Abstract
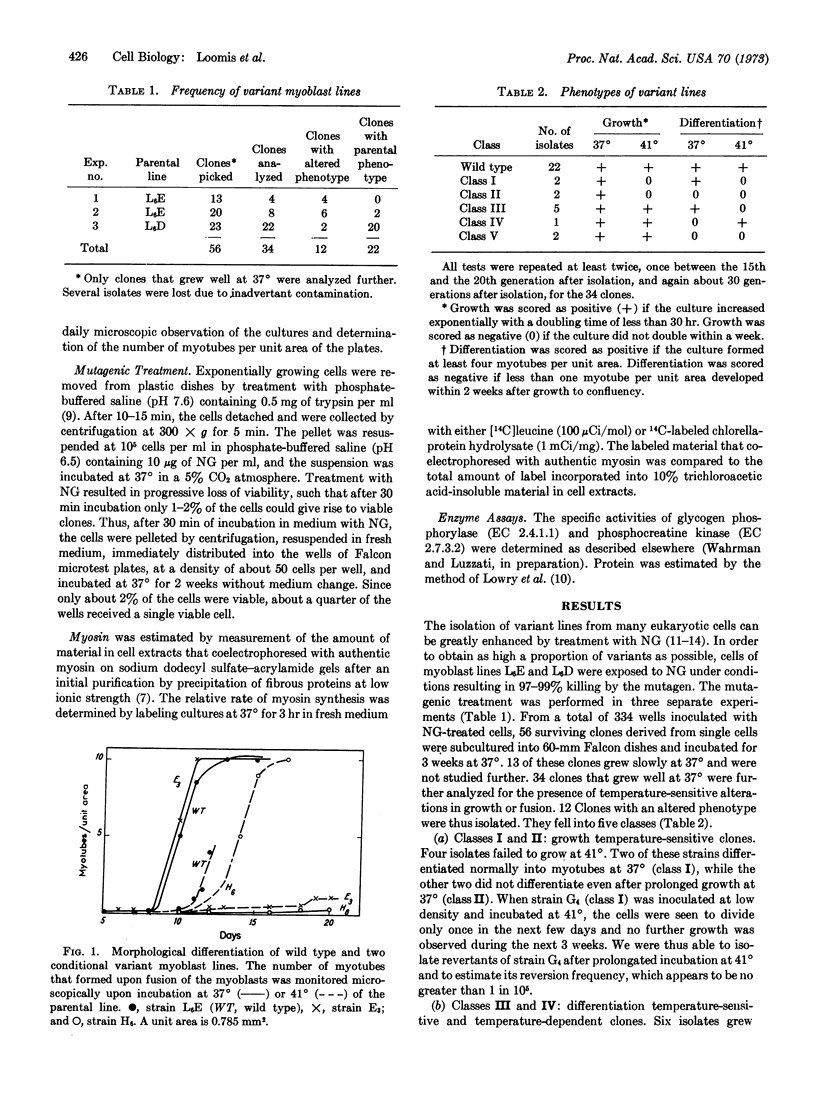
Upon reaching confluency, mononucleated myoblasts fuse into multinucleated myotubes and concomitantly accumulate various characteristic muscle proteins, including myosin, actin, and several enzymes. We have approached the problem of determining the relationship between morphological and biochemical differentiation of muscle cells by isolating a series of temperature-sensitive clones from the established myoblast line, L6.
Twelve phenotypically variant clones were isolated from mutagenized populations of myoblasts. These fell into five classes, distinguishing conditional growth variants from conditional developmental variants. The phenotype of these strains, at least for the more extensively studied ones, was stable for more than 80 generations.
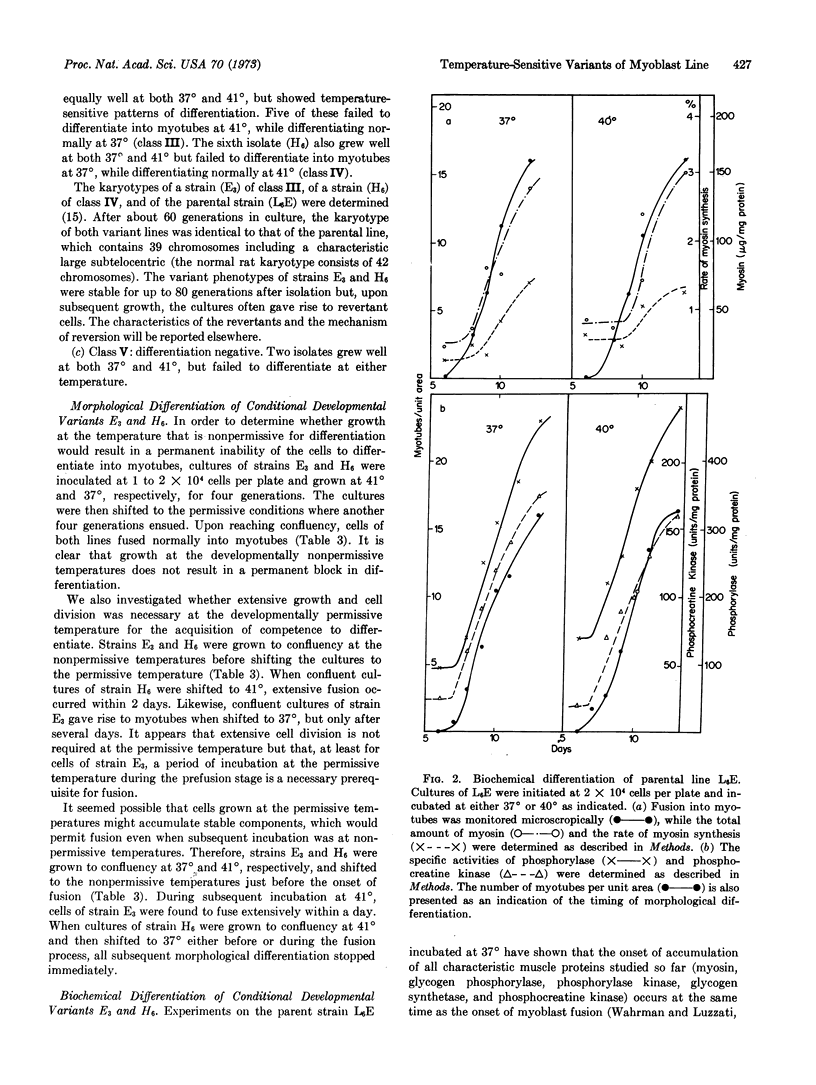
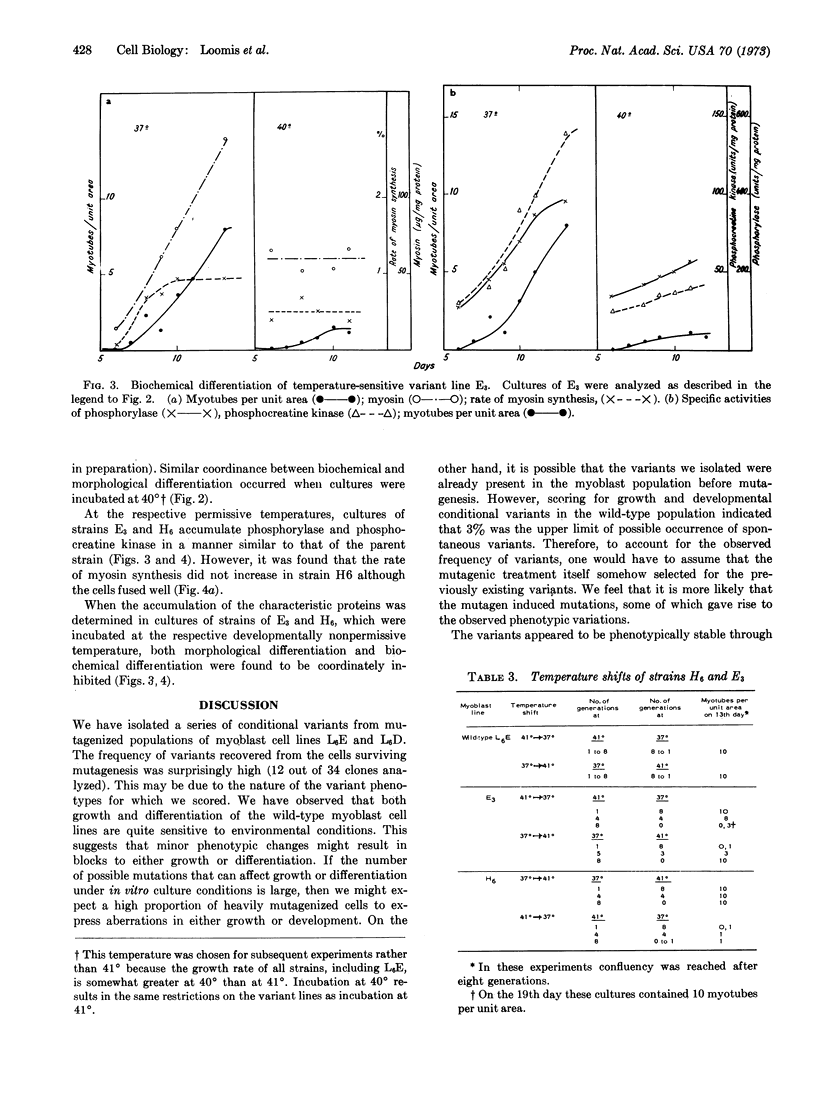
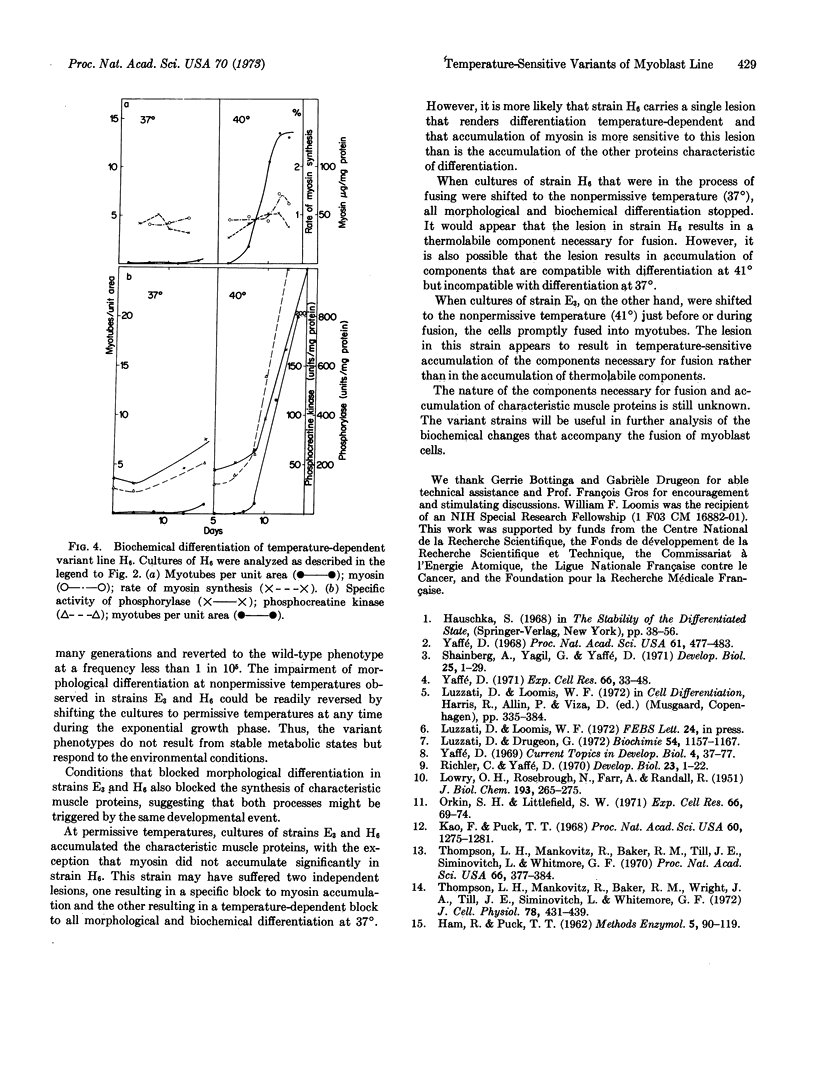
Synthesis of characteristic proteins such as myosin, glycogen phosphorylase (EC 2.4.1.1), and phosphocreatine kinase (EC 2.7.3.2) has been studied in two conditional developmental mutants. One mutant, E3, fuses into myotubes at 37° but not at 40°; the other, H6, does not fuse into myotubes at 37° but does so at 40°. At permissive temperatures the enzymes accumulated in mutant cells with the same time course as in the parent cell line. Myosin accumulated in strain E3 but not in strain H6. At nonpermissive temperatures neither fusion into myotubes nor accumulation of any of the proteins occured in the cells of these two variant lines.
Keywords: myoblast differentiation, myosin, phosphorylase, phosphocreatine kinase, nitrosoguanidine
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells, VII. Induction and isolation of nutritional mutants in Chinese hamster cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1275–1281. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luzzati D., Drugeon G. The effect of actinomycin D on RNA and protein synthesis during differentiation of myoblasts of line L 6 E: the half-life of functional myosin messenger. Biochimie. 1972;54(9):1157–1167. doi: 10.1016/s0300-9084(72)80020-8. [DOI] [PubMed] [Google Scholar]
- Richler C., Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970 Sep;23(1):1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Mankovitz R., Baker R. M., Till J. E., Siminovitch L., Whitmore G. F. Isolation of temperature-sensitive mutants of L-cells. Proc Natl Acad Sci U S A. 1970 Jun;66(2):377–384. doi: 10.1073/pnas.66.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Mankovitz R., Baker R. M., Wright J. A., Till J. E., Siminovitch L., Whitmore G. F. Selective and nonselective isolation of temperature-sensitive mutants of mouse L-cells and their characterization. J Cell Physiol. 1971 Dec;78(3):431–440. doi: 10.1002/jcp.1040780312. [DOI] [PubMed] [Google Scholar]


