Abstract
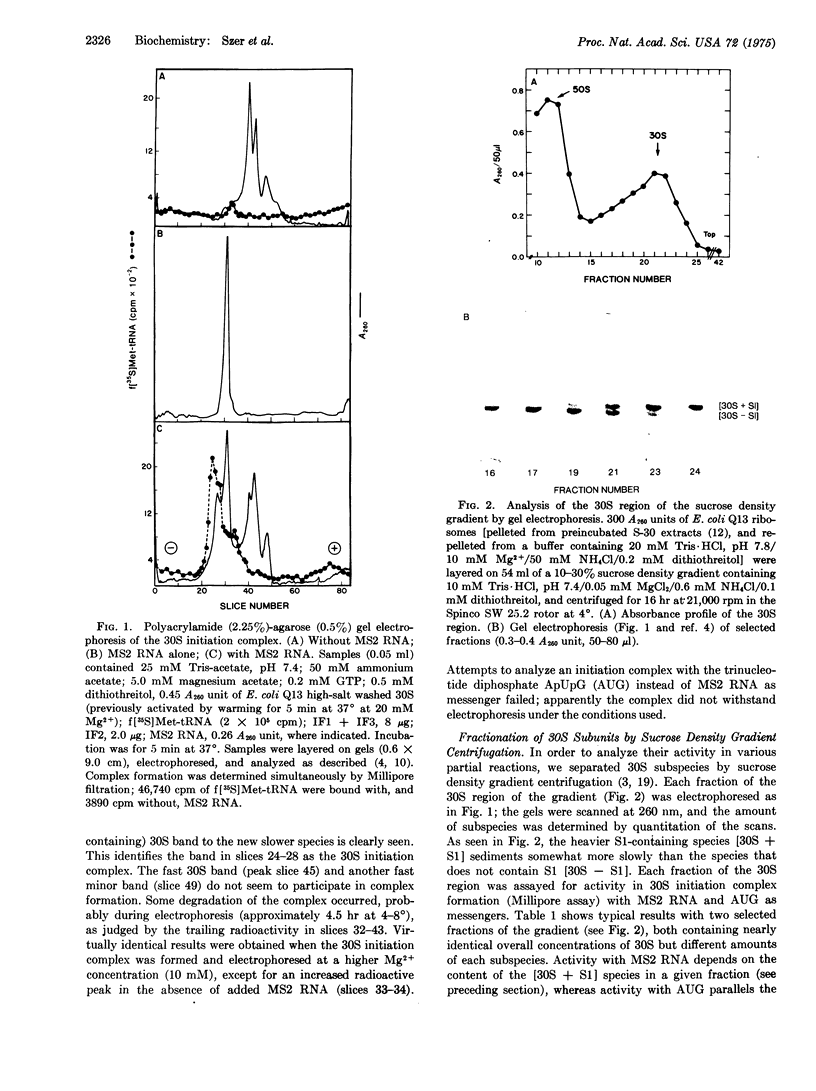
Among several subspecies of 30S subunits of Escherichia coli observed by polyacrylamide-agarose gel electrophoresis, only the slow-moving, protein S1-containing subspecies participates in the formation of the 30S initiation complex with coliphage MS2 RNA as mRNA; the other subspecies retain activity with AUG as mRNA; they are also active in the poly(U)-directed binding of Phe-tRNA. Protein S1 from Caulobacter crescentus substitutes for E. coli S1 despite the fact that C. crescentus ribosomes do not bind MS2 RNA. Under appropriate conditions, the entire population of E. coli 30S subunits can be isolated as the S1-containing subspecies. Protein S1 is lost by salt treatment of ribosomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dahlberg A. E., Dingman C. W., Peacock A. C. Electrophoretic characterization of bacterial polyribosomes in agarose-acrylamide composite gels. J Mol Biol. 1969 Apr 14;41(1):139–147. doi: 10.1016/0022-2836(69)90131-4. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E. Two forms of the 30 S ribosomal subunit of Escherichia coli. J Biol Chem. 1974 Dec 10;249(23):7673–7678. [PubMed] [Google Scholar]
- Groner Y., Pollack Y., Berissi H., Revel M. Cistron specific translation control protein in Escherichia coli. Nat New Biol. 1972 Sep 6;239(88):16–19. doi: 10.1038/newbio239016a0. [DOI] [PubMed] [Google Scholar]
- Held W. A., Gette W. R., Nomura M. Role of 16S ribosomal ribonucleic acid and the 30S ribosomal protein S12 in the initiation of natural messenger ribonucleic acid translation. Biochemistry. 1974 May 7;13(10):2115–2122. doi: 10.1021/bi00707a019. [DOI] [PubMed] [Google Scholar]
- Hermoso J. M., Szer W. Replacement of ribosomal protein S1 by interference factor ialpha in ribosomal binding of phage Ms2 RNA. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4708–4712. doi: 10.1073/pnas.71.12.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Pollack Y., Petre J. Physical and functional homology between ribosomal protein S1 and interference factor i. Eur J Biochem. 1974 Jun 1;45(1):109–117. doi: 10.1111/j.1432-1033.1974.tb03535.x. [DOI] [PubMed] [Google Scholar]
- Jay G., Kaempfer R. Host interference with viral gene expression: mode of action of bacterial factor i. J Mol Biol. 1974 Jan 15;82(2):193–212. doi: 10.1016/0022-2836(74)90341-6. [DOI] [PubMed] [Google Scholar]
- Kurland C. G., Voynow P., Hardy S. J., Randall L., Lutter L. Physical and functional heterogeneity of E. coli ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:17–24. doi: 10.1101/sqb.1969.034.01.006. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S., Ochoa S. Purification and properties of two messenger-discriminating species of E. coli initiation factor 3. Arch Biochem Biophys. 1973 May;156(1):84–96. doi: 10.1016/0003-9861(73)90344-5. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S., Ochoa S. Specific inhibitors of MS2 and late T4 RNA translation in E. coli. Biochem Biophys Res Commun. 1972 Oct 17;49(2):371–376. doi: 10.1016/0006-291x(72)90420-2. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S., Sillero M. A., Ochoa S. Isolation and properties of crystalline initiation factor F1 from Escherichia coli ribosomes. Eur J Biochem. 1971 Feb;18(4):536–543. doi: 10.1111/j.1432-1033.1971.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Leffler S., Szer W. Messenger selection by bacterial ribosomes. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2364–2368. doi: 10.1073/pnas.70.8.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlechild J., Spencer M. Stability and homogeneity of preparations of ribosomal particles from Escherichia coli. Biochemistry. 1973 Jul 31;12(16):3102–3108. doi: 10.1021/bi00740a025. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Specificity in bacterial protein synthesis: role of initiation factors and ribosomal subunits. Nature. 1970 May 23;226(5247):705–707. doi: 10.1038/226705a0. [DOI] [PubMed] [Google Scholar]
- Mazumder R. Initiation factor 2-dependent ribosomal binding of N-formylmethionyl-transfer RNA without added guanosine triphosphate. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2770–2773. doi: 10.1073/pnas.69.10.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W., Brenowitz J. Translation of MS2 RNA by ribosomes from different bacterial species. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1154–1160. doi: 10.1016/0006-291x(70)90360-8. [DOI] [PubMed] [Google Scholar]
- Szer W., Leffler S. Interaction of Escherichia coli 30S ribosomal subunits with MS2 phage RNA in the absence of initiation factors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3611–3615. doi: 10.1073/pnas.71.9.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M., Aviram M., Kanarek A., Weiss A. Polyuridylic acid binding and translating by Escherichia coli ribosomes: stimulation by protein I, inhibition by aurintricarboxylic acid. Biochim Biophys Acta. 1972 Oct 27;281(3):381–392. doi: 10.1016/0005-2787(72)90452-2. [DOI] [PubMed] [Google Scholar]
- Van Dieijen G., Van Der Laken C. J., Van Knippenberg P. H., Van Duin J. Function of Escherichia coli ribosomal protein S1 in translation of natural and synthetic messenger RNA. J Mol Biol. 1975 Apr 15;93(3):351–366. doi: 10.1016/0022-2836(75)90282-x. [DOI] [PubMed] [Google Scholar]
- Wahba A. J., Miller M. J., Niveleau A., Landers T. A., Carmichael G. G., Weber K., Hawley D. A., Slobin L. I. Subunit I of G beta replicase and 30 S ribosomal protein S1 of Escherichia coli. Evidence for the identity of the two proteins. J Biol Chem. 1974 May 25;249(10):3314–3316. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zamir A., Miskin R., Elson D. Inactivation and reactivation of ribosomal subunits: amino acyl-transfer RNA binding activity of the 30 s subunit of Escherichia coli. J Mol Biol. 1971 Sep 14;60(2):347–364. doi: 10.1016/0022-2836(71)90299-3. [DOI] [PubMed] [Google Scholar]