Abstract
Although opioids are known to affect neurogenesis in vivo, it is uncertain the extent to which opioids directly or indirectly effect the proliferation, differentiation or death of neuronal precursors. To address these questions, the intrinsic role of the opioid system in neurogenesis was systematically explored in cerebellar external granular layer (EGL) neuronal precursors isolated from postnatal mice and maintained in vitro. Isolated neuronal precursors expressed proenkephalin-derived peptides, as well as specific μ and δ, but negligible κ, opioid receptors. The developmental effects of opioids were highly selective. Morphine-induced μ receptor activation inhibited DNA synthesis, while a preferential δ2-receptor agonist ([D-Ala2]-deltorphin II) or Met-enkephalin, but not the δ1 agonist [D-Pen2, D-Pen5]-enkephalin, inhibited differentiation within the same neuronal population. If similar patterns occur in the developing cerebellum, spatiotemporal differences in endogenous μ and δ opioid ligand-receptor interactions may coordinate distinct aspects of granule neuron maturation. The data additionally suggest that perinatal exposure to opiate drugs of abuse directly interfere with cerebellar maturation by disrupting normal opioid signaling and inhibiting the proliferation of granule neuron precursors.
Keywords: opiate drug abuse, endogenous opioid system, cerebellar development, apoptosis, cell division, neuronal differentiation, μ opioid receptors, δ opioid receptors
Introduction
The endogenous opioid system (endogenous opioids and opioid receptors) is widely expressed by developing cells of neural and non-neural origin (Vértes et al., 1982; Zagon et al., 1985; McDowell & Kitchen 1987; Spruce et al., 1990; Leslie & Loughlin 1993; Böttger & Spruce 1995; Leslie et al., 1998; Zhu et al., 1998), as well as in neoplastic cells (Bostwick et al., 1987; Maneckjee & Minna 1990; Rosen & Bar-Shavit 1994; Böttger & Spruce 1995; Pan et al., 1998; Panagiotou et al., 1998). In adults, opioid neuropeptides regulate numerous physiological functions, including pain and central reward pathways (Nestler 1996; Kreek & Koob 1998), digestion, respiration and tissue oxygen levels (Yeadon & Kitchen 1989), immune (Sharp et al., 1998), and endocrine systems (e.g., thyroid and growth hormone axes) (Akil et al., 1984; Millan & Herz 1985). During development, however, opioids additionally regulate neural maturation (Zagon & McLaughlin 1983; Hammer, Jr. 1993; Hauser & Mangoura 1998). Understanding how opioids modulate neuronal maturation is important, because of ubiquity of endogenous opioids during normal development and because of the potential impact of opiate drug abuse on neural development in children exposed in utero (Hutchings 1982; Chasnoff et al., 1986; Pasto et al., 1993; Bunikowski et al., 1998).
Contributing to the lack of understanding of how opioids affect cellular maturation is the complexity of the opioid system itself. Multiple opioid peptides and receptors exist. Each follows unique spatial, temporal and cell-type specific patterns of expression during development (Evans 1993; Leslie et al., 1998; Zhu et al., 1998). A second challenge is the diversity of cell targets and opioid actions during development. Opioids affect neuronal (Zagon & McLaughlin 1983; Zagon & McLaughlin 1987), astroglial (Gurwell et al., 1996; Hauser et al., 1996; Hauser & Mangoura 1998), and oligodendroglial maturation (Knapp et al., 1998). Thus, even within a single brain region, opioids may directly affect multiple cell targets. Lastly, as mentioned above, opioids have many indirect effects that can in turn affect brain development (Kuhn et al., 1991).
Opioids have been reported to inhibit neuroblast replication and differentiation in the developing cerebellum in vivo (Zagon & McLaughlin 1983; Zagon & McLaughlin 1987; Hauser & Mangoura 1998). However, the conclusions regarding cell division are based largely on reductions in [3H]-thymidine labeling indices, without assessing whether opioids affect cell turnover, the cell cycle, or programmed cell death. Because of the complexity of opioid actions and targets, surprisingly few studies have systematically examined the intrinsic effect of opioids on neuronal maturation [see (Hauser & Mangoura 1998; Reznikov et al., 1999)]. To test whether opioids intrinsically affect granule neurogenesis, we examined the effects of opioids on the development of isolated cerebellar external granular layer (EGL) neuronal precursors (Hatten 1987; Gao et al., 1991; Opanashuk & Hauser 1998). The EGL is a transient layer of granule neuron precursors (Ramón y Cajal 1960; Miale & Sidman 1961; Altman 1972; Hallonet et al., 1990; Zhang & Goldman 1996; Yang et al., 1996; Hallonet & Alvarado-Mallart 1997), that express naloxone binding sites and/or δ or ζ opioid receptors (Kinney & White, 1991; Zagon et al., 1992; Mansour et al., 1994) and proenkephalin peptides in vivo (Zagon et al., 1985; Osborne et al., 1993), and whose development is affected by opioids in vivo (Zagon & McLaughlin 1983; Zagon et al., 1985; Zagon & McLaughlin 1986; Zagon & McLaughlin 1987; Hauser et al., 1989; Kinney & White 1991; Zagon et al., 1992; Osborne et al., 1993). Our findings indicate that opioids intrinsically affect granule cell proliferation and differentiation in a highly selective manner.
Materials and Methods
Cell Culture
Enriched cultures of EGL neuronal precursors were established in re-aggregate culture largely using procedures described before (Hatten 1985; Hatten 1987; Gao et al., 1991). Briefly, postnatal (P) day 5 or 6 mice were euthanized by ether anesthesia followed by decapitation. The meninges were carefully removed and whole cerebella washed in Ca2+ and Mg2+-free Tyrode’s solution and dissociated into single cells. Cerebellar cells were filtered through 33 μm mesh nylon (Tetko, Elmsford, NY, USA), and separated by centrifugation by layering cells onto a step density gradient of Percoll (35/60% in Ca2+ and Mg2+-free Tyrode’s solution containing 2 mM EDTA) (Pharmacia, Piscataway, NJ, USA) and centrifuging at 2,000 x g for 10 min at 5°C. EGL neuroblasts were gently removed, washed and repelleted (50 x g) before plating on poly-D-lysine (15 min). Three panning cycles, each consisting of pre-plating (15 min) followed by 30 sec agitation, were used to further enrich the proportion of EGL neurons. EGL neuronal precursors were allowed to re-aggregate in suspension in serum-containing basal medium for about 20–24 h to generate neurospheres with cluster sizes of 20–50 cells. This medium consisted of Dulbecco’s minimal essential medium with KCl (5 mM), glutamine (292 μg/ml), glucose (9 mg/ml), donor horse serum (10%), and fetal bovine serum (5%). After 20–24 h, neurospheres were place into suspension and cell density was determined by dissociating a small sample of the neurosphere clusters using Ca2+ and Mg2+-free Tyrode’s solution. The neurospheres were transferred to 12 well plates (3 x 105 cells 1 ml−1 per well) at the beginning of each experiment. Each well contained a centrally positioned 16 mm diameter glass or plastic coverslip. The spatial geometry is such that the 1 ml volume of liquid is sufficiently large to allow the neurospheres to be uniformly distributed onto the surface of the 16-mm diameter coverslips placed within the 25 mm diameter wells (minimizing the effect of the culture fluid meniscus). With these culture conditions we did not notice differences in the cells in wells at perimeter of the culture plate (“edge effect”) nor were there differences in the behavior or density of cells at the periphery of well. Cells were maintained in serum-free medium consisting of Dulbecco’s minimal essential medium supplemented with (final concentrations): KCl (5 mM), glutamine (292 μg/ml), glucose (9 mg/ml), B-27 supplement (2% v/v), and N-2 supplement (1% v/v). Cell culture reagents were obtained from Gibco, Life Technologies, Gaithersburg, MD, USA.
Immunocytochemical localization of Opioid Peptides and Receptors
Cultures were incubated in Zamboni’s fixative (Zamboni & De Martino 1967) for 30 min, pH 7.2 at 4°C) and rinsed in phosphate buffered saline (PBS). Monoclonal antibodies against a common epitope of the proenkephalin precursor protein (PE-18 or PE-25) were used to detect enkephalin precursor in immature EGL neurons (Böttger et al., 1995). Cultures were incubated in PE-18 or PE-25 antibodies diluted 1:1200 at 4°C on an orbital shaker (40–60 rpm) for 24 h. Secondary, biotinylated donkey-anti-mouse antibodies conjugated to avidin-peroxidase were used as directed (Vectastain-ABC kit, Vector Laboratories, Burlingame, CA, USA) to detect PE-18 and PE-25 antibodies. Nickel-intensified diaminobenzidine (DAB) consisting of 2.5% nickel ammonium sulfate, 0.35% DAB, and 0.012% H2O2 in 0.1-M sodium acetate (pH 6.0) was used as a substrate for peroxidase. To assess opioid receptor immunoreactivity, cultures were incubated in PBS containing crystalline bovine serum albumin (BSA) (1%), goat serum (1%), and Triton X 100 (0.1%). Purified rabbit-antisera directed against μ (MOR1 (Arvidsson et al., 1995a)), δ2 (DOR442 (Arvidsson et al., 1995b)), or κ (KOR (Arvidsson et al., 1995c)) opioid receptors were diluted, 1:5000, 1:5000, or 1:2500, respectively, in PBS containing Triton-X 100 (0.1%) and BSA (0.1%) (Calbiochem, San Diego, CA, USA). Cultures were incubated in diluted primary antiserum at 4°C on an orbital shaker (40–60 rpm) for 24 h. Secondary, biotinylated goat-anti-rabbit antibodies conjugated to avidin-peroxidase were used as directed (Vectastain-ABC kit) to detect MOR1, DOR1 or KOR primary antibodies. Nickel-intensified DAB was used as a substrate for peroxidase in all the above reactions (as described earlier). Preabsorbed controls (against μ, δ and κ epitopes) and/or controls lacking primary antiserum were included to assure the specificity of the reactions.
Electron Microscopy
For electron microscopy, neurosphere clusters were plated on poly-D-lysine-coated ACLAR plastic coverslips. Cells were fixed in situ (on their coverslips) for 30 min at room temperature in 4% paraformaldehyde (v/v), 2% glutaraldehyde (v/v) in Sorenson’s phosphate buffer, pH 7.2 and transferred into ice-cold fixative for an additional 3.5 h. After fixation, coverslips with neurospheres were rinsed 3 times (20 min per rinse) in ice-cold PBS, post fixed for 1 h in 1% OsO4 in 0.1 M phosphate buffer at 4°C, dehydrated in graded methanol:water and propylene oxide, and embedded in epoxy resin. After the epoxy was polymerized, ACLAR coverslips were peeled away from epoxy-embedded neurospheres. Neurospheres and cells in monolayers were sectioned on a LKB ultramicrotome and examined using a Hitachi H-7000 transmission electron microscope at 75 kV.
Opioid Treatment
In all experiments, the developmental effects of opioids were compared in cultures containing identical numbers of EGL cells (3 x 105 1 ml−1 per well). Immediately after plating, EGL cells were continuously exposed to opioid agonists and/or the antagonist naloxone for 24 or 48 h. Met-enkephalin (Sigma, St. Louis, MO), the preferential δ1 agonist [D-Pen2, D-Pen5]-enkephalin (DPDPE; Chiron, San Diego, CA) (Mosberg et al., 1983), the preferential δ2 agonist [D-Ala2]-deltorphin II (deltorphin II; Bachem, King of Prussia, PA, USA) (Erspamer 1992), morphine sulfate (Sigma), and naloxone (E.I. Dupont, Wilmington, DE) were dissolved in serum free medium immediately before use.
DNA Content
Total DNA content was assessed at 48 h following continuous opioid treatment using the CyQuant DNA assay (Molecular Probes, Eugene, OR) as described by the manufacturer. Cells were harvested following opioid treatment by removing the medium from the wells and freezing the cell culture plates at −80°C. To assay DNA content, cells were thawed and resuspended in lysis buffer containing EDTA (1 mM) and DNAse-free RNAse (Sigma) was then added to a final concentration of 1 μg/ml for 1 hr at room temperature. CyQuant dye was added for 5 min at room temperature and DNA concentration measured by fluorescence spectroscopy at 480 nM excitation and 520 nM emission wavelengths using a CytoFluor 2300 microplate reader (Millipore). The amount of DNA in each culture was extrapolated by linear regression using bacteriophage DNA as a standard.
DNA Synthesis
DNA synthesis was assessed at 24 h following continuous opioid exposure by determining the portion of EGL cells that incorporated 5′-bromodeoxyuridine (BrdU). EGL cells were grown on glass coverslips inserted into the cell culture wells. Cultures were exposed to BrdU (50 μM) for 14 h immediately before harvesting (Hauser 1992). Cultures were harvested by fixing in Zamboni’s fluid for 30 min (Zamboni & De Martino 1967). Cells were rinsed in PBS, pretreated with ice-cold ethanol (70%) for 24–48 h, HCl (0.5 N) for 30 min, and rinsed in PBS. Cells were incubated in mouse anti-BrdU IgG1 monoclonal antibodies (MAB1467, Chemicon, Temecula, CA, USA) at a 1:1000 dilution (from ascites fluid stock) for 24 h at 4°C. Cell cultures were rinsed in PBS and incubated in affinity purified biotinylated donkey anti-mouse IgG1 conjugated to avidin-peroxidase as directed (Vector ABC kit, Burlingame, CA, USA). Peroxidase-conjugated BrdU antibody complexes were visualized with nickel-intensified DAB (see above). Coverslips with attached cells and cell clusters were removed from culture plates and mounted on microscope slides using Permount (Fisher, Pittsburgh, PA, USA). The BrdU labeling index (% BrdU labeled cells/total cells) was determined by counting cells using a 100x oil-immersion objective aided by a 10 x 10 square-lattice eyepiece reticule (Hauser 1992). About 2000 randomly chosen cells from multiple neurospheres were counted per culture. To arbitrarily sample cells, the x-axis microscope stage controller was moved along an equatorial line through the center of the coverslip and all cell clusters entering the field of vision were sampled. At least 6 cultures, each consisting of cells from separate mice were assessed per experimental group.
Neuron Viability
Neuron viability was assessed at 24 h following continuous opioid treatment. Viability was assessed using a commercially available kit (Live-Dead Assay; Molecular Probes, Eugene, OR, USA) (Opanashuk & Hauser 1998). Neurospheres were plated on to poly-L-lysine-coated (1 mg/ml) glass coverslips for 2 h. Following opioid treatment, cultures were rinsed and incubated in DPBS containing ethidium homodimer (3.5 μM) and calcein-AM (4 μM) (Molecular Probes) for 40 min at 35°C in 5% CO2/95% air (Opanashuk & Hauser 1998). Cells were rinsed with DPBS, stored at 4°C, and counted within 48 h. Red ethidium fluorescence was detected at 535-nm excitation and 590-nm emission wavelengths; green calcein fluorescence was detected at 485-nm excitation and 530-nm emission wavelengths. Coverslips with attached cells and cell clusters were removed from culture plates and mounted on microscope slides using Prolong Antifade (Molecular Probes). The proportion of dead neurons was determined as a percentage of the total number of neurons using a Nikon Diaphot fluorescent microscope (60x objective). About 600 neurons were counted in each culture. To arbitrarily sample cells, the x-axis microscope stage controller was moved along an equatorial line through the center of the coverslip and all cell clusters entering the field of vision were sampled. Four cultures were counted per group, with each culture consisting of cells from separate mice.
Neurite Growth
Neurite growth was assessed in the subpopulation of granule neurons that failed to reaggregate into neurospheres. Immature granule neurons and neuritic processes were labeled immunocytochemically-using antibodies against neuronal ubiquitin. Anti-neuronal ubiquitin antiserum (PGP 9.5; Ultraclone Ltd., Cambridge, UK) was used at 1:750 dilutions, and detected using secondary rabbit anti-human IgG antibodies conjugated to peroxidase (Wilkinson et al., 1989). Nickel-intensified DAB was used as a substrate (as described earlier). Coverslips with attached cells and cell clusters were removed from culture plates and mounted on microscope slides using Permount (Fisher, Pittsburgh, PA, USA). Neuritic length was determined in PGP 9.5-immunoreactive neurites using a calibrated eyepiece reticule with concentric circles as previously described (Sholl 1953; Volkmar & Greenough 1972; Hauser et al., 1989), except measurements were made using a 100x oil-immersion objective and phase contrast optics (Nikon, Diaphot). About 25 randomly chosen, granule neurons that were located away from the neurospheres were sampled from each culture, and at least 4 cultures were assessed per experimental group. Each culture consisted of cells from separate mice. The isolated neurons expressed μ and δ opioid receptor phenotypes in the same proportion as those present in the neurospheres, and their numbers were unaffected by opioids. There are significant spatiotemporal differences in the behavior of cerebellar granule neurons (Raetzman & Siegel 1999). For this reason, the isolated neurons and neurons within clusters are likely to behave differently, and the effects of opioids on each of the two populations may need to be considered independently.
Results
High rates of BrdU incorporation occurred 24–72 h following re-aggregation. At 96 h, neuronal processes were evident on the surface of the clusters as well as in the outgrowth. The outgrowth consisted of some neuronal cell bodies as well as extensive neuritic processes. In addition, some isolated EGL cells were also present outside of the zone of the outgrowth. These cells were uniformly distributed as a monolayer on the surface of the culture dish. Presumably, the isolated EGL cells failed to re-aggregate or detach from the clusters during or after plating. Importantly, less than 0.5% of the cells in these cultures were GFAP immunoreactive astrocytes (data not shown). Physical contacts with astrocyte plasma membranes inhibit DNA synthesis in EGL neuroblasts (Gao et al., 1991).
Opioid Receptor Immunoreactivity
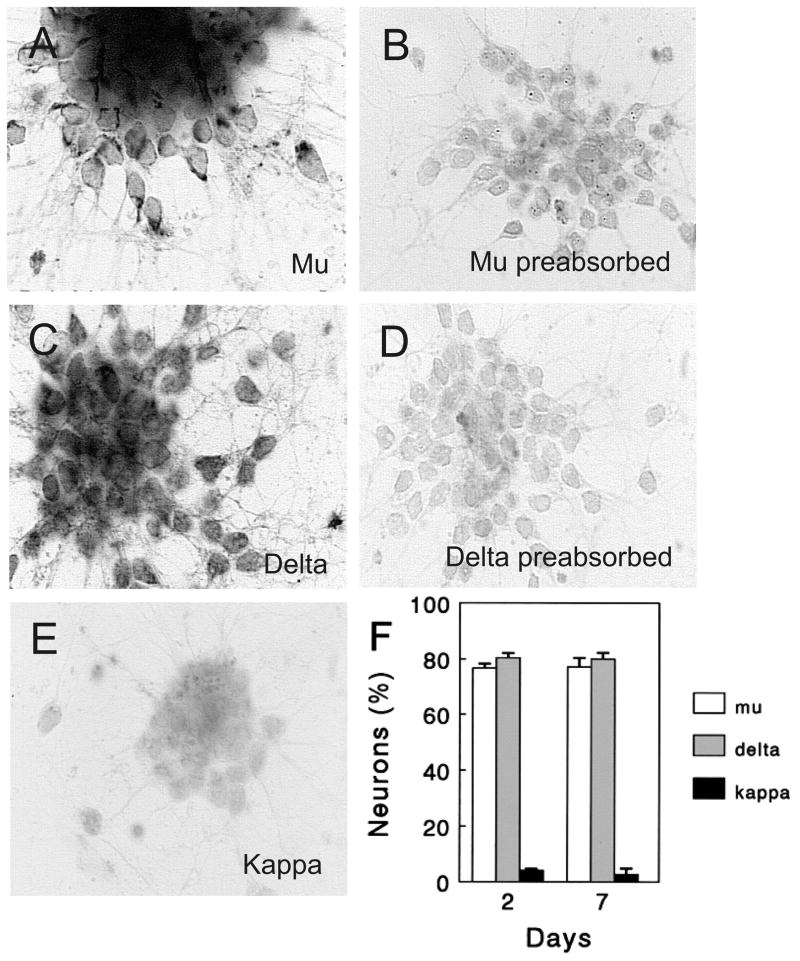
Purified populations of EGL neuroblasts expressed μ and δ receptor immunoreactivity; however, few cells were κ immunoreactive (Figs. 1A–E). μ-Opioid receptor immunoreactivity was punctate and tended to be polarized within the cell body, whereas the δ immunoproduct was more diffuse and uniformly distributed throughout the cell body and neurites (Fig. 1). About 75–80% of the EGL neurons expressed μ opioid receptor immunoreactivity, and about the same proportion expressed δ receptor immunoreactivity suggesting many EGL cells co-expressed both μ and δ receptor types (Fig. 1F). The proportion of cells expressing either μ or δ immunoreactivity remained relatively consistent at 2 and 7 days in vitro (Fig. 1F; mean ± SEM of 4 experiments). Specific μ, δ, or κ (κ not shown) receptor immunoreactivity was not evident in preabsorbed controls (Figs. 1B,C).
Fig. 1.
Opioid receptor immunoreactivity in neuronal precursors. (A–E) Cerebellar external granule layer (EGL) neuronal precursors expressed μ (A) and δ (C) opioid receptor immunoreactivity in brightfield photomicrographs; however, few, if any, cells were κ immunoreactive (E) at 48 h in vitro. Specific immunoreactivity was not present in μ (B) or δ (E) preabsorbed controls. Scale bar = 15 μm. (F) The proportion of EGL cells expressing μ and δ opioid receptor immunoreactivity was similar at 48 h and 7 days in vitro.
Intrinsic Opioid Neuropeptides
We examined whether proenkephalin-derived peptides were present in EGL neuronal precursors cultured in serum free medium. Monoclonal antibodies that recognize the partially processed proenkephalin protein precursor were used to identify proenkephalin immunoreactivity (Böttger et al., 1995; Böttger & Spruce 1995). Proenkephalin-derived peptides were detected in EGL cells using monoclonal antibodies PE-18 and PE-25 (Fig. 2). Proenkephalin immunoreactivity, as detected with PE-18 and PE-25, was located predominantly within cell bodies, with some immunoreactivity additionally extending into the axon and dendrites of more mature neurons (Fig. 2).
Fig. 2.
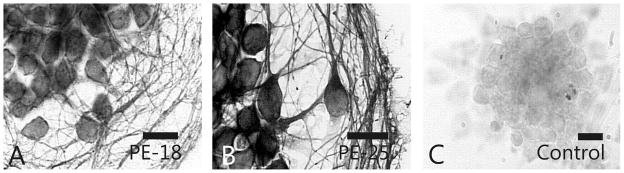
Proenkephalin immunoreactivity in neuronal precursors. Proenkephalin precursor protein immunoreactivity was detected in cerebellar external granular layer (EGL) neuronal precursors using PE-18 or PE-25 monoclonal antibodies. Scale bars = 10 μm.
HPLC-RIA was used to detect enkephalin within the EGL neuron cultures and spent medium. Neuron clusters were plated so that each culture contained 3 x 105 cells per 1-ml of serum free medium. Enkephalin levels were 39.5 ± 6.9-pg/culture/24 h. These antibodies detect Met-and Leu-enkephalin. Enkephalins were not detected in serum free medium and the limit of detection by HPLC-RIA was 1 pg/ml.
DNA content
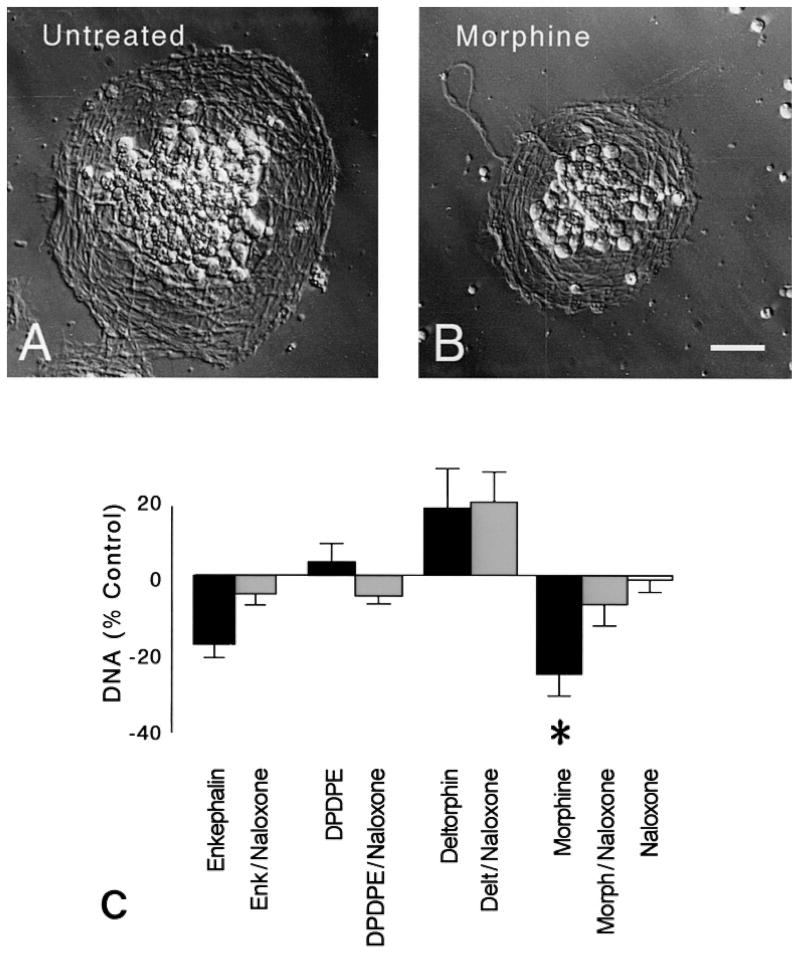
DNA content was assessed to estimate the effect of opioids on cell number. DNA content was assessed at 48 h following opioid treatment to allow sufficient time for changes in DNA synthesis (determined at 24 h) to be manifest as actual changes in cell numbers. DNA content was significantly altered following 48 h of continuous opioid treatment at 3 days in vitro (P < 0.02; ANOVA) (Fig. 3). The effects of opioids were highly selective. Following treatment with Met-enkephalin, a preferential δ receptor agonist, there was a trend toward reduced amounts of DNA. However, this trend was not significant (P = 0.065) and was not mimicked by other δ opioid receptor agonists. In contrast, treatment with morphine significantly reduced DNA content. Untreated control cultures contained 26.7 ± 8.3 μg DNA. Because the number of cells was identical in each culture at the start of each experiment prior to morphine treatment, and morphine did not affect the rate of cell death (Table 1), suggested that the change in DNA content resulted from reductions in the rate or proportion of dividing cells. Examining DNA synthesis in individual neuronal precursors further assessed this question.
Fig. 3.
Effect opioids on neuron number. (A–B) Compared to untreated controls, external granular layer (EGL) neurosphere clusters were reduced the size following 48 h of morphine treatment (B). Hoffman-modulation contrast optics; scale bar = 25 μm. (C) DNA content was significantly reduced following morphine exposure (*P < 0.05 vs. untreated controls). The effects of morphine were prevented by co-administering naloxone. Neither the δ opioid receptor agonists (Met-enkephalin, DPDPE, or deltorphin II) nor naloxone affected DNA content. Met-enkephalin (Enk), [D-Pen2,5]-enkephalin (DPDPE), [D-Ala2]-deltorphin II (Delt), Morphine (Morph).
Table 1.
Opioids did not affect the Survival of Neuronal Precursors from the Cerebellar External Granular Layer (EGL)a
| Treatment | Non-Viable Neurons (%)b |
|---|---|
| Met-enkephalin | 5.3 ± 1.2 |
| Met-enkephalin + naloxone | 4.8 ± 1.1 |
|
| |
| DPDPE | 6.0 ± 0.7 |
| DPDPE + naloxone | 7.5 ± 1.3 |
|
| |
| Deltorphin II | 6.0 ± 1.7 |
| Deltorphin II + naloxone | 4.5 ± 0.9 |
|
| |
| Morphine | 4.2 ± 1.3 |
| Morphine + naloxone | 5.1 ± 1.2 |
|
| |
| Naloxone | 6.6 ± 1.1 |
EGL neurons were continuously exposed to opioid agonists and/or antagonists for 24 h in vitro.
No significant effects on survival were noted following opioid treatment (ANOVA).
DNA Synthesis
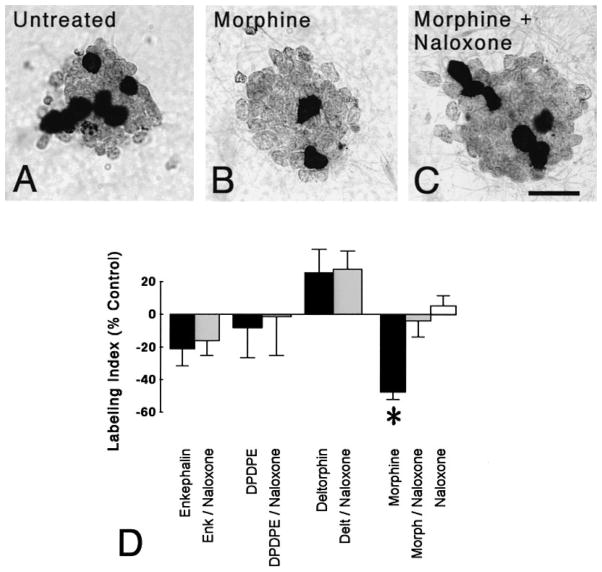
BrdU incorporation by EGL neuroblasts was affected by opioid treatment at 24 h following drug treatment (P < 0.015; ANOVA) (Fig. 4A–D). Treatment with the endogenous δ receptor agonist Met-enkephalin (1 μM) for 24 h did not affect the percentage of EGL neuroblasts incorporating BrdU (Fig. 4D). Similarly, incubation with more selective δ1 (DPDPE, 1 μM) or δ2 (deltorphin II, 1 μM) opioid receptor agonists did not alter the BrdU labeling index (Fig. 4D). In contrast, continuous exposure to morphine (1 μM), a preferential μ receptor agonist, markedly reduced the proportion of neuronal precursors incorporating BrdU (P < 0.025; Newman-Keuls) (Fig. 4D). Importantly, the effects of morphine were attenuated by co-administering naloxone (3 μM) suggesting morphine’s actions were mediated by specific opioid receptors. Although enkephalins are present in these cultures, exposure to naloxone alone had no effect on the BrdU labeling index.
Fig. 4.
Effect of opioids on bromodeoxyuridine (BrdU) incorporation in granule neuron precursors. (A–C) Compared to untreated controls (A), continuous morphine treatment (1 μM) for 24 h reduced the proportion of neuroblasts incorporating BrdU (B). The inhibitory effects of morphine were prevented by concurrent administration of naloxone (3 μM) (C). Neuroblasts incorporating BrdU were densely labeled (arrows) and daughter cells often remained in close proximity after dividing. Scale bar = 25 μm. (D) Morphine-treatment (24 h) markedly reduced the proportion of neuroblasts incorporating BrdU (*P > 0.025 vs. untreated controls). The effect of morphine was antagonized by naloxone, while naloxone alone did not alter the BrdU-labeling index. Mean ± SEM of 4–6 experiments. Met-enkephalin (Enk), [D-Pen2,5]-enkephalin (DPDPE), [D-Ala2]-deltorphin II (Delt), Morphine (Morph).
Neuron Viability
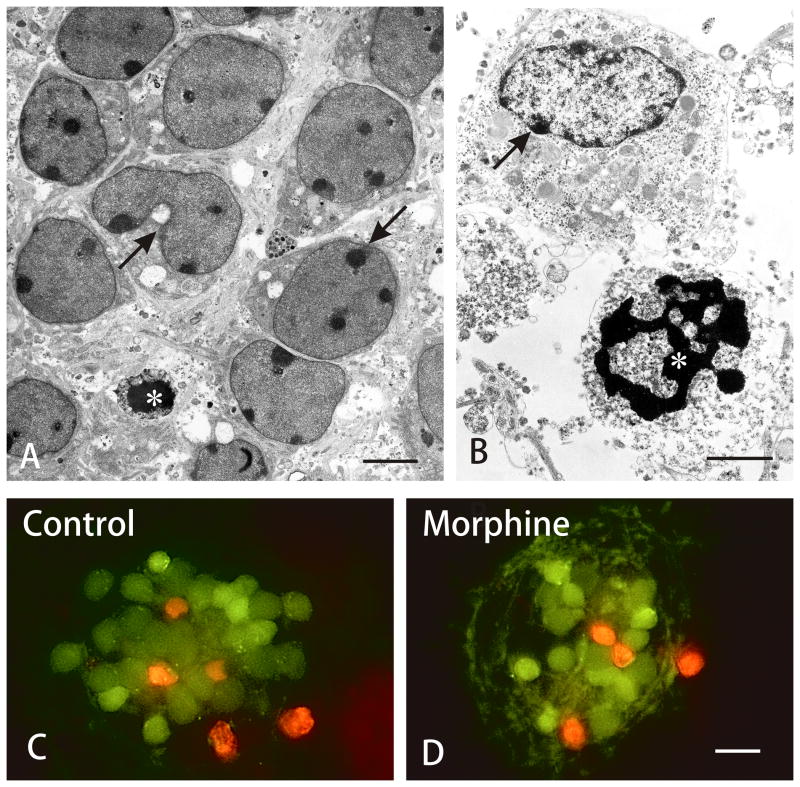
Apoptotic granule cell death occurs during normal cerebellar development in vivo (Wood et al., 1993) and in the re-aggregate EGL cell cultures (Gao et al., 1991) (Figs. 5A–B). To determine whether opioids affected EGL neuron survival, the proportion of viable neurons was determined following 24 h of continuous opioid exposure at 48-h in vitro. Living and non-viable EGL cells were readily discernable ultrastructurally and by using the viability assay (Fig. 5). Viable and non-viable cell markers did not overlap in the same cell; all cells were labeled as living or dead (Figs. 5C–D).
Fig. 5.
Effect of opioids on neuron precursor survival. (A–B) Electron micrographs showing both viable and degenerating neuronal precursors within external granular layer (EGL) cells in untreated cultures. (A) Compromised neurons displayed cytoplasmic vacuoles and swollen mitochondria (arrows); a pyknotic cell with shrunken and densely packed heterochromatin (*). (B) A neuron with accumulated cytoplasmic vacuoles and marginal heterochromatin (arrow), and a cell with chromatin fragmentation patterns characteristic of apoptosis (*). Scale bars = 5 μm (A) and 10 μm (B). (C–D) Fluorescent images of living (green) and non-viable (red) immature neurons from the cerebellar external granular layer (EGL) in untreated control (A) and morphine-treated (B) neurospheres. Neither morphine nor exposure to other opioids affected the proportion of dying cells (see Table 1). Scale bar = 20 μm.
Cell viability was unaffected by opioid exposure (Table 1). Survival rates were determined following 24 h of continuous opioid exposure at 48-h in vitro. Importantly, morphine, which caused significant declines in BrdU incorporation and DNA content, did not affect survival. The proportion of dying EGL cells in untreated control cultures was 6.0 ± 1.2%.
Differentiation
To explore the effect of opioids on granule cell differentiation, the effects of 48 h of continuous opioid exposure were assessed at 6 days in vitro. At 4–6 days in vitro, a zone of neuritic outgrowth surrounded the re-aggregate neurospheres and some differentiated granule neurons were present. During this time, some immature granule neurons were located away from the neurosphere clusters. These neurons were postmitotic (i.e., did not incorporate BrdU) and displayed rapid increases in neuritic outgrowth with 1–5 partially differentiated neurites. The number of isolated granule neurons present was unaffected by opioids (data not shown).
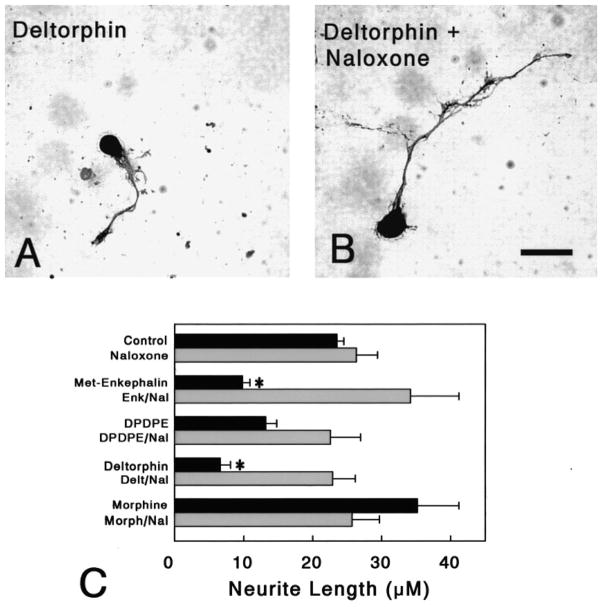
Neurite length was significantly reduced following opioid treatment and the effects were highly selective (P < 0.01; ANOVA) (Fig. 6). Following treatment with Met-enkephalin (1 μM), a preferential δ receptor agonist, there was a significant reduction in neuritic length (P < 0.05; Newman-Keuls) (Fig. 5C). Marked reductions were seen following deltorphin II (P < 0.015; Newman-Keuls), a δ2 agonist, but not following treatment with the δ1 agonist DPDPE (Mosberg et al., 1983; Erspamer 1992; Rossi et al., 1997). This suggests that the δ2 opioid receptor subtype specifically affects neuritic growth and Met-enkephalin is activating δ2 receptors. In contrast, treatment with morphine had no effect on neuritic length. Treatment with naloxone alone also had no effect on neuritic length.
Fig. 6.
Effect of opioids on neurite outgrowth. (A–B) Brightfield photomicrographs showing PGP 9.5-immunoreactive neurons in cultures treated with deltorphin II with or without naloxone. Deltorphin II-exposed neurons had reduced neuritic fields (A,B). Scale bar = 10 μm. (C) Met-enkephalin (*P < 0.04) and deltorphin II (*P < 0.015) treatment significantly reduced the length of neurites (*); co-treating with naloxone prevented the significant effects of the δ agonists. Neither agonists (morphine or DPDPE), nor the antagonist naloxone, affected the elaboration of neurites. Met-enkephalin (Enk), [D-Pen2,5]-enkephalin (DPDPE), [D-Ala2]-deltorphin II (Delt), Morphine (Morph).
Discussion
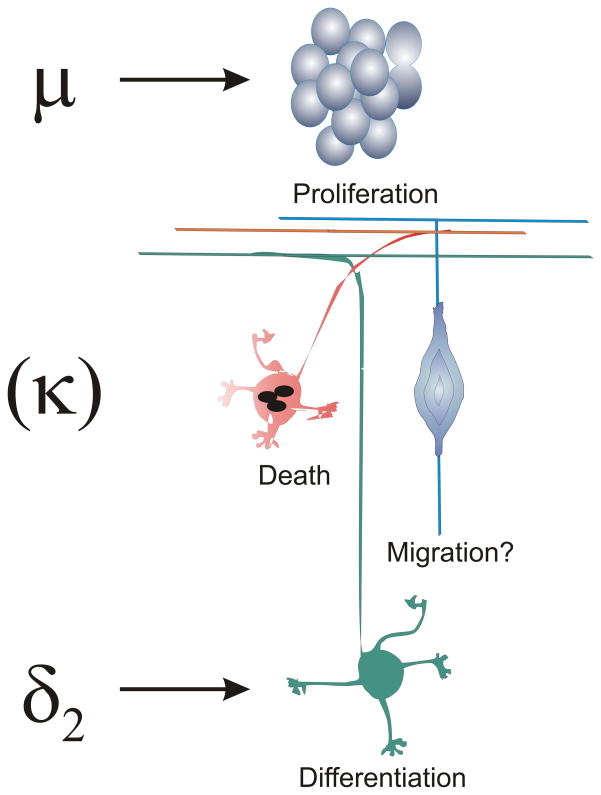
Our results show that opioids intrinsically affect the maturation of EGL neuroblasts and their progeny, and are likely to be of fundamental importance during cerebellar development. EGL neuronal precursors express μ and δ, but few express κ receptors in vitro and developmental effects of opioids were segregated across different receptor types. μ-Opioid receptor activation inhibited neuroblast proliferation, while δ opioid receptor agonists preferentially inhibited neuritic differentiation (Fig. 7).
Fig. 7.
Summary of the effects of opioids on granule neuron development. Activation of μ opioid receptor activation inhibited the proliferation of granule neuronal precursors, while activation of preferential δ2 agonists inhibited neuronal differentiation. Granule neurons did not express κ opioid receptors. Opioids did not affect neuronal viability. Whether opioids affect migration was not assessed.
Opioid Receptors
The cerebellum is traditionally viewed as a region devoid of opioid receptors, since opioid receptor mRNA, protein, or radioligand binding sites are largely absent from mature cerebellum (Mansour et al., 1994). In contrast, high levels μ, δ, and putative ζ opioid receptor binding sites during early postnatal maturation (Kinney & White 1991; Zagon et al., 1992; Leslie et al., 1998). Opioid receptor abundance is highly correlated with the transitory arrival of the EGL (Kinney & White 1991; Zagon et al., 1992). Few studies have systematically assessed μ, δ or κ receptor mRNA expression in the postnatal EGL (Georges et al., 1998; Leslie et al., 1998; Zhu et al., 1998). Adult granule neurons express low levels of δ receptor mRNA (Mansour et al., 1994). Although putative ζ opioid receptor binding coincides with the advent of the EGL (Zagon et al., 1992), the precise nature of ζ binding sites (including their relationship to δ2 sites) is not known and awaits molecular characterization.
Opioids and Cell Proliferation
Our findings that μ receptors inhibit neuroblast proliferation are consistent with findings suggesting that opiates inhibit division in other neural cell types (Vernadakis et al., 1982; Sakellaridis & Vernadakis 1986; Kornblum et al., 1987; Hammer, Jr.1993; Bartolome et al., 1997; Mangoura 1997). Because the reduction in the BrdU labeling index at 24 h was followed by reductions in DNA content (cell numbers) at 48 h without alterations in cell viability, suggests that morphine inhibited neuroblast proliferation. However, based on this evidence alone, it is uncertain whether morphine is causing greater numbers of neuroblasts to exit the mitotic pool (reducing the growth fraction) or whether morphine is altering the duration of the cell cycle (Korr 1980; Reznikov et al., 1999). Morphine was used in the present study because it is a preferential μ agonist, but more importantly because of its role as a drug of abuse, since heroin is largely deacetylated to morphine and 6-mono-acetyl morphine before acting on the brain. Endogenous μ agonist peptides, such as β-endorphin and several of the casomorphin-related peptides, are present during development (Bayon et al., 1979), and inhibit cell proliferation in the brain (Bartolome et al., 1991) and in cell lines (Kampa et al., 1997). Endomorphins, a recently discovered novel class of endogenous μ agonists (Zadina et al., 1997), are also expressed during perinatal development in rats (Barr & Zadina 1998).
Mitogenic signaling has been traditionally associated with tyrosine kinase receptors. However, recent evidence suggests that heterotrimeric G-protein coupled receptors such as μ, δ, and κ opioid receptors activate specific phosphotyrosine pathways (Mangoura & Dawson 1993; Burt et al., 1996; Georgoussi et al., 1997; Mangoura 1997; Wilson et al., 1997). μ or δ opioid receptors can activate p21ras/mitogen-activated protein kinase (MAP-kinase) (Burt et al., 1996; Georgoussi et al., 1997; Wilson et al., 1997; Mullaney et al., 1997; Belcheva et al., 1998; Ignatova et al., 1999). Downstream μ-activation may stimulate phosphatidylinositol (PI) turnover, influencing PI-3-kinase and/or Ca2+-mobilization (Barg et al., 1992; Barg et al., 1993; Hauser et al., 1996; Polakiewicz et al., 1998). The ability to stimulate either traditional opioid signaling pathways [see (Huang 1995)] and/or MAP-kinase may explain how opioids can have varied effects on cell division (Lee & Wurster 1994; Law & Bergsbaken 1995; Hauser & Mangoura 1998; Knapp et al., 1998).
The lack of significant effects with Met-enkephalin on cell division was unexpected and prompted us to additionally assess the actions of more selective δ receptor agonists (DPDPE and deltorphin II). Enkephalin immunoreactivity is localized in germinal zones in the cerebellum (Zagon et al., 1985; Osborne et al., 1993) and treatment with Met-enkephalin reportedly decreases DNA synthesis in EGL neuroblasts in vivo (Zagon & McLaughlin 1991). Alternatively, other investigators suggest that opioids are strategically positioned to affect differentiation, since enkephalins (i.e., Met- or Leu-enkephalin) and opioid receptor-expressing cells can be found immediately outside germinative zones (Huntley et al., 1988; Tecott et al., 1989; Casini et al., 1995; Leslie et al., 1998; Zhu et al., 1998), which agrees with our present findings. In actuality, however, the effect of enkephalins on cell replication may vary greatly depending on neuroblast type (Davila-Garcia & Azmitia 1989; DiCicco-Bloom et al., 1990; Zagon & McLaughlin 1991; Hauser & Mangoura 1998). Although our findings discount an involvement of δ receptors in proliferation, Met-enkephalin might act via alternative mechanisms that do not involve traditional δ opioid receptors. Besides cell surface receptors, the intracellular trafficking of proenkephalin to the nucleus correlates with growth arrest and differentiation (Brooks et al., 1993; Böttger & Spruce 1995), and enkephalins may act as transcriptional regulators (Bakalkin et al., 1991; Bakalkin et al., 1995).
Opioids and Neuronal Differentiation
Our findings suggest the involvement of δ2-receptor subtypes in granule cell neuritic growth. Opioids have been shown to affect neuritic differentiation in vivo and in vitro (Hauser et al., 1989; Hammer, Jr. 1993; Hauser & Stiene-Martin 1993; Sklair-Tavron et al., 1996). Met-enkephalin, a δ agonist, significantly increases the phosphorylation of the Src kinase substrate cortactin causing dramatic changes its localization and movement to the cytosol, and accompanying phosphorylation of vinculin at focal adhesion sites (Mangoura 1997). As noted in the methods, however, isolated neurons may behave differently to opioids than the neurons in neurosphere clusters, because of potential developmental constraints imposed by each unique microenvironment. Thus, μ agonists may potentially affect the differentiation of granule neurons in neurospheres, whereas δ2 agonists may have fewer effects on neurons in clusters.
Findings that EGL cells transiently and coordinately express both opioid receptors and peptides in this and other studies suggests paracrine or autocrine interactions occur among developing granule cells (Zagon et al., 1985; Osborne et al., 1993). Despite this, differentiation was unaffected by naloxone alone in the present study, suggesting that the amount of Met-enkephalin present within our cultures was insufficient to inhibit neuritic outgrowth. The relatively large volume of medium in our cultures may dilute the small quantities of opioids released by the EGL cells and was a consistent finding even if enkephalinase (neutral endopeptidase EC 3.4.24.11) inhibitors were added (data not shown). This might be different in the intact central nervous system, where enkephalin is released within a confined extracellular space. Alternatively, Purkinje cells, cerebellar astrocytes, as well as enkephalin-containing mossy fibers, also express enkephalins within the developing cerebellum (Williams & Dockray 1983; Walker & King 1989; Spruce et al., 1990; Osborne et al., 1993).
Opioids and Neuronal Survival
Although opioids did not affect EGL cell viability, morphine or Met-enkephalin can inhibit neuronal cell death in the avian ciliary ganglion (Meriney et al., 1985; Meriney et al., 1991). In a cell line transfected with μ opioid receptors, the μ agonist D-Ala2-MePhe4, Gly-ol5-enkephalin (DAMGO) activates Akt-induced neuroprotection (Polakiewicz et al., 1998). Alternatively, in other studies, opioids only enhanced cell losses if apoptosis was induced by other factors, such as staurosporine or wortmannin (Dawson et al., 1997; Goswami et al., 1998). Together, the above findings suggest that the effects of opioids on cell death are variable and cell type specific.
In summary, our findings provide strong evidence that multiple opioid receptor types regulate different aspects of granule neuron maturation. When the effects of opioids on neurons, astroglia, and oligodendroglia are considered together, it is apparent that μ, δ, and κ opioid receptors control maturation in each cell-type differently (Hauser & Mangoura 1998; Knapp et al., 1998). This diversity may strategically position the opioid system to coordinate the development of granule neurons with the maturation of other neuronal and glial cell types.
Acknowledgments
We thank Dr. Mary E. Hatten and Dr. Nam K. Cho for providing detailed protocols and advice concerning EGL neuroblast culture, and Dr. Barbara A. Spruce for providing proenkephalin antibodies. We also thank Ms. Jane Foldes, Mr. Kenneth Martin, Dr. Lisa Opanashuk, and Ms. Ann Pahk for technical assistance. Supported by NIH DA06204.
Abbreviations
- BrdU
5′-bromodeoxyuridine
- DAMGO
D-Ala2-MePhe4, Gly-ol5-enkephalin
- DAB
diaminobenzidine
- Delt or deltorphin
[D-Ala2]-deltorphin II
- DPDPE
[D-Pen2, D-Pen5]-enkephalin
- EGL
external granular layer
- Enk
Met-enkephalin
- Morph
morphine
- G1
pre-DNA synthesis phase of the cell cycle
- MAP-kinase
mitogen-activated protein-kinase
- Nal
naloxone
- PBS
phosphate buffered saline
- PI-3-kinase
phosphatidylinositol-3-kinase
- PGP 9.5
protein gene product 9.5 (neuronal ubiquitin)
- S-phase
DNA synthesis phase of the cell cycle
References
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous Opioids: Biology and Function. Ann Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972;145:353–398. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Dado RJ, Riedl M, Lee JH, Law PY, Loh HH, Elde R, Wessendorf MW. δ-opioid receptor immunoreactivity: Distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J Neurosci. 1995b;15:1215–1235. doi: 10.1523/JNEUROSCI.15-02-01215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a μ-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995a;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee JH, Nakano AH, Lin X, Loh HH, Law PY, Wessendorf MW, Elde R. The kappa-opioid receptor is primarily postsynaptic: Combined immunohistochemical localization of the receptor and endogenous opioids. Proc Natl Acad Sci USA. 1995c;92:5062–5066. doi: 10.1073/pnas.92.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalkin G, Telkov M, Yakovleva T, Terenius L. [Leu5]enkephalin-encoding sequences are targets for a specific DNA-binding factor. Proc Natl Acad Sci USA. 1995;92:9024–9028. doi: 10.1073/pnas.92.20.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalkin GY, Ponomariev D, Sarkisyan RA, Terenius L. Sequence similarity between opioid peptide precursors and DNA- binding proteins. FEBS Lett. 1991;282:175–177. doi: 10.1016/0014-5793(91)80471-e. [DOI] [PubMed] [Google Scholar]
- Barg J, Belcheva M, McHale R, Levy R, Vogel Z, Coscia CJ. Beta-endorphin is a potent inhibitor of thymidine incorporation into DNA via mu- and kappa-opioid receptors in fetal rat brain cell aggregates in culture. J Neurochem. 1993;60:765–767. doi: 10.1111/j.1471-4159.1993.tb03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg J, Belcheva MM, Coscia CJ. Evidence for the implication of phosphoinositol signal transduction in μ-opioid inhibition of DNA synthesis. J Neurochem. 1992;59:1145–1152. doi: 10.1111/j.1471-4159.1992.tb08357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA, Zadina J. Maturation of endomorphin-2-like imunoreactivity in the rat. International Narcotics Research Conference Abstracts. 1998;29:36–36. Ref Type: Abstract. [Google Scholar]
- Bartolome JV, Alicke B, Bartolome MB. Central administration of morphine inhibits brain and liver ornithine decarboxylase activity in neonatal rats: involvement of transcription- and non-transcription-dependent mechanisms. Eur J Pharmacol. 1997;331:145–153. doi: 10.1016/s0014-2999(97)01045-5. [DOI] [PubMed] [Google Scholar]
- Bartolome JV, Bartolome MB, Lorber BA, Dileo SJ, Schanberg SM. Effects of central administration of beta-endorphin on brain and liver DNA synthesis in preweaning rats. Neurosci. 1991;40:289–294. doi: 10.1016/0306-4522(91)90191-p. [DOI] [PubMed] [Google Scholar]
- Bayon A, Shoemaker WJ, Bloom FE, Mauss A, Guillemin R. Perinatal development of the endorphin and enkephalin containing systems of the rat brain. Brain Res. 1979;179:93–101. doi: 10.1016/0006-8993(79)90493-1. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Vogel Z, Ignatova E, Avidor-Reiss T, Zippel R, Levy R, Young EC, Barg J, Coscia CJ. Opioid modulation of extracellular signal-regulated protein kinase activity is ras-dependent and involves Gβγ subunits. J Neurochem. 1998;70:635–645. doi: 10.1046/j.1471-4159.1998.70020635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostwick DG, Null WE, Holmes D, Weber E, Barchas JD, Bensch KG. Expression of opioid peptides in tumors. N Engl J Med. 1987;317:1439–1443. doi: 10.1056/NEJM198712033172304. [DOI] [PubMed] [Google Scholar]
- Böttger A, Spruce BA. Proenkephalin is a nuclear protein responsive to growth arrest and differentiation signals. J Cell Biol. 1995;130:1251–1262. doi: 10.1083/jcb.130.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttger V, Böttger A, Lane EB, Spruce BA. Comprehensive epitope analysis of monoclonal anti-proenkephalin antibodies using phage display libraries and synthetic peptides: Revelation of antibody fine specificities caused by somatic mutations in the variable region genes. J Mol Biol. 1995;247:932–946. doi: 10.1006/jmbi.1995.0191. [DOI] [PubMed] [Google Scholar]
- Brooks PJ, Funabashi T, Kleopoulos SP, Mobbs CV, Pfaff DW. Cell-specific expression of preproenkephalin intronic heteronuclear RNA in the rat forebrain. Brain Res Mol Brain Res. 1993;19:22–30. doi: 10.1016/0169-328x(93)90144-e. [DOI] [PubMed] [Google Scholar]
- Bunikowski R, Grimmer I, Heiser A, Metze B, Schafer A, Obladen M. Neurodevelopmental outcome after prenatal exposure to opiates. Eur J Pediatr. 1998;157:724–730. doi: 10.1007/s004310050923. [DOI] [PubMed] [Google Scholar]
- Burt AR, Carr IC, Mullaney I, Anderson NG, Milligan G. Agonist activation of p42 and p44 mitogen-activated protein kinases following expression of the mouse δ opioid receptor in Rat-1 fibroblasts: Effects of receptor expression levels and comparisons with G-protein activation. Biochem J. 1996;320:227–235. doi: 10.1042/bj3200227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini G, Molnar M, Davis BM, Bagnoli P. Posthatching development of preproenkephalin mRNA-expressing cell populations in the pigeon telencephalon. Dev Brain Res. 1995;84:233–244. doi: 10.1016/0165-3806(94)00176-z. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Burns KA, Burns WJ, Schnoll SH. Prenatal drug exposure: effects on neonatal and infant growth and development. Neurobehav Toxicol Teratol. 1986;8:357–362. [PubMed] [Google Scholar]
- Davila-Garcia MI, Azmitia EC. Effects of acute and chronic administration of leu-enkephalin on cultured serotonergic neurons: evidence for opioids as inhibitory neuronal growth factors. Dev Brain Res. 1989;49:97–103. doi: 10.1016/0165-3806(89)90062-x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Dawson SA, Goswami R. Chronic exposure to kappa-opioids enhances the susceptibility of immortalized neurons (F-11kappa 7) to apoptosis-inducing drugs by a mechanism that may involve ceramide. J Neurochem. 1997;68:2363–2370. doi: 10.1046/j.1471-4159.1997.68062363.x. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Townes-Anderson E, Black IB. Neuroblast mitosis in dissociated culture: regulation and relationship to differentiation. J Cell Biol. 1990;110:2073–2086. doi: 10.1083/jcb.110.6.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V. The opioid peptides of the amphibian skin. Int J Dev Neurosci. 1992;10:3–30. doi: 10.1016/0736-5748(92)90003-i. [DOI] [PubMed] [Google Scholar]
- Evans CJ. Diversity among the opioid receptors. In: Korenman SG, Barchas JD, editors. Biological Basis of Substance Abuse. Oxford University Press; New York: 1993. pp. 31–48. [Google Scholar]
- Gao WQ, Heintz N, Hatten ME. Cerebellar granule cell neurogenesis is regulated by cell-cell interactions in vitro. Neuron. 1991;6:705–715. doi: 10.1016/0896-6273(91)90168-y. [DOI] [PubMed] [Google Scholar]
- Georges F, Normand E, Bloch B, Le MC. Opioid receptor gene expression in the rat brain during ontogeny, with special reference to the mesostriatal system: an in situ hybridization study. Brain Res Dev Brain Res. 1998;109:187–199. doi: 10.1016/s0165-3806(98)00082-0. [DOI] [PubMed] [Google Scholar]
- Georgoussi Z, Merkouris M, Mullaney I, Megaritis G, Carr C, Zioudrou C, Milligan G. Selective interactions of mu-opioid receptors with pertussis toxin- sensitive G proteins: involvement of the third intracellular loop and the c-terminal tail in coupling. Biochim Biophys Acta. 1997;1359:263–274. doi: 10.1016/s0167-4889(97)00097-9. [DOI] [PubMed] [Google Scholar]
- Goswami R, Dawson SA, Dawson G. Cyclic AMP protects against staurosporine and wortmannin-induced apoptosis and opioid-enhanced apoptosis in both embryonic and immortalized (F-11kappa7) neurons. J Neurochem. 1998;70:1376–1382. doi: 10.1046/j.1471-4159.1998.70041376.x. [DOI] [PubMed] [Google Scholar]
- Gurwell JA, Duncan MJ, Maderspach K, Stiene-Martin A, Elde RP, Hauser KF. κ-Opioid receptor expression defines a phenotypically distinct subpopulation of astroglia: relationship to Ca2+ mobilization, development, and the antiproliferative effect of opioids. Brain Res. 1996;737:175–187. doi: 10.1016/0006-8993(96)00728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallonet M, Alvarado-Mallart RM. The chick/quail chimeric system: a model for early cerebellar development. Perspect Dev Neurobiol. 1997;5:17–31. [PubMed] [Google Scholar]
- Hallonet ME, Teillet MA, Le DN. A new approach to the development of the cerebellum provided by the quail-chick marker system. Development. 1990;108:19–31. doi: 10.1242/dev.108.1.19. [DOI] [PubMed] [Google Scholar]
- Hammer RP., Jr . Effects of opioids on the developing brain. In: Hammer RP Jr, editor. The Neurobiology of Opiates. CRC Press; Boca Raton, Florida: 1993. pp. 1–21. [Google Scholar]
- Hatten ME. Neuronal regulation of astroglial morphology and proliferation in vitro. J Cell Biol. 1985;100:384–396. doi: 10.1083/jcb.100.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten ME. Neuronal inhibition of astroglial cell proliferation is membrane mediated. J Cell Biol. 1987;104:1353–1360. doi: 10.1083/jcb.104.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF. Morphine regulates DNA synthesis in cerebellar neuroblasts in vitro. Dev Brain Res. 1992;70:291–297. doi: 10.1016/0165-3806(92)90210-n. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Mangoura D. Diversity of the endogenous opioid system in development: novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect Dev Neurobiol. 1998;5:337–449. [PubMed] [Google Scholar]
- Hauser KF, McLaughlin PJ, Zagon IS. Endogenous opioid systems and the regulation of dendritic growth and spine formation. J Comp Neurol. 1989;281:13–22. doi: 10.1002/cne.902810103. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Stiene-Martin A. Opiates and the regulation of nervous system development: Evidence from in vitro studies. In: Hammer RP Jr, editor. Neurobiology of Opiates. CRC Press; Boca Raton, Florida: 1993. pp. 23–61. [Google Scholar]
- Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC. μ-Opioid receptor-induced Ca2+ mobilization and astroglial development: Morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca2+ -dependent mechanism. Brain Res. 1996;720:191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L-YM. Cellular mechanisms of excitatory and inhibitory actions of opioids. In: Tseng LF, editor. The Pharmacology of Opioid Peptides. Harwood Academic Publishers; 1995. pp. 131–149. [Google Scholar]
- Huntley GW, Hendry SHC, Killackey HP, Chalupa LM, Jones EG. Temporal sequence of neurotransmitter expression by developing neurons of fetal monkey visual cortex. Dev Brain Res. 1988;43:69–96. doi: 10.1016/0165-3806(88)90154-x. [DOI] [PubMed] [Google Scholar]
- Hutchings DE. Methadone and heroin during pregnancy: a review of behavioral effects in human and animal offspring. Neurobehvr Toxicol Teratol. 1982;4:429–434. [PubMed] [Google Scholar]
- Ignatova EG, Belcheva MM, Bohn LM, Neuman MC, Coscia CJ. Requirement of receptor internalization for opioid stimulation of mitogen-activated protein kinase: biochemical and immunofluorescence confocal microscopic evidence. J Neurosci. 1999;19:56–63. doi: 10.1523/JNEUROSCI.19-01-00056.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa M, Bakogeorgou E, Hatzoglou A, Damianaki A, Martin PM, Castanas E. Opioid alkaloids and casomorphin peptides decrease the proliferation of prostatic cancer cell lines (LNCaP, PC3 and DU145) through a partial interaction with opioid receptors. Eur J Pharmacol. 1997;335:255–265. doi: 10.1016/s0014-2999(97)01213-2. [DOI] [PubMed] [Google Scholar]
- Kinney HC, White WF. Opioid receptors localize to the external granular cell layer of the developing human cerebellum. Neuroscience. 1991;45:13–21. doi: 10.1016/0306-4522(91)90099-a. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: μ and κ opioid receptors mediate differential mitogenic and growth responses. Glia. 1998;22:189–201. doi: 10.1002/(sici)1098-1136(199802)22:2<189::aid-glia10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Kornblum HI, Loughlin SE, Leslie FM. Effects of morphine on DNA synthesis in neonatal rat brain. Dev Brain Res. 1987;31:45–52. doi: 10.1016/0165-3806(87)90081-2. [DOI] [PubMed] [Google Scholar]
- Korr H. Advances Anat, Embryol, Cell Biol. Vol. 61. Springer-Verlag; New Yrok: 1980. Proliferation of different cell types in the brain; pp. 1–72. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Ignar D, Windh R. Methodological Issues in Controlled Studies on the Effects of Prenatal Exposure to Drug Abuse. NIDA Res. Monog. Vol. 114. USPHS, NIDA; Rockville, MD: 1991. Endocrine function as a target of perinatal drug effects: methodological issues; pp. 206–232. [PubMed] [Google Scholar]
- Law PY, Bergsbaken C. Properties of delta opioid receptor in neuroblastoma NS20Y: receptor activation and neuroblastoma proliferation. J Pharmacol Exp Ther. 1995;272:322–332. [PubMed] [Google Scholar]
- Lee YS, Wurster RD. Differential effects of methionine enkephalin on the growth of brain tumor cells. J Neurooncol. 1994;19:11–15. doi: 10.1007/BF01051044. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Chen Y, Winzer-Serhan UH. Opioid receptor and peptide mRNA expression in proliferative zones of fetal rat central nervous system. Can J Physiol Pharmacol. 1998;76:284–293. [PubMed] [Google Scholar]
- Leslie FM, Loughlin SE. Ontogeny and plasticity of opioid systems. In: Hammer RP Jr, editor. The Neurobiology of Opiates. CRC Press; Boca Raton, FL: 1993. pp. 85–123. [Google Scholar]
- Maneckjee R, Minna JD. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc Natl Acad Sci USA. 1990;87:3294–3298. doi: 10.1073/pnas.87.9.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoura D. mu-Opioids activate tyrosine kinase focal adhesion kinase and regulate cortical cytoskeleton proteins cortactin and vinculin in chick embryonic neurons. J Neurosci Res. 1997;50:391–401. doi: 10.1002/(SICI)1097-4547(19971101)50:3<391::AID-JNR5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Mangoura D, Dawson G. Opioid peptides activate phospholipase D and protein kinase C-ε in chicken embryo neuron cultures. Proc Natl Acad Sci USA. 1993;90:2915–2919. doi: 10.1073/pnas.90.7.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: An in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- McDowell J, Kitchen I. Development of opioid systems: peptides, receptors, and pharmacology. Brain Res Rev. 1987;12:397–421. doi: 10.1016/0165-0173(87)90006-3. [DOI] [PubMed] [Google Scholar]
- Meriney SD, Ford MJ, Oliva D, Pilar G. Endogenous opioids modulate neuronal survival in the developing avian ciliary ganglion. J Neurosci. 1991;11:3705–3717. doi: 10.1523/JNEUROSCI.11-12-03705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriney SD, Gray DB, Pilar G. Morphine-induced delay of normal cell death in the avian ciliary ganglion. Science. 1985;228:1451–1453. doi: 10.1126/science.2990029. [DOI] [PubMed] [Google Scholar]
- Miale IL, Sidman RL. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol. 1961;4:277–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Herz A. The endocrinology of opioids. Int Rev Neurobiol. 1985;26:1–83. doi: 10.1016/s0074-7742(08)60072-0. [DOI] [PubMed] [Google Scholar]
- Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HI, Galligan JJ, Burks TF. Bis-penicillamine enkephalins show pronounced delta enkephalin selectivity. Proc Natl Acad Sci (USA) 1983;80:5871–5880. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaney I, Carr IC, Burt AR, Wilson M, Anderson NG, Milligan G. Agonist-mediated tyrosine phosphorylation of isoforms of the shc adapter protein by the delta opioid receptor. Cell Signal. 1997;9:423–429. doi: 10.1016/s0898-6568(96)00188-x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Under siege: The brain on opiates. Neuron. 1996;16:897–900. doi: 10.1016/s0896-6273(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Opanashuk LA, Hauser KF. Opposing actions of the EGF family and opioids: Heparin binding-epidermal growth factor (HB-EGF) protects mouse cerebellar neuroblasts against the antiproliferative effect of morphine. Brain Res. 1998;804:87–94. doi: 10.1016/s0006-8993(98)00647-7. [DOI] [PubMed] [Google Scholar]
- Osborne JG, Kindy MS, Spruce BA, Hauser KF. Ontogeny of proenkephalin mRNA and enkephalin peptide expression in the cerebellar cortex of the rat: Spatial and temporal patterns of expression follow maturational gradients in the external granular layer and in Purkinje cells. Dev Brain Res. 1993;76:1–12. doi: 10.1016/0165-3806(93)90117-s. [DOI] [PubMed] [Google Scholar]
- Pan EC, Bohn LM, Belcheva MM, Thomas GE, Manepalli AN, Mamone JY, Johnson FE, Coscia CJ. Kappa-opioid receptor binding varies inversely with tumor grade in human gliomas. Cancer. 1998;83:2561–2566. doi: 10.1002/(sici)1097-0142(19981215)83:12<2561::aid-cncr23>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Panagiotou S, Hatzoglou A, Calvo F, Martin PM, Castanas E. Modulation of the estrogen-regulated proteins cathepsin D and pS2 by opioid agonists in hormone-sensitive breast cancer cell lines (MCF7 and T47D): evidence for an interaction between the two systems [In Process Citation] J Cell Biochem. 1998;71:416–428. doi: 10.1002/(sici)1097-4644(19981201)71:3<416::aid-jcb10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Pasto ME, Graziani LJ, Tunis SL, Deiling JM, Kurtz AB, Goldberg B, Finnegan LP. Ventricular configuration and cerebral growth in infants born to drug-dependent mothers. Pediatr Radiol1985. 1993;15:77–81. doi: 10.1007/BF02388706. [DOI] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J Biol Chem. 1998;273:23534–23541. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Siegel RE. Immature granule neurons from cerebella of different ages exhibit distinct developmental potentials. J Neurobiol. 1999;38:559–570. [PubMed] [Google Scholar]
- Ramón y Cajal S. Studies on Vertebrate Neurogenesis. Charles C. Thomas; Springfield, Il: 1960. [Google Scholar]
- Reznikov K, Hauser KF, Nazarevskaja G, Trunova Y, Derjabin V, Bakalkin G. Opioids modulate cell division in the germinal zone of the late embryonic neocortex. European Journal of Neuroscience. 1999 Aug 1;:2711–2719. doi: 10.1046/j.1460-9568.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- Rosen H, Bar-Shavit Z. Dual role of osteoblastic proenkephalin derived peptides in skeletal tissues. J Cell Biochem. 1994;55:334–339. doi: 10.1002/jcb.240550310. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Su W, Leventhal L, Su H, Pasternak GW. Antisense mapping DOR-1 in mice: further support for delta receptor subtypes. Brain Res. 1997;753:176–179. doi: 10.1016/s0006-8993(97)00081-4. [DOI] [PubMed] [Google Scholar]
- Sakellaridis N, Vernadakis A. An unconventional response of adenylate cyclase to morphine and naloxone in the chicken during early development. Proc Natl Acad Sci (USA) 1986;83:2738–2742. doi: 10.1073/pnas.83.8.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp BM, Roy S, Bidlack JM. Evidence for opioid receptors on cells involved in host defense and the immune system. J Neuroimmunol. 1998;83:45–56. [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat (London) 1953;87:387–407. [PMC free article] [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci USA. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce BA, Curtis R, Wilkin GP, Glover DM. A neuropeptide precursor in cerebellum: Proenkephalin exists in subpopulations of both neurons and astrocytes. EMBO J. 1990;9:1787–1795. doi: 10.1002/j.1460-2075.1990.tb08303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Rubenstein JLR, Paxinos G, Evans CJ, Eberwine JH, Valentino KL. Developmental expression of proenkephalin mRNA and peptides in rat striatum. Dev Brain Res. 1989;49:75–86. doi: 10.1016/0165-3806(89)90060-6. [DOI] [PubMed] [Google Scholar]
- Vernadakis A, Estin C, Gibson DA, Amott S. Effects of methadone on ornithine decarboxylase and cyclic nucleotide phosphohydrolase in neuronal and glial cell cultures. J Neurosci Res. 1982;7:111–117. doi: 10.1002/jnr.490070203. [DOI] [PubMed] [Google Scholar]
- Vértes Z, Melegh G, Vértes M, Kovács S. Effect of naloxone and D-Met2-Pro5-enkephalinamide treatment on the DNA synthesis in the developing rat brain. Life Sci. 1982;31:119–126. doi: 10.1016/0024-3205(82)90423-4. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Greenough WT. Rearing complexity affects branching of cortical dendrites in the visual cortex of the rat. Science. 1972;176:1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- Walker JJ, King JS. Ontogenesis of enkephalinergic afferent systems in the opossum cerebellum. Dev Brain Res. 1989;48:35–58. doi: 10.1016/0165-3806(89)90092-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson KD, Lee K, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- Williams RG, Dockray GJ. Distribution of enkephalin-related peptides in rat brain: immunohistochemical studies using antisera to met-enkephalin and met-enkephalin Arg6Phe7. Neuroscience. 1983;9:563–586. doi: 10.1016/0306-4522(83)90175-6. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Burt AR, Milligan G, Anderson NG. Mitogenic signalling by delta opioid receptors expressed in rat-1 fibroblasts involves activation of the p70s6k/p85s6k S6 kinase. Biochem J. 1997;325:217–222. doi: 10.1042/bj3250217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KA, Dipasquale B, Youle RJ. In situ labeling of granule cells for apoptosis-associated DNA fragmentation reveals different mechanisms of cell loss in developing cerebellum. Neuron. 1993;11:621–632. doi: 10.1016/0896-6273(93)90074-2. [DOI] [PubMed] [Google Scholar]
- Yang XW, Zhong R, Heintz N. Granule cell specification in the developing mouse brain as defined by expression of the zinc finger transcription factor RU49. Development. 1996;122:555–566. doi: 10.1242/dev.122.2.555. [DOI] [PubMed] [Google Scholar]
- Yeadon M, Kitchen I. Opioids and respiration. Prog Neurobiol. 1989;33:1–16. doi: 10.1016/0301-0082(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Gibo DM, McLaughlin PJ. Ontogeny of zeta (ζ), the opioid growth factor receptor, in the rat brain. Brain Res. 1992;596:149–156. doi: 10.1016/0006-8993(92)91542-m. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Increased brain size and cellular content in infant rats treated with an opioid antagonist. Science. 1983;221:1179–1180. doi: 10.1126/science.6612331. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Opioid antagonist (naltrexone) modulation of cerebellar development: histological and morphometric studies. J Neurosci. 1986;6:1424–1432. doi: 10.1523/JNEUROSCI.06-05-01424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Endogenous opioid systems regulate cell proliferation in the developing rat brain. Brain Res. 1987;412:68–72. doi: 10.1016/0006-8993(87)91440-5. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Identification of opioid peptides regulating proliferation of neurons and glia in the developing nervous system. Brain Res. 1991;542:318–323. doi: 10.1016/0006-8993(91)91585-o. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Rhodes RE, McLaughlin PJ. Distribution of enkephalin immunoreactivity in germinative cells of developing rat cerebellum. Science. 1985;227:1049–1051. doi: 10.1126/science.3883485. [DOI] [PubMed] [Google Scholar]
- Zamboni L, De Martino C. Buffered picric acid formaldehyde: a new rapid fixative for electron microscopy. J Cell Biol. 1967;35:148A. [Google Scholar]
- Zhang L, Goldman JE. Generation of cerebellar interneurons from dividing precursors in white matter. Neuron. 1996;16:47–54. doi: 10.1016/s0896-6273(00)80022-7. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hsu MS, Pintar JE. Developmental expression of the mu, kappa, and delta opioid receptor mRNAs in mouse. J Neurosci. 1998;18:2538–2549. doi: 10.1523/JNEUROSCI.18-07-02538.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]