Abstract
Few options are available for treating patients with advanced prostate cancer (PC). As PC is a slow growing disease and accessible by ultrasound, gene therapy could provide a viable option for this neoplasm. Conditionally replication-competent adenoviruses (CRCAs) represent potentially useful reagents for treating PC. We previously constructed a CRCA, cancer terminator virus (CTV), which showed efficacy both in vitro and in vivo for PC. The CTV was generated on a serotype 5-background (Ad.5-CTV) with infectivity depending on Coxsackie-Adenovirus Receptors (CARs). CARs are frequently reduced in many tumor types, including PCs thereby limiting effective Ad-mediated therapy. Using serotype chimerism, a novel CTV (Ad.5/3-CTV) was created by replacing the Ad.5 fiber knob with the Ad.3 fiber knob thereby facilitating infection in a CAR-independent manner. We evaluated Ad.5/3-CTV in comparison with Ad.5-CTV in low CAR human PC cells, demonstrating higher efficiency in inhibiting cell viability in vitro. Moreover, Ad.5/3-CTV potently suppressed in vivo tumor growth in a nude mouse xenograft model and in a spontaneously induced PC that develops in Hi-myc transgenic mice. Considering the significant responses in a Phase I clinical trial of a non-replicating Ad.5-mda-7 in advanced cancers, Ad.5/3-CTV may exert improved therapeutic benefit in a clinical setting.
Prostate cancer (PC) is the most frequently diagnosed cancer and is the second leading cause of cancer death among men in the United States (Damber and Aus, 2008; Siegel et al., 2012). It is estimated that 238,590 new PC cases will be diagnosed in 2013 and 29,720 men will die of PC. Patients with localized disease may be treated with surgery or radiation, whereas the treatment options for patients with metastatic disease is purely palliative. Current therapies include hormonal therapy, radiotherapy, and cytotoxic chemotherapeutic agents (Sternberg, 2002; Siegel et al., 2012). Although existing approaches are beneficial in men with various stages of PC, the complications frequently associated with these conventional treatment options diminish positive clinical outcomes. Consequently, more efficient and innovative treatments are mandatory, and genetic therapies represent promising approaches for the treatment of this neoplasm.
Using subtraction hybridization combined with induction of cancer cell terminal differentiation, our laboratory cloned melanoma differentiation-associated gene-7/interleukin-24 (mda-7/IL-24) (Jiang et al., 1995, 1996), a novel member of the IL-10-related cytokine gene family (Sarkar et al., 2002a; Wolk et al., 2002; Sauane et al., 2003; Dash et al., 2010a). Subsequent studies documented that mda-7/IL-24 displays almost ubiquitous anti-tumor properties in vitro and in vivo, leading to its rapid entry into the clinic, where its safety and clinical efficacy, when administered by adenovirus (Ad.mda-7; INGN 241), was observed in a phase I clinical trial in humans with advanced carcinomas and melanomas (Jiang et al., 1996; Fisher et al., 2003, 2007; Cunningham et al., 2005; Tong et al., 2005; Lebedeva et al., 2007a,c). mda-7/IL-24 preferentially induces apoptosis in cancer cells while exerting no discernible toxic effects toward normal cells (Sarkar et al., 2002b; Sauane et al., 2008; Dash et al., 2010a, 2011a) and it also elicits potent “anti-tumor bystander activity” in distant cancer cells as a consequence of autocrine and paracrine secretion of MDA-7/IL-24 (Su et al., 2001a, 2005a; Fisher, 2005; Lebedeva et al., 2007a,b; Sauane et al., 2008; Dash et al., 2010b).
Since PC is generally a relatively slow-growing disease, it may require repeated gene therapy treatments, with single or multiple genes, over the lifespan of the patient. Conditionally replication-competent adenoviruses (CRCAs) provide a potentially valuable reagent for gene therapy (Curiel and Fisher, 2012). Using subtraction hybridization we cloned a novel rodent gene, progression elevated gene-3 (PEG-3), in the context of tumor progression in transformed rat embryo cells (Su et al., 1997). PEG-3: (i) displays elevated expression as a function of oncogenic transformation (by diverse oncogenes) (Su et al., 1997, 2002); (ii) induces an aggressive cancer phenotype without promoting transformation when expressed in normal cells (Su et al., 1999, 2002); and (iii) the gene promoter (PEG-Prom) has been isolated and shown to display elevated expression in both rodent and human tumors (including PC), with negligible expression in normal cells (including human prostate epithelium) (Su et al., 2001b, 2005b; Sarkar et al., 2005a,b, 2006a,b, 2007b, 2008; Bhang et al., 2011; Das et al., 2012). Considering the cancer-specific expression aspects of the PEG-Prom, we constructed a bipartite serotype 5 CRCA (called a cancer terminator virus, Ad.5-CTV) in which the expression of E1A and E1B genes of Ad, necessary for replication, is controlled by the PEG-Prom (Sarkar et al., 2005a, 2006, 2007b, 2008). This novel Ad.5-CTV also expressed mda-7/IL-24 from the E3 region (Ad.PEG-E1A-mda-7). The ability of Ad.5-CTV to infect and express MDA-7/IL-24 in PC cells depends on the presence of Coxsackie-Adenovirus Receptors (CARs) on their surface. Ad.5-CTV is capable of efficiently infecting high CAR cells (such as DU-145) and expressing robust levels of mda-7/IL-24, whereas infection is restricted and expression of MDA-7/IL-24 is minimal in low CAR cells, such as PC-3 (Dash et al., 2010b, 2011b).
An approach to circumvent the low efficiency of Ad.5 infection of tumor cells involves “tropism modification” in which virus capsid proteins that normally associate with CAR are modified, permitting both CAR-dependent and CAR-independent infectivity of tumor cells. Studies using various tumor cell types have shown that inclusion of the infective type 3 Ad sequence within the Ad type 5 virus knob (Ad.5/3 recombinant virus) promotes viral infectivity in tumor cells displaying reduced or no CAR expression (Hamed et al., 2010; Dash et al., 2010b; Eulitt et al., 2011; Park et al., 2011; Azab et al., 2012). It is worth noting that Ad.5/3 also retains high infectivity in CAR-expressing tumor cells showing equal efficacy when compared with Ad.5, thereby providing an expanded range of utility for Ad.5/3, in both low and high CAR-expressing tumor cells.
In the present study, we constructed and evaluated the in vitro and in vivo efficacy in low and high CAR PCs of a novel tropism-modified CTV in which the virus capsid proteins that normally associate with CAR were modified, Ad.5/3-CTV, permitting CAR-independent infectivity of tumor cells. In low CAR PC-3 cells, Ad.5/3-CTV is more efficient than Ad.5-CTV in infecting tumor cells, delivering a transgene (mda-7/IL-24), expressing MDA-7/IL-24 protein and inducing cancer-specific apoptosis. In an in vivo context, Ad.5/3-CTV is superior to the Ad.5-CTV in inhibiting in vivo tumor growth and exerting an anti-tumor “bystander” effect in nude mouse human PC xenografts and Ad.5/3-CTV potently suppresses PC development in an immunocompetent Hi-Myc transgenic mouse model of PC.
Materials and Methods
Cell lines, culture conditions, and viability assays
DU-145 and PC-3 PC cells were obtained from the American Type Culture Collection and cultured as described (Lebedeva et al., 2003). Construction and characterization of PC-3 over-expressing Bcl-2, PC-3-Bcl-2, and control clones containing the neomycin vector, PC-3-Neo, were described previously (Lebedeva et al., 2003). Cell viability was determined by standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays (Lebedeva et al., 2003). Cell cultures were routinely tested for mycoplasma using a kit from Sigma (MP-0025) and only mycoplasma free cells were used for these studies.
Construction of Ad.5/3-CTV
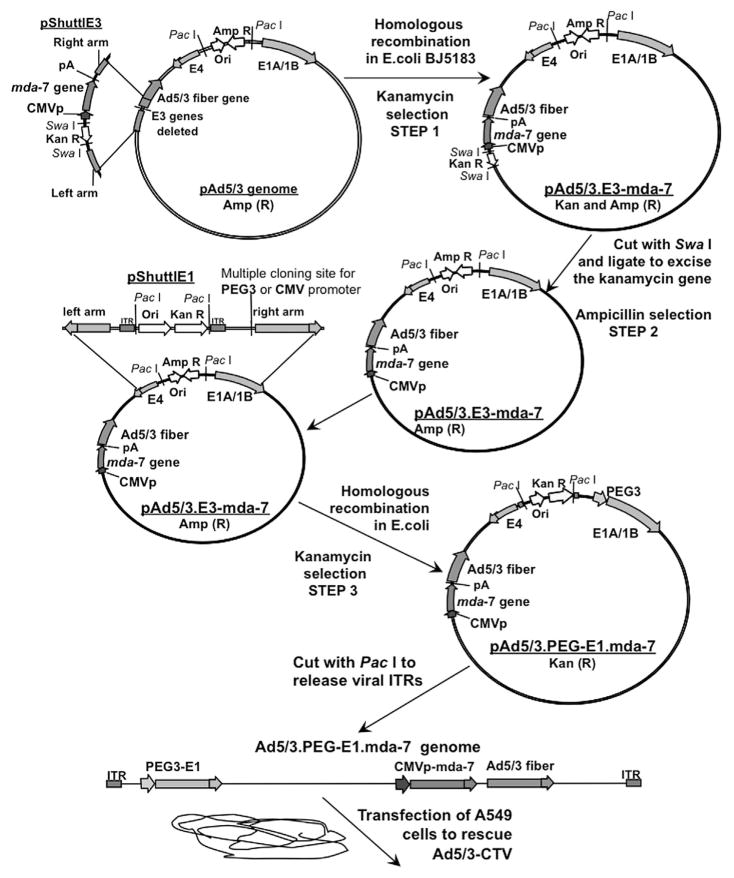
The genome of Ad.5/3-PEG-E1-mda-7 was generated in three consecutive steps (Fig. 1). (1) Homologous recombination of pAd.5/3 genomic plasmid with pShuttlE3 plasmid containing the mda-7/IL-24 expression cassette and kanamycin selection results in the pAd.5/3-E3-mda-7 genome. (2) pAd.5/3-E3-mda-7 was cut with SwaI to excise the kanamycin resistance gene. (3) The resultant pAd.5/3-E3-mda-7 plasmid was recombined with pShuttlE1 plasmid containing E1A and E1B genes under control of the PEG-3 promoter resulting in Ad.5/3-PEG-E1-mda-7 (Ad.5/3-CTV) genomic plasmid. This plasmid was digested with PacI to release viral ITRs and transfected in A549 cells to rescue the CRCA, Ad.5/3-CTV.
Fig. 1.
Generation of a tropism-modified cancer terminator virus (Ad.5/3-CTV). Schematic representation outlining the construction of a tropism-modified cancer terminator virus for delivery of mda-7/IL-24. The detailed procedures are described in Materials and Methods Section.
Preparation of whole-cell lysates and Western blot analyses
Preparation of whole-cell lysates and Western blot analyses were performed as previously described (Sarkar et al., 2005b). The primary antibodies used were anti-MDA-7/IL-24 (Gen Hunter Corporation, Nashville, TN), anti-EF1α (1:1,000; mouse mono-clonal; Millipore, Lake Placid, NY), anti-Mcl-1 (1:500; mouse monoclonal; Santa Cruz, Santa Cruz, CA), anti-BiP/GRP78 (1:500; rabbit monoclonal; Santa Cruz, CA), anti-GRP94 (1:1,000; rabbit monoclonal; Sigma, St Louis, MO), and anti-PARP (1:1,000; rabbit monoclonal; Cell Signaling, Danvers, MA).
Human PC xenografts in athymic nude mice
PC-3-Bcl-2 cells (2 × 106) were injected s.c. in 100 μl of 1:1 PBS and Matrigel in the left and right flanks of male athymic nude mice (NCRnu/nu, 6–8 weeks old, ~20 g body weight) (Sarkar et al., 2005a). After establishing palpable tumors of ~100 mm3, requiring ~7–10 days, intratumoral injections of different Ads were given only to the tumors on the left flank at a dose of 1 × 1010 viral particles in 100 μl. The injections were given twice a week for four weeks. A minimum of five animals was used per experimental point. Tumor volume was calculated using the formula: π/6 × larger diameter × (smaller diameter)2. At the end of the experiment, the animals were sacrificed, and the tumors were removed and weighed.
Hi-Myc mice and animal husbandry protocols
The VCU Institutional Animal Care and Use Committee approved the experimental protocol used in this study and the animals were cared for in accordance with institutional guidelines. This study used Hi-Myc transgenic mice in which prostate-specific expression of human c-Myc is driven by the rat probasin promoter with two androgen response elements (ARR2/probasin promoter) (Ellwood-Yen et al., 2003). Mice were obtained from the Mouse Repository of the National Cancer Institute Mouse Models of Human Cancer Consortium at NCI Frederick, MD, USA. Mouse-tail DNA was isolated using the DNeasy Blood & Tissue Kit from QIAGEN (Valencia, CA) and subjected to a PCR-based screening assay for genotyping. For genotyping Hi-Myc mice, the upstream primer (located within the ARR2-PB promoter), 5′-AAACATGATGACTACCAAGCTTGGC-3′ and the downstream primer (within the MYC cDNA sequence) 5′-ATGATAGCATCTTGTTCTTAGTCTTTTTCTTAATAGGG-3′ were used to generate a PCR product of 177 base pairs.
Preparation of microbubbles (MBs), ultrasound (US) platform, ultrasound-targeted microbubble destruction (UTMD), and BI-97C1 (Sabutoclax)
Preparation of MBs followed by UTMD for delivery of mda-7/IL-24 expressing Ads has been described previously (Dash et al., 2011a). Targeson (Targeson) custom synthesis US contrast agent (perfluorocarbon MBs, encapsulated by a lipid coat and poly (ethyleneglycol) stabilizer) were obtained. MBs were reconstituted in the presence or absence of 1 ml of 1 × 1011 viral particles of indicated Ads and unenclosed surface-associated Ads were treated with complement as previously described (Cianfriglia et al., 1999; Howard et al., 2006). For in vivo experiments, US exposure was achieved with a Micro-Maxx SonoSite (SonoSite, Oceanside, CA) US machine equipped with the transducer L25 set at 0.7 Mechanical Index, 1.8 MPa for 10 min. Mice were sedated in an IMPAQ6 anesthesia apparatus (VetEquip, Pleasanton, CA) that was saturated with 3–5% isofluorane and 10–15% oxygen with the aid of a precision vaporizer to deliver the appropriate amount of anesthetic and to induce anesthesia. For MB/Ad injection a 27-gauge needle with a heparin lock was placed within a lateral tail vein for administration of contrast material. The mice received injections of 100 μl of MBs with Ads through the tail vein eight times in the span of 4 weeks. Ultrasound (sonoporation) was performed with a SonoSite scanner (SonoSite) equipped with the transducer L25 set at 0.7 Mechanical Index, 1.8 MPa for 10 min in the ventral side of mice in the prostatic area. BI-97C1 (Sabutoclax) was administered intraperitoneally at a dose level of 3 mg/kg three times a week for the duration of the study (total 12 injections). Compounds dissolved in 500 μl of solvent (ethanol/Cremophor EL/saline = 10:10:80) were injected intraperitoneally. At the end of the experiment, the Hi-Myc mice were sacrificed and the prostate was dissected. The harvested prostate was preserved in neutral buffered formalin at 4°C before embedding in paraffin for immunohistochemical analysis.
Immunohistochemical staining
For immunohistochemical (IHC) analysis, formalin-fixed and paraffin-embedded specimens were sectioned 3–4-μm thick. Sections were deparaffinized, re-hydrated and then quenched in 3% H2O2 for 20 min. Sections were washed with PBS and blocked in PBS containing 1% BSA for 20 min at 37°C. Monoclonal anti-MDA-7/IL-24 (1:200) was incubated for 3 h at room temperature and then washed three times in PBS. Sections were incubated with an avidin–biotin–peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) and then washed two times in PBS. The immunoreactivity was determined using diaminobenzidine (DAB) as the final chromogen. Finally, sections were counterstained with Meyer’s Hematoxylin, dehydrated through a sequence of increasing concentrations of alcohol, cleared in xylene and mounted with epoxydic medium. Sections were also processed for hematoxylin and eosin (H&E) staining.
Determination of apoptotic cells by TUNEL assay
For TUNEL assays, we used the DeadEnd Colorimetric TUNEL Assay kit (Promega, Madison, WI) performed according to the manufacturer’s instructions. Briefly, paraffin-embedded slides were deparaffinized and rehydrated. Pre-equilibrated slides were labeled with a labeling DNA-strand break solution containing a biotinylated nucleotide mix (60 min at 37°C). After several washes in two times SSC and PBS, slides were blocked with hydrogen peroxide (3–5 min at room temperature). After several washes in PBS, the slides were mounted with mounting solution with DAPI. Apoptotic cells on the slides were observed under an Olympus epifluorescence microscope (X10 magnification; Olympus, Center Valley, PA) in randomly chosen fields. For detection of apoptosis in a time-dependent manner in vitro, PC-3 cells were grown in microscopic slide culture chambers (BD Bioscience, San Jose, CA) and cells were treated with Ad.5/3-CTV and BI-97C1 (Sabutoclax, Sanford Burnham Medical Research Institute, La Jolla, CA) after which the cells were fixed with 4% formaldehyde at the indicated time and TUNEL assays were performed as per the manufacturer’s instruction using an Olympus epifluorescence microscope (X10 magnification; Olympus, Center Valley, PA) (Dash et al., 2011a).
Statistical analysis
Statistical analysis was done using Student’s t-test, followed by Fisher’s protected least significant difference analysis. P < 0.05 was considered significant.
Results
Ad.5/3-CTV displays enhanced mda-7/IL-24 expression and inhibition of cell viability in low CAR PC cells
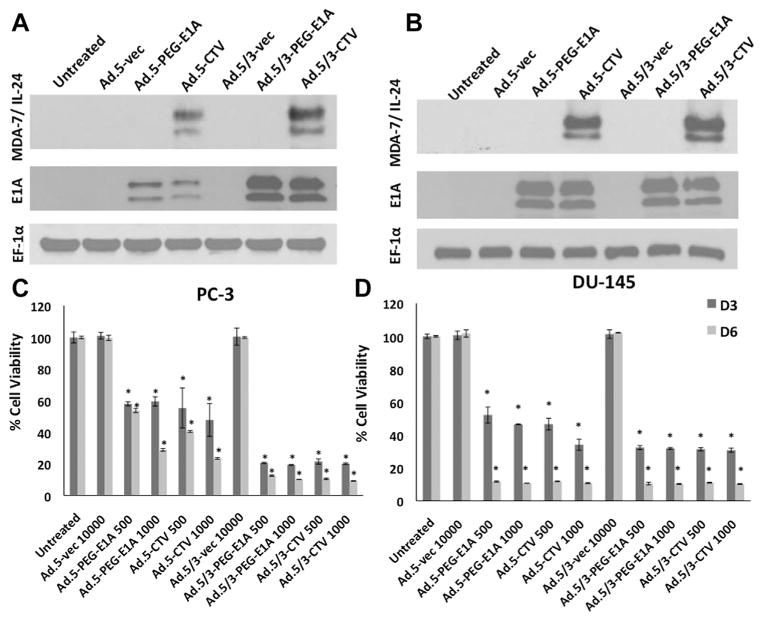
The scheme for constructing Ad.5/3-CTV, in which viral replication is controlled by the PEG-Prom and which also expresses mda-7/IL-24 in an Ad.5/3 background, is shown in Figure 1 and described in detail in Materials and Methods Section. As controls we used Ad.5-vec (replication-incompetent empty Ad.5), Ad.5/3-vec (replication-incompetent empty Ad.5/3), Ad.5-PEG-E1A, in which viral replication is controlled by the PEG-Prom in an Ad.5 background, and Ad.5/3-PEG-E1A, in which viral replication is controlled by the PEG-Prom in an Ad.5/3 background. We compared MDA-7/IL-24 expression upon infection of Ad.5/3-CTV and Ad.5-CTV in PC cells that contain low or high CAR on their surface. For this purpose, we used PC-3, which have a reduced level of CAR (D value 0.32) in comparison with DU-145, which express a high level of CAR (D value 0.92) (Lebedeva et al., 2003; Dash et al., 2010b). In PC-3, MDA-7/IL-24 expression was significantly higher upon infection with Ad.5/3-CTV, as compared to Ad.5-CTV, whereas infection with both Ad.5/3-CTV and Ad.5-CTV in DU-145 resulted in comparable expression of MDA-7/IL-24 protein (Fig. 2A and B). Infection with control Ad vectors did not result in MDA-7/IL-24 expression. These findings indicate that infection with Ad.5/3-CTV promotes enhanced transgene delivery in low CAR containing PC cells compared to Ad.5-CTV, and both CTVs are comparable in transgene delivery in high CAR PC cells. An analogous finding was evident when analyzing Ad replication by monitoring E1A protein levels in PC-3 and DU-145 cells (Fig. 2A and B). In DU-145 cells, a similar pattern of virus replication was apparent following infection with Ad.5 or Ad.5/3 background viruses, while in PC-3 cells replication of Ad.5/3 was significantly elevated in comparison with infection with Ad.5 (Fig. 2A and B).
Fig. 2.
Ad.5/3-CTV enhances mda-7/IL-24 expression and inhibition of cell viability in low CAR prostate cancer cells. A and B: DU-145 and PC-3 cells were infected with the indicated vp/cell of Ad.5-vec, Ad.5-PEG-E1A, Ad.5-CTV, Ad.5/3-vec, Ad.5/3-PEG-E1A, and Ad.5/3-CTV for 48 h and total proteins were isolated. The expression of MDA-7/IL-24 (23.8 kDa protein, with 35–40 kDa glycosylated species detected on the gel), E1A and EF-1α (as a loading control) proteins were analyzed by Western blot analyses. C and D: Cell viability using the MTT assay was quantified after 3 and 6 days with the indicated doses of vp/cell of Ad.5-CTV, Ad.5/3-CTV and their respective controls. Results are the mean ± SD (n = 3) *P < 0.05.
The efficacy of Ad.5/3-CTV and Ad.5-CTV in reducing cell proliferation of PC cells was evaluated in vitro by MTT assays. Ad.5/3-CTV infection resulted in enhanced reduction in the viability of PC-3 cells as compared to Ad.5-CTV infection at m.o. i.’s of 500 and 1,000 VP/cell on day 3-post and day 6-post infection (Fig. 2C). In DU-145 cells both Ad.5/3-CTV and Ad.5-CTV showed parallel efficiencies in reducing growth when assayed using equivalent viral titers and evaluated at parallel time points (Fig. 2D). It should be noted that Ad.5-PEG-E1A and Ad.5/3-PEG-E1A was as effective as Ad.5-CTV and Ad.5/3-CTV in reducing cell viability in both cell lines indicating that the profound effect of Ad replication in inhibiting cell viability might mask the in vitro growth inhibitory effects of mda-7/IL-24. In these contexts, in vivo evaluation of CTV is mandatory to confirm the “anti-tumor bystander” effect exerted by MDA-7/IL-24.
Ad.5/3-CTV, but not Ad.5-CTV, induces ER stress and apoptosis, and overcomes therapy resistance in PC-3-Bcl-2 tumor cells
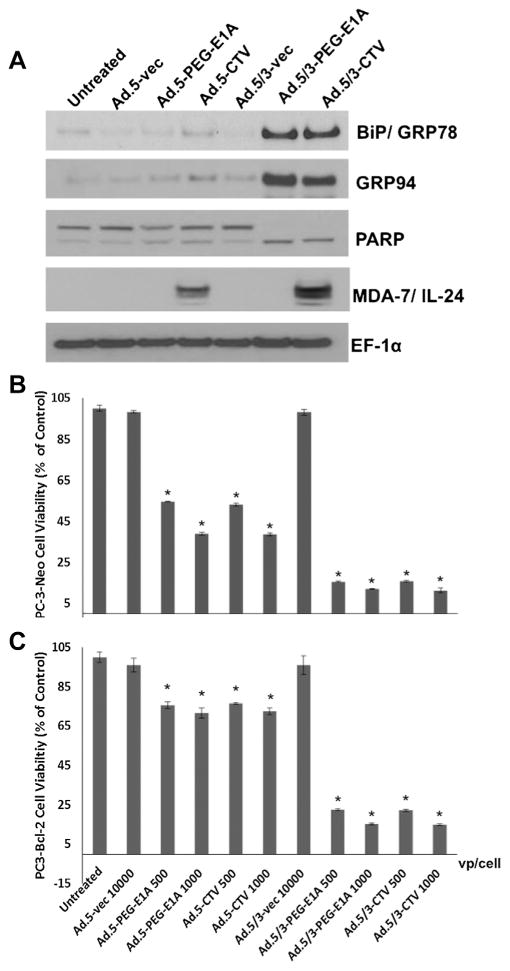
We next analyzed the expression of mda-7/IL-24-downstream genes and signals that confer its tumor suppressor properties upon infection with Ad.5/3-CTV and Ad.5-CTV in low CAR PC-3 cells. Ad.5/3-CTV induces an ER stress response (unfolded-protein response) and we therefore determined the expression levels of ER-stress markers. In PC-3-Neo cells, the levels of BiP/GRP78 and GRP94 were significantly higher upon infection with Ad.5/3-CTV as compared with Ad.5-CTV. Ad.5/3-CTV also efficiently induced apoptosis as evidenced by increased cleavage of PARP (Fig. 3A). It should be noted, that infection with the conditionally replication competent Ad.5/3, Ad.5/3-PEG-E1A, also induced a stress response, as did Ad.5/3-CTV, as indicated by enhanced BiP/GRP78, GRP94 and PARP cleavage (Fig. 3A). However, this effect was not evident with Ad.5-PEG-E1A or Ad.5-CTV infection.
Fig. 3.
Ad.5/3-CTV, but not Ad.5-CTV, induce ER stress and apoptosis, and overcome therapy resistance in PC-3-Bcl-2 tumor cells. A: Changes in BiP/GRP78, GRP94, and activation of PARP were detected by Western blot analysis after 2 days of treatment of PC-3 cells with the indicated Ads. B + C: Cell viability using the MTT assay was quantified after 6 days with the indicated doses of vp/cell of Ad.5-CTV, Ad.5/3-CTV, and their respective controls. Results are the mean ± SD (n = 3).*P < 0.05 with the Ad.5-vec 10,000 vp/cell-infected group.
The Bcl-2 gene family plays a central role in PC and over expression of Bcl-2 gene family members confers resistance to specific cancer therapeutics (Lebedeva et al., 2003). In this context, we evaluated the efficacy of Ad.5/3-CTV versus Ad.5-CTV in PC-3-Bcl-2 cells (PC-3 cells that stably over express Bcl-2), which are resistant to mda-7/IL-24-mediated killing (Lebedeva et al., 2003). As control, we used PC-3-Neo cells that are stably transformed with the same vector expressing only the neomycin-resistance gene and not the Bcl-2 gene. In MTT assays, Ad.5-PEG-E1A and Ad.5-CTV were less effective in inhibiting cell proliferation (viability) of PC-3-Bcl-2, whereas PC3-Neo cells were sensitive to these viruses (Fig. 3B and C). Accordingly, the effect of Ad.5-CTV was more robust in suppressing growth of PC-3-Neo as compared to PC-3-Bcl-2 cells, whereas the Ad.5/3-CTV displayed equivalent vigorous in vitro anti-proliferative activity in both cell types (Fig. 3B and C). These findings support the enhanced potential therapeutic application of Ad.5/3-CTV versus Ad.5-CTV in PC patients frequently showing Bcl-2 over expression and down-regulation of CAR.
Ad.5/3-CTV eradicates primary and inhibits distant PC-3-Bcl-2 xenografts in nude mice
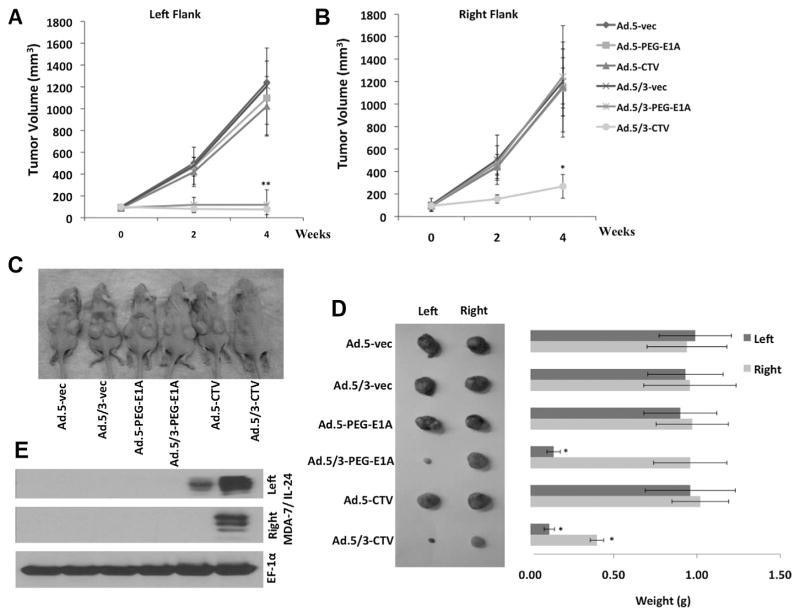
Experiments were performed to determine if the enhanced in vitro activity of Ad.5/3-CTV compared to Ad.5-CTV in low CAR PC-3-Bcl-2 cells translates into enhanced in vivo activity. PC-3-Bcl-2 tumor cells were inoculated in both the right and left flanks of athymic nude mice. After ~7–10 days palpable tumor xenografts of ~100 mm3 developed and the mice received eight intratumoral injections only in the left flank tumors over a 4-week period with 1 × 1010 viral particles per 100 μl. The Ads used for this study included Ad.5-vec, Ad.5/3-vec, Ad.5-PEG-E1A, Ad.5/3-PEG-E1A, Ad.5-CTV, and Ad.5/3-CTV. No injections were administrated to right flank tumors. PC-3-Bcl-2 formed large, aggressive and actively proliferating tumors on both flanks that were not affected by treatment with Ad.5-vec, Ad.5/3-vec, Ad.5-PEG-E1A, or Ad.5-CTV. Although Ad.5/3-PEG-E1A inhibited the growth of tumors on the left flank, it had no effect on the distant tumors on the right flank (Fig. 4A–D). In contrast, Ad.5/3-CTV dramatically inhibited tumor growth on the injected left flank and markedly inhibited tumor growth on the right flank, exceeding the therapeutic effect of any other viral treatment. These results provide definitive evidence for enhanced therapeutic efficacy of Ad.5/3-CTV as compared to Ad.5-CTV in prostate tumor cells with reduced CAR, and highlight the potent “bystander anti-cancer” activity of mda-7/IL-24 resulting in growth inhibition of right-side, non-injected tumors. The effectiveness of Ad.5/3-CTV and Ad.5-CTV in transducing MDA-7/IL-24 in vivo was confirmed by Western blotting using total protein extracts from the harvested tumors and probing with MDA-7/IL-24 antibody. Ad.5/3-CTV generated more MDA-7/IL-24 protein in both flanks, validating the previously reported “bystander anti-tumor” effect of MDA-7/IL-24 (Su et al., 2001a, 2005a; Chada et al., 2004; Lebedeva et al., 2007a; Sarkar et al., 2007b). In contrast, only weak MDA-7/IL-24 protein expression was evident in Ad.5-CTV injected PC-3-Bcl-2 tumor (left flank) and no protein expression was evident on the right flank tumor (Fig. 4E). These findings demonstrate that in low CAR PC cells, Ad.5/3-CTV can generate robust expression of MDA-7/IL-24 protein that is sufficient to inhibit tumor cell proliferation, and exert “bystander anti-tumor” activity mediated by MDA-7/IL-24 in distant tumors.
Fig. 4.
Ad.5/3-CTV eradicates primary and inhibits distant PC-3-Bcl-2 xenografts in nude mice. Tumor xenografts with PC-3-Bcl-2 cells were established in athymic nude mice in both right and left flanks; and only tumors on the left side were injected with the indicated Ads over a 4-week period (total of nine injections). Measurements of PC-3-Bcl-2 xenograft tumor volumes on (A) left and (B) right flanks; points, average (with a minimum of five mice in each group); bars ± SD. Inset contains a photograph of the animals of each representative group. C: Photograph of the PC-3-Bcl-2 xenograft tumor at the end of the study. D: Measurement of tumor weight at the end of the study; columns, mean (with at least five mice in each group); bars ± SD. E: Western blot analysis of protein extracts from representative PC-3-Bcl-2 tumor samples treated with Ad.5-vec, Ad.5/3-vec, Ad.5-PEG-E1A, Ad.5/3-PEG-E1A, Ad.5-CTV, Ad.5/3-CTV. The immunoblot was reacted with anti-MDA-7/IL-24.
Combination treatment of Ad.5/3-CTV and BI-97C1 (Sabutoclax) potentiates inhibition of prostate tumor growth in vivo in immune competent animals
Because PC is a relatively slow-growing disease, repeated systemic gene therapy applications in combination with anti-tumor chemotherapeutic agents over the life span of the patient may be necessary to provide enduring clinical responses (Di Lorenzo and De Placido, 2006; Damber and Aus, 2008). Previous studies demonstrated that BI-97C1 (Sabutoclax), which is a pure optical derivative of Apogossypol (Wei et al., 2010), has significant activity as a single agent against PC cells in vitro and in vivo in nude mouse xenograft studies. Apogossypol derivatives antagonize the anti-apoptotic Bcl-2 family members including Bcl-2 and myeloid cell leukemia-1 (Mcl-1) (Wei et al., 2009). MDA-7/IL-24 induces cancer-specific apoptosis through the translational inhibition of Mcl-1 (Dash et al., 2010c). BI-97C1 (Sabutoclax) sensitizes PC cells to mda-7/IL-24-mediated toxicity in vitro and in vivo (Dash et al., 2011a).
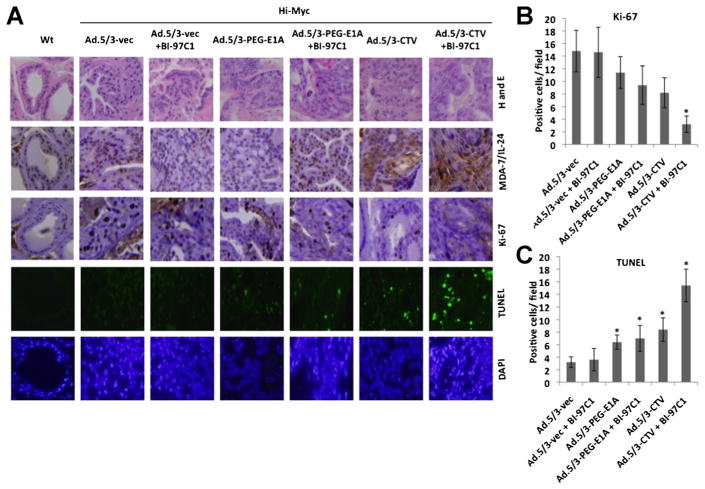
Experiments were performed to determine if Ad.5/3-CTV in combination with BI-97C1 could also inhibit prostate tumor growth in vivo. For this analysis we used an immunocompetent transgenic mouse model of PC (the Hi-Myc mouse) that spontaneously develops PC. In Hi-Myc mice, prostate-specific c-Myc gene expression is controlled through the rat probasin promoter with two androgen response elements (ARR2/probasin promoter). Hi-Myc mice develop prostatic intraepithelial neoplasia (mPIN) as early as 2–4 weeks of age and invasive adenocarcinoma of the prostate at 6 months (Ellwood-Yen et al., 2003). Treatment was initiated at 22 weeks of age. The ability to deliver adenoviruses systemically is limited by sequestering of the virus in the liver and clearance of the virus by the immune system (Muruve, 2004; Koizumi et al., 2007; Schenk et al., 2010). To overcome these formidable problems we employed a microbubble-targeted ultrasound destruction (UTMD) approach (Lu et al., 2003; Howard et al., 2006; Greco et al., 2010; Das et al., 2012) in which MBs incorporating adenoviruses are targeted to release therapeutic viruses at the tumor site using US. Using US to sonicate the MBs creates transient non-lethal perforations in cells and other membranes. In this way, systemic and targeted delivery of mda-7/IL-24 to the prostate of Hi-Myc mice was performed by tail vein injection of MBs incorporating Ad.5/3-vec, Ad.5/3-PEG-E1A, or Ad.5/3-CTV followed by sonoporation in the prostatic area (Dash et al., 2011a). A total of eight tail vein injections of each adenovirus were administered over a 4-week period. BI-97C1 was administered intraperitoneally (i.p.) in each group at 3 mg/kg three times a week throughout the study. The prostates of Hi-myc mice were sectioned and stained for MDA-7/IL-24 and Ki-67, proliferation marker, and apoptosis induction was analyzed by TUNEL assay. MDA-7/IL-24 expression was accompanied by increased TUNEL positive cells and decreased Ki-67 positive cells in the Ad.5/3-CTV and BI-97C1-treated group compared to the control groups (Fig. 5). Although, Ad.5/3-CTV alone induced significant apoptosis it was markedly augmented when used in combination with BI-97C1.
Fig. 5.
Combination treatment of Ad.5/3-CTV and BI-97C1 (Sabutoclax) potentiates inhibition of prostate tumor growth in vivo in immune competent animals. A: The prostatic region of 22-week-old male Hi-Myc mice were sonoporated for 10 min following tail-vein injection of the indicated complement-treated MB/Ad complexes and treated as described in the Materials and Methods Section for 4 weeks (total eight injections of indicated viruses). BI-97C1 (Sabutoclax) was administered intraperitoneally (i.p.) in each group at 3 mg/kg three times a week throughout the study. At the end of the experiment, the mice were sacrificed and the prostates were collected. The paraffin-embedded sections were obtained from the prostate and immunohistochemistry was performed to measure systemic transgene delivery by staining with anti-MDA-7/IL-24. TUNEL assay and Ki-67 staining detected apoptosis and cell proliferation in the prostate sections. Nuclei were visualized with DAPI. Wild type mice of the same strain that do not develop prostate cancer served as a control for these experiments. (B) Quantification of microvessel density in prostate section per field followed by Ki-67 staining and (C) Quantification of TUNEL positive signals in the prostate section (*P < 0.05 between the indicated groups). Data represent mean ± SD (n = 3).
Discussion
The progression of PC is often slow and different therapeutics may be required during various stages of disease progression and at multiple times during the lifespan of the patient. In the context of gene therapy, it may be necessary to employ different genes, used alone or in combination, and systemic viral delivery approaches over extended time frames (Damber and Aus, 2008; Dash et al., 2011b). PC clinical trials have been performed with conditionally replicating adenoviruses (DeWeese et al., 2001; Small et al., 2006; Fukuhara et al., 2010), resulting in partial clinical responses. CG706 (created by inserting the PSE, a minimal promoter construct of human PSA gene to drive the E1A) and CG7870 (rat probasin promoter-driven E1A gene and the PSE-driven E1B gene) were tested in respective phase I trials, in which patients with local recurrence received an intraprostatic injection of the viruses following primary radiotherapy. Liver function toxicities with CV706 administration were not observed (DeWeese et al., 2001). The responses of patients were dose-dependent and there was a reduction of 50% of PSA. With CG7870 a mild liver inflammation was observed, but approximately 25–49% reduction in PSA was observed (Small et al., 2006). While this conditional replicating viral approach has potential it needs further validation as a frontline therapy for patients with PC, including monitoring changes in PSA and evaluating disease progression over time.
To enhance therapeutic efficacy of Ad gene therapy for PC, we have presently used a number of strategies. We earlier constructed a bipartite Ad.5 where viral replication is controlled by the minimal active region of the promoter of the PEG-3 gene (Su et al., 1997, 2005b), restricting viral replication to cancer cells with limited activity in normal cells, and mda-7/IL-24 is driven by a CMV promoter from the E3 region of Ad.5 (Sarkar et al., 2005a, 2006, 2007b, 2008). These viruses, termed CTVs (reviewed in Das et al., 2012), have displayed profound activity in athymic nude mouse human xenograft models, including breast carcinomas, PC (including therapy-resistant PCs over expressing Bcl-2 or Bcl-XL) and metastatic melanoma (Sarkar et al., 2005a, 2006, 2007b, 2008; Greco et al., 2010).
To enhance viral entry into CAR deficient cancer cells, we engineered chimeric adenoviruses containing the Ad.3 sequence within the Ad.5 virus knob (Ad.5/3) (Dash et al., 2010b; Azab et al., 2012), which redirects binding of the vector to the Ad.3 receptor, desmoglein 2 (Wang et al., 2011). In this study, we document that Ad.5/3-CTV eradicates, thereby further verifying the anti-cancer potency of this therapeutic virus not only in primary-injected tumors but also distant non-injected tumors derived from a resistant PC cell line in a nude mouse xenograft model. As discussed, Ad.5/3-CTV is capable of infecting cancer cells regardless of their cell surface CAR status, which makes it potentially more efficacious than Ad.5-CTV that fails to efficiently infect and deliver the therapeutic genes in low CAR PC cells. The Ad.5/3 chimeras are directed to CD46 and CD80/CD86 receptors that are highly expressed on tumor cells significantly improving anti-tumor efficacy (Sirena et al., 2004; Ulasov et al., 2007). Tropism modified Ad.5/3 conditional replication has also been used to express GMCSF and this approach was also found to be safe and efficacious in treating cancer patients (Koski et al., 2010). Additionally, it has been reported that Ad.5/3 serotype chimerism could also be a viable strategy for circumventing the generation of neutralizing antibodies in patients receiving multiple rounds of conditionally replicating viruses (Raki et al., 2011).
Systematic delivery of Ads is challenging because of sequestration of viruses in the liver restricting efficient delivery to disseminated tumors (Koizumi et al., 2007) and neutralization of viruses by the immune system (Curiel and Fisher, 2012). To prevent trapping of CTV in the liver and elimination of viruses by the immune system we have developed an innovative approach that involves the use of perfluorocarbon MBs and US (Greco et al., 2010; Dash et al., 2011a). This approach is called ultrasound-targeted microbubble destruction (UTMD). We have applied the UTMD approach using a tropism-modified Ad.5/3-CTV in Hi-Myc transgenic mice, which develop PC (Ellwood-Yen et al., 2003; Dash et al., 2011a). Ad.5/3-CTV in complement-treated MBs were administered systemically through the tail vein of mice and released in the prostate area through US in this syngeneic immunocompetent PC mouse model (Hi-Myc mouse) using UTMD (Dash et al., 2011a). Hi-Myc mice develop, with high penetrance, prostatic intraepithelial neoplasia (PIN) that advances over time to invasive adenocarcinomas in all lobes of the prostate gland (Ellwood-Yen et al., 2003). In the present study, we show the combinatorial anti-cancer effect of Ad.5/3-CTV and the novel Mcl-1 antagonist, BI-97C1 (Sabutoclax), which significantly inhibits PC in Hi-Myc transgenic mice. For combination studies we chose an Mcl-1 antagonist based on our previous observations where we demonstrated that suppression of the pro-survival Bcl-2 family member, Mcl-1, is required for mda-7/IL-24-mediated apoptosis of prostate carcinomas (Dash et al., 2010c, 2011a). Here, we demonstrate that pharmacological inhibition of Mcl-1 expression with the novel Apogossypol derivative BI-97C1 is sufficient to sensitize prostate tumors to mda-7/IL-24-induced (Ad.5/3-CTV) apoptosis.
In summary, if specific technical impediments can be overcome, gene therapy for PC holds substantial promise in treating this disease (Curiel and Fisher, 2012; Das et al., 2012). (1) The prostate gland is dispensable and not vital for survival, and it is accessible by US. (2) Ad.5/3-CTV can be either directly injected into the primary tumor or delivered through UTMD with complement-treated MBs incorporating Ads thereby resulting in cancer cell lysis and expression of MDA-7/IL-24. (3) The replication of this virus and the expression of the therapeutic genes can be directly targeted in cancer cells without non-specific expression in normal cells by using the cancer-specific PEG-3 promoter. (4) Disease progression can be monitored (Cookson, 2001; Gopalkrishnan et al., 2001). In these contexts, the use of Ad.5/3-CTV to administer the therapeutic and cancer-specific cytotoxic MDA-7/IL-24 protein to selectively induce cytolysis and apoptosis in prostate tumor cells may represent a potentially viable treatment option (Anderson, 1998; Sarkar et al., 2007a; Das et al., 2012).
Acknowledgments
Contract grant sponsor: NIH;
Contract grant numbers: R01CA097318, R01CA127641, P01CA104177.
Contract grant sponsor: National Foundation for Cancer Research.
Contract grant sponsor: NIH;
Contract grant number: R01 CA138540.
Contract grant sponsor: James S. McDonnell Foundation.
Contract grant sponsor: Dana Foundation.
Contract grant sponsor: A. David Mazzone Prostate Cancer Foundation Challenge Award.
The present study was supported in part by NIH grants R01 CA097318, R01 CA127641, and P01 CA104177, and the National Foundation for Cancer Research to P.B. Fisher; NIH grant R01 CA138540, the James S. McDonnell Foundation, and an A. David Mazzone Prostate Cancer Foundation Challenge Award to M.G. Pomper and P.B. Fisher the Dana Foundation to D. Sarkar. D. Sarkar and X.-Y. Wang are Harrison Scholars in Cancer Research and D. Sarkar is a Blick Scholar. P.B. Fisher holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center.
Literature Cited
- Anderson WF. Human gene therapy. Nature. 1998;392:25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- Azab B, Dash R, Das SK, Bhutia SK, Shen XN, Quinn BA, Sarkar S, Wang XY, Hedvat M, Dmitriev IP, Curiel DT, Grant S, Dent P, Reed JC, Pellecchia M, Sarkar D, Fisher PB. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the Apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. J Cell Physiol. 2012;227:2145–2153. doi: 10.1002/jcp.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhang HE, Gabrielson KL, Laterra J, Fisher PB, Pomper MG. Tumor-specific imaging through progression elevated gene-3 promoter-driven gene expression. Nat Med. 2011;17:123–129. doi: 10.1038/nm.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada S, Mhashilkar AM, Ramesh R, Mumm JB, Sutton RB, Bocangel D, Zheng M, Grimm EA, Ekmekcioglu S. Bystander activity of Ad-mda7: Human MDA-7 protein kills melanoma cells via an IL-20 receptor-dependent but STAT3-independent mechanism. Mol Ther. 2004;10:1085–1095. doi: 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Cianfriglia F, Di Gregorio DA, Manieri A. Multiple primary tumours in patients with oral squamous cell carcinoma. Oral Oncol. 1999;35:157–163. doi: 10.1016/s1368-8375(98)00105-5. [DOI] [PubMed] [Google Scholar]
- Cookson MM. Prostate cancer: Screening and early detection. Cancer Control. 2001;8:133–140. doi: 10.1177/107327480100800203. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, Hood J, Coffee K, Nemunaitis J. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: A phase I study. Mol Ther. 2005;11:149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Curiel DT, Fisher PB, editors. Applications of viruses for cancer therapy. Adv Cancer Res. 2012;115:1–334. [Google Scholar]
- Damber JE, Aus G. Prostate cancer. Lancet. 2008;371:1710–1721. doi: 10.1016/S0140-6736(08)60729-1. [DOI] [PubMed] [Google Scholar]
- Das SK, Sarkar S, Dash R, Dent P, Wang XY, Sarkar D, Fisher PB. Cancer terminator viruses and approaches for enhancing therapeutic outcomes. Adv Cancer Res. 2012;115:1–38. doi: 10.1016/B978-0-12-398342-8.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelmen TP, Das SK, Kim K, Lee SG, Park MA, Yacoub A, Rahmani M, Emdad L, Dmitriev IP, Wang XY, Sarkar D, Grant S, Dent P, Curiel DT, Fisher PB. mda-7/IL-24: A unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev. 2010a;21:381–391. doi: 10.1016/j.cytogfr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Dmitriev I, Su ZZ, Bhutia SK, Azab B, Vozhilla N, Yacoub A, Dent P, Curiel DT, Sarkar D, Fisher PB. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) improves therapeutic efficacy in low CAR prostate cancer cells. Cancer Gene Ther. 2010b;17:447–456. doi: 10.1038/cgt.2009.91. [DOI] [PubMed] [Google Scholar]
- Dash R, Richards JE, Su ZZ, Bhutia SK, Azab B, Rahmani M, Dasmahapatra G, Yacoub A, Dent P, Dmitriev IP, Curiel DT, Grant S, Pellecchia M, Reed JC, Sarkar D, Fisher PB. Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine. Cancer Res. 2010c;70:5034–5045. doi: 10.1158/0008-5472.CAN-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Azab B, Quinn BA, Shen X, Wang XY, Das SK, Rahmani M, Wei J, Hedvat M, Dent P, Dmitriev IP, Curiel DT, Grant S, Wu B, Stebbins JL, Pellecchia M, Reed JC, Sarkar D, Fisher PB. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proc Natl Acad Sci USA. 2011a;108:8785–8790. doi: 10.1073/pnas.1100769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Azab B, Shen XN, Sokhi UK, Sarkar S, Su ZZ, Wang XY, Claudio PP, Dent P, Dmitriev IP, Curiel DT, Grant S, Sarkar D, Fisher PB. Developing an effective gene therapy for prostate cancer: New technologies with potential to translate from the laboratory into the clinic. Discov Med. 2011b;11:46–56. [PMC free article] [PubMed] [Google Scholar]
- DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, Hamper U, DeJong R, Detorie N, Rodriguez R, Haulk T, DeMarzo AM, Piantadosi S, Yu DC, Chen Y, Henderson DR, Carducci MA, Nelson WG, Simons JW. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- Di Lorenzo G, De Placido S. Hormone refractory prostate cancer (HRPC): Present and future approaches of therapy. Int J Immunopathol Pharmacol. 2006;19:11–34. [PubMed] [Google Scholar]
- Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Eulitt PJ, Park MA, Hossein H, Cruikshanks N, Yang C, Dmitriev IP, Yacoub A, Curiel DT, Fisher PB, Dent P. Enhancing mda-7/IL-24 therapy in renal carcinoma cells by inhibiting multiple protective signaling pathways using sorafenib and by Ad.5/3 gene delivery. Cancer Biol Ther. 2011;10:1290–1305. doi: 10.4161/cbt.10.12.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65:10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: From the laboratory into the clinic. Cancer Biol Ther. 2003;2(Suppl 1):S23–S37. [PubMed] [Google Scholar]
- Fisher PB, Sarkar D, Lebedeva IV, Emdad L, Gupta P, Sauane M, Su ZZ, Grant S, Dent P, Curiel DT, Senzer N, Nemunaitis J. Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): Novel gene therapeutic for metastatic melanoma. Toxicol Appl Pharmacol. 2007;224:300–307. doi: 10.1016/j.taap.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara H, Homma Y, Todo T. Oncolytic virus therapy for prostate cancer. Int J Urol. 2010;17:20–30. doi: 10.1111/j.1442-2042.2009.02383.x. [DOI] [PubMed] [Google Scholar]
- Gopalkrishnan RV, Kang DC, Fisher PB. Molecular markers and determinants of prostate cancer metastasis. J Cell Physiol. 2001;189:245–256. doi: 10.1002/jcp.10023. [DOI] [PubMed] [Google Scholar]
- Greco A, Di Benedetto A, Howard CM, Kelly S, Nande R, Dementieva Y, Miranda M, Brunetti A, Salvatore M, Claudio L, Sarkar D, Dent P, Curiel DT, Fisher PB, Claudio PP. Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Mol Ther. 2010;18:295–306. doi: 10.1038/mt.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed HA, Yacoub A, Park MA, Eulitt PJ, Dash R, Sarkar D, Dmitriev IP, Lesniak MS, Shah K, Grant S, Curiel DT, Fisher PB, Dent P. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol Ther. 2010;18:1130–1142. doi: 10.1038/mt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Howard CM, Forsberg F, Minimo C, Liu JB, Merton DA, Claudio PP. Ultrasound guided site specific gene delivery system using adenoviral vectors and commercial ultrasound contrast agents. J Cell Physiol. 2006;209:413–421. doi: 10.1002/jcp.20736. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–2486. [PubMed] [Google Scholar]
- Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi N, Yamaguchi T, Kawabata K, Sakurai F, Sasaki T, Watanabe Y, Hayakawa T, Mizuguchi H. Fiber-modified adenovirus vectors decrease liver toxicity through reduced IL-6 production. J Immunol. 2007;178:1767–1773. doi: 10.4049/jimmunol.178.3.1767. [DOI] [PubMed] [Google Scholar]
- Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I, Nokisalmi P, Raki M, Rajecki M, Guse K, Ranki T, Oksanen M, Holm SL, Haavisto E, Karioja-Kallio A, Laasonen L, Partanen K, Ugolini M, Helminen A, Karli E, Hannuksela P, Pesonen S, Joensuu T, Kanerva A, Hemminki A. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther. 2010;18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, Reed JC, Fisher PB. Bcl-2 and Bcl-x(L) differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene. 2003;22:8758–8773. doi: 10.1038/sj.onc.1206891. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, Staudt MR, Liu SJ, Taher MM, Xiao R, Barral P, Lee SG, Wang D, Vozhilla N, Park ES, Chatman L, Boukerche H, Ramesh R, Inoue S, Chada S, Li R, De Pass AL, Mahasreshti PJ, Dmitriev IP, Curiel DT, Yacoub A, Grant S, Dent P, Senzer N, Nemunaitis JJ, Fisher PB. mda-7/IL-24, novel anticancer cytokine: Focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (review) Int J Oncol. 2007a;31:985–1007. [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Emdad L, Kolomeyer A, Sarkar D, Kitada S, Waxman S, Reed JC, Fisher PB. Targeting inhibition of K-ras enhances Ad.mda-7-induced growth suppression and apoptosis in mutant K-ras colorectal cancer cells. Oncogene. 2007b;26:733–744. doi: 10.1038/sj.onc.1209813. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Washington I, Sarkar D, Clark JA, Fine RL, Dent P, Curiel DT, Turro NJ, Fisher PB. Strategy for reversing resistance to a single anticancer agent in human prostate and pancreatic carcinomas. Proc Natl Acad Sci USA. 2007c;104:3484–3489. doi: 10.1073/pnas.0700042104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QL, Liang HD, Partridge T, Blomley MJ. Microbubble ultrasound improves the efficiency of gene transduction in skeletal muscle in vivo with reduced tissue damage. Gene Ther. 2003;10:396–405. doi: 10.1038/sj.gt.3301913. [DOI] [PubMed] [Google Scholar]
- Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- Park MA, Hamed HA, Mitchell C, Cruickshanks N, Dash R, Allegood J, Dmitriev IP, Tye G, Ogretmen B, Spiegel S, Yacoub A, Grant S, Curiel DT, Fisher PB, Dent P. A serotype 5/3 adenovirus expressing MDA-7/IL-24 infects renal carcinoma cells and promotes toxicity of agents that increase ROS and ceramide levels. Mol Pharmacol. 2011;79:368–380. doi: 10.1124/mol.110.069484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raki M, Sarkioja M, Escutenaire S, Kangasniemi L, Haavisto E, Kanerva A, Cerullo V, Joensuu T, Oksanen M, Pesonen S, Hemminki A. Switching the fiber knob of oncolytic adenoviruses to avoid neutralizing antibodies in human cancer patients. J Gene Med. 2011;13:253–261. doi: 10.1002/jgm.1565. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Dent P, Fisher PB. mda-7 (IL-24): Signaling and functional roles. Biotechniques Suppl. 2002a;33:30–39. [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, Dent P, Fisher PB. mda-7 (IL-24) mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci USA. 2002b;99:10054–10059. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci USA. 2005a;102:14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Randolph A, Valerie K, Fisher PB. Targeted virus replication plus immunotherapy eradicates primary and distant pancreatic tumors in nude mice. Cancer Res. 2005b;65:9056–9063. doi: 10.1158/0008-5472.CAN-05-1261. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Fisher PB. Unique conditionally replication competent bipartite adenoviruses-cancer terminator viruses (CTV): Efficacious reagents for cancer gene therapy. Cell Cycle. 2006;5:1531–1536. doi: 10.4161/cc.5.14.3095. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, Curiel DT, Fisher PB. Melanoma differentiation associated gene-7 (mda-7)/IL-24: A ‘magic bullet’ for cancer therapy? Expert Opin Biol Ther. 2007a;7:577–586. doi: 10.1517/14712598.7.5.577. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Su ZZ, Park ES, Chatman L, Vozhilla N, Dent P, Curiel DT, Fisher PB. Eradication of therapy-resistant human prostate tumors using a cancer terminator virus. Cancer Res. 2007b;67:5434–5442. doi: 10.1158/0008-5472.CAN-07-0195. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Park ES, Vozhilla N, Dent P, Curiel DT, Fisher PB. A cancer terminator virus eradicates both primary and distant human melanomas. Cancer Gene Ther. 2008;15:293–302. doi: 10.1038/cgt.2008.14. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Lebedeva IV, Dent P, Pestka S, Fisher PB. MDA-7/IL-24: Novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 2003;14:35–51. doi: 10.1016/s1359-6101(02)00074-6. [DOI] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, Fisher PB. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc Natl Acad Sci USA. 2008;105:9763–9768. doi: 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk E, Essand M, Bangma CH, Consortium GF, Barber C, Behr JP, Briggs S, Carlisle R, Cheng WS, Danielsson A, Dautzenberg IJ, Dzojic H, Erbacher P, Fisher K, Frazier A, Georgopoulos LJ, Hoeben R, Kochanek S, Koppers-Lalic D, Kraaij R, Kreppel F, Lindholm L, Magnusson M, Maitland N, Neuberg P, Nilsson B, Ogris M, Remy JS, Scaife M, Schooten E, Seymour L, Totterman T, Uil TG, Ulbrich K, Veldhoven-Zweistra JL, de Vrij J, van Weerden W, Wagner E, Willemsen R. Clinical adenoviral gene therapy for prostate cancer. Hum Gene Ther. 2010;21:807–813. doi: 10.1089/hum.2009.206. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Sirena D, Lilienfeld B, Eisenhut M, Kalin S, Boucke K, Beerli RR, Vogt L, Ruedl C, Bachmann MF, Greber UF, Hemmi S. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J Virol. 2004;78:4454–4462. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, Yu DC, Aimi J, Ando D, Working P, Kirn D, Wilding G. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Sternberg CN. Highlights of contemporary issues in the medical management of prostate cancer. Crit Rev Oncol Hematol. 2002;43:105–121. doi: 10.1016/s1040-8428(02)00023-9. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Shi Y, Fisher PB. Subtraction hybridization identifies a transformation progression-associated gene PEG-3 with sequence homology to a growth arrest and DNA damage-inducible gene. Proc Natl Acad Sci USA. 1997;94:9125–9130. doi: 10.1073/pnas.94.17.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Goldstein NI, Jiang H, Wang MN, Duigou GJ, Young CS, Fisher PB. PEG-3, a nontransforming cancer progression gene, is a positive regulator of cancer aggressiveness and angiogenesis. Proc Natl Acad Sci USA. 1999;96:15115–15120. doi: 10.1073/pnas.96.26.15115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Shi Y, Fisher PB. Cooperation between AP1 and PEA3 sites within the progression elevated gene-3 (PEG-3) promoter regulate basal and differential expression of PEG-3 during progression of the oncogenic phenotype in transformed rat embryo cells. Oncogene. 2000;19:3411–3421. doi: 10.1038/sj.onc.1203666. [DOI] [PubMed] [Google Scholar]
- Su Z, Lebedeva IV, Gopalkrishnan RV, Goldstein NI, Stein CA, Reed JC, Dent P, Fisher PB. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci USA. 2001a;98:10332–10337. doi: 10.1073/pnas.171315198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Shi Y, Friedman R, Qiao L, McKinstry R, Hinman D, Dent P, Fisher PB. PEA3 sites within the progression elevated gene-3 (PEG-3) promoter and mitogen-activated protein kinase contribute to differential PEG-3 expression in Ha-ras and v-raf oncogene transformed rat embryo cells. Nucleic Acids Res. 2001b;29:1661–1671. doi: 10.1093/nar/29.8.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Gopalkrishnan RV, Narayan G, Dent P, Fisher PB. Progression elevated gene-3, PEG-3, induces genomic instability in rodent and human tumor cells. J Cell Physiol. 2002;192:34–44. doi: 10.1002/jcp.10114. [DOI] [PubMed] [Google Scholar]
- Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, James CD, Randolph A, Valerie K, Walter MR, Dent P, Fisher PB. Unique aspects of mda-7/IL-24 antitumor bystander activity: Establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005a;24:7552–7566. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Sarkar D, Emdad L, Duigou GJ, Young CS, Ware J, Randolph A, Valerie K, Fisher PB. Targeting gene expression selectively in cancer cells by using the progression-elevated gene-3 promoter. Proc Natl Acad Sci USA. 2005b;102:1059–1064. doi: 10.1073/pnas.0409141102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, Coffee K, Ramesh R, Ekmekcioglu S, Grimm EA, van Wart Hood J, Merritt J, Chada S. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): Biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Ulasov IV, Rivera AA, Han Y, Curiel DT, Zhu ZB, Lesniak MS. Targeting adenovirus to CD80 and CD86 receptors increases gene transfer efficiency to malignant glioma cells. J Neurosurg. 2007;107:617–627. doi: 10.3171/JNS-07/09/0617. [DOI] [PubMed] [Google Scholar]
- Wang H, Li Z, Yumul R, Lara S, Hemminki A, Fender P, Lieber A. Multimerization of adenovirus serotype 3 fiber knob domains is required for efficient binding of virus to desmoglein 2 and subsequent opening of epithelial junctions. J Virol. 2011;85:6390–6402. doi: 10.1128/JVI.00514-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Kitada S, Rega MF, Stebbins JL, Zhai D, Cellitti J, Yuan H, Emdadi A, Dahl R, Zhang Z, Yang L, Reed JC, Pellecchia M. Apogossypol derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2009;52:4511–4523. doi: 10.1021/jm900472s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Stebbins JL, Kitada S, Dash R, Placzek W, Rega MF, Wu B, Cellitti J, Zhai D, Yang L, Dahl R, Fisher PB, Reed JC, Pellecchia M. BI-97C1, an optically pure Apogossypol derivative as pan-active inhibitor of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2010;53:4166–4176. doi: 10.1021/jm1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: Immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]