodendroglia with further maturation, we speculate that cells committed to an oligodendroglial fate may be preferentially vulnerable to combined opiate-HIV-1 toxicity. Interestingly, there was a trend toward greater numbers of dying oligodendrocytes with combined morphine-Tat1–72 exposure compared to vehicle-treated cells (Table 2), although oligodendrocyte losses were not statistically significant at 96 h. As mentioned elsewhere, since morphine and Tat in combination are killing O-2A cells, significant oligodendrocyte losses might become apparent as precursor pools become depleted with more prolonged exposure (Fig. 6). Also, as noted earlier, the expression of some cell type specific markers might be selectively lost or attenuated in dying cells and this should be considered when interpreting the results. A more detailed assessment of the effects of opioids-HIV in oligodendrocytes at different stages of development using multiple immunological markers for oligodendrocyte in addition to O4 might reveal particular stages during development when oligodendria are especially vulnerable to opioids and HIV. Assuming glial precursors and immature oligodendria are affected in vivo similarly, chronic opiate abuse is likely to have devastating consequence on the long-term maintenance of glial populations and CNS function.
White matter pathology has been reported with heroin use (Rizzuto et al., 1997; Buttner et al., 2000; Zheng & Zhang., 2001; Barnett et al., 2001). Immature oligodendria express µ-opioid receptors when they are proliferating (Knapp et al., 1998; Stiene-Martin et al., 2001), but levels of µ- receptor expression decrease with oligodendrocyte differentiation (Tryoen-Toth et al., 2000). Oligodendrocytes do not express δ-opioid receptors in vitro, while κ-opioid receptors are expressed by more mature, non-dividing cells (Knapp et al., 1998). This infers that opiate drugs, which preferentially activate µ-opioid receptors, are more likely to affect immature oligodendrocytes.
The effects of HIV on oligodendria have been less well characterized than astrocytes. Oligodendrocytes can reportedly become infected (An et al., 1999) and may subsequently undergo degenerative and/or apoptotic changes (Schmidbauer et al., 1992; Cosenza et al., 2002). HIV-1 encodes two sequences with considerable homology to the response element of the hnRNP A2-RNA trafficking factor present in oligodendrocytes (Mouland et al., 2001). Although white mater pallor is seen frequently in HIV infected patients, it is commonly thought to be secondary to the breakdown of the blood brain barrier (Petito & Cash., 1992; Power et al., 1993; Buttner et al., 1996).
Depending on dosage, opioids can have opposing actions through the same receptor type (Crain & Shen., 1990; Gintzler & Xu., 1991). Similarly, as a function of dose, µ-opioid agonists can prevent or exacerbate cell death (Meriney et al., 1985; Singhal et al., 1997; Singhal et al., 1998; Polakiewicz et al., 1998). Opioid drug abusers reportedly tolerate opioid blood levels that are several to 100-fold greater than those seen therapeutically (Gurwell et al., 2001). Restated, the morphine concentrations and pharmacodynamic parameters used to exacerbate Tat1–72 toxicity in the present study are likely to be achieved with chronic drug abuse, but are unlikely to be realized with therapeutic dosages. Unlike drug abuse, therapeutic dosages of opiates are likely to parallel the actions of endogenous opioid peptides whose role in the pathogenesis of neuro-AIDS is not understood.
Our findings indicate that opiate drug abuse can exacerbate the pathogenesis of HIV through independent actions in separate glial subpopulations in vitro. Although our in vitro studies represent an important first step in identifying potential targets of opiate-HIV interactions in the CNS, additional studies need to be performed before the present findings can be generalized to the adult brain. The consequences of destroying glial precursors are uncertain. Fated glial precursors may possess life spans lasting years, suggesting that deficits in gliogenesis would take time to become evident. A speculative notion is that the progressive destruction of glial precursors and ensuing predicted loss in total glial numbers might contribute to the accelerated neurocognitive defects seen in chronic opiate abusing-HIV infected individuals. Interestingly, disruptions in neurogenesis have been implicated in the etiology of Alzheimer’s disease (Haughey et al., 2002), traumatic brain injury (Braun et al., 2002; Chirumamilla et al., 2002), seizure disorders (Ribak & Dashtipour., 2002; Parent & Lowenstein., 2002), and neuropsychiatric disorders (Jacobs et al., 2000; Kempermann 2002; McEwen 2002), and may accompany opiate exposure (Dodge Miller et al., 1982; Eisch et al., 2000; Stiene-Martin et al., 2001; Duman et al., 2001).
Acknowledgements
This work was supported by NIH DA13559. Monoclonal antibodies developed by Drs. Thomas M. Jessell and Jane Dodd (E-NCAM), and Dr. Susan Hokfield (nestin), were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHHD and maintained by the University of Iowa, Department of Biological Sciences. GD3 monoclonal IgG3 were obtained from the National Cancer Institute BRB Preclinical Repository, Bethesda, MD). We thank Mr. Kenneth Martin for valued technical assistance and Dr. Robin J. Goody for helpful discussions.
Abbreviations
- Akt
protein kinase B
- β-FNA
β-funaltrexamine hydrochloride
- DPBS
Dulbecco’s phosphate buffered saline
- E-NCAM
polysialyated neural cell adhesion molecule
- GFAP
glial fibrillary acidic protein
- GRP
glial restricted precursor
- HIV
Human Immunodeficiency Virus
- µOR
µ-opioid receptor
- Mor
morphine
- Nal
naloxone
- NeuN
neuronal nuclear marker
- O-2A
oligodendrocyte-type 2 astrocyte precursor
- PI3-kinase
phosphoinositide-3 kinase
References
- An SF, Groves M, Giometto B, Beckett AA, Scaravilli F. Detection and localisation of HIV-1 DNA and RNA in fixed adult AIDS brain by polymerase chain reaction/in situ hybridisation technique. Acta Neuropathol.(Berl) 1999;98:481–487. doi: 10.1007/s004010051113. [DOI] [PubMed] [Google Scholar]
- Arora PK. Morphine-induced immune modulation: does it predispose to HIV infection? NIDA.Res.Monogr. 1990;96:150–165. [PubMed] [Google Scholar]
- Atwood WJ, Tornatore CS, Meyers K, Major EO. HIV-1 mRNA transcripts from persistently infected human fetal astrocytes. Ann N Y Acad Sci. 1993;693:324–325. doi: 10.1111/j.1749-6632.1993.tb26298.x. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Bansal R, Warrington A, Gard A, Ranscht B, Pfeiffer S. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mab used in the analysis of oligodendrocyte development. J.Neurosci.Res. 1989;24:548–557. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]
- Barnett MH, Miller LA, Reddel SW, Davies L. Reversible delayed leukoencephalopathy following intravenous heroin overdose. J.Clin.Neurosci. 2001;8:165–167. doi: 10.1054/jocn.2000.0769. [DOI] [PubMed] [Google Scholar]
- Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121:2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- Bell JE, Donaldson YK, Lowrie S, McKenzie CA, Elton RA, Chiswick A, Brettle RP, Ironside JW, Simmonds P. Influence of risk group and zidovudine therapy on the development of HIV encephalitis and cognitive impairment in AIDS patients. AIDS. 1996;10:493–499. doi: 10.1097/00002030-199605000-00007. [DOI] [PubMed] [Google Scholar]
- Berger JR, Nath A. HIV dementia and the basal ganglia. Intervirology. 1997;40:122–131. doi: 10.1159/000150539. [DOI] [PubMed] [Google Scholar]
- Braun H, Schafer K, Hollt V. BetaIII tubulin-expressing neurons reveal enhanced neurogenesis in hippocampal and cortical structures after a contusion trauma in rats. J.Neurotrauma. 2002;19:975–983. doi: 10.1089/089771502320317122. [DOI] [PubMed] [Google Scholar]
- Buttner A, Mall G, Penning R, Weis S. The neuropathology of heroin abuse. Forensic Sci.Int. 2000;113:435–442. doi: 10.1016/s0379-0738(00)00204-8. [DOI] [PubMed] [Google Scholar]
- Buttner A, Mehraein P, Weis S. Vascular changes in the cerebral cortex in HIV-1 infection. II. An immunohistochemical and lectinhistochemical investigation. Acta Neuropathol.(Berl) 1996;92:35–41. doi: 10.1007/s004010050486. [DOI] [PubMed] [Google Scholar]
- Carr DJ, Serou M. Exogenous and endogenous opioids as biological response modifiers. Immunopharmacology. 1995;31:59–71. doi: 10.1016/0162-3109(95)00033-6. [DOI] [PubMed] [Google Scholar]
- Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 tat protein exits from cells via a leaderless secretory pathway and binds to extracellelar matrix-associated heparan sulfate proteoglycan through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Sheng WS, Hu S, Tsang M, Peterson PK. Priming effect of morphine on the production of tumor necrosis factor-a by microglia: Implications in respiratory burst activity and human immunodeficiency virus-1 expression. J.Pharmacol.Exp.Ther. 1994;269:198–203. [PubMed] [Google Scholar]
- Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J.Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- Cosenza MA, Zhao ML, Shankar SL, Shafit-Zagardo B, Lee SC. Up-regulation of MAP2e–expressing oligodendrocytes in the white matter of patients with HIV-1 encephalitis. Neuropathol.Appl.Neurobiol. 2002;28:480–488. doi: 10.1046/j.1365-2990.2002.00420.x. [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Opioids can evoke direct receptor-mediated excitatory effects on sensory neurons. Trends.Pharmacol.Sci. 1990;11:77–81. doi: 10.1016/0165-6147(90)90322-y. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Noble M, Mayer-Proschel M. Characterization of A2B5+ glial precursor cells from cryopreserved human fetal brain progenitor cells. Glia. 2002;40:65–77. doi: 10.1002/glia.10116. [DOI] [PubMed] [Google Scholar]
- Dodge Miller CR, O’Steen WK, Deadwyler SA. Effect of morphine on 3H-thymidine incorporation in the subependyma of the rat: an autoradiographic study. J.Comp.Neurol. 1982;208:209–214. doi: 10.1002/cne.902080209. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Falek A. Neuroimmunomodulation by opiates and other drugs of abuse: relationship to HIV infection and AIDS. Adv.Biochem.Psychopharmacol. 1988;44:145–158. [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J.Pharmacol.Exp.Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc.Natl.Acad.Sci.U.S.A. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan R, Wingfield P, Gallo R. Release, uptake, and effects of extracellular human immunodeficiency virus type-1 Tat protein on cell growth and viral replication. Journal of Virology. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LG, Gendelman HE. Human immunodeficiency virus type 1 infection of the nervous system: pathogenic mechanisms. Ann.Neurol. 1993;33:429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Hansson E, Rönnbäck L. Mu and delta opiate receptors in neuronal and astroglial primary cultures from various regions of the brain-coupling with adenylate cyclase, localisation on the same neurones and association with dopamine (D1) receptor adenylate cyclase. Neuropharmacology. 1991;30:1233–1239. doi: 10.1016/0028-3908(91)90170-g. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Fulton BP, Burne JF, Raff MC. Visualization of O-2A progenitor cells in developing and adult rat optic nerve by quisqualate-stimulated cobalt uptake. J.Neurosci. 1992;12:4816–4833. doi: 10.1523/JNEUROSCI.12-12-04816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Two proliferative stages of the oligodendrocyte lineage (A2B5+O4− and O4+GalC−) under different mitogenic control. Neuron. 1990;5:615–625. doi: 10.1016/0896-6273(90)90216-3. [DOI] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D’Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J.Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekker G, Lokensgard JR, Peterson PK. Naltrexone potentiates anti-HIV-1 activity of antiretroviral drugs in CD4(+) lymphocyte cultures. Drug Alcohol Depend. 2001;64:257–263. doi: 10.1016/s0376-8716(01)00140-5. [DOI] [PubMed] [Google Scholar]
- Ghezzi S, Noonan DM, Aluigi MG, Vallanti G, Cota M, Benelli R, Morini M, Reeves JD, Vicenzi E, Poli G, Albini A. Inhibition of CXCR4-dependent HIV-1 infection by extracellular HIV-1 Tat. Biochem.Biophys.Res.Commun. 2000;270:992–996. doi: 10.1006/bbrc.2000.2523. [DOI] [PubMed] [Google Scholar]
- Gintzler AR, Xu H. Different G proteins mediate the opioid inhibition or enhancement of evoked [5-methionine]enkephalin release. Proc.Natl.Acad.Sci.(USA) 1991;88:4741–4745. doi: 10.1073/pnas.88.11.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL, Selnes OA, McArthur JC. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- Goldman JE, Reynolds R. A reappraisal of ganglioside GD3 expression in the CNS. Glia. 1996;16:291–295. doi: 10.1002/(SICI)1098-1136(199604)16:4<291::AID-GLIA1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, Hauser KF. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer’s disease. Neuromolecular.Med. 2002;1:125–135. doi: 10.1385/NMM:1:2:125. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J.Acquir.Immune.Defic.Syndr. 2002;31(Suppl 2):S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Harris-White ME, Jackson JA, Opanashuk LA, Carney JM. Opioids disrupt Ca2+ homeostasis and induce carbonyl oxyradical production in mouse astrocytes in vitro: transient increases and adaptation to sustained exposure. Exp.Neurol. 1998;151:70–76. doi: 10.1006/exnr.1998.6788. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Houdi AA, Turbek CS, Elde RP, Maxson W., III Opioids intrinsically inhibit the genesis of mouse cerebellar granule cell precursors in vitro: Differential impact of µ and δ receptor activation on proliferation and neurite elongation. Eur.J.Neurosci. 2000;12:1281–1293. doi: 10.1046/j.1460-9568.2000.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Stiene-Martin A. Characterization of opioid-dependent glial development in dissociated and organotypic cultures of mouse central nervous system: Critical periods and target specificity. Dev.Brain Res. 1991;62:245–255. doi: 10.1016/0165-3806(91)90172-f. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC. µ-Opioid receptor-induced Ca2+ mobilization and astroglial development: Morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca2+-dependent mechanism. Brain Res. 1996;720:191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghtling RA, Mellon RD, Tan RJ, Bayer BM. Acute effects of morphine on blood lymphocyte proliferation and plasma IL-6 levels. Ann.N.Y.Acad.Sci. 2000;917:771–177. doi: 10.1111/j.1749-6632.2000.tb05442.x. 771–777. [DOI] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. Detection of human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirology. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol.Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Jones M, Olafson K, Del Bigio MR, Peeling J, Nath A. Intraventricular injection of human immunodeficiency virus type 1 (HIV- 1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J.Neuropathol.Exp.Neurol. 1998;57:563–570. doi: 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Regulation of adult hippocampal neurogenesis - implications for novel theories of major depression. Bipolar.Disord. 2002;4:17–33. doi: 10.1034/j.1399-5618.2002.40101.x. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Itkis OS, Zhang L, Spruce BA, Bakalkin G, Hauser KF. Endogenous opioids and oligodendroglial function: possible autocrine/paracrine effects on cell survival and development. Glia. 2001;35:156–165. doi: 10.1002/glia.1080. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: µ and κ opioid receptors mediate differential mitogenic and growth responses. Glia. 1998;22:189–201. doi: 10.1002/(sici)1098-1136(199802)22:2<189::aid-glia10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann.N.Y.Acad.Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Krum JM, Rosenstein JM. VEGF mRNA and its receptor flt-1 are expressed in reactive astrocytes following neural grafting and tumor cell implantation in the adult CNS. Exp.Neurol. 1998;154:57–65. doi: 10.1006/exnr.1998.6930. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Maragos WF, Chan SL, Jones M, Rangnekar VM, Jakel RJ, Mattson MP. Evidence that Par-4 participates in the pathogenesis of HIV encephalitis. Am.J.Pathol. 1999;155:39–46. doi: 10.1016/S0002-9440(10)65096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp.Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat.Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu Y, Lee JC, Xue H, Pevny LH, Kaprielian Z, Rao MS. Oligodendrocyte and astrocyte development in rodents: An in situ and immunohistological analysis during embryonic development. Glia. 2002;40:25–43. doi: 10.1002/glia.10111. [DOI] [PubMed] [Google Scholar]
- Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J.Virol. 1997;71:2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, Cullen BR. HIV-1 structural gene expression requires the binding of multiple Rev monomers to the viral RRE: implications for HIV-1 latency. Cell. 1991;65:241–8. doi: 10.1016/0092-8674(91)90158-u. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: An in situ hybridization study. J.Comp.Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Marechal V, Arenzana-Seisdedos F, Heard JM, Schwartz O. Opposite effects of SDF-1 on human immunodeficiency virus type 1 replication. J.Virol. 1999;73:3608–3615. doi: 10.1128/jvi.73.5.3608-3615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann.Neurol. 1992a;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim CL, Hansen LA, Wiley CA. Selective neuronal vulnerability in HIV encephalitis. J Neuropathol Exp Neurol. 1992b;51:585–93. doi: 10.1097/00005072-199211000-00003. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Mucke L. Pathogenesis of HIV-1 associated neurodegeneration. Crit Rev.Neurobiol. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol.Aging. 2002;23:921. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Meriney SD, Gray DB, Pilar G. Morphine-induced delay of normal cell death in the avian ciliary ganglion. Science. 1985;228:1451–1453. doi: 10.1126/science.2990029. [DOI] [PubMed] [Google Scholar]
- Milner R, Relvas JB, Fawcett J, ffrench-Constant C. Developmental regulation of alphav integrins produces functional changes in astrocyte behavior. Mol.Cell Neurosci. 2001;18:108–118. doi: 10.1006/mcne.2001.1003. [DOI] [PubMed] [Google Scholar]
- Mouland AJ, Xu H, Cui H, Krueger W, Munro TP, Prasol M, Mercier J, Rekosh D, Smith R, Barbarese E, Cohen EA, Carson JH. RNA trafficking signals in human immunodeficiency virus type 1. Mol.Cell Biol. 2001;21:2133–2143. doi: 10.1128/MCB.21.6.2133-2143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MP, Schwartz SA, Polasani R, Hou J, Sweet A, Chadha KC. Immunoregulatory effects of morphine on human lymphocytes. Clin.Diagn.Lab Immunol. 1997;4:127–132. doi: 10.1128/cdli.4.2.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. Pathobiology of human immunodeficiency virus dementia. Semin.Neurol. 1999;19:113–127. doi: 10.1055/s-2008-1040830. [DOI] [PubMed] [Google Scholar]
- Nath A. Human Immunodeficiency Virus (HIV) Proteins in Neuropathogenesis of HIV Dementia. J.Infect.Dis. 2002;186(Suppl 2):S193–S198. doi: 10.1086/344528. S193-S198. [DOI] [PubMed] [Google Scholar]
- Nath A, Geiger J. Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Prog.Neurobiol. 1998;54:19–33. doi: 10.1016/s0301-0082(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J.Acquir.Immune.Defic.Syndr. 2002;31(Suppl 2):S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nath A, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopamine systems associated with AIDS dementia. Psychopharmacol. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J.Virol. 1996;70:1475–1480. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II Neuropathology. Ann.Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Parent JM, Lowenstein DH. Seizure-induced neurogenesis: are more new neurons good for an adult brain? Prog.Brain Res. 2002;135:121–131. doi: 10.1016/S0079-6123(02)35012-X. 121–131. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Eriksson PS. Opioid-induced proliferation through the MAPK pathway in cultures of adult hippocampal progenitors. Mol.Cell Neurosci. 2003a;23:360–372. doi: 10.1016/s1044-7431(03)00061-7. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, Eriksson PS. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur.J Neurosci. 2003b;17:1159–1172. doi: 10.1046/j.1460-9568.2003.02538.x. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Schut R, Hu S, Balfour HH, Jr, Chao CC. Enhancement of HIV-1 replication by opiates and cocaine: The cytokine connection. Adv.Exp.Med.Biol. 1993;335:181–188. doi: 10.1007/978-1-4615-2980-4_26. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J.Neuroimmunol. 1998;83:63–69. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp BM, Gekker G, Jackson B, Balfour HH., Jr Opiates, human peripheral blood mononuclear cells, and HIV. Adv.Exp.Med.Biol. 1991;288:171–178. doi: 10.1007/978-1-4684-5925-8_19. [DOI] [PubMed] [Google Scholar]
- Petito CK, Cash KS. Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol. 1992;32:658–66. doi: 10.1002/ana.410320509. [DOI] [PubMed] [Google Scholar]
- Petito CK, Kerza-Kwiatecki AP, Gendelman HE, McCarthy M, Nath A, Podack ER, Shapshak P, Wiley CA. Review: neuronal injury in HIV infection. J.Neurovirol. 1999;5:327–341. doi: 10.3109/13550289909029474. [DOI] [PubMed] [Google Scholar]
- Petito CK, Roberts B. Evidence of apoptotic cell death in HIV encephalitis [see comments] Am J Pathol. 1995;146:1121–30. [PMC free article] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J Biol.Chem. 1998;273:23534–23541. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- Power C, Kong PA, Crawford TO, Wesselingh S, Glass JD, McArthur JC, Trapp BD. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann.Neurol. 1993;34:339–350. doi: 10.1002/ana.410340307. [DOI] [PubMed] [Google Scholar]
- Raff MC, Abney EA, Miller A. Two glial cell lineages diverge prenatally in rat optic nerve. Dev.Biol. 1984;106:53–60. doi: 10.1016/0012-1606(84)90060-5. [DOI] [PubMed] [Google Scholar]
- Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on the culture. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Rappaport J, Joseph J, Croul S, Alexander G, Del Valle L, Amini S, Khalili K. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. J.Leukoc.Biol. 1999;65:458–465. doi: 10.1002/jlb.65.4.458. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Dashtipour K. Neuroplasticity in the damaged dentate gyrus of the epileptic brain. Prog.Brain Res. 2002;136:319–328. doi: 10.1016/s0079-6123(02)36027-8. 319–328. [DOI] [PubMed] [Google Scholar]
- Rizzuto N, Morbin M, Ferrari S, Cavallaro T, Sparaco M, Boso G, Gaetti L. Delayed spongiform leukoencephalopathy after heroin abuse. Acta Neuropathol.(Berl) 1997;94:87–90. doi: 10.1007/s004010050676. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 2003;24:116–121. doi: 10.1016/s1471-4906(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Rouveix B. Opiates and immune function. Consequences on infectious diseases with special reference to AIDS. Therapie. 1992;47:503–512. [PubMed] [Google Scholar]
- Sabatier JM, Vives E, Marbrouk K, al e. Evidence for neurotoxicity of tat from HIV. Journal of Virology. 1991;65:961–967. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidbauer M, Huemer M, Cristina S, Trabattoni GR, Budka H. Morphological spectrum, distribution and clinical correlation of white matter lesions in AIDS brains. Neuropathol.Appl.Neurobiol. 1992;18:489–501. doi: 10.1111/j.1365-2990.1992.tb00816.x. [DOI] [PubMed] [Google Scholar]
- Scorziello A, Florio T, Bajetto A, Schettini G. Intracellular signalling mediating HIV-1 gp120 neurotoxicity. Cell Signal. 1998;10:75–84. doi: 10.1016/s0898-6568(97)00093-4. [DOI] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, Nath A, Hauser KF. Apoptotic death of striatal neurons induced by HIV-1 Tat and gp120: differential involvement of caspase-3 and endonuclease G. J.Neurovirol. 2004 doi: 10.1080/13550280490441103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Goebel SM, Martin KM, Knapp PE, Bakalkin G, Hauser KF. Dynorphin A (1–17) induces apoptosis in striatal neurons thorugh a-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor-mediated cytochrome c release and caspase-3 activation. Neurosci. 2003;122:1013–1023. doi: 10.1016/j.neuroscience.2003.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal PC, Bhaskaran M, Patel J, Patel K, Kasinath BS, Duraisamy S, Franki N, Reddy K, Kapasi AA. Role of p38 mitogen-activated protein kinase phosphorylation and Fas-Fas ligand interaction in morphine-induced macrophage apoptosis. J.Immunol. 2002;168:4025–4033. doi: 10.4049/jimmunol.168.8.4025. [DOI] [PubMed] [Google Scholar]
- Singhal PC, Reddy K, Franki N, Sanwal V, Gibbons N. Morphine induces splenocyte apoptosis and enhanced mRNA expression of cathepsin-B. Inflammation. 1997;21:609–617. doi: 10.1023/a:1027334122387. [DOI] [PubMed] [Google Scholar]
- Singhal PC, Sharma P, Kapasi AA, Reddy K, Franki N, Gibbons N. Morphine enhances macrophage apoptosis. J.Immunol. 1998;160:1886–1893. [PubMed] [Google Scholar]
- Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces. An immunocytochemical study in the central nervous system. Dev.Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Gurwell JA, Hauser KF. Morphine alters astrocyte growth in primary cultures of mouse glial cells: Evidence for a direct effect of opiates on neural maturation . Dev.Brain Res. 1991;60:1–7. doi: 10.1016/0165-3806(91)90149-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Knapp PE, Martin KM, Gurwell JA, Ryan S, Thornton SR, Smith FL, Hauser KF. Opioid system diversity in developing neurons, astroglia, and oligodendria in the subventricular zone and striatum: impact on gliogenesis in vivo . Glia. 2001;36:78–88. [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in µ, δ, and κ-opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22:249–259. [PMC free article] [PubMed] [Google Scholar]
- Stoll-Keller F, Schmitt C, Thumann C, Schmitt MP, Caussin C, Kirn A. Effects of morphine on purified human blood monocytes. Modifications of properties involved in antiviral defences. Int.J.Immunopharmacol. 1997;19:95–100. doi: 10.1016/s0192-0561(97)00017-9. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Carlos MP, Chuang LF, Torres JV, Doi RH, Chuang RY. Methadone induces CCR5 and promotes AIDS virus infection. FEBS Lett. 2002a;519:173–177. doi: 10.1016/s0014-5793(02)02746-1. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang AJ, Chuang LF, Doi RH, Chuang RY. Morphine promotes simian acquired immunodeficiency syndrome virus replication in monkey peripheral mononuclear cells: induction of CC chemokine receptor 5 expression for virus entry. J.Infect.Dis. 2002b;185:1826–1829. doi: 10.1086/340816. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang TK, Chuang LF, Doi RH, Chuang RY. Morphine upregulates kappa-opioid receptors of human lymphocytes. Adv.Exp.Med.Biol. 2001;493:81–87. doi: 10.1007/0-306-47611-8_10. 81–87. [DOI] [PubMed] [Google Scholar]
- Tardieu M, Hery C, Peudenier S, Boespflug O, Montagnier L. Human immunodeficiency virus type 1-infected monocytic cells can destroy human neural cells after cell-to-cell adhesion. Ann.Neurol. 1992;32:11–17. doi: 10.1002/ana.410320104. [DOI] [PubMed] [Google Scholar]
- Thompson KA, McArthur JC, Wesselingh SL. Correlation between neurological progression and astrocyte apoptosis in HIV-associated dementia. Ann.Neurol. 2001;49:745–752. doi: 10.1002/ana.1011. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Doran JF, Jackson P, Dhillon AP, Rode J. PGP 9.5 - A new marker for vertebrate neurons and neuroendocrine cells. Brain Res. 1983;278:224–228. doi: 10.1016/0006-8993(83)90241-x. [DOI] [PubMed] [Google Scholar]
- Tryoen-Toth P, Gaveriaux-Ruff C, Labourdette G. Down-regulation of mu-opioid receptor expression in rat oligodendrocytes during their development in vitro. J.Neurosci.Res. 2000;60:10–20. doi: 10.1002/(SICI)1097-4547(20000401)60:1<10::AID-JNR2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Turchan J, Anderson C, Hauser KF, Sun Q, Zhang J, Liu Y, Wise PM, Kruman I, Maragos W, Mattson MP, Booze R, Nath A. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC.Neurosci. 2001;2:3. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselingh SL, Thompson KA. Immunopathogenesis of HIV-associated dementia. Curr.Opin.Neurol. 2001;14:375–379. doi: 10.1097/00019052-200106000-00018. [DOI] [PubMed] [Google Scholar]
- Wilkinson KD, Lee K, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc.Natl.Acad.Sci.U.S.A. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhang X. Characteristics of spongiform leukoencephalopathy induced by heroin: MRI detection. Chin Med.J.(Engl.) 2001;114:1193–1195. [PubMed] [Google Scholar]