Abstract
Mitochondrial function is altered with age, and variants in mitochondrial DNA (mtDNA) modulate risk for several age-related disease states. However, the association of mtDNA copy number, a readily available marker which reflects mitochondrial depletion, energy reserves and oxidative stress, on aging and mortality in the general population has not been addressed. To assess the association between mtDNA copy number and two primary outcomes—prevalent frailty and all-cause mortality, we utilize data from participants were from two multi-center, multi-ethnic, community-based, prospective studies—the Cardiovascular Health Study (CHS) (1989-2006) and the Atherosclerosis Risk in Communities study (ARIC) (1987-2013). A total of 4,892 participants (43.3% men) from CHS and 11,509 participants (44.9% men) from ARIC self-identifying as white or black were included in the analysis. mtDNA copy number, the trait of interest, was measured using a qPCR-based method in CHS and an array-based method in ARIC from DNA isolated from whole blood in participants from both cohorts.
In race-stratified meta-analyses, we observe a significant inverse association of mtDNA copy number with age, and higher mtDNA copy number in women relative to men. Lower mtDNA copy number was also significantly associated with prevalent frailty in white participants from CHS (OR 0.91, 95% CI, 0.85-0.97). Additionally, mtDNA copy number was a strong independent predictor of all-cause mortality in an age and sex-adjusted, race-stratified analysis of 16,401 participants from both cohorts with a pooled hazard ratio of 1.47 (95% CI, 1.33-1.62) for the lowest quintile of mtDNA copy number relative to the highest quintile.
Keywords: Mitochondria, Mortality, Aging, Frailty
Introduction
Age-related declines in mitochondrial function have long been hypothesized to underlie multiple biological changes that increase vulnerability to multiple disease states, functional and cognitive decline, and ultimately, mortality[1–3]. The mechanisms contributing to age-related mitochondrial functional change encompass multiple domains, including declines in energy (ATP) production/energy reserves[4, 5], increased free radical production[6], altered rates of apoptosis and mitophagy, and altered fusion/fission[7]. Alterations in these crucial intracellular processes lead to dysfunctional cells, altered tissues, and increased risk of disease [8–10]. The link between age-related changes in mitochondrial function and altered phenotypes and disease states is bolstered by the observation that mice with deficiency of the proofreading mechanism of the mitochondrial polymerase display a premature aging phenotype[11, 12] and that mitochondrial dysfunction is a core component of several neurodegenerative disorders in humans[13–15].
The role of mitochondrial DNA (mtDNA) in aging and late life decline has also been studied, with evidence that mtDNA variants modulate risk of several age-associated diseases[10, 13, 16–19]. We have previously implicated a specific mitochondrial genetic variant in frailty[20], a clinical syndrome prevalent in older individuals characterized by broad decline in resilience and increased risk for disability and all-cause mortality[21]. The variant was located in the control region (D-loop), which plays a key role in mitochondrial replication, and suggests the possibility of affecting the levels of mitochondrial DNA. We therefore hypothesized that mtDNA copy number, which is a marker of mitochondrial replication and cellular energy reserves, with low levels of mtDNA copy number likely reflecting mitochondrial depletion, is likely to play an important role in the aging process. While the role of mitochondrial depletion in severe disorders, such as MDS (mtDNA depletion syndrome) is well established, its effect on aging and mortality in the general population is less understood. Several studies have examined the correlation between age and mtDNA copy number with often ambiguous and conflicting results[22–26]. To address this gap in the literature, we examined mtDNA copy number in two large multi-center prospective studies—the Cardiovascular Health Study (CHS) and the Atherosclerosis Risk in Communities (ARIC) study—in a total of 16,401 samples of European and African descent.
Methods
Ethics
The ARIC and CHS studies have been approved by the Institutional Review Boards (IRB) of all participating institutions, including the IRBs of the University of Minnesota, Johns Hopkins University, University of North Carolina, University of Mississippi Medical Center, Wake Forest University, University of Pittsburgh, and University of California Davis, and all participants provided written informed consent.
Participants
CHS is a prospective multi-center study comprising of 5,888 older individuals aged 65 years and above (15.69% African American, 42.37% female), drawn from 4 US communities[31, 32] with initial enrollment in 1989-90, and follow-up recruitment of a minority cohort comprising 687 participants in 1992-93. Participants were followed by annual telephone interviews and clinic visits through 1998-99 and semi-annual telephone interviews subsequently. Mortality information was obtained via contact with next of kin, death certificates, autopsy and coroner's reports. DNA was extracted by salt precipitation following proteinase K digestion of the buffy coat from whole blood. Only participants self-identifying as white or black were included in this analysis. Participants were included only if they consented to use of their DNA for studies of cardiovascular disease outcomes. DNA used for qPCR assay (see below) came from the first visit the participant entered the study.
ARIC is a prospective study of 15,792 individuals, 45-65 years of age, from 4 different US communities[33]. The first visit was carried out in 1987-89, with four subsequent in-person visits and annual telephone interviews after initial visit. DNA was isolated from whole blood using the Gentra Puregene Blood Kit (Qiagen)[34]. Mortality was tracked via telephone follow-ups, hospitalization records, state records, and the National Death Index. Cause of death was determined using cause of death on the death certificate (ICD-9 code). Only samples with a self-reported race of white or black were included in this analysis. DNA used for array-based genotyping was isolated at different visits, with majority of the samples coming from visit2 (1990-92) (detailed breakdown in Table S3).
Frailty and SF-12 metrics
We operationalized frailty in CHS participants as detailed previously by Fried et al.[21]. Briefly, participants were scored on a 0-1 scale (1 being at risk and 0 being not at risk for frailty) for 5 characteristics—slowness, exhaustion, shrinking, weakness, and low activity, and classified as robust (0 characteristics), pre-frail (1 or 2 characteristics), or frail (>=3 characteristics).
Frailty was not measured in ARIC. However the SF12v2 questionnaire, one of the most commonly used measures of general health, was administered at visit 5 (2011-13). The SF12 physical and mental component scores (PCS and MCS respectively) are determined from self-reported answers on physical issues, pain, energy levels, and mental wellness[35]. The scores are on a 0-100 scale with higher scores corresponding to higher physical or mental wellness.
MtDNA Copy Number qPCR Assay
mtDNA copy number in the CHS samples was determined using a multiplexed real time quantitative polymerase chain reaction (qPCR) utilizing ABI TaqMan chemistry (Applied Biosystems). Each well consisted of a VIC labeled, primer-limited assay specific to a mitochondrial target (ND1) (Assay ID Hs02596873_s1), and a FAM labeled assay specific to a region of the nuclear genome selected for being non-repetitive with no known alternative splicing events (RPPH1) (Assay ID Hs03297761_s1). Each sample was run in triplicate on a 384 well plate in a 10μL reaction containing 20ng of DNA. The cycle threshold (Ct) value was determined from the amplification curve for each target by the ABI Viia7 software. A ΔCt value was computed for each well as the difference between the Ct for the RPPH1 target and the Ct for the ND1 target, as a measure of mtDNA copy number relative to nuclear DNA copy number. For samples with standard deviation of ΔCt values of the three replicates > 0.5, an outlier replicate was detected and excluded from analysis. If sample ΔCt standard deviation remained >0.5 post replicate exclusion, the sample was excluded completely from further analyses. Replicates with values of Ct for ND1 >28, Ct for RPPH1 >5 standard deviations from the mean, or ΔCt value >3 standard deviations from the mean, were removed from each plate. Additionally, we observed a linear increase in ΔCt value by order in which the replicate was pipetted onto the plate. This effect was adjusted for using a linear regression, and ΔCt values corrected for pipetting order were used for all subsequent analyses.
MtDNA Copy Number from Microarray Intensities
13,444 ARIC samples were genotyped on the Affymetrix Genome-Wide Human SNP Array 6.0. Genotypes were called using Birdseed (version 2) as implemented in the Affymetrix Power Tools software[36]. In addition to determining genotype calls, the software was used to compute probe intensities for each of the two alleles at every SNP (A and B alleles).
To determine mtDNA copy number, data for 119 mitochondrial SNPs were collected across all samples. For mitochondrial SNPs, the software assumes haploidy and hence all genotype calls are homozygous. At a SNP with genotype call AA, probe intensity corresponding to the A allele is considered the true signal, and probe intensity for B allele is considered background. At each SNP, the overall signal intensity was calculated as the absolute difference of the probe intensities of the two alleles (|A-B|). The median probe intensity difference across all mitochondrial SNPs was taken as a measure of the relative mtDNA copy number for each sample.
Additionally, we generated principal components (PC) on probe intensities for both alleles of a randomly chosen subset of 1,000 autosomal SNPs. PCs generated from these data allow for correction of both technical artifacts (plate and batch effects) and population substructure. The mtDNA copy number was adjusted for the first 20 PCs, age, sex, and collection site using a linear model. Standardized residuals generated from this model were used for all subsequent analyses.
Statistical Analysis
All statistical analyses were performed using R version 3.0.1. For the qPCR based assay, across plate normalization was performed using quantile normalization as implemented in the R package ‘qpcrNorm’[37]. Plate layouts used were non-random with respect to race, requiring all analyses post-normalization and post-removal of plate effects to be stratified by race. Mean ΔCt value was calculated per sample and adjusted for age, sex and collection site using a linear regression model. Standardized residuals were used as the measure of mtDNA copy number. Effect estimates are expressed in terms of standard deviation units (sd) of mtDNA copy number. In CHS, this corresponds to ~0.82 ΔCt units following across plate normalization (sd for whites=0.82 [mean=6.64]; sd for blacks=0.83 [mean=6.63]). In ARIC, the raw probe intensities obtained from the array-based method used to determine copy number, cannot be interpreted without adjusting for PCs accounting for plate and batch artifacts.
All analyses were conducted initially in CHS and validated in ARIC. The frailty characteristics were treated as binary variables and overall frailty was treated as an ordered variable (0, 1, 2). The association with mtDNA copy number was determined using a logistic regression model for the individual frailty characteristics, and a proportional odds model for overall frailty. Prevalence ratios for the individual frailty components were estimated using marginal standardization of the logistic models as implemented by the ‘prLogisticBootMarg’ function in R package ‘prLogistic’ v1.2[38].
To assess the association of mtDNA copy number with mortality, a Cox proportional-hazards model was used, adjusting for age, sex, and collection site, as the baseline model. A secondary multivariate mortality analysis was run including age, sex, collection site, body mass index (BMI), high-density lipoprotein (HDL), total cholesterol, prevalent hypertension (defined by elevated systolic or diastolic blood pressure, or hypertension medication intake), and smoking status as covariates, and excluding participants with prevalent coronary heart disease (CHD), diabetes, or history of myocardial infarction (MI).
For our analyses, baseline was defined as time at which the blood sample that was used to determine mtDNA copy number was collected. Age, follow-up time, and other variables were adjusted accordingly. Samples for which time of DNA extraction was unavailable were excluded. Quintiles were calculated using residuals from age, sex, collection site (for both cohorts), and PCs (for ARIC) adjusted mtDNA copy number. The hazard ratios from both cohorts were pooled using a random effects, inverse-variance weighted meta-analysis, as implemented by the ‘metagen’ function in R package ‘meta’ (version 3.1-2).
Sample Exclusions
In CHS, a total of 996 samples were excluded from the final analysis, primarily due to insufficient amount of DNA to run the assay (442 samples) and concerns about data quality (554 samples). In ARIC, array genotyping data was available on 13,444 of the 15,792 total participants. Further, sample exclusions based on sample quality and relatedness have been previously described[39]. Additionally, samples not self-identifying as either black or white, in either cohort were excluded (39 participants in CHS and 48 in ARIC). Differences between included and excluded participants are available in Table S2.
Results
Sample characteristics
The baseline characteristics of the 4,892 participants (4108 whites, 784 blacks) from the CHS cohort included in the current analysis after sample exclusions and stratified by age-,sex- and collection site- adjusted quintiles, are detailed in Table 1 (Also see Table S1 for unadjusted quintiles). We observed an inverse association between mtDNA copy number and age at time of DNA collection in both racial groups—a reduction of 0.14 (95% CI, 0.08-0.19, P<0.001) and 0.19 (95% CI, 0.06-0.31, P=0.002) sd over 10 years in whites and blacks, respectively. Additionally, we noted a higher mtDNA copy number in women relative to men, (OR=1.21 for women relative to men, 95% CI, 1.14-1.28, P<0.001) in whites, with a consistent, but not statistically significant effect in blacks (OR=1.14 for women relative to men, 95% CI, 0.99-1.31, P=0.08).
Table 1.
Sample characteristics stratified by age-, sex-, and collection site-adjusted quintiles
| CHS-Whites | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Pval | |
| No. of samples | 821 | 822 | 821 | 822 | 822 | |
| Age (in yrs) | 72.4 ± 5.4 | 72.9 ± 5.6 | 72.3 ± 5.3 | 72.6 ± 5.6 | 72.3 ± 5.4 | |
| Number of males--no (%) | 351 (42.7) | 380 (46.2) | 382 (46.5) | 356 (43.3) | 347 (42.2) | |
| Follow up time (in yrs) | 11.6 ±4.66 | 11.7 ± 4.78 | 12.06 ± 4.99 | 12.41 ± 4.93 | 12.67 ± 5.04 | <0.001 |
| No. of deaths--no (%) | 551 (67.1) | 539 (65.6) | 492 (60.0) | 491 (59.7) | 458 (55.7) | <0.001 |
| Mean age at death (in yrs) | 82.85 ± 5.39 | 83.59 ± 5.42 | 83.04 ± 5.50 | 83.88 ± 5.48 | 83.69 ± 5.72 | 0.002 |
| CHS-Blacks | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Pval | |
| No. of samples | 156 | 157 | 156 | 157 | 157 | |
| Age (in yrs) | 73.1 ± 6.1 | 73.0 ± 5.3 | 72.6 ± 5.6 | 72.6 ± 6.0 | 73.1 ± 5.8 | |
| Number of males--no (%) | 68 (43.6) | 59 (37.6) | 48 (30.8) | 59 (37.6) | 68 (43.3) | |
| Follow up time (in yrs) | 9.853 ± 4.46 | 10.59 ± 4.28 | 10.2 ± 4.43 | 10.81 ± 4.26 | 10.41 ± 4.39 | 0.3 |
| No. of deaths--no (%) | 96 (61.5) | 79 (50.3) | 88 (56.4) | 81 (51.6) | 85 (54.1) | 0.47 |
| Mean age at death (in yrs) | 81.97 ± 6.02 | 82.13 ± 5.84 | 81.81 ± 5.79 | 81.86 ± 6.21 | 82.08 ± 6.12 | 0.87 |
| ARIC-Whites | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Pval | |
| No. of samples | 1804 | 1804 | 1805 | 1804 | 1805 | |
| Age (in yrs) | 58.4 ± 6.0 | 58.0 ± 5.9 | 58.1 ± 5.9 | 58.2 ± 6.0 | 58.1 ± 6.1 | |
| Number of males--no (%) | 863 (47.8) | 844 (46.8) | 837 (46.4) | 829 (46) | 869 (48.1) | |
| Follow up time (in yrs) | 15.82 ± 4.50 | 16.69 ± 4.46 | 16.59 ± 4.84 | 17 ± 4.83 | 16.95 ± 5.62 | <0.001 |
| No. of deaths--no (%) | 628 (34.8) | 468 (25.9) | 496 (27.5) | 423 (23.4) | 419 (23.2) | <0.001 |
| Mean age at death (in yrs) | 71.71 ± 6.54 | 72.44 ± 6.54 | 72.95 ± 6.47 | 72.32 ± 6.64 | 72.78 ± 7.05 | 0.01 |
| ARIC-Blacks | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Pval | |
| No. of s amples | 496 | 496 | 497 | 496 | 497 | |
| Age (in yrs) | 57.5 ± 5.9 | 57.2 ± 6.0 | 57.2 ± 5.8 | 57.7 ± 6.0 | 57.2 ± 6.1 | |
| Number of males--no (%) | 182 (36.7) | 181 (36.5) | 191 (38.4) | 190 (38.3) | 177 (35.6) | |
| Follow up time (in yrs) | 14.67 ± 5.16 | 15.46 ± 5.22 | 15.66 ± 5.43 | 15.84 ± 5.63 | 16.12 ± 6.15 | <0.001 |
| No. of deaths--no (%) | 213 (42.9) | 193 (38.9) | 188 (37.8) | 174 (35.1) | 158 (31.8) | <0.001 |
| Mean age at death (in yrs) | 68.87 ± 6.48 | 69.64 ± 6.82 | 70.37 ± 6.30 | 70.54 ± 6.66 | 70.35 ± 7.45 | 0.1 |
Data are presented as Mean±SD. Quintiles were calculated from age, sex, collection site adjusted mtDNA copy number (details in Methods). ‘Pval for trend’ is the pvalue for effect of trait on age, sex, collection site standardized mtDNA copy number as a continuous variable.
We used 11,509 samples (9,025 whites, 2,484 blacks) from ARIC to validate our initial findings from CHS (Table 1 and S1). As in CHS, we observed an inverse association of mtDNA copy number with baseline age with a reduction of 0.11 sd (95% CI, 0.07-0.14, P<0.001) in whites and 0.11 sd (95% CI, 0.04-0.17, P=0.001) in blacks, over a 10 year period, and a significantly higher mtDNA copy number in women relative to men (whites OR=1.52 for women relative to men, 95% CI 1.46-1.59, P<0.001; blacks OR=1.42 for women relative to men, 95% CI 1.31-1.54, P<0.001).
Frailty
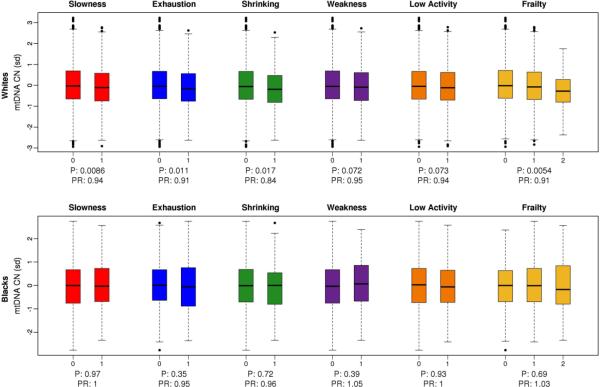
In a race-stratified analysis of samples from CHS, we observed a statistically significant association between lower mtDNA copy number and frailty, adjusted for age and sex, in whites (OR 0.91, 95% CI, 0.85-0.97, P=0.005). Furthermore, this association was not driven by any single component of the frailty phenotype, with three out of five frailty characteristics showing statistically significant association with lower mtDNA copy number in whites (Figure 1), and a similar trend of association for the remaining characteristics. While we observed this association in whites, we see no association of mtDNA copy number on any of the frailty characteristics in CHS blacks.
Figure 1. Frailty components in CHS.
Association between age, sex and collection site adjusted mitochondrial copy number and frailty components in white samples (top panel) and black samples (bottom panel) from CHS. MtDNA copy number is expressed in terms of standard deviation units. Participants were scored as being at risk (1) or not at risk (0) for each characteristic of frailty. Overall frailty was scored in terms of number of characteristics that each participant was at risk for—robust 0 characteristics, pre-frail 1-2 characteristics and frail >2 characteristics.
Effect size estimates are reported as prevalence ratios (details in Methods).
While frailty characteristics were not measured in ARIC participants, the latest visit (2011-2013) included the SF12v2 mental component score (MCS) and physical component score PCS. Of the ARIC participants included in our study 4,961 (4,046 whites and 915 blacks) participants were interviewed at visit 5 with a mean MCS of 46.35 in whites and 43.94 in blacks. In white participants from ARIC we observe a significant association between higher PCS, adjusted for age at visit 5, sex, and collection site, and mtDNA copy number, with an increase of 0.51 PCS units per sd unit increase in mtDNA copy number (95% CI, 0.17-0.84, P=0.003). The same model in blacks showed a similar association with an increase of 0.76 PCS units per sd unit increase in mtDNA copy number (95% CI, 0.02-1.50, P=0.04). Secondary analyses adjusting for additional covariates--prevalent diabetes, CHD or hypertension at time of DNA collection— showed the same trend of association between high PCS score and mtDNA copy number with effect estimates of 0.42 PCS units in whites (95% CI, 0.09-0.75, P=0.01), and 0.83 PCS units in blacks (95% CI, 0.11-1.56, P=0.02).
Mortality
A total of 2,961 deaths (60.4% samples) were observed in the CHS participants during 26,770 person-years of follow-up. In an age, sex, and collection site adjusted, race-stratified analysis, we observed a statistically significant association between lower mtDNA copy number and mortality, with overall hazard ratio of 1.39 (95% CI, 1.23-1.58, P<0.001) for the lowest quintile of copy number relative to the highest quintile in whites (Figure 2; Model 1 from Table 2). A more stringent multivariate model adjusted for age, sex, collection center, BMI, HDL, total cholesterol, prevalent hypertension, and smoking status, and excluding all samples with prevalent CHD, diabetes or previous history of MI, yielded a hazard ratio of 1.33 (95% CI, 1.13-1.56, P<0.001) (Model 2 from Table 2). When stratified by sex, we observed no significant difference in the inverse association between mtDNA copy number and mortality in men and women (P for interaction=0.80). As in frailty, we fail to observe a statistically significant association between mtDNA copy number and risk for mortality in CHS blacks. (Table 2).
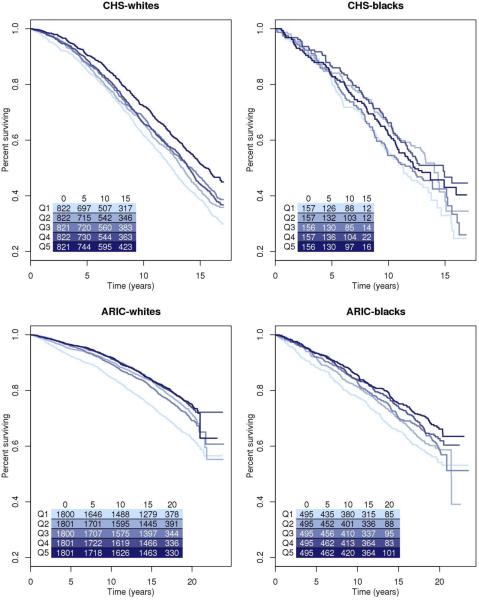
Figure 2. Kaplan-Meier survival curves by quintiles of mtDNA copy number.
Kaplan-Meier estimates for all-cause mortality by quintile of mtDNA copy number were calculated for both race groups in CHS and ARIC. Table indicates the total number of people in the model at each time point.
Table 2.
Lower mtDNA copy number is associated with increased risk for all-cause mortality
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Overall | Pval for trend | ||
|---|---|---|---|---|---|---|---|---|
| CHS | ||||||||
| Whites | Model 1 | 821(551) | 822(539) | 821(492) | 822(491) | 822(458) | 4109(2532) | |
| 1.39 (1.23-1.58) | 1.29 (1.14-1.46) | 1.17 (1.03-1.33) | 1.09 (0.96-1.23) | 1 | 0.89 (0.85-0.92) | <0.001 | ||
| Model 2 | 509(302) | 590(359) | 586(314) | 624(339) | 610(301) | 2902(1607) | ||
| 1.33 (1.13-1.56) | 1.38 (1.18-1.61) | 1.17 (1-1.37) | 1.09 (0.93-1.27) | 1 | 0.89 (0.85-0.94) | <0.001 | ||
| Blacks | Model 1 | 156(96) | 157(79) | 156(88) | 157(81) | 157(85) | 784(429) | |
| 1.25 (0.93-1.68) | 0.92 (0.68-1.26) | 1.21 (0.9-1.63) | 0.94 (0.7-1.28) | 1 | 0.96 (0.87-1.05) | 0.35 | ||
| Model 2 | 84(41) | 100(48) | 99(47) | 102(49) | 89(44) | 469(227) | ||
| 0.98 (0.63-1.51) | 0.88 (0.58-1.33) | 1.16 (0.76-1.79) | 0.88 (0.58-1.34) | 1 | 1.03 (0.9-1.19) | 0.65 | ||
| ARIC | ||||||||
| Whites | Model 1 | 1800(629) | 1801(467) | 1800(496) | 1801(423) | 1801(416) | 9004(2431) | |
| 1.63 (1.44-1.84) | 1.18 (1.03-1.34) | 1.28 (1.13-1.46) | 1.02 (0.89-1.17) | 1 | 0.84 (0.81-0.88) | <0.001 | ||
| Model 2 | 1356(365) | 1441(294) | 1504(342) | 1534(309) | 1526(296) | 7352(1603) | ||
| 1.38 (1.19-1.61) | 1.07 (0.91-1.25) | 1.23 (1.05-1.44) | 1.03 (0.87-1.2) | 1 | 0.89 (0.85-0.94) | <0.001 | ||
| Blacks | Model 1 | 495(213) | 495(195) | 495(189) | 495(173) | 495(156) | 2476(926) | |
| 1.47 (1.19-1.81) | 1.36 (1.1-1.67) | 1.28 (1.03-1.58) | 1.08 (0.87-1.34) | 1 | 0.86 (0.8-0.91) | <0.001 | ||
| Model 2 | 313(101) | 334(102) | 348(102) | 339(93) | 360(95) | 1678(485) | ||
| 1.25 (0.95-1.66) | 1.15 (0.87-1.52) | 1.09 (0.82-1.44) | 0.98 (0.74-1.31) | 1 | 0.91 (0.83-1) | 0.04 |
Numbers of events are presented as total number of subjects, followed by number of events in parentheses, for each quintile. Effect estimates are reported as hazards ratio for each quintile, followed by 95% confidence interval for the estimate of hazards ratio in parentheses.
Model 1 was the baseline model adjusted for age, sex and collection site.
Model 2 was more stringent model that included age, sex, collection site, BMI, HDL, total cholesterol, prevalent hypertension, and smoking status as covariates, and excluded samples with prevalent CHD, diabetes or previous history of MI.
We observed a similar inverse association of mtDNA copy number with mortality in ARIC (3,362 deaths, 188,377 person-years of follow-up), as seen in CHS, with a hazard ratio of 1.63 (95% CI, 1.44-1.84, P<0.001) for the lowest quintile of mtDNA copy number relative to the highest quintile, in whites in an age, sex, and center adjusted analysis (Table 2). We also observed a significantly higher risk of mortality in blacks with hazard ratio of 1.47 (95% CI, 1.19-1.81, P<0.001) for the lowest quintile of copy number relative to the highest quintile. In the subsequent multivariate analyses, low copy number remained strongly associated with increased risk for mortality in whites (hazard ratio=1.38, 95% CI, 1.19-1.61, P<0.001). We observe a similar, albeit not statistically significant, inverse association in blacks (hazard ratio=1.25, 95% CI 0.95-1.66, P=0.056).
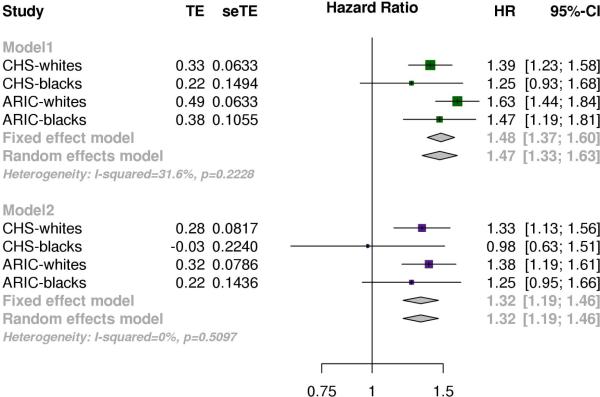
An inverse-variance weighted meta-analysis of race-stratified results from both cohorts for the age, sex and collection site adjusted effect of the lowest quintile relative to the highest quintile on mortality, yielded an overall hazard ratio of 1.47 (95% CI, 1.33-1.62, P<0.001), with no significant heterogeneity between the subgroups (P=0.26) (Model1 from Figure 3). A subsequent meta-analysis of the results from a more stringent multivariate model, gave a meta-analyzed hazard ratio of 1.32 (95% CI, 1.19-1.46, P<0.001) (Model2 from Figure 3). Additionally, we evaluated the associations of mtDNA copy number with cause-specific mortality, and observed a consistent association of low mtDNA copy number in death due to diseases of the circulatory system, respiratory system or neoplasms (Figure S1). Heterogeneity between effect estimates from cause of death subgroups was determined to be non-significant (P=0.21) using a random-effects model.
Figure 3. Meta-analysis of effects mtDNA copy number on mortality.
Effects of highest copy quintile of copy number relative to lowest quintile from race stratified analyses in each cohort were meta-analyzed using an inverse-variance weighted approach. Model 1 was the baseline model adjusted for age, sex and collection site. Model 2 was more stringent model that included age, sex, collection site, BMI, HDL, total cholesterol, hypertension, and smoking status as covariates, and excluded samples with prevalent CHD, diabetes or previous history of MI.
Discussion
We demonstrate that low mtDNA copy number is strongly associated with age, sex, and frailty, and an independent predictor of mortality in 16,401 samples from two large multi-ethnic cohorts, even after adjustment for traditional mortality risk factors and exclusion of prevalent disease states associated with high risk of mortality. The secondary analyses excluding participants with prevalent disease states at baseline allows us to eliminate the concern that these conditions lead to altered mtDNA copy number and hence drive the association with mortality (i.e. reverse causation). Furthermore, the fact that we see consistent effect estimates from both cohorts using independent methods of ascertaining the mtDNA copy number demonstrates the robustness of our findings. While the qPCR-based metric is well established in the literature, we believe that the measure derived from >100 mitochondrial markers on the genotyping array is likely to be a more accurate measure of copy number. Also given that majority of modern large-scale genotyping arrays include mitochondrial markers, this measure can be easily generated from other large cohorts with genotyping data.
Our results demonstrating a strong inverse association between age and mtDNA copy number are in line with previous studies that have shown decreased mtDNA copy number with age in different tissue types[24, 26]. Recently, Mengel-From and colleagues report a marginal association between high mtDNA copy number, and better health and survival in 1,067 Danish samples[27]. In a much larger sample size from two independent cohorts, we replicate their findings on the protective effect of high mtDNA copy number with respect to survival and increased energy reserves. Additionally, our data indicating a higher mtDNA copy number in women relative to men across all the subgroups might suggest that a mito-protective effect may account for the disparity in life expectancy between men and women.
Frailty has been previously shown to be predictive of both incident disability and mortality[21]. While there has been considerable debate about what drives the onset of frailty, our findings add to the evidence of a role for mitochondria in this process. Given that energy utilization forms a core feature of the phenotype, and low copy number is associated with overall frailty and several of its components, it is not surprising that mtDNA levels might form part of the biological component of the phenotype. While we were unable to assess the frailty phenotype in both cohorts, in ARIC we show a striking association between the physical component score of the SF-12 metric, and mtDNA copy number measured 15-20 years prior. Interestingly, several groups have published a link between mtDNA and cognitive function in the elderly[27–29], however we do not observe any association between mtDNA copy number and the cognitive component of the SF-12 (P for both race groups > 0.4) in ARIC participants.
Several limitations to the study should be noted. First, the mtDNA copy number used in this study is derived from a single time-point, and thus does not take into account the dynamic nature of mtDNA copy number during the life of an individual. Second, while mtDNA copy number has been associated with ATP production rate[26], it is an indirect measure, and further, does not account for acquired mutational burden-a mechanism that forms a critical part of the mitochondrial theory of aging. Third, while we are able to comment on differences between men and women with respect to mtDNA copy number, we cannot do so for race due to technical limitations of study design (see Methods Statistical analysis). This is an important issue, given the significant disparities in health outcomes in the U.S. between whites and blacks[30]. Finally, we were measuring mtDNA copy number in DNA derived from whole blood, which is not necessarily the relevant tissue with respect to many aging-related diseases.
In conclusion, while mitochondria have a central role in energy production, and thus the biological hypothesis for involvement in aging related decline (with energy utilization serving as a core feature of the phenotype) is readily apparent, this has been a neglected area of research with respect to general health outcomes. With recent changes in technology, including the ability to readily assess mtDNA copy number from existing genotyping array data, this is likely to become a rapidly emerging area of research. We highlight that a single, easily implemented, measure of mtDNA copy number, isolated from whole blood decades before the event of interest (death), is predictive of physical function later in life and all-cause mortality.
Supplementary Material
Key Messages.
Mitochondrial DNA (mtDNA) copy number is associated with age and sex.
Lower mtDNA copy number is also associated with prevalent frailty.
mtDNA copy number is a significant predictor of all-cause mortality in a multi-ethnic population.
Acknowledgements
This work was supported by the Johns Hopkins University Claude D. Pepper Older Americans Independence Center, National Institute on Aging, P30-AG021334.
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. The authors thank the staff and participants of the ARIC study for their important contributions. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research.
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHSNHLBI.org.
The funding agencies had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest
FNA, AM and DEA declare that a United States Patent Application relating to the use of mtDNA as a biomarker has been filed, and is pending, on behalf of The Johns Hopkins University. All other authors declare no financial, personal, or professional conflicts of interest.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Bibliography
- 1.ERNSTER L, LOW H, NORDENBRAND K, ERNSTER B. A component promoting oxidative phosphorylation, released from mitochondria during aging. Exp Cell Res. 1955;9:348–349. doi: 10.1016/0014-4827(55)90108-7. [DOI] [PubMed] [Google Scholar]
- 2.Honjo I, Ozawa K, Kitamura O, et al. Rapid change of phospholipid in pancreas mitochondria during aging. J Biochem (Tokyo) 1968;64:311–320. doi: 10.1093/oxfordjournals.jbchem.a128897. [DOI] [PubMed] [Google Scholar]
- 3.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 4.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol 526 Pt. 2000;1:203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial Dysfunction in the Elderly: Possible Role in Insulin Resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui H, Kong Y, Zhang H. Oxidative Stress, Mitochondrial Dysfunction, and Aging. J Signal Transduct. 2011 doi: 10.1155/2012/646354. doi: 10.1155/2012/646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan DC. Mitochondria: Dynamic Organelles in Disease, Aging, and Development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Kabekkodu SP, Bhat S, Mascarenhas R, et al. Mitochondrial DNA variation analysis in cervical cancer. Mitochondrion. 2013 doi: 10.1016/j.mito.2013.07.001. doi: 10.1016/j.mito.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Liou C-W, Chen J-B, Tiao M-M, et al. Mitochondrial DNA coding and control region variants as genetic risk factors for type 2 diabetes. Diabetes. 2012;61:2642–2651. doi: 10.2337/db11-1369. doi: 10.2337/db11-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenney MC, Hertzog D, Chak G, et al. Mitochondrial DNA haplogroups confer differences in risk for age-related macular degeneration: a case control study. BMC Med Genet. 2013;14:4. doi: 10.1186/1471-2350-14-4. doi: 10.1186/1471-2350-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kujoth GC, Hiona A, Pugh TD, et al. Mitochondrial DNA Mutations, Oxidative Stress, and Apoptosis in Mammalian Aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 12.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 13.Lezi E, Swerdlow RH. Mitochondria in neurodegeneration. Adv Exp Med Biol. 2012;942:269–286. doi: 10.1007/978-94-007-2869-1_12. doi: 10.1007/978-94-007-2869-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DuBoff B, Feany M, Götz J. Why size matters – balancing mitochondrial dynamics in Alzheimer’s disease. Trends Neurosci. 2013;36:325–335. doi: 10.1016/j.tins.2013.03.002. doi: 10.1016/j.tins.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Gaweda-Walerych K, Zekanowski C. Integrated pathways of parkin control over mitochondrial maintenance - relevance to Parkinson's disease pathogenesis. Acta Neurobiol Exp (Warsz) 2013;73:199–224. doi: 10.55782/ane-2013-1931. [DOI] [PubMed] [Google Scholar]
- 16.Van der Walt JM, Nicodemus KK, Martin ER, et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am J Hum Genet. 2003;72:804–811. doi: 10.1086/373937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Walt JM, Dementieva YA, Martin ER, et al. Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neurosci Lett. 2004;365:28–32. doi: 10.1016/j.neulet.2004.04.051. doi: 10.1016/j.neulet.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 18.Hudson G, Nalls M, Evans JR, et al. Two-stage association study and meta-analysis of mitochondrial DNA variants in Parkinson disease. Neurology. 2013;80:2042–2048. doi: 10.1212/WNL.0b013e318294b434. doi: 10.1212/WNL.0b013e318294b434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thyagarajan B, Wang R, Nelson H, et al. Mitochondrial DNA copy number is associated with breast cancer risk. PloS One. 2013;8:e65968. doi: 10.1371/journal.pone.0065968. doi: 10.1371/journal.pone.0065968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore AZ, Biggs ML, Matteini A, et al. Polymorphisms in the Mitochondrial DNA Control Region and Frailty in Older Adults. PLoS ONE. 2010;5:e11069. doi: 10.1371/journal.pone.0011069. doi: 10.1371/journal.pone.0011069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 22.Clay Montier LL, Deng JJ, Bai Y. Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics Yi Chuan Xue Bao. 2009;36:125–131. doi: 10.1016/S1673-8527(08)60099-5. doi: 10.1016/S1673-8527(08)60099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu JC, Tsai JC, Kuo JC, et al. Oxidative Stress-related Alteration of the Copy Number of Mitochondrial DNA in Human Leukocytes. Free Radic Res. 2003;37:1307–1317. doi: 10.1080/10715760310001621342. [DOI] [PubMed] [Google Scholar]
- 24.Welle S, Bhatt K, Shah B, et al. Reduced amount of mitochondrial DNA in aged human muscle. J Appl Physiol. 2003;94:1479–1484. doi: 10.1152/japplphysiol.01061.2002. doi: 10.1152/japplphysiol.01061.2002. [DOI] [PubMed] [Google Scholar]
- 25.Hebert SL, Lanza IR, Nair KS. Mitochondrial DNA alterations and reduced mitochondrial function in aging. Mech Ageing Dev. 2010;131:451–462. doi: 10.1016/j.mad.2010.03.007. doi: 10.1016/j.mad.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mengel-From J, Thinggaard M, Dalgård C, et al. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum Genet. 2014;133:1149–1159. doi: 10.1007/s00439-014-1458-9. doi: 10.1007/s00439-014-1458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J-W, Park KD, Im J-A, et al. Mitochondrial DNA copy number in peripheral blood is associated with cognitive function in apparently healthy elderly women. Clin Chim Acta. 2010;411:592–596. doi: 10.1016/j.cca.2010.01.024. doi: 10.1016/j.cca.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Kim M-Y, Lee J-W, Kang H-C, et al. Leukocyte mitochondrial DNA (mtDNA) content is associated with depression in old women. Arch Gerontol Geriatr. 2011;53:e218–221. doi: 10.1016/j.archger.2010.11.019. doi: 10.1016/j.archger.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Kington RS, Smith JP. Socioeconomic status and racial and ethnic differences in functional status associated with chronic diseases. Am J Public Health. 1997;87:805–810. doi: 10.2105/ajph.87.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fried LP, Borhani NO, Enright P, et al. The cardiovascular health study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. doi: 10.1016/1047-2797(91)90005-W. [DOI] [PubMed] [Google Scholar]
- 32.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 33.Investigators TA. The Atherosclerosis Risk in Communit (aric) Stui)y: Design and Objectwes. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 34.ARIC Carotid MRI Study Investigators Atheroschlerosis Risk in Communities Carotid MRI Study. Manual 7 DNA Determinations [Google Scholar]
- 35.Fleishman JA, Selim AJ, Kazis LE. Deriving SF-12v2 physical and mental health summary scores: a comparison of different scoring algorithms. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2010;19:231–241. doi: 10.1007/s11136-009-9582-z. doi: 10.1007/s11136-009-9582-z. [DOI] [PubMed] [Google Scholar]
- 36.Korn JM, Kuruvilla FG, McCarroll SA, et al. Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat Genet. 2008;40:1253–1260. doi: 10.1038/ng.237. doi: 10.1038/ng.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mar JC, Kimura Y, Schroder K, et al. Data-driven normalization strategies for high- throughput quantitative RT-PCR. BMC Bioinformatics. 2009;10:110. doi: 10.1186/1471-2105-10-110. doi: 10.1186/1471-2105-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amorim RO and LD. prLogistic: Estimation of Prevalence Ratios using Logistic Models. 2013 [Google Scholar]
- 39.Arking DE, Reinier K, Post W, et al. Genome-Wide Association Study Identifies GPC5 as a Novel Genetic Locus Protective against Sudden Cardiac Arrest. PLoS ONE. 2010;5:e9879. doi: 10.1371/journal.pone.0009879. doi: 10.1371/journal.pone.0009879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.