Abstract
We have cocrystallized the drug ethidium bromide with the dinucleoside monophosphate 5-iodouridylyl(3'-5')adenosine and have solved the three-dimensional structure to atomic resolution by x-ray crystallography. This has allowed the direct visualization of intercalative binding by this drug to a fragment of a nucleic acid double helix.
Full text
PDF
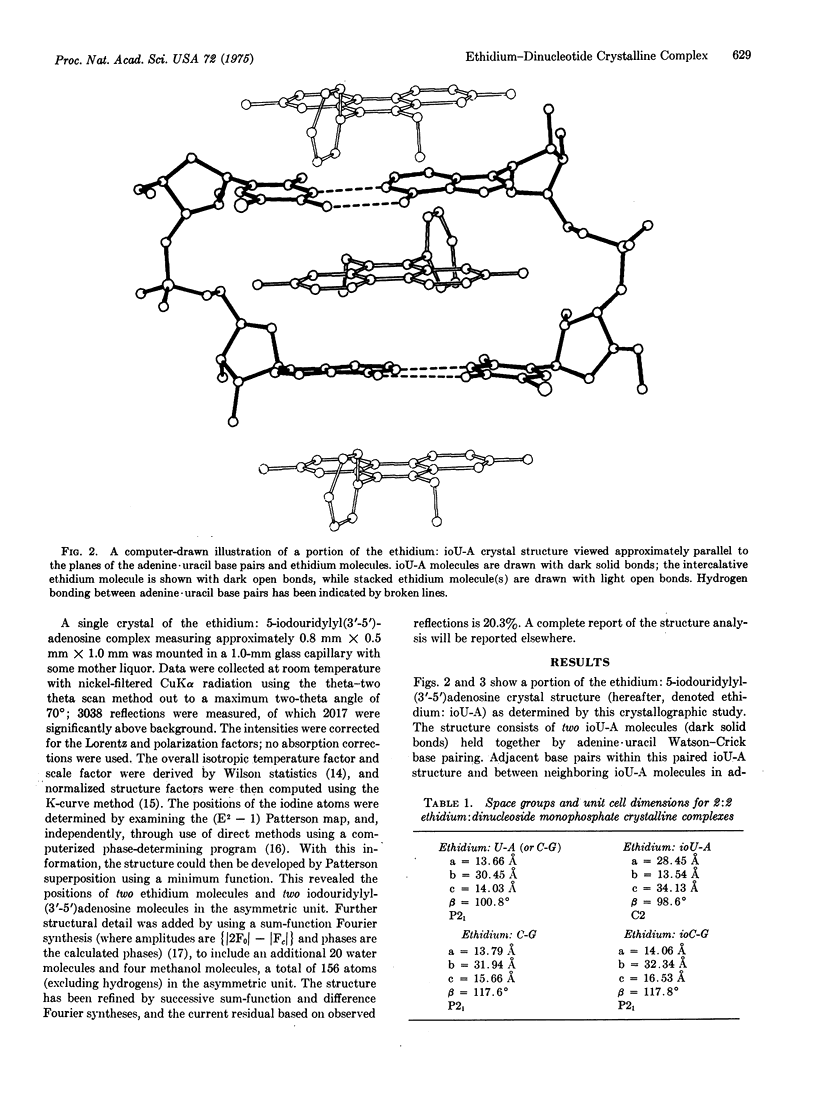
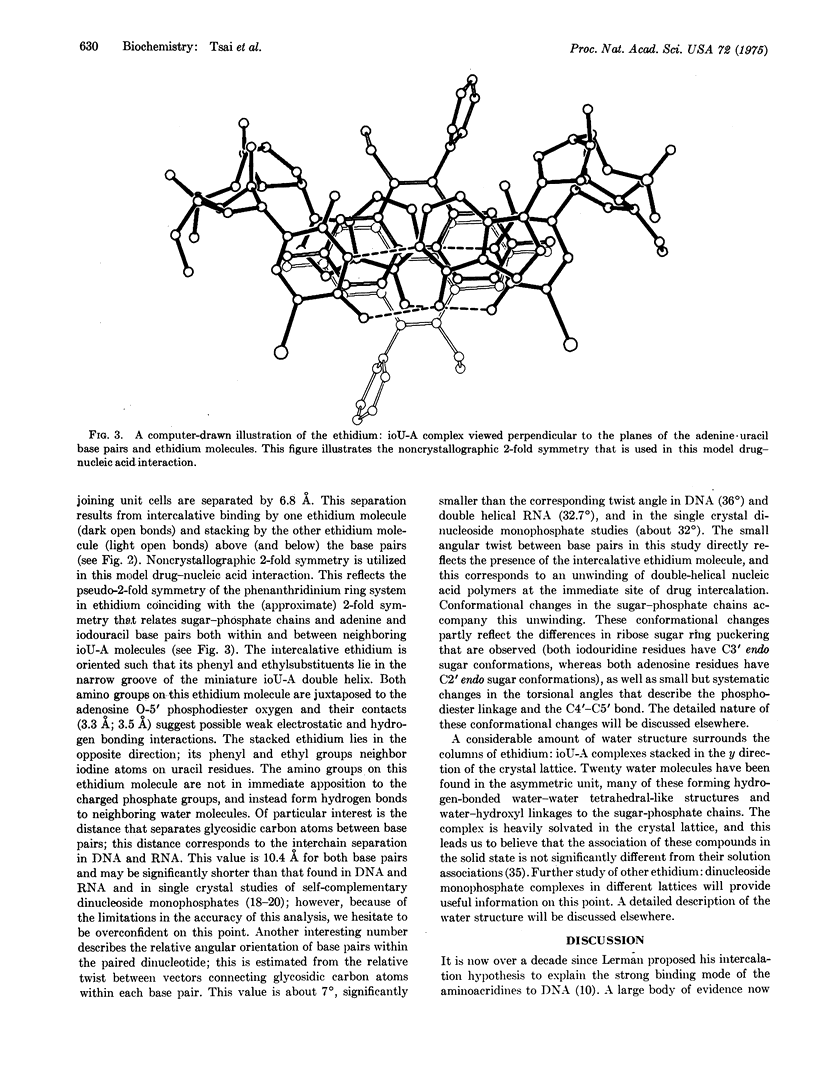


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. Interaction of closed circular DNA with intercalative dyes. II. The free energy of superhelix formation in SV40 DNA. J Mol Biol. 1970 Feb 14;47(3):419–435. doi: 10.1016/0022-2836(70)90312-8. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Waring M. J. Supercoiling of polyoma virus DNA measured by its interaction with ethidium bromide. J Mol Biol. 1967 Apr 14;25(1):23–30. doi: 10.1016/0022-2836(67)90276-8. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Livingston D. C., Ward D. C. The synthesis and enzymatic polymerization of nucleotides containing mercury: potential tools for nucleic acid sequencing and structural analysis. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2238–2242. doi: 10.1073/pnas.70.8.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. O., Seeman N. C., Rosenberg J. M., Rich A. A crystalline fragment of the double helix: the structure of the dinucleoside phosphate guanylyl-3',5'-cytidine. Proc Natl Acad Sci U S A. 1973 Mar;70(3):849–853. doi: 10.1073/pnas.70.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Electron microscopic study of the ethidium bromide-DNA complex. J Mol Biol. 1971 Sep 14;60(2):401–403. doi: 10.1016/0022-2836(71)90303-2. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M. Multiple repressor binding at the operators in bacteriophage lambda. Proc Natl Acad Sci U S A. 1973 May;70(5):1531–1535. doi: 10.1073/pnas.70.5.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Studies of the binding of actinomycin and related compounds to DNA. J Mol Biol. 1968 Jul 28;35(2):251–290. doi: 10.1016/s0022-2836(68)80024-5. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. MECHANISMS OF ACTION OF PHENANTHRIDINE AND AMINOQUINALDINE TRYPANOCIDES. Adv Chemother. 1964;25:35–83. doi: 10.1016/b978-1-4831-9929-0.50008-5. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Allison J. L., Hahn F. E. Evidence for intercalation of chloroquine into DNA. Biochim Biophys Acta. 1966 Dec 21;129(3):622–624. doi: 10.1016/0005-2787(66)90078-5. [DOI] [PubMed] [Google Scholar]
- Pigram W. J., Fuller W., Hamilton L. D. Stereochemistry of intercalation: interaction of daunomycin with DNA. Nat New Biol. 1972 Jan 5;235(53):17–19. doi: 10.1038/newbio235017a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. M., Seeman N. C., Kim J. J., Suddath F. L., Nicholas H. B., Rich A. Double helix at atomic resolution. Nature. 1973 May 18;243(5403):150–154. doi: 10.1038/243150a0. [DOI] [PubMed] [Google Scholar]
- Saucier J. M., Wang J. C. Angular alteration of the DNA helix by E. coli RNA polymerase. Nat New Biol. 1972 Oct 11;239(93):167–170. doi: 10.1038/newbio239167a0. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Jain S. C. Stereochemistry of actinomycin binding to DNA. II. Detailed molecular model of actinomycin-DNA complex and its implications. J Mol Biol. 1972 Jul 14;68(1):21–34. doi: 10.1016/0022-2836(72)90259-8. [DOI] [PubMed] [Google Scholar]
- WARING M. J. COMPLEX FORMATION WITH DNA AND INHIBITION OF ESCHERICHIA COLI RNA POLYMERASE BY ETHIDIUM BROMIDE. Biochim Biophys Acta. 1964 Jun 22;87:358–361. doi: 10.1016/0926-6550(64)90238-5. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Complex formation between ethidium bromide and nucleic acids. J Mol Biol. 1965 Aug;13(1):269–282. doi: 10.1016/s0022-2836(65)80096-1. [DOI] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E. Studies on the binding of actinomycin D to DNA and DNA model polymers. J Mol Biol. 1970 Apr 28;49(2):319–342. doi: 10.1016/0022-2836(70)90248-2. [DOI] [PubMed] [Google Scholar]
- Zylber E., Vesco C., Penman S. Selective inhibition of the synthesis of mitochondria-associated RNA by ethidium bromide. J Mol Biol. 1969 Aug 28;44(1):195–204. doi: 10.1016/0022-2836(69)90414-8. [DOI] [PubMed] [Google Scholar]


