Abstract
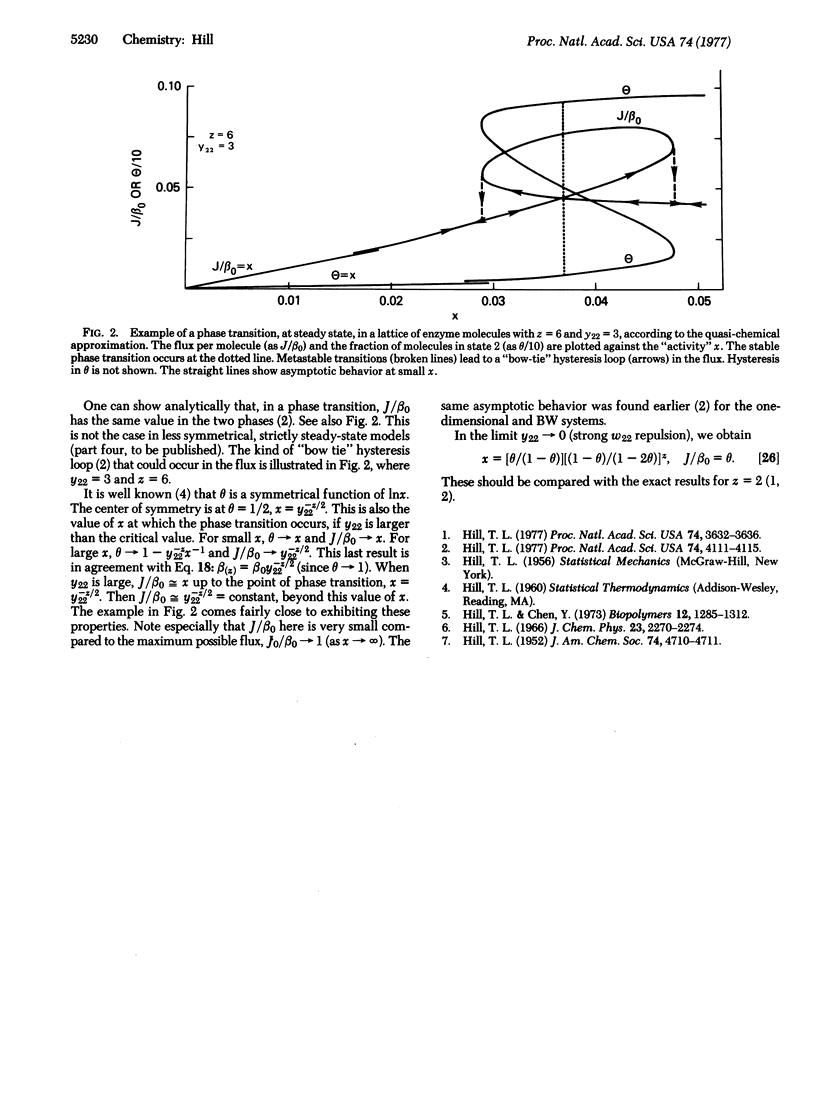
Two examples of enzyme systems with interactions, at steady state, are treated here. In both cases, the enzyme cycle has two states and quasi-equilibrium in spatial distributions obtains at steady state (because f alpha + f beta = 1). The first example is a dilute solution of enzyme molecules in a solvent. The flux (turnover) per molecule is expanded in powers of the enzyme concentration (a "viral" expansion). Aggregation of the enzyme molecules in solution is considered as a special case. In the second example, we treat an arbitrary lattice of enzyme molecules, with nearest-neighbor interactions, using the well-known quasi-chemical approximation. The flux per molecule is obtained. Critical behavior and hysteresis are illustrated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hill T. L. Further study of the effect of enzyme-enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4111–4115. doi: 10.1073/pnas.74.10.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Theoretical study of the effect of enzyme-enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3632–3636. doi: 10.1073/pnas.74.9.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]


