Abstract
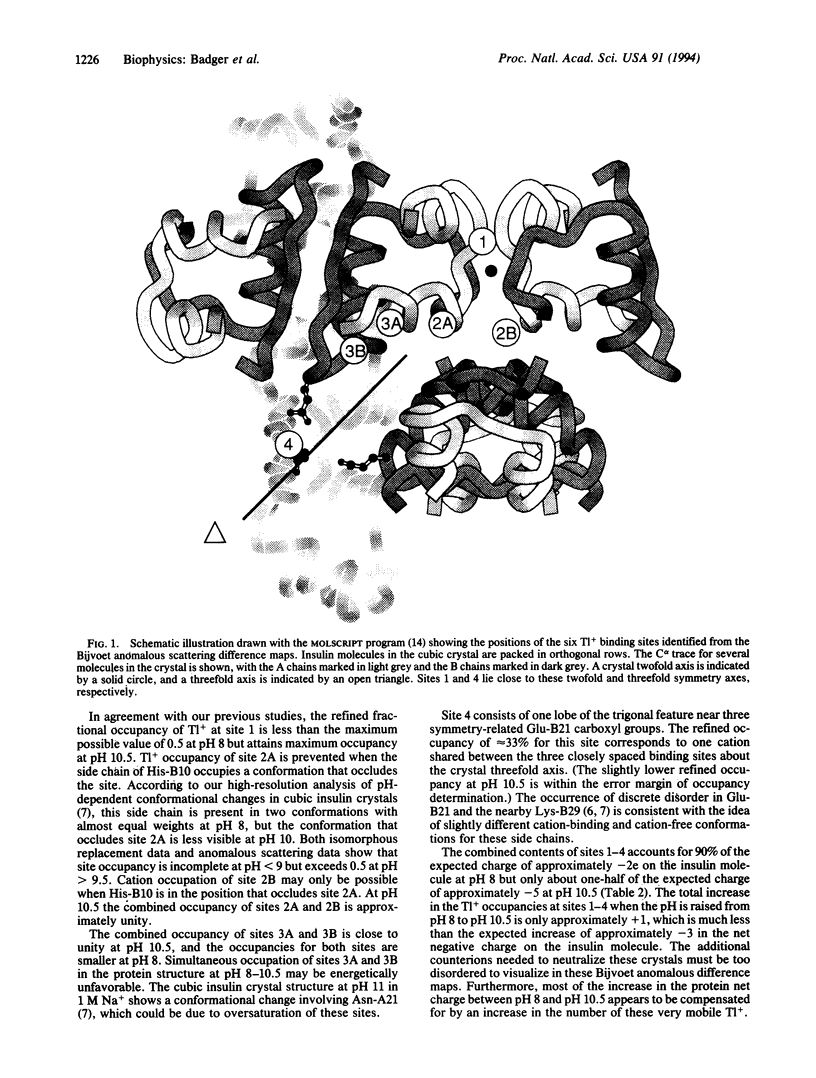
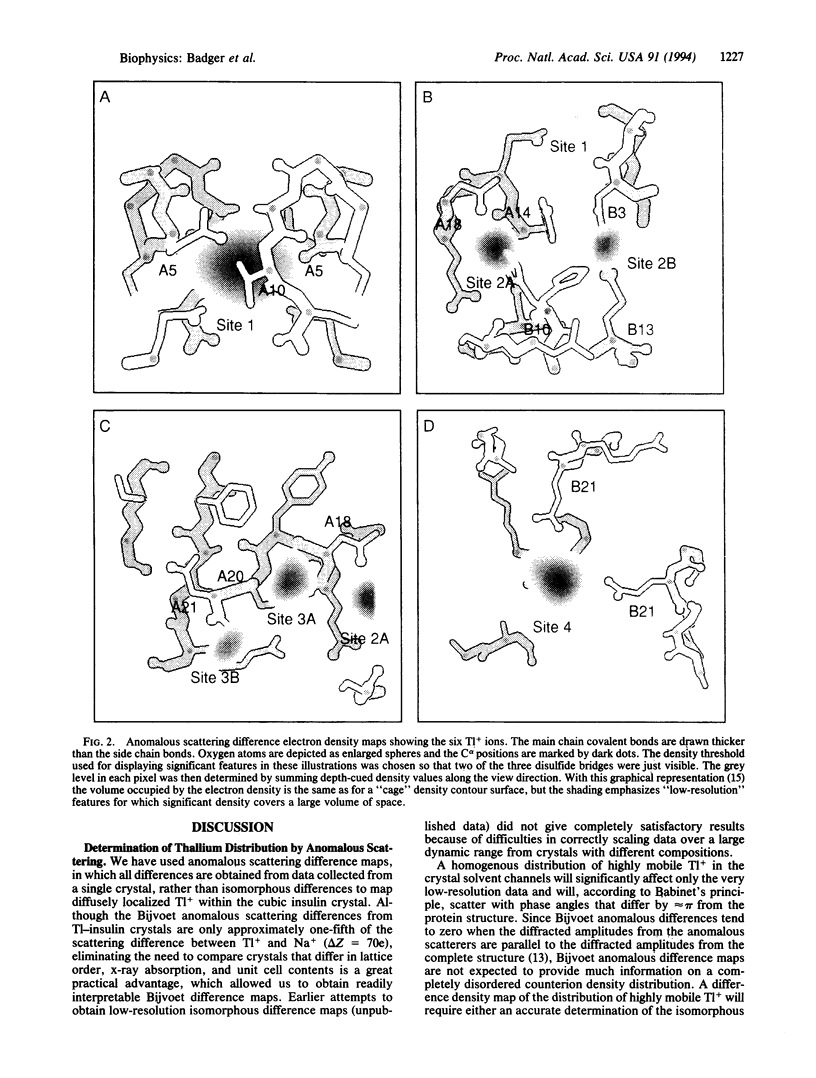
To determine the distribution of monovalent cations around a protein we have measured anomalous scattering diffraction data from Tl-containing cubic insulin crystals at pH 8 and pH 10.5. The differences between Bijvoet reflection pairs within each set of data were used to calculate anomalous scattering difference maps. Both maps show the same six Tl+ sites, which include two well-ordered Tl+ ions previously identified from isomorphous exchange experiments. The other four sites constitute a second class of cations, which, while much more mobile than the protein atoms, are associated with particular ligating groups. Three of the six Tl+ sites are created exclusively by protein main and side chain carbonyl dipoles rather than negatively charged groups. All of the Tl+ ions are positioned so as to interact with both protein atoms and water molecules. The Tl+ occupancies appear to depend in a complex way on interactions with each other and flexibility in the protein structure. The combined occupancies of these cations are slightly less than is required to neutralize the net protein charge of approximately -2e at pH 8 but account for only about half of the approximately -5e protein charge at pH 10.5. Thus, more disordered counterions, not seen in these Bijvoet anomalous scattering difference maps, are more numerous at higher protein net charge.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger J., Harris M. R., Reynolds C. D., Evans A. C., Dodson E. J., Dodson G. G., North A. C. Structure of the pig insulin dimer in the cubic crystal. Acta Crystallogr B. 1991 Feb 1;47(Pt 1):127–136. doi: 10.1107/s0108768190009570. [DOI] [PubMed] [Google Scholar]
- Dodson E. J., Dodson G. G., Lewitova A., Sabesan M. Zinc-free cubic pig insulin: crystallization and structure determination. J Mol Biol. 1978 Nov 5;125(3):387–396. doi: 10.1016/0022-2836(78)90409-6. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Dani J. A. An introduction to molecular architecture and permeability of ion channels. Annu Rev Biophys Biophys Chem. 1987;16:205–226. doi: 10.1146/annurev.bb.16.060187.001225. [DOI] [PubMed] [Google Scholar]
- Getzoff E. D., Cabelli D. E., Fisher C. L., Parge H. E., Viezzoli M. S., Banci L., Hallewell R. A. Faster superoxide dismutase mutants designed by enhancing electrostatic guidance. Nature. 1992 Jul 23;358(6384):347–351. doi: 10.1038/358347a0. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. H. Calculation of electrostatic potentials in an enzyme active site. Nature. 1987 Nov 5;330(6143):84–86. doi: 10.1038/330084a0. [DOI] [PubMed] [Google Scholar]
- Gursky O., Badger J., Li Y., Caspar D. L. Conformational changes in cubic insulin crystals in the pH range 7-11. Biophys J. 1992 Nov;63(5):1210–1220. doi: 10.1016/S0006-3495(92)81697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursky O., Li Y., Badger J., Caspar D. L. Monovalent cation binding to cubic insulin crystals. Biophys J. 1992 Mar;61(3):604–611. doi: 10.1016/S0006-3495(92)81865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W. A. Determination of macromolecular structures from anomalous diffraction of synchrotron radiation. Science. 1991 Oct 4;254(5028):51–58. doi: 10.1126/science.1925561. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Hayes F. R., Russell A. J., Thomas P. G., Fersht A. R. Prediction of electrostatic effects of engineering of protein charges. Nature. 1987 Nov 5;330(6143):86–88. doi: 10.1038/330086a0. [DOI] [PubMed] [Google Scholar]
- Yamashita M. M., Wesson L., Eisenman G., Eisenberg D. Where metal ions bind in proteins. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5648–5652. doi: 10.1073/pnas.87.15.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]




