Abstract
Adipose tissue plays a critical role in energy and metabolic homeostasis, but it is challenging to adapt techniques to modulate adipose function in vivo. Here we develop an in vivo, systemic method of gene transfer specifically targeting adipose tissue using adeno-associated virus (AAV) vectors. We constructed AAV vectors containing CMV promoter regulated reporter genes, intravenously injected adult mice with vectors using multiple AAV serotypes, and determined that AAV2/8 best targeted adipose tissue. Altering vectors to contain adiponectin promoter/enhancer elements and liver specific microRNA-122 target sites restricted reporter gene expression to adipose tissue. As proof of efficacy, the leptin gene was incorporated into the adipose-targeted expression vector, package into AAV2/8, and administered intravenously to 9-10 week old ob/ob mice. Phenotypic changes were measured over an eight week period. Leptin mRNA and protein were expressed in adipose and leptin protein was secreted into plasma. Mice responded with reversal of weight gain, decreased hyperinsulinemia, and improved glucose tolerance. AAV2/8-mediated systemic delivery of an adipose-targeted expression vector can replace a gene lacking in adipose tissue and correct a mouse model of human disease, demonstrating experimental application and therapeutic potential in disorders of adipose.
Keywords: Gene Therapy, Adipose, AAV, Leptin, Obesity
Adipose tissue has emerged not just as an energy storage depot but also as an active endocrine and paracrine organ that mediates homeostatic and patho-physiological actions in health and disease.1, 2 Because of its high lipid content and broad distribution in multiple depots throughout the body, it is difficult to adapt traditional techniques to modulate adipose function selectively in vivo for experimental and therapeutic applications.
Adeno-associated viruses (AAVs) are promising tools in the development of gene therapy for genetic disorders in humans and for studying disease and therapeutics in animal models.3 Unlike other viral vectors, such as adenoviruses, which tend to have immunogenic adverse effects, AAVs are nonpathogenic and have low immunogenicity, thus making them a safer alternative for therapeutic use in humans and suitable for chronic studies in animal models.3 AAVs target both dividing and quiescent cells and, despite predominantly remaining episomal in host cells, are able to induce relatively stable transgene expression.3, 4 AAVs have induced sustained transgene expression in small animal models,5 large animal models,6 and humans.7 In animal models, liver, heart, and skeletal muscle have been safely and successfully targeted for gene transfer.6, 8, 9 In Europe, AAV-based gene therapy to muscle was recently approved for patients with severe lipoprotein lipase (LPL) deficiency.10
Little work, however, has focused on targeting adipose. Adipose tissue is a secretory organ, and mature adipocytes are terminally differentiated, non-dividing cells, thus making adipose an attractive target for non-integrating gene expression vectors.3 Prior to AAV, a few studies used more immunogenic viruses such as adenovirus, retrovirus, and lentivirus to target adipose or adipocytes in culture for gene transfer.11, 12 More recently, a couple of groups have targeted adipose tissue with AAVs by directly injecting visceral or subcutaneous adipose in obese mice with virus in combination with Pluronics.13, 14 However, local delivery is limiting for translation and, to date, no studies have used systemically administered AAV to target multiple adipose tissue depots.
In this work, we developed a systemic AAV delivered vector to selectively target multiple adipose tissue depots. We show that recombinant AAV serotype 2/8 (AAV2/8) vectors target subcutaneous, brown, gonadal, and omental adipose tissues and that use of regulatory elements from the adiponectin promoter inhibits transgene expression in most other tissues that AAV2/8 transduce except for liver. Adipose specificity was further increased by inserting synthetic microRNA-122 (miR-122) target sites into the 3’UTR of the expression cassette. This liver specific microRNA (miRNA)15, 16 provides a tool for attenuation of expression of genes even when delivered to liver by AAV.17, 18 As proof of principle, we show that adipose targeting via AAV2/8 vectors can deliver the leptin gene to adipose tissue in ob/ob mice and correct the metabolic defects in adipose leptin deficiency.
Results
Recombinant adeno-associated virus serotype 2/8 transduces adipose tissue
To test efficacy of multiple serotypes targeting adipose tissue in vivo, we constructed an AAV2-based targeting vector containing a reporter gene regulated by a ubiquitous CMV promoter (Supplement Figure 1A). For initial serotype qualitative screening, the luciferase gene was used as reporter, i.e., AAV-CMV-Luciferase vector, and multiple AAV serotypes, including 2, 7, and 8 (e.g., AAV2/8 where “8” refers to the capsid serotype derived from AAV8) were administered to 2-3 month old C57BL/6J mice at a dose of 1×1012 GC, a dose that has been shown to be safe in mice19 and used to target cardiac tissue.17, 18 Two weeks after virus administration, relative to other vectors, luciferase reporter activity was highest in gonadal, omental, subcutaneous, and brown adipose tissues of mice that received the AAV2/8 reporter vector (Supplemental Figure 1B). As expected using a CMV promoter, luciferase activity was detected at high levels in heart, liver and other tissues (Supplement Figure 1B). Delivery of AAV2/8-CMV-enhanced green fluorescent protein (eGFP) vector also revealed green fluorescence in subcutaneous, omental, and gonadal adipose tissue and in liver (Supplemental Figure 1C). Although there was some heterogeneity in adipose depot expression of GFP compared to luciferase, these experiments qualitatively corroborate findings with AAV2/8-CMV-Luciferase, and demonstrate that these vectors transduce adipose tissue.
Human adiponectin enhancer/promoter enhances selectivity for adipose tissue
To enhance adipose targeting, the promiscuous CMV promoter was replaced with an adipose specific promoter. We cloned the adiponectin distal enhancer and proximal promoter as described20 and generated vector AAV2/8-Adipo-eGFP (Figure 1 A). Two weeks after C57BL/6J mice were administered AAV2/8-Adipo-eGFP (1×1012 GC). Qualitative imaging of fresh unfixed tissue detected eGFP fluorescence in subcutaneous, brown, omental, and gonadal adipose (Figure 1Bi-iv, respectively). Although these data were not designed to be quantitative, compared to mice receiving AAV2/8-CMV-eGFP, those receiving AAV2/8-Adipo-eGFP still had eGFP expression in adipose tissue (Figure 1C), but eGFP was not detected in heart (Figure 1C), or other organs, e.g., lung, kidney, and pancreas (Supplemental Figure 2). To our disappointment however, eGFP expression was not suppressed in liver (Figure 1Bv and Figure 1C respectively).
Figure 1. Transcriptional regulatory elements from human adiponectin gene enhance adipose selectivity of AAV2/8-induced transgene expression.
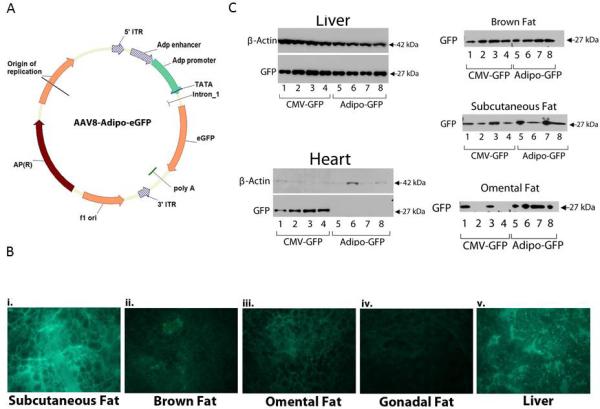
(a) Schematic illustration of the AAV packaging plasmid (AAV2/8-Adipo-eGFP) containing eGFP gene and adiponectin distal enhancer and proximal promoter. (b) Fluorescent microscopy imaging of eGFP expression in adipose and liver tissues. C57BL/6J mice were injected with AAV2/8-Adipo-GFP (1×1012 GC). For qualitative screening, fresh, unfixed adipose and liver tissue sections were visualized using a fluorescent microscope. Images were taken using the same exposure time for tissue samples per individual mouse. Representative microphotographs for adipose tissues (i subcutaneous, ii. brown, iii. omental, and iv. gonadal and liver v.) are depicted. (c) Immunoblot analyses of eGFP expression in mouse tissue. Lanes correspond to tissue samples from individual mice (see also, supplemental Figure 2).
MicroRNA-122 Eliminates Hepatic Transgene Expression
In order to inhibit liver expression of the reporter gene, we incorporated the miR-122 target sequence (miR-122T) into the 3’UTR of the AAV2/8-Adipo-eGFP vector to make AAV2/8-Adipo-eGFPmiR122(x) vectors where “x” is target sequence copy number (Figure 2A). As controls, AAV2/8-Adipo-eGFPScr(x) vectors were generated. Initially, 8 copies of miR-122T or scrambled sequence were used. Mice were injected with AAV2/8-Adipo-eGFPmiR122(8x), AAV2/8-Adipo-eGFPScr(8x), or AAV2/8-Adipo-eGFP. Transduction, based on qualitative western blot screening, with all three vectors induced eGFP expression in subcutaneous, gonadal, omental, and brown adipose (Figure 2B) but eGFP was clearly not detected in livers from mice administered AAV2/8-Adipo-eGFPmiR122(8x) while it was detected in mice receiving AAV2/8-Adipo-eGFPScr(8x) (Figure 2B). These data indicate that miR-122 directly inhibited expression of the eGFP transgene in liver.
Figure 2. miR-122-regulated AAV2/8 vectors eliminate liver expression.
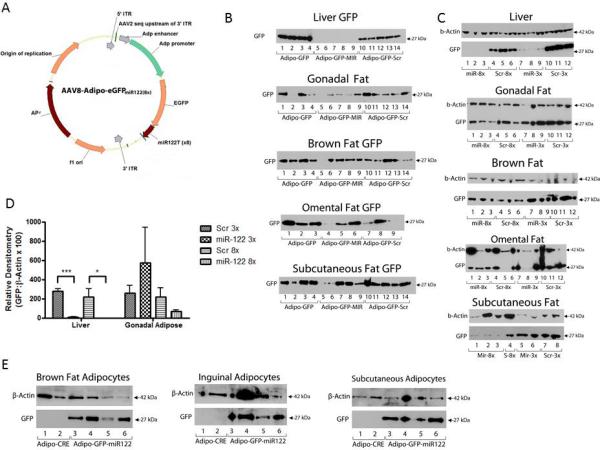
(a) Schematic illustration of AAV packaging plasmid, AAV2/8-Adipo-eGFPmiR122(x), containing an expression cassette with eGFP, human adiponectin promoter elements, and variable(x) 3’UTR miR-122 target sequences. (b) Immunoblot analyses of eGFP expression in liver and adipose tissues. Mice were injected with 1×1012 GC of AAV2/8-Adipo-eGFP, AAV2/8-Adipo-eGFPmiR122(8x) (8 target sequence repeats), or AAV2/8-Adipo-eGFPScr(8x) (8 scrambled target sequence repeats). Two weeks post-virus, organs were dissected and flash frozen before immunoblot analyses. (c) Immunoblots comparing effects of number of repeats of miR-122 target sequence on eGFP expression in liver and adipose from mice administered AAV2/8 vectors with either 3 or 8 copies of miR-122 target sequences or matching scrambled sequences. (d) Semi-quantitative densitometry analyses of eGFP protein expression relative to β-actin expression from liver and gonadal adipose tissues. Data are mean ± s.e., n=3, * P<0.05. (e) eGFP immunoblots of isolated mature adipocytes from mice receiving AAV2/8-Adipo-eGFPmiR122(8x) or control AAV2/8 without eGFP. Lanes represent tissue samples from individual mice.
Next, we tested the ability of fewer miR-122T repeats to inhibit reporter gene expression in the liver. In mice receiving AAV2/8-Adipo-GFPmiR122(3x), hepatic eGFP expression was highly repressed but there still was minor residual expression (Figure 2C). Semi-quantitative densitometry analyses of western blots in Figure 2C confirmed that both 3x and 8x repeats of miR-122T highly repressed eGFP expression in the liver, while adipose tissue continued to express eGFP in the presence of miR-122T (Figure 2D). Adipocytes isolated from brown, subcutaneous, and gonadal adipose treated with AAV2/8-Adipo-GFPmiR122(8x) expressed eGFP (Figure 2E). These data show that systemic delivery of an AAV2/8 vector containing a reporter gene regulated by an adiponectin enhancer/promoter and liver-specific miRNA target sites induced transgene expression selectively in mouse adipose tissue.
Adipose-targeted AAV2/8 delivery of leptin attenuates the ob/ob mouse phenotype
We replaced the eGFP gene in the AAV2/8-Adipo-eGFPmiR122(8x) vector with human leptin (AAV2/8-Adipo-LeptinmiR122(8x)) and administered this via tail vein injection to nine-week old leptin deficient ob/ob mice. Control ob/ob mice received AAV2/8-Adipo-eGFPmiR122(8x). The human leptin gene was used in order to distinguish between transgene mRNA and endogenous mouse leptin mRNA which is still transcribed in ob/ob mice. As expected, ob/ob mice were obese, hyperphagic, and had elevated glucose and insulin levels.
Within 7 days of AAV2/8-Adipo-LeptinmiR122(8x) administration, plasma leptin levels ranged from 0.1 to 0.4 ng/mL and then increased slightly through 42 days post viral treatment (Figure 3Ai). The peak average leptin level, 0.3 ± 0.05 ng/mL, was approximately 7% of the level we observed in a one-time measurement of age-matched WT C57/BL/6J mice (average 4.4 ng/mL) (Figure 3Aii) and wild-type rodent levels reported by others (3-7 ng/mL).21, 22 These leptin levels, however, were sufficient to have marked and rapid effects on appetite, weight and glucose homeostasis. By 4-7 days post-virus administration, the leptin-treated group already consumed less food than control ob/ob mice (4.4 ± 0.6 g/day vs. 6.7 ± 0.4 g/day, p <0.001). After 25 days, average food consumption of the leptin treated group had decreased to amounts near the measured range (3.5 to 3.9 g/day) of aged matched wild type C57BL/6J mice (Figure 3B). On average, the leptin treated ob/ob mice ate at least 2 g/day less food than the control mice. With decreased food consumption, AAV2/8-Adipo-LeptinmiR122(8x) treated ob/ob mice lost weight gradually while control ob/ob mice continued to gain weight. After 50 days, AAV-leptin treated ob/ob mice average weight was 14 g less than control mice (36.8 ± 1.1 g vs. 51.0 ± 1.1 g, p <0.001) and weighed 3.5 g less than baseline whereas control ob/ob mice weighed 11.1 g more than baseline (Figure 3C).
Figure 3. Adipose-targeted AAV2/8 delivery of leptin gene in ob/ob mice alleviates the leptin deficient phenotype.
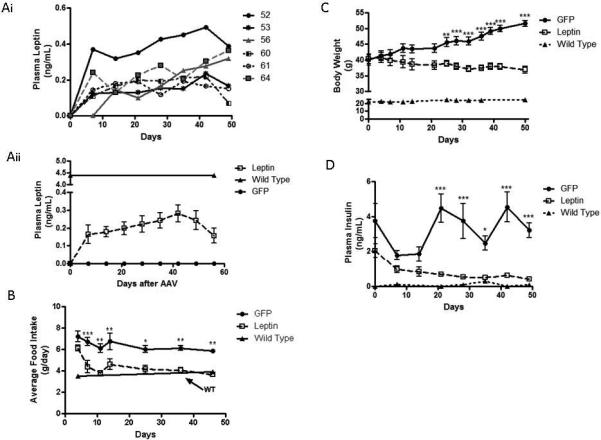
Ob/ob mice (9-10 week old) were administered 1×1012 GC of AAV2/8-Adipo-LeptinmiR122(8x) or AAV2/8-Adipo-eGFPmiR122(8x), designated as “Leptin” or “GFP”, respectively. Food intake and body weight were measured periodically and plasma samples were taken weekly from 5 hr-fasted mice. Data are mean ± s.e., n=5-6, except where indicated. * P<0.05, **P<0.01, and **P<0.001, significant difference between Leptin and GFP groups at indicated time points. (ai) Plasma leptin levels in individual mice receiving the leptin gene are displayed. Leptin was not detected in control mice. (aii) Average leptin levels in AAV2/8-Adipo-LeptinmiR122(8x) treated mice relative to a one-time measurement of average leptin levels in age-matched wild type mice. (b) Periodic average daily food consumption in Leptin and GFP groups relative to age-matched wild type mice. (c) Body weight in Leptin and GFP ob/ob mice and age matched wild type mice. (d) Plasma insulin in Leptin and GFP ob/ob mice and age matched wild type mice.
With increasing obesity, ob/ob mice become more hyperinsulinemic and fasting insulin levels were >10-fold higher in control vector-treated ob/ob mice (1.1-6.6 ng/mL) compared to WT mice (Figure 3D). In contrast, fasting insulin levels in ob/ob mice receiving AAV2/8-Adipo-LeptinmiR122(8x) dropped by 75-80% and approached levels observed in age-matched WT mice (0.4-0.5 ng/mL for leptin-treated vs. 0.1 ng/mL for WT mice). Prior to administration of AAV, glucose tolerance testing (GTT) revealed a similar degree of glucose intolerance in both groups of ob/ob mice (Figure 4A). At 2 (Figure 4B), 4 (Figure 4C) and 7 (Figure 4D) weeks after AAV, mice receiving AAV2/8-Adipo-LeptinmiR122(8x) had improved glucose tolerance compared to control vector treated ob/ob mice, with statistically significant improvements in glucose AUC by 4 and 7 weeks post AAV; the GTT AUC profile approached that of WT mice by 7-weeks post AAV.
Figure 4. Adipose targeted AAV2/8 delivery of leptin gene in ob/ob mice improves glucose tolerance.
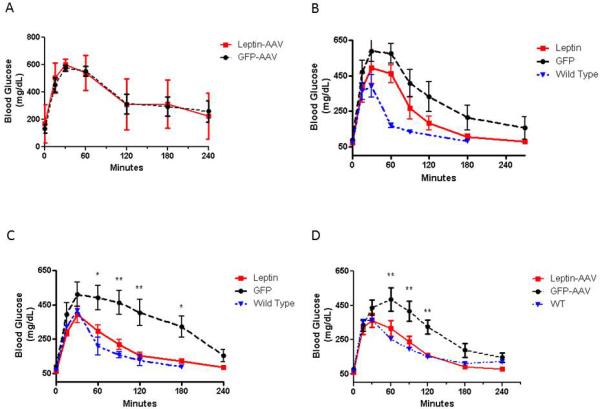
Ob/ob mice received AAV2/8-Adipo-LeptinmiR122(8x) or AAV2/8-Adipo-eGFPmiR122(8x), designated as “Leptin” or “GFP”, respectively, in legend. Glucose tolerance tests (GTT) were performed at baseline (a), 2 (b), 4 (c), and 7 (d) weeks post AAV administration on 16-hour fasted mice. Data are mean ± s.e., n=5-6. * P<0.05, and **P<0.01, significant difference between leptin and GFP groups.
We assessed the degree of adipose selectivity in leptin gene expression. At sacrifice, eight weeks after AAV delivery, human leptin mRNA levels in subcutaneous, brown, and omental adipose tissues were several hundred fold higher than levels in lungs and at least five fold higher than levels detected in heart and skeletal muscle (Figure 5A). Biodistribution assays, which quantified average viral DNA copy number in total tissue DNA, revealed liver samples contained 100-, 280-, and 220-fold more copies of AAV DNA than brown, gonadal, and omental adipose samples, respectively (data not shown). Despite this vastly disparate delivery of viral DNA, relative mRNA levels in livers were comparable to adipose tissue levels, demonstrating that in addition to the differential activity of the adiponectin promoter/enhancer in liver vs. adipose, RNA expression in liver was strongly attenuated by the miR-122T. Indeed, the amount of leptin protein, normalized to total protein per tissue, was highest in adipose tissues compared to all other tissues tested (e.g., 34 and 39 pg leptin/mg protein in subcutaneous and brown fat respectively compared to 10 pg/mg in liver) (Figure 5B). These data also show that some tissues, e.g. lung, had higher leptin protein levels than expected based on mRNA expression data, suggesting the possibility of tissue contamination from circulating leptin.
Figure 5. Human leptin mRNA and protein expression in ob/ob mice tissues.
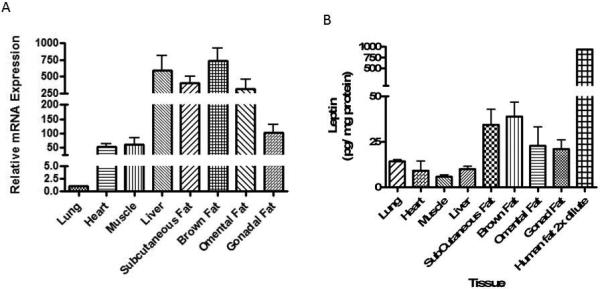
Eight weeks post virus administration, tissue samples from AAV2/8-Adipo-LeptinmiR122(8x) treated mice were analyzed for human leptin mRNA and protein. Data are shown as mean ± s.e., n=5-6. No human leptin mRNA or protein was detected in control treated ob/ob mice. (a) Human leptin mRNA relative expression, normalized to that tissue's respective β-Actin mRNA expression. Expression in each tissue is displayed relative to lung (set to 1). (b) Human leptin protein expression in mouse tissue supernatants, normalized to total protein in tissue supernatants.
Discussion
Targeted genetic manipulation in adipose is important for understanding adipose physiology, its role in disease, and for development of therapeutics. We tested gene transfer using several AAV types and determined that the recombinant AAV2/8 serotype was most effective at targeting adipose, but also transduced other tissues. Adipose selectivity was enhanced by incorporating proximal promoter and distal enhancer elements from the adiponectin gene into the AAV vector, yet expression persisted in liver. Addition of multiple copies of the liver-specific miR-122T into the 3’UTR of the expression cassette, however, inhibited GFP protein expression in liver to undetectable levels. As proof of principle, the human leptin gene was inserted in the AAV expression vector and administered to ob/ob mice. Human leptin mRNA and protein were expressed in multiple adipose depots and plasma leptin was readily detectable, albeit at low levels. These levels, however, reversed weight gain, decreased hyperinsulinemia and improved glucose tolerance indicating that in vivo AAV2/8 delivery of our adipose targeting vector rescued metabolic phenotypes in a rodent model of human disease. These studies represent the first time in which sustained and largely selective gene transfer to multiple adipose tissue depots was accomplished using a single dose of a systemically administered gene expression vector.
Adipose tissue is critical for normal physiology including energy intake and expenditure, thermoregulation, and insulin and glucose homeostasis. The complexity of adipose biology, the gravity of pathology23 arising from genetic defects in adipose24-26 and the epidemic of obesity and type 2 diabetes underscore the importance of developing methods to selectively target adipose for gene transfer. Adipose targeting for genetic manipulation in vivo is challenging, however, even in mouse models and often relies on conditional knockout of genes of interest. Gene transfer with AAV offers the potential for higher throughput studies and the ability to knock-down, replace or overexpress genes in adipose coupled to the unique potential for therapeutic applications. AAVs are nonpathogenic and elicit only low innate immune and inflammatory responses in animals and humans, conferring a major advantage over other viral-mediated gene therapy modalities.27 In fact, the Committee on Human Medicinal Products in Europe recently approved the first human gene therapy treatment, Glybera (uniQure), utilizing AAV to deliver a variant of lipoprotein lipase (LPL) to skeletal muscle for patients with LPL deficiency.10
In order to confer greater adipose selectivity to the AAV2/8 vector, we included regulatory regions from the human adiponectin promoter, expressed specifically in adipocytes. To account for AAV packaging size constraints, a modified 700 bp enhancer/promoter construct20 from the adiponectin gene increased the adipose selectivity of the AAV 2/8 vector. In our initial experiments of increasing adipose selectivity, we were interested primarily in crude screening using GFP presence in adipose and other tissues, so we probed for β-actin only selectively, knowing that we were going to further modify the vectors. Despite transcriptional control by this adipose specific enhancer/promoter, our initial targeting vector still induced significant liver transgene expression. One possibility is that the enhancer unit from the adiponectin promoter leads to expression in the liver. A similar phenomenon was observed with muscle-specific promoters with a strong myosin heavy chain (MHC) enhancer.18 The adiponectin enhancer contains two completely conserved CCAAT boxes that bind the transcription factor CCAAT/enhancer binding protein alpha,20 which regulates gene expression in many cell types including hepatocytes. Thus, in liver, which is highly transduced by AAV after intravascular administration,28-30 leaky adiponectin enhancer activity and appreciable transgene expression is not unexpected.
In order to prevent liver transgene expression, we used miRNA-dependent knockdown.31 miR-122 is an evolutionary conserved15 and highly abundant15, 16 liver specific miRNA. Three copies of miR-122T drastically inhibited GFP expression in liver, and eight copies reduced GFP expression to undetectable levels. It is noteworthy that increasing the copy number of miR-122T may have caused some destabilization of reporter gene mRNA. Decreases in liver expression were observed when increasing scrambled miR-T copies from 3 to 8, which resulted in a longer 3’ UTR by approximately 130 bp. It has been previously documented that increasing the length of the 3’ UTR can lead to decreased reporter gene expression32 and potential nonsense mediated RNA decay due to changes in secondary structure.33 In studies parallel to ours, Qiao et al. and Geisler et al. took similar strategies and used miR-122T in AAV9 vectors targeting cardiac tissue to inhibit aberrant transgene expression in liver.17, 18 In our studies, we did not directly examine possibly toxicities, e.g., hepatic steatosis, hepatitis, or hepatocellular carcinoma that have been observed in miR-122 knockout models34 and that, theoretically, may arise from exogenous miR-122T within a highly expressed transgene competing with endogenous miR-122T for miR122. This possibility is, however, very unlikely because miR-122 is so abundant in the liver that saturation is improbable. Indeed, published work suggests that significant concentrations of a perfectly complementary miRNA target sequence do not appreciably affect the regulation of imperfect targets to their respective miRNA.31 Further, Geisler et al. found an absence of biochemical and histological liver side effects after administration of AAV containing miR-122T and also found no change in expression of Gys1, a protein regulated by miR-122.17, 35 The dose they used, 7.5 × 10^11 GC,17 was only 25% less than the 1.0 × 10^12 GC used in our studies, a dose that has been used in other studies without inducing significant liver toxicity.19 These doses in mice are approximately 35 times larger per kg body mass than a 1 × 10^12 GC/kg dose of alipogene tiparvovec (Glybera) that was demonstrated to be safe and effective in humans,36 thus raising questions about the dosing of our vector that would be required for adipose directed studies in humans and suggests that further enhancements in vector design may be required for human translation as discussed subsequently.
Leptin mRNA expression was higher in adipose than all other tissues, indicating that the adiponectin promoter/enhancer elements in the AAV vector were able to hinder leptin expression in non-adipose tissue. Based on our vector screening data, we would have expected to still detect substantial leptin mRNA in the liver despite its transcription being suppressed by the adiponectin promoter/enhancer. However, noting that biodistribution assays revealed hundreds fold more AAV genome copies in liver compared to adipose tissues, the leptin mRNA levels in liver were remarkably low. This reduced level of human leptin mRNA indicate the miR-122T in the vector leads to mRNA degradation as expected37 and agrees with results showing degradation of mRNA in liver when using miR-122 to increase cardiac specificity of an AAV vector.17 Inhibition of translation of the intact leptin mRNA in the liver also could lead to further suppression of extraneous leptin protein synthesis.38 Indeed, we found that subcutaneous and brown adipose expressed ~3-fold higher leptin protein than liver suggesting possible translation inhibition of leptin mRNA. However, we recognize that liver expression was not fully ablated.
Leptin, a protein hormone with a cytokine-like structure produced almost exclusively by adipocytes, activates leptin receptors in the hypothalamus to reduce food intake and enhance thermogenesis and metabolic rate.39 Although rare, congenital leptin deficiencies in humans result in hyperphagia, hyperinsulinemia, and severe obesity.24 The ob/ob mouse which has a total loss of leptin protein has strikingly similar phenotype to congenital leptin deficiency in human, and therefore represents a cogent model of human disease to test the therapeutic potential of our adipose targeted AAV vector. Recombinant leptin therapy through daily subcutaneous injections significantly reduces appetite, body fat, hyperinsulinemia, and hyperlipidemia in leptin-deficient humans.40 The phenotype of ob/ob mice is also corrected by exogenous leptin introduced through injections,41 transplantation of wild type fat pads,42 or systemic adenovirus mediated leptin gene transfer.43 Additionally, AAV-mediated intramuscular leptin gene transfer rescued the obese phenotype22 because leptin is secreted and is biologically active even if expressed ectopically. However, no prior gene therapy based study has sought to reintroduce the leptin gene in its native tissue.
Adipose and plasma levels of human leptin in AAV2/8-Leptin-miR122 treated ob/ob mice were less than 10% of leptin protein levels in WT mice and in humans. However, this concentration of circulating leptin was able to correct hyperphagia, induce weight loss or prevent weight gain, alleviate hyperinsulinemia, and increase glucose tolerance to near wild type levels. Similar results are observed in leptin deficient patients when leptin levels are restored to 10% of normal levels using recombinant leptin therapy.44 Our data is consistent with clinical studies of genetic deficiencies in factor VIII, factor IX, and LPL where profound corrections of physiologic aberrancies in response to AAV gene therapy were associated with very small corrections of physiological levels (5-10% of normal levels) of the deficient gene product.6, 10, 45 Thus, the ability to replace a defective adipose secreted protein and correct the physiological defect with an adipose-targeted AAV vector in a rodent model of human disease is encouraging for translation and therapeutics.
Several challenges, however, remain for wider application. Leptin can have biological effects at levels less than 10% of biologically normal, whereas other defective genes may need to be replaced to a greater extent with the corrected gene product to be therapeutically beneficial. The low expression levels of leptin that were achieved indicate that use of this AAV8 adiponectin/miR122 vector to replace defective genes in adipose tissue to treat other diseases including different forms of lipodystrophy that result from defective transcription factors or enzymes24-26 may be limited where higher expression of the corrected gene is necessary. This current limitation of the vector may affect the ability to express adequate levels of a gene of interest or rapidly generate adipose-specific conditional knockouts, (i.e. expressing CRE in adipose of floxed mice) for testing biological hypotheses. Further, despite use of eight copies of miR-122T there was modest residual expression of the leptin transgene in the liver, as has been also observed by others.18 Residual ectopic expression may be difficult to eliminate but might not be essential in studying the biology or therapeutic potential of gene transfer. In order to address these limitations, however, future studies should assess the efficacy of AAV2/9, which may have lesser tropism for liver, add additional enhancer elements from the adiponectin promoter, and evaluate a wider range of miR-122T repeats with these alternative vectors.
This study represents the first time multiple adipose tissue depots have been targeted with selectivity for gene transfer with one systemic administration of an AAV targeting vector. With the growing obesity epidemic, further refinement and application of adipose-targeted AAV vectors will provide tools to better understand the role of adipose in metabolic homeostasis and disease and facilitate therapeutic advances for adipose related disorders.
Materials and Methods
Rodent studies and in vivo gene transfer
All procedures involving the use and care of animals were performed according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and according to guidelines set for forth by the University of Pennsylvania's Institutional Animal Care and Use Committee. For initial AAV testing, 2-3 month old C57BL/6J (Jackson Laboratories, Bar Harbor, ME) male mice were injected intravenously (IV) via tail vein with 1×1012 genome content (GC) of AAV containing vectors of choice in phosphate buffer saline (PBS). After 2 weeks, mice were sacrificed, fresh adipose and liver tissue slices prepared for GFP fluorescent microscopy, and remaining tissue frozen in liquid nitrogen.
For leptin therapy studies, 6-week old male ob/ob mice (Jackson Laboratories) were singly housed in and body weight and tail blood glucose levels were measured twice weekly for 3 weeks. Weight-matched ob/ob were then divided into 2 groups and treated with 1×1012 GC of AAV expressing leptin or eGFP (n=5-6/group). Over eight weeks, body weight, food intake, and 5 hour-fasted blood glucose levels were measured twice weekly with a Contour glucometer (Bayer, Leverkusen, Germany). Retro-orbital blood was obtained weekly for measurement of plasma leptin and insulin by ELISA. Glucose tolerance testing (GTT) was performed at two-week intervals by measuring blood glucose levels over time after intraperitoneally administered D-glucose (2g/kg body weight).
Construction of AAV packaging (cis) plasmid for AAV production
The promoter and distal enhancer of adiponectin (hAdp) were constructed by SOEing PCR (Polymerase Chain Reaction-Splicing by Overlapping Extension) using 2 sets of primers and human genomic DNA as template. In short, primer set, Adp-Bgl-F1: 5’-AGCTAAGATCTCTCTTTCCACATGACGGCCTTTG -3’), and Adp-SOE-R: 5’-CTGAATTCTTAAAGCTGGCGCGCCGCTGTAGCTATTGCACAAGGTGGAATAAC -3’) were used to PCR amplify the distal enhancer (−2664-2507). A second set of primers, Adp-SOE-F (5’-GTGCAATAGCTACAGCGGCGCGCCAGCTTTAAGAATTCAGGGCCT -3’) and Adp-Mlu-R1 (5’-AGCTAACGCGTCCCTCTGGTATGGAATCAG -3’), were used to amplify the promoter (−540 to +77). The PCR products from these 2 reactions served as templates for amplification using primers: Adp-Bgl-F1, and Adp-Mlu-R1. The final SOEing PCR product was then cloned into pZac2.1, an AAV packaging plasmid, in between MluI and BglIII sites, to replace CMV promoter to generate pENN.AAV.hAdp. The plasmid, pAAV.hAdp.EGFP-miR122T.SV40, encoding the reporter EGFP and tandem repeats of miR-122 target sequences in 3’UTR was then created by sequential insertion of EGFP and 3 or 8 copies of the synthetic miR-122a sequences (CAAACACCATTCAACACTCCA) downstream of the hAdp promoter. The control vector expressing the reporter and encoding the repeats of scrambled miRNA target sequences (GGAGCTCCACCGCGGTGGCAT) was made using the same strategy.
Recombinant AAV production
AAV vectors were generated by triple transfection using: (1) an AAV cis plasmid carrying a transgene expression cassette flanked by the viral ITRs (2) an AAV trans plasmid encoding the AAV2 rep and AAV8 (or other serotypes) capsid genes and (3) a plasmid encoding adenoviral genes providing helper functions for AAV replication. The plasmids were used to transfect subconfluent HEK293 cell (ATCC) grown in 6 cell stack factories using PEI–Max (Polysciences, Warrington, PA) and the vectors purified from cell lysates and supernatant as described previously.46
Adipocyte Isolation
Adipose tissue samples from leptin or control treated ob/ob mice (500 mg), were prepared as described47 using 1 mg/mL collagenase (Roche, Cat. #: 11213857001). Digested tissue was filtered through a 200 μm filter (Becton Dickinson, Franklin Lakes, NJ) and collagenase neutralized by adding 9 mL DMEM with 10% FBS. The filtrate was centrifuged for 10 min at 500 × g to separate the floating mature adipocytes from the stromal vascular fraction. The mature adipocytes were pipetted off the top, lysed with RIPA buffer, and subjected to immunoblot analyses.
ELISA Analyses
Plasma levels of mouse insulin and leptin were measured by sandwich ELISA using ALPCO's Mouse Ultrasensitive Insulin ELISA (Salem, NH) and Millipore's Human Leptin “Dual Range” ELISA (EMD Millipore, Billerica, MA) according to manufacturer's instructions. The leptin ELISA was adapted to measure tissue leptin levels using supernatant from tissue samples homogenized with a TissueLyser in RIPA buffer containing 1% Triton X-100. The lysate was used in place of plasma in the ELISA kit, leptin concentrations normalized to tissue protein content, and results were compared to serially diluted human adipose lysate samples assayed in parallel.
Luciferase Activity Assay
Supernatants from tissues (25–100mg) were homogenized in luciferase lysis buffer and analyzed for luciferase activity using the Luciferase Assay System (Promega, Madison, WI) according to manufacturer's protocol with activity normalized to protein content.
Real-time PCR Analysis
RNA was isolated from tissues using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. First strand cDNA was reverse transcribed from 1000 ng RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems (ABI), Foster City, CA). RT reactions were performed using standard methods (ABI) and real-time PCR analysis was performed using Taqman normalized to beta actin (ABI Prism 7900 Sequence Detection System; Applied Biosystems). Taqman premixed primer/probe sets were human leptin (Hs00174877_m1), mouse leptin (Mn00434759_m1) and mouse beta actin (4352341E).
Immunoblot Analysis
Tissue or cell samples (~100mg) were analyzed by standard SDS-PAGE electrophoresis immunoblotting using primary rabbit antibodies against GFP (D5.1) and beta actin (13E5) (Cell Signaling Technology, Danvers, MA) and secondary hrp-goat anti-rabbit antibody (Invitrogen), and visualized by chemiluminescence.
Quality control and Biodistribution of AAV vectors
Genome titers (i.e., genome copies [GC]/ml) of purified AAV vectors were determined by real-time PCR using a primer–probe set corresponding to the poly(A) region of the vector and linearized plasmid standards. The primer sequences for SV40 poly A TaqMan analysis are as follows: forward primer, 5’-AGCAATAGCATCACAAATTTCACAA-3’, reverse primer, 5’ CCAGACATGATAAGATACATTGATGAGTT-3’ and probe, 5’-6FAMAGCATTTTTTTCACTGCATTCTAGTTGTGGTTTGTC –TAMRA. For biodistribution, DNA was extracted from mouse tissue using QIAamp DNA mini kit (Qiagen) according to manufacturer's protocol. AAV genome was detected by detecting SV40 polyA sequence in the vector as described above. For each sample, 3 reactions were conducted including one reaction spiked in with 10E+6 copies of linearized plasmid encoding the target sequence for monitoring reaction efficiency. The observed values of spiked reaction must be greater than 20% of expected values to pass assay acceptance criteria.
Statistical Analysis
Data are expressed as mean ± standard error of mean (s.e.m.). To test for statistical significance, an unpaired Student's t-test was applied or 1 way ANOVA for grouped data with Bonferroni post-hoc tests when ANOVA revealed statistical differences between groups. For statistical significance in GTTs or time course studies, 2-way ANOVA was used with Bonferroni post-hoc testing as above.
Supplementary Material
Acknowledgments
We thank the University of Pennsylvania Vector Core for providing the vectors and conducting the biodistribution analyses. This work was supported by research awards from the National Institutes of Health (NIH) to the University of Pennsylvania including R01-DK-090505 (to Muredach Reilly), a NIDDK Diabetes and Endocrine Research Center award (P20-DK 019525), and by a NIDDK Molecular Therapy P30 Center Grant (P30-DK-047757). Sean O'Neill received support from a NRSA grant 5-T32-HL07748 funded by the National Heart, Lung, and Blood Institute. We acknowledge the Diabetes Research Center Mouse Phenotyping Core for their work supported by P30-DK19525. Muredach Reilly is supported also by R01-DK071224, U01-HL108636, and K24-HL107643 from the NIH.
Footnotes
Conflicts of Interest: There are no conflicts of interest to report.
Supplementary information is available at Gene Therapy's website.
References
- 1.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29(1):109–17. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- 2.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao H, Molday RS, Hu J. Gene therapy: light is finally in the tunnel. Protein & cell. 2011;2(12):973–89. doi: 10.1007/s13238-011-1126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter BJ. Adeno-associated virus and the development of adeno-associated virus vectors: a historical perspective. Molecular therapy : the journal of the American Society of Gene Therapy. 2004;10(6):981–9. doi: 10.1016/j.ymthe.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Wang B, Li J, Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(25):13714–9. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arruda VR, Stedman HH, Nichols TC, Haskins ME, Nicholson M, Herzog RW, et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105(9):3458–64. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18(3):643–50. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(18):11854–9. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo YJ, Zhang JC, Taylor MD, Cohen JE, Hsu VM, Sweeney HL. One year transgene expression with adeno-associated virus cardiac gene transfer. International journal of cardiology. 2005;100(3):421–6. doi: 10.1016/j.ijcard.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Yla-Herttuala S. Endgame: glybera finally recommended for approval as the first gene therapy drug in the European union. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20(10):1831–2. doi: 10.1038/mt.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morizono K, De Ugarte DA, Zhu M, Zuk P, Elbarbary A, Ashjian P, et al. Multilineage cells from adipose tissue as gene delivery vehicles. Human gene therapy. 2003;14(1):59–66. doi: 10.1089/10430340360464714. [DOI] [PubMed] [Google Scholar]
- 12.Nagamatsu S, Nakamichi Y, Ohara-Imaizumi M, Ozawa S, Katahira H, Watanabe T, et al. Adenovirus-mediated preproinsulin gene transfer into adipose tissues ameliorates hyperglycemia in obese diabetic KKA(y) mice. FEBS letters. 2001;509(1):106–10. doi: 10.1016/s0014-5793(01)03146-5. [DOI] [PubMed] [Google Scholar]
- 13.Mizukami H, Mimuro J, Ogura T, Okada T, Urabe M, Kume A, et al. Adipose tissue as a novel target for in vivo gene transfer by adeno-associated viral vectors. Human gene therapy. 2006;17(9):921–8. doi: 10.1089/hum.2006.17.921. [DOI] [PubMed] [Google Scholar]
- 14.Zhang FL, Jia SQ, Zheng SP, Ding W. Celastrol enhances AAV1-mediated gene expression in mice adipose tissues. Gene therapy. 2011;18(2):128–34. doi: 10.1038/gt.2010.120. [DOI] [PubMed] [Google Scholar]
- 15.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA biology. 2004;1(2):106–13. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 16.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–8. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 17.Geisler A, Jungmann A, Kurreck J, Poller W, Katus HA, Vetter R, et al. microRNA122-regulated transgene expression increases specificity of cardiac gene transfer upon intravenous delivery of AAV9 vectors. Gene therapy. 2011;18(2):199–209. doi: 10.1038/gt.2010.141. [DOI] [PubMed] [Google Scholar]
- 18.Qiao C, Yuan Z, Li J, He B, Zheng H, Mayer C, et al. Liver-specific microRNA-122 target sequences incorporated in AAV vectors efficiently inhibits transgene expression in the liver. Gene therapy. 2011;18(4):403–10. doi: 10.1038/gt.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kassim SH, Li H, Bell P, Somanathan S, Lagor W, Jacobs F, et al. Adeno-associated virus serotype 8 gene therapy leads to significant lowering of plasma cholesterol levels in humanized mouse models of homozygous and heterozygous familial hypercholesterolemia. Human gene therapy. 2013;24(1):19–26. doi: 10.1089/hum.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segawa K, Matsuda M, Fukuhara A, Morita K, Okuno Y, Komuro R, et al. Identification of a novel distal enhancer in human adiponectin gene. The Journal of endocrinology. 2009;200(1):107–16. doi: 10.1677/JOE-08-0376. [DOI] [PubMed] [Google Scholar]
- 21.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature medicine. 1995;1(11):1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 22.Murphy JE, Zhou S, Giese K, Williams LT, Escobedo JA, Dwarki VJ. Long-term correction of obesity and diabetes in genetically obese mice by a single intramuscular injection of recombinant adeno-associated virus encoding mouse leptin. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):13921–6. doi: 10.1073/pnas.94.25.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg A. Acquired and inherited lipodystrophies. The New England journal of medicine. 2004;350(12):1220–34. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 24.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 25.Agostini M, Schoenmakers E, Mitchell C, Szatmari I, Savage D, Smith A, et al. Non-DNA binding, dominant-negative, human PPARgamma mutations cause lipodystrophic insulin resistance. Cell metabolism. 2006;4(4):303–11. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes VA, Curtis DE, Sukumaran S, Shao X, Parameswara V, Rashid S, et al. Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell metabolism. 2009;9(2):165–76. doi: 10.1016/j.cmet.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch ML, Green L, Porteus MH, Samulski RJ. Self-complementary AAV mediates gene targeting and enhances endonuclease delivery for double-strand break repair. Gene therapy. 2010;17(9):1175–80. doi: 10.1038/gt.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunningham SC, Dane AP, Spinoulas A, Logan GJ, Alexander IE. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16(6):1081–8. doi: 10.1038/mt.2008.72. [DOI] [PubMed] [Google Scholar]
- 29.Grimm D, Pandey K, Nakai H, Storm TA, Kay MA. Liver transduction with recombinant adeno-associated virus is primarily restricted by capsid serotype not vector genotype. Journal of virology. 2006;80(1):426–39. doi: 10.1128/JVI.80.1.426-439.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nature biotechnology. 2005;23(3):321–8. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 31.Brown BD, Gentner B, Cantore A, Colleoni S, Amendola M, Zingale A, et al. Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state. Nature biotechnology. 2007;25(12):1457–67. doi: 10.1038/nbt1372. [DOI] [PubMed] [Google Scholar]
- 32.Tanguay RL, Gallie DR. Translational efficiency is regulated by the length of the 3' untranslated region. Mol Cell Biol. 1996;16(1):146–56. doi: 10.1128/mcb.16.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eberle AB, Stalder L, Mathys H, Orozco RZ, Muhlemann O. Posttranscriptional gene regulation by spatial rearrangement of the 3′ untranslated region. PLoS biology. 2008;6(4):e92. doi: 10.1371/journal.pbio.0060092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen J, Friedman JR. miR-122 regulates hepatic lipid metabolism and tumor suppression. The Journal of clinical investigation. 2012;122(8):2773–6. doi: 10.1172/JCI63966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell metabolism. 2006;3(2):87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Gaudet D, Methot J, Dery S, Brisson D, Essiembre C, Tremblay G, et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene therapy. 2013;20(4):361–9. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 38.Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, et al. Widespread translational inhibition by plant miRNAs and siRNAs. Science. 2008;320(5880):1185–90. doi: 10.1126/science.1159151. [DOI] [PubMed] [Google Scholar]
- 39.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 40.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. The Journal of clinical investigation. 2002;110(8):1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 42.Klebanov S, Astle CM, DeSimone O, Ablamunits V, Harrison DE. Adipose tissue transplantation protects ob/ob mice from obesity, normalizes insulin sensitivity and restores fertility. The Journal of endocrinology. 2005;186(1):203–11. doi: 10.1677/joe.1.06150. [DOI] [PubMed] [Google Scholar]
- 43.Muzzin P, Eisensmith RC, Copeland KC, Woo SL. Correction of obesity and diabetes in genetically obese mice by leptin gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(25):14804–8. doi: 10.1073/pnas.93.25.14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. The New England journal of medicine. 1999;341(12):879–84. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 45.Peyvandi F, Palla R, Menegatti M, Siboni SM, Halimeh S, Faeser B, et al. Coagulation factor activity and clinical bleeding severity in rare bleeding disorders: results from the European Network of Rare Bleeding Disorders. Journal of thrombosis and haemostasis : JTH. 2012;10(4):615–21. doi: 10.1111/j.1538-7836.2012.04653.x. [DOI] [PubMed] [Google Scholar]
- 46.Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z, et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Human gene therapy. 2010;21(10):1259–71. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117(1):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


