Abstract
Prenatal ethanol exposure (PE) is one of the developmental factors leading to increased addiction propensity (risk). However, the neuronal mechanisms underlying this effect remain unknown. We examined whether increased excitatory synaptic transmission in ventral tegmental area (VTA) dopamine (DA) neurons, which is associated with drug addiction, was impacted by PE. Pregnant rats were exposed to ethanol (0 or 6 g/kg/day) via intragastric intubation from gestational day 8–20. Amphetamine self-administration, whole-cell recordings, and electron microscopy were performed in male offspring between 2 and 12-week-old. The results showed enhanced amphetamine self-administration in PE animals. In PE animals, we observed a persistent augmentation in calcium-permeable AMPA receptor (CP-AMPAR) expression, indicated by increased rectification and reduced decay time of AMPAR-mediated excitatory postsynaptic currents (AMPAR-EPSCs), enhanced depression of AMPAR-EPSCs by NASPM (a selective CP-AMPAR antagonist), and increased GluA3 subunits in VTA DA neuron dendrites. Increased CP-AMPAR expression in PE animals led to enhanced excitatory synaptic strength and the induction of CP-AMPAR-dependent long-term potentiation (LTP), an anti-Hebbian form of LTP. These observations suggest that, in PE animals, increased excitatory synaptic strength in VTA DA neurons might be susceptible to further strengthening even in the absence of impulse flow. The PE-induced persistent increase in CP-AMPAR expression, the resulting enhancement in excitatory synaptic strength, and CP-AMPAR-dependent LTP are similar to effects observed after repeated exposure to drugs of abuse, conditions known to increase addiction risk. Therefore, these mechanisms could be important neuronal substrates underlying PE-induced enhancement in amphetamine self-administration and increased addiction risk in individuals with fetal alcohol spectrum disorders.
INTRODUCTION
There are individual differences in drug addiction propensity (risk). Only a proportion of individuals develop drug addiction after using drugs repeatedly (Everitt et al, 2008). Current evidence suggests that prenatal exposure to drugs of abuse (eg, ethanol, psychostimulants, opiates, cannabinoids, nicotine) could increase addiction risk (Malanga and Kosofsky, 2003). Indeed, clinical studies have shown that prenatal ethanol exposure (PE) increases addiction risk (Famy et al, 1998; Baer et al, 2003; Alati et al, 2006). Results from animal studies show that PE leads to behavioral phenotypes associated with increased addiction risk such as enhanced locomotor activity to novelty (Bond, 1981; Abel, 1984; Kelly et al, 1988; Thomas et al, 2008), anxiety/depression-like behavior (Hellemans et al, 2008; Weinberg et al, 2008), behavioral sensitization to stimulants (Blanchard et al, 1987; Barbier et al, 2009), and augmented HPA axis reactivity (Lee et al, 2008; Weinberg et al, 2008). Furthermore, PE facilitates the learning of drug cues in the conditioned place preference paradigm (Spear and Molina, 2005; Barbier et al, 2008, 2009). However, the cellular mechanisms mediating PE-induced increase in addiction risk are not well understood.
The mesolimbic/cortical dopamine (DA) systems are the major targets for drugs of abuse (Koob and Le Moal, 1997) and have a critical role in addictive behaviors. Specifically, DA neurons located in the ventral tegmental area (VTA) are important in mediating addictive behaviors or increased addiction risk. For example, intra-VTA psychostimulant administration enhances the subsequent cocaine self-administration (Suto et al, 2003) and reinstates drug-seeking behavior (Shaham et al, 2003). These effects could be mediated by enhanced excitatory synaptic transmission in the VTA because augmenting and blocking glutamate receptor expression in the VTA can increase responding to drugs (Carlezon et al, 2000) and prevent psychostimulant self-administration (Suto et al, 2003), respectively. Furthermore, numerous studies report increased excitatory synaptic strength in VTA DA neurons after cocaine, ethanol, or stress exposure, conditions known to increase addiction risk. Specifically, increased excitatory synaptic strength in VTA DA neurons appears to be mediated by increased calcium-permeable AMPA receptor (CP-AMPAR) expression in VTA DA neurons (Bellone and Luscher, 2006; Argilli et al, 2008). The CP-AMPARs have larger conductance than calcium-impermeable AMPARs (CI-AMPARs) (Cull-Candy et al, 2006). Therefore, the enhanced expression of CP-AMPARs in VTA DA neurons could contribute to the overall increase in excitatory synaptic strength after exposure to drugs of abuse or stress and mediates increased addiction risk. In previous in vivo studies, we have shown that PE leads to a long-lasting increase in the excitation in VTA DA neurons (Shen et al, 1999; Xu and Shen, 2001; Choong and Shen, 2004a; Shen and Choong, 2006), supporting the possibility that PE can enhance the excitatory synaptic strength in VTA DA neurons via the augmentation of CP-AMPARs expression. This possibility was investigated in the present study.
MATERIALS AND METHODS
All experimental procedures were carried out in accordance with the University at Buffalo Institutional Animal Care and Use Committee guidelines.
PE and Cross Fostering
The procedure of PE exposure and cross fostering are described in detail in previous studies (Choong and Shen, 2004a). Breeding of Sprague-Dawley rats (Harlan, Indianapolis, IN) was performed to control the prenatal environment. Ethanol (15% w/v) was administered via intragastric intubation from gestation day 8 to 20 (two intubations at 0 or 3 g/kg ethanol/day) during weekdays. A single dose (0 or 4 g/kg) was given during weekends. The control dams received sucrose solution (22.5% w/v in 0.9% saline) to equate for ethanol's calories and were pair-fed with ethanol-treated dams. Dams received thiamine injections twice per week (8 mg/kg; i.m.) to compensate ethanol intake-induced thiamine deficiency, which could cause dysfunction in neurons. To control for postnatal rearing conditions such as disrupted maternal behaviors in PE dams due to ethanol withdrawal, on postnatal day 1, pups from the ethanol groups were culled to 10 per litter and transferred to dams that did not receive any treatment during pregnancy and gave birth 2 days earlier. Pups from the control litters were cross-fostered with each other (switch dams). One to three male offspring per litter were used.
Amphetamine Self-Administration
Jugular vein surgery
Seven-week-old male offspring were anesthetized with ketamine/xylazine (65 mg/kg/15 mg/kg; i.p.). Buprenorphine (0.025 mg/kg, s.c.) was applied as the preoperative analgesic. The external jugular vein was implanted with a heparinized cannula (Instech, Plymouth Meeting, PA) connected to an injection port in a harness (Instech). Animals were allowed to recover for at least 5 days after surgery. The catheters were flushed daily with 0.1–0.2 ml solution of enrofloxacin (4 mg/ml) and heparinized saline (50 IU/ml) to further preserve catheter patency.
Self-administration
After a 5–7 days of recovery period, animals were trained daily in operant chambers (Med Associates, St Albans, VT) to self-administer amphetamine intravenously (0.02 or 0.1 mg/kg/infusion, salt weight, over 5–7 s; ∼0.12 ml/infusion) under a fixed ratio (FR) 1 schedule for 3 h. A priming dose of amphetamine was automatically delivered to the rats. Additional nine infusions per day were allowed. After animals self-administered nine infusions for two consecutive days under FR 1, they were trained on FR 2 schedule. When animals reached nine infusions under FR 2, they were tested under a progressive ratio (PR) schedule (ratio values: 5 × EXP(0.25 × infusion number−5); maximal infusions per day: 14) (Richardson and Roberts, 1996) without the priming infusion for 6 days. Animals that did not reach the FR 2 criteria (nine self-administered infusions) within eight daily sessions were not tested on the PR schedule. The above method is an established behavioral paradigm to examine group differences in addiction risk (Suto et al, 2003). Data analysis was conducted only in rats with patent cannula at the end of the self-administration experiments.
In Vitro Patch Clamp Experiments
Slice preparation
Midbrain slices were prepared from 2 to 12-week-old male offspring (Haj-Dahmane and Shen, 2010). Rats were anesthetized with isoflurane and decapitated. The brain was placed in cold modified artificial cerebrospinal fluid (in mM): 110 choline Cl, 2.5 KCl, 0.5 CaCl2, 7 MgSO4, 1.25 NaH2PO4, 26.2 NaHCO3, 11.6 sodium L-ascorbate, 3.1 sodium pyruvate, 25 glucose, and saturated with 95% O2/5% CO2. Horizontal slices (250 μm) were cut using a Vibratome (VT1200, Leica Biosystems, St Louis, MO). Slices were incubated for 45–60 min at 35 °C in standard artificial cerebrospinal fluid (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, 11 glucose saturated with 95% O2/5% CO2. One hour later, a slice was transferred to the recording chamber at 30±1 °C. In animals >6-week-old, slices were incubated in artificial cerebrospinal fluid containing kynurenic acid (0.5 mM) to improve the viability of DA neurons. Recordings were obtained after extensive (>30 min) wash. Previous studies have shown incubation in kynurenic acid might alter the basic membrane functions. This manipulation did not alter the excitatory synaptic neurotransmission in VTA DA neurons. We observed no changes in rectification index (RI) after kynurenic acid incubation in 3–4-week-old control animals (without kynurenic acid: RI=1.71±0.13, n=6; with kynurenic acid: RI=1.64±0.24; n=5).
Whole-Cell Recordings
Neurons were visualized using an upright microscope equipped with a differential interference contrast/infra-red imaging system (Olympus BX 51, Tokyo, Japan). All recordings were performed in picrotoxin (100 μM) to block GABAA receptors. Whole-cell recordings were obtained with patch electrodes (3–5 MΩ) containing: (in mM) 120 potassium gluconate, 10 Na2-phosphocreatine, 10 KCl, 10 HEPES, 1 MgCl2; 1 EGTA; 2 Na2-ATP; 0.25 Na-GTP (pH: 7.3), except in current–voltage (I–V) relationship of AMPA receptor-excitatory postsynaptic currents (EPSCs) and AMPAR/NMDAR ratio experiments, in which cesium methanesulfonate-based intracellular solution was used (in mM: 120 cesium methanesulfonic acid, 5 TEA-Cl, 10 Na2-phosphocreatine, 10 HEPES; 1 QX-314; 1 MgCl2; 1 EGTA; 2 Na2-ATP, 0.25 Na-GTP, 0.1 spermine; pH: 7.3). In some AMPAR/NMDAR ratio experiments, spermine was omitted from the methanesulfonate-based internal solution. Biocytin (0.2%) was added in the internal solution in some recordings.
Putative DA neurons were identified by the presence of the hyperpolarization-activated cation current (Ih). Membrane potentials recorded were corrected for the liquid junction potential.
Stimulation and Recording
A patch pipette was placed rostral the recorded neuron to evoke EPSCs or excitatory postsynaptic potentials (EPSPs) by square-pulses (duration: 100–200 μs; 0.1 Hz). Stimulation intensity was adjusted to evoke 50–70% of the maximal response (EPSC amplitude: up to 300 pA). The EPSC amplitude was determined by subtracting EPSC peak current from the baseline current. Signals were amplified with an Axoclamp 2B or Multiclamp 700 B amplifier and acquired using the pClamp 10 software (Molecular Devices, Union City, CA). Access resistance (10–20 mΩ) was continuously monitored. Recordings with access resistance increased >0% were discarded.
To examine the I–V relationships and RI, neurons were voltage-clamped at −70, 0, and +50 mV (30 traces/membrane potential). The I–V curves were constructed by normalizing AMPAR-EPSC amplitude at 0 and +50 mV to amplitude at −70 mV. The RI was calculated using the following formula: RI=[I−70 mV/(−70 mV-Erev)]/[I+50 mV/(50 mV-Erev)], where I−70 mV is the average amplitude of AMPAR-EPSCs at −70 mV, I+50 mV is the amplitude of AMPAR-EPSCs at +50 mV, and Erev is the reversal potential AMPAR-EPSCs. Linearity corresponded to RI=1.4 (Liu and Cull-Candy, 2002). To analyze EPSC decay time constant (τ), 30 EPSCs per neuron were randomly selected and fitted with the exponential equation: y=y0+A1*exp−(t-t0)/τ, where A1 represents peak EPSC amplitude, y0 baseline current, and τ time constant.
To determine the AMPAR/NMDAR ratio, neurons were voltage-clamped at +50 mV and EPSCs were recorded in the absence (mixed EPSCs) and presence of APV (50 μM; AMPAR-EPSCs). NMDAR-EPSCs were determined as the difference between AMPAR-EPSCs and mixed EPSCs. The AMPAR/NMDAR ratio was obtained as average AMPAR-EPSC amplitude (30 traces)/average NMDAR-EPSC amplitude (30 traces). To construct the input-output curves, stimulation was delivered at 1, 2, 4, 8, 16, and 32 V (10 stimulation per intensity at 10 s intervals) via the stimulation electrode consistently placed ∼100 μM rostral to the recorded neuron.
To examine the spike-timing dependent (STD) plasticity, neurons were recorded in the current clamp mode and held at −70 mV by DC current injection. The induction of STD long-term potentiation (LTP) was achieved by pairing five bursts of three pairs of presynaptic stimulation with postsynaptic hyperpolarization or depolarization pulses (5 ms, 1 nA; see Figure 6 for details). This stimulation paradigm was repeated every 5 s for 15 times.
Cell Identification
Some brain slices used in recordings were fixed (4% paraformaldehyde) and incubated with Alexa Fluor 594 for biocytin, followed by antibody for tyrosine hydroxylase (TH) and goat antimouse IgG Alexa Fluor 488. Neurons were visualized with a Zeiss Axioimager microscope.
Electron microscopy
Animals were perfused (4% paraformaldehyde, 0.1% glutaraldehyde). Horizontal vibratome sections (500 μM) containing VTA areas were collected. VTA areas were dissected out and imbedded. Two blocks per rat were sectioned (80 nM) and labeled with TH antibody, followed by antibody against GluA1, GluA2, or GluA3. Sections were secondarily labeled with a 15 nm colloidal gold mouse IgG (for TH) and a 6 nm colloidal gold rabbit IgG (for AMPAR subunits). Aurion blocking system (Electron Microscopy Sciences, Hatfield, PA) was used to further cut down non-specific binding. Sections were viewed at × 50 000 with a JEOL 100 CXII electron microscope. Subunits of AMPAR were counted only in TH-positive dendrites. The TH and GluA3 antibodies have been cited in previous studies (He et al, 1998; Blanco et al, 2012). Antibodies against AMPAR subunits were selected because they were affinity-purified and the specificity was confirmed with western blot and by negative binding on tissue sections when the antibody was previously exposed to antigen peptide. A pilot study was executed in which 40 um frozen sections were labeled with TH and the GluA1, 2, and 3 antibodies followed by secondary colloidal gold labels and silver enhancement. Antibody against TH only labeled areas that contained DA neurons. GluA2 was the most prevalent label, whereas GluA1 was not as widely distributed. On the other hand, GluR3 was the lowest label. This observation is consistent with that in the literature (Bassani et al, 2009) and supports the specificity of the GluA1, GluA2, and GluA3 antibodies.
Chemicals and Drugs
Most chemicals and antibodies were obtained from Sigma-Aldrich (St Louis, MO; mouse monoclonal TH antibody cat number: T2928, rabbit polyclonal GluA1 antibody cat number: SAB4501293; rabbit polyclonal GluA2 antibody cat number: SAB4501295). Antibody against GluA3 was monoclonal-raised in rabbit from Cell Applications Inc. (San Diego, CA; Cat number: CG1180). Picrotoxin, D-(-)-2-Amino-5-phosphonopentanic acid (APV), kynurenic acid, and N-[3-[[4-[(3-minopropyl) amino]butyl]amino]propyl]-1-naphthaleneacetamide trihydrochloride (NASPM) were purchased from R&D Systems (Minneapolis, MN). Colloidal gold mouse and rabbit IgG were from Electron Microscopy Sciences (Hatfield, PA). Fluorescent dyes were from Life Technologies (Grand Island, NY).
Data Analysis
Results are presented as mean±SEM. Statistical analyses were conducted using ANOVA with or without repeated measures followed by Tukey LSD post hoc test. The group difference in the proportion of animals entering into the PR schedule in amphetamine self-administration experiment was analyzed by Chi-square analysis. In NASPM (Figure 2), AMPAR/NMDAR ratio (Figure 5), and STD LTP (Figure 6) experiments, planned comparison was used for examining the differences between control and PE groups or differences across two time points (eg, 10–20 min after induction) after ANOVA. EPSC decay time constants (τ) were compared by the Kolmogorov–Smirnov test because of skewed distribution. The results from electron microscope studies were analyzed with independent t-test. The level of significance was set at 0.05.
RESULTS
Birth Outcome
Seventy-four dams (24 control, 25 PE, and 25 foster) were used. There were no differences in litter size (control: 13.8±0.7; PE: 13.2±0.7), pup number by gender (control male: 7.0±0.6; control female 6.8±0.5; PE male: 6.7±0.6, PE female: 6.5±0.6), or male pup weight on postnatal day 1 (control: 6.92±0.17 g; PE male: 6.63±0.17 g). However, PE female pups (6.19±0.15 g) had lower weight compared with control female pups (6.60±0.17 g; t-test, t47=2.45, P<0.05). The above results indicate that the PE treatment did not lead to major teratogenic effects.
Amphetamine Self-Administration
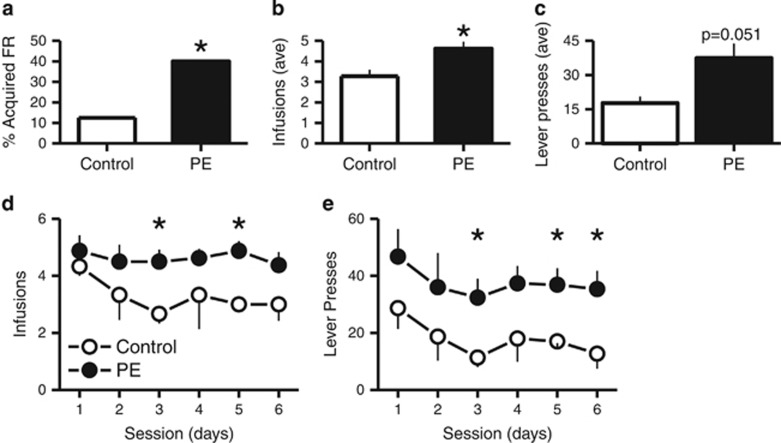
Not all animals acquired self-administration under the FR schedule within eight sessions. At the 0.02 mg/kg/infusion dose of amphetamine tested, a higher proportion of PE animals reached the acquisition criterion under the FR schedule (Control: 3/24, 13% PE: 8/20, 40% χ21=4.4, P<0.05; Figure 1a). No group differences were detected at the higher amphetamine dose tested (0.1 mg/kg/infusion), although a greater proportion of animals from both the control and PE groups tested at this higher dose reached criterion (Control:12/17, 71% PE: 7/13, 54%) compared with those tested with the lower dose.
Figure 1.
Number of amphetamine infusions and responses (lever presses) for PE and control animals during six PR schedule self-administration sessions. Animals self-administered amphetamine at 0.02 mg/kg per infusion. *P<0.05, differences between control and PE animals.
Under the PR schedule, PE animals self-administered more amphetamine than controls when the amphetamine dose was set at 0.02 mg/kg/infusion (t-test, t9=2.26, P<0.05; Figure 1b and d). The responses (lever presses) were also increased in PE animals (t-test, t9=1.81, P=0.051; Figure 1c and e). No group differences were detected in the number of infusions or lever presses emitted at the higher dose of amphetamine tested (0.1 mg/kg/infusion). The effect observed at the lower dose of amphetamine tested (Figure 1) resembles those observed in amphetamine-sensitized rats that show a greater propensity to acquire self-administration behaviors for lower but not higher doses of the drug (Pierre and Vezina, 1997; Vezina, 2004). Notably, the present findings show that PE exposure can also lead to enhanced work output and drug taking for 6 days of testing on a PR schedule of reinforcement (Figure 1b–e). Together, these data indicate that the PE paradigm used in the present study produced strong behavioral effects that could place individuals at increased risk for drug addiction.
DA Neuron Identification
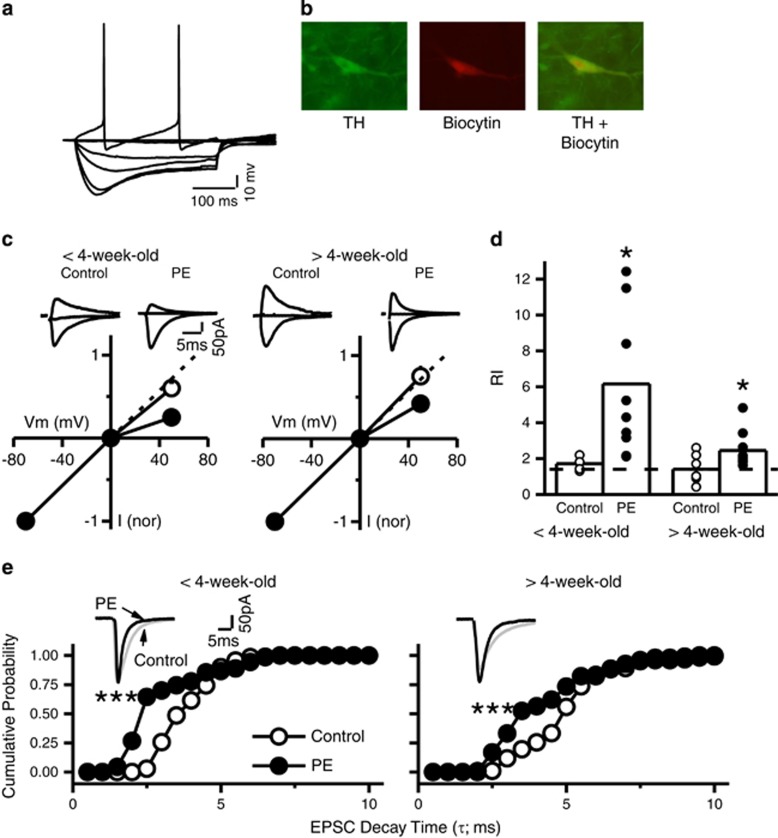
We recovered 30 putative VTA DA neurons identified with Ih (Figure 2a) and filled with biocytin. Twenty-six (87%) were TH-positive (Figure 2b), indicating a high level of positive identification of DA neurons using Ih. This result is similar to that reported by one study (Mao et al, 2011) but different from another (Margolis et al, 2006). However, in the study by Margolis et al. (2006), regions not traditionally confined to VTA areas are included. We only recorded areas adjacent and medial to the medial lemniscus.
Figure 2.
Prenatal ethanol exposure (PE) leads to a persistent increase in CP-AMPAR expression in VTA DA neurons. (a) Typical voltage response of a putative VTA DA neuron to hyperpolarizing and depolarizing current pulses. Note the prominent voltage sag induced by membrane hyperpolarization, reflecting the activation of the Ih current. (b) Post hoc immunocytochemistry confirmed the identity of DA neurons by TH staining co-labeled with biocytin injected in the cell during recording. This method revealed using Ih to identify VTA DA neurons reached 87% accuracy. (c) Prenatal ethanol exposure increased the rectification of AMPAR-EPSCs in animals <4-week-old (35 days) and >4-week-old. Left panel illustrates the I–V curves of AMPAR-EPSCs in control (○) and PE (●)-exposed animals <4-week-old. Note the profound rectification of AMPAR-EPSCs at positive potentials in PE rats. Dashed line indicates linearity (without rectification). Upper traces are superimposed sample AMPAR-EPSCs traces recorded at −70, 0, and 50 mV. Right panel illustrates I–V curves of AMPAR-EPSCs obtained in control (○) and PE (●) animals >4 weeks old. Note that PE-induced increase in rectification persisted. (d) A summary graph depicting increased RI of AMPAR-EPSCs in PE animals < and >4 weeks old. Dashed indicates linearity of RI at 1.4. (e) Prenatal ethanol exposure reduced the decay time constant τ of AMPAR-EPSCs. Left panel illustrates the cumulative distribution of τ obtained in control (○) and PE (●) animals <4 weeks old. Right panel is the cumulative distribution of τ obtained in animals >4 weeks old. Note that PE induced a significant decrease of τ in both age groups. *P<0.05; ***P<0.001, differences between control and PE animals.
Prenatal Ethanol Exposure Persistently Increases CP-AMPAR Expression
Electrophysiology
We examined the I–V curves and RI of AMPAR-EPSCs in control and PE animals at different ages. Both group (F1,27=9.91; P<0.05; 2-way ANOVA) and age (F1,27=5.07; P<0.05) influenced the results. In control animals <4-week-old (35 days), the I–V curve exhibited a linear relationship with little inward rectification at +50 mV (Figure 2c). In contrast, in age-matched PE rats, the I–V curve exhibited inward rectification and was non-linear (Figure 2c). In PE animals, the RI was greater (5.86±1.32; 350% increase; n=9) than that in control animals (1.71±0.13, Tukey HSD post hoc comparison, P<0.05; n=6; Figure 2d).
Similar results were obtained in animals >4-week-old (control RI: 1.41±0.29, n=7; PE RI: 2.45±0.35; n=9; 75% increase in PE animals; P<0.05; Figure 2c and d). Furthermore, in PE rats, the RI in animals <4-week-old was greater (40% increase) compared with that in animals >4-week-old (P<0.05), suggesting an age-dependent decrease in CP-AMPAR expression.
CP-AMPAR EPSCs exhibit a faster decay time constant (τ) than CI-AMPARs (Toth et al, 2000). We examined τ of AMPAR-EPSCs from neurons recorded in the above experiment. As shown in Figure 2e, the cumulative probability curves of τ were shifted to the left in VTA DA neurons recorded from PE animals in both age groups (Kolmogorov–Smirnov test; <4-week-old, P<0.001; >4-week-old, P<0.001).
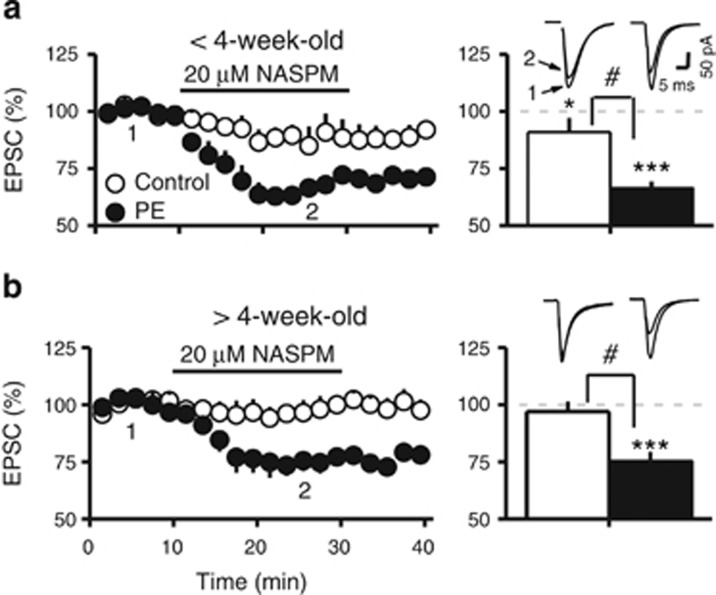
We next examined the effects of the specific CP-AMPAR antagonist NASPM (20 μM) on AMPAR-EPSCs. The results revealed that PE potentiated the inhibition of AMPAR-EPSCs by NASPM (F1,28=33.74; 3-way ANOVA with repeated measures, P<0.001). Age also impacted NASPM-induced inhibition (F1,28=2.49; P<0.05) but only when control and PE animals were considered together. As shown in Figure 3a, in animals <4-week-old, NASPM reduced the AMPAR-EPSC amplitude in both control (89.5±4.6% of baseline; 10.5% decrease 15 min after NASPM; planned comparison following 3-way ANOVA, P<0.05) and PE animals (63.26±4.27% of baseline; 36.7% decrease; P<0.001). The effect of NASPM was greater in PE animals (P<0.01).
Figure 3.
Prenatal ethanol exposure enhances the depression of AMPAR-EPSCs induced by CP-AMPAR antagonist NASPM in VTA DA neurons. (a) Left panel is a summary graph of the effects of NASPM (20 μM) on AMPAR-EPSC amplitude (expressed as percentage of baseline) obtained in control (○) and PE (●) animals <4-week-old. Right panel depicts the significant depression in average amplitude of AMPAR-EPSCs during NASPM application in control (open bar) and PE animals (filled bar). Note that PE significantly enhanced the NASPM-induced depression of AMPAR-EPSCs. Sample AMPAR-EPSCs collected at the time points indicated by numbers in the left panel are depicting on top of the right panel. (b) The NASPM-induced depression on AMPAR-EPSCs persisted in PE animals >4-week-old. Left panel illustrates the effects of NASPM obtained in control (○) and PE (●) animals. Right panel is a summary graph of AMPAR-EPSCs measured during NASPM application. Note that NASPM reduced the amplitude of AMPAR-EPSCs only in PE animals. Sample EPSC traces are depicted on the top. #P<0.05, differences in NASPM-induced depression between control and PE animals; *P<0.05, ***P<0.001, differences in EPSC amplitude before and after NASPM (between points 1 and 2).
In animals >4-week-old, the difference between control and PE groups remained (P<0.05). However, NASPM reduced EPSC amplitude only in PE (73.5±4.7% of baseline; 26.5% decrease; P<0.001) but not in control animals (96.2±4.1% of baseline; Figure 3b).
Electron microscopy
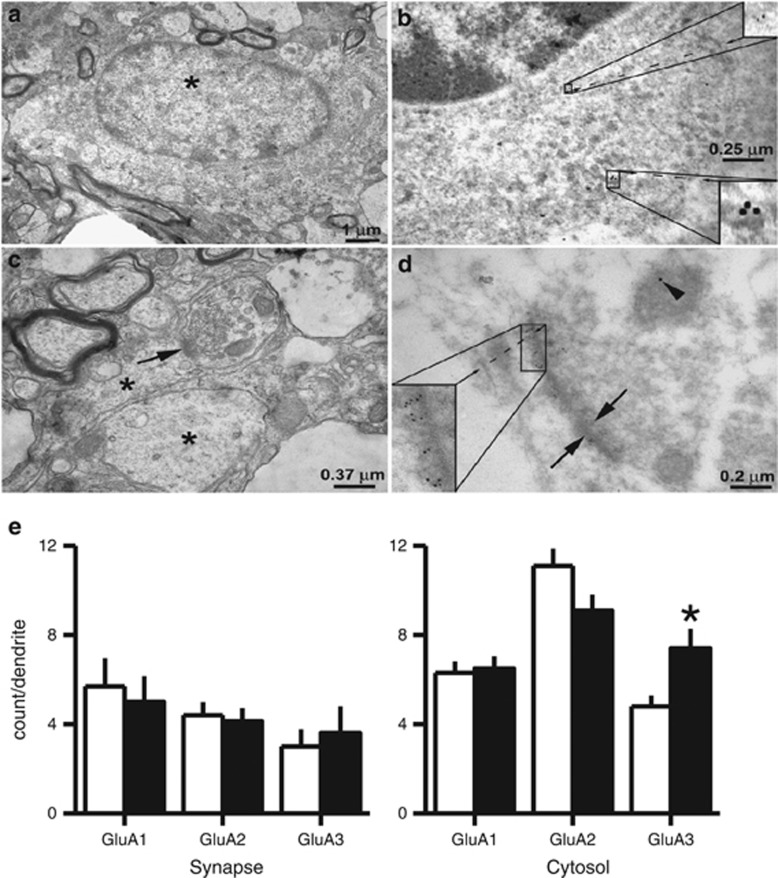
The results were obtained in 6–8-week-old animals. The data are presented as number of AMPAR subunit-positive particles/dendrite. In PE animals, there was a significant increase in GluA3-positive particles in the cytosol/TH-positive dendrite (Control: 4.8±0.5/dendrite, n=81; PE: 7.4±0.9/dendrite, n=100; t-test, t179=2.40, P<0.05; Figure 4). No differences were found in GluA1-positive or GluA2-positive particles (Figure 4). We did not find any differences in AMPAR subunits in the synapses of TH+ dendrites. This could be due to relatively few synapses (<30 per subunit) were found in our EM sections. The result from the EM study supports the increase of CP-AMPARs in PE animals and suggests GluRA3-containing AMPARs are added in VTA DA neurons.
Figure 4.
Electron microscopy of the VTA showing PE increases the expression of GluA3 subunits in VTA DA neuron dendrites. (a) illustrates an electron micrograph of a VTA neuron soma with nucleus. (b) Immunoreactivity for GluA1 and TH. Inset at the lower right shows TH labeling with 15 nm particles. Inset at upper right shows GluA1 labeling with 6 nm particles. (c) Electron micrograph of dendrites (asterisks) and a synapse (arrow) in a TH-positive neuron. (d) GluA2 immunoreactivity (6 nm particles shown at the inset in the lower left) in a synapse (arrows) located in a TH-positive dendrite (arrowhead). (e) Bar graphs showing the average GluA1, GluA2, and GluA3 subunit count/dendrite. In PE animals, the count of GluA3 subunits is significantly higher in the cytosol than that in control animals (*P<0.05, differences between control and PE animals).
Prenatal Ethanol Exposure Enhances Excitatory Synaptic Strength
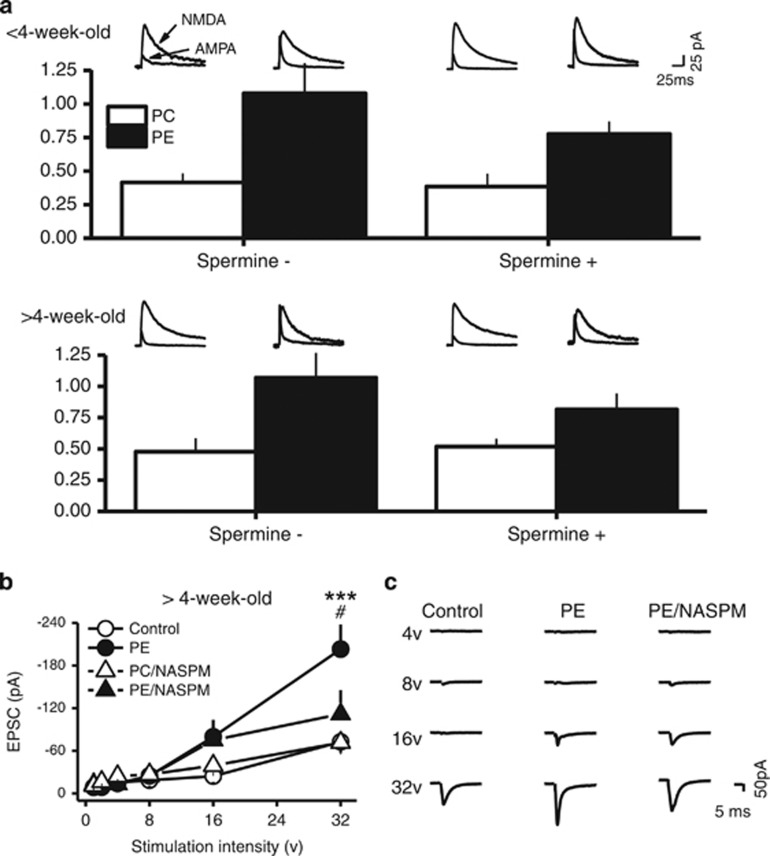
To examine the excitatory synaptic strength, we investigated the AMPAR/NMDAR ratio. The effect was examined using internal solution without spermine and with spermine. The results showed a significant main effect of prenatal ethanol treatment (3-way ANOVA, F1,43=27.51; P<0.001). There were no differences due to age or internal solutions. When spermine-free internal solution was used, the AMPAR/NMDAR ratio was greater in PE animals <4-week-old (control: 0.42±0.12, n=7; PE: 1.08±0.13, n=6; planned comparison, P<0.001; Figure 5a) as well in PE animals >4-week-old (control: 0.48±0.14, n=5; PE: 1.07±0.13; n=6; P<0.05; Figure 5a). When spermine-containing internal solution was used, the AMPAR/NMDAR ratio was also greater in PE animals <4-week-old, (control: 0.38±0.10, n=7; PE: 0.75±0.09, n=7; P<0.05; Figure 5a) as well in PE animals >4-week-old (control: 0.52±0.06, n=7; PE: 0.82±0.13; n=6; P<0.05; Figure 5a). These results suggest PE leads to an increase in excitatory synaptic strength. However, because there were no differences in AMPAR/NMDAR ratio caused by spermine-free and spermine-containing internal solutions (although a trend of decrease was observed when spermine-containing solution was used), the results suggest that VTA DA neurons could contain endogenous polyamines that lead to rectification (reduction) of CP-AMPAR-dependent EPSCs at depolarized membrane potentials reported in previous studies (Rozov and Burnashev, 1999; Shin et al, 2005). Under this condition, the precise contribution of CP-AMPARs on excitatory synaptic strength cannot be accurately accessed by AMPAR/NMDAR ratio using NASPM.
Figure 5.
Prenatal ethanol exposure increases the strength of excitatory synapses of VTA DA neurons. (a) PE enhanced AMPAR/NMDAR ratio in animals <4-week-old and >4-week-old. Bar graph depicting the average AMPAR/NMDAR ratios obtained in control and PE animals. Note an increase in AMPAR/NMDAR ratio in PE animals in both age groups and this effect was not altered by the absence or presence of spermine in the internal solution, suggesting endogenous polyamines in VTA DA neurons. Superimposed AMPAR- and NMDAR-EPSCs traces recorded at +50 mV in control (left traces) and PE (right traces) animals are presented above the bar graphs. (b) Input-output curves of AMPR-EPSCs obtained in control and PE-exposed animals >4-week-old. Prenatal ethanol exposure significantly shifted the input-output I-O curve to the left. Specifically, PE led to increased AMPAR-EPSC amplitude when stimulation intensity reached 32 V. This effect was no longer observed in the presence of NASPM. (c) Sample traces of evoked AMPAR-EPSCs at different stimulation intensities in control and PE animals and in the presence of NASPM in PE animals. *P<0.05; **P<0.01, differences between control and PE groups. #P<0.05, differences between vehicle and NASPM in PE animals.
Therefore, we used another approach to examine the excitatory synaptic strength—the input-output curves (in animals >4-week-old) and the effect of NASPM. This experiment was conducted at −70 mV. The contribution of CP-AMPARs could be evaluated more accurately without rectification. We found a significant interaction between group and stimulation intensity (3-way ANOVA; F5,135=7.17; P<0.001), confirming a left shift in PE animals (Figure 5b and c). Bath-applied NASPM also influenced the input-output curves (3-way ANOVA; F5,135=2.30; P<0.05). Specifically, AMPAR-EPSC amplitude from PE animals was higher than control animals when the stimulation intensity was at 32 mV (control: 72.67±15.42 pA, n=11; PE: 203.08±34.72 pA, n=10; planned comparison, P<0.05; Figure 5b and c). Bath-applied NASPM had no effects in control but decreased the AMPAR-EPSC amplitude in PE animals close to control levels (in NASPM, control: 71.52±16.66 pA, n=5; PE: 111.81±33.64 pA, n=5; P<0.05 between PE in vehicle and PE in NASPM; no differences were observed between PE in NASPM and control in vehicle or NASPM; Figure 5b and c). The result also suggests that CP-AMPAR leads to increased excitatory synaptic strength in PE animals due to an addition instead of a switch from CI-AMPARs to CP-AMPARs. The overall increase in AMPAR population could be the reason of consistently increased AMPAR/NMDAR ratio even with reduced amplitude of CP-AMPAR-mediated EPSCs due to rectification at positive membrane potentials.
Prenatal Ethanol Exposure-Induced Increase in CP-AMPAR Expression Facilitates CP-AMPAR-Dependent (anti-Hebbian) LTP
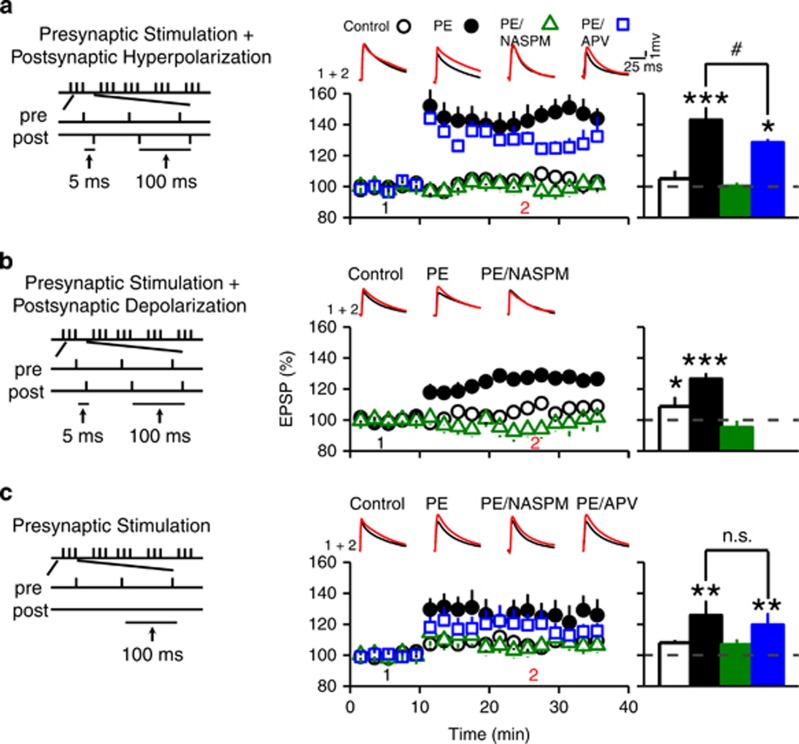
The activation of CP-AMPARs can lead to CP-AMPAR-dependent LTP (Mahanty and Sah, 1998; Lamsa et al, 2007; Mameli et al, 2011), which is also called anti-Hebbian LTP because it could be induced by pairing presynaptic stimulation with postsynaptic membrane hyperpolarization instead of depolarization. We examined CP-AMPAR-dependent, anti-Hebbian LTP in animals >4-week-old.
We first paired presynaptic stimulation with postsynaptic hyperpolarization during induction. The results showed a significant group and LTP induction interaction (3-way ANOVA with repeated measure, F3,16=5.06, P<0.01; Figure 6a). In control animals, the induction protocol failed (EPSP amplitude 4.1±6.8% above baseline 15 min after induction; n=5; planned comparison). In PE animals, the induction was successful (53.4±15.6% above baseline; n=8; P<0.001). When recordings were conducted in the presence of NASPM, LTP induction in PE animals failed. (3.2±5.5% above baseline; n=3; Figure 6a). Conversely, NMDA receptor blocker APV did not block LTP induction, but reduced LTP magnitude (31.2±4.5% above baseline, P<0.05, n=4) in PE animals. These results demonstrated that PE-induced increase in CP-AMPAR facilitates anti-Hebbian LTP at excitatory synapses of VTA DA neurons.
Figure 6.
Prenatal ethanol exposure promotes CP-AMPAR-dependent STD LTP in VTA DA neurons. (a) Prenatal ethanol exposure facilitates the induction of anti-Hebbian LTP in VTA DA neurons. Left panel depicts the stimulation protocol used for the induction of anti-Hebbian LTP (delivered every 5 s for 15 times). Middle panel depicts summary graphs of AMPAR-EPSP amplitude recorded before (time point 1) and after (time point 2) the induction protocol in control (○), PE animals (●), PE animals with NASPM (Δ), and PE animals with APV (□). Note that the pairing protocol elicited a profound LTP in PE but not in control animals. This anti-Hebbian LTP was blocked by the CP-AMPAR antagonist NASPM (20 μM), but not by the NMDAR antagonist APV (50 μM). However, APV decreased the magnitude of LTP in PE animals. Upper traces illustrate sample EPSP traces taken before and during LTP. Right panel depicts summary graph of the magnitude of LTP obtained in various conditions described in the middle panel. (b) Prenatal ethanol exposure enhances the STD LTP induced by pairing presynaptic stimulation with postsynaptic depolarization. Left panel depicts the stimulation protocol. Middle panel illustrates the magnitude and time course of STD LTP obtained in control (○), PE (●) animals, and PE animals with NASPM (Δ). Upper graph depicts sample EPSP traces. Right panel is a summary bar graph of average STD LTP magnitude. Note that PE significantly enhanced the magnitude of STD LTP, which was blocked by NASPM. A weak STD LTP was also observed in control rats. (c) Presynaptic stimulation alone was sufficient to induce LTP in PE animals. Left panel illustrates the stimulation protocol using only presynaptic stimulation. Middle panel depicts the magnitude and time course of the LTP in control animals (○), PE animals (●), PE animals with NASPM (Δ), and PE animals with APV (□). In PE animals, LTP induction was abolished by NASPM but not by APV. Upper panel depicts sample EPSP traces. Right graph is a summary of LTP magnitude in conditions depicted in the middle panel. *P<0.05; ***P<0.001, differences before and after the stimulation protocol; #P<0.05, differences between PE and PE with APV treatment; n.s., no significant differences.
We next paired presynaptic stimulation with postsynaptic depolarization during LTP induction. The results showed a significant group and LTP induction interaction (3-way ANOVA, F2,27=3.62; P<0.05; Figure 6b). In control animals, the induction protocol caused a weak LTP (12.9±4.3% above baseline, n=11; P<0.05; Figure 6b). In PE animals, the LTP was greater (25.2±6.3% above baseline, n=14; Figure 6b; P<0.001). Application of NASPM completely abolished the LTP in PE animals (6.0±4.6% below baseline; n=5). The results indicate that CP-AMPARs also facilitate ‘regular' STD LTP induction in PE animals.
Next, LTP induction was carried out without postsynaptic stimulation. The results showed a significant LTP induction effect (3-way ANOVA, F1,23=20.0; P<0.001; Figure 6c). We did not observe LTP in control animals (6.2±2.1% above baseline; n=9; Figure 6c). In PE animals, LTP was successfully induced (25.2±12.2% above baseline; P<0.01; n=5; Figure 6c) and blocked by NASPM (4.4±5.8% above baseline; n=6; Figure 6c) but not by APV (19.5±7.0% above baseline; P<0.01; Figure 6c). When LTP magnitudes using different induction protocols were compared in PE animals, pairing presynaptic stimulation with postsynaptic hyperpolarization led to greater LTP than pairing presynaptic stimulation with postsynaptic depolarization or using presynaptic stimulation only (significant interaction between induction protocol and LTP level; 3-way ANOVA, F1, 25=5.46, P<0.05). This pattern of response is similar to anti-Hebbian LTP and supports that the presence of endogenous polyamines underlies the voltage-dependence of the anti-Hebbian LTP (Rozov and Burnashev, 1999; Shin et al, 2005; Lamsa et al, 2007).
DISCUSSION
Increased Amphetamine Self-Administration in PE Animals
The results from the present study show that PE produces strong behavioral effects that could place individuals at an increased risk for drug addiction during adulthood. This effect is demonstrated first by the finding that a higher proportion of PE animals acquire amphetamine self-administration, and second, by the additional finding that PE leads to enhanced work output and drug taking for 6 days of testing on a PR schedule of reinforcement. The finding that these effects are observed at the lower dose of self-administered amphetamine tested is consistent with those of other reports showing that amphetamine-sensitized rats are more likely to acquire self-administration behaviors for lower but not higher doses of the drug (Pierre and Vezina, 1997, 2004).
Persistent Increases in CP-AMPAR Expression in PE Animals
A major finding in the present study is a persistent increase in CP-AMPAR expression in VTA DA neurons in PE animals. This effect can be observed in animals as old as 12 weeks. At the similar age (10–11-week-old), PE rats show enhanced amphetamine self-administration. Therefore, there is a strong association between increased CP-AMPAR function in VTA DA neurons and increased addiction risk after PE. The increased CP-AMPAR expression is supported by confirming the unique electrophysiological properties of CP-AMPARs including the non-linear I–V relationship due to intracellular polyamine block of channel pores at positive membrane potentials (Isaac et al, 2007) and rapidly decaying EPSCs (Toth et al, 2000). The PE-induced increase in CP-AMPARs is further supported by enhanced depression of AMPAR-EPSCs by specific CP-AMPAR antagonist NASPM and increased GluA3 AMPAR subunits in TH-positive dendrites from the EM experiments. The results also demonstrate that VTA DA neurons express a mixture of CP-AMPARs and CI-AMPARs in both control and PE animals <4-week-old. However, CP-AMPAR expression is higher in PE animals. Results from the NASPM experiments show that in PE rats, 37% and 27% of total AMPAR-EPSC amplitude is mediated by CP-AMPARs in animals <4-week-old and >4-week-old, respectively. In control animals, the levels are 10% and 4% (not significantly different from 0%) in animals <4-week-old and >4-week-old, respectively. Previous studies have shown that in VTA DA neurons and cerebellar neurons, the regulation of AMPAR expression might be input-specific (Liu and Cull-Candy, 2000; Toth et al, 2000; Bellone and Luscher, 2005; Lamsa et al, 2007; Good and Lupica, 2010). In VTA DA neurons, increases in CP-AMPARs after cocaine treatment is observed in synapses receiving local VTA projections and the pedunculopontine nucleus in 2-week-old rats (Good and Lupica, 2010). Our preparation only evaluates the excitatory synapses receiving local projections; it remains to be determined whether synapses receiving projections from the pedunculopontine express increased CP-AMPARs in PE animals.
The PE-induced increase in CP-AMPAR is a persistent effect which is observed in 2–12-week-old animals. Many studies demonstrate that CP-AMPARs expression is transient and developmentally regulated. For example, in neocortical neurons, high levels of CP-AMPARs are switched to CI-AMPARs between the first and second week of age in rats (Brill and Huguenard, 2008). The switch is triggered by synaptic activity and the activation of CP-AMPARs, resulting in the incorporation of GluA2-subunits into AMPARs to form CI-AMPRs (Liu and Cull-Candy, 2000; Cull-Candy et al, 2006; Isaac et al, 2007). A similar developmental switch is described in VTA DA neurons in mice during the first 2 weeks of age (Bellone et al, 2011). Therefore, the increased CP-AMPARs in PE animals could be caused by delayed and/or abnormal developmental switch of AMPARs. This possibility is supported by the observation of age-dependent decrease of CP-AMPARs in PE animals. In the present study, we recorded from animals as old as 12 weeks. It would be interesting to know whether CP-AMPARs level will continue to decline in PE animals. A previous study showing increased CP-AMPAR expression in prenatal cocaine-exposed mice is reduced to control levels when animals reach 90 days of age (Bellone et al, 2011).
Evidence has shown that PE alters the morphology of dendrites and cell bodies of VTA DA neurons (Shetty et al, 1993). It is also possible that these morphological changes disrupt the excitatory synaptic activity and interfere with the normal activation of CP-AMPARs and their consequential switch to CI-AMPARs. In VTA DA neurons, the switch from CP-AMPARs to CI-AMPARs neurons during early development also requires the activation of type I metabotropic glutamate receptors (Bellone and Luscher, 2005) and/or NMDA receptors (Mameli et al, 2011). Morphological changes in VTA DA neurons in PE animals could also impact the activation of these receptors and impair the switch from CP-AMPARs to CI-AMPARs, leading to higher levels of CP-AMPARs throughout development in PE animals.
Increased Excitatory Synaptic Strength Caused by CP-AMPARs in PE Animals
Owing to the larger conductance of CP-AMPARs (Cull-Candy et al, 2006), we predict the excitatory synaptic strength is increased in VTA DA neurons in PE animals. This is confirmed by the input/output curve experiments showing increased AMPAR-EPSC amplitude in PE animals which is reduced to control levels by NASPM. The observation suggests that increased excitatory synaptic strength in PE animals is caused by adding CP-AMPARs to the AMPAR population instead of switching CI-AMPARs with CP-AMPARs. Under this condition, the persistently enhanced AMPAR/NMDAR ratio in PE animals could be due to an overall increase in AMPAR population. We have examined the AMPAR/NMDAR ratio with spermine-free and spermine-containing internal solutions. The results show a lack of difference in AMPAR/NMDAR ratio caused by the presence of spermine and suggest VTA DA neurons might contain endogenous polyamines as observed in other neurons in previous studies (Rozov and Burnashev, 1999; Shin et al, 2005). Although AMPAR/NMDAR ratio can also be influenced by changes in NMDAR function (Mameli et al, 2011; Yuan et al, 2013), our analysis of NMDAR-EPSC decay time constant does not show differences between control and PE animals (data not shown) and does not support that there are changes in NMDAR function at this time.
Anti-Hebbian LTP in PE Animals
Another important finding in the present study is that PE-induced increase in CP-AMPAR expression directly leads to CP-AMPAR-dependent LTP. This finding is consistent with previous studies demonstrating CP-AMPAR-dependent LTP in hippocampal neurons in developing rats (Mahanty and Sah, 1998; Lamsa et al, 2007) and in VTA DA neurons after cocaine exposure in developing mice (Mameli et al, 2011). The CP-AMPAR-dependent LTP induction is mediated by calcium entry from CP-AMPARs, not NMDARs (Mahanty and Sah, 1998). The CP-AMPAR-dependent LTP is termed anti-Hebbian LTP because pairing presynaptic stimulation with postsynaptic hyperpolarization instead of depolarization is more effective in LTP induction due to the depolarization-induced polyamine blockade of CP-AMPARs (Rozov and Burnashev, 1999; Shin et al, 2005; Lamsa et al, 2007). In the present study, the internal solution used for the STD LTP experiments does not contain spermine, supporting the presence of endogenous polyamines in VTA DA neurons. This notion is consistent with the conclusion from the AMPAR/NMDAR ratio experiment in the present study and with a previous study demonstrating anti-Hebbian LTP in VTA DA neurons in developing mice using spermine-free internal solution (Mameli et al, 2011).
In the present study, we have demonstrated the CP-AMPAR-dependent, anti-Hebbian form of LTP induced by pairing presynaptic stimulation and postsynaptic hyperpolarization in PE animals. One interesting observation is that NMDAR antagonist APV administration induces a decrease in LTP amplitude in PE animals suggesting that NMDARs might have a minor role in anti-Hebbian LTP induction. Future studies are needed to define the role of NMDARs in detail. We also find that in PE animals, CP-AMPAR-dependent LTP can be induced with only presynaptic stimulation or pairing presynaptic stimulation with postsynaptic depolarization. However, under these conditions, the level of LTP expression is lower than the level of anti-Hebbian LTP. These observations suggest that in PE animals, the already enhanced excitatory synaptic strength could be prone to further strengthening even in the absence of impulse activity of VTA DA neurons.
The PE-induced increase in CP-AMPAR expression and AMPAR/NMDAR ratio are similar to the effects after exposure to cocaine. However, CP-AMPAR-dependent LTP using STD induction has not been consistently reported. One study reports LTP induction failure after cocaine exposure in rats (Argilli et al, 2008). Another study shows that CP-AMPAR-dependent LTP can be induced when STD LTP induction utilizes postsynaptic hyperpolarization, but not with postsynaptic depolarization after cocaine exposure in mice (Mameli et al, 2011). The cause of the discrepancy is unclear. It is possible that the variation in the level of CP-AMPAR expression caused by different cocaine exposure and LTP induction protocols and animal species involving different developmental time courses could contribute to the success or failure of LTP induction (Argilli et al, 2008; Mameli et al, 2011).
Functional Implications
Increased CP-AMPAR expression and excitatory synaptic strength in VTA DA neurons have long been proposed as a critical mechanism for addictive behaviors. Exposure to psychostimulants, ethanol, or stress has been shown to increase addiction risk as well as CP-AMPAR expression in VTA DA neurons (Bellone and Luscher, 2006; Argilli et al, 2008). Results from behavioral studies demonstrate that enhancing GluRA1 expression that could facilitate CP-AMPAR expression in the VTA leads to increased responding to drug reward (Carlezon et al, 2000), whereas blocking glutamate receptors in the VTA during psychostimulant pre-exposure prevents enhanced psychostimulant self-administration (Suto et al, 2003). Therefore, PE-induced increase in addiction risk (Baer et al, 1998, 2003; Famy et al, 1998; Alati et al, 2006) could be mediated by enhanced excitatory synaptic strength caused by increased CP-AMPAR expression.
How can increased CP-AMPAR expression and the resulting augmentation in excitatory synaptic strength/CP-AMPAR-dependent LTP alter the impulse activity and function of VTA DA neurons? Several studies have investigated the spontaneous impulse activity of VTA DA neurons after PE. An in vitro patch clamp study (Wang et al, 2006) shows that PE does not alter the impulse activity of VTA DA neurons. Conversely, a series of in vivo studies demonstrate that PE persistently decreases the impulse activity in VTA DA neurons (Shen et al, 1999; Xu and Shen, 2001; Choong and Shen, 2004a; Shen and Choong, 2006). Interestingly, the decreased activity is reflected in the number of VTA DA neurons displaying spontaneous impulse activity (the number of spontaneously active VTA DA neurons). In other words, fewer VTA DA neurons are firing action potentials. However, the firing rate of these spontaneously active neurons remains unchanged. These observations are similar to that observed after repeated antipsychotic treatment first reported by Grace and Bunney (Bunney and Grace, 1978; Grace et al, 1997), who demonstrate that the reduced number of spontaneously active DA neurons is caused by over-excitation instead of inhibition, leading to depolarization block and the cessation of impulse activity in a proportion of VTA DA neurons. Such a conclusion is inferred from the observations that the decreased number of spontaneously active VTA DA neurons can be reversed (normalized) by enhancing the inhibitory tone to these neurons, such as acute apomorphine administration that activates D2-like somatodendritic autoreceptors. Using the same strategy, it is also demonstrated that PE-induced decrease in the spontaneously active VTA DA neurons is indeed caused by over-excitation/depolarization block (Shen et al, 1999; Shen and Choong, 2006).
An interesting observation is that acute psychostimulant administration can also provide the inhibitory tone and normalize VTA DA neuron impulse activity in vivo in PE animals (Choong and Shen, 2004b; Xu and Shen, 2001), resulting in a net increase in impulse activity which could lead to augmented impulse-dependent DA release in the terminal area. Therefore, the over-excitation in VTA DA neurons might contribute to the increased sensitivity/responding to drugs of abuse and enhanced addiction risk after PE. In PE animals, depolarization block/decreased number of spontaneously active VTA DA neurons begins when rats approach 4 weeks of age and is observable in 16-month-old rats (Shen et al, 1999; Choong and Shen, 2004a). The increased CP-AMPAR expression is detected in 2–12-week-old PE rats in the present study. In addition, we demonstrate in the present study that increased CP-AMPAR expression leads to enhancement in CP-AMPAR-dependent, anti-Hebbian LTP which can be induced in the absence of impulse activity. Therefore, increased CP-AMPAR expression could contribute to the development and maintenance of depolarization block/over-excitation in VTA DA neurons, leading to increased responses to psychostimulants and enhanced addiction risk. Future studies utilizing pharmacological agents capable of normalizing CP-AMPARs (eg, mGluR1 agonists) can further confirm the role of CP-AMPARs in VTA DA neuron function and addictive behaviors.
It is worth noting that over-excitation/decreased number of spontaneously active VTA DA neurons is also a persistent effect following repeated exposure to drugs of abuse such as amphetamine, cocaine, methylphenidate, nicotine, and ethanol during adolescence and adulthood in rats (Shen and Choong, 2006; Shen et al, 2007). The effect is observable 60 days after the cessation of drug treatment and could be a result of a time-dependent increase in the excitation in VTA DA neurons. Supporting evidence for the time-dependent increase in excitation comes from a transient increase in firing rate and number of spontaneously active VTA DA neurons observed within 14 days after withdrawal from repeated amphetamine or cocaine treatment, or cocaine self-administration in rats (White and Wang, 1984; Henry et al, 1989; Marinelli et al, 2003). This transient increase in VTA DA neuron activity is linked to enhanced addiction risk (for review see Wolf and Tseng, 2012). Interestingly, a later study demonstrates that after repeated methylphenidate treatment, the activity of VTA DA neurons increases first followed by depolarization inactivation/reduced number spontaneously active neurons after prolonged withdrawal (>30 days), during which the firing rate of the remaining spontaneously active VTA DA neurons remains unchanged (Shen and Choong, 2006). This study demonstrates that, instead of a transient increase in excitation, the excitation in VTA DA neurons could continue to increase and parallel the time course of increased excitatory synaptic strength reported following prolonged withdrawal from cocaine self-administration in rats (Chen et al, 2008) and mice (Mameli et al, 2009).
Taken together, the increased CP-AMPAR expression could be a critical and general cellular mechanism for increased addiction risk. The development of pharmacological tools aiming at normalizing CP-AMPAR expression in VTA DA neurons might be a useful approach to ameliorate addiction risk in highly vulnerable individuals.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
The work is supported by AA12435 (RS) and AA019482 (RS). We thank Ms Marita Paredez and Mr Ruixang Wang for technical support.
References
- Abel EL. Prenatal effects of alcohol. Drug Alcohol Depend. 1984;14:1–10. doi: 10.1016/0376-8716(84)90012-7. [DOI] [PubMed] [Google Scholar]
- Alati R, Al Mamun A, Williams GM, O'Callaghan M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Arch Gen Psychiatry. 2006;63:1009–1016. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol. 1998;59:533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60:377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Barbier E, Houchi H, Warnault V, Pierrefiche O, Daoust M, Naassila M. Effects of prenatal and postnatal maternal ethanol on offspring response to alcohol and psychostimulants in long evans rats. Neuroscience. 2009;161:427–440. doi: 10.1016/j.neuroscience.2009.03.076. [DOI] [PubMed] [Google Scholar]
- Barbier E, Pierrefiche O, Vaudry D, Vaudry H, Daoust M, Naassila M. Long-term alterations in vulnerability to addiction to drugs of abuse and in brain gene expression after early life ethanol exposure. Neuropharmacology. 2008;55:1199–1211. doi: 10.1016/j.neuropharm.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Bassani S, Valnegri P, Beretta F, Passafaro M. The GLUR2 subunit of AMPA receptors: synaptic role. Neuroscience. 2009;158:55–61. doi: 10.1016/j.neuroscience.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Bellone C, Mameli M, Luscher C. In utero exposure to cocaine delays postnatal synaptic maturation of glutamatergic transmission in the VTA. Nat Neurosci. 2011;14:1439–1446. doi: 10.1038/nn.2930. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Hannigan JH, Riley EP. Amphetamine-induced activity after fetal alcohol exposure and undernutrition in rats. Neurotoxicol Teratol. 1987;9:113–119. doi: 10.1016/0892-0362(87)90087-0. [DOI] [PubMed] [Google Scholar]
- Blanco E, Bilbao A, Luque-Rojas MJ, Palomino A, Bermudez-Silva FJ, Suarez J, et al. Attenuation of cocaine-induced conditioned locomotion is associated with altered expression of hippocampal glutamate receptors in mice lacking LPA1 receptors. Psychopharmacology (Berl) 2012;220:27–42. doi: 10.1007/s00213-011-2446-6. [DOI] [PubMed] [Google Scholar]
- Bond NW. Prenatal alcohol exposure in rodents: a review of its effects on offspring activity and learning ability. Aust J Psychol. 1981;33:331–344. [Google Scholar]
- Brill J, Huguenard JR. Sequential changes in AMPA receptor targeting in the developing neocortical excitatory circuit. J Neurosci. 2008;28:13918–13928. doi: 10.1523/JNEUROSCI.3229-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BS, Grace AA. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci. 1978;23:1715–1727. doi: 10.1016/0024-3205(78)90471-x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, et al. Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. J Neurosci. 2000;20:RC62. doi: 10.1523/JNEUROSCI.20-05-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong K, Shen RY. Prenatal ethanol exposure alters the postnatal development of the spontaneous electrical activity of dopamine neurons in the ventral tegmental area. Neuroscience. 2004;126:1083–1091. doi: 10.1016/j.neuroscience.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Choong KC, Shen RY. Methylphenidate restores ventral tegmental area dopamine neuron activity in prenatal ethanol-exposed rats by augmenting dopamine neurotransmission. J Pharmacol Exp Ther. 2004;309:444–451. doi: 10.1124/jpet.103.060657. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famy C, Streissguth AP, Unis AS. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am J Psychiatry. 1998;155:552–554. doi: 10.1176/ajp.155.4.552. [DOI] [PubMed] [Google Scholar]
- Good CH, Lupica CR. Afferent-specific AMPA receptor subunit composition and regulation of synaptic plasticity in midbrain dopamine neurons by abused drugs. J Neurosci. 2010;30:7900–7909. doi: 10.1523/JNEUROSCI.1507-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS, Moore H, Todd CL. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends Neurosci. 1997;20:31–37. doi: 10.1016/S0166-2236(96)10064-3. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. Regulation of plasticity of glutamate synapses by endocannabinoids and the cyclic-AMP/protein kinase A pathway in midbrain dopamine neurons. J Physiol. 2010;588 (Pt 14:2589–2604. doi: 10.1113/jphysiol.2010.190066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XP, Patel M, Whitney KD, Janumpalli S, Tenner A, McNamara JO. Glutamate receptor GluR3 antibodies and death of cortical cells. Neuron. 1998;20:153–163. doi: 10.1016/s0896-6273(00)80443-2. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–175. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J Pharmacol Exp Ther. 1989;251:833–839. [PubMed] [Google Scholar]
- Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Goodlett CR, Hulsether SA, West JR. Impaired spatial navigation in adult female but not adult male rats exposed to alcohol during the brain growth spurt. Behav Brain Res. 1988;27:247–257. doi: 10.1016/0166-4328(88)90121-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science. 2007;315:1262–1266. doi: 10.1126/science.1137450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi I, Kang S, Rivier C. Role of various neurotransmitters in mediating the long-term endocrine consequences of prenatal alcohol exposure. Ann N Y Acad Sci. 2008;1144:176–188. doi: 10.1196/annals.1418.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Cull-Candy SG. Activity-dependent change in AMPA receptor properties in cerebellar stellate cells. J Neurosci. 2002;22:3881–3889. doi: 10.1523/JNEUROSCI.22-10-03881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Malanga CJ, Kosofsky BE. Does drug abuse beget drug abuse? Behavioral analysis of addiction liability in animal models of prenatal drug exposure. Brain Res Dev Brain Res. 2003;147:47–57. doi: 10.1016/j.devbrainres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Mameli M, Bellone C, Brown MT, Luscher C. Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat Neurosci. 2011;14:414–416. doi: 10.1038/nn.2763. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Mao D, Gallagher K, McGehee DS. Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J Neurosci. 2011;31:6710–6720. doi: 10.1523/JNEUROSCI.5671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons. J Physiol. 2006;577 (Pt 3:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Cooper DC, Baker LK, White FJ. Impulse activity of midbrain dopamine neurons modulates drug-seeking behavior. Psychopharmacology (Berl) 2003;168:84–98. doi: 10.1007/s00213-003-1491-1. [DOI] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berl) 1997;129:277–284. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature. 1999;401:594–598. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shen RY, Choong KC. Different adaptations in ventral tegmental area dopamine neurons in control and ethanol exposed rats after methylphenidate treatment. Biol Psychiatry. 2006;59:635–642. doi: 10.1016/j.biopsych.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Shen RY, Choong KC, Thompson AC. Long-term reduction in ventral tegmental area dopamine neuron population activity following repeated stimulant or ethanol treatment. Biol Psychiatry. 2007;61:93–100. doi: 10.1016/j.biopsych.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Shen RY, Hannigan JH, Kapatos G. Prenatal ethanol reduces the activity of adult midbrain dopamine neurons. Alcoholism Clin Exp Res. 1999;23:1801–1807. [PubMed] [Google Scholar]
- Shetty AK, Burrows RC, Phillips DE. Alterations in neuronal development in the substantia nigra pars compacta following in utero ethanol exposure: immunohistochemical and Golgi studies. Neuroscience. 1993;52:311–322. doi: 10.1016/0306-4522(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Shin J, Shen F, Huguenard JR. Polyamines modulate AMPA receptor-dependent synaptic responses in immature layer v pyramidal neurons. J Neurophysiol. 2005;93:2634–2643. doi: 10.1152/jn.01054.2004. [DOI] [PubMed] [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in an NMDA, AMPA/kainate, and metabotropic glutamate receptor-dependent manner. Neuropsychopharmacology. 2003;28:629–639. doi: 10.1038/sj.npp.1300075. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Sather TM, Whinery LA. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav Neurosci. 2008;122:1264–1273. doi: 10.1037/a0013271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Haj-Dahmane S, Shen RY. Effects of prenatal ethanol exposure on the excitability of ventral tegmental area dopamine neurons in vitro. J Pharmacol Exp Ther. 2006;319:857–863. doi: 10.1124/jpet.106.109041. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Wang RY. Electrophysiological evidence for A10 dopamine autoreceptor subsensitivity following chronic D-amphetamine treatment. Brain Res. 1984;309:283–292. doi: 10.1016/0006-8993(84)90594-8. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why. Front Mol Neurosci. 2012;5:1–16. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Shen RY. Amphetamine normalizes the electrical activity of dopamine neurons in the ventral tegmental area following prenatal ethanol exposure. J Pharmacol Exp Ther. 2001;297:746–752. [PubMed] [Google Scholar]
- Yuan T, Mameli M, O' Connor EC, Dey PN, Verpelli C, Sala C, et al. Expression of cocaine-evoked synaptic plasticity by GluN3A-containing NMDA receptors. Neuron. 2013;80:1025–1038. doi: 10.1016/j.neuron.2013.07.050. [DOI] [PubMed] [Google Scholar]