Abstract
Cholesterol modification of Hedgehog (Hh) ligands is fundamental for the activity of Hh signaling, and cholesterol biosynthesis is also required for intracellular Hh signaling transduction. Here, we investigated the roles and underlying mechanism of Hh signaling in metabolism of cholesterol. The main components of the Hh pathway are abundantly expressed in both human cytotrophoblasts and trophoblast-like cells. Activation of Hh signaling induces the conversion of cholesterol to progesterone (P4) and estradiol (E2) through up-regulating the expression of steroidogenic enzymes including P450 cholesterol side chain cleavage enzyme (P450scc), 3β-hydroxysteroid dehydrogenase type 1 (3β-HSD1), and aromatase. Moreover, inhibition of Hh signaling attenuates not only Hh-induced expression of steroidogenic enzymes but also the conversion of cholesterol to P4 and E2. Whereas Gli3 is required for Hh-induced P450scc expression, Gli2 mediates the induction of 3β-HSD1 and aromatase. Finally, in ovariectomized nude mice, systemic inhibition of Hh signaling by cyclopamine suppresses circulating P4 and E2 levels derived from a trophoblast-like choricarcinoma xenograft, and attenuates uterine response to P4 and E2. Together these results uncover a hitherto uncharacterized role of Hh signaling in metabolism of cholesterol.
Keywords: Cholesterol, Hedgehog signaling, Steroids
1. Introduction
Hedgehog (Hh) signaling has conserved roles in the development of various organs in metazoans ranging from Drosophila to humans [1, 2]. The mammalian Hh family of secreted proteins consists of sonic hedgehog (Shh), indian hedgehog (Ihh) and desert hedgehog (Dhh). In vertebrates, in the absence of Hh, patched 1 (Ptc1) receptor represses smoothened (Smo) activity, and the Gli transcription factors (Gli2 and Gli3) are proteolytically cleaved into repressors within the primary cilium. The cleavage requires the activities of suppressor of fused (SuFu) and kinesin family member 7 (Kif7) and is mediated by phosphorylation of Gli2 or Gli3 by protein kinase A (PKA), casein kinase 1 (CK1), and glycogen synthase kinase-3β (GSK3β). In the presence of Hh, binding of Hh to Ptc1 relieves the inhibition of Smo, resulting in activation of the Gli transcriptional factors that induce transcription of target genes including cyclin D, cyclin E, myc as well as Ptc1 and Gli1[3, 4]. Overall, the conserved effect of Hh is to switch the Gli transcription factors from repressors into activators and allow for well-coordinated transcriptional events [2].
Hh ligands are precursor proteins that are translocated to endoplasmic reticulum, and undergo the post-translationally covalent-modification including palmityl residue attachment at the N-terminus mediated by the palmityl transferase and cholesteryl residue attachment at the C-terminus by autocatalytic modification [5, 6]. These two lipid modifications occur independently and are both essential for activity of Hh signaling [5, 7]. Moreover, metabolites of cholesterol and cholesterol biosynthetic pathway intermediates including oxysterols have been shown to play an intracellular role in Shh signal transduction, inhibition of their biosynthesis at different steps by pharmacological inhibitors suppresses Shh signaling, and leads to defective responses to Shh, both in vitro and in vivo [8–11]. Finally, defect in either 17β-hydroxysteroid dehydrogenase 7 (17β-HSD7) or 3β-HSD, the enzymes of the cholesterol biosynthetic pathway, leads to severe developmental abnormalities in several tissues, resulting from an abnormal sterol profile and deficient responses to Hh signaling [12, 13]. Thus, cholesterol modification of Hh ligands is fundamental for Hh activity, and cholesterol biosynthesis is also required for intracellular Hh signal transduction. To date, there is no evidence that Hh signaling regulates the cholesterol metabolism.
Human placenta is a crucial site for the conversion of cholesterol into steroids, and biosynthesis of placental steroids is essential for pregnancy maintenance and embryo development. Among the cholesterol-derived steroids, progesterone (P4) and estradiol (E2) are the most important hormones that are fundamentally involved in the regulation of the menstrual cycle and in the establishment and maintenance of pregnancy [14]. Placental trophoblasts convert the cholesterol to pregnenolone (P5), the first step in the synthesis of all steroids, and then to P4 by P450 cholesterol side chain cleavage enzyme (P450scc) and 3β-HSD1, respectively [15, 16]. On the other hand, in fetal adrenal, maternal P5 and P4 are converted into C19 androgens (dehydroepiandrosterone, DHEA) which are further utilized by placental trophoblasts to synthesize the E2 through sequential reactions catalyzed either by 3β-HSD1 and aromatase via the intermediate A-dione, or by aromatase and 17β-HSD1 via C18 estrone [15, 17].
In the present study, we have demonstrated that Hh signaling stimulates cholesterol conversion to P4 and E2 by inducing the expression of key steroidogenic enzymes. We further identify the genes encoding these enzymes as direct transcriptional targets of either Gli2 or Gli3. This work establishes Hh signaling as an essential mechanism in conversion of cholesterol into its steroid metabolites.
2. Materials and methods
2.1. Cell culture
All the cell lines used in the present study were obtained from ATCC (Manassas, VA). JEG-3 and BeWo choriocarcinoma cells were cultured as described previously [15], 293 EcR Shh cells (Shh-expressing cells) and control HEK 293 cells were used for the production of biologically active murine Shh conditional medium (SM) and control medium (CM), respectively, in the presence of ecdysone [18]. Human placentas were obtained from uncomplicated normal term (38~40 W) pregnancy after elective cesarean section without labor, following a protocol approved by the Ethics Committee of School of Medicine of Zhejiang University. The primary cytotrophoblasts were purified by using a 5~65% Percoll (Sigma) gradient at step increments of 5%, and cultured to allow syncytialization in vitro as described previously [19].
2.2. Oligonucleotides, plasmids, viruses, and infections
The primers for regular RT-PCR and quantitative RT-PCR were listed in the Supplement Data (Supplementary Table 1 and 2). Retroviruses expressing Smo, hGli2, hGli3 and their constitutively active forms including ca-Smo, ΔN-Gli2 and ΔN-Gli3 were all as previously described [20, 21]. Lentiviruses expressing Gli1-, Gli2- and Gli3-shRNA were generated by co-transfecting the 293FT packaging cells with lentiviral shRNA expression vector, Pll3.7, inserted with the hairpin shRNA templates of complementary oligonucleotides (Supplementary Table 4) at the sites of XbaI and NotI. Either the retrovirus-containing or the lentiviruses-containing supernatants with the titers greater than 1×106 cfu/ml was used for infection of JEG-3 cells in the presence of 8 μg/ml polybrene (Sigma) as described previously [16]. The promoter regions of P450scc (nt −2497/+141), 3β-HSD1 (nt −2416/+207), and aromatase (nt −2487/+7), and their deletion mutants potentially interacting with Glis were amplified by PCR (primers in the Supplementary Table 3) in the presence of genomic DNA from JEG-3 cells, and the PCR products were subcloned into pGL3-Basic vector (Promega) to generate the luciferase reporter constructs as described previously [16]. The mutations at potential binding sites of Glis were introduced by site-directed mutagenesis. All of these constructs were verified by DNA a sequencer.
2.3. Antibodies, proteins, and chemicals
Human Gli1 (ab92611), Gli2 (ab26056), Gli3 (ab69838), and Smo (ab72130) antibodies were from Abcam (Cambridge, UK), human Ptc1 (06-1102) and Shh (06-1106) were from Millipore (Billerica, MA), and antibodies for human aromatase (sc-30086), P450scc (sc-292456), 3β-HSD1 (sc-30820), and β-actin (sc-69879) were from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant active human ΔN-Shh (1845-SH) was from R&D Systems, and cyclopamine was obtained from Sigma (St. Louis, MO).
2.4. RT-PCR, quantitative RT-PCR, and chip-PCR
RT-PCR was performed to detect the expression of main components of Hh signaling pathway including Dhh, Ihh, Shh, Ptc1, Smo, Gli1, Gli2, and Gli3, and quantitative RT-PCR was performed to measure the expression of steroidogenic enzymes including P450scc, 3β-HSD1, aromatase, 17β-HSD1, 17β-HSD2, 17β-HSD5, and hSTS as described previously [15, 16]. Chromatin immunoprecipitation (Chip) was conducted by using a commercial kit (Millipore, Billerica, MA), and a method modified from the manufacturer’s protocol as described previously [19]. Briefly, the shearing of chromatin DNA was performed by sonication to produce an approximate 500 bp of input DNA, and was subjected to immunoprecipitation with either Gli antibodies or control IgG. After the immunoprecipitates were incubated with protein A agarose/salmon sperm DNA, the antibody-protein-DNA-agarose complex was washed and harvested for subsequent reverse cross-linking. The sheared DNA fragments from reverse cross-linking was extracted with a DNA extraction kit for further PCR amplification by using the primers listed in the Supplemental Data (Supplementary Table 5).
2.5. Transient transfections and duel-luciferase assays
Transient transfections in JEG-3 cells were performed by using Lipofectamine 2000 reagent (Invitrogen) as per the manufacturer’s instructions. In each well of 24-well plates, the cells were transfected with 2 μl Lipofectamine 2000 reagent, 2 μg luciferase reporter constructs, and 0.02 μg Renilla luciferase constructs (Promega) for 8 hrs in the absence of serum, then, the cells were cultured in the media containing either CM or SM for 48 hrs. In some experiments, the cells were infected with the viruses for 24 hrs, and then transfected with the luciferase reporters for 8 hrs, and finally cultured in the media containing either CM or SM for 48 hrs. After transfection and treatment, the cellular lysates were prepared in reporter lysis buffer (Promega), and the supernatants were used for dual-luciferase assay according to the manufacturer’s instructions (Promega). The firefly luciferase activities were normalized to Renilla luciferase levels.
2.6. Western blots and EIA
Western blots were performed using standard protocols, and the intensity of protein bands (n=3) and statistical analysis was undertaken by NIH ImageJ. EIA assays for P4 and E2 were performed in serum-free culture supernatants harvested from JEG-3 cells and cytotrophobalsts, which were incubated with the substrates of steroidogenic enzymes (25-hydroxycholesterol or testosterone) for 4 hrs, as described previously [15, 22].
2.7. Ovariectomy, JEG-3 choriocarcinoma xenografts, and treatments
Female Balb/c-nu/nu mice (6-week-old) were ovariectomized (OVX) and rested for a week, and tumor-bearing mice model was established by subcutaneous inoculation with xenografts of human choriocarcinoma JEG-3 cells (5×106 cells/site) into both the left and right armpit. After the tumors grew to a size of ≈250 mm3 (6~7 d), mice were received subcutaneous injections of either cyclopamine (25 mg/kg/d or 50 mg/kg/d) or vehicle for 15 d. The tumor volumes at different stages were calculated by the standard protocol, and after the mice were sacrificed, the tumors and uterus were harvested for further analysis including determination of serum murine and human E2 and P4 levels by EIA, examination of uterine response to P4 and E2 by H&E staining of paraffin-embeded uterine sections, and measurements of proliferation and apoptosis of tumor cells by flow cytometery and TUNEL staining, respectively. All animal care and handling procedures were approved by the Institutional Animal Care and Use Committee of Zhejiang University.
2.8. Statistics
All the numerous data were expressed as mean ± S.D., and were analyzed by one-way ANOVA and Tukey-Kramer multiple comparison test (SPSS 13.0J software; SPSS, Inc., Chicago, IL). Statistical significance was assessed at p <0.05 and p< 0.01. Experiments were independently triplicated, and results were qualitatively identical. Representative experiments are shown.
3. Results
3.1. Conversion of cholesterol induced by Hh in primary cytotrophoblasts and trophoblast-like cells
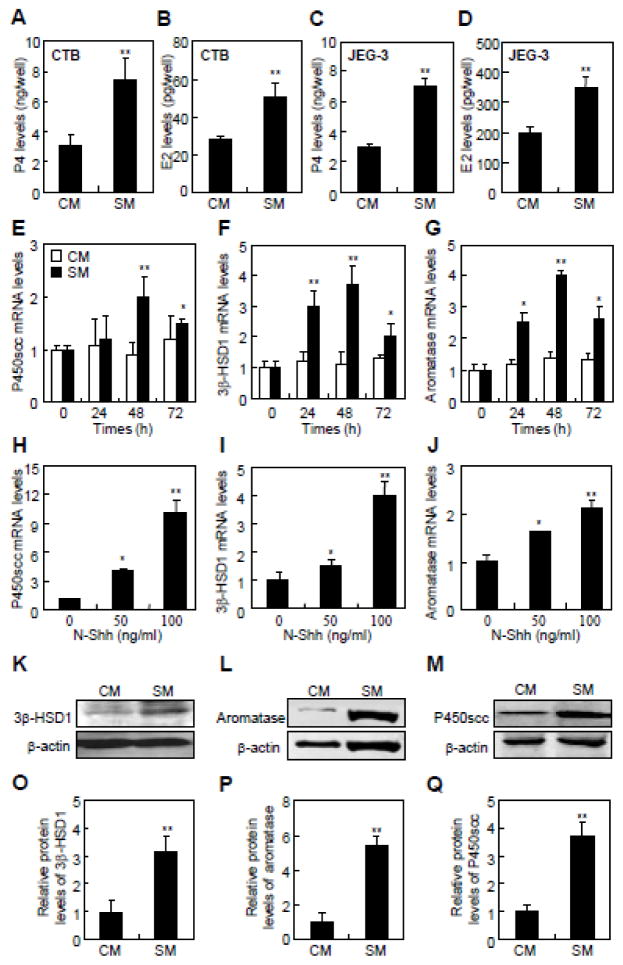
Human primary cytotrophoblasts (CTBs) and trophoblast-like cell linesare valuable models to study the conversion of cholesterol to steroids [22, 23]. To gain quantitative information about the expression of the main components of Hh pathway, we performed RT-PCR assays in primary CTBs and trophoblast-like JEG-3 and BeWo cell lines. The main components of Hh signaling were abundantly expressed in both the primary CTBs and trophoblast-like cells lines (Supplementary Fig. 1A and B). CTBs expressed relatively higher levels of Ihh, Shh, Smo, Gli2, and Gli3 than Dhh, Ptc1, and Gli1. On the other hand, JEG-3 and BeWo cells expressed more Dhh, Ihh, Ptc1, Smo, Gli2, and Gli3 than Shh and Gli1 (Supplementary Fig. 1A and B). Shh conditional medium (SM), control conditional medium (CM), and human N-Shh recombinant protein (N-Shh) have been widely used to activate Hh signaling in vitro (Supplementary Fig. 1C and D) [18, 24]. To examine the conversion of cholesterol to steroids, we performed enzyme immunoassay (EIA) assays to determine the P4 and E2 levels in the cells incubated with 25-hydroxycholesterol and testosterone, respectively. SM increased the levels of both P4 and E2 in JEG-3 cells (2.3- and 1.7- fold, respectively) and cytotrophoblasts (2.4- and 1.8-fold, respectively) after 48 hrs of stimulation (Fig. 1A–D). To investigate whether Hh induced the expression of steroidogenic enzymes, we performed quantitative RT-PCR and western blot assays in JEG-3 cells after stimulation with either SM or N-Shh. SM stimulated the mRNA levels of P450scc, 3β-HSD1, and aromatase in a time-dependent manner, reaching 2–4 fold over the control at 48 hrs (Fig. 1E–G). Similarly, N-Shh significantly induced these mRNAs in a dose-dependent manner, reaching approximately 2–9 fold over the control at 100 ng/ml (Fig. 1H–J). In contrast, SM did not affect the mRNA levels of steroid sulfatase (STS) or 17β-HSD1, 2 and 5 (Supplementary Fig. 1E–H). Moreover, SM increased the protein levels of P450scc, 3β-HSD1, and aromatase by 3.8-, 3.2-, and 5.5-fold, respectively, over the control after 48 hrs (Fig. 1K–Q). Thus, Hh stimulates the conversion of cholesterol to P4 and E2 likely through up-regulation of key steroidogenic enzymes.
Fig. 1.
Hh signaling stimulates cholesterol conversion and steroidogenic enzymes expression in primary cytotrophoblasts and trophoblast-like JEG-3 cells. (A–D) P4 and E2 levels in the culture media of cytotrophoblasts (CTBs) and JEG-3 cells containing 25-hydroxycholesterol and testosterone, respectively, after 48 hrs of either control medium (CM) or Shh conditional medium (SM) treatment. (E–J) Determination of mRNA levels of P450scc, 3β-HSD1, and aromatase by quantitative RT-PCR in JEG-3 cells following CM, SM or Shh recombinant protein (N-Shh) treatments. (K–M) Measurements of the expression of P450scc, 3β-HSD1, and aromatase by western blots in JEG-3 cells following the same treatments. (O–Q) Quantification via densitometry (n=3) and statistical analysis of bands of K–M, respectively. The data shown represent average fold of Shh-treatment groups compared with the control groups. RNA and protein abundance normalized to β-actin, respectively. **p<0.01, *p<0.05; n=6, error bar, SD.
3.2. Involvement of Smo in Hh-induced conversion of cholesterol
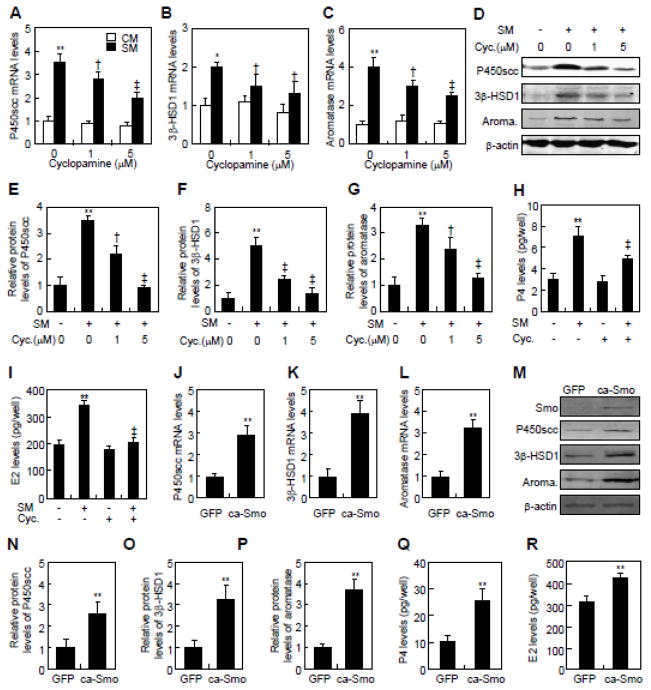
To examine whether Hh-induced conversion of cholesterol was Smo-dependent, we inhibited Smo activity with cyclopamine, or activated it by over-expressing a constitutively active form of Smo (ca-Smo) [25, 26]. Cyclopamine dose-dependently attenuated both mRNA and protein levels of P450scc, 3β-HSD1 and aromatase in JEG-3 cells in response to SM. At 5 μM, cyclopamine reduced the SM-induced mRNA levels of steroidogenic enzymes by approximately 40~45% (Fig. 2A–C). The same treatment completely abolished the induction of P450scc protein, and reduced the induction of 3β-HSD1 and aromatase proteins by 65% and 59%, respectively (Fig. 2D–G). Moreover, cyclopamine significantly suppressed the SM-induced conversion of cholesterol to P4 and E2, even though it did not affect the basal levels of P4 and E2 (Fig. 2H and I). Conversely, ca-Smo expression in JEG-3 cells by retroviral infection increased the mRNA levels of P450scc, 3β-HSD1, and aromatase by 2.0- to 3.2-fold, and induced the protein levels by 2.8- to 3.7-fold over the control (green fluorescent protein, GFP) (Fig. 2J–P). Expression of ca-Smo also increased the conversion of cholesterol to P4 and E2 by approximately 2.5- and 1.5-fold, respectively (Fig. 2Q, R). Thus, suppression of Smo activity attenuates Shh-induced conversion of cholesterol to P4 and E2, and constitutive activation of Smo alone is sufficient to induce the cholesterol-derived steroidogenesis in JEG-3 cells.
Fig. 2.
Smo is involved in Hh-induced conversion of cholesterol in JEG-3 cells. (A–C) P450scc, 3β-HSD1, and aromatase mRNA levels in JEG-3 cells in response to control medium (CM) and Shh conditional medium (SM) in the presence of indicated concentrations of cyclopamine. (D–F) Quantification via densitometry (n=3) and statistical analysis of bands of G. The data shown represent average fold of each group compared with the control group. (G) P450scc, 3β-HSD1 and aromatase protein levels in JEG-3 cells in response to control medium (−) or Shh conditional medium (SM) in the presence of indicated concentrations of cyclopamine. (H, I) P4 and E2 levels in JEG-3 cells, after 48 hrs of treatments with control medium (−) and Shh conditional medium (S.M., +) in either the presence (+) or the absence (−) of 5 μM cyclopamine (Cyc). (J–L) P450scc, 3β-HSD1 and aromatase mRNA levels in JEG-3 cells, after infection with either control or constitutively active form of Smo (ca-Smo)-expressing lentiviruses for 48 hrs. (M) P450scc, 3β-HSD1 and aromatase protein levels in JEG-3 cells, after infection with either control or ca-Smo-expressing lentiviruses for 48 h. (N–P) Quantification via densitometry (n=3) and statistical analysis of bands of M. The data shown represent average fold of ca-Smo-transfected groups compared with the GFP-transfected groups. (Q, R) P4 and E2 levels in JEG-3 cells, after infection with either control or ca-Smo-expressing lentiviruses for 48 hrs. RNA and protein abundance normalized to β-actin, respectively. **, ‡p<0.01, *, † p<0.05; n=3, error bar, SD.
3.3. Distinct roles of Gli transcriptional factors in conversion of cholesterol
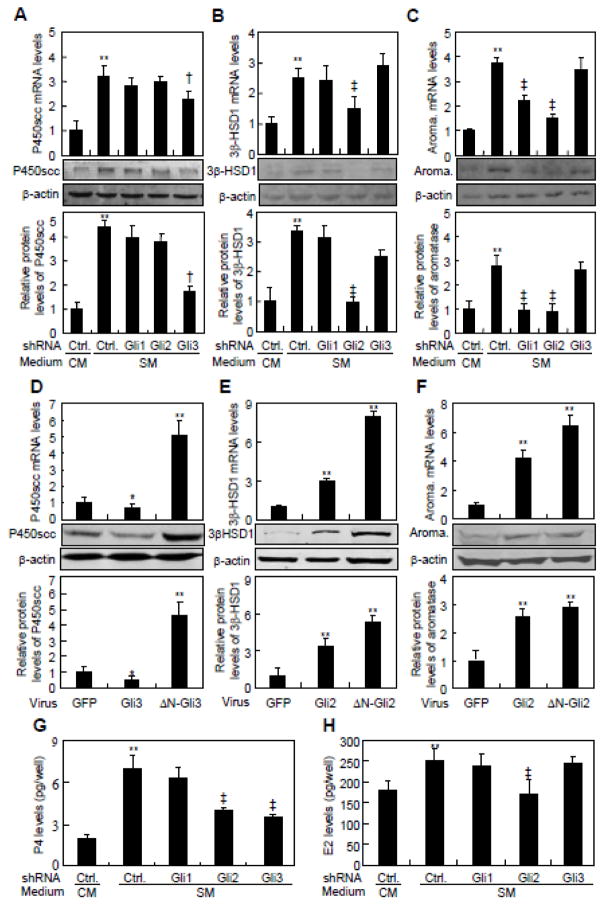
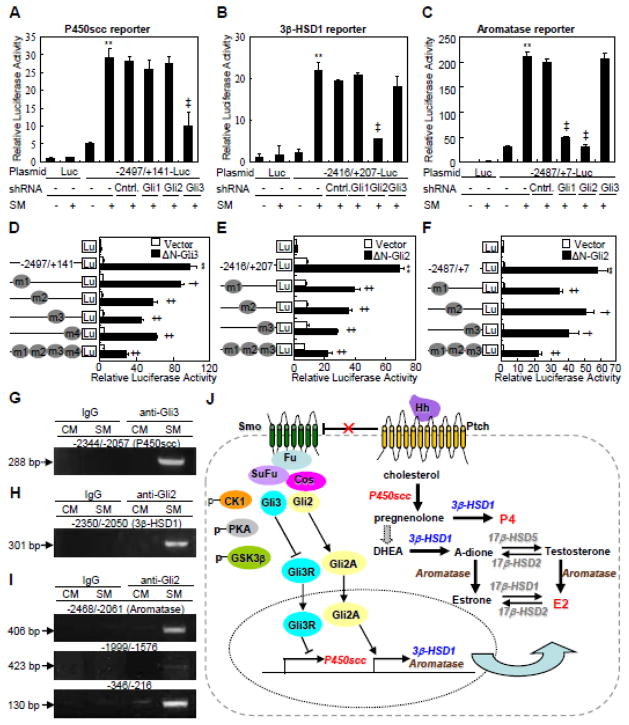
In order to assess the potential involvement of Gli transcriptional factors in the Hh-induced conversion of cholesterol, we generated lentiviruses expressing Gli1-, Gli2-, or Gli3-shRNA which knocked down the expression of Gli1, Gli2, and Gli3 by as much as 60~100% at either mRNA or protein levels (Supplementary Fig. 1I–K). Knockdown of Gli3 but not Gli1 or Gli2 reduced SM-induced P450scc mRNA by 30% and protein by 65% (Fig. 3A). On the other hand, knockdown of Gli2 but not Gli1 and Gli3 suppressed SM-induced 3β-HSD1 mRNA and protein levels, by 45% and 73%, respectively (Fig. 3B). Finally, knockdown of either Gli1 or Gli2 but not Gli3 diminished aromatase expression by 40~70% at mRNA and protein levels (Fig. 3C). In keeping with the regulation of the enzymes, knockdown of either Gli2 or Gli3 but not Gli1 significantly suppressed SM-induced P4 production, whereas knockdown of Gli2 but not Gli1 and Gli3 greatly suppressed SM-induced E2 production (Fig. 3G and H). Thus, the Gli proteins play distinct roles in mediating Hh induction of various steroidogenic enzymes to regulate the conversion of cholesterol.
Fig. 3.
Gli2 and Gli3 are involved in Hh-induced conversion of cholesterol in JEG-3 cells. (A–C) P450scc, 3β-HSD1 and aromatase expression in JEG-3 cells, after infection with Gli1-, Gli2- or Gli3-shRNA-expressing lentivirus and treatment with either control medium (CM) or Shh conditional medium (SM) for 48 hrs. Quantification via densitometry (n=3) and statistical analysis of bands were also performed. (D–F) P450scc, 3β-HSD1 and aromatase expression in JEG-3 cells, after infection with either constitutively active form of Gli2- (ΔN-Gli2) or Gli3 (Gli3-ΔC)-expressing lentivirus for 48 h. (G, H) P4 and E2 levels in JEG-3 cells, after infection with Gli1-, Gli2- or Gli3-shRNA-expressing lentivirus and treatment with either control medium (CM) or Shh conditional medium (SM) for 48 hrs. RNA and protein abundance normalized to β-actin, respectively. **, ‡p<0.01, † p<0.05; n=3, error bar, SD.
To further investigate the effects of Gli proteins on Hh-induced steroidogenesis, we generated retroviruses expressing either full-length or N-terminally truncated active forms of Gli2 (ΔN-Gli2) and Gli3 (ΔN-Gli3) [27, 28]. JEG-3 cells were infected with the various retroviruses for 48 hrs before harvest. Full-length Gli3 decreased the P450scc mRNA and protein levels by 40% and 20%, respectively, whereas ΔN-Gli3 induced P450scc expression at both mRNA (5-fold) protein (4.6-fold) levels (Fig. 3D). Gli2 and ΔN-Gli2 both induced 3β-HSD1 mRNA (3- and 8-fold, respectively) and protein (3- and 6-fold, respectively) (Fig. 3E). Finally, Gli2 and ΔN-Gli2 induced aromatase mRNA by 4- and 6-fold, and protein by 2.5- and 3.0-fold, respectively (Fig. 3F). Thus, Gli transcriptional activators are sufficient to stimulate the expression of steroidogenic enzymes.
3.4. Direct activation of the expression of steroidogenic enzymes by Gli2 and Gli3 in response to Hh
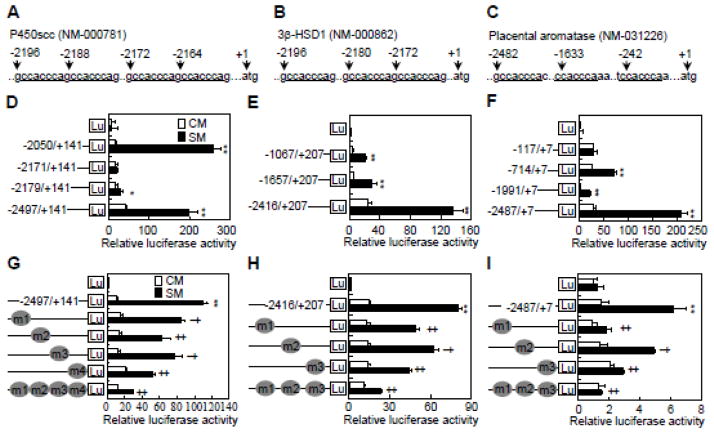
To investigate the potential direct regulation of P450scc, 3β-HSD1 and aromatase by Hh, we cloned their promoter regions containing the potential Gli binding sequence 5′-CCACCCA-3′ (GRE) [29, 30], and generated luciferase reporter constructs driven by each promoter region (nt −2497/+141 for P450scc, nt −2416/+207 for 3β-HSD1, and nt −2487/+7 for aromatase) or truncated sequences lacking one or more GRE (Fig. 4A–C). After transfection with the pRL-null plasmid and the different reporter constructs, JEG-3 cells were stimulated with either SM or CM for 48 hrs. The full-length constructs all exhibited a robust response to SM, but almost all truncation mutants exhibited a significantly lower response (Fig. 4D–F). P450scc (nt −2050/+141) was the only truncation construct that showed a similar response to SM as the full-length construct (Fig. 4D). To further examine the functionality of GREs, we generated P450scc (nt −2497/+141), 3β-HSD1 (nt −2416/+207) and aromatase (nt −2487/+7) reporter variants that harbored mutations at individual or multiple GRE (triple or quadruple mutant). Each individual GRE mutation significantly decreased the response to SM, but the triple or quadruple mutations virtually abolished the response altogether (Fig. 4G–I). Thus, Hh signaling induces gene expression of key steroidogenic enzymes likely through direct binding of Gli transcriptional factors to GREs located within the promoter regions.
Fig. 4.
Hh transactivates P450scc, 3β-HSD1 and aromatase in Gli response elements-dependent manners in JEG-3 cells. (A–C) Schematic presentation of 5′-untranscriptional region and the potential Gli binding sites on P450scc, 3β-HSD1 and aromatase promoters. (D–F) Dual-luciferase assays in JEG-3 cells, after transient transfection with reporter constructs of wild type or deletion mutants for P450scc, 3β-HSD1 and aromatase, and treatment with either control medium (CM) or Shh conditional medium (SM) for 48 h. (G–I) Dual-luciferase assays in JEG-3 cells, after transient transfection with reporter constructs of wild type or Gli binding site mutants for P450scc, 3β-HSD1 and aromatase. **, ‡p<0.01, *,† p<0.05; n=6, error bar, SD.
To confirm the functionality of GREs in Hh-Gli regulation, we examined the response of the luciferase reporters to various manipulations of the Hh signaling pathway. Cyclopamine robustly suppressed SM-induced P450scc, 3β-HSD1 and aromatase reporter activities (Supplementary Fig. 2A, D, G), whereas ca-Smo stimulated the expression of all three constructs (Supplementary Fig. 2B, E, H). Knockdown of Gli1, Gli2 or Gli3 differentially attenuated SM-induced expression of these reporters (Fig. 5A–C). Consistent with our earlier results, ΔN-Gli2 and ΔN-Gli3 exhibited differential stimulation of the different reporters (Supplementary Fig. 2B, E, H). Notably, deletion of either one or more GREs in the luciferase constructs greatly attenuated the induction by either ΔN-Gli3 or ΔN-Gli2 (Supplementary Fig. 2C, F, I). Each individual GRE mutation significantly decreased the reporter activities induced by either ΔN-Gli3 or ΔN-Gli2, but the triple or quadruple mutations exhibited greater suppression (Fig. 5D–F). These results further support that Gli3 directly activates P450scc (CYP11A1), whereas Gli2 directly activates 3β-HSD1(HSD3B1) and aromatase (CYP19) in response to Hh.
Fig. 5.
Glis mediate Hh-induced transactivation of P450scc, 3β-HSD1 and aromatase in JEG-3 cells. (A–C) Dual-luciferase assays in JEG-3 cells, after infection with Gli-shRNA-expressing lentivirus, transient transfection with P450scc, 3β-HSD1 or aromatase reporter constructs, and treatment with either control medium (−) or Shh conditional medium (SM, +) for 48 hrs. (D–F) Dual-luciferase assays in JEG-3 cells, 48 hrs after transient co-transfection with constitutively active forms of Gli (Gli3-ΔC, ΔN-Gli2) and reporter constructs of either wild type or Gli binding site mutants for P450scc, 3β-HSD1 and aromatase. (G–I) Detection of the interactions between Glis and DNA of P450scc, 3β-HSD1 and aromatase by ChIP assays in JEG-3 cells, after 48 hrs of treatment with either control medium (CM) or Shh conditional medium (SM). (J) A model for Hh-induced steroidogenesis in human trophoblasts. **, ‡p<0.01, *,† p<0.05; n=6, error bar, SD.
To examine the physical interaction between Gli and GRE located at the promoter regions of steroidogenic enzyme genes, we performed chromatin immunoprecipitation followed by PCR (ChIP-PCR) in JEG-3 cells by using antibodies against Gli2 and Gli3. The PCR primers were designed to amplify specific genomic DNA fragments of nt −2344/−2057 for P450scc, nt −2350/−2050 for 3β-HSD1, and nt −2468/−2061, nt −1999/−1576, nt −346/−216 for aromatase. Gli3 bound to the fragment of nt −2344/−2057 in P450scc in the presence of SM but not CM (Fig. 5G). Similarly, Gli2 bound to the fragment of nt −2350/−2050 in 3β-HSD1 in the presence of SM but not CM (Fig. 5H). In contrast, Gli2 bound to the fragment of nt −346/−216 in aromatase both with or without SM, but the binding was much stronger with SM (Fig. 5I, low band). Gli2 also bound to nt −2468/−2061, but only weakly to nt −1999/−1576 in aromatase in response to SM (Fig. 5I, upper and middle bands). Thus, we have identified P450scc, 3β-HSD1 and aromatase as direct transcriptional target genes of Hh-Gli signaling.
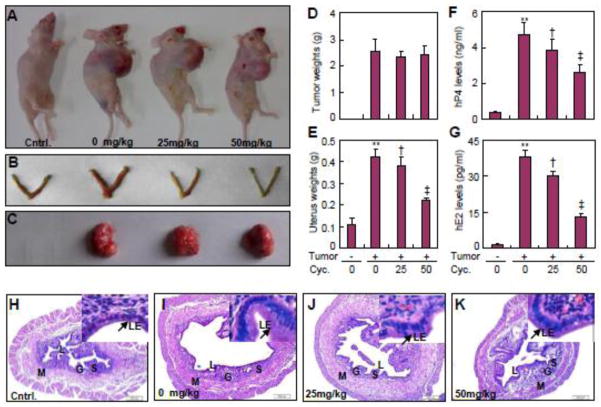
3.5. Cyclopamine diminishes P4 and E2 production by JEG-3 choriocarcinoma xenografts in ovariectomized nude mice
To examine the functional relevance of Hh-dependent conversion of cholesterol to P4 and E2 by trophoblasts, we generated JEG-3 choriocarcinoma xenografts in ovariectomized nude mice and monitored the uterine responses in the presence or absence of cyclopamine administration. Cyclopamine administration did not cause morphological changes in the uterus of ovariectomized nude mice without xenografts (data not shown). However, with or without cyclopamine, overiectomy led to trace serum levels of murine P4 and E2 (Supplementary Fig. 3C and D), whereas JEG-3 choriocarcinoma xenografts in the overiectomized mice produced abundant human P4 and E2 in the serum, whose levels were comparable to physiological serum concentrations in mice [31] (Fig. 6F, G). As predicted, ovariectomy led to uterine hypoplasia and endometrial regression indicated by a significant reduction in uterine diameter, a thin epithelial layer as well as a diminished number of glands and stromal cells (Fig. 6H; Supplementary Fig. 3I–K). In contrast, the JEG-3 xenografs in ovariectomized nude mice resulted in enlarged uterus, flattened but thick epithelial layer, crowded and distended endometrial glands, and decidualized stromal cells (Fig. 6I; Supplementary Fig. 3I–K). Cyclopamine at 25 and 50 mg/kg did not affect the volumes (Supplementary Fig. 3B) and weights of the xenografts at different stages of post inoculation (Fig. 6A, C, D), nor did it change the proliferation or apoptosis status of the xenograft cells (Supplementary Fig. 3E–H). However, cyclopamine markedly and dose-dependently suppressed the level of human P4 and E2 derived from xenografts; at 50 mg/kg cyclopamine decreased P4 and E2 levels by approximately 45% and 75%, respectively (Fig. 6F, G). Moreover, cyclopamine at 25 and 50 mg/kg decreased the uterine wet weight, a classical marker for P4 and E2 exposure, by approximately 45% and 75%, respectively (Fig. 6B, E). Cyclopamine also significantly reduced the thickness of the epithelial layer, the number of stromal cells, as well as the size and number of endometrial glands (Fig. 6J, K; Supplementary Fig. 3I–K). Thus, systemic inhibition of Hh signaling reduced the conversion of cholesterol to P4 and E2 by JEG-3 choriocarcinoma xenografts in the mouse, resulting in a lesser uterine response.
Fig. 6.
Cyclopamine suppresses endometrial response to P4 and E2 produced from JEG-3 choriocarcinoma xenografts in ovariectomized nude mice. (A–C) Representative gross morphology of uterus and xenografts from ovariectomized nude mice subcutaneously inoculated with JEG-3 cells, and treated with indicated dosages of cyclopamine for 12 d after 10 d of inoculation. (D, E) Tumor and uterine weights in ovariectomized nude mice with same treatments. (F, G) Human P4 and E2 levels in circulation of ovariectomized nude mice with same treatments. (H–K) HE staining for uterus from ovariectomized nude mice with same treatments. G, glandularepithelium; L, lumen; LE, luminal epithelium; M, myometrium; S, stroma.**, ‡p<0.01, † p<0.05; n=6, error bar, SD.
4. Discussion
In the present study, by using both biochemical approaches and a xenograft model, we have uncovered the important roles of Hh signaling in conversion of cholesterol into steroids in human primary CTBs and trophoblast-like cell line. At the molecular level, Hh induces the transcription of 3β-HSD1 and aromatase through Gli2, and P450scc via Gli3, thereby increasing the conversion of cholesterol to P4 and E2 (Fig. 5J). Our results therefore identify Hh as a critical signal in supporting the physiological function of cholesterol metabolism.
Cholesterol modification is essential for the activity of Hh ligands and cholesterol biosynthesis is necessary for intracellular signal transduction of Hh pathway [32], conversely, the current study indicated that activation of Hh signaling is essential for the metabolism of cholesterol. Although the present work deals only with conversion of cholesterol to steroid by the trophoblasts, our finding further reveals the mutual interplay between the Hh signaling and cholesterol and expands the list of sterodogenic endocrine cells influenced by Hh signaling. Since the first step in the synthesis of all steroid hormones is the conversion of cholesterol into pregnenolone [33], Hh signaling induces the P450scc expression and stimulates the conversion of cholesterol into pregnenolone, implicating the possibly extensive effects of Hh signaling on the production of steroid hormones other than P4 and E2.
Considering that the main components of Hh pathway are abundantly expressed in the primary CTBs and trophoblast-like cell lines, our data suggest that Hh proteins may signal through both autocrine and paracrine mechanisms to regulate the functions of trophoblasts, consistent with previous findings in other endocrine tissues, for instance, Shh and Dhh are produced by adrenocortical cells in the adrenal gland, and sertoli cells in the testis, respectively, whereas Dhh and Ihh are secreted by granulosa cells in the ovary [34–36]. These tissue-specific ligands are believed to act upon the respective Hh-responding cells to regulate their differentiation [37]. Beyond the conversion of cholesterol to steroids in the trophoblasts as reported here, Hh signaling has been implicated in hormonal production by other cells and tissues. These include the production of peptide hormones including adrenocorticotropic hormone (ACTH), growth hormone (GH) and prolactin in human cells derived from corticotrophinoma, somatotrophinoma or prolactinoma [38, 39]. Other examples are P4 production in granulosa cells, androstenedione in theca cells and insulin in rat β-cell line INS-1 [40–42]. However, how Hh regulates the production of these hormones is not clear from those studies. Here we provide evidence that Hh stimulates the conversion of cholesterol to P4 and E2 through up-regulation of key enzymes, and that the up-regulation is at least partly due to direct transcriptional activation of the genes by the Gli family of transcription factors. Specifically, whereas Gli2 is responsible for Hh-induced 3β-HSD1 and aromatase expression, Gli3 appears to be the main mediator for P450scc gene transcription in response to Hh. It’s worth noting that full-length Gli3 suppressed P455scc expression whereas ΔNGli3 activated it in our transfection studies. Although Gli3 is commonly known as a transcriptional repressor via the truncated form (Gli3R), an activator function has also been detected in certain settings [43]. Thus, the induction of P450scc transcription by Hh may reflect both depression of the Gli3 repressor and activation by the Gli3 activator.
In in vitro experiments, we added the exogenous cholesterol to the culture medium and observed the intracellular conversion of cholesterol to P4 and E2 in response to Hh. However, in in vivo experiments, we supposed that autocrine production of Shh, Ihh or Dhh by JEG-3 xenografts could stimulate the intratumoral conversion of circulating cholesterol to hP4 and hE2. Uterus is an important target organ for steroid hormones, both P4 and E2 are the primary hormones responsible for preparing the uterine endometrium for implantation. E2 is essential for the proliferation of the uterine luminal and glandular epithelium and also sensitizes it to P4 by inducing the expression of P4 receptor [44]. P4 plays important roles in the proliferation, differentiation, and maintenance of the stroma, and is critical for initiation and maintenance of the decidual reaction of the uterine stroma as well [45]. Considering that uterine decidualization is a generally accepted approach to readout the changes in cholesterol-derived P4 and E2 levels in vivo [44, 45], we took advantages of xenografts in ovariectomized nude mice, and demonstrated that systemic inhibition of Hh signaling reduced P4 and E2 production and resulted in the attenuation of uterine response to P4 and E2, providing further in vivo evidence of Hh signaling in the conversion of cholesterol into P4 and E2.
Cholesterol and its derivatives have profound effects on the Hh pathway, and are important components for the function of the Hh signaling cascade [32]. Mutations in enzymes for cholesterol biosynthesis are associated with a number of human diseases, such as Smith-Lemli-Opitz syndrome (SLOS) and lathosterolosis characterized by accumulation of the cholesterol precursors 7-dehydrocholesterol and lathosterol, and decreased cholesterol concentration, which impairs the Shh pathway at the level of Smo [46]. Holoprosencephaly (HPE), the most severe form of SLOS, results from impairment of Shh signaling secondary to abnormal cholesterol metabolism [47, 48]. Hh signaling is essential for the conversion of cholesterol to P4 and E2 in trophoblasts, and P4 and E2 are fundamentally involved in the establishment and maintenance of pregnancy, thus, by demonstrating the role of Hh signaling in the normal functions of human trophoblasts, the present study suggests that abnormal Hh signaling may cause pregnancy-related clinical disorders.
5. Conclusions
Cholesterol modification is essential for Hh ligands activities and intracellular transduction of Hh signaling. In this manuscript, we have uncovered that activation of Hh signaling triggers sequential conversion of cholesterol to its metabolites, progesterone and estradiol, by induction the expression of downstream target genes including Gli3-controlled P450scc and Gli2-controlled 3β-HSD1 and aromatase in human trophoblasts. Our results are not only consistent with the importance of Hh signaling in the embryonic development of placenta, but we also identify the important roles of Hh signaling in maintenance of mature placental functions, and possibly of human pregnancy.
Supplementary Material
Supplementary Fig. 1. (A, B) RT-PCR assays for Hh ligands and the main components of Hh signaling in trophoblast-like cells, primary cytotrophoblasts and human placentas. (C, D) Gli-luciferase activities and Gli1 protein levels in JEG-3 cells in response to Shh conditional medium (SM) and control medium (CM). (E–H) Messenger RNA expression of hSTS, 17β-HSD2 and 17β-HSD1 and 17β-HSD5 in JEG-3 cells, after treatment with CM or SM for 48 hrs. ** p<0.01, n=3, error bar, SD. (I–K) Validation of Gli1-shRNA-, Gli2-shRNA-, and Gli3-shRNA-expressing lentivirus targeted to sequence 1 and 2, respectively. RNA and protein abundance normalized to β-actin, respectively. ** p<0.01, * p<0.05; n=3, error bar, SD.
Supplementary Fig. 2. Smo and Glis mediate Hh-induced transactivation of P450scc, 3β-HSD1 and aromatase genes in JEG-3 cells. (A) Cyclopamine at 5 μM (Cyc., +) inhibits Shh conditional medium (SM, +)-induced P450scc reporter activities in JEG-3 cells. (B) ΔN-Gli2, ca-Smo, Gli3 and Gli3-ΔC induce P450scc reporter activities in JEG-3 cells. (C) Gli3-ΔC is more active than Gli3 in induction of P450scc reporter activities in JEG-3 cells. (D) Cyclopamine at 5 μM (Cyc., +) inhibits Shh conditional medium (SM, +)-induced 3β-HSD1 reporter activities in JEG-3 cells. (E) ΔN-Gli2, Gli2 and ca-Smo but not Gli3 and Gli3-ΔC induce 3β-HSD1 reporter activities in JEG-3 cells. (F) ΔN-Gli2 is more active than Gli2 in induction of 3β-HSD1 reporter activities in JEG-3 cells. (G) Cyclopamine inhibits Shh conditional medium (SM)-induced aromatase reporter activities in JEG-3 cells. (H) ca-Smo induces aromatase reporter activities in JEG-3 cells. (I) Silence of Gli3 expression neither affects Shh conditional medium (SM)-induced aromatase reporter activities in JEG-3 cells. (J) ΔN-Gli2 induces aromatase reporter activities in JEG-3 cells. **, ‡p<0.01; n=3, error bar, SD.
Supplementary Fig. 3. Cyclopamine neither affects the proliferation and apoptosis of tumor cells, the mP4 and mE2 levels of circulation in ovariectomized nude mice bearing JEG-3 choriocarcinoma xenografts. (A) Body weights of ovariectomized nude mice bearing with xenografts, after administration with vehicle or different dosages of cyclopamine for indicated times. (B) Volume of tumors from ovariectomized nude mice with the same treatments. (C) Murine P4 levels in circulation of ovariectomized nude mice with the same treatments. (D) Murine E2 levels in circulation of ovariectomized nude with the same treatments. (E, E′, F, F′) Flow cytometric analysis of the proliferation rates in tumor cells labeled with BrdU. (G, G′, H, H′) TUNEL staining for detection of apoptosis in tumor cells. Scale bar, 10 μM. (I–K) Semi-quantification of uterine morphological changes in JEG-3 choriocarcinoma xenografts-bearing ovariectomized mice administrated with indicated dosages of cyclopamine. (I) Luminar heights in the uterus. (J) Uterine diameter. (K) Glands number per μM2.
Highlights.
A novel role of Hedgehog signaling in metabolism of cholesterol is uncovered.
Activation of Hh signaling triggers conversion of cholesterol to P4 and E2.
Activation of Hh signaling induces the expression of steroidogenic enzymes.
Steroidogenic enzyme genes are the downstream targets of Hedgehog signaling.
Acknowledgments
We thank Dr. Sasaki for his generous donation of plasmid. This work is supported by National Institutes of Health (R01 DK065789) to F.L., and by 973 Program (No. 2011CB944403), National Natural Science Foundation of China (No. 30973580, No. 31071292, No. 81370713), and Natural Science Foundation of Zhejiang Province, China (No. R2110269) to X.W..
Abbreviations
- ACTH
Adrenocorticotropic hormone
- caSmo
Constitutively active form of Smo
- CK1
Casein kinase 1
- DHEA
Dehydroepiandrosterone
- E2
Estradiol
- GH
Growth hormone
- GSK3β
Glycogen synthase kinase-3β
- Hh
Hedgehog
- Kif7
Kinesin family member 7
- PKA
Protein kinase A
- P4
Progesterone
- P450scc
P450 cholesterol side chain cleavage enzyme
- P5
Pregnenolone
- Smo
Smoothened
- STS
Steroid sulfatase
- SuFu
Suppressor of fused
- 3β-HSD1
3β-hydroxysteroid dehydrogenase type 1
- 17β-HSD
17β-hydroxysteroid dehydrogenase
Footnotes
Supplementary information includes five tables and three figures, can be found with this article online.
Conflict of interest
The authors have no conflicts of interest.
Contributors
C.T. designed and performed the experiments; Y.P., H.L. and W.X. performed the supplementary experiments of Western Blotting; H.Z. and H.R. performed the supplementary experiments of qRT-PCR; J.W. and C.Z. performed the supplementary experiments of Luciferase Assay; L.T. and T.I. performed the statistical analysis; F.L. and X.W. wrote the manuscript. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–563. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- 2.Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 3.Robbins DJ, Fei DL, Riobo NA. The hedgehog signal transduction network. Sci Signal. 2012;5:re6. doi: 10.1126/scisignal.2002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varjosalo M, Taipale J. Hedgehog: Functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 5.Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- 6.Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 7.Traiffort E, Dubourg C, Faure H, Rognan D, Odent S, Durou MR, David V, Ruat M. Functional characterization of sonic hedgehog mutations associated with holoprosencephaly. J Biol Chem. 2004;279:42889–42897. doi: 10.1074/jbc.M405161200. [DOI] [PubMed] [Google Scholar]
- 8.Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, Rohatgi R. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol. 2012;8:211–220. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, Parhami F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem. 2007;282:8959–8968. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- 11.Nedelcu D, Liu J, Xu Y, Jao C, Salic A. Oxysterol binding to the extracellular domain of Smoothened in Hedgehog signaling. Nat Chem Biol. 2013;9:557–64. doi: 10.1038/nchembio.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stottmann RW, Turbe-Doan A, Tran P, Kratz LE, Moran JL, Kelley RI, Beier DR. Cholesterolmetabolism is required for intracellular hedgehog signal transduction in vivo. PLoS Genet. 2011;7:e1002224. doi: 10.1371/journal.pgen.1002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konig A, Happle R, Bornholdt D, Engel H, Grzeschik KH. Mutations in the NSDHL gene, encoding a 3beta-hydroxysteroid dehydrogenase, cause CHILD syndrome. Am J Med Genet. 2000;90:339–346. [PubMed] [Google Scholar]
- 14.Albrecht E, Pepe G. Placental steroid hormone biosynthesis in primate pregnancy. Endocr Rev. 1990;11:124–150. doi: 10.1210/edrv-11-1-124. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Iguchi T, Itoh N, Okamoto K, Takagi T, Tanaka K, Nakanishi T. Ascorbic acid transported by sodium-dependent vitamin C transporter 2 stimulates steroidogenesis in human choriocarcinoma cells. Endocrinology. 2008;149:73–83. doi: 10.1210/en.2007-0262. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Zhu H, Pan Y, Tang C, Watanabe M, Ruan H, Wang Y, Wang J, Yao HY, Iguchi T. Ascorbic acid uptaken by sodium-dependent vitamin C transporter 2 induces βhCG expression through Sp1 and TFAP2A transcription factors in human choriocarcinoma cells. J Clin Endocrinol Metab. 2012;97:E1667–E1676. doi: 10.1210/jc.2012-1753. [DOI] [PubMed] [Google Scholar]
- 17.Payne A, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 18.Favaro R, Valotta M, Ferri AL, Latorre E, Mariani J, Giachino C, Lancini C, Tosetti V, Ottolenghi S, Taylor V, Nicolis SK. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12:1248–56. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- 19.Li JN, Ge YC, Yang Z, Guo CM, Duan T, Myatt L, Guan H, Yang K, Sun K. The Sp1 transcription factor is crucial for the expressionof 11b-hydroxysteroid dehydrogenase type 2 in humanplacental trophoblasts. J Clin Endocrinol Metab. 2011;96:E899–E907. doi: 10.1210/jc.2010-2852. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of β-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X, Zeng LH, Taniguchi T, Xie QM. Activation of PKA and phosphorylation of sodium-dependent vitamin C transporter 2 by prostaglandin E2 promote osteoblast-like differentiation in MC3T3-E1 cells. Cell Death Differ. 2007;14:1792–1801. doi: 10.1038/sj.cdd.4402190. [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi T, Kohroki J, Suzuki S, Ishizaki J, Hiromori Y, Takasuga S, Itoh N, Watanabe Y, Utoguchi N, Tanaka K. Trialkyltin compounds enhance human CG secretion and aromatase activity in human placental choriocarcinoma cells. J Clin Endocrinol Metab. 2002;87:2830–2837. doi: 10.1210/jcem.87.6.8540. [DOI] [PubMed] [Google Scholar]
- 23.Weiss U, Cervar M, Puerstner P, Schmut O, Haas J, Mauschitz R, Arikan G, Desoye G. Hyperglycaemia in vitro alters the proliferation and mitochondrial activity of the choriocarcinoma cell lines BeWo, JAR and JEG-3 as models for human first-trimester trophoblast. Diabetologia. 2001;44:209–219. doi: 10.1007/s001250051601. [DOI] [PubMed] [Google Scholar]
- 24.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 25.Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 26.Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- 27.Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem. 1999;274:8143–8152. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- 29.Pavletich NP, Pabo CO. Crystal structure of a five-finger GLI-DNA complex: new perspectives on zinc fingers. Science. 1993;261:1701–1707. doi: 10.1126/science.8378770. [DOI] [PubMed] [Google Scholar]
- 30.Kinzler KW, Vogelstein B. The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol Cell Biol. 1990;10:634–642. doi: 10.1128/mcb.10.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood GA, Fata JE, Watson KLM, Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction. 2007;133:1035–1044. doi: 10.1530/REP-06-0302. [DOI] [PubMed] [Google Scholar]
- 32.Tukachinsky H, Kuzmickas RP, Jao CY, Liu J, Salic A. Dispatched and scube mediate the efficient secretion of the cholesterol-modified hedgehog ligand. Cell Rep. 2012;2:308–320. doi: 10.1016/j.celrep.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malassinè A, Frendo JL, Evain-Brion D. A comparison of placental development and endocrine functions between the human and mouse model. Hum Reprod Update. 2003;9:531–539. doi: 10.1093/humupd/dmg043. [DOI] [PubMed] [Google Scholar]
- 34.King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci USA. 2009;106:21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park SY, Meeks JJ, Raverot G, Pfaff LE, Weiss J, Hammer GD, Jameson JL. Nuclear receptors Sf1 and Dax1 function cooperatively to mediate somatic cell differentiation during testis development. Development. 2005;132:2415–2423. doi: 10.1242/dev.01826. [DOI] [PubMed] [Google Scholar]
- 36.Wijgerde M, Ooms M, Hoogerbrugge JW, Grootegoed JA. Hedgehog signaling in mouse ovary: Indian hedgehog and desert hedgehog from granulosa cells induce target gene expression in developing theca cells. Endocrinology. 2005;146:3558–3566. doi: 10.1210/en.2005-0311. [DOI] [PubMed] [Google Scholar]
- 37.Huang CJ, Yao HH. Diverse functions of hedgehog signaling in formation and physiology of steroidogenic organs. Mol Reprod Dev. 2010;77:489–496. doi: 10.1002/mrd.21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vila G, Papazoglou M, Stalla J, Theodoropoulou M, Stalla GK, Holsboer F, Paez-Pereda M. Sonic hedgehog regulates CRH signal transduction in the adult pituitary. FASEB J. 2005;19:281–283. doi: 10.1096/fj.04-2138fje. [DOI] [PubMed] [Google Scholar]
- 39.Vila G, Theodoropoulou M, Stalla J, Tonn JC, Losa M, Renner U, Stalla GK, Paez-Pereda M. Expression and function of sonic hedgehog pathway components in pituitary adenomas: evidence for a direct role in hormone secretion and cell proliferation. J Clin Endocrinol Metab. 2005;90:6687–6694. doi: 10.1210/jc.2005-1014. [DOI] [PubMed] [Google Scholar]
- 40.Thomas MK, Lee JH, Rastalsky N, Habener JF. Hedgehog signaling regulation of homeodomain protein islet duodenum homeobox-1 expression in pancreatic beta-cells. Endocrinology. 2001;142:1033–1040. doi: 10.1210/endo.142.3.8007. [DOI] [PubMed] [Google Scholar]
- 41.Thomas MK, Rastalsky N, Lee JH, Habener JF. Hedgehogsignalingregulation of insulin production by pancreatic beta-cells. Diabetes. 2000;49:2039–2047. doi: 10.2337/diabetes.49.12.2039. [DOI] [PubMed] [Google Scholar]
- 42.Russell MC, Cowan RG, Harman RM, Walker AL, Quirk SM. The hedgehog signaling pathway in the mouse ovary. Biol Reprod. 2007;85:77226–77236. doi: 10.1095/biolreprod.106.053629. [DOI] [PubMed] [Google Scholar]
- 43.Buttitta L, Mo R, Hui CC, Fan CM. Interplays of Gli2 and Gli3 and their requirement in mediating Shh-dependent sclerotome induction. Development. 2003;130:6233–6243. doi: 10.1242/dev.00851. [DOI] [PubMed] [Google Scholar]
- 44.Groothuis PG, Dassen HHNM, Romano A, Punyadeera C. Estrogen and the endometrium: lessons learned from gene expression profiling in rodents and human. Hum Reprod Update. 2007;13:405–417. doi: 10.1093/humupd/dmm009. [DOI] [PubMed] [Google Scholar]
- 45.Heryanto B, Rogers PAW. Regulation of endometrial endothelial cell proliferation by oestrogen and progesterone in the ovariectomized mouse. Reproduction. 2002;123:107–113. doi: 10.1530/rep.0.1230107. [DOI] [PubMed] [Google Scholar]
- 46.Cooper M, Wassif C, Krakowiak P, Taipale J, Gong R. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 47.Weaver D, Solomon B, Akin-Samson K, Kelley R, Muenke M. Cyclopia (synophthalmia) in Smith-Lemli-Opitz syndrome: First reported case and consideration of mechanism. Am J Med Genet C Semin Med Genet. 2010;154C:142–145. doi: 10.1002/ajmg.c.30241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haas D, Muenke M. Abnormal sterol metabolism in holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:102–108. doi: 10.1002/ajmg.c.30243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. (A, B) RT-PCR assays for Hh ligands and the main components of Hh signaling in trophoblast-like cells, primary cytotrophoblasts and human placentas. (C, D) Gli-luciferase activities and Gli1 protein levels in JEG-3 cells in response to Shh conditional medium (SM) and control medium (CM). (E–H) Messenger RNA expression of hSTS, 17β-HSD2 and 17β-HSD1 and 17β-HSD5 in JEG-3 cells, after treatment with CM or SM for 48 hrs. ** p<0.01, n=3, error bar, SD. (I–K) Validation of Gli1-shRNA-, Gli2-shRNA-, and Gli3-shRNA-expressing lentivirus targeted to sequence 1 and 2, respectively. RNA and protein abundance normalized to β-actin, respectively. ** p<0.01, * p<0.05; n=3, error bar, SD.
Supplementary Fig. 2. Smo and Glis mediate Hh-induced transactivation of P450scc, 3β-HSD1 and aromatase genes in JEG-3 cells. (A) Cyclopamine at 5 μM (Cyc., +) inhibits Shh conditional medium (SM, +)-induced P450scc reporter activities in JEG-3 cells. (B) ΔN-Gli2, ca-Smo, Gli3 and Gli3-ΔC induce P450scc reporter activities in JEG-3 cells. (C) Gli3-ΔC is more active than Gli3 in induction of P450scc reporter activities in JEG-3 cells. (D) Cyclopamine at 5 μM (Cyc., +) inhibits Shh conditional medium (SM, +)-induced 3β-HSD1 reporter activities in JEG-3 cells. (E) ΔN-Gli2, Gli2 and ca-Smo but not Gli3 and Gli3-ΔC induce 3β-HSD1 reporter activities in JEG-3 cells. (F) ΔN-Gli2 is more active than Gli2 in induction of 3β-HSD1 reporter activities in JEG-3 cells. (G) Cyclopamine inhibits Shh conditional medium (SM)-induced aromatase reporter activities in JEG-3 cells. (H) ca-Smo induces aromatase reporter activities in JEG-3 cells. (I) Silence of Gli3 expression neither affects Shh conditional medium (SM)-induced aromatase reporter activities in JEG-3 cells. (J) ΔN-Gli2 induces aromatase reporter activities in JEG-3 cells. **, ‡p<0.01; n=3, error bar, SD.
Supplementary Fig. 3. Cyclopamine neither affects the proliferation and apoptosis of tumor cells, the mP4 and mE2 levels of circulation in ovariectomized nude mice bearing JEG-3 choriocarcinoma xenografts. (A) Body weights of ovariectomized nude mice bearing with xenografts, after administration with vehicle or different dosages of cyclopamine for indicated times. (B) Volume of tumors from ovariectomized nude mice with the same treatments. (C) Murine P4 levels in circulation of ovariectomized nude mice with the same treatments. (D) Murine E2 levels in circulation of ovariectomized nude with the same treatments. (E, E′, F, F′) Flow cytometric analysis of the proliferation rates in tumor cells labeled with BrdU. (G, G′, H, H′) TUNEL staining for detection of apoptosis in tumor cells. Scale bar, 10 μM. (I–K) Semi-quantification of uterine morphological changes in JEG-3 choriocarcinoma xenografts-bearing ovariectomized mice administrated with indicated dosages of cyclopamine. (I) Luminar heights in the uterus. (J) Uterine diameter. (K) Glands number per μM2.