Abstract
Depression is characterized by disturbed sleep and eating, a variety of other, nonspecific somatic symptoms, and significant somatic comorbidities. Why there is such close association between cognitive and somatic dysfunction in depression is nonetheless poorly understood. An explosion of research in the area of interoception—the perception and interpretation of bodily signals—over the last decade nonetheless holds promise for illuminating what have until now been obscure links between the social, cognitive-affective, and somatic features of depression. This paper reviews rapidly accumulating evidence that both somatic signaling and interoception are frequently altered in depression. This includes comparative studies showing vagus-mediated effects on depression-like behaviors in rodent models as well as studies in humans indicating both dysfunction in the neural substrates for interoception (e.g., vagus, insula, anterior cingulate cortex) and reduced sensitivity to bodily stimuli in depression. An integrative framework for organizing and interpreting this evidence is put forward which incorporates (a) multiple potential pathways to interoceptive dysfunction; (b) interaction with individual, gender, and cultural differences in interoception; and (c) a developmental psychobiological systems perspective, emphasizing likely differential susceptibility to somatic and interoceptive dysfunction across the lifespan. Combined with current theory and evidence, it is suggested that core symptoms of depression (e.g., anhedonia, social deficits) may be products of disturbed interoceptive-exteroceptive integration. More research is nonetheless needed to fully elucidate the relationship between mind, body, and social context in depression.
Keywords: interoception, somatic signals, cenesthopathy, mind-body relationships, major depression
The close connection between mind, body, and depression1 is a longstanding problem (e.g., Burton, 1621; Fuchs, 2005; Micali, 2011; Pollitt, 1965). A number of chronic medical conditions, such as cardiac disease and pain are, for example, highly comorbid with depression (e.g., Bair, Robinson, Katon, & Kroenke, 2003; Glassman, 2007; Katon, 2003). Depression is also a common correlate of extreme homeostatic disturbance, as occurs in starvation (Brozek, Guetzkow, Vig Baldwin, & Cranston, 1951; Fliederbaum, Heller, Zweibaum, & Zarchi, 1979) and anorexia nervosa (e.g., Halmi et al., 1991). At the same time, depression involves conspicuous somatic symptoms of appetite and weight change, sleep disturbance, and sexual dysfunction (DSM-5; APA, 2013; Beck, 1967; Devlin & Walsh, 1989; Paykel, 1977; Schuyler, 1974) as well as varied non-specific complaints such as fatigue, dizziness, pain, and headache (Jain, 2009; Kapfhammer, 2006; Simon et al., 1999). The latter are the primary indicators of depression in many cultures (Kim, 2010; Kleinman, 2004; Simon et al., 1999; Yusim et al., 2010) and are among the more common indicators of depression in children (McCauley, Carlson, & Calderon, 1991; Ryan et al., 1987). Somatic symptoms can be the first sign of a depressive episode (Beck, 1967) and are often the first and only symptoms presented in primary care settings (Kirmayer, 2001; Tylee & Ghandi, 2005). In addition, the presence and disappearance of somatic symptoms often correlates with the severity and remittance of depression (Beck, 1967; Casper et al., 1985; Fava et al., 1997; Paykel et al., 1995).
Despite such close association, cognitive approaches have tended to privilege the cognitive and behavioral over the somatic features of depression (e.g., Gotlib & Joormann, 2010). Neurobiological and neuropsychiatric approaches have similarly tended toward strict encephalocentrism—assuming that the most important causes of disorder necessarily operate at the level of the brain and the neurons, circuits, and synapses therein—often to the exclusion of large portions of the physiological systems of interest (cf. Blakely, 2001; Kleinman & Becker, 1998; Kuo, 1967; Le Magnen, 1971). For example, the bulk of the serotonin system can be found only below the neck—in the gut, enteric nervous system, and vasculature (see Gershon, 1998). Serotoninergic theories of depression and antidepressant drug action nonetheless focused almost exclusively on disruptions in serotonin functioning in the brain (e.g., Elhwuegi, 2004; Ressler & Nemeroff, 2000; see Hale, Raison, & Lowry, 2013, for an exception).
Common to cognitive-behavioral and neuropsychiatric approaches is the assumption that in the complex system of brain, body, and context, the seat of control and thus causal primacy—the philosopher’s hegemonikon—is localizable squarely in the head.2 Such an assumption is, however, out of sync not only with ecological views of perception and action (see Gibson, 1979; Turvey, 1992; Warren, 2006), but with a number of other advances in cognitive science (e.g., Chiel & Beer, 1997; Clark, 2008; E. Smith & Semin, 2007; Thelen & L. Smith, 1994). Control is increasingly viewed as distributed or distributable across brain-body-environment systems (e.g., Fernandez-Leon, 2012; Kirchhoff & Newsome, 2012) and cognition is increasingly viewed as embodied (e.g., Chiel & Beer, 1997; Clark, 1999; Lakoff & Johnson, 1999), situated (see E. Smith & Semin, 2007), and even extended (see Clark, 1996, 2008). For example, bodies and effectors (e.g., muscles, limbs) provide information, constraints, and “calculations” critical for generating adaptive behavior (e.g., Chiel & Beer, 1997; Thelen & L. Smith, 1994). Cognition is also “scaffolded” by and often wholly offloaded to human-made artifacts, other social agents, and the affordances provided thereby (Clark, 1996, 2008; Gigerenzer, 2000; E. Smith, 2008; E. Smith & Semin, 2007). At the same time, somatic signals or “visceral factors” are increasingly viewed as playing a critical role in social, affective, and decision-making processes in fields as diverse as social psychology, neuroscience, economics, marketing, and law (e.g., Craig, 2003b; Critchley & Harrison, 2013; Damasio, 1994; Danziger et al., 2011; Kang et al., 2011; Lamm & Singer, 2010; Loewenstein, 1996). Such approaches have, however, seen little application to problems in clinical psychology and psychiatry (see Eigsti, 2013; Fuchs & Schlimme, 2009; Michalak, Burg, & Heidenreich, 2012; for exceptions).
Accumulating evidence points to the centrality of the body in depression. This evidence is of two kinds. The first comes from studies indicating that bodily signals significantly impact depression-related symptoms. A number of recent studies have, for example, related both gut microbiome and inflammatory manipulations to depression- and anxiety-like behavior in mice—effects which in many cases are mediated by somatic signals transmitted to the brain via the vagus nerve (e.g., Bravo et al., 2011; Lyte et al., 2006), one of the major neural relays between brain and body (Zagon, 2001). Vagal nerve stimulation, in humans, has moreover been found to significantly improve mood and to be an effective treatment for refractory cases of depression (e.g., Elger et al., 2000; Sackeim et al., 2001). The second sort of evidence comes from studies indicating abnormalities of interoception—the internal sense of the condition of the body3—and in the neural systems underlying interoception in depression. Interoception plays a central role in homeostasis, motivated behavior, and emotional processing (e.g., Cameron, 2009; Craig, 2009; Critchley & Nagai, 2012; Herbert & Pollatos, 2012; Wiens, 2005) and is assumed to be disturbed in many models of depression, based on neural evidence alone (e.g., Andréasson, Arborelius, Erlanson-Albertsson, Lekander, 2007; Krishnan & Nestler, 2010). For example, the insula, often considered primary interoceptive cortex—responsible for translating “raw” somatic signals into consciously accessible feelings (Craig, 2002, 2009)—is among the regions most frequently associated with depression in imaging studies (Fitzgerald, Laird, Maller, & Daskalakis, 2008; Sliz & Hayley, 2012).
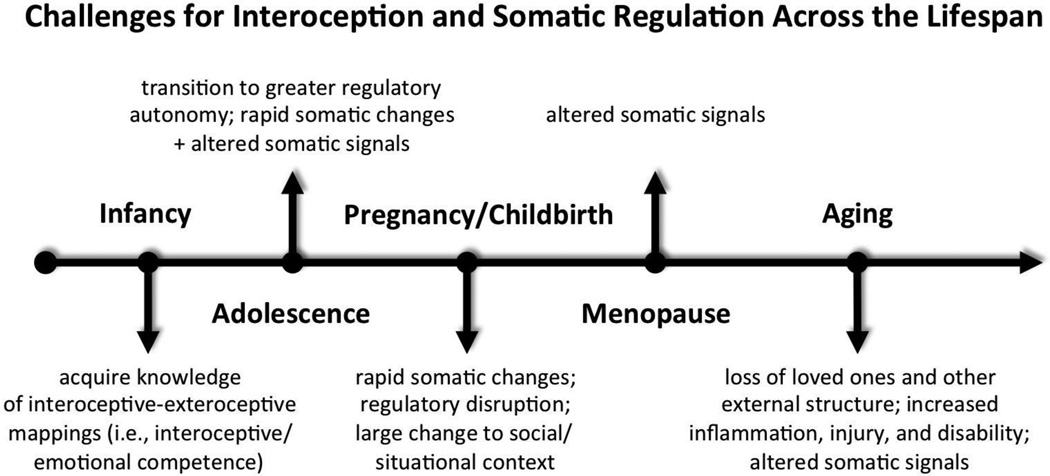
The aim of this paper is to provide a needed review of this rapidly growing literature, while putting forward a framework to guide future studies of interoceptive dysfunction (ID) in depression. The framework is grounded in both developmental psychobiological systems metatheory and a broad review of the literature on interoception and somatic perception. The two overarching assumptions of the framework are that (a) social and regulatory systems4 are deeply interrelated in highly social species such as ours (Hart, 1988; Hofer, 1984) and (b) the interpretation of somatic signals is both context-dependent and error prone (Cioffi, 1991; Mechanic, 1972). From this perspective, we rely on somatic signals to navigate the vagaries of our social worlds (Damasio, 1994, 1999) and to guide our appraisals of external stimuli generally (e.g., Cabanac, 1971). However, we also rely heavily on the environment outside our skins, particularly our social surrounds, to disambiguate our inner perceptual worlds (cf. Cioffi, 1991; Schachter, 1959). This framework distinguishes itself from current models by emphasizing both multiple pathways to ID and that ID is likely to be a function of somatic and regulatory challenges faced across the lifespan, interacting with individual, gender, and cultural differences in interoception. For example, psychophysiological findings suggest that males and females often differ dramatically in their reliance upon and sensitivity to changes in external cues used for disambiguating somatic signals (Pennebaker, 1995). A focus on interoception thus provides a novel means of elucidating not only the poorly understood connection between mind, body, and psychosocial context but also the gender bias in the epidemiology of depression.
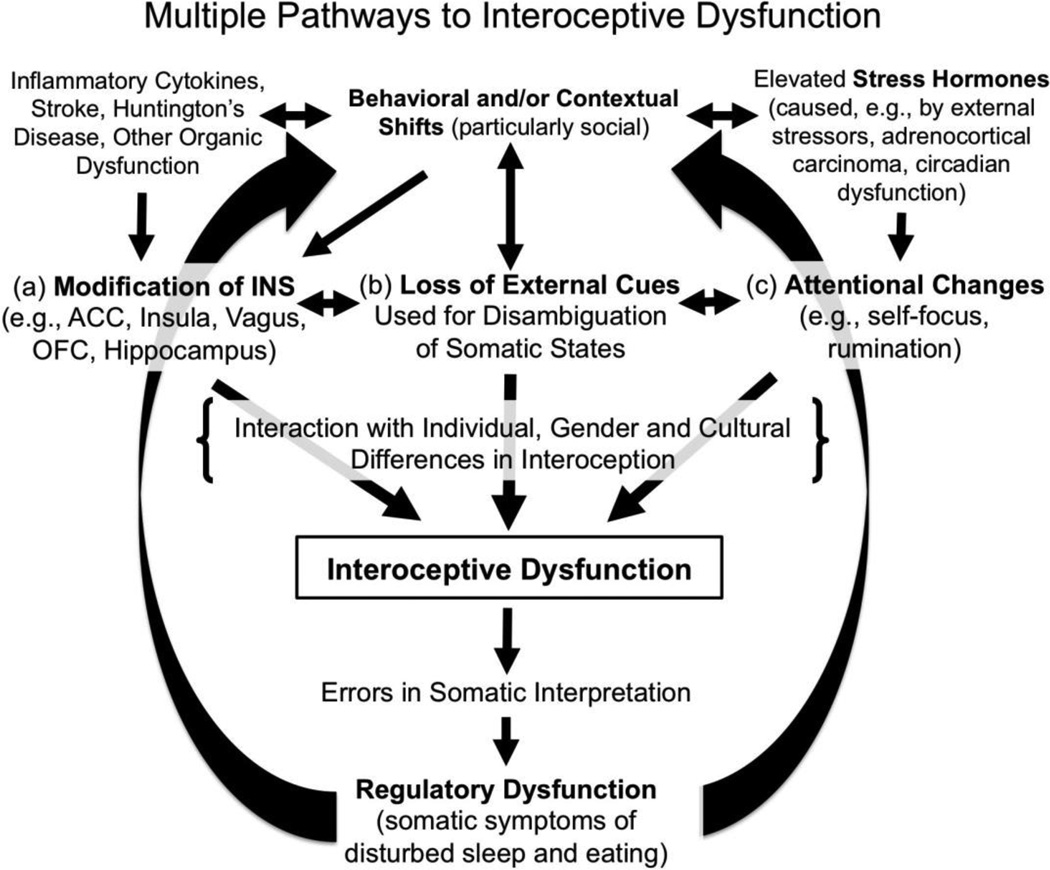
Three distinct pathways through which ID is likely to arise in depression are suggested by current literature. These include: (a) alteration of the neural substrates for interoception (e.g., by stress hormones, pro-inflammatory cytokines); (b), the loss of social and other exteroceptive cues ordinarily used to disambiguate somatic signals; and (c) shifts in attention, due to cognitive tendencies like rumination. Although particular forms of ID are certainly transdiagnostic—anxiety and panic, for example, involve heightened interoceptive sensitivity (e.g., Domschke, Stevens, Pfleiderer, & Gerlach, 2010; Stevens et al., 2011) and ID is thought to play a role in a number of other disorders, including autism and schizophrenia (e.g., Uddin & Menon, 2009; Wylie & Tregellas, 2010)—depression may involve any or all of these pathways.
The review is divided into five sections. The first two are a primer on interoception and its neural substrates, respectively; including definitions of relevant terminology, a taxonomy of ID, and a brief review of the role of interoception in social and emotional cognition. The central section reviews evidence implicating somatic signals and ID in depression. Next, existing models of ID in depression are reviewed and the three main pathways to ID explicated. The final section integrates these with the broader literature on interoception, focusing on the potential for individual, gender, and cultural differences in the presentation of ID in depression.
Interoception and Interoceptive Dysfunction: An Introduction
Interoceptive-Exteroceptive Penetrance and Integration
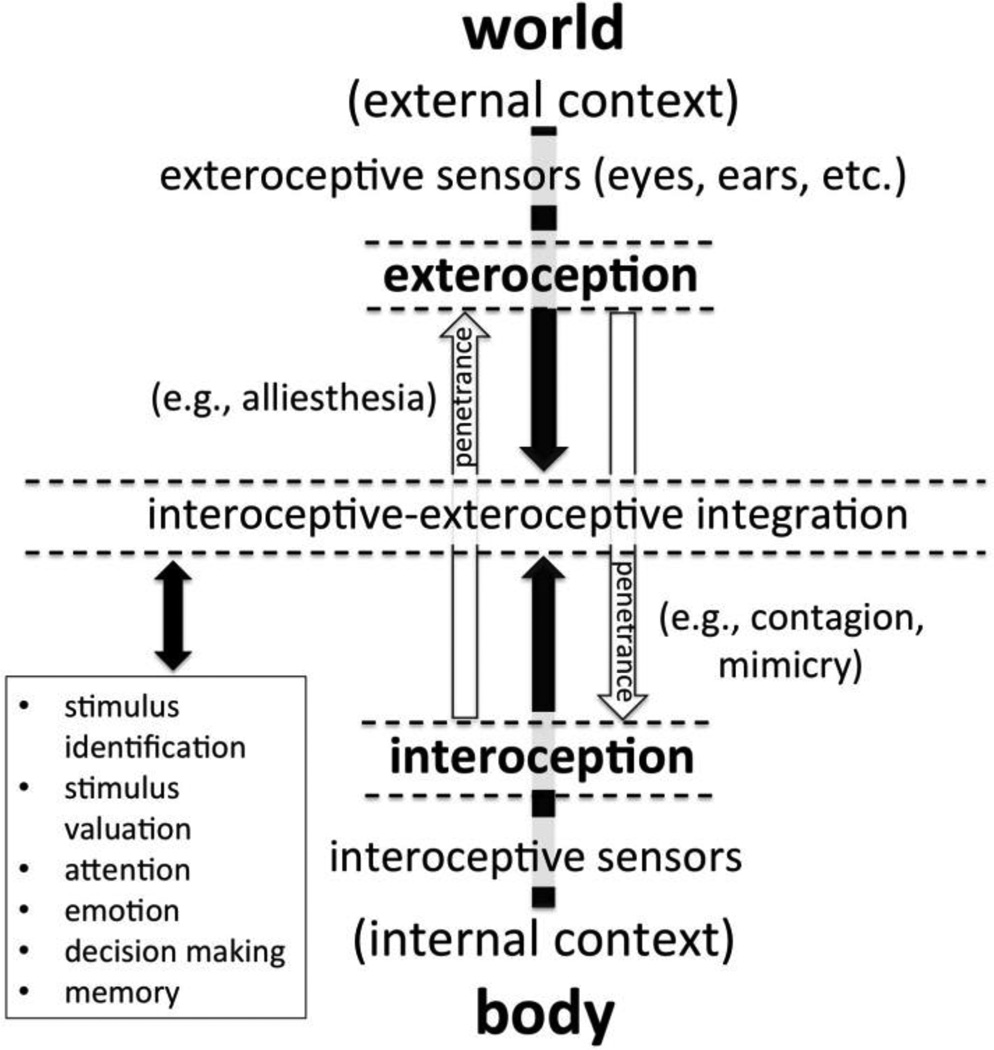
The internal and external senses interact continuously. As illustrated in Figure 1, both provide information to neuro-cognitive systems critical for mediating the relationship between internal, bodily condition and outside world (e.g., attention, memory, decision-making). Such systems necessarily rely on both interoceptive and exteroceptive inputs to promote short-term survival and long-term homeostasis (Critchley & Harrison, 2013). That is, the brain must continuously adjust to the ever changing needs of the body, to promote adaptive responses to external stimuli, while at the same time somatic adjustments must be made continuously, to ensure readiness for changing situational demands. Rather than being entirely insulated, there is thus mutual influence or bidirectional penetrance between the interoceptive and exteroceptive senses (see Stokes, 2012; Fig. 1).5 An apple or a juicy steak, for example, is generally far more attractive after a 12 hr fast than after a large meal. Likewise, a glass of water is ordinarily far more appealing after vigorous exercise than just after drinking several glasses of water. This modulation of the attractiveness of external stimuli by the homeostatic condition of the body is termed alliesthesia (see Brondel & Cabanac, 2007; Cabanac, 1971).
Figure 1.
A depiction of the integration and bidirectional “penetrance” of interoception and exteroception. A number of cognitive processes (closed box) necessarily rely upon the integration of interoceptive and exteroceptive information. For example, the homeostatic condition of the body is imperative for judging whether or not to approach, avoid, or even attend to a given stimulus. Penetrance describes “spill over” or bleed-through from one perceptual system to another—from interoception to exteroception or vice versa. For example, alliesthesia occurs when bodily signals shift the valence or attractiveness of an exteroceptive stimulus (Cabanac, 1971), whereas contagion occurs when an exteroceptive stimulus overrides representation of bodily signals. Interoceptive dysfunction can thus potentially influence a large number of cognitive processes, particularly those typically involving alliesthesia and contagion (e.g., motivational behavior, social cognition).
Numerous additional effects of interoception on exteroception are observable. A number of classic “New Look” studies, for example, found that somatic signals can (a) affect detection thresholds for and reaction times to orectic (need-related, appetitive) stimuli (e.g., Gilchrist & Nesberg, 1952; Lazarus, Yousem, & Arenberg, 1953); (b) bias the interpretation of ambiguous stimuli (e.g., Epstein, 1961; Levine, Chein, & Murphy, 1942; Sanford, 1936, 1937); (c) prime orectic thoughts and associations (e.g., Sanford, 1936, 1937), as well as increase both (d) the recall of orectic words (e.g., Epstein & Levitt, 1962) and (e) spontaneous production of orectic imagery (e.g., Giddan, 1966).6 Such findings have been corroborated by a number of recent reports (e.g., Aarts, Dijksterhuis, & De Vries, 2001). Hunger, for example, has been found to bias attention, such that food-related stimuli gain preferential processing (e.g., Mogg et al., 1998; Piech, Pastorino, & Zald, 2010). Radel and Clément-Guillotin (2012) similarly found that hunger altered perceptual sensitivity to food-related words and Changizi and Hall (2001) found that thirst increased the probability that ambiguous stimuli were perceived to be transparent or water-like. It has thus been hypothesized that bodily information enters into visual processing even at early stages of object identification, as it provides information critical for evaluating the homeostatic relevance, value, or relative utility of exteroceptive stimuli (Barrett & Bar, 2009).7
On the other side of the coin, exteroceptive stimuli can influence and in some cases override aspects of interoception. A friend laughs and I laugh, sometimes even if a joke is not funny. A person across the table from me yawns and I yawn, often even if I didn’t previously feel sleepy. Such instances of contagion are among the more mysterious features of social life (Freedman & Perlick, 1979; Guggisberg, Mathis, Schnider, & Hess, 2010). Social contagion can moreover take numerous forms, including emotional or mood contagion (e.g., Neumann & Strack, 2000), goal contagion (e.g., Aarts, Gollwitzer, & Hassin, 2004), consummatory mimicry (e.g., Hermans et al., 2012), itch contagion (Holle et al., 2012), and even pupillary contagion, wherein the size of one’s pupils is modulated by that of a social partner (Harrison et al., 2006). The ubiquity of contagion indicates not only that exteroceptive inputs penetrate (or enter into) interoceptive processing, but that core neural systems rely upon integrated interoceptive-exteroceptive representations (cf. Ainley, Tajadura-Jiménez, Fotopoulou, & Tsakiris, 2012; Tsakiris, Tajadura-Jiménez, & Costantini, 2011) and these, in many cases, automatically drive efferent responses (cf. Bargh & Chartrand, 1999).
When overt, visible behaviors are involved, “mirror neurons” and the “mirror neuron system” are often invoked (see Gallese & Goldman, 1998). A mirror neuron, in the strictest sense, is a neuron whose activity is specific to a particular action, regardless of whether the action is produced by an individual themselves or observed, performed by another. Originally discovered in monkeys (Gallese, Fadiga, Fogassi, & Rizzolatti, 1996), neurons with mirroring properties have now been repeatedly demonstrated in humans (e.g., Chong et al., 2008; Zaki, Weber, Bolger, & Ochsner, 2009). The existence of neurons with a high degree of specificity for particular actions nonetheless implies sophisticated mechanisms for integrating exteroceptive and somatic information (see de Waal & Ferrari, 2010; Demiris, Aziz-Zadeh, & Bonaiuto, 2014). Although little is known about how such integration is achieved, mirroring activity has been found in an impressively large number of brain regions in humans. A recent meta-analysis of 125 relevant fMRI studies, for example, showed activation in 34 different Brodmann areas, including both motor regions and regions critical for interoception and stimulus evaluation, such as the insula, cingulate, and amygdala (Molenberghs, Cunnington, & Mattingley, 2012).
Social behavior appears to rely heavily on such mirroring. Mimicry of the posture, facial expressions, and mannerisms of social partners, for example, occurs automatically, even among strangers (e.g., Chartrand & Bargh, 1999; van Baaren, Holland, Kawakami, & van Knippenberg, 2004; Y. Wang, Newport, & Hamilton, 2011). Such unconscious mimicry may be essential to social reward and part of the “glue” of social bonding (e.g., Cacioppo et al., 2014; Lakin, Jefferis, Cheng, & Chartrand, 2003). For example, the capacity for spontaneous, unconscious mimicry is impaired in autism and schizophrenia (e.g., McIntosh et al., 2006; Oberman, Winkielman, & Ramachandran, 2009; Varcin, Bailey, & Henry, 2010) and diminished by negative mood induction (Van Baaren et al., 2006). A recent study moreover found that sensitivity to bodily signals, as measured via heartbeat perception (described below), correlated positively with automatic mimicry of finger movements (Ainley, Brass, & Tsakris, 2014). Such findings point to a potentially deep connection between interoceptive-exteroceptive integration and social cognitive abilities (cf. Adolphs & Damasio, 2001; Damasio, 1994).
Exteroception as Context for Interoception
Despite their importance, interoceptive stimuli are almost universally described as vague and diffuse; lacking the discreteness, temporally and spatially, of external stimuli (e.g., Aziz et al., 2000; Craig, 1996, 2004; Hölzl, Erasmus, & Moltner, 1996).8 With few exceptions—the tempo of breathing is, for example, perceptible via multiple senses—they also typically lack the useful redundancy characteristic of exteroceptive stimuli (see Bahrick & Lickliter, 2012). A number of authors have thus argued that bodily stimuli are always interpreted based on a combination of cognitive set (expectations, biases, schemas) and exteroceptive context (cf. Barsky, 1992; Cioffi, 1991; Mechanic, 1972; Pennebaker, 1992, 1995). Cioffi (1991), for example, writes that bodily sensations, “…are as often socially influenced interpretations as they are the direct output of a biological system” (p. 25). Barsky (1992) similarly writes that, “We infer what we are perceiving from what we think we ought to be perceiving, and this depends largely on our circumstances…Situational context furnishes clues that are used to infer the meaning and to decide on the significance of a bodily symptom” (p. 29).
The idea that social and situational cues, along with the top-down influence of beliefs, thoughts, and expectations, have a powerful influence on the interpretation of bodily signals and physical symptoms is a cornerstone of many theories of emotion (e.g., Gross & Thompson, 2007; Russell, 2003) and also important in cognitive approaches to anxiety (e.g., performance anxiety) treatment which focus on teaching reappraisal of the situational and somatic correlates of arousal (see Webb, Miles, & Sheeran, 2012). This view is also supported by data from both classic “misattribution” (e.g., Brodt & Zimbardo, 1981; Dutton & Aron, 1974; Schachter & Singer, 1962) and excitation-transfer (Cantor, Zillmann, & Bryant, 1975; Zillmann, 1988, 1996) paradigms, as well as modern placebo and nocebo studies (see Benedetti, 2013).9 For example, nocebo responses are common in medical and psychiatric settings (e.g., Mitsikostas, Mantonakis, & Chalarakis, 2014) and occur when an inert or sham treatment induces undesirable side-effects purely as the result of expectations about the occurrence of such symptoms (Benedetti, 2013).
Interoception as Context for Exteroception
At the same time that we rely on social and other contextual cues to disambiguate somatic signals (Cioffi, 1991; Mechanic, 1972), we appear to rely heavily on internal signals to navigate our social worlds (Anderson et al., 1999; Ariely & Loewenstein, 2006; Damasio, 1994, 1996). Projection bias as seen in the hot-cold empathy gap, in which people are better at empathizing with and thus predicting the behavior of those who are experiencing a similar state (e.g. hunger, thirst, fatigue, arousal) as themselves—including their own future behavior—provides one example of this principle (e.g., Loewenstein, 1996; 2005; Nordgren, van der Pligt, & van Harreveld, 2006, 2007). For example, Nordgren et al. (2007) found that when participants were hungry, fatigued, or aroused, they were more likely to excuse the impulsive behavior of those in a similar state. Participants also appear to rely upon and project their current states of hunger and thirst when making predictions about others’ experiences of deprivation from food and water (O’Brien & Ellsworth, 2012; Van Boven & Loewenstein, 2003).
Such penetrance generally occurs outside of conscious awareness (Bargh & Chartrand, 1999; Critchley & Harrison, 2013; Loewenstein, 1996). For example, a simple thermal manipulation—having participants hold either a warm or a cold cup during a short elevator ride—significantly impacted participants’ judgments about the personality of a social target, as well as their likelihood of making a prosocial decision (Williams and Bargh, 2008). Ijzerman and Semin (2009) found that thermal manipulations (e.g., holding a warm or cool cup) impacted both social proximity and relational language use. A number of studies have similarly found that both hunger and glucose depletion can modulate prosocial decision-making, resulting in less altruistic behavior (e.g., Briers, Pandelaere, Dewitte, & Warlop 2006; DeWall, Baumeister, Gailliot, & Maner, 2008; although cf. Aarøe & Petersen, 2013). Danziger, Levav, and Avnaim-Pesso (2011) moreover found that judges in a set of parole cases were more likely to rule against defendants immediately before than after each of their daily food breaks. Judges and lawyers alike were unaware of this, despite the large magnitude of the effect10 (Danziger et al., 2011).
Interoceptive-Exteroceptive Integration: A Summary
Based on the evidence reviewed, the human brain appears to contain circuitry highly permissive of interoceptive-exteroceptive penetrance, particularly in the domains of motivated behavior and social-emotional cognition (cf. Damasio, 1994; Nauta, 1971; Seth, 2013). Bodily stimuli are clearly capable of modulating the representation and apprehension of outside stimuli, likely beginning at early stages of processing (cf. Barrett & Bar, 2009; Stokes, 2012). Such penetrance gives rise to alliesthesia, the hot-cold empathy gap, and a host of other nameless effects, often viewed as “noise” or “extraneous influences” from a traditional, dis-embodied view of cognition (e.g., Danziger et al., 2011). Exteroceptive stimuli can also modulate the perception and interpretation of somatic signals (cf. Cioffi, 1991; Mechanic, 1972) and can override such signals, as occurs in contagion (e.g., Aarts et al., 2004; Holle et al., 2012). Meta-analytic results suggest that large portions of the brain participate in interoceptive-exteroceptive integration (e.g., Molenberghs et al., 2012)—a function long ascribed to “limbic” (Mayberg, 1997) and related regions, such as the basal ganglia and interoceptive cortex (Alexander, DeLong, & Strick, 1986; Craig, 2009; Critchley et al., 2002, 2004; Seth, Suzuki, & Critchley, 2012). Arguments have been made that such integration is both central to the perception of “self” and feelings of body ownership (e.g., Craig, 2010; Seth, 2013) and subserves critical aspects of social cognition (e.g., Damasio, 1994; Dijksterhuis, 2005; Lakin et al., 2003).
Anatomy and Function of the Interoceptive Nervous System
Interoception begins with the body. Somatic stimuli affect the CNS—giving rise to interoception—via detection by free nerve endings, specialized receptors (e.g., nociceptors, chemoreceptors), and the hypothalamus and circumventricular organs (see Cameron, 2002, 2009; Critchley & Harrison, 2013). Nevertheless, the transmission of somatic information to and its processing and representation by the brain, its integration with exteroceptive sensory channels and influence on reward and decision-making systems, as well as higher-level disambiguation and interpretation of somatic information are also important facets of interoception (cf. Cioffi, 1991; Mechanic, 1972; Vaitl, 1996). The major neuroanatomical structures and circuits underlying interoception—here referred to collectively as the Interoceptive Nervous System (INS)—and their known functionality are depicted in Figure 2.
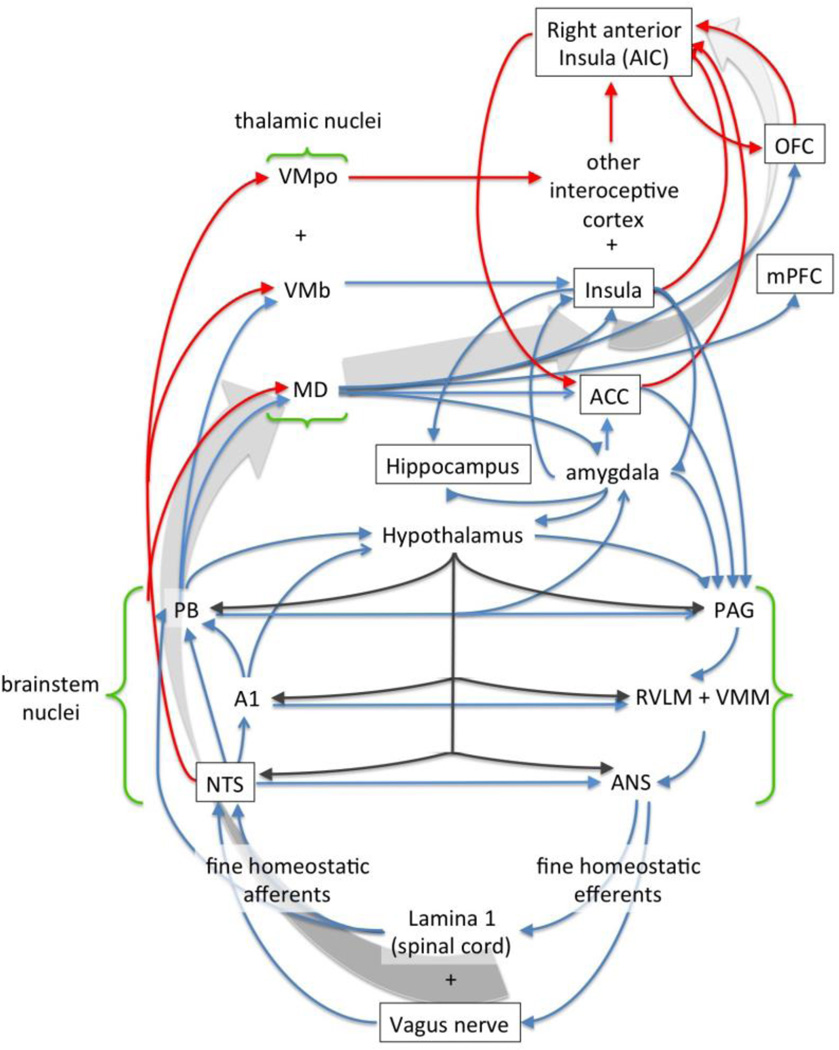
Figure 2.
A simplified illustration of the major connections and many feedback loops involved in interoception in humans and other primates; adapted with permission from Craig (2003a), with additional connections added based on the work of others (e.g., Mufson et al., 1981; Ray & Price, 1993). Red lines indicate pathways that are phylogenetically novel in primates. Larger grey arrows indicate the general flow of bodily information from the vagus and brain stem upward to the right anterior insula—the theoretical nexus of consciously accessible feelings of the body and “self” (see Craig, 2003a). A1 = brainstem area 1; ACC = anterior cingulate cortex; ANS = autonomic nervous system; MD = medial dorsal nucleus of the thalamus; NTS = nucleus of the solitary tract; OFC = orbitofrontal cortex; PAG = periaqueductal gray; PB = parabrachial nucleus; mPFC = medial prefrontal cortex; RVLM = rostral ventrolateral medulla; VMb = basal ventral medial nucleus of the thalamus; VMpo = posterior ventral medial nucleus of the thalamus; VMM = ventral medial medulla.
The Vagus Nerve and Interoception
Among the many nerves that relay bodily signals to the brain, the vagus figures prominently, as it carries “broadband” information from nearly every somatic tissue to the brainstem (Cameron, 2002; Zagon, 2001). This includes a veritable plethora of signals (Leichnetz, 1972), including information about mechanical and chemical stimulation (Powley & Phillips, 2002), inflammation and sickness (e.g., Holzer, 2009), pain and temperature (e.g., Busch et al., 2013), hunger and satiety (e.g., Li, Wu, & Owyang, 2004), as well as signals modulating sleep and arousal (e.g., Peñaloza-Rojas, Barrera-Mera, & Kubli-Garfias, 1969). Vagal afferents terminate at the nucleus of the solitary tract (NTS), from which second-order neurons project widely to a number of brainstem, forebrain and limbic structures, including the thalamus, amygdala, and hippocampus (Cameron, 2002, 2009; Vaitl, 1996). These afferents also project to a number of prefrontal areas, including the anterior cingulate (ACC), medial prefrontal (mPFC), orbitofrontal (OFC), and insular cortex (Cameron, 2002, 2009).
Interoception and the Brain
Hippocampus
Most well known for its role in learning, memory, and spatial cognition, the hippocampus is also involved in aspects of interoception, particularly “contextualizing,” or the use of interoceptive signals as contextual cues for memory storage and retrieval (e.g., state-dependent memory; e.g., McIntyre et al., 1985). As aptly stated by Kennedy and Shapiro (2009), “Motivational states arising from interoceptive cues provide an internal context that modulates the relative significance, meaning, or organization of events in memory…motivational states such as hunger and thirst define internal, contextual cues that can specify behavioral goals and inform memory retrieval” (p. 10805). For example, bodily states such as hunger can function as Pavlovian occasion setters, modulating the strength of CS-US associations (e.g., Davidson, 1987; 1998, 2000). The hippocampus is central to this process (e.g., Davidson, 1993; Davidson et al., 2010; Kennedy & Shapiro, 2009) and plays a role in the utilization of hunger and other motivational signals as contextual cues for the regulation of ingestion (e.g., Jarrard, 1973; Kennedy & Shapiro, 2004; Lathe, 2001; Tracy, Jarrard, & Davidson, 2001).
Anterior cingulate cortex
The cingulate gyrus borders the corpus callosum, the prominent white matter tracts connecting the two halves of the brain. The anterior cingulate cortex (ACC) is a structurally and functionally heterogeneous hub, densely connected with limbic, paralimbic, and frontoparietal regions—with more cognitive and more affective-visceral subdivisions (Koski & Paus, 2000; Margulies et al., 2007). The ACC is thus involved in a large number of processes, including attention, motivation, response initiation, and goal-directed behavior (e.g., Devinsky, Morrell, & Vogt, 1995). The ACC is also part of a stimulus evaluation or salience network, including the medial prefrontal cortex (mPFC), insula, and amygdala (e.g., Chaudhry et al., 2009; Devinsky et al., 1995). This network appears to be heavily involved in assessing stressful or negative, fear- and pain-related stimuli (e.g., J. Fan et al., 2011). The subgenual ACC (sgACC), in particular, sends and receives projections to and from numerous regions, including the OFC, mPFC, insula, amygdala, and entorhinal-parahippocampal cortices (Hamani et al., 2011), and is prominently implicated in sadness and depression (e.g., Greicius et al., 2007; Mayberg et al., 1999). In addition to stimulus evaluation, the ACC (particularly dorsal ACC or dACC) and mPFC are involved in generating both internal autonomic and overt expressive emotional responses (e.g., Gianaros et al., 2005).
Insular Cortex
Tracing the somatosensory cortex downward, into the Sylvian fissure, the island of tissue known as insular cortex or the insula can be found, hidden beneath portions of the parietal and temporal cortices (a.k.a. operculum). The insula has long been known to be involved in visceromotor processes—giving rise both to somatic and visceral sensations as well as autonomic adjustments (e.g., Penfield & Faulk, 1955). The insula is comprised of neurons responsive to every known class of interoceptive stimulus (Ibañez, Gleichgerrcht, & Manes, 2010). This includes neurons whose activity correlates with proprioception (Karnath & Baier, 2010), arousal (e.g., Critchley et al., 2000; Georgiadis & Holstege, 2005; King, Menon, Hachinski, & Cechetto, 1999), temperature (e.g., Craig, Chen, Bandy & Reiman, 2000; Olausson et al., 2005), pain (e.g., Ploghaus et al., 1999), itch (Darsow et al., 2000), and air hunger (i.e., the need for oxygen; Liotti et al., 2001). Insular neurons also respond to flavor and other qualities of food (e.g., de Araujo & Rolls, 2004; King et al., 1999; Small, 2010), in addition to alimentary signals of hunger and thirst (e.g., de Araujo, Kringelbach, Rolls, & McGlone, 2003; Hollis et al., 2008; Siep et al., 2009; Tataranni et al., 1999). The insula is thus massively multimodal and, with portions of the operculum, considered primary interoceptive cortex (Craig, 2003a; Ibañez et al., 2010). Grey matter volume (GMV) and activity in the right anterior insular cortex (AIC) and operculum, for example, correlate with measures of sensitivity to interoceptive stimuli (Critchley et al., 2004). The insula also plays a role in the seemingly disparate domains of episodic memory (Xie et al., 2012), temporal perception (e.g., Wittmann, van Wassenhove, Craig, & Paulus, 2010), music appreciation (e.g., Blood & Zatorre, 2001), emotional experience (e.g., Zaki, Davis, & Ochsner, 2012), and social cognition (e.g., Y. Fan et al., 2011; Lamm & Singer, 2010; Singer, Critchley, & Preuschoff, 2009; Wicker et al., 2003).
There are a number of competing theories of insula function. Most view the insula as underlying the generation of conscious feelings and subjective awareness (Ibañez et al., 2010; Seth, et al., 2012; Singer et al., 2009). Craig (2002, 2009, 2010) has nonetheless put forward the most comprehensive model of insula function. In Craig’s model, bodily stimuli are processed by the insula—with lateral differences—in a posterior-to-anterior direction: posterior insula being the locus for primary interoceptive representations, mid-insula being involved in the integration of these with information from other brain regions, and AIC being the locus of further integrated re-representations of interoceptive information (Craig, 2009). The latter neurons, particularly in the right AIC, instantiate a unified representation of all feelings at a given moment that is taken to underlie the conscious, subjective experience of an embodied “self” (Craig, 2004, 2010; cf. Damasio, 2003b). Craig’s model also posits that AIC neurons serve as a kind of experiential or phenomenological buffer, in which a finite number of successive “emotional moments” are stored, including representations of both current and prior moments, as well as predictions about future moments (see Craig, 2009; cf. Seth et al., 2012). This model can thus account for insula involvement in temporal and musical perception, episodic memory, and error detection and anxiety (see Craig, 2009; Seth, 2013; Singer et al., 2009).
Orbitofrontal cortex
Located above the eye sockets, the orbitofrontal cortex (OFC) is intimately connected to adjacent PFC and plays a role both in the computation of the subjective incentive value of stimuli (e.g., Chaudhry et al., 2009; Padoa-Schioppa & Cai, 2011; Roberts, 2006) as well as behavioral flexibility (Reekie, Braesicke, Man, & Roberts, 2008; Roberts, 2006). In general, insula activity is associated more with the intensity of stimuli whereas OFC and ACC activity is associated more with the pleasantness or reward-value of stimuli (e.g., Yanagihara, 2012; cf. Lorenz, Minoshima, & Casey, 2003). For example, OFC activation correlates with ratings of the subjective pleasantness of foods and liquids (de Araujo et al., 2003; Kringelbach et al., 2003) as well as with aesthetic judgments more generally (Brown et al., 2011). Food deprivation and gastric distention also cause state-dependent changes in OFC and a number of other regions, including parahippocampal gyrus, ventral striatum, insula, and amygdala (e.g., Goldstone et al., 2009; LaBar et al., 2001; Morris & Dolan, 2001; Rolls, Sienkiewicz, & Yaxley, 1989; Tataranni et al., 1999; J.-G. Wang et al., 2008). Collectively, these facts implicate OFC—particularly medial OFC—and ACC in alliesthesia (see Brown et al., 2011; Kringelbach, 2005; Kringelbach, Stein, & van Hartevelt, 2012; Small et al., 2007).
Ventromedial prefrontal cortex
The ventromedial prefrontal cortex (vmPFC) has long been seen as ideally suited for the integration of interoceptive and exteroceptive information, as well as a likely source for interoceptive influence on decision-making processes (see Damasio, 1994; Nauta, 1971). Early studies found that PFC damage resulted in mood and personality changes, deficits in error processing and goal-directed behavior, and abnormal perseverative tendencies (Nauta, 1971). More recently, vmPFC has been characterized as a component of the stimulus valuation network—with the ventral striatum, hippocampus, medial OFC, and posterior cingulate (PCC)—which automatically generates reward values for encountered stimuli (Lebreton et al., 2009; Peters & Büchel, 2010). The vmPFC is thus critical for social and moral cognition. For example, the vmPFC plays a role in social contagion and mimicry (e.g., Lebreton et al., 2012; Nahab, Hattori, Saad, & Hallett, 2009) and connectivity between right vmPFC and dorsolateral PFC (dlPFC) appears to underlie compliance with social norms (Baumgartner et al., 2011). A large literature also links vmPFC damage to disturbed social and moral cognition; for example, deficits in skills such as empathy and theory of mind (e.g., Eslinger, Flaherty-Craig, & Benton, 2004; Shamay-Tsoory et al., 2005) as well as moral reasoning (e.g., Thomas, Croft, & Tranel, 2011) are common, particularly if damage is sustained early in development (e.g., Anderson et al., 1999; Young et al., 2010).
Antonio Damasio’s (1994, 1996, 1999) somatic marker hypothesis is the most well known attempt to explicate the relationship between the vmPFC, somatic representation, and social-emotional cognition (cf. Critchley, 2005). The hypothesis has its origins in observations of patients with vmPFC damage, including a reanalysis of the case of Phineas Gage (H. Damasio et al., 1994). Despite an apparently full recovery from his accident, for example, the once reliable and hard working Gage floundered in his everyday decision-making, as do many vmPFC-damaged patients (Damasio, 1994). Such deficits, in Damasio’s view, are the result of an inability of individuals with PFC damage to call upon representations of bodily signals when weighing out and choosing between alternatives (Damasio, 1996; cf. Nauta, 1971).
Interoception, Emotion, and Social Cognition
The dual role of the vmPFC in social and emotional cognition is representative of the INS as a whole. For example, the vagus is linked to both emotion and sociality (e.g., Porges, 1997, 2009, 2011; Porges et al., 1994) and has been characterized as a core component of a social engagement system, responsible for regulating both outward displays of affect and autonomic adjustments (see Porges, 2007, 2011). The AIC is similarly implicated in both consciously accessible feelings of emotion (Craig, 2008; Critchley et al., 2004) and core facets of social cognition, such as empathy and contagion (e.g., Carr et al., 2003; Ernst et al., 2013; Holle et al., 2012; Jabbi, Swartz, & Keysers, 2007; Jackson, Meltzoff, & Decety, 2005; Lamm & Singer, 2010; Mazzola et al., 2010; Singer et al., 2009). The ACC likewise plays an important role in both emotional and social cognition (e.g., Allman et al., 2001; Somerville, Heatherton, & Kelley, 2006). ACC damage is, for example, associated with aberrant social behavior, including sociopathy (Devinsky et al., 1995). Insular damage likewise results not only in cardiac and other autonomic abnormalities (Cechetto, 1994) but deficits in both the subjective experience and identification of emotions such as disgust in others (see Ibañez et al., 2010). Individual empathy scores have also been found to correlate with AIC and adjacent frontal operculum activity during emotion observation (e.g., Jabbi et al., 2007). These same regions (AIC and ACC) are also active during both the first-person and vicarious experience of disgust and pain (e.g., Jackson et al., 2005; Wicker et al., 2003). Such findings suggest broad overlap in circuitry underlying the representation of our own bodily states and the “simulation” and apprehension of such states in others (see Bar-On, Tranel, Denburg, & Bechara, 2003; Decety, 2011; Jabbi et al., 2007; Keysers & Gazzola, 2006; Keysers, Kaas, & Gazzola, 2010; Lamm & Singer, 2010; Oberman & Ramachandran, 2007; Singer et al., 2009).
A Taxonomy of Interoceptive Dysfunction
Interoception is neither a unitary nor a simple sixth sense (cf. Zagon, 2001), as it involves the transduction and processing of numerous and diverse signals (Cameron, 2002). Dysfunction may occur across any of these, involving any number of the structures described above. A full taxonomy of interoceptive dysfunction (ID) would thus have to include at least the fourteen facets of interoception outlined in Table 1, along with abnormalities in the major classes of somatic signals (i.e., hormones, cytokines, etc.). A number of terms are currently used in the literature to refer to particular domains in which ID is thought to occur, particularly deficits in “awareness” and “sensitivity,” as well as problems with the “contextualization” and “amplification” of somatic signals. Nevertheless, these terms are often poorly defined and inconsistently employed. A review of the most common and important of these follows.
Table 1.
A Taxonomy of Interoceptive Dysfunction
| Type of Interoceptive Deficit/Disturbance |
Description and Example(s) of Deficits |
|---|---|
| Sensory | Abnormal transduction of somatic stimuli (Cameron, 2002; Vaitl, 1996). For example, congenital insensitivity to pain involves sensory degeneration and a lack of sensitivity to stimuli that ordinarily result in pain (see Indo, 2009). |
| Transmission | Abnormal encoding and/or transmission of interoceptive stimuli. For example, a number of peripheral neuropathies affect myelination and thus transmission of somatic information (Cameron, 2002; Vaitl, 1996; Wernicke, 1906). |
| Representation | Abnormal cortical representation of interoceptive stimuli (see Craig, 2002, 2009, 2010). In theory, entirely illusory or hallucinatory perception of interoceptive signals is also possible given abnormal spontaneous activity in interoceptive regions or abnormally large top-down modulation of interoception (see Wiens, 2005). Such a process could, for example, underlie the excessive ingestion of fluids (i.e., polydipsia) and cenesthopathy frequently observed in severe schizophrenia (e.g., Adetoki et al., 2013). |
| Affective response | Abnormally heightened or diminished affective response to interoceptive stimuli. For example, an interoceptive asymbolia would entail intact sensory processing but abnormal affective response to or salience of somatic signals. Such a condition could result in depersonalization (see Sierra & Berrios, 1997; Sedeño et al., 2014) or an interoceptive homologue to Capgras syndrome (cf. Graux et al., 2011; Hirstein & Ramachandran, 1997). |
| Integration/Penetrance | Abnormal integration or binding of interoceptive and exteroceptive information. |
| Alliesthesia | Abnormally heightened or diminished modulation of exteroceptive processing by somatic stimuli. For example, hyperalliesthesia would entail abnormally large modulation of exteroceptive perception by somatic signals or state —a concept implied in descriptions of alliesthetic processes in addiction (cf. Paulus, Tapert, & Schulteis, 2009; Verdejo-Garcia, Clark, & Dunn, 2012). In theory, extreme hyperalliesthesia could result inexteroceptive hallucinations (c.f. Forrer, 1960a, b), similar to movement-or proprioceptively-induced hallucinations observed in experimental blindness (see Pascual-Leone & Hamilton, 2001). |
| Contagion | Abnormally heightened or diminished modulation of interoceptive processing by exteroceptive stimuli. Hyper-contagion, for example, might entail abnormally heightened tendencies for non-intentional mimicry and/or echolalia (e.g., as occurs in some cases of autism and other developmental disorders). |
| Awareness | Abnormal proneness to direct attention to bodily sensations (e.g., as occurs in hypochondriasis). Garfinkel and Critchley (2013) refer to this aspect of interoception as “interoceptive sensibility” and use “interoceptive awareness” to refer to the meta cognitive awareness of interoceptive sensitivity. |
| Amplification | Somatosensory amplification entails increased attention to somatic signals, particularly those that are relatively weak and infrequent, with a bias toward interpreting such signals as abnormal, noxious, and/or symptomatic of disease (Barsky, 1992). |
| Somatization | A tendency to experience and communicate physical symptoms when the cause of distress is psychological or psychosocial and no physical illness is present (Lipowski, 1988). Note that “somatization” can be culturally normative and thus ought not be taken as a necessary indicator of pathology (see Kirmayer, 2001). |
| Consciousness | Abnormally intensified or diminished consciousness of somatic stimuli. Such a disorder might, for example, resemble an interoceptive blind sight or a dissociation of somatic signals from awareness; such a dissociation, theoretically, occurs nightly, to prevent somatic stimuli from causing premature waking (cf. Ungredda, Gluck, & Geliebter, 2012). |
| Memory/Knowledge | Abnormal memory or knowledge of somatic signals. For example, an inability to utilize interoceptive signals as contextual cues for storage/retrieval (Davidson & Jarrard, 1993; Jarrard, 1973; Nauta, 1971) or an interoceptive amnesia, as was displayed in the case study described by Sidis and Goodhart (1905). |
| Somatic interpretation | |
| Disambiguation | Disturbance in the ability to use interoceptive signals to disambiguate exteroceptive stimuli (i.e., as cues for recognition) and/or the ability to utilize exteroceptive cues to disambiguate or “contextualize” interoceptive signals (Cioffi, 1991; Mechanic, 1972; Zucker & Harshaw, 2012). |
| Discrimination | Difficulty distinguishing somatic signals from each other and/or from emotional signals. For example, an inability to distinguish tiredness from hunger or emotional upset (e.g., Bruch, 1961, 1969). |
| Communication | Impaired ability to communicate about bodily signals and/or feelings. For example, alexithymia involves difficulty identifying and describing emotional and somatic states (Lesser, 1981; Messina, Beadle, & Paradiso, 2014). |
| Decision-making | A deficit in utilizing somatic information in decision-making (Damasio, 1994, 1999; Nauta, 1971). |
Note. This taxonomy is necessarily incomplete, but nonetheless represents the most thorough tabulation of interoceptive dysfunction available.
Interoceptive sensitivity
Interoceptive sensitivity refers to the threshold at which an interoceptive stimulus of a particular intensity is detected. Importantly, sensitivity (i.e., “there is a stimulus present,” “something is happening”) is not equivalent to accuracy identifying a stimulus (Cioffi, 1991; Vaitl, 1996). This is the sense in which interoceptive sensitivity is used in the psychophysiological literature, and heartbeat perception (HBP) tasks are widely employed as measures of interoceptive sensitivity (e.g., Barrett et al., 2004). Several variants of HBP tasks have been employed (e.g., Jones, 1994; McFarland, 1975; Schandry, 1981; Whitehead, Drescher, Heiman, & Blackwell, 1977; Wiens & Palmer, 2001). The most common is the Schandry (1981) method, which involves participants tracking their own heartbeats over a series of fixed temporal intervals without exteroceptive aid (e.g., feeling their own pulse), while an objective measure is also obtained (e.g., via electrocardiogram). An individual error score is then calculated by subtracting the counted from the actual number of beats and then dividing this by the actual number of beats (Schandry, 1981). The method of constant stimuli, in contrast, involves a periodic pulse of light or a tone delivered at various delays following the R-wave of the participants’ heartbeat. Participants then judge whether the pulse is synchronous with their heartbeat (Whitehead et al., 1977; Yates, Jones, Marie, & Hogben, 1985).11
A perennial question is whether HBP tasks can be taken as objective measures of sensitivity to somatic stimuli, given that the intensity of the stimulus—the beating heart—cannot be varied systematically, as would be typical in a psychophysical paradigm (e.g., Eichler & Katkin, 1994; Herbert et al., 2010). Studies have nonetheless found significant correlation between HBP performance and accuracy detecting gastrointestinal stimuli, which have been varied systematically (Herbert, Muth, Pollatos, & Herbert, 2012; Whitehead & Drescher, 1980). HBP tasks thus appear to provide a valid measure of general sensitivity to somatic stimuli (but see Pennebaker, 1982). This argument is bolstered by findings indicating that (a) increases in autonomic arousal following isometric exercise and mental stress correlate with right AIC/ACC activity (Critchley et al., 2000) and (b) both GMV (Critchley et al., 2004) and right AIC-opercular activity during HBP tasks correlate positively with HBP accuracy (Critchley et al., 2004; Pollatos, Schandry, Auer, & Kaufmann, 2007).12 As will be discussed later, there are large individual, gender, and potentially cultural and age differences in sensitivity to somatic stimuli (see Jones, 1994; Ma-Kellams et al., 2012; Pennebaker, 1995).
Interoceptive awareness
The concept of abnormal interoceptive awareness has its origin in early work on anorexia nervosa and developmental obesity (Bruch, 1961, 1969). Bruch employed the term to refer to a deficit that was both perceptual and conceptual in nature—the former referring to difficulty detecting, identifying, and distinguishing somatic signals of hunger and satiety from other somatic and affective signals; the latter involving interpreting and responding to such signals (e.g., Bruch, 1961). In recent literature, “interoceptive awareness” nonetheless refers only to a score on the Interoceptive Awareness (IA) subscale of the Eating Disorders Inventory (EDI), a self-report scale measuring confidence in interoceptive and emotional abilities (Garner, Olmstead, & Polivy, 1983). “Interoceptive awareness” is also often used, incorrectly, to refer to abilities measured by HBP tasks (e.g., Critchley et al., 2004; Pollatos, Gramann, & Schandry, 2007), which involve a manipulation rather than measure of awareness. There are thus several distinct uses of interoceptive awareness in the literature,13 none of which adequately capture the meaning conveyed when the term is undefined—as it often is—something akin to general “consciousness of and/or proneness to direct attention toward bodily stimuli.” Applying such a definition, an individual with low interoceptive awareness would be someone who, in the course of daily life, only rarely attends to or has their attention drawn to somatic stimuli.14 If interoceptive awareness is defined in this way then neither the IA scale of the EDI nor HBP tasks can be taken as direct measures of the construct, although the IA and similar scales such as the Body Perception Questionnaire (BPQ; Porges, 1993) may correlate with awareness.15
Attention and distraction
Somatic stimuli are generally thought to attract attention only if and when they are more salient than other available stimuli (e.g., Herbert et al., 2010; Vaitl, 1996). Under conditions of health, the vast majority of bodily signals thus go unnoticed (cf. Critchley & Harrison, 2013). Although relatively little is known about how attention affects interoception (cf. Barrett et al., 2007), interoceptive and exteroceptive stimuli are often thought to utilize and compete for the same limited attentional resources (Vaitl, 1996). Matthias, Schandry, Duschek, and Pollatos (2009), for example, found that HBP correlated with visual selective and divided attention performance. Recent studies comparing attention to interoceptive and exteroceptive stimuli have nonetheless found the two types of attention to be dissociable (Farb, Segal, & Anderson, 2013; Wiebking et al., 2014). For example, posterior insula activity appears to better predict internally oriented attention whereas AIC activation appears to better predict externally oriented attention (Farb et al. 2013; cf. Sliz & Hayley, 2012).
Attention can also amplify the perceived intensity of pain and other somatic sensations (e.g., Aziz et al., 2000; Bantick et al., 2002). For example, Pennebaker and Lightner (1980) found that manipulations of both attention and external cues altered perceptions of fatigue and other physical symptoms during running—increasing and decreasing somatic perceptions when attention was directed inwardly and outwardly, respectively. Perception of alimentary signals like hunger is also modulated by both attention (e.g., Hebb, 1949; Herman, Ostovich, Polivy, 1999) and exposure to external cues (Fedoroff, Polivy, & Herman, 1997; Herman et al., 1999; Rogers & Hill, 1989). Herman et al. (1999), for example, found that manipulating attentional focus via exposure to videos that contained palatable food cues vs. content that was simply cognitively engrossing significantly increased and decreased, respectively, participants’ ratings of hunger. Brunstrom and Mitchell (2006) similarly found that distraction affected the rate at which negative alliesthesia for a food being consumed developed. Such evidence indicates that attention and context are both powerful modulators of interoception, with attention modulating the “gain” or intensity of interoceptive stimuli (Farb et al., 2013) and exteroceptive cues serving as information about internal states (Cioffi, 1991).
Anatomy and Function of the INS: Summary and Implications for Depression
In summary, interoception is central to the regulation of bodily homeostasis and thus to motivated behavior, emotion, and sociality. Each of the neural structures highlighted can be characterized as mediating some form of adaptive penetrance or interoceptive-exteroceptive integration (see Fig. 1). That is, these structures orchestrate the simultaneous tuning of cognition to the needs of the body and the readiness of the body for meeting situational demands. Core components of the INS, such as the insula and ACC thus play a role both in stimulus valuation or salience determination and the triggering of rapid, autonomic adjustments; others, such as the OFC and hippocampus play roles in alliesthesia and state-dependent memory, respectively. As illustrated in Figure 2, the circuitry underlying interoception is complex, involving feedback loops at multiple levels (see Craig, 2003a). Pathology can occur at any level of the INS, producing a large array of potential deficits (see Table 1). Despite the paucity of terms currently in use, there are thus numerous possible forms of ID with varied and distinct consequences for cognition, affect, and decision-making—not simply deficits in sensitivity and awareness. With these facts in mind, we are in an excellent position to approach the question of ID in depression.
Evidence Implicating Somatic Signals and Interoceptive Dysfunction in Depression
Somatic symptoms and comorbidities (see Beck, 1975; Glassman et al., 2007; Jain, 2009) provide prima facie evidence that depression often involves ID. Rapidly accumulating evidence not only supports this claim, but suggests that such symptoms and comorbidities are correlated with abnormalities in key components of the INS (cf. Avery et al., 2014). As stated at the outset, this evidence is of two broad kinds. The first consists of evidence of abnormalities in somatic signals (e.g., cytokines, stress hormones), and the second, in evidence of INS dysfunction in depression. Both of these will now be reviewed.
Evidence of Interoceptive Dysfunction at the Level of the Vagus
Sickness, cytokines, and depression
Cytokines are a class of somatic signal increasingly recognized as significant to depression (see Dantzer & Kelley, 2007; Raison & Miller, 2013). Studies of cytokines have even given rise to a uniquely somatocentric theory of depression—malaise theory—which argues that inflammation-induced malaise and its behavioral correlates constitute the biological core of depression (Charlton, 2000). Cytokines are chemical messengers—released by immune cells in response to pathogens, damage, and stress—that trigger a suite of adaptive responses (Hart, 1988; Steptoe, Hamer, & Chida, 2007). On the physiological side, this typically includes fever, reduced plasma iron levels, hypothalamus-pituitary-adrenal (HPA) axis activation, increased heart rate, and lowered heart rate variability (Dantzer, 2009; Fairchild et al., 2011; Hart, 1988). The behavioral side of the response, known as sickness behavior, includes reduced grooming, eating, and fluid intake, social withdrawal, sleep alterations, anhedonia (e.g., reduced sucrose consumption), as well as reductions in sexual responsivity that are specific to females (Avitsur & Yirmiya, 1999; Hart, 1988; Larson & Dunn, 2001). In humans, sickness includes most of these symptoms plus malaise or fatigue, lowered mood, and impaired memory and concentration (DellaGioia & Hannestad, 2010). Sickness behavior thus overlaps substantially with the symptomatology of depression (see DellaGioia & Hannestad, 2010; Hart, 1988; Slavich & Irwin, 2014). Cytokines reach the brain and trigger such responses via peripheral nerves such as the vagus (e.g., Hansen, Taishi, Chen, & Krueger, 1998) and receptors in the circumventricular organs and choroid plexus of the brain (Dantzer, 2009). Severing or otherwise inactivating the vagus thus blocks many of the effects of intraperitoneally administered lipopolysaccharide (LPS)—a molecule that tricks the body into mounting an inflammatory response—including changes in eating and social behavior (e.g., Bluthé et al., 1994; Bret-Dibat et al., 1995; Hansen et al., 1998; Marvel et al., 2004).
Sickness has been remarkably well validated as a model for the somatic or “vegetative” features of depression (cf. Dantzer et al., 2008). For example, meta-analyses find a reliable, positive correlation between inflammatory markers (e.g., interleukin-6, tumor necrosis factor-α) and depressed mood in humans (see Howren, Lamkin, & Suls, 2009). Heart rate variability has moreover been shown to predict inflammatory response to LPS, suggesting bidirectional interplay between vagal and immune function (Marsland et al., 2007). A number of studies have, additionally, found elevated levels of inflammatory markers in otherwise healthy persons with MDD (e.g., DellaGioia & Hannestad, 2010; Dowlati et al., 2010). The causal implications of such correlations have been confirmed in studies employing LPS and vaccine injections (see Schedlowski, Engler, & Grigoleit, 2014). Immune-activating injections result in significant decrements in mood, feelings of social connectedness, and social interest—changes that correlate with the magnitude of immune response (e.g., Eisenberger, Inagaki, Marshal, & Irwin, 2010; Hannestad et al., 2012; Reichenberg et al., 2001; Wright, Strike, Brydon, & Steptoe, 2005). For example, Reichenberg et al. (2001) found that LPS injection resulted in significant increase in anxiety followed by significant increase in depressed mood in healthy participants. Given the close relation between inflammatory response and depressive symptoms it has been suggested that depression may, in cases, derive from abnormally prolonged or severe inflammatory processes in the body (see Charlton, 2000; Dantzer, 2009; Viljoen & Panzer, 2005).
Given the role of interoception in bodily homeostasis, it is not surprising that cytokines modify the activity of many cortical components of the INS (see Wan et al., 1995). A number of studies, for example, have found that experimentally induced inflammation modifies activity in insular and cingulate cortices—particularly right AIC, right ACC, dACC, and sgACC (Capuron et al., 2005; Hannestad et al., 2012; Harrison et al., 2009a, 2009b). Interestingly, whereas mood changes following injection have been found to be predicted by sgACC activity during the viewing of emotional faces (Harrison et al., 2009a), changes in social interest were found to be predicted by insula activation, with participants showing the least change in insula activation showing the greatest changes in social interest (Hannestad et al., 2012). In another study, inflammatory markers were found to correlate with bilateral AIC and posterior insula, as well as mPFC and dmPFC activation during social exclusion; right AIC and left posterior insula activity, in particular, were found to correlate with depressed mood (Eisenberger et al., 2009).
Gut-brain axis and depression
Paralleling knowledge of brain-immune interaction, the gut-brain axis and gut-brain signaling are increasingly recognized as playing an important role in cognition, emotion, and even psychopathology (Cryan & O’Mahony, 2011; Dinan & Cryan, 2013; Forsythe et al., 2010; Foster & Neufeld, 2013; Gershon, 1998; Goehler, Lyte, & Gaykema, 2007; Mayer, 2011). Bidirectional interplay between anxiety and gastrointestinal (GI) disorders is well known (e.g., Mayer & Tillisch, 2011; Wilhelmsen, 2000). The impact of the gut microbiome—the massive community of microbes inhabiting the gut—on cognition and behavior is a newer and rapidly growing field of research (see Dinan & Cryan, 2013; Farmer, Randall, & Aziz, in press). It is becoming increasingly clear that gut microbiota can impact immune regulation and inflammatory response (Bäckhed et al., 2005; Cebra, 1999), influence HPA-axis programming (see Dinan & Cryan, 2012), and alter critical aspects of brain and behavior (see Borre et al., 2014; Collins, Surette, & Bercik, 2012; Dinan & Cryan, 2013).
Microbiome-HPA-axis interaction is characterized by bidirectional influence; stress can adversely impact the microbiome (e.g., Bailey et al., 2010; Bendtsen et al., 2012) and increase circulating inflammatory markers (e.g., Bailey et al., 2011), whereas probiotics (e.g., Lactobacillus rhamnosus) appear to blunt HPA-axis responsivity to stress (e.g., Ait-Belgnaoui et al., 2012). A number of measures of anxiety- and depression-related behavior in rodent models, including behavior on the elevated plus maze, stress-induced hyperthermia, and forced-swim—a measure of proneness to adopt passive responding and “give up” attempts to escape an aversive situation—are thus affected by the makeup of the microbiome (e.g., Bravo et al., 2011; Neufeld et al., 2011). Mice raised in the complete absence of microbiota (i.e., germ free) paradoxically exhibit both HPA-axis hyperresponsivity (Sudo et al., 2004) and reduced anxiety (e.g., Heijtz et al., 2011; Neufeld et al., 2011) as well as deficits in social behavior that appear to be specific to males (Desbonnet et al., 2014). Probiotics have been found to decrease anxiety- and depression-like behaviors (e.g., Bravo et al., 2011; Desbonnet et al., 2009, 2010) as well as visceral pain (e.g., McKernan, Fitzgerald, Dinan, & Cryan, 2010). Bravo et al. (2011), for example, found that administration of L. rhamnosus modified both HPA-axis reactivity to stress and GABAergic expression in a number of brain regions (e.g., cingulate cortex), while reducing anxiety and depression-like behavior in mice. Acute GI infection (e.g., Campylobacter jejuni), in contrast, has been shown to increase anxiety- and depression-like behavior in rodents, even in the absence of elevated cytokines and other overt signs of sickness (e.g., Goehler et al., 2005, 2008). Such effects appear to be mediated in large part by vagal afferents (Lyte et al., 2006). For example, in mice, application of L. rhamnosus to the surface of the gut significantly increases the firing of vagal afferents (Perez-Burgos et al., 2013) and severing the vagus reverses many of the effects of microbiome manipulation and GI infection on brain and behavior (e.g., Bercik et al., 2011; Bravo et al., 2011; X. Wang et al., 2002).
Knowledge of gut-brain communication and its relevance to depression and anxiety in humans is currently limited, but nonetheless the focus of a rapidly growing field. For example, a recent study reported significant gut microbiome abnormalities in clinically depressed persons as compared to healthy age- and gender-matched controls (Naseribafrouei et al., 2014). Probiotics also appear to improve anxiety and symptomatology in IBS and chronic fatigue syndrome (Rao et al., 2009; Silk et al., 2008) and have been found to improve depression and anxiety in non-clinical samples (Messaoudi et al., 2011; Owen et al., 2014) and mood in a general population sample, specifically in participants who displayed low mood upon entering the study (Benton, Williams, & Brown, 2007). In another line of research, altered gut-brain feedback in Crohn’s disease (CD)—an inflammatory bowel disease with high anxiety and depression comorbidity (Graff, Walker, & Bernstein, 2009)—has been found to up-regulate the intensity of negative emotions (Vianna et al., 2006). Specifically, individuals with active CD experienced more intense negative affect while viewing negative, emotionally charged film clips than both controls and individuals with inactive CD (Vianna et al., 2006). Phillips et al. (2003) similarly found that non-painful esophageal stimulation fused with negative emotional context (the presentation of fearful faces), amplifying both the discomfort experienced as a result of the procedure and feelings of anxiety, which appeared to be mediated by heightened dACC, AIC, and PCC activity.
Vagal tone and heart rate variability in depression
A number of additional sources of evidence point to vagal dysregulation in depression. For example, an extensive literature links cardiac vagal control and vagal tone or tonic parasympathetic activation to anxiety and depression (see Porges, 2011). The most common measures employed in these studies are heart rate variability (HRV) and respiratory sinus arrhythmia (RSA). Low HRV and RSA have been linked to poorer (a) emotion regulation (see Thayer & Lane, 2009; Thayer & Brosschot, 2005; Thompson, Lewis, & Calkins, 2008); (b) self regulation (e.g., Reynard et al., 2011); and (c) social functioning (e.g., Egizio et al., 2008; Patriquin, Lorenzi, Scarpa, & Bell, 2014; Porges, 2011); as well as (d) higher rates of unexplained somatic symptoms (e.g., Tak et al., 2010) and (e) increased risk for depression (e.g., Gentzler, Santucci, Kovacs, & Fox, 2009; Porges, 2007; Vaccarino et al., 2008). For example, HRV correlates with a number of aspects of responsivity to faces, including emotion recognition (e.g., Park et al., 2012, 2013). Depression appears to involve lower RSA and HRV (e.g., Kemp et al., 2010; Rottenberg, Clift, Bolden, Salomon, 2007) and these moreover have been found to predict treatment response (see Taylor, 2010).
A number of rodent models of depression have also found that HRV and RSA changes correlate with depression-like behavior (e.g., Grippo, Beltz, & Johnson, 2003; Grippo, Moffitt, & Johnson, 2008; Grippo et al., 2009, 2012). For example, Grippo et al. (2003) found that four weeks of chronic mild stress in rats induced anhedonia (i.e., reduced sucrose intake), reduced motor activity, reductions in HRV, and elevated sympathetic tone. Social isolation results in both RSA/HRV changes and depression-like behavior in voles and rats (Carnevali et al., 2012; Grippo et al., 2011; Grippo, Cushing, & Carter, 2007; Grippo, Lamb, Carter, & Porges, 2007)—changes which are blocked by administration of the hormone oxytocin (Grippo et al., 2007). Such findings indicate a close coupling between the social milieu, vagal regulation, and depressive symptomatology (cf. Porges, 2011). In support for this, a recent study in humans found that depressed participants with high RSA reported fewer symptoms of depression than those with low RSA six months post-evaluation only under conditions of high social support (Hopp et al., 2013). A number of treatment studies have also investigated RSA and HRV biofeedback—an approach that has produced significant improvement in both anxiety and depression (Beckham et al., 2013; Karavidas et al., 2007; Siepmann et al., 2008).
Vagal nerve stimulation
Direct stimulation of the vagus nerve16 (Vagal Nerve Stimulation; VNS) was originally developed as a treatment for otherwise untreatable epilepsy (see Terry, Tarver, & Zabara, 1990). However, epileptic patients who received VNS showed unexpected improvements in mood (Elger et al., 2000; George et al., 2000) and VNS was subsequently tested in treatment-resistant depression (TRD; e.g., Rizvi et al., 2011; Rush et al., 2000; Sackeim et al., 2001; Schlaepfer et al., 2008). A number of studies have established the efficacy of VNS for TRD (see Table 2). For example, in a meta-analysis of six prospective studies including over 1,000 patients receiving VNS plus standard treatment and 400 patients receiving standard treatment, VNS significantly improved treatment response and remission rates for TRD (Berry et al., 2013). In rodent models, VNS similarly has antidepressant-like effects (Krahl, Senanayake, Pekary, & Sattin, 2004) and has been shown to increase neurogenesis and hippocampal plasticity (e.g., Biggio et al., 2009).
Table 2.
Studies on Vagal Nerve Stimulation as Treatment for Refractory Depression
| Study | Comparison Groups and Ns | Outcome Measures(s) | Main Finding(s) |
|---|---|---|---|
| George et al. (2005) | TRD receiving 12-month TAU (N = 124); TRD receiving 12-month TAU + VNS (N = 205; described in Rush et al., 2005) | CGI-I; HAMD; IDS-SR | Greater decrease in depression scores and higher response rate in VNS + TAU than in TAU only group (IDS-SR and HAMD). Three times as many participants in VNS + TAU were rated as “much improved” or “very much improved” than in the TAU only group (CGI-I). More participants in the VNS + TAU group showed sustained response to treatment (IDS-SR). |
| Olin et al. (2012) | TRD receiving TAU (N = 309) or TAU + VNS (N = 373) for 2–3 years; Participants self-selected into treatment groups (non-random). | AOS;CGI-I; MADRS Item 10; QIDS-SR | Reduced risk of suicidality in VNS + TAU compared to TAU only group. |
| Rush et al. (2005) | TRD receiving 10 weeks VNS (N = 112) or Sham VNS (N = 110) | CGI-I; HAMD; MADRS; IDS-SR | Higher response rates to active than sham VNS, according to secondary (IDS-SR) but not primary (HAMD) outcome measure. |
Note. Studies on the efficacy of Vagal Nerve Stimulation (VNS) in depression. Only studies including Treatment as Usual (TAU) or Sham VNS controls are included (see Berry et al., 2013; for a broader meta-analysis). That is, case studies (e.g., Borckardt et al., 2006; Critchley et al., 2007), studies of VNS efficacy without controls (e.g., Christmas et al., 2013; Cristancho et al., 2011), studies comparing only various levels of VNS intensity (e.g., Aaronson et al., 2013), and studies focused solely on specific biological and cognitive consequences of VNS in depression, such as effects on pain perception, cravings, and flavor perception (e.g., Borckardt et al., 2005; Sperling et al., 2011) are not included. All findings noted are statistically significant. It is important to note that all three of the listed studies were funded by or conducted by employees of Cyberonics, Inc. (Houston, TX), the manufacturer of the VNS devices used in these studies.
AOS = Assessment of Suicidality; CGI-I = Clinical Global Impression-Improvement; HAMD = Hamilton Rating Scale-Depression; IDS-SR = Inventory of Depressive Symptomatology – Self-Report; MADRS = Montgomery Asberg Depression Rating Scale; QIDS-SR = Quick Inventory of Depressive Symptomatology Self-Report; TRD = Treatment resistant depression;
In humans, VNS has been found to have salutary effects on HPA-axis functioning (O’Keane, Dinan, Scott, & Corcoran, 2005) and result in a number of changes in brain regions upstream of the vagus (Nahas et al., 2006; Pardo et al., 2008). The latter includes the thalamus, hypothalamus, hippocampus, amygdala, as well as orbitofrontal, cingulate, insular, and temporal cortices (e.g., Bohning et al., 2001; Conway et al., 2006, 2012b; Henry et al., 1998, 2004; Kosel et al., 2011).17 Changes in cortical components of the INS appear moreover to mediate the beneficial effects of VNS in depression. For example, a longitudinal study of depressed persons receiving VNS found decreased right AIC activity over time, which correlated with changes in severity of depression (Nahas et al., 2007). In another longitudinal PET study, baseline glucose metabolism in AIC correlated positively and in OFC correlated negatively with response to VNS treatment (i.e., change in HAM-D score) after 12 months (Conway et al., 2012a).18
Evidence of Interoceptive Dysfunction at the Level of the Brain
Beyond the vagus, every major component of the INS has been found to exhibit abnormal morphology and/or functionality in depression. This includes the hippocampus (e.g., Du et al., 2012; MacQueen et al., 2003; Sheline et al., 1996), ACC (e.g., Auer et al, 2000; Bora, Fornito, Pantelis, & Yücel, 2012; Du et al., 2012; Skaf et al., 2002), OFC (e.g., Bremner et al., 2002; Lacerda et al., 2004), vmPFC (e.g., Portella et al., 2011), and insula (e.g., Bora et al., 2012; Cullen et al., 2009; Giesecke et al., 2005; Herwig et al., 2010; Sprengelmeyer et al., 2011; T. Takahashi et al., 2010; Wiebking et al., 2010). The insula is in fact one of the regions most consistently associated with depression in imaging studies (see Fitzgerald et al., 2008). Lai and Wu (2014), for example, found significantly reduced left insular and frontal GMV in cases of medication-naïve, first-episode depression compared to controls. Insula GMV has also been found to predict depression relapse (Soriano-Mas et al., 2011). Avery et al. (2014) reported reduced dorsal mid-insula activation in persons with MDD in a task requiring attention to interoceptive signals. In addition, dorsal mid insula activity during this task was negatively correlated both with depression severity and severity of somatic symptoms (Avery et al., 2014). Given the large number of studies indicating insula abnormalities in depression (see Sliz & Hayley, 2012, for a review), a representative sampling of these are shown in Table 3.
Table 3.
Studies Investigating Insula Morphology and Functioning in Depression
| Study | Comparison Groups and Ns | Imaging Method(s); Task(s) | Main Finding(s) |
|---|---|---|---|
| Anand et al. (2005) | Unmedicated unipolar MD (N = 15), Healthy controls (N = 15) | fMRI BOLD signal changes during emotional picture viewing (IAPS); HDRS; | Greater activation in amygdala, insula, pallidostriatum, ACC, and amPFC during negative compared to neutral picture viewing in MDD. |
| Beauregard et al. (2006) | Unmedicated unipolar MD (N = 12), Healthy controls (N = 12) | fMRI BOLD signal changes during a task involving the down-regulation of sadness | Greater activation of right insula, amygdala, anterior temporal pole, and dACC during emotion down-regulation in MD. |
| Biver et al. (1997) | Unipolar MD (N = 8), Healthy controls (N = 22) | PET imaging + selective radioligand [18F]altanserin, to investigate 5-HT2 receptor binding | Reduced serotoninergic binding in right AIC and OFC in MD. |
| Brody et al. (2001a) | MDD (N = 39), No controls | FDG-PET imaging pre- and post-12-week treatment with either paroxetine or psychotherapy; examined correlations with HAMD and PoMS factors | Changes in all factors with treatment correlated with ventral frontal lobe changes; Changes on the anxiety/somatization factor of the HAMD and tension factor of the PoMS correlated with lowered right vACC and bilateral AIC activity. PoMS fatigue subscale correlated with left AIC changes. |
| Brody et al. (2001b) | MDD (N = 24), Healthy controls (N = 16) | FDG-PET imaging pre- and post- 12-week treatment with either paroxetine or psychotherapy | At baseline: higher metabolism in PFC (bilateral), dorsal caudate and thalamus in MDD. After treatment: increases in left insula and bilateral inferior temporal lobe; left insular changes were largest effect overall; paroxetine had largest effect in right insula. |
| Connolly et al. (2013) | Medication naïve, adolescents with first-episode MDD (N = 30); Healthy controls (N = 45) | fMRI; resting state functional connectivity (iFC); CDRS-R; BDI II; RRS; | MDD group showed heightened iFC between sgACC and left precuneus and mid insula, and right AIC. MDD group also showed increased iFC between the pgACC and right amygdala; BDI-II scores were correlated with iFC between sgACC and left precuneus; CDRS-R scores correlated with iFC between sgACC and left mid frontal gyrus. |
| Grimm et al. (2008) | MDD (N = 20), Healthy controls (N = 30) | fMRI during positive and negative emotional picture viewing (IAPS) and judgments of emotion | During judgments of emotion MDD showed greater activation in right dlPFC, right insula, medial occipital and right parietal cortex; controls showed greater activation in left dlPFC, PCC/MPC, bilateral occipital cortex, and left premotor cortex. Signal change in right dlPFC during emotional judgment correlated with BDI scores. |
| Grimm et al. (2009) | MDD (N = 25), Healthy controls (N = 25) | fMRI during positive and negative emotional picture viewing (IAPS) and judgments of self-relatedness | Lower BOLD signal levels in MDD during judgment of pictures in dmPFC, sACC, precuneus, bilateral DMT, VS, left dlPFC, and insula; during positive pictures; changes in insula activation correlated positively with self-relatedness ratings; an opposite (neg) correlation was found in controls. |
| Horn et al. (2010) | Medicated, MDD (N = 22), Healthy controls (N = 22) | MRS; resting state fMRI | In severe MDD, funct. connectivity between pgACC and AIC predicted by glutamatergic activity in pgACC. |
| Kennedy et al. (2006) | MDD (N = 14), Healthy controls (N = 14) | PET imaging + [11C]carfentanil, to investigate μ-opioid receptor binding under neutral and sadness conditions; plasma cortisol and corticotropin | Greater μ-opioid receptor activation in right anterior temporal and anterior insular cortices, left inferior temporal cortex, and right ventral basal ganglia, thalamus and amygdala during sadness in MDD than in controls. Greater deactivation of μ-opioid receptors in controls in ACC, hypothalamus, left amygdala, and a number of other regions. |
| Kong et al. (2014) | Single episode, unmedicated MDD (N = 28), Healthy controls (N = 28) | MRI pre- and post-treatment with fluoxetine (8 weeks) | Prior to treatment, MDD had decreased GMV in right dlPFC and left mid frontal gyrus, and increased GMV in left thalamus and right insula compared to controls. Treatment increased GMV in left mid frontal gyrus and right OFC. |
| Lai & Wu (2014) | First episode, unmedicated MDD (N = 38), Healthy controls (N = 27) | MRI; HAM-D | Lower GMV in left insula, mid frontal gyrus, medial frontal gyrus, and bilateral superior frontal gyri in MDD. Negative correlation between total GMV and HAM-D scores in MDD. |
| Lane et al. (2013) | MDD (N = 8), Healthy controls (N = 11) | fMRI; ECG for measurement of cardiac vagal control; Emotional Counting Stroop | Magnitude of RSA change during Stroop correlated with bilateral sgACC, left BA47, and left AIC in healthy controls. These correlations were absent in MDD. |
| Liu et al. (2010) | MDD (N = 15), first-degree relatives of MDD group (N = 15), Healthy controls (N = 15) | fMRI resting state functional connectivity using ReHo | Reduced ReHo in right insula and lentiform nucleus, left mid frontal gyrus, cerebellum, superior temporal gyrus, and precentral/postcentral gyri and increased ReHo in left inferior frontal gyrus and inferior parietal lobule in MDD compared to controls. Reduced ReHo in right insula and left cerebellum in first-degree relatives of MDD. |
| Liu et al. (2014) | MDD (N = 19), Recovered from MDD (N = 19), Healthy controls (N = 19); all participants were female | MRI; HAM-D | Current MDD group had lower GMV in right dorsal anterior insula and left dorsal insula than controls; Recovered MDD group had lower GMV in left dorsal insula than controls. Right dorsal anterior insula GMV correlated positively with number of depressive episodes in current MDD group. |
| Manoliu et al. (2014) | MDD (N = 25), Healthy controls (N = 25) | MRI; intrinsic functional connectivity (iFC) via resting state fMRI; HAM-D; BDI | MDD group showed decreased iFC between posterior DMN and dorsal CEN, and increased iFC between the SN and inferior posterior DMN. Intra-iFC was decreased in bilateral anterior insula in MDD; there was a significant negative correlation between intra-iFC for right anterior insula and severity of depression, as measured by both BDI and HAM-D. |
| Mayberg et al. (1997) | Unipolar MD (N = 18), Healthy controls (N = 15) | FDG-PET; HAM-D; Response to Tx with antidepressants, assessed at 6 weeks; | Responders had increased rACC metabolism, whereas non-responders had decreased rACC metabolism relative to controls. Non-responders had decreased left PFC metabolism compared to controls; Responders and non-responders also differed in dlPFC, premotor and basal ventral frontal cortex, and anterior insular metabolism. |
| Peng et al. (2011) | First episode MDD (N = 22), Healthy controls (N = 30) | MRI; HAM-D | MDD group showed reduced GMV in bilateral inferior/middle frontal gyri, temporal poles, mid temporal gyri, and anterior insula, right OFC, left parahippocampal gyrus, and left cerebellum. A negative correlation between HAM-D scores and right dlPFC GMV was also found. |
| Ramasubbu et al. (2014) | MDD (N = 55), Healthy controls (N = 19) | MRI; intrinsic functional connectivity (iFC) via resting state fMRI; HAM-D; HAM-A; | MDD group showed decreased iFC between left amygdala and a number of regions, including vlPFC, motor cortex, caudate, precuneus, and right insula. Right amygdala showed decreased iFC with left caudate, and right cuneus and insula. Amygdala iFC with these regions did not correlate with HAM- and HAM-A scores; however, iFC between left amygdala and right temporal pole correlated negatively with HAM-D and HAM-A scores. |
| Soriano-Mas et al. (2011) | MDD (N = 70), Healthy controls (N = 40) | MRI pre- and post-2 year treatment; HAM-D; | No differences in global measures of GMV or WMV between patients and controls. MDD group showed reductions in left posterior insula and WMV increases in brainstem tegmentum. Males with MDD showed reduced GMV in right thalamus. Left posterior insula GMV correlated negatively with days to symptom remission. “Core HAM-D” symptoms correlated positively with left posterior cingulate GMV. |
| Sprengelmeyer et al. (2011) | Medicated, chronic (>2 years) MDD (N = 27), Healthy controls (N = 51) | MRI; fMRI during Ekman 60 faces and Emotion Hexagon (EH) tasks; HAM-D; BDI-II; SHAPS; | Reduced discrimination of disgust on 60 faces test in MDD; reduced accuracy for sadness, disgust, and anger on EH task in MDD. Reduced GMV in right amygdala, superior frontal gyrus, and parahippocampal gyrus, left insula, mid temporal and fusiform gyri in MDD relative to controls. In MDD, GMV in right anterior insula, ACC, and mid temporal gyrus, left inferior frontal gyrus correlated with HAM-D score. Similar finding with BDI-II, however, bilateral insula and left ACC GMV correlated with BDI-II scores; Bilateral insula and left mPFC GMV correlated with discrimination of disgust. |
| Tahmasian et al. (2013) | Recurrent MDD (N = 21), Healthy controls (N = 20) | MRI; intrinsic functional connectivity (iFC) via resting state fMRI; HAM-D; BDI; GAF; | MDD group showed decreased hippocampal and amygdala GMV. Reduced iFC between amygdala and dmPFC, inferior frontal gyrus, and fronto-insular operculum in MDD. |
| T. Takahashi et al. (2010) | Current MDD (N = 29), Recovered from MDD (N = 27) Healthy controls (N = 33) | MRI; | Left anterior insula GMV was reduced in both current and remitted MDD compared to controls. Medication status had no effect on insula GMV. A significant negative correlation between insular GMV and age of onset of MDD for both current and remitted MDD was also found. |
| van Tol et al. (2014) | Acute MDD (N = 20) Healthy controls (N = 20) | MRI; resting state functional connectivity (iFC) via fMRI; fALFF; HAM-D; | MDD group showed lower cortical thickness in right posterior mid cingulate, dlPFC, dmPFC, and superior temporal gyrus. No correlation between regional thickness and HAM-D scores was found. MDD group also showed reduced iFC between dmPFC and left anterior insula and mid frontal gyrus, and decreased iFC with left mid temporal gyrus, right temporal pole, and PCC/precuneus. |
| Veer et al. (2010) | MDD (N = 19) Healthy controls (N = 19) | fMRI; resting state functional connectivity (iFC); MADRS; | No differences in GMV were detected between MDD group and controls. Decreased iFC between bilateral amygdala and left insula in affective network. Reduced iFC of left frontal pole in attention/working memory network. No relationship was found between MADRS scores and any iFC measure. |
| L. Wang et al. (2014) | MDD with history of childhood neglect (N = 19), MDD without such history (N = 21), Healthy controls (N = 20) | MRI; resting state connectivity strength (FFC) for every voxel; HAM-D; CTQ; | Both MDD groups showed reduction in FCS in bilateral vmPFC and vACC. The MDD with neglect group also showed reduced FCS for dmPFC, vlPFC, insula, caudate, thalamus, parahippocampal gyrus, hippocampus, amygdala and cerebellum. In the MDD with neglect group, negative correlation between the emotional neglect subscale of the CTQ and FCS for bilateral thalamus, dmPFC, and dlPFC was found. |
| Wiebking et al. (2010) | MDD (N = 17), Healthy controls (N = 17) | fMRI during Schandry (1981) HBP and exteroceptive tone-counting tasks; BDI; BPQ; MADRS; | Higher scores on all BPQ subscales except “awareness” in MDD. BDI scores correlated positively with BPQ scores. MDD group had less change in insula activation during HBP task than controls, due to heightened activation during rest periods. Deactivation of insula during rest correlated with BPQ scores in controls, but not MDD. |
| Yao et al. (2009) | MDD (N = 26), Healthy controls (N = 26) | fMRI resting state functional connectivity using ReHo; HAM-D; | MDD group showed reduced ReHo in right OFC, vACC, insula, fusiform gyrus, and PCC, and left dACC and lentiform nucleus. ReHo in OFC and dACC correlated positively with Cognitive Disturbance (HAM-D); ReHo in right vACC and insula correlated with Hopelessness; ReHo in left dACC correlated with Sleep disturbance; ReHo in PCC and right insula correlated with Retardation; Anxiety also correlated with right insula ReHo. |
| Zhang et al. (2014) | Unmedicated, first-episode MDD (N = 32), Healthy controls (N = 35) | fMRI resting state functional connectivity (iFC) and ALFF; CES-D; | MDD group showed reduced ALFF in bilateral OFC and increased ALFF in bilateral temporal gyrus and insula, and left fusiform and mid occipital gyri. MDD group also showed reduced iFC between left amygdala and left OFC. No correlations between iFC or ALFF and CES-D scores were found. |
Note. Functional and structural neuroimaging studies focused on fronto-insular functioning or featuring insula abnormalities as a major finding detected in depression compared to non-depressed controls (see Fitzgerald et al., 2008; L. Wang, Hermens, & Lagopoulos, 2012; for reviews). All findings noted are statistically significant.
AIC = anterior insular cortex; ACC = anterior cingulate cortex; pgACC = pregenual ACC; rACC = rostral ACC; sACC = supragenual ACC; vACC = ventral ACC; ALFF = amplitude of low-frequency fluctuations; fALFF = fractional ALFF; BDI = Beck Depression Inventory; BOLD = Blood oxygen-level dependent; BPQ: Body perception questionnaire; CDRS-R = Children’s Depression Rating Scale-Revised; CES-D = Center for Epidemiological Studies Depression Scale; CTQ = Childhood Trauma Questionnaire; DMN = Default Mode Network; DMT = dorsomedial thalamus; FDG = [18F]fluorodeoxyglucose; GAF = Global Assessment of Functioning; GMV = grey matter volume; HAM-A = Hamilton Anxiety Rating Scale; HAM-D = Hamilton Depression Rating Scale; IAPS = International Affective Picture System; MADRS = Montgomery–Asberg Depression Rating Scale; MD = Major Depression; MDD = Major Depressive Disorder; MRI = Magnetic Resonance Imaging; fMRI = functional MRI; OFC = Orbitofrontal cortex; PCC = posterior cingulate cortex; PET = Positron Emission Tomography; PFC = prefrontal cortex; amPFC = anteromedial PFC; dlPFC = dorsolateral PFC; dmPFC = dorsomedial PFC; vlPFC = ventrolateral PFC; PoMS = Profile of Mood States; ReHo = regional homogeneity; RRS = Rumination Response Scale; SHAPS = Snaith-Hamilton Pleasure Scale; SN = Salience Network; VS = ventral striatum; WMV = white matter volume.
The anterior insula and anterior cingulate form a closely connected circuit, nearly always being co-activated in studies of emotion (see Craig, 2009, 2010; Medford & Critchley, 2010; Taylor, Seminowicz, & Davis, 2009). Altered functioning of the ACC-AIC circuit is thus likely to play a role in any ID involving the insulae (e.g., Horn et al., 2010; Mayberg et al., 1999). Mayberg et al. (1999), for example, found that inducing sadness in healthy volunteers caused increased sgACC and AIC activation, and that recovery from MDD was associated with a lowering of activation in both regions. Lane et al. (2013) similarly found high correlations between RSA changes during an emotional Stroop task and sgACC and left AIC activity in healthy subjects, but not in depressed participants.
A number of meta-analyses of emotion- and pain-related neural functioning indicate AIC-ACC involvement in depression. Hamilton et al. (2012), for example, performed a meta-analysis of fMRI studies examining the processing of negative emotional stimuli in depression, finding greater activity in the pulvinar nuclei of the hypothalamus during the resting state, and greater activity in the amygdala, dACC, insula, and superior temporal gyrus during exposure to negative emotional stimuli. In a similar meta-analysis of studies examining the processing of positive and negative stimuli, Groenewold et al. (2013) found that relative to controls depressed persons exhibited increased activation of the mid cingulate and ACC while processing negative emotional stimuli and decreased ACC, insula, striatum, and thalamus activation while processing positive emotional stimuli. Mutschler, Ball, Wankerl, and Strigo (2012) performed a combined meta-analysis of studies of pain processing in healthy individuals and emotional processing in MDD. Depressed persons exhibited increased pain-related processing as well as a shift, within the insula, toward processing emotional stimuli using the dorsal AIC—the area maximally associated with pain processing in healthy participants (Mutschler et al., 2012).
Such studies suggest a connection between the negative cognitive-affective biases characteristic of depression and disturbances in interoceptive and reward-related regions of the brain. In a remarkably thorough study indicating connections between INS components and affective and social disturbances in MDD, Sprengelmeyer et al. (2011) found reduced GMV in depressed persons in a number of areas, including the amygdala, insula, ventral and medial temporal lobes, and parahippocampal and hippocampal cortices. GMV in bilateral insula, ACC, inferior frontal gyrus, middle temporal gyrus, and parahippocampal gyrus moreover correlated negatively with depression severity, as measured by the BDI II and Hamilton Depression Rating Scale (Hamilton, 1960). Impaired recognition of facial expressions of disgust was also found in participants with MDD—the magnitude of which correlated with GMV in bilateral AIC and mPFC (Sprengelmeyer et al., 2011). In a healthy, nondepressed sample, Wiebking et al. (2014) found that change in insula activation during HBP correlated negatively with scores on the Beck Hopelessness Scale.
Evidence of Interoceptive Dysfunction at the Level of Sensitivity and Awareness
Given the reviewed evidence indicating INS dysfunction, changes in sensitivity to and/or awareness of bodily signals might be expected in depression. Comparatively few studies have addressed this question. There is some evidence that depressed persons have reduced confidence in their interoceptive abilities, as measured by the IA subscale of the EDI (e.g., Fava et al., 1997; Kopp, 2009; C. Smith & Steiner, 1992). However, such results say little about interoceptive sensitivity and awareness (Pollatos et al., 2008). One study, employing the BPQ—which contains a set of questions focused on general awareness of bodily symptoms and autonomic responsivity (Porges, 1993)—found that depression appears to involve heightened awareness of somatic signals (Wiebking et al., 2010). The Body Awareness scale of the BPQ is nevertheless not highly correlated with interoceptive sensitivity (see Fairclough & Goodwin, 2007; Schulz et al., 2013). Furthermore, heightened awareness of bodily stimuli does not imply accuracy interpreting and responding to such stimuli, particularly under noisy, real-world conditions (cf. Cioffi, 1991; Wilhelmsen, 2000).
A number of studies have examined interoceptive sensitivity in depression using variants of the Schandry (1981) HBP task (see Table 4). Ehlers and Breuer (1992), for example, found that depressed persons displayed poorer HBP performance than did patients with panic disorder and generalized anxiety. Anxiety and panic nonetheless involve abnormally heightened HBP (Domschke et al., 2010; Stevens et al., 2011), so these represent poor reference groups for assessing interoception in depression. Van der Does, Van Dyck, and Spinhoven (1997) obtained similar results, but additionally found that depressed persons had poorer HBP—although not significant so—than a control sample.19 Mussgay, Klinkenberg, and Rüddel (1999) compared performance on a modified HBP task across a number of psychiatric diagnoses, and found significantly reduced performance in comparison to controls only in personality disorders; depressed participants exhibited somewhat poorer performance than controls, though the difference, again, was not statistically significant.
Table 4.
Studies Investigating Interoceptive Sensitivity in Depression
| Study | Comparison Groups and Ns | Task; Method | Main Finding(s) |
|---|---|---|---|
| Ehlers & Breuer (1992) | compared participants with depression (N = 16; 8 males) to panic disorder (N = 13; 8 males) and generalized anxiety disorder (N = 15; 9 males) | HBP; Schandry method | poorer HBP in panic and GAD than depression; no comparison with healthy controls, but higher error rates in depression compared to controls in separate experiment of same study.* |
| van der Does et al. (1997) | compared participants with depression or dysthymia (combined; N = 16; 3 males) to panic disorder (PD; N = 23; 8 males) and healthy controls (N = 21; 8 males) | HBP; Schandry method | depressed participants had the highest HBP error rate and the lowest % of accurate HB perceivers, and were significantly worse than PD but not controls. |
| Dunn et al. (2007) | compared healthy controls (N = 18; 4 males) to a mild-to-moderately depressed community sample (N = 18; 5 males), to a severely depressed clinical sample (N = 18; 5 males) | HBP; Schandry method | a significant negative correlation between HBP error scores and BDI was found only for the moderately depressed community sample; a significant difference from controls was found only in the moderately depressed sample; overall, a U-shaped relationship between HBP and depression severity was found. |
| Dunn et al. (2010) | examined correlations across a range of depressed and non-depressed community volunteers (N = 113; 31 males) | HBP; computerized version of Schandry method | found no relationship between HBP and trait anxiety, nor between HBP and BDI; found that the relation between HBP and BDI was modulated by trait anxiety, and the relation between HBP and anxious arousal was modulated by anhedonia. |
| Herbert et al. (2011) | examined correlations in a minimally depressed, non-clinical (under-graduate) sample (N = 155; 67 males) | HBP; Schandry method | found a significant negative correlation between BDI-2 and HBP scores. |
| Mussgay et al. (1999) | compared a number of psychiatric diagnoses (inpatients), including neurotic depression (N = 141; 19 males) and reactive depression (N = 106; 24 males) to healthy controls (N = 48; 19 males) | HBP; Schandry method | a significant difference from controls was found only for personality disorders although there was a trend (p = .06) toward significantly poorer HBP in reactive depression. |
| Pollatos et al. (2009) | examined correlations in a minimally depressed, non-clinical (under-graduate) sample (N = 119; 21 males) | HBP; Schandry method | found a significant negative correlation between BDI and HBP, and a significant positive correlation between anxiety and HBP; the relationship between BDI and HBP was modulated by anxiety. |
| Terhaar et al. (2012) | compared moderately depressed participants with MDD (N =16; 3 males) to age- and gender-matched controls (N = 16) | HBP; Schandry method + exteroceptive tone tracking task | depressed participants had significantly lower HBP than controls, but did not differ on the tone tracking task. |
| EEG (examined heartbeat evoked potentials; HEP) | reduced HEP amplitudes in depression compared to controls. | ||
| Furman et al. (2013) | compared female participants with MDD and no comorbid anxiety (N = 25) to healthy controls with no history of depression (N = 36) | HBP; Schandry method + Affect Intensity Measure (AIM) + Structured Clinical Interview for DSM-IV (SCID) | HBP accuracy was significantly lower in MDD than in controls; there was also a trend toward a significant negative correlation between overall BDI score and HBP (r = −.321, p = .056). For the MDD group but not controls, positive affectivity as measured by the AIM was positively correlated with HBP accuracy. MDD with decision-making difficulty, as measured by the SCID, was associated with lower HBP. |
Note. Schandry method refers to the method described in Schandry (1981).
based on visual inspection of the reported data; no statistical analysis was performed.
BDI = Beck Depression Inventory (Beck et al., 1988); EEG = Electroencephalogram; GAD = General anxiety disorder; HEP = Heart beat evoked potential; HB = Heart beat; HBP = Heart beat perception;
A number of recent studies employing improved designs have found evidence of a deficit in interoceptive sensitivity in depression. Dunn, Dalgleish, Ogilvie, and Lawrence (2007), for example, examined HBP and self-reported awareness of bodily signals using the Body-Consciousness Questionnaire (BCQ; Miller, Murphy, & Buss, 1981) in a moderately depressed community sample (BDI = 22.2 ± 1.9),20 a clinically depressed group (BDI = 28.3 ± 2.1), and controls (BDI = 4.3 ± .7). No differences in BCQ scores were found; however, the moderately but not severely depressed group showed significantly poorer HBP than controls. Further analysis revealed an inverted-U relationship between HBP and BDI score: intermediate BDI scores were associated with impaired HBP whereas high and low scores were associated with better HBP (Dunn et al., 2007). Furman et al. (2013) argued that a high rate of anxiety comorbidity (greater than 50%) in Dunn et al. (2007) may have produced the null finding in Dunn et al.’s clinically depressed sample. Furman et al. (2013) thus measured HBP and BDI-2 both in a clinical sample with MDD and no anxiety comorbidity (BDI = 25.0 ± 1.8) and a healthy sample with no history of depression (BDI = 3.1 ± .5), finding significantly poorer HBP in the depressed sample than controls. Terhaar, Viola, Bär, and Debener (2012) similarly examined a moderately depressed sample—comparable to that used in Dunn et al. (2007)—diagnosed with MDD and no comorbid panic (BDI = 22.6 ± 2.5) and healthy controls (BDI = 2.31 ± .7). Terhaar et al. (2012) measured HBP and performance on an exteroceptive tone counting task, while also obtaining heartbeat-evoked potentials (HEP) using EEG. Depressed persons were found to exhibit significantly reduced HEP and significantly poorer HBP than controls, but did not differ on the exteroceptive tone counting task (Terhaar et al., 2012).
A number of studies have examined the correlation between depression and interoceptive sensitivity in non-clinical samples. For example, Pollatos et al. (2009) examined the relationship between depression, anxiety, and HBP in a non-depressed college sample (BDI-2 = 3.49 ± .3) and found a significant (a) negative correlation between BDI and HBP performance (r = −.19, p < .05), (b) positive correlation between anxiety and HBP (r = .18, p < .05) and (c) positive correlation between anxiety and depression (r = .33, p < .001). Employing a similar sample of healthy college students (BDI-2 = 3.52 ± .3), Herbert, Herbert, and Pollatos (2011) replicated the finding of a significant negative correlation (r = −.21, p < .05) between BDI-2 and HBP scores. Furman et al. (2013) similarly found a trend toward a significant negative correlation between HBP and BDI in their control sample (r = −.32, p = .056). In contrast, Dunn et al. (2010) examined HBP in a community sample that included a number of mild to moderately depressed and anxious individuals (BDI = 8.8 ± .7) and found no significant relationship between HBP and either BDI or anxiety measures. Instead, a complex interaction between anxiety, anhedonia, and HBP was found: high anhedonia being associated with poorer HBP, irrespective of anxiety status (cf. Ferguson & Katkin, 1996), and lower levels of anhedonia associated with progressively better HBP performance in individuals with higher levels of anxiety (Dunn et al., 2010).
Taken together, studies of interoceptive sensitivity in depression present a complex, yet increasingly coherent picture: several studies indicate that depressed persons show moderately impaired interoceptive sensitivity, particularly in the range of mild to moderately severe depression (Dunn et al., 2007; Ehlers & Breuer, 1992; Mussgay et al., 1999; Pollatos et al., 2009; Terhaar et al., 2012; Van der Does et al., 1997). Other studies suggest that severely depressed persons may exhibit equivalent or even improved HBP relative to controls—similar to the superior HBP typically found in anxiety and panic (e.g., Critchley et al., 2004; Ehlers & Breuer, 1992)—and thus that there is U-shaped relationship between depression severity and interoceptive sensitivity (Dunn et al., 2007). Such complexity appears to be mediated, in part, by the fact that anxiety and depression—although correlated and highly comorbid—have opposite associations with interoceptive sensitivity (e.g., Pollatos et al., 2009). Studies reporting more complex relationships between interoceptive sensitivity and depression have generally failed to control for anxiety status (e.g., Dunn et al., 2007, 2010). Studies that have controlled for anxiety have more consistently reported significantly lower interoceptive sensitivity in depression (Furman et al., 2013; Terhaar et al., 2012). Additional support for reduced interoceptive sensitivity in depression is provided by negative correlations between HBP and BDI scores in non-clinical samples (Furman et al., 2013; Herbert et al., 2011; Pollatos et al., 2009).
There are additional issues to consider when interpreting the available studies, including the variable medication status of depressed participants. Few studies to date have examined the effects of SSRIs and anxiolytics on somatic sensitivity. Mussgay et al. (1999), for example, found no significant effect of medication status (collapsing all medications) on HBP of depressed persons, although there was a trend toward poorer HBP when on medication—a finding difficult to interpret given that medication status likely covaries with depression severity. Terhaar et al. (2012) similarly found no significant difference in HBP between medicated and unmediated depressed persons, but did not likely have enough statistical power for such a comparison.21 In contrast, a number of studies have examined the effects of SSRIs (e.g., fluoxetine, paroxetine) on brain activity using fMRI or PET, finding both that SSRIs result in decreased insula activation (e.g., Arce et al., 2008; Kennedy et al., 2001; Kraus et al., 2014; Mayberg et al., 2002, 2009; Simmons et al., 2009) and that changes in insula activity (in addition to decreases in sgACC and hippocampus, and increases in ACC, PFC, and PCC) correlate with response to drug treatment (Ernst et al., 2014; Mayberg et al., 2009).22 Combined with the fact that serotonin is vasoactive and directly impacts the cardiovascular system, it is thus likely that SSRIs impact measures of interoceptive sensitivity (Mayberg et al., 2009).
Somatic and Interoceptive Dysfunction in Depression: A Summary
In summary, accumulating evidence implicates abnormal somatic signaling and ID in depression. Inflammatory cytokines and gut signals are, for example, increasingly recognized as capable of inducing core affective, cognitive, and behavioral features of depression (see Dantzer & Kelley, 2007; Dinan & Cryan, 2013; Foster & Neufeld, 2013). All of the major components of the INS highlighted in Figure 2, including the vagus, insula (see Table 3), and ACC moreover show changes in morphology and/or functioning in depression. For example, measures of vagal tone such (e.g., HRV and RSA) are reliably associated with anxiety and depression (see Porges, 2011) and the vagus has proven to be a valuable point of intervention for the treatment of refractory cases of depression (see Table 2). In addition, studies employing HBP paradigms are beginning to converge on finding significantly reduced interoceptive sensitivity in depression, when anxiety comorbidity is controlled (see Furman et al., 2013). The latter finding is predicted from neuroimaging results, given that insula GMV correlates with HBP (Critchley et al., 2004) and depression appears to involve reduced insula GMV (e.g., Lai & Wu, 2014; T. Takahashi et al., 2010). Taken as a whole, this evidence provides support for the thesis that depression is often characterized by disturbed somatic signaling and ID.
Models of Interoceptive Dysfunction in Depression
Not surprisingly given the evidence, a number of neurobiological models of depression take interoceptive dysfunction as a given—something that requires explanation and integration with other data on depression. In general these models fail to specify what role(s) interoception and ID might play within the overall constellation of symptoms characteristic of the disorder (e.g., Andréasson et al., 2007; Krishnan & Nestler, 2010; Mayberg, 2009). A few models nonetheless explicitly relate ID to specific features of depression (see Table 5). For example, Paulus (2007) speculated that disturbed somatic processing could result in altered valuation of exteroceptive stimuli, contributing to anhedonia and decision-making deficits in depression. Paulus and Stein (2010) argue that individuals at risk for anxiety and depression experience a reduced signal-to-noise ratio in interoceptive processing—essentially, that bodily signals are noisier in depression, rendering them more difficult to detect and interpret. The Paulus and Stein (2006, 2010) model also posits altered alliesthesia for external stimuli, as well as amplification of and exaggerated emotional responses to bodily signals, based on beliefs about and top-down attempts to control such signals. Paulus and Stein (2010) avoid outlining specific causal pathways (p. 458) and make few predictions, except that anxious and depressed persons with ID should show communicative deficits (i.e. alexithymia) and impairments on tasks requiring the use of somatic signals, such as the Iowa Gambling Task (e.g., Must et al., 2006).
Table 5.
Core Features of Current Models of Interoceptive Dysfunction (ID) in Depression
| Paulus & Stein (2010) | Northoff et al. (2011) | Current framework |
|---|---|---|
|
|
|
Northoff, Wiebking, Feinberg, and Panksepp (2011), in contrast, attempt to account for both interoceptive and exteroceptive changes in depression. Reduced sensitivity to flavors and odors have, for example, been documented in depression—changes which both correlate with depression severity (see Canbeyli, 2010) and are associated with reduced insula responsivity (Croy et al., 2014). The Northoff et al. (2011) model, illustrated in Figure 3, thus posits resting-state or baseline abnormalities in a number of systems in depression, including the ‘interoceptive system’ (insula, periaqueductal grey, and tectum). Northoff et al. argue that depression involves abnormally high resting-state activity in the insula and other limbic and subcortical regions (see Drevets, 2000) and hypoactivity in higher-level cortical regions (e.g., dlPFC). Such activity is argued to result in an imbalance in interoceptive-exteroceptive processing; that is, simultaneous hyper-responsivity to internal, bodily stimuli and hypo-responsivity to external stimuli (e.g., Wiebking et al., 2010), leading to the increased self-focus characteristic of depression (e.g., Grimm et al., 2009). In addition, such imbalance is argued to give rise to a number of other core symptoms of depression, including negative biases, dysphoria, anhedonia, hopelessness, apathy, and working memory deficits (Northoff et al., 2011).
Figure 3.
An illustration of the Northoff et al. (2011) model of interoception in depression. An imbalance between interoceptive and exteroceptive processing is central to this model. Neurally, this is manifest as simultaneous hyperactivity in cortical-midline structures (e.g., interoceptive regions such as insula and related regions involved in temporal perception, affect and reward, such as the pACC, vmPFC, and amygdala) and hypoactivity in “lateral ring” structures (e.g., dlPFC and sensorimotor cortices). A number of potential causes of such imbalance are noted by Northoff et al., including genetic, neurochemical, endocrine, immune, and social factors. A variety of downstream consequences of interoceptive-exteroceptive imbalance, which overlap with symptoms of depression, are shown. A number of other symptoms not shown, such as negative biases, negative mood, anhedonia, psychomotor retardation, decreased goal orientation and working memory, are noted by Northoff et al. (2011). These are nonetheless related by Northoff et al. to changes in functioning of specific brain regions/networks rather than specifically to interoceptive-exteroceptive imbalance.
Multiple Pathways to Interoceptive Dysfunction in Depression: A Framework
Although each of these models links ID to a limited set of features or symptoms of depression, no attempt has yet been made to provide a comprehensive account of the relation between ID and depressive symptomatology. For example, somatic symptoms of depression, such as disturbed sleep and eating, are absent, as are links to the social context of depression. There are additional weaknesses to these models, including a lack of specificity regarding causal pathways (e.g., Paulus & Stein, 2010) and a lack of integration with the wider literature on interoception. Additionally, these models fail to relate ID to gender and developmental biases in the epidemiology of depression.
The framework put forward here attempts to remedy these shortcomings without challenging the specifics of these models.23 As illustrated in Figure 4, both equifinality24 and likelihood for overdetermination of ID is acknowledged. That is, several distinct pathways to ID are theoretically possible, any or all of which may be operative in a given case of depression or at a given point in a depressive episode. These can be broadly divided into three main routes to ID, caused by (a) direct modification of one or more components of the INS, by stress, stroke, or other causes; (b) the loss of situational cues ordinarily used to disambiguate interoceptive signals, due to situational or behavioral changes, like withdrawal; and (c) shifts in attention or awareness, due to cognitive tendencies like analytic self-focus and rumination. This framework, additionally, emphasizes interaction of these pathways with individual, gender, and cultural differences in interoception, and likelihood for increased occurrence of ID during particular phases of the lifespan. At a minimum, the net outcome of ID is likely to include somatic dysregulation—significantly changed sleep and/or eating—which can, theoretically, feedback and cause further ID (see Fig. 4). Each of the major pathways to ID are reviewed below.
Figure 4.
A framework for understanding interoceptive dysfunction (ID) in depression. The topmost row indicates common proximal causes of ID (e.g., stroke, stress, pro-inflammatory cytokines, behavioral or contextual changes). The next row illustrates the three main pathways by which these are likely to give rise to ID: (a) modification to neural systems underlying interoception, or the interoceptive nervous system (INS); (b) loss of exteroceptive cues ordinarily used to disambiguate interoceptive signals; and (c) attentional changes. Arrows indicate known interactions and likely causal influences, although these are not exhaustively illustrated (e.g., inflammatory cytokines, stroke, etc., may also influence stress hormone levels and vice versa). As is indicated in brackets, all such pathways ultimately interact with individual, gender, and cultural differences in interoception in giving rise to ID. ID is moreover likely to give rise to errors in somatic interpretation or missed somatic signals, contributing to the somatic symptoms (e.g., disrupted sleep and eating) typical of depression (cf. Healy & Williams, 1988).
Fight or Flight, Stress, and Interoception
The “fight or flight” system evolved to aid behavioral response in emergencies, rapidly mobilizing resources for processes vital to survival, while diverting resources from processes of less immediate importance, like digestion (see Sapolsky, 1999). Intuitively, such a system should include a means of blunting or dissociating from awareness the perception of potentially distracting somatic signals—even regulatory signals like pain and hunger which, in the long-run, might themselves indicate threats to survival. That stress hormones have such an effect is indicated by the occurrence of stress-induced analgesia, in which stress both blunts affective reactions to pain and raises pain thresholds (Horvath & Kekesi, 2006; Sapolsky, 1992)—effects which have been shown to involve activation of somatosensory cortices, as well as AIC and ACC (Yilmaz et al., 2010).25 Glucocorticoids (e.g., cortisol, corticosterone) are known to have both analgesic effects (e.g., Hargreaves et al., 1987; Lim, Oei, & Funder, 1983) as well as variety of effects on motivated behaviors such as eating and drinking (e.g., Erickson, Drevets, & Schulkin, 2003; la Fleur, 2006)—with both low and high levels associated with reduced hunger (e.g., Dallman et al., 1993; Leibowitz, 1995).
Although few studies have examined the impact of stress on interoceptive sensitivity and the results from these are equivocal (see Fairclough & Goodwin, 2007; Schulz et al., 2013), there is overwhelming evidence that the neural systems for interoception are impacted by HPA-axis activation and stress (cf. Mayer, Naliboff, & Munakata, 1999). In addition to affecting a number of limbic structures, including the amygdala (see Roozendaal, McEwen, & Chattarji, 2009), stress and HPA-axis activity have been shown to modify the morphology and/or functioning of core components of the INS, including the vagus (e.g., Cho, Qui, & Bruce, 1996; Dale et al., 2009; Porges, 1995), hippocampus (e.g., Bremner, 1999; Sala et al., 2004), ACC (e.g., Gianaros et al., 2007), OFC (e.g., Gianaros et al., 2007; Pruessner et al, 2008), and insula (e.g., Gianaros et al., 2005; Liston, McEwen, & Casey 2009; Stein, Simmons, Feinstein, & Paulus, 2007; J. Wang et al., 2005, 2007). Liston et al. (2009), for example, found that a month of psychosocial stress resulted in reversible decoupling of the PFC with the frontoparietal attention-shifting network, which includes the ACC, PCC, and insula.
Loss of Exteroceptive Scaffolding
If social cognition is viewed as both fundamentally embodied (cf. Damasio, 1994, 1999; Kaschak & Maner, 2009; Nauta, 1971; Williams & Bargh, 2008) and deeply situated, in what for humans is a more or less complex social milieu (Barrett, Mesquita, & E. Smith, 2010; E. Smith, 2008; E. Smith & Semin, 2007), then the question, “What is the context for cognition?” becomes paramount. In the case of depression, the answer is well documented: as a consequence of a variety of maladaptive social tendencies, depressed persons tend to have relatively impoverished social networks (Joiner, 2002; Pettit & Joiner, 2006; Segrin, 2000). Nevertheless, depression can be both cause and consequence of such social dysfunction (Segrin, 2000). On the one hand, depression can be precipitated by major change to the social milieu, like loss from moving, rejection, or death of a loved one (e.g., Bruce, Kim, Leaf, & Jacobs, 1990; Slavich et al., 2010). On the other hand, depressed persons have a decidedly negative bias when interpreting social signals, including facial expressions (see Bistricky, Ingram, & Atchley, 2011, for a review), and likely experience a diminished capacity for prosocial behavior generally (e.g., Baron, Inman, Kao, & Logan, 1992; Likowski et al., 2011; Van Baaren et al., 2006; Vrijsen, Lange, Becker, & Rinck, 2010). Even non-depressed persons, for example, show reduced facial mimicry of social partners following a negative mood induction (Van Baaren et al., 2006).
The negative biases and social deficits of depressed persons can produce incongruence with social partners that can strain relationships and increase the likelihood for interpersonal strife (e.g., Bos et al., 2007; Coyne, 1976; Strack & Coyne, 1983). Such dynamics can also lead to depression contagion or the spreading of depression between people (Joiner, 1994; Joiner & Katz, 1999)—a phenomenon that has been documented both in close romantic relationships (Katz, Beach, & Joiner, 1999; Kouros & Cummings, 2010) and among roommates (Joiner, 1994). Depression can thus be corrosive to relationships and erode support (e.g., Stice, Ragan, & Randall, 2004). Many children and adults, additionally, withdraw socially when stressed and/or depressed (e.g., Beck, Steer, & Carbin, 1988; Bell-Dolan, Reaven, & Peterson, 1993)—a tendency that closely resembles sickness behavior (see Dantzer & Kelley, 2007).
Importantly, drastic change to the social milieu necessarily entails a loss of exteroceptive scaffolding for interoception. That is, a critical information source for disambiguating somatic signals is suddenly absent (cf. Cioffi, 1991; Hofer, 1984). From the perspective of the present framework, this necessarily increases the likelihood that somatic signals will go unnoticed or be misinterpreted, and thus the probability of regulatory dysfunction (i.e., disrupted sleep and/or eating). It is well established that social stimuli can function as zeitgebers and zeitstorers—stimuli that set/entrain or disrupt biological rhythms, respectively (see Favreau et al., 2009; Mistlberger & Skene, 2004, for reviews). Among these, a social activity can itself act as a cue for entrainment or else be correlated with exposure to other zeitgebers (e.g., light, food). The number of social interactions an individual engages in, for example, is significantly correlated with their total light exposure—often considered the most critical zeitgeber—and has been found to mediate the relationship between light exposure and depression (Haynes, Ancoli-Israel, & McQuaid, 2005). Food intake—an activity often highly social and socially influenced—similarly functions as one of the most important biological zeitgebers (Stephan, 2002).
The loss or change of social partners and/or disruption of social rhythmicity due to other shifts in behavior (e.g., because of illness) can thus result in significant disruption to biological rhythms. This point was emphasized by Hofer (1984), who hypothesized that many of the biological and psychological features of bereavement or depression triggered by loss may be the consequence of a loss of social zeitgebers. Hofer pointed out that circadian rhythms can be disrupted both because a loved one is suddenly gone—removing all of the zeitgebers woven into the fabric of that relationship—and because grieving and depressed persons often withdraw socially, removing even more zeitgebers. Ehlers, Frank, and Kupfer (1988) elaborated upon Hofer’s hypothesis in their social zeitgeber theory, which posits that many of the somatic symptoms of depression stem from disrupted circadian rhythms, as a consequence of a loss or disruption of social zeitgebers—a theory that has received increasing support, at least as applied to bipolar depression (e.g., Frank et al., 2005; Grandin, Alloy, & Abramson, 2006).
Within the current framework, one of the key mechanisms by which the disruption of social zeitgebers is likely to impact circadian rhythmicity is through modifying the capacity for interpreting somatic signals. For vulnerable individuals, particularly those who rely heavily upon social and other contextual cues to disambiguate such signals and lack well-developed self-regulation, any large shift in habit and/or the social milieu has the potential to produce regulatory and circadian dysfunction. Regular mealtimes are among the zeitgebers frequently disrupted by the loss of a loved one (Ehlers et al., 1988) and are also frequently lacking in depression (Beck, 1975). As Beck (1975) noted, depressed people often “forget” to eat and are thus prone to unintentionally skip meals. In light of the pervasive influence of social stimuli on food intake (Herman & Polivy, 2005) it is thus likely that social context of depression is important for understanding both ID and the somatic symptomatology characteristic of depression.
Shifts in Attention or Awareness
In addition to stress and changes in exteroceptive context, depression often involves intense self-focus (e.g., Northoff et al., 2011; T. Smith & Greenberg, 1981)—a feature that may underlie the heightened somatic awareness and “somatizing” common in depression (Wiebking et al., 2010). Self-focus can nonetheless takes several distinct forms; some adaptive, others not (Teasdale, 1999). Depressed persons are more prone to maladaptive, analytic and ruminative self-focus (Nolen-Hoeksema, 1991). Rumination is defined as highly repetitive thinking, rigidly focused on negative thoughts, events, and feelings (Nolen-Hoeksema, 1991). Depressed persons also frequently experience feelings of helplessness and lack of control (e.g., Alloy & Abramson, 1982; Benassi, Sweeney, & Dufour, 1988), and are prone to endorse external rather than internal events and factors as causes of their depression (Hansson, Chotai, & Bodlund, 2010).
Under such conditions, bodily signals may lose out both in the competition for attentional resources (Pennebaker & Lightner, 1980) and as explanations for somatically-generated negative affect (cf. Healy & Williams, 1988). Tendencies like inward self-focus and rumination—while they may indiscriminately amplify a range of somatic symptoms—may thus paradoxically result in regulatory dysfunction. For example, rumination is associated with poorer sleep quality in non-depressed samples (e.g., Carney, Harris, Moss, & Edinger, 2010; Guastella & Moulds, 2007) and appears to mediate the relation between disordered eating and depressive symptoms in non-clinical samples (Harrell & Jackson, 2008).26 Rumination is also correlated with the length and severity of negative affect during and greater difficulty recovering from depression (e.g., Lyubomirsky & Tkach, 2003; Nolen-Hoeksema, 1991).
Three Pathways of Interoceptive Dysfunction: Summary and Consequences for Depression
In summary, there are at least three potential pathways to ID in depression. Given the well-established bidirectional association between stress and depression (e.g., Hammen, Hazel, Brennan, & Najman, 2012), the first of these—direct modification of the INS—is likely to be at play in a great many cases. This is corroborated by the many studies linking functional and morphological changes in AIC, ACC, and other INS components to depression (e.g., Mayberg, 1999; Sprengelmeyer et al., 2011). The second pathway—the loss of cues used to disambiguate bodily signals—is also likely at play in many cases given that social withdrawal and erosion of social support are common in both depression (e.g., Hofer, 1984; Stice et al., 2004) and sickness (Hart, 1988). The third pathway—shifts in attention and/or awareness—is similarly likely to be at play in many cases, particularly those involving rumination and self-focus. Within the framework outlined here, each of these pathways will tend to give rise to both ID and a heightened chance of disrupted sleep and/or eating, particularly in those who rely more heavily on external than internal cues for disambiguating bodily signals and self-regulation. The fact that all three pathways may be active simultaneously means that ID and somatic disturbance will likely be overdetermined in many cases of depression. As illustrated in Figure 4, susceptibility to these pathways will nonetheless vary depending on individual, gender, and cultural differences in interoception, interacting with differential vulnerabilities over the lifespan.
Individual, Gender, and Cultural Differences in Interoception
Individual Differences in Interoception
Individual variability in sensitivity to interoceptive stimuli is a common finding in psychophysiological studies (see Jones, 1994; Katkin, 1985). For example, HBP varies greatly between individuals and only 10–40% of participants typically perform better than chance on HBP tasks (e.g., Jones et al., 1987; Schandry, Sparrer, & Weitkunat, 1986). One source of variability is Body Mass Index (BMI), in that leaner participants tend to show greater HBP accuracy (e.g., Rouse, Jones, & Jones, 1988). Cerebral lateralization has also been associated with individual differences in HBP; right hemispheric dominance being associated with better HBP (e.g., Hantas, Katkin, & Reed, 1984). Insula and ACC morphology also vary widely between individuals (Fornito et al., 2006; Johnson, Buchanan, Morris, & Fobbs, 2009; Pujol et al., 2002). Variation in insula is in fact so large that identifying the same sulci and gyri between individuals is often not possible (Johnson et al., 2009). The ACC similarly exhibits great variability in the presence of specific sulci and gyri (Buda et al., 2011; Fornito et al., 2006). Such anatomical differences imply functional differences (cf. Craig, 2010; Fornito et al., 2006)—an assertion supported by findings that right AIC/operculum GMV and activity during HBP correlate with interoceptive sensitivity (Critchley et al., 2004).
Individual differences in interoceptive sensitivity also appear to covary with other facets of interoception. For example, poorer interoceptive sensitivity is correlated with alexithymia or difficulty identifying and communicate about internal signals and emotional states (e.g., Herbert et al., 2011; Kano et al., 2007; Näring & Van der Staak, 1995). Sensitivity to interoceptive stimuli also appears to correlate negatively with important aspects of “self” perception, including “self-objectification” or the tendency to view the body as an object (Ainley & Tsakiris, 2013) and the susceptibility of the sense of “body ownership” to exteroceptive influence (Tsakiris et al., 2011). In a study of the Rubber Hand illusion, for example, Tsakiris et al. (2011) found that interoceptive sensitivity correlated negatively with susceptibility to the illusion of ownership of the rubber hand, in that those with poorer HBP were more susceptible to the illusion.
Gender Differences in Interoception
A number of sources of evidence point to gender differences in somatic perception. For example, females are more likely to report somatic symptoms in general and thus tend to score higher on measures of somatization than males (see Kroenke & Spitzer, 1998; Wool & Barsky, 1994). Measures of sexual concordance—the degree of match between physiological and self-report measures of sexual arousal—similarly show reliable gender differences, with females exhibiting significantly lower concordance than males (see Chivers et al., 2010).27 In most laboratory studies of interoceptive sensitivity, females also appear relatively insensitive to bodily signals compared to males (see Jones, 1994; Pennebaker, 1995; Pennebaker & Roberts, 1992; Vaitl, 1996). This includes measures of sensitivity to heartbeat (e.g., Ehlers & Breuer, 1992; Katkin et al., 1982; Pennebaker & Hoover, 1984; Whitehead et al., 1977), blood pressure (Pennebaker & Watson, 1988), respiratory air flow (Harver, Katkin, & Bloch, 1993), stomach contractions (Whitehead & Drescher, 1980), and blood-glucose levels (e.g., Cox et al., 1985).
Such findings nevertheless do not appear to generalize to performance under naturalistic conditions (Cox et al., 1985; V. Smith, 1986). For example, in a study of blood glucose estimation in Type I Diabetics, men were significantly better than women at estimating their blood glucose levels in a hospital setting, wherein blood glucose was automatically monitored and adjusted intravenously (Cox et al., 1985). However, when participants made the same estimations under non-hospital, real-world conditions—conditions in which they had access to both interoceptive and exteroceptive cues—this gender difference disappeared (Cox et al., 1985). Females thus appear to be more vulnerable to the loss of exteroceptive, situational cues than males and, as a result, underperform in laboratory tests of interoceptive sensitivity (see Pennebaker & Roberts, 1992; Roberts & Pennebaker, 1994). Unfortunately, few studies have attempted to replicate the findings of Cox et al. (1985) or compared performance on measures of interoception both in and outside of the lab (or with and without access to typical real-world, exteroceptive cues). The same general pattern is nonetheless apparent across the literature when considering these two classes of studies. That is, studies employing laboratory measures of interoception—devoid of the situational cues typically available outside of the lab—tend to report a gender difference in interoceptive sensitivity (e.g., Ehlers & Breuer, 1992; Frankum & Ogden, 2005; Pennebaker & Watson, 1988) whereas studies that provide naturalistic access to exteroceptive cues during interoception fail to find gender differences (e.g., V. Smith, 1986).
Relatively few studies have explored gender differences in the anatomy and/or functioning of the INS. Females generally exhibit lower HRV than males—a difference that disappears by age fifty (see De Meersman & Stein, 2007). Females also appear to have higher cingulate cortex GMV than males—a difference that may increase with age (Chen, Sachdev, Wen, & Anstey, 2007; Paus et al., 1996; R. Takahashi, Ishii, Kakigi, & Yokoyama, 2011). Such findings imply differences in interoception, given that measures of HRV (Knapp-Kline & Kline, 2005) and cingulate GMV (e.g., Critchley et al., 2004) have both been found to correlate with measures of interoceptive sensitivity. A PET study also found that males showed higher resting-state glucose metabolism in right insula, mid temporal gyrus, and medial frontal lobe than females, whereas females displayed higher hypothalamic metabolism than males (Kawachi et al., 2002). Functional imaging studies indicate that males and females likely differ in cortical representation of somatic signals (e.g., Kern et al., 2001; Naliboff et al., 2003; Hong et al., 2013). Kern et al. (2001), for example, found that females showed activation of the AIC, ACC, and PFC in response to colorectal distension, whereas males exhibited activation only in parietooccipital and somatosensory cortices. A number of studies have nonetheless found that both sexes show insular and ACC activation to colonic distension, but males either exhibit or trend toward enhanced activation of these regions relative to females (e.g., Berman et al., 2000, 2006). Some of the differences between studies are nonetheless likely accounted for by gender differences in pain thresholds (e.g., Moulton et al., 2006) and anticipatory response to noxious stimulation (e.g., Berman et al., 2006; Kano et al., 2013).28
In a related vein, there are gender differences in HPA-axis functioning and stress responsivity (see Darnall & Suarez, 2009; Kudielka & Kirschbaum, 2005; Nirupa et al., 2014) that are also relevant to gender differences in interoception, given that stress and inflammation can modify functioning of core components of the INS. For example, females generally show blunted HPA-axis activation to laboratory stressors and pain compared to men, although such differences vary depending on a number of factors, including menstrual phase and stressor type (see Kudielka & Kirschbaum, 2005). Gender differences in behavioral and neural response to laboratory stressors have also been reported in a number of studies (e.g., Lighthall et al., 2012; J. Wang et al., 2007). For example, Lighthall et al. (2012) found that cold-pressor stress modulated left dorsal striatum and AIC activity differently for males and females. Such stress led to increased activity in these regions in females and decreased activity in males—differences that also correlated with gender differences in performance on a decision-making task post-stressor (Lighthall et al., 2012). Wang et al. (2007), on the other hand, found that males show little increase in activation of limbic regions following a laboratory stressor, whereas females exhibit significantly increased activation of the insula, ventral striatum, ACC, and PCC, with differences in ACC and PCC persisting following exposure.
Such results imply gender dimorphism in the modulation of interoception by stress. In the sole study specifically addressing the question, Fairclough and Goodwin (2007) found no baseline differences in HBP between males and females, after controlling for BMI, but found that females exhibited significantly lower accuracy than males following exposure to a stressor (i.e., a difficult math task). Females also show more vigorous immune responses to pathogens than men (see Fish, 2008) and a number of studies have documented sexually dimorphic inflammatory response to stressors (see Darnall & Suarez, 2009). There are also large gender differences in behavioral response to stress (e.g., Taylor et al., 2000). For example, women are more likely than men to utilize social support when stressed (see Taylor et al., 2000) and low levels of social support is a greater risk factor for depression in women (e.g., Edwards, Nazroo, & Brown, 1998; Kendler, Myers, & Prescott, 2005; Olstad, Sexton, & Søgaard, 2001). For example, in a large prospective study of over 1,000 pairs of opposite-sex dizygotic twins, self-reported social support predicted future episodes of MDD for women, irrespective of their depression history, but not for their male twins (Kendler et al., 2005; Kendler & Gardner, 2014). Collectively, these facts imply that females may be at a greater risk of ID following stress and/or inflammation. Nevertheless, whether differences in interoception—particularly the use of contextual cues for interoception—mediate the differential effects of social support on depression risk for males and females remains an open question.
Cultural Differences in Interoception
Cultures vary widely in their construal of the relation between mind and body, as well as their use of bodily referents and somatic metaphor when communicating about emotional states (see Kirmayer, 2001; Lewis-Fernández & Kleinman, 1994).29 Data addressing the question of cultural differences in the perception of bodily signals are nonetheless limited. Chentsova-Dutton and Dzokoto (in press) examined interoception and body awareness in West Africans and European Americans, finding that West Africans showed higher awareness of bodily symptoms, as measured by the Body Awareness Questionnaire (Shields, Mallory, & Simon, 1989), but lower interoceptive sensitivity, as measured using a modified HBP task involving moment-to-moment ratings of changes in HR. Ma-Kellams et al. (2012) similarly examined HBP accuracy in East Asian and Caucasian undergraduates, finding that East Asians had poorer interoceptive sensitivity than Caucasians. Drawing on a series of additional experiments, Ma-Kellams et al. (2012) argued that this difference was mediated by a greater sensitivity to contextual cues in East Asian participants. The findings from these experiments30 nonetheless suggest a difference in integration of interoceptive and exteroceptive stimuli, given that East Asians displayed both greater alliesthesia—indicating modulation of exteroception by bodily signals—and greater modulation of interoception by exteroceptive cues (see Ma-Kellams et al., 2012).
Maister and Tsakiris (2014), in contrast, failed to replicate the finding of a significant difference in HBP between East Asian and Caucasian undergraduates in a study examining HBP under conditions in which participants viewed either a blank screen, an image of their own face, or an image of another same-ethnicity face. Instead, a significant interaction between ethnicity and HBP change in response to participants being shown an image of their own face was found, with Caucasians classified as having poor baseline HBP showing improved HBP when tested under these conditions—an effect not seen in East Asians. The interpretation of Maister and Tsakiris’s (2014) results is nonetheless not straightforward given that the HBP performance of all participants classified as having good baseline HBP was hindered, regardless of ethnicity, by being shown images of faces during HBP, indicating likely interference between exteroceptive stimulation and HBP in this paradigm.
A Lifespan View of Interoception
The existence of gender- and culture-specific patterns of interoception raises the question of how such differences arise—a question to which a number of biological, developmental, and cultural answers can be plausibly offered (see Pennebaker & Roberts, 1992). Nevertheless, few studies to date have examined the development of interoception. Little is known, for example, about how interoceptive sensitivity and awareness develop or are modified over the course of the lifespan (e.g., Koch & Pollatos, 2014). The approach adopted here is grounded in developmental psychobiological systems metatheory, which views individual development as a series of adaptations to a series of distinct ontogenetic niches, each with its own unique set of resources, constraints, and challenges, as well as potential for mismatch with adaptions acquired at earlier stages (see Alberts, 2008; Alberts & Harshaw, 2014; Bateson & Martin, 2000; Gottlieb, 2001; West & King, 1987). As illustrated in Figure 5, the framework assumes that different phases of ontogeny entail unique interoceptive and regulatory challenges and that major transitions (cf. Birch & Anzman, 2010)—particularly as impact the somatic and social milieus—likely place vulnerable individuals at increased risk for developing ID, potentially contributing to risk for anxiety and depression. What is necessarily a partial sketch of what is known about these transitions and their impact on interoception follows.
Figure 5.
A schematic illustration of common interoceptive and regulatory challenges across the lifespan, from a female and Western-biased cultural perspective. The framework put forward here assumes that over the course of the lifespan major somatic changes and regulatory transitions (e.g., from childhood to adolescence) likely place unique strain on regulatory and interoceptive capacities alike. Such challenges are nonetheless likely to be highly culture-specific (e.g., menopause is not universally viewed negatively). For vulnerable individuals, ensuing ID and somatic dysregulation may, theoretically, contribute to the development of anxiety and/or depression.
Early development
The first transition of note is that of birth—from the symbiosis of intrauterine life to the highly social, postnatal regulatory milieu (see Harshaw, 2008). In contrast to the “blooming, buzzing confusion” envisaged by James (1890), the perception of regulatory signals is often assumed to be “basic,” “primordial,” or “innate” (e.g., Damasio, 1994, 2003a; Damasio & Carvalho, 2013; Denton, 2006; Denton, McKinley, Farrell, & Egan, 2009; Greene, 2007; Hurley, Dennett, & Adams, 2011). Nevertheless, there is little empirical backing for such claims (see Blumberg, 2005; Harshaw, 2008) and a number of eminent researchers and theorists have argued that the perception of alimentary signals of hunger and thirst, in particular, develop during the individual lifespan; that is, that infants must acquire knowledge that particular internal signals indicate needs for specific resources having particular exteroceptively perceptible sensory qualities and requiring particular behaviors to satisfy (Baldwin, 1896; Bruch, 1969, 1970; Buck, 1989a, 1989b; Craig, 1912, 1918; Hebb, 1949; Hall, Arnold, & Myers, 2000).
Hebb (1949) argued that much of the confusion in the literature on eating in his day was the result of the neglect of cognitive and developmental influences on hunger and eating. Bruch (e.g., 1969, 1970) argued that early interactions with caregivers in feeding contexts could shape the perception and interpretation of alimentary signals in adolescence and adulthood. Buck (e.g., 1989a) similarly points out that the process of learning to identify and label internal sensations differs fundamentally from that by which we learn about objects in the outside world (cf. Schachter, Goldman, & Gordon, 1968). In Buck’s model of emotional and communicative development, caregivers function as living biofeedback machines in that they provide infants and children exteroceptive feedback about internal states by perceiving, interpreting, and responding to infant and child cues and emotional displays (Buck, 1989a). In this way, children are socialized to exhibit varying degrees of competence at identifying, labeling, and responding to motivational and emotional signals (Buck, Goldman, Easton, & Smith, 1998). Such models provide a ready avenue for the socialization of gender differences in interoception, somatic interpretation, and alexithymia—a process that would depend largely upon culture-specific gender norms and parenting practices (Pennebaker & Roberts, 1992).
Adolescence
Parental feeding practices vary greatly. For example, parents exert varying degrees of control over feeding, and there is strong evidence that such variability impacts the emerging self-regulatory capacities of children (see Frankel et al., 2012). Despite such variation, most children in Western cultures nonetheless receive substantial external scaffolding of their sleep and eating during childhood, but are provided increasing opportunities to self-regulate during adolescence (Bruch, 1969). The next major transition is thus from the highly structured, externally-scaffolded regulatory niche of childhood to fully independent and autonomous self-regulation. Prior to adolescence, even profound disturbances in self-regulation can, in theory, be masked by the social scaffolding provided by adults (see Bruch, 1969).
That this transition is often fraught with difficulty is indicated by the high rate of sleep disturbance in adolescents (e.g., Kirmil-Gray, Eaglston, Gibson, & Thoresen, 1984; Laberge et al., 2001; Morrison, McGee, & Stanton, 1992). A steep rise in both clinical eating disorders (Levine & Smolak, 1992; Patton, 1988) and sub-clinical abnormalities like meal skipping (e.g., Shaw, 1998) also occurs during this period. Within the present framework, children with latent interoceptive and/or self-regulatory deficits may be more vulnerable to regulatory disturbance and depression during adolescence (cf. Bruch, 1969). The available data lend support to this claim. For example, sub-clinical eating pathology during adolescence is associated with both increased symptoms of depression (Fulkerson et al., 2004) and risk for suicidality (Crow, Eisenberg, Story, & Neumark-Sztainer, 2008). Sleep disturbance is similarly associated with both anxiety and depression during adolescence (e.g., Marks & Monroe, 1976; Morrison et al., 1992). Prospective studies moreover find that the strength of the relationship between sleep disturbance and subsequent depression increases from childhood to adolescence (Alfano et al., 2009; Gregory & O’Connor, 2002; Johnson, Chilcoat, & Breslau, 2000).
Pregnancy and childbirth
Although no research to date has examined the effects of pregnancy and childbirth on interoception, the large somatic and contextual changes involved inevitably entail interoceptive change if not challenge. Persinger (2001) hypothesized that interoceptive changes during the menstrual cycle, pregnancy, and menopause (e.g., altered cravings and strange somatic sensations) occur as a result of somatic signals affecting the vagus and insula. Pregnancy and lactation are correlated with changes in the cingulate and related regions in rodents (e.g., Salmaso, Cossette, & Woodside, 2011). However, almost nothing is known about pregnancy- and parturition-related changes in interoception in humans, apart from pregnancy involving reduced HRV and altered sympathovagal balance (e.g., Ekholm, Hartiala, & Huikuri, 1997; Speranza, Verlato, & Albiero, 1998). The timing of labor and delivery can induce transient circadian disruption and sleep loss, with consequent negative impact on mood (Wilkie & Shapiro, 1992). Childbirth also generally marks a transition to a stressful and highly disrupted schedule, as caretakers grapple with the needs of an initially acircardian neonate. Coping with an infant’s sleep patterns can result in disrupted sleep and fatigue, which are associated with postpartum depression (see Ross, Murray, & Steiner, 2005). From the perspective of the current framework, social and contextual changes during pregnancy and following childbirth (cf. Hopkins, Marcus, & Campbell, 1984; Paykel et al., 1980) are nonetheless likely to be as important as biological changes as risk factors for ID.
Menopause
Data relevant to interoception during menopause are similarly limited. The perimenopausal period involves significant somatic change (see Steiner, Dunn, & Born, 2003) and frequently both sleep disruption (e.g., Nowakowski, Meliska, Martinez, & Parry, 2009) and conspicuous, abnormal somatosensations such as hot flashes, sweating, flushing, numbness, itching, and even “burning mouth syndrome” (Persinger, 2001; T. Takahashi et al., 2001). A recent PET study of eighteen perimenopausal women found that the occurrence of hot flashes was associated with lowered regional glucose metabolism in both hypothalamic thermoregulatory centers and insular cortex (Joffe et al., 2012), supporting the idea that abnormal somatic sensations during menopause are indicative of ID (Persinger, 2001).
Aging
A number of biological, social, and contextual factors may contribute to increased levels of ID with aging (cf. Mendes, 2010). These include (a) major contextual changes and stressors (e.g., retirement, disability, role loss; Rosow, 1973); (b) the loss of loved ones (e.g., Bruce et al., 1990; Onrust & Cuijpers, 2006); and (c) increased incidence of inflammation-triggering injury and disease (Alexopoulos & Morimoto, 2011). Comparative studies have found that older animals show exaggerated and prolonged sickness behavior in response to infection (e.g., Huang et al., 2008; Kelley et al., 2013) and human studies have found upregulated inflammatory activity with aging (Bruunsgaard, Pedersen, & Pedersen, 2001)—the degree of which correlates with depression (Baune et al., 2012).
Aging also involves both upregulated HPA-axis activity (e.g., Seeman, Singer, Wilkinson, & McEwen, 2001) and a gradual decline in HRV (see De Meersman & Stein, 2007). Gradual decline in interoceptive sensitivity, as measured via HBP, has also been reported (Khalsa, Rudrauf, & Tranel, 2009). Cenesthopathy or reports of abnormal bodily sensations (e.g., parasitosis or delusions of infestation by parasites or bugs) are relatively common in old age and senility and these may be particularly likely in females (e.g., T. Takahashi et al., 2001). An overall reduction in both white and grey matter also occurs with aging (e.g., Giorgio et al., 2010); however, relatively greater reductions in GMV have been observed in a number of regions, including insular and cingulate cortices—particularly AIC and ACC (e.g., Good et al., 2001; Tisserand et al., 2004; Vaidya et al., 2007). Decreased metabolism in bilateral OFC and right AIC with age has also been reported (Hsieh et al., 2012). A recent study moreover found a gradual decline in bilateral posterior insula and left ACC activation to soft, pleasurable touch over the course of adulthood (May et al., 2014).
Based on such evidence, it has been suggested that the connection between mind and body gradually deteriorates with age, impacting decision-making and other processes that rely upon representations of somatic signals (see Mendes, 2010). This idea was supported by the results of Khalsa et al. (2009), as well as findings indicating a gradual decline in proprioception (see Goble et al., 2009) and performance on tasks thought to rely upon the use of somatic signals with age (e.g., Denburg, Tranel, & Bechara, 2005). It is nonetheless possible that such changes occur not because of a decline in sensitivity but rather in affective responsivity to somatic signals—that is, an interoceptive asymbolia (see Table 1). In a recent study, for example, the largest age-related changes in functional connectivity with the amygdala during the viewing of novel images were found for the AIC, although changes in amygdala connectivity with hippocampus and OFC were also observed (Moriguchi et al., 2011). This shift in amygdala-insula connectivity could explain both the results of Denburg et al. (2005) and the general decline in responsivity to negative emotional stimuli with age (see Mather, 2012).
Mendes (2010) also speculates that deteriorating interoception leads older adults to rely more heavily upon external cues than do younger adults. There is currently no data specifically addressing this argument. If Mendes is correct, older adults may be particularly vulnerable to developing somatic symptomatology as a consequence of social and contextual perturbations. Aging is characterized by shifts in roles and expectations far exceeding those experienced during the transition from childhood to adolescence: that is, a transition from an exteroceptive milieu highly structured by the demands of work, social obligations, etc., to one of almost total, unstructured regulatory “freedom” during retirement (see Rosow, 1973). Evidence suggests that such contextual shifts are both a source of stress (Rosow, 1973) and associated with depression (e.g., LaGory & Fitzpatrick, 1992).31 With increasing age it is nonetheless typical that increasing amounts of external scaffolding are provided—in the form of various kinds of assistive care—which, in theory, may buffer older adults from the consequences of ID.
Stress, infection, and inflammation during development
In addition to phase-specific challenges, modifications of interoception by stress, infection, and inflammation are possible across the lifespan. For example, vagal tone and vagal reactivity can be modified by early life stress (see Field & Diego, 2008; Porges, 2011)—changes which mediate the effects of early adverse experiences on outcomes like depression (e.g., Cyranowski et al., 2011; Gentzler et al., 2012; Thayer & Brosschot, 2005). A number of studies have similarly found long-term changes in insula activity and morphology following early stress, trauma, and abuse (e.g., Ansell et al., 2012; Mueller et al., 2010). Similar changes have been documented in ACC (e.g., Ansell et al., 2012; Mueller et al., 2010) and hippocampus (e.g., Arabadzisz et al., 2010; Bremner et al., 1999). Ansell et al. (2012), for example, found cumulative stressful life events to be negatively correlated with GMV in insula, sgACC, and mPFC. A recent study of depressed persons with a history of childhood maltreatment moreover found altered functional connectivity between the vmPFC/ACC and a number of regions, including insula, amygdala, caudate, thalamus, and hippocampus (L. Wang et al., 2014).
The implication of such findings is that individuals at risk for depression as a result of early life stress and/or inflammation may exhibit trait-like differences in interoception. It is well established that exposure to stress hormones during early development significantly impacts later depression susceptibility (e.g., Brunton & Russell, 2010; Kofman, 2002; Zagron & Weinstock, 2006). Early stress appears to sensitize individuals such that they become more vulnerable to stressors experienced later in life (e.g., Espejo et al., 2006; Hammen, Henry, & Daley, 2000). The causal mechanisms underlying such sensitization are poorly understood (Hammen, 2005). A focus on interoceptive pathways and ID nonetheless suggests a number of potential mechanisms. For example, early stress can cause enduring effects on insula, ACC, or other INS components (e.g., Ansell et al., 2012; Field & Diego, 2008; Porges, 2011) and tonic changes and/or circadian abnormalities in HPA-axis activation (Heim & Nemeroff, 2001; Heim et al., 2008; Lupien, McEwen, Gunnar, & Heim, 2009)—changes which may result in altered interoceptive awareness, interoceptive sensitivity, and/or interoceptive-exteroceptive integration.
Individual, Gender, Cultural, and Lifespan Differences in Interoception: A Summary
In summary, interoception appears to vary significantly from person-to-person and by gender as well as potentially culture and age. Specifically, women and possibly East Asians, West Africans, and older adults may rely more heavily upon contextual cues for interoception than males, persons of European decent, and younger adults, respectively. From the perspective of the framework outlined here (see Fig. 4), it is predicted that the former groups and individuals with similar interoceptive profiles—i.e. those who rely more upon social and other contextual cues for disambiguating bodily signals—should be at greater risk for somatic dysregulation and depression following large shifts in context and/or behavior. In addition to potential for greater vulnerability in the face of contextual shifts, women are more prone to rumination than men (see Nolen-Hoeksema, 2012)—a difference that has been shown to partially mediate the relationship between gender and depression (Nolen-Hoeksema & Aldao, 2011). Females may thus be both more prone to developing regulatory dysfunction and more vulnerable to the potential mood-lowering consequences of such dysfunction than males. Such vulnerability is nonetheless predicted to interact with somatic and regulatory challenges faced across the lifespan in giving rise to ID and somatic dysregulation.
That females report more somatic symptomatology (e.g., Haug, Mykletun, & Dahl, 2004) and are also at heightened risk for depression (see Hankin & Abramson, 2001; Weissman et al., 1993) is well established. Gender differences in depression are nonetheless not evident in childhood, but arise suddenly during adolescence (Nolen-Hoeksema & Girgus, 1994; Wade, Cairney, & Pevalin, 2002). As reviewed, adolescence is a time of transition—from a highly scaffolded regulatory niche to one of autonomous self-regulation—that may be particularly difficult for children who rely more strongly on social and contextual cues for interoception and self-regulation (cf. Bruch, 1969). Given that females appear to rely more on contextual cues for somatic perception (see Pennebaker, 1995) and also undergo significant somatic change (e.g., menarche) not experienced by males, female adolescents might be predicted to suffer more ID and somatic symptomatology during this transition. Gender differences in the reporting of unexplained functional somatic symptoms (e.g., headaches, abdominal pain, fatigue) do appear suddenly in adolescence, with female adolescents showing higher reporting of functional somatic symptoms than males (see J.E. Beck, 2008). Female adolescents are also more likely than males to exhibit both eating disturbances like habitually skipping breakfast (e.g., Berkey et al., 2003; Shaw, 1998; Timlin, Pereira, Story, Neumark-Sztainer, 2008) and sleep problems (e.g., Vallido, Jackson, & O’Brien, 2009; Yarcheski & Mahon, 1994). In the case of eating disturbances, these have been linked in prospective studies to heightened risk for depression in female adolescents (e.g., Stice et al., 2000; Stice & Bearman, 2001).
Other major somatic transitions faced by women (e.g., pregnancy, childbirth, menopause) may similarly place women at heightened risk for ID, somatic dysregulation, and depression relative to men. In support of this claim, the occurrence of hot flashes and other somatosensory abnormalities—indicators of somatic change and potential ID (e.g., Joffe et al., 2012)—is one of the strongest predictors of depression during menopause (e.g., Cohen et al., 2006; Freeman, Sammel, Lin, & Nelson, 2006). This relationship moreover appears to be strongly correlated with differences in insomnia and subjective sleep quality that do not appear to be caused by hot flashes simply waking women during the night (see Joffe et al., 2009).
Only a few studies have examined cross-cultural differences in interoception. The two studies comparing interoception in East Asian and Caucasian undergraduates suggest potential differences in the integration of interoceptive and exteroceptive information (Ma-Kellams et al., 2012; Maister & Tsakiris, 2014) and possibly greater dependency of interoceptive sensitivity on context in East Asians (Ma-Kellams et al., 2012). Nevertheless, these studies were conducted on college undergraduate samples and did not consistently find impaired HBP in East Asians. The generalizability of these findings is thus unclear. A great deal more research will likely be needed to clarify cross-cultural differences in interoception and whether these mediate any meaningful differences in susceptibility to ID, somatic symptomatology, and/or depression.
Similarly, there is only a small literature on the question of interoception and aging, containing some evidence that interoception may gradually deteriorate in late adulthood (e.g., Khalsa et al., 2009; May et al., 2014). With respect to the effects of aging on depression risk, several studies have found that, after controlling for other factors, depression risk is either stable or else gradually declines after mid-adulthood (e.g., Jorm, 2000; Roberts, Kaplan, Shema, & Strawbridge, 1997; Scott et al., 2008). Multiple risk factors for depression (e.g., disability, inflammation, low social support) nevertheless tend to increase with age (see Lewinsohn, Rohde, Seeley, & Fischer, 1991; Vink, Aartsen, & Schoevers, 2008), which may result in a higher depressive symptomatology rather than prevalence of clinical depression after the age of 75 (e.g., van't Veer-Tazelaar et al., 2008). Although many of these risk factors have the potential to impact interoception—and there is some evidence of deterioration or changes in interoception with aging (see Mendes, 2010)—evidence directly linking such changes to heightened susceptibility to somatic symptomatology and depression in old age is currently lacking.
Discussion and Future Directions
A wide range of evidence implicating somatic signals and interoceptive dysfunction (ID) in depression was reviewed. Somatic signals, including stress hormones and cytokines, along with alterations of brain-body signalling via the vagus, are capable of significantly impacting mood and depression. Based on neural evidence alone, it is also clear that depression frequently involves some ID, particularly given that changes in insula—the region of the brain most closely associated with conscious perception of bodily signals—are reliably associated with depression (see Fitzgerald et al., 2008; see Table 3). A number of other regions involved in interoceptive processing and interoceptive-exteroceptive integration (e.g., hippocampus, ACC, mPFC) are nonetheless also reliably modified in depression. Evidence is similarly accumulating that depression involves not only lowered confidence in interoceptive abilities (e.g., Kopp, 2009) but some degree of lowered sensitivity to bodily stimuli, as measured by HBP tasks (e.g., Furman et al., 2013; see Table 4). Anxiety comorbidity nonetheless appears to complicate this relationship (cf. Furman et al., 2013), given that anxiety is associated with heightened awareness and sensitivity to somatic signals (e.g., Domschke et al., 2010). Although more research is needed to elucidate the prevalence and specific subtypes of ID typical in depression (see Table 1), the evidence reviewed supports the conclusion that depression often involves ID.
Many neurobiological models of depression thus include components of interoceptive dysfunction (e.g., Andréasson et al., 2007; Mayberg, 2009; Northoff et al., 2011; Paulus & Stein, 2010). As reviewed, there are a variety of shortcomings to existing models, particularly a lack of attention to the question of causality. That is, how do interoceptive deficits come about in the first place? The framework presented here outlines three broad pathways by which ID can arise in the context of depression and related disorders (see Fig. 4). As reviewed, females appear to be more susceptible to at least two of these pathways, in that they are more vulnerable to shifts in context (see Pennebaker, 1995) and more prone to rumination than males (see Nolen-Hoeksema, 2012). A focus on interoception and ID thus provides a novel means of potentially shedding light on gender bias in the epidemiology of depression.
Beyond this, the framework predicts that susceptibility to ID will be a joint function of individual, gender, and cultural differences in somatic perception, interacting with interoceptive and regulatory challenges faced across the lifespan (e.g., Fig. 5). For example, a case can be made that the higher risk of depression in adolescence (Hankin & Abramson, 2001) may be at least partially tied to unique interoceptive and regulatory challenges faced during this period. Adolescence involves both rapid somatic change and transition from high levels of social scaffolding of sleep and eating to greater levels of autonomous self-regulation (cf. Bruch, 1969). Although prospective studies indicate a reliable association between sleep disturbance and subsequent depression across the lifespan (see Baglioni et al., 2011; Gregory & Sadeh, 2012), the strength of this association increases during adolescence relative to childhood (Alfano et al., 2009; Gregory & O’Connor, 2002; Johnson et al., 2000).
Women may nonetheless be at heightened risk for ID and somatic dysregulation given the larger number of major somatic and regulatory transitions faced by women (e.g., menarche, pregnancy, childbirth, menopause). For example, female adolescents appear more likely to suffer from both eating and sleep disturbance than males (see Timlin et al., 2008; Vallido et al., 2009) and these—particularly eating disturbances—have been prospectively linked to risk for depression in studies of female adolescents (e.g., Stice et al., 2000; Stice & Bearman, 2001). Prospective studies have similarly linked somatic symptoms—particularly sleep disturbance—to subsequent depression during pregnancy (e.g., Kamysheva et al., 2010; Wolfson, Crowley, Anwer, & Bassett, 2003). The available data on ID during menopause also indicate a high correlation between somatic symptoms (i.e., vasomotor symptoms, such as hot flashes), sleep disturbance, and depression in perimenopausal women (e.g., Joffe et al., 2009). Collectively, these facts provide tentative support for a connection between heightened risk for depression and heightened vulnerability to ID and regulatory disturbance in females.32 Nevertheless, relatively little is known regarding the effects of the major somatic and regulatory transitions faced by women on interoception, and more research is thus necessary to disentangle what is likely to be complex bidirectional interplay between ID, somatic symptomatology, and depression.
It is important to note that many of these transitions—and with them, expectations for effects on mind and body—are culturally constructed, defined, and elaborated. For example, the construct of “menopause” is not universal in that the transition to a non-reproductive phase is not universally cast as a negative. Likewise, aberrant somatic sensations, such as hot flashes, are not universally experienced as aversive (see Lock, 1994). Despite the large literature on cultural differences in the construal of the relation between mind and body, reflected in differences in “somatization” among other things (e.g., Csordas, 1993; Kirmayer, 2001; Kirmayer & Young, 1998; Simon et al., 1999), there is a paucity of data on cultural differences in interoception (e.g., Ma-Kellams et al., 2012; Maister & Tsakiris, 2014). Given that the available literature is too sparse to warrant any generalization, it is important to note that sensitivity to cultural distinct modes of interoception and communication about the body will likely be critical for scientific progress in this area (cf. Kirmayer, 2001). For example, laboratory-based measures of interoceptive sensitivity, such as HBP, may be ill-suited for gauging sensitivity in cultures that stress reliance on social and exteroceptive cues for interoception (e.g., Chentsova-Dutton & Dzokoto, in press; Ma-Kellams et al., 2012). That is, these cultures may arrive at a qualitatively different solution to the problem of somatic perception and interpretation, and different tools may thus be needed to sensitively quantify interoception within these cultures. Likewise, constructs such as “somatization” are likely counterproductive given that somatization is entirely normative in many cultures (see Kirmayer, 2001; Kirmayer & Young, 1998).
As reviewed, interoception is not a single, unitary sense. Numerous forms of ID are thus possible, each with unique implications for depressive symptomatology (see Table 1). Most studies to date have nonetheless been limited to interoceptive sensitivity and at best examined correlations between overall depression ratings (e.g., BDI scores) and HBP or insula activation (e.g., Dunn et al., 2007, 2009; Pollatos et al., 2009). Beyond this, few studies have tested models relating specific forms of ID to specific symptoms of depression (e.g., Dunn et al., 2010; Furman et al., 2013; Mitterschiffthaler et al., 2003). The framework put forward here suggests only that ID is likely to give rise to typical somatic symptoms of depression such as disrupted sleep and eating, which can potentially result in further ID (e.g., by disrupting circadian rhythms; cf. Healy & Williams, 1988; Hofer, 1984). Recent findings provide support for this hypothesis given that change in dorsal mid-insula activity during HBP appears to correlate negatively with both somatic symptoms and depression severity (Avery et al., 2014). The strong correlation between ID, somatic symptoms, and depression during menopause similarly provides support for this hypothesis (e.g., Freeman et al., 2006; Joffe et al., 2012).
Interoceptive dysfunction is nonetheless potentially relevant to understanding a number of other symptoms of depression (cf. Paulus & Stein, 2010; Northoff et al., 2011; Table 5). For example, there is large overlap in the neural systems underlying interoception, social-emotional cognition, and decision-making (e.g., Damasio, 1996; Porges, 2011). Social cognition, in particular, appears to draw heavily on interoceptive-exteroceptive integration and “mirroring” capabilities (e.g., Lakin et al., 2003). Many forms of ID are thus likely to be accompanied by social and emotional deficits. Several symptoms of depression can, for example, be viewed as disorders of penetrance (see Stokes, 2012) rather than as the result of a simple “imbalance” in processing (cf. Northoff et al., 2011). The anhedonia characteristic of depression may thus be the result of an overgeneralized negative alliesthesia; social deficits may result from negative alliesthesia for social stimulation (cf. Paulus & Stein, 2010) and/or a diminished capacity for contagion or mimicry, due to unavailability of INS circuitry for interoceptive-exteroceptive integration needed for social mirroring (cf. Adolphs & Damasio, 2001). In support for this possibility, anhedonia has been associated both with increased insula and ACC activity while viewing positively valenced images (Mitterschiffthaler et al., 2003) and with reduced HBP accuracy (Dunn et al., 2010). Reduced interoceptive sensitivity has also been linked to impaired social-emotional processing, higher externally-oriented thinking, and alexithymia (e.g., Herbert et al., 2011; Pollatos & Schandry, 2008). Furman et al. (2013) similarly found that decision-making deficits in MDD are associated with reduced HBP. A focus on interoception and ID thus holds the promise of permitting the construction of models in which somatic, cognitive-affective, and social features of depression are causally interlinked (e.g., Fig. 4).
In summary, evidence implicating somatic signals and interoceptive dysfunction in depression was reviewed and a framework for organizing this evidence presented. Three distinct pathways through which interoception is likely to be modified in depression were outlined, and these situated within the broader literature on interoception and somatic interpretation. A key tenet of the framework presented is that social and other contextual cues are ordinarily employed to disambiguate bodily signals (cf. Cioffi, 1991; Mechanic, 1972) and that this may render depressed persons—particularly those who rely more heavily upon such cues for self-regulation under ordinary circumstances—vulnerable to errors in somatic interpretation given large shifts in context or behavior (cf. Hofer, 1984). Depression may nonetheless involve a range of distinct forms of interoceptive dysfunction (e.g., when comorbid with anxiety), each with potentially distinct consequences for depressive symptomatology. Although much about the perception of the body in depression remains unknown, it is hoped that this framework will prove useful for hypothesis generation in this new and promising area of research.
Acknowledgments
I am indebted to Robert Lickliter, Dolf Zillmann, Nancy Zucker, Kelly Hammond, and several anonymous reviewers, for helpful feedback on earlier drafts of this manuscript. The completion of this paper was also partially funded by NIMH grant RO1-MH082019 to Jeffrey Alberts.
Footnotes
Although much of the material reviewed in the present paper is relevant to Major Depressive Disorder (MDD), “depression” will be used throughout to refer to the broad spectrum of depression phenotypes, as measured by tools such as the Beck Depression Inventory (BDI; Beck et al., 1988; BDI-2; Beck, Steer, & Brown, 1996).
A view championed by Herophilus, Erasistratus, Hippocrates, and later Galen (see Gross, 1995; Rocca, 2003).
In this paper “interoception” is used in its most inclusive sense, to refer to the perception of all stimuli originating within the body (Craig, 2004). Coenesthesia, coenaesthesia, and Gemeingefühl occur in the older philosophical and psychiatric literature, often to mean a general bodily sense, separate from the skin senses (Fuchs, 1995)—a meaning similar to the broad definition of interoception advocated by Craig (2004). Coenestopathic and cenesthopathy similarly have meanings approximating the subjective consequences of interoceptive dysfunction, as employed here. The latter terms remain current in the psychiatric literature, particularly as pertains to schizophrenia (Graux, Lemoine, Gaillard, & Camus, 2011).
“Regulatory” is used here and throughout to refer to the core systems involved in the maintenance of homeostasis (e.g., immune, thermoregulatory, activity-rest, alimentary).
“Penetrance” is employed here and throughout to refer to the penetration or incursion of activity from one perceptual system into that of another (see Stokes, 2012).
Although Saugstad (1966) and others (e.g., Francis, 2012) have offered critiques of a number of such “New Look” studies, no studies contradicting the main conclusions of these have appeared.
Stokes’ (2012) “orectic penetration hypothesis” bears some resemblance Barrett and Bar’s (2009) suggestion.
It is possible that spatiotemporally precise information may be present at lower levels of processing, yet remain inaccessible to conscious awareness (see Vaitl, 1996). Hölzl et al. (1996) nonetheless report that, at least in studies employing gastric distension, spatial localization is only possible at stimulus intensities that also trigger conscious awareness, suggesting a fundamental difference between interoception and exteroception.
Although there are certainly many caveats to specific points argued by Schachter and Singer (1962) and in other classic attribution work—including that unexplained physiological arousal tends to be experienced as inherently negative (e.g., Marshall & Zimbardo, 1979) and that the precise timing of events matters a great deal for misattribution effects (see Zillmann, 1996)—the general point that arousal can intensify emotional reactions depending on available situational cues and top-beliefs and expectations is well-supported by the literature (see Reisenzein, 1983; Zillmann, 1988, 1996).
There was an approximately 65% chance, on average, of a favorable ruling for the defendant at the beginning of each decision period, compared to a 0–10% chance of a favorable ruling at the end of each period (i.e. prior to the two food breaks and at the end of the day; Danziger et al., 2011).
Important differences have been noted between these two paradigms (e.g., Knoll & Hodapp, 1992). For example, a recent study found that cold-pressor stress increased accuracy in the Schandry task but reduced accuracy in the Whitehead task (Schulz et al., 2013), possibly because attention is partially diverted to an exteroceptive stimulus in the latter (Pennebaker, 1982; Schulz et al., 2013).
Critchley et al. (2004) employed a signal detection paradigm similar to the Whitehead paradigm, whereas Pollatos et al. (2007) employed the Schandry (1981) method.
Given the several uses of “interoceptive awareness” in the literature, when reviewing the literature I employ the terminology laid out here rather than that employed by the authors of the particular studies in question. The similar, though less rigorously delimited, notion of “body awareness”—referring to a combination of sensitivity and attentiveness to bodily signals—has gained in popularity in recent years, particularly in the context of mindfulness-based therapies (see Mehling et al., 2009; Ginzburg, Tsur, Barak-Nahum, & Defrin, 2014).
This is a distinctly different construct from (a) confidence in interoceptive ability; (b) interoceptive sensitivity; and (c) reactivity or proneness to overreact to somatic signals and/or interpret them in a negatively biased way (e.g., Barsky et al., 1988).
In general there appears to be little to no correlation between self-report measures of confidence in interoceptive ability and objective measures of interoceptive sensitivity (Ehlers & Breuer, 1992; Pollatos et al., 2008; Whitehead et al., 1977), which is not surprising given that the former provide only an indirect measure of awareness rather than sensitivity.
Electrical stimulation of the cervical portion of the left vagus, generally via an implanted, pacemaker-like device (Terry et al., 1990). A trans-cutaneous approach to VNS has also been developed (Kraus et al., 2007).
The specific parameters of the electrical pulses (e.g., frequency, intensity, pulse duration) delivered to the vagus have a significant impact on which brain regions are affected (e.g., Lomarev et al., 2002; Mu et al., 2004).
Acute VNS has also been found to result in deactivation of the right AIC in mild to moderate depression, but higher activation of the AIC in more severe depression, suggesting a qualitative shift in central response to somatic input in more severe depression (Nahas et al., 2007) that may explain some of the variation in interoceptive sensitivity tied to severity of depression (Dunn et al., 2007).
Whereas nearly 10% of controls and 30% of panic disorder patients were classified as accurate perceivers, 0% of the depressed sample were classified as such. Similarly, whereas nearly 24% of controls and 4% of PD patients with panic were classified as “non-responders” (subjects who reported not feeling any heartbeats), roughly 37% of depressed persons were classified as such (Van der Does et al., 1997).
All values in parentheses are means ± standard errors.
Terhaar et al. (2012) fail to report sufficient information to calculate the actual power of the t-test they performed; however, the fact that the comparison involved 11 medicated and 5 unmedicated depressed persons, all else equal, would entail that the study had sufficient power to detect a only very large effect of medication status on HBP.
Comparable results, particularly with respect to hippocampal and insular deactivation, were found in a study examining the neural consequences of fluoxetine administration in rats subject to forced swim (Jang et al., 2009).
A great deal more empirical work will be necessary to ascertain which model(s) or which features of these models are in fact operative in depression. It is likely that elements of both the Paulus and Stein (2010) and Northoff et al. (2011) models will be supported.
Equifinality is the notion that in a complex system a single endpoint can be arrived at via varied and diverse pathways (von Bertalanffy, 1968; Cicchetti & Rogosch, 1996).
The classic portrayal of eating by “unrestrained” eaters was, for similar reasons, a lowering of intake under conditions of stress or anxiety (e.g., Herman & Polivy, 1975).
Rumination in depression also involves activation of a number of brain regions, including rostral ACC and insula (e.g., Cooney et al., 2010).
The available evidence suggests that sexual concordance is not significantly related to performance on typical laboratory measures of interoceptive sensitivity such as HBP (Suschinsky & Lalumière, 2012, 2014). No studies to date have, however, examined the correlation between sexual concordance and performance on real-world measures of interoceptive sensitivity (e.g., Cox et al., 1985).
Kano et al. (2013), for example, found differences in response to painful oesophageal distension that differed between actual pain and anticipation conditions; with females showing greater decrease in amygdala activation during anticipation and greater increase in activation of the mid cingulate and AIC during actual pain than males.
For example, “somatization” is entirely normative in many cultures but considered pathological in Western cultures (see Kirmayer, 2001).
These two experiments were a virtual version of Dutton and Aron’s (1974) classic scary bridge study—adapted for the laboratory and modern virtual reality equipment—and a version of a classic “false feedback” paradigm (e.g., Vallins, 1966), in which participants were tested for the effects of false feedback about their own heart rates on affective judgments about a set of images from the International Affective Picture System (IAPS). Ma-Kellams et al. (2012) found that East Asian participants displayed greater exteroceptive biasing of their affective judgments of IAPS images in the false-feedback paradigm and greater misattribution of arousal (i.e. attraction to an opposite-sex avatar) in the virtual scary-bridge experiment.
If Mendes is correct, laboratory HBP paradigms are also likely to underestimate the interoceptive sensitivity of older adults (e.g., Khalsa et al., 2009).
Although some evidence also suggests higher rates of a “somatic subtype” of depression in women than in men (e.g., Silverstein, 2002; Silverstein et al., 2013), absolute rates of this subtype appear to be low and thus cannot fully explain gender difference in rates of depression (Delisle et al., 2012). Additionally, some have suggested that sex differences in the epidemiology of depression may be partly related to the absence of “male-typical,” externalizing symptoms from standard diagnostic criteria (Martin, Neighbors, & Griffith, 2013; Rice et al., in press).
References
- Aarøe L, Petersen MB. Hunger games: Fluctuations in blood glucose levels influence support for social welfare. Psychological Science. 2013;24:2550–2556. doi: 10.1177/0956797613495244. [DOI] [PubMed] [Google Scholar]
- Aaronson ST, Carpenter LL, Conway CR, Reimherr FW, Lisanby SH, Schwartz TL, Bunker M. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: Acute and chronic effects. Brain Stimulation. 2013;6:631–640. doi: 10.1016/j.brs.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Aarts H, Dijksterhuis A, Vries P. On the psychology of drinking: Being thirsty and perceptually ready. British Journal of Psychology. 2001;92:631–642. doi: 10.1348/000712601162383. [DOI] [PubMed] [Google Scholar]
- Aarts H, Gollwitzer PM, Hassin RR. Goal contagion: Perceiving is for pursuing. Journal of Personality and Social Psychology. 2004;87:23–37. doi: 10.1037/0022-3514.87.1.23. [DOI] [PubMed] [Google Scholar]
- Ádám G. Visceral perception: Understanding internal cognition. New York: Plenum Press; 1998. [Google Scholar]
- Adetoki A, Evans R, Cassidy G. Polydipsia with water intoxication in treatment-resistant schizophrenia. Progress in Neurology and Psychiatry. 2013;17:20–23. [Google Scholar]
- Adolphs R, Damasio AR. The interaction of affect and cognition: A neurobiological perspective. In: Forgas JP, editor. Handbook of Affect and Social Cognition. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 27–49. [Google Scholar]
- Ainley V, Tsakiris M. Body conscious? Interoceptive awareness, measured by heartbeat perception, is negatively correlated with self-objectification. PloS ONE. 2013;8:e55568. doi: 10.1371/journal.pone.0055568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainley V, Brass M, Tsakiris M. Heartfelt imitation: High interoceptive awareness is linked to greater automatic imitation. Neuropsychologia. 2014;60:21–28. doi: 10.1016/j.neuropsychologia.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Ainley V, Tajadura-Jiménez A, Fotopoulou A, Tsakiris M. Looking into myself: Changes in interoceptive sensitivity during mirror self-observation. Psychophysiology. 2012;49:1672–1676. doi: 10.1111/j.1469-8986.2012.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Alberts JR. The nature of nurturant niches in ontogeny. Philosophical Psychology. 2008;21(3):295–303. [Google Scholar]
- Alberts JR, Harshaw C. Behavioral development and ontogenetic adaptation. In: Yasukawa K, Tang-Martinez Z, editors. Animal Behavior: How and Why Animals Do the Things They Do Vol. 1: History, Causes, and Development. Praeger; 2014. pp. 289–324. [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. International Journal of Geriatric Psychiatry. 2011;26:1109–1118. doi: 10.1002/gps.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano CA, Zakem AH, Costa NM, Taylor LK, Weems CF. Sleep problems and their relation to cognitive factors, anxiety, and depressive symptoms in children and adolescents. Depression and Anxiety. 2009;26:503–512. doi: 10.1002/da.20443. [DOI] [PubMed] [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex: The evolution of an interface between emotion and cognition. Annals of the New York Academy of Sciences. 2001;935:107–117. [PubMed] [Google Scholar]
- Alloy LB, Abramson LY. Learned helplessness, depression, and the illusion of control. Journal of Personality and Social Psychology. 1982;42:1114–1126. doi: 10.1037//0022-3514.42.6.1114. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5 th edition: DSM-5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in the human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Andréasson A, Arborelius L, Erlanson-Albertsson C, Lekander M. A putative role for cytokines in the impaired appetite in depression. Brain, Behavior and Immunity. 2007;21:147–152. doi: 10.1016/j.bbi.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biological Psychiatry. 2012;72:57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabadzisz D, Diaz-Heijtz R, Knuesel I, Weber E, Pilloud S, Dettling AC, Pryce CR. Primate early life stress leads to long-term mild hippocampal decreases in corticosteroid receptor expression. Biological Psychiatry. 2010;67:1106–1109. doi: 10.1016/j.biopsych.2009.12.016. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. The Journal of Neuroscience. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. Journal of Neurophysiology. 2003;90:1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology. 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariely D, Loewenstein G. The heat of the moment: The effect of sexual arousal on sexual decision making. Journal of Behavioral Decision Making. 2006;19:87–98. [Google Scholar]
- Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: An in vivo proton magnetic resonance spectroscopy study. Biological Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Avery J, Drevets WC, Moseman S, Bodurka J, Barcalow J, Kyle Simmons W. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biological Psychiatry. 2014;76:258–266. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Yirmiya R. The immunobiology of sexual behavior: Gender differences in the suppression of sexual activity during illness. Pharmacology Biochemistry and Behavior. 1999;64:787–796. doi: 10.1016/s0091-3057(99)00165-3. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Schnitzler A, Enck P. Functional neuroimaging of visceral sensation. Journal of Clinical Neurophysiology. 2000;17:604–612. doi: 10.1097/00004691-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Riemann D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders. 2011;135:10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Bahrick LE, Lickliter R. The role of intersensory redundancy in early perceptual, cognitive, and social development. In: Bremner AJ, Lewkowicz DJ, Spence C, editors. Multisensory development. Oxford University Press; 2012. pp. 183–206. [Google Scholar]
- Bailey MT, Dowd SE, Parry N, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infection and Immunity. 2010;78:1509–1519. doi: 10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain, Behavior, and Immunity. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: Aliterature review. Archives of Internal Medicine. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Baldwin JM. Instinct. Science. 1896;3:669. doi: 10.1126/science.3.70.669. [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;123:1790–1800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Chartrand TA. The unbearable automaticity of being. American Psychologist. 1999;54:462–479. [Google Scholar]
- Baron RS, Inman ML, Kao CF, Logan H. Negative emotion and superficial social processing. Motivation and Emotion. 1992;16:323–346. [Google Scholar]
- Barrett LF, Bar M. See it with feeling: affective predictions during object perception. Philosophical Transactions of the Royal Society B. 2009;364:1325–1334. doi: 10.1098/rstb.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Smith ER. The context principle. In: Mesquita B, Barrett LF, Smith ER, editors. The mind in context. New York/London: Guilford Press; 2010. pp. 1–22. [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Quigley K, Bliss-Moreau E, Aronson KR. Interoceptive sensitivity and self-reports of emotional experience. Journal of Personality and Social Psychology. 2004;87:684–697. doi: 10.1037/0022-3514.87.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsky AJ. Amplification, somatization, and the somatoform disorders. Psychosomatics. 1992;33:28–34. doi: 10.1016/S0033-3182(92)72018-0. [DOI] [PubMed] [Google Scholar]
- Barsky AJ, Goodson JD, Lane BS, Cleary PD. The amplification of somatic symptoms. Psychosomatic Medicine. 1988;50:510–519. doi: 10.1097/00006842-198809000-00007. [DOI] [PubMed] [Google Scholar]
- Bateson PPG, Martin PR. Design for a Life: How behavior and personality develop. New York: Simon & Schuster; 2000. [Google Scholar]
- Baumgartner T, Knoch D, Hotz P, Eisenegger C, Fehr E. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nature Neuroscience. 2011;14:1468–1474. doi: 10.1038/nn.2933. [DOI] [PubMed] [Google Scholar]
- Baune BT, Smith E, Reppermund S, Air T, Samaras K, Lux O, Trollor JN. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: The prospective Sydney memory and aging study. Psychoneuroendocrinology. 2012;37:1521–1530. doi: 10.1016/j.psyneuen.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Paquette V, Levesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17:843–846. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: Clinical, experimental, and theoretical aspects. New York: Harper & Row; 1967. [Google Scholar]
- Beck AT. Depression: Causes and treatment. University of Pennsylvania Press; 1975. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck JE. A developmental perspective on functional somatic symptoms. Journal of Pediatric Psychology. 2008;33:547–562. doi: 10.1093/jpepsy/jsm113. [DOI] [PubMed] [Google Scholar]
- Beckham AJ, Greene TB, Meltzer-Brody S. A pilot study of heart rate variability biofeedback therapy in the treatment of perinatal depression on a specialized perinatal psychiatry inpatient unit. Archives of Women's Mental Health. 2013;16:59–65. doi: 10.1007/s00737-012-0318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Dolan DJ, Reaven NM, Peterson L. Depression and social functioning: A multidimensional study of the linkages. Journal of Clinical Child & Adolescent Psychology. 1993;22:306–315. [Google Scholar]
- Benassi VA, Sweeney PD, Dufour CL. Is there a relation between locus of control orientation and depression? Journal of Abnormal Psychology. 1988;97:357–367. doi: 10.1037//0021-843x.97.3.357. [DOI] [PubMed] [Google Scholar]
- Bendtsen KMB, Krych L, Sørensen DB, Pang W, Nielsen DS, Josefsen K, Hansen AK. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PloS ONE. 2012;7:e46231. doi: 10.1371/journal.pone.0046231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F. Placebo and the new physiology of the doctor-patient relationship. Physiological Reviews. 2013;93:1207–1246. doi: 10.1152/physrev.00043.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. European Journal of Clinical Nutrition. 2007;61:355–361. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterology & Motility. 2011;23:1132-e544. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkey CS, Rockett HRH, Gillman MW, Field AE, Colditz GA. Longitudinal study of skipping breakfast and weight change in adolescents. International Journal of Obesity. 2003;27:1258–1266. doi: 10.1038/sj.ijo.0802402. [DOI] [PubMed] [Google Scholar]
- Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman S, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. European Journal of Pain. 2000;4:157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Bueller JA, Mayer EA. Sex differences in regional brain response to aversive pelvic visceral stimuli. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2006;291:R268–R276. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- Berry SM, Broglio K, Bunker M, Jayewardene A, Olin B, Rush AJ. A patient-level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Medical Devices. 2013;6:17–35. doi: 10.2147/MDER.S41017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bertalanffy L. General systems theory. New York: Braziller; 1968. [Google Scholar]
- Biggio F, Gorini G, Utzeri C, Olla P, Marrosu F, Mocchetti I, Follesa P. Chronic vagus nerve stimulation induces neuronal plasticity in the rat hippocampus. International Journal of Neuropsychopharmacology. 2009;12:1209–1221. doi: 10.1017/S1461145709000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL, Anzman SL. Learning to eat in an obesogenic environment: A developmental systems perspective on childhood obesity. Child Development Perspectives. 2010;4:138–143. [Google Scholar]
- Bistricky SL, Ingram RE, Atchley RA. Facial affect processing and depression susceptibility: Cognitive biases and cognitive neuroscience. Psychological Bulletin. 2011;137:998–1028. doi: 10.1037/a0025348. [DOI] [PubMed] [Google Scholar]
- Biver F, Wikler D, Lotstra F, Damhaut P, Goldman S, Mendlewicz J. Serotonin 5-HT2 receptor imaging in major depression: focal changes in orbito-insular cortex. The British Journal of Psychiatry. 1997;171:444–448. doi: 10.1192/bjp.171.5.444. [DOI] [PubMed] [Google Scholar]
- Blakely RD. Physiological genomics of antidepressant targets: Keeping the periphery in mind. Journal of Neuroscience. 2001;21:8319–8323. doi: 10.1523/JNEUROSCI.21-21-08319.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proceedings of the National Academy of Sciences. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS. Basic instinct: The genesis of behavior. Thunder’s Mouth Press; 2005. [Google Scholar]
- Bluthé RM, Bret-Dibat JL, Layé S, Walter V, Parnet P, Lestage J, Dantzer R. Cytokines induce sickness behaviour by a vagal mediated mechanism. Journal of Neuroimmunology. 1994;54:160. [Google Scholar]
- Bohning DE, Lomarev MP, Denslow S, Nahas Z, Shastri A, George MS. Feasibility of vagus nerve stimulation-synchronized blood oxygenation level-dependent functional MRI. Investigative Radiology. 2001;36:470–479. doi: 10.1097/00004424-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in Major Depressive Disorder: A meta-analysis of voxel based morphometry studies. Journal of Affective Disorders. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Anderson B, Andrew Kozel F, Nahas Z, Richard Smith A, Jackson Thomas K, George MS. Acute and long-term VNS effects on pain perception in a case of treatment-resistant depression. Neurocase. 2006;12:216–220. doi: 10.1080/13554790600788094. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Kozel FA, Anderson B, Walker A, George MS. Vagus nerve stimulation affects pain perception in depressed adults. Pain Research & Management. 2005;10:9–14. doi: 10.1155/2005/256472. [DOI] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends in Molecular Medicine. 2014;20:509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Bos EH, Bouhuys AL, Geerts E, van Os TWPD, Ormel J. Stressful life events as a link between problems in nonverbal communication and recurrence of depression. Journal of Affective Disorders. 2007;97:161–169. doi: 10.1016/j.jad.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Bostwick JM, Pankratz VS. Affective disorders and suicide risk: A reexamination. American Journal of Psychiatry. 2000;157:1925–1932. doi: 10.1176/appi.ajp.157.12.1925. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. Does stress damage the brain? Biological Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Charney DS. Reduced volume of orbitofrontal cortex in major depression. Biological Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Bret-Dibat JL, Bluthe RM, Kent S, Kelley KW, Dantzer R. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behavior and Immunity. 1995;9:242–246. doi: 10.1006/brbi.1995.1023. [DOI] [PubMed] [Google Scholar]
- Briers B, Pandelaere M, Dewitte S, Warlop L. Hungry for money the desire for caloric resources increases the desire for financial resources and vice versa. Psychological Science. 2006;17:939–943. doi: 10.1111/j.1467-9280.2006.01808.x. [DOI] [PubMed] [Google Scholar]
- Brodt SE, Zimbardo PG. Modifying shyness-related social behavior through symptom misattribution. Journal of Personality and Social Psychology. 1981;41:437–449. doi: 10.1037//0022-3514.41.3.437. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Mandelkern MA, Fairbanks LA, Ho ML, Baxter LR., Jr Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biological Psychiatry. 2001a;50:171–178. doi: 10.1016/s0006-3223(01)01117-9. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Baxter LR. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: Preliminary findings. Archives of General Psychiatry. 2001b;58:631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- Brondel L, Cabanac M. Alliesthesia in visual and auditory sensations from environmental signals. Physiology & Behavior. 2007;91:196–201. doi: 10.1016/j.physbeh.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Brown S, Gao X, Tisdelle L, Eickhoff SB, Liotti M. Naturalizing aesthetics: brain areas for aesthetic appraisal across sensory modalities. NeuroImage. 2011;58:250–258. doi: 10.1016/j.neuroimage.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozek J, Guetzkow H, Vig Baldwin M, Cranston R. A quantitative study of perception and association in experimental semistarvation. Journal of Personality. 1951;19:245–264. doi: 10.1111/j.1467-6494.1951.tb01100.x. [DOI] [PubMed] [Google Scholar]
- Bruce ML, Kim K, Leaf PJ, Jacobs S. Depressive episodes and dysphoria resulting from conjugal bereavement in a prospective community sample. American Journal of Psychiatry. 1990;147:608–611. doi: 10.1176/ajp.147.5.608. [DOI] [PubMed] [Google Scholar]
- Bruch H. Conceptual confusion in eating disorders. Journal of Nervous and Mental Disease. 1961;133:46–54. [Google Scholar]
- Bruch H. Hunger and instinct. Journal of Nervous and Mental Disease. 1969;149:91–114. doi: 10.1097/00005053-196908000-00002. [DOI] [PubMed] [Google Scholar]
- Bruch H. Instinct and interpersonal experience. Comprehensive Psychiatry. 1970;11:495–506. doi: 10.1016/0010-440x(70)90011-8. [DOI] [PubMed] [Google Scholar]
- Brunstrom JM, Mitchell GL. Effects of distraction on the development of satiety. British Journal of Nutrition. 2006;96:761–769. [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: Sex specific effects. Journal of Neuroendocrinology. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Current Opinion in Hematology. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Buck R. Emotional communication in personal relationships: A developmental interactionist view. In: Hendrick C, editor. Close relationships. Newbury Park, CA: Sage; 1989a. pp. 144–163. [Google Scholar]
- Buck R. Subjective, expressive, and peripheral bodily components of emotion. In: Wagner H, Manstead A, editors. Handbook of social psychophysiology. New York: John Wiley & Sons; 1989b. pp. 199–221. [Google Scholar]
- Buck R, Goldman CK, Easton CJ, Smith NN. Social learning and emotional education: Emotional expression and communication in behaviorally disordered children and schizophrenic patients. In: Flack WF, Laird JD, editors. Emotions in psychopathology: Theory and research. New York: Oxford University Press; 1998. pp. 298–314. [Google Scholar]
- Busch V, Zeman F, Heckel A, Menne F, Ellrich J, Eichhammer P. The effect of transcutaneous vagus nerve stimulation on pain perception–An experimental study. Brain Stimulation. 2013;6:202–209. doi: 10.1016/j.brs.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Buda M, Fornito A, Bergström ZM, Simons JS. A specific brain structural basis for individual differences in reality monitoring. Journal of Neuroscience. 2011;31:14308–14313. doi: 10.1523/JNEUROSCI.3595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanac M. The physiological role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Cacioppo S, Zhou H, Monteleone G, Majka EA, Quinn KA, Ball AB, Cacioppo JT. You are in sync with me: Neural correlates of interpersonal synchrony with a partner. Neuroscience. 2014;277:842–858. doi: 10.1016/j.neuroscience.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Cameron OG. Visceral sensory neuroscience: Interoception. Oxford/New York: Oxford University Press; 2002. [Google Scholar]
- Cameron OG. Visceral brain–body information transfer. NeuroImage. 2009;47:787–794. doi: 10.1016/j.neuroimage.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Canbeyli R. Sensorimotor modulation of mood and depression: An integrative review. Behavioural Brain Research. 2010;207:249–264. doi: 10.1016/j.bbr.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Cantor JR, Zillmann D, Bryant J. Enhancement of experienced sexual arousal in response to erotic stimuli through misattribution of unrelated residual excitation. Journal of Personality and Social Psychology. 1975;32:69–75. doi: 10.1037/h0076784. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH. Anterior cingulate activation and error processing during interferon-alpha treatment. Biological Psychiatry. 2005;58:190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CE, Harris AL, Moss TG, Edinger JD. Distinguishing rumination from worry in clinical insomnia. Behaviour Research and Therapy. 2010;48:540–546. doi: 10.1016/j.brat.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper RC, Redmond DE, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder: Presence and relationship to the classification of depression. Archives of General Psychiatry. 1985;42:1098–1104. doi: 10.1001/archpsyc.1985.01790340082012. [DOI] [PubMed] [Google Scholar]
- Cebra JJ. Influences of microbiota on intestinal immune system development. The American Journal of Clinical Nutrition. 1999;69:1046s–1051s. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- Cechetto DF. Identification of a cortical site for stress-induced cardiovascular dysfunction. Integrative Physiological and Behavioral Science. 1994;29:362–373. doi: 10.1007/BF02691356. [DOI] [PubMed] [Google Scholar]
- Changizi MA, Hall WG. Thirst modulates a perception. Perception. 2001;30:1489–1498. doi: 10.1068/p3266. [DOI] [PubMed] [Google Scholar]
- Charlton BG. The malaise theory of depression: Major depressive disorder is sickness behavior and antidepressants are analgesic. Medical Hypotheses. 2000;54:126–130. doi: 10.1054/mehy.1999.0986. [DOI] [PubMed] [Google Scholar]
- Chartrand TL, Bargh JA. The chameleon effect: The perception–behavior link and social interaction. Journal of Personality and Social Psychology. 1999;76:893–910. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- Chaudhry AM, Parkinson JA, Hinton EC, Owen AM, Roberts AC. Preference judgements involve a network of structures within frontal, cingulate and insula cortices. European Journal of Neuroscience. 2009;29:1047–1055. doi: 10.1111/j.1460-9568.2009.06646.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Sachdev PS, Wen W, Anstey KJ. Sex differences in regional gray matter in healthy individuals aged 44–48 years: A voxel-based morphometric study. NeuroImage. 2007;36:691–699. doi: 10.1016/j.neuroimage.2007.03.063. [DOI] [PubMed] [Google Scholar]
- Chentsova-Dutton YE, Dzokoto V. Listen to your heart: The cultural shaping of interoceptive awareness and accuracy. Emotion. doi: 10.1037/a0036193. (in press). [DOI] [PubMed] [Google Scholar]
- Chiel HJ, Beer RD. The brain has a body: Adaptive behavior emerges from interactions of nervous system, body and environment. Trends in Neurosciences. 1997;20:553–557. doi: 10.1016/s0166-2236(97)01149-1. [DOI] [PubMed] [Google Scholar]
- Chivers ML, Seto MC, Lalumiere ML, Laan E, Grimbos T. Agreement of self-reported and genital measures of sexual arousal in men and women: A meta-analysis. Archives of Sexual Behavior. 2010;39:5–56. doi: 10.1007/s10508-009-9556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C, Qui B, Bruce I. Vagal hyperactivity in stress induced gastric ulceration in rats. Journal of Gastroenterology and Hepatology. 1996;11:125–128. doi: 10.1111/j.1440-1746.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Chong TTJ, Cunnington R, Williams MA, Kanwisher N, Mattingley JB. fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Current Biology. 2008;18:1576–1580. doi: 10.1016/j.cub.2008.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmas D, Steele JD, Tolomeo S, Eljamel MS, Matthews K. Vagus nerve stimulation for chronic major depressive disorder: 12-month outcomes in highly treatment-refractory patients. Journal of Affective Disorders. 2013;150:1221–1225. doi: 10.1016/j.jad.2013.05.080. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996;8:597–600. [Google Scholar]
- Cioffi D. Beyond attentional strategies: A cognitive-perceptual model of somatic interpretation. Psychological Bulletin. 1991;109:25–41. doi: 10.1037/0033-2909.109.1.25. [DOI] [PubMed] [Google Scholar]
- Clark A. Being there: Brain, body, and world together again. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- Clark A. An embodied cognitive science? Trends in Cognitive Sciences. 1999;3:345–351. doi: 10.1016/s1364-6613(99)01361-3. [DOI] [PubMed] [Google Scholar]
- Clark A. Supersizing the mind: Embodiment, action, and cognitive extension. Oxford University Press; 2008. [Google Scholar]
- Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: The Harvard study of moods and cycles. Archives of General Psychiatry. 2006;63:385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Conway CR, Sheline YI, Chibnall JT, George MS, Fletcher JW, Mintun MA. Cerebral blood flow changes during vagus nerve stimulation for depression. Psychiatry Research: Neuroimaging. 2006;146:179–184. doi: 10.1016/j.pscychresns.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Conway CR, Chibnall JT, Gangwani S, Mintun MA, Price JL, Hershey T, Sheline YI. Pretreatment cerebral metabolic activity correlates with antidepressant efficacy of vagus nerve stimulation in treatment-resistant major depression: A potential marker for response? Journal of Affective Disorders. 2012a;139:283–290. doi: 10.1016/j.jad.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CR, Sheline YI, Chibnall JT, Bucholz RD, Price JL, Gangwani S, Mintun MA. Brain blood-flow change with acute vagus nerve stimulation in treatment-refractory major depressive disorder. Brain Stimulation. 2012b;5:163–171. doi: 10.1016/j.brs.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Yang TT. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugène F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DJ, Clarke WL, Gonder-Frederick L, Pohl S, Hoover C, Snyder A, Pennebaker J. Accuracy of perceiving blood glucose in IDDM. Diabetes Care. 1985;8:529–36. doi: 10.2337/diacare.8.6.529. [DOI] [PubMed] [Google Scholar]
- Coyne JC. Depression and the response of others. Journal of Abnormal Psychology. 1976;85:186–193. doi: 10.1037//0021-843x.85.2.186. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nature Neuroscience. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: The sense of the physiological condition of the body. Current Opinions in Neurobiology. 2003a;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. A new view of pain as a homeostatic emotion. TRENDS in Neurosciences. 2003b;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: Why are some more aware than others? TRENDS in Cognitive Sciences. 2004;8:239–241. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Craig AD. An ascending general homeostatic afferent pathway originating in lamina I. Progress in Brain Research. 1996;107:225–242. doi: 10.1016/s0079-6123(08)61867-1. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception and emotion: A neuroanatomical perspective. In: Lewis M, Haviland-Jones JM, Feldman Barrett L, editors. Handbook of emotions. 3 rd ed. New York: Guilford Press; 2008. pp. 272–290. [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain Structure and Function. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Craig W. Observations on doves learning to drink. Journal of Animal Behavior. 1912;2:273–278. [Google Scholar]
- Craig W. Appetites and aversions as constituents of instincts. Biological Bulletin. 1918;34:91–107. [Google Scholar]
- Cristancho P, Cristancho MA, Baltuch GH, Thase ME, O'Reardon JP. Effectiveness and safety of vagus nerve stimulation for severe treatment-resistant major depression in clinical practice after FDA approval: Outcomes at 1 year. Journal of Clinical Psychiatry. 2011;72:1376–1382. doi: 10.4088/JCP.09m05888blu. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA. Visceral Influences on Brain and Behavior. Neuron. 2013;77:624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Nagai Y. How emotions are shaped by bodily states. Emotion Review. 2012;4:163–168. [Google Scholar]
- Critchley HD, Corfield DL, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. Journal of Physiology. 2000;523:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: The influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33:653–663. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtien P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Lewis PA, Orth M, Josephs O, Deichmann R, Trimble MR, Dolan RJ. Vagus nerve stimulation for treatment-resistant depression: Behavioural and neural effects on encoding negative material. Psychosomatic Medicine. 2007;69:17–22. doi: 10.1097/PSY.0b013e31802e106d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow S, Eisenberg ME, Story M, Neumark-Sztainer D. Suicidal behavior in adolescents: Relationship to weight status, weight control behaviors, and body dissatisfaction. International Journal of Eating Disorders. 2008;41:82–87. doi: 10.1002/eat.20466. [DOI] [PubMed] [Google Scholar]
- Croy I, Symmank A, Schellong J, Hummel C, Gerber J, Peter J, Thomas H. Olfaction as a marker for depression in humans. Journal of Affective Disorders. 2014;160:80–86. doi: 10.1016/j.jad.2013.12.026. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterology & Motility. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- Csordas TJ. Somatic modes of attention. Cultural Anthropology. 1993;8:135–156. [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougana B, Gabbay V, Hulvershorn L, Mueller BA, Milham MP. A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Swartz HA, Salomon K, Gianaros PJ. Cardiac vagal control in nonmedicated depressed women and nondepressed controls: Impact of depression status, lifetime trauma history, and respiratory factors. Psychosomatic Medicine. 2011;73:336–343. doi: 10.1097/PSY.0b013e318213925d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LP, Carroll LE, Galen G, Hayes JA, Webb KW, Porges SW. Abuse history is related to autonomic regulation to mild exercise and psychological wellbeing. Applied Psychophysiology and Biofeedback. 2009;34:299–308. doi: 10.1007/s10484-009-9111-4. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M. Feast and famine: Critical role of glucocorticoids with insulin in daily energy flow. Frontiers in Neuroendocrinology. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- Damasio A. Descartes’ error: Emotion, reason, and the human brain. Penguin; 1994. [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio A. The feeling of what happens: Body and emotion in the making of consciousness. Harcourt; 1999. [Google Scholar]
- Damasio A. Looking for Spinoza. Harcourt; 2003a. [Google Scholar]
- Damasio A. Feelings of emotion and the self. Annals of the New York Academy of Sciences. 2003b;1001:253–261. doi: 10.1196/annals.1279.014. [DOI] [PubMed] [Google Scholar]
- Damasio A. Self comes to mind. Pantheon Books; 2010. [Google Scholar]
- Damasio A, Carvalho GB. The nature of feelings: Evolutionary and neurobiological origins. Nature Reviews Neuroscience. 2013;14:143–152. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowksi T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: Clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunology and Allergy Clinics of North America. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior, and Immunity. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger S, Levav J, Avnaim-Pesso L. Extraneous factors in judicial decisions. Proceedings of the National Academy of Sciences. 2011;108:6889–6892. doi: 10.1073/pnas.1018033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall BD, Suarez EC. Sex and gender in psychoneuroimmunology research: Past, present and future. Brain, Behavior, and Immunity. 2009;23:595–604. doi: 10.1016/j.bbi.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow U, Drzezga A, Frisch M, Munz F, Weilke F, Bartenstein P, Ring J. Processing of histamine-induced itch in the human cerebral cortex: A correlation analysis with dermal reactions. Journal of Investigative Dermatology. 2000;115:1029–1033. doi: 10.1046/j.1523-1747.2000.00193.x. [DOI] [PubMed] [Google Scholar]
- Davidson TL. Learning about deprivation intensity stimuli. Behavioral Neuroscience. 1987;101:198–208. doi: 10.1037//0735-7044.101.2.198. [DOI] [PubMed] [Google Scholar]
- Davidson TL. The nature and function of interoceptive signals to feed: Toward integration of physiological and learning perspectives. Psychological Review. 1993;100:640–657. doi: 10.1037/0033-295x.100.4.640. [DOI] [PubMed] [Google Scholar]
- Davidson TL. Hunger cues as modulatory stimuli. In: Schmajuk N, Holland P, editors. Occasion setting: Data and theory. American Psychological Association Press; 1998. pp. 223–248. [Google Scholar]
- Davidson TL. Pavlovian occasion setting: A link between physiological change and appetitive behavior. Appetite. 2000;35:271–272. doi: 10.1006/appe.2000.0355. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Jarrard LE. A role for hippocampus in the utilization of hunger signals. Behavioral and Neural Biology. 1993;59:167–171. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behavioral Neuroscience. 2010;124:97–105. doi: 10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meersman RE, Stein PK. Vagal modulation and aging. Biological Psychology. 2007;74:165–173. doi: 10.1016/j.biopsycho.2006.04.008. [DOI] [PubMed] [Google Scholar]
- de Waal F, Ferrari PF. Towards a bottom-up perspective on animal and human cognition. Trends in Cognitive Sciences. 2010;14:201–207. doi: 10.1016/j.tics.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Decety J. The neuroevolution of empathy. Annals of the New York Academy of Sciences. 2011;1231:35–45. doi: 10.1111/j.1749-6632.2011.06027.x. [DOI] [PubMed] [Google Scholar]
- Delisle VC, Beck AT, Dobson KS, Dozois DJ, Thombs BD. Revisiting gender differences in somatic symptoms of depression: Much ado about nothing? PloS ONE. 2012;7:e32490. doi: 10.1371/journal.pone.0032490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neuroscience & Biobehavioral Reviews. 2010;34:130–143. doi: 10.1016/j.neubiorev.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiris Y, Aziz-Zadeh L, Bonaiuto J. Information processing in the mirror neuron system in primates and machines. Neuroinformatics. 2014;12:63–91. doi: 10.1007/s12021-013-9200-7. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Tranel D, Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005;43:1099–1106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Denton DA. The primordial emotions: The dawning of consciousness. Oxford University Press; 2006. [Google Scholar]
- Denton DA, McKinley MJ, Farrell M, Egan GF. The role of primordial emotions in the evolution of consciousness. Consciousness and Cognition. 2009;18:500–514. doi: 10.1016/j.concog.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. Journal of Psychiatric Research. 2009;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Molecular Psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Devlin MJ, Walsh BT. Eating disorders and depression. Psychiatric Annals. 1989;19:473–476. [Google Scholar]
- DeWall CN, Baumeister RF, Gailliot MT, Maner JK. Depletion makes the heart grow less helpful: Helping as a function of self-regulatory energy and genetic relatedness. Personality and Social Psychology Bulletin. 2008;34:1653–1662. doi: 10.1177/0146167208323981. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A. Why we are social animals: The high road to imitation as social glue. In: Hurley S, Chater N, editors. Perspectives on Imitation: From Neuroscience to Social Science. Cambridge, MA: MIT Press; 2005. pp. 207–220. [Google Scholar]
- Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF. Melancholic microbes: A link between gut microbiota and depression? Neurogastroenterology & Motility. 2013;25:713–719. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: An overview and integration of neurobiological findings. Clinical Psychology Review. 2010;30:1–11. doi: 10.1016/j.cpr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biological Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Du M-Y, Wu Q-Z, Yue Q, Li J, Liao Y, Kuang W-H, Gong QY. Voxel wise meta-analysis of gray matter reduction in major depressive disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012;36:11–16. doi: 10.1016/j.pnpbp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Ogilvie AD, Lawrence AD. Heartbeat perception in depression. Behaviour Research and Therapy. 2007;45:1921–1930. doi: 10.1016/j.brat.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Stefanovitch I, Evans D, Oliver C, Hawkins A, Dalgleish T. Can you feel the beat? Interoceptive awareness is an interactive function of anxiety- and depression-specific symptom dimensions. Behaviour Research and Therapy. 2010;48:1133–1138. doi: 10.1016/j.brat.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton DG, Aron AP. Some evidence for heightened sexual attraction under conditions of high anxiety. Journal of Personality and Social Psychology. 1974;30:510–517. doi: 10.1037/h0037031. [DOI] [PubMed] [Google Scholar]
- Egizio VB, Jennings JR, Christie IC, Sheu LK, Matthews KA, Gianaros PJ. Cardiac vagal activity during psychological stress varies with social functioning in older women. Psychophysiology. 2008;45:1046–1054. doi: 10.1111/j.1469-8986.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Breuer P. Increased cardiac awareness in panic disorder. Journal of Abnormal Psychology. 1992;101:371–382. doi: 10.1037//0021-843x.101.3.371. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms: A unified approach to understanding the etiology of depression. Archives of General Psychiatry. 1988;45:948–952. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- Eichler S, Katkin ES. The relationship between cardiovascular reactivity and heartbeat detection. Psychophysiology. 1994;31:229–234. doi: 10.1111/j.1469-8986.1994.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Eigsti IM. A review of embodiment in autism spectrum disorders. Frontiers in Psychology. 2013;4:224. doi: 10.3389/fpsyg.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: An inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, Behavior, and Immunity. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. NeuroImage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm EM, Hartiala J, Huikuri HV. Circadian rhythm of frequency-domain measures of heart rate variability in pregnancy. BJOG: An International Journal of Obstetrics & Gynaecology. 1997;104:825–828. doi: 10.1111/j.1471-0528.1997.tb12027.x. [DOI] [PubMed] [Google Scholar]
- Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Research. 2000;42:203–210. doi: 10.1016/s0920-1211(00)00181-9. [DOI] [PubMed] [Google Scholar]
- Elhwuegi AS. Central monoamines and their role in major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28:435–451. doi: 10.1016/j.pnpbp.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Epstein S. Food-related responses to ambiguous stimuli as a function of hunger and ego strength. Journal of Consulting Psychology. 1961;25:463. doi: 10.1037/h0044029. [DOI] [PubMed] [Google Scholar]
- Epstein S, Levitt H. The influence of hunger on the learning and recall of food related words. Journal of Abnormal and Social Psychology. 1962;64:130–135. doi: 10.1037/h0040920. [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets W, Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neuroscience and Biobehavioral Reviews. 2003;27:233–246. doi: 10.1016/s0149-7634(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Ernst J, Northoff G, Böker H, Seifritz E, Grimm S. Interoceptive awareness enhances neural activity during empathy. Human Brain Mapping. 2013;34:1615–1624. doi: 10.1002/hbm.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, Boeker H, Seifritz E, Northoff G, Grimm S. Changes in the association of neural activity during interoceptive awareness with neurotransmitter concentrations in the course of depression [Paper presented at the annual meeting of the Society of Biological Psychiatry] Biological Psychiatry. 2014;75:367S–367S. [Google Scholar]
- Eslinger PJ, Flaherty-Craig CV, Benton AL. Developmental outcomes after early prefrontal cortex damage. Brain and Cognition. 2004;55:84–103. doi: 10.1016/S0278-2626(03)00281-1. [DOI] [PubMed] [Google Scholar]
- Espejo EP, Hammen CL, Connolly NP, Brennan PA, Najman JM, Bor W. Stress sensitization and adolescent depressive severity as a function of childhood adversity: A link to anxiety disorders. Journal of Abnormal Child Psychology. 2006;35:287–299. doi: 10.1007/s10802-006-9090-3. [DOI] [PubMed] [Google Scholar]
- Fairchild KD, Srinivasan V, Moorman JR, Gaykema RP, Goehler LE. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2011;300:R330–R339. doi: 10.1152/ajpregu.00487.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough SH, Goodwin L. The effect of psychological stress and relaxation on interoceptive accuracy: Implications for symptom perception. Journal of Psychosomatic Research. 2007;62:289–295. doi: 10.1016/j.jpsychores.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Fan J, Gu X, Liu X, Guise KG, Park Y, Martin L, Hof PR. Involvement of the anterior cingulate and frontoinsular cortices in rapid processing of salient facial emotional information. NeuroImage. 2011;54:2539–2546. doi: 10.1016/j.neuroimage.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35:903–911. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Anderson AK. Attentional modulation of primary interoceptive and exteroceptive cortices. Cerebral Cortex. 2013;23:114–126. doi: 10.1093/cercor/bhr385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer AD, Randall HA, Aziz Q. It's a gut feeling-How the gut microbiota affects the state of mind. The Journal of Physiology. doi: 10.1113/jphysiol.2013.270389. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Abraham M, Clancy-Colecchi K, Pava JA, Matthews J, Rosenbaum JF. Eating disorder symptomatology in major depression. Journal of Nervous and Mental Disease. 1997;185:140–144. doi: 10.1097/00005053-199703000-00002. [DOI] [PubMed] [Google Scholar]
- Favreau A, Richard-Yris M-A, Bertin A, Houdelier C, Lumineau S. Social influences on circadian behavioural rhythms in vertebrates. Animal Behaviour. 2009;77:983–989. [Google Scholar]
- Fedoroff IC, Polivy J, Herman CP. The effect of pre-exposure to food cues on the eating behavior of restrained and unrestrained eaters. Appetite. 1997;28:33–47. doi: 10.1006/appe.1996.0057. [DOI] [PubMed] [Google Scholar]
- Ferguson MJ, Bargh JA. How social perception can automatically influence behavior. TRENDS in Cognitive Sciences. 2004;8:33–39. doi: 10.1016/j.tics.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Ferguson ML, Katkin ES. Visceral perception, anhedonia, and emotion. Biological Psychology. 1996;42:131–145. doi: 10.1016/0301-0511(95)05151-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Leon JA. Behavioral robustness: An emergent phenomenon by means of distributed mechanisms and neurodynamic determinacy. Biosystems. 2012;107:34–51. doi: 10.1016/j.biosystems.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M. Vagal activity, early growth and emotional development. Infant Behavior & Development. 2008;31:361–373. doi: 10.1016/j.infbeh.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EN. The X-files in immunity: Sex-based differences predispose immune responses. Nature Reviews Immunology. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Human Brain Mapping. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliederbaum J, Heller A, Zweibaum K, Zarchi J. Clinical aspects of hunger disease in adults. In: Winick M, editor; Osnos M, translator. Hunger disease: Studies by the Jewish physicians in the Warsaw ghetto. 1979. pp. 11–36. Originally published (1946). [Google Scholar]
- la Fleur SE. The effects of glucocorticoids on feeding behavior in rats. Physiology & Behavior. 2006;89:110–114. doi: 10.1016/j.physbeh.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Fornito A, Whittle S, Wood SJ, Velakoulis D, Pantelis C, Yücel M. The influence of sulcal variability on morphometry of the human anterior cingulate and paracingulate cortex. NeuroImage. 2006;33:843–854. doi: 10.1016/j.neuroimage.2006.06.061. [DOI] [PubMed] [Google Scholar]
- Forrer GR. Effect of oral activity on hallucinations. Archives of General Psychiatry. 1960a;2:100–103. doi: 10.1001/archpsyc.1960.03590070102012. [DOI] [PubMed] [Google Scholar]
- Forrer GR. Benign auditory and visual hallucinations. Archives of General Psychiatry. 1960b;3:95–98. doi: 10.1001/archpsyc.1960.01710010097012. [DOI] [PubMed] [Google Scholar]
- Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain, Behavior, and Immunity. 2010;24:9–16. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- Foster JA, Neufeld K-AM. Gut–brain axis: How the microbiome influences anxiety and depression. Trends in Neurosciences. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, Monk T. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Archives of General Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- Frankel LA, Hughes SO, O'Connor TM, Power TG, Fisher JO, Hazen NL. Parental influences on children's self-regulation of energy intake: Insights from developmental literature on emotion regulation. Journal of Obesity. 2012;327259 doi: 10.1155/2012/327259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankum S, Ogden J. Estimation of blood glucose levels by people with diabetes: a cross-sectional study. British Journal of General Practice. 2005;55:944–948. [PMC free article] [PubMed] [Google Scholar]
- Freedman J, Perlick D. Crowding, contagion, and laughter. Journal of Experimental Social Psychology. 1979;15:295–303. [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Archives of General Psychiatry. 2006;63:375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- Fuchs T. Corporealized and disembodied minds: A phenomenological view of the body in melancholia and schizophrenia. Philosophy, Psychiatry, & Psychology. 2005;12:95–107. [Google Scholar]
- Fuchs T, Schlimme JE. Embodiment and psychopathology: A phenomenological perspective. Current Opinion in Psychiatry. 2009;22:570–575. doi: 10.1097/YCO.0b013e3283318e5c. [DOI] [PubMed] [Google Scholar]
- Fulkerson JA, Sherwood NE, Perry CL, Neumark-Sztainer D, Story M. Depressive symptoms and adolescent eating and health behaviors: A multifaceted view in a population-based sample. Preventive Medicine. 2004;38:865–875. doi: 10.1016/j.ypmed.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Furman DJ, Waugh CE, Bhattacharjee K, Thompson RJ, Gotlib IH. Interoceptive awareness, positive affect, and decision making in Major Depressive Disorder. Journal of Affective Disorders. 2013;151:780–785. doi: 10.1016/j.jad.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Critchley HD. Interoception, emotion and brain: New insights link internal physiology to social behaviour. Commentary on: “Anterior insular cortex mediates bodily sensibility and social anxiety” by Terasawa et al. (2012) Social Cognitive and Affective Neuroscience. 2013;8:231–234. doi: 10.1093/scan/nss140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner DM, Olmstead MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. International Journal of Eating Disorders. 1983;2:15–34. [Google Scholar]
- Gentzler AL, Rottenberg J, Kovacs M, George CJ, Morey JN. A typical development of resting respiratory sinus arrhythmia in children at high risk for depression. Developmental Psychobiology. 2012;54:556–567. doi: 10.1002/dev.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox NA. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology. 2009;82:156–163. doi: 10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, Ballenger JC. Vagus nerve stimulation: A new tool for brain research and therapy. Biological Psychiatry. 2000;47:287–295. doi: 10.1016/s0006-3223(99)00308-x. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR, Holstege G. Human brain activation during sexual stimulation of the penis. Journal of Comparative Neurology. 2005;493:33–38. doi: 10.1002/cne.20735. [DOI] [PubMed] [Google Scholar]
- Gershon M. The second brain. New York: Harper Collins; 1998. [Google Scholar]
- Gianaros PJ, Derbyshire SWG, May C, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42:627–635. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, Hariri AR. Perigenual anterior cingulate morphologycovaries with perceived social standing. Social Cognitive and Affective Neuroscience. 2007;2:161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JJ. The ecological approach to visual perception. Boston: Houghton Mifflin; 1979. [Google Scholar]
- Giddan NS. Effect of thirst on stimulus recovery and spontaneous imagery. Perceptual and Motor Skills. 1966;23:631–638. [Google Scholar]
- Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis & Rheumatism. 2005;52:1577–1584. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- Gigerenzer G. Adaptive thinking. Oxford: Oxford University Press; 2000. [Google Scholar]
- Gilchrist JC, Nesberg LS. Need and perceptual change in need-related objects. Journal of Experimental Psychology. 1952;44:369–376. doi: 10.1037/h0061823. [DOI] [PubMed] [Google Scholar]
- Ginzburg K, Tsur N, Barak-Nahum A, Defrin R. Body awareness: differentiating between sensitivity to and monitoring of bodily signals. Journal of Behavioral Medicine. 2014;37:564–575. doi: 10.1007/s10865-013-9514-9. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. NeuroImage. 2010;51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH. Depression and cardiovascular comorbidity. Dialogues in Clinical Neuroscience. 2007;9:9–17. doi: 10.31887/DCNS.2007.9.1/ahglassman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Wenderoth N, Van Impe A, Swinnen SP. Proprioceptive sensibility in the elderly: Degeneration, functional consequences and plastic-adaptive processes. Neuroscience & Biobehavioral Reviews. 2009;33:271–278. doi: 10.1016/j.neubiorev.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Lyte M, Gaykema R. Infection-induced viscerosensory signals from the gut enhance anxiety: Implications for psychoneuroimmunology. Brain, Behavior, and Immunity. 2007;21:721–726. doi: 10.1016/j.bbi.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema R, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: Early responses to intestinal infection with Campylobacter jejuni. Brain, Behavior, and Immunity. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RPA. Campylobacter jejuni infection increases anxiety-like behavior in the hole board: Possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain, Behavior, and Immunity. 2008;22:354–366. doi: 10.1016/j.bbi.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, Bell JD. Fasting biases brain reward systems towards high-calorie foods. European Journal of Neuroscience. 2009;30:1625–1635. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. Individual development and evolution: The genesis of novel behavior. Lawrence Erlbaum; 2001. [Google Scholar]
- Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: A review of comorbidity and management. Inflammatory Bowel Diseases. 2009;15:1105–1118. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- Grandin LD, Alloy LB, Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: Review and evaluation. Clinical Psychology Review. 2006;26:679–694. doi: 10.1016/j.cpr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Graux J, Lemoine M, Gaillard P, Camus V. Les cénesthopathies: Un trouble des émotions d’arrière plan. Regards croisés des sciences cognitives et de la phénoménologie. L'Encéphale. 2011;37:361–370. doi: 10.1016/j.encep.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Greene JD. The secret joke of Kant's soul. In: Sinnott-Armstrong W, editor. Moral Psychology, Vol. 3: The Neuroscience of Morality: Emotion, Disease, and Development. Cambridge, MA: MIT Press; 2007. pp. 35–79. [Google Scholar]
- Gregory AM, O'Connor TG. Sleep problems in childhood: A longitudinal study of developmental change and association with behavioral problems. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:964–971. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Sadeh A. Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Medicine Reviews. 2012;16:129–136. doi: 10.1016/j.smrv.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Schatzberg AF. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biological Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, Northoff G. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Human Brain Mapping. 2009;30:2617–2627. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiology & Behavior. 2003;78:703–710. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic Medicine. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Johnson AK. Evaluation of baroreceptor reflex function in the chronic mild stress rodent model of depression. Psychosomatic Medicine. 2008;70:435–443. doi: 10.1097/PSY.0b013e31816ff7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Cardiac regulation in the socially monogamous prairie vole. Physiology & Behavior. 2007;90:386–393. doi: 10.1016/j.physbeh.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, II, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Carter CS, McNeal N, Chandler DL, LaRocca MA, Bates SL, Porges SW. 24-hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: Implications for the study of depression and cardiovascular disease. Psychosomatic Medicine. 2011;73:59–66. doi: 10.1097/PSY.0b013e31820019e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Sgoifo A, Jepson AJ, Bates SL, Chandler DL, Preihs K. The integration of depressive behaviors and cardiac dysfunction during an operational measure of depression: Investigating the role of negative social experiences in an animal model. Psychosomatic Medicine. 2012;74:612–619. doi: 10.1097/PSY.0b013e31825ca8e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: Evidence from a meta-analysis of fMRI studies. Neuroscience & Biobehavioral Reviews. 2013;37:152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Gross CG. Aristotle and the brain. The Neuroscientist. 1995;1:245–250. [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. New York, NY: Guilford Press; 2007. pp. 3–24. [Google Scholar]
- Guastella AJ, Moulds ML. The impact of rumination on sleep quality following a stressful life event. Personality and Individual Differences. 2007;42:1151–1162. [Google Scholar]
- Guggisberg AG, Mathis J, Schnider A, Hess CW. Why do we yawn? Neuroscience and Biobehavioral Reviews. 2010;34:1267–1276. doi: 10.1016/j.neubiorev.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Hale MW, Raison CL, Lowry CA. Integrative physiology of depression and antidepressant drug action: Implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression. Pharmacology & Therapeutics. 2013;137:108–118. doi: 10.1016/j.pharmthera.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Hall WG, Arnold HM, Myers KP. The acquisition of an appetite. Psychological Science. 2000;11:101–105. doi: 10.1111/1467-9280.00223. [DOI] [PubMed] [Google Scholar]
- Halmi KA, Eckert E, Marchi P, Sampugnaro V, Apple R, Cohen J. Comorbidity of psychiatric diagnoses in anorexia nervosa. Archives of General Psychiatry. 1991;48:712–718. doi: 10.1001/archpsyc.1991.01810320036006. [DOI] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biological Psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: A meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hammen C, Hazel NA, Brennan PA, Najman J. Intergenerational transmission and continuity of stress and depression: Depressed women and their offspring in 20 years of follow-up. Psychological Medicine. 2012;42:931–942. doi: 10.1017/S0033291711001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Henry R, Daley SE. Depression and sensitization to stressors among young women as a function of childhood adversity. Journal of Consulting and Clinical Psychology. 2000;68:782–787. [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Subramanyam K, DellaGioia N, Planeta-Wilson B, Weinzimmer D, Pittman B, Carson RE. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. Journal of Nuclear Medicine. 2012;53:601–607. doi: 10.2967/jnumed.111.097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MK, Taishi P, Chen Z, Krueger JM. Vagotomy blocks the induction of interleukin-1β (IL-1β) mRNA in the brain of rats in response to systemic IL-1β. The Journal of Neuroscience. 1998;18:2247–2253. doi: 10.1523/JNEUROSCI.18-06-02247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M, Chotai J, Bodlund O. Patients' beliefs about the cause of their depression. Journal of Affective Disorders. 2010;124:54–59. doi: 10.1016/j.jad.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Hantas M, Katkin ES, Reed SD. Cerebral lateralization and heartbeat discrimination. Psychophysiology. 1984;21:274–278. doi: 10.1111/j.1469-8986.1984.tb02934.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Mueller GP, Dubner R, Goldstein D, Dionne RA. Corticotropin-releasing factor (CRF) produces analgesia in humans and rats. Brain Research. 1987;422:154–157. doi: 10.1016/0006-8993(87)90550-6. [DOI] [PubMed] [Google Scholar]
- Harrell ZA, Jackson B. Thinking fat and feeling blue: Eating behaviors, ruminative coping, and depressive symptoms in college women. Sex Roles. 2008;58:658–665. [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neuroscience & Biobehavioral Reviews. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Singer T, Rotshtein P, Dolan RJ, Critchley HD. Pupillary contagion: Central mechanisms engaged in sadness processing. Social Cognitive and Affective Neuroscience. 2006;1:5–17. doi: 10.1093/scan/nsl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry. 2009a;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biological Psychiatry. 2009b;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshaw C. Alimentary epigenetics: A developmental psychobiological systems view of the perception of hunger, thirst and satiety. Developmental Review. 2008;28:541–569. doi: 10.1016/j.dr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harver A, Katkin ES, Bloch E. Signal-detection outcomes on heartbeat and respiratory resistance detection tasks in male and female subjects. Psychophysiology. 1993;30:223–230. doi: 10.1111/j.1469-8986.1993.tb03347.x. [DOI] [PubMed] [Google Scholar]
- Haug TT, Mykletun A, Dahl AA. The association between anxiety, depression, and somatic symptoms in a large population: The HUNT-II study. Psychosomatic Medicine. 2004;66:845–851. doi: 10.1097/01.psy.0000145823.85658.0c. [DOI] [PubMed] [Google Scholar]
- Haynes PL, Ancoli-Israel S, McQuaid J. Illuminating the impact of habitual behaviors on depression. Chronobiology International. 2005;22:279–297. doi: 10.1081/cbi-200053546. [DOI] [PubMed] [Google Scholar]
- Healy D, Williams JMG. Dysrhythmia, dysphoria, and depression: The interaction of learned helplessness and circadian dysrhythmia in the pathogenesis of depression. Psychological Bulletin. 1988;103:163–178. doi: 10.1037/0033-2909.103.2.163. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior: A neuropsychological theory. New York/London/Sydney: John Wiley & Sons, Inc.; 1949. [Google Scholar]
- Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Henry TR, Bakay RA, Votaw JR, Pennell PB, Epstein CM, Faber TL, Hoffman JM. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39:983–990. doi: 10.1111/j.1528-1157.1998.tb01448.x. [DOI] [PubMed] [Google Scholar]
- Henry TR, Bakay RA, Pennell PB, Epstein CM, Votaw JR. Brain blood-flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: II. Prolonged effects at high and low levels of stimulation. Epilepsia. 2004;45:1064–1070. doi: 10.1111/j.0013-9580.2004.03104.x. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Pollatos O. The body in the mind: On the relationship between interoception and embodiment. Topics in Cognitive Science. 2012;4:692–704. doi: 10.1111/j.1756-8765.2012.01189.x. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Herbert C, Pollatos O. On the relationship between interoceptive awareness and alexithymia: Is interoceptive awareness related to emotional awareness? Journal of Personality. 2011;79:1149–1175. doi: 10.1111/j.1467-6494.2011.00717.x. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Muth ER, Pollatos O, Herbert C. Interoception across modalities: On the relationship between cardiac awareness and the sensitivity for gastric functions. PloS ONE. 2012;7:e36646. doi: 10.1371/journal.pone.0036646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert BM, Pollatos O, Flor H, Enck P, Schandry R. Cardiac awareness and autonomic cardiac reactivity during emotional picture viewing and mental stress. Psychophysiology. 2010;47:342–354. doi: 10.1111/j.1469-8986.2009.00931.x. [DOI] [PubMed] [Google Scholar]
- Herman CP, Polivy J. Anxiety, restraint, and eating behavior. Journal of Abnormal Psychology. 1975;84:666–672. [PubMed] [Google Scholar]
- Herman CP, Polivy J. Normative influences on food intake. Physiology & Behavior. 2005;86:762–772. doi: 10.1016/j.physbeh.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Herman CP, Ostovich JM, Polivy J. Effects of attentional focus on subjective hunger ratings. Appetite. 1999;33:181–193. doi: 10.1006/appe.1999.0243. [DOI] [PubMed] [Google Scholar]
- Hermans RC, Lichtwarck-Aschoff A, Bevelander KE, Herman CP, Larsen JK, Engels RC. Mimicry of food intake: The dynamic interplay between eating companions. PloS One. 2012;7:e31027. doi: 10.1371/journal.pone.0031027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Brühl AB, Kaffenberger1 T, Baumgartner T, Boeker H, Jäncke L. Neural correlates of ‘pessimistic’ attitude in depression. Psychological Medicine. 2010;40:789–800. doi: 10.1017/S0033291709991073. [DOI] [PubMed] [Google Scholar]
- Hirstein W, Ramachandran VS. Capgras syndrome: A novel probe for understanding the neural representation of the identity and familiarity of persons. Proceedings of the Royal Society of London. Series B: Biological Sciences. 1997;264:437–444. doi: 10.1098/rspb.1997.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA. Relationships as regulators: A psychobiological perspective on bereavement. Psychosomatic Medicine. 1984;46:183–197. doi: 10.1097/00006842-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Holle H, Warne K, Seth AK, Critchley HD, Ward J. Neural basis of contagious itch and why some people are more prone to it. Proceedings of the National Academy of Sciences. 2012;109:19816–19821. doi: 10.1073/pnas.1216160109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis JH, McKinley MJ, D’Souza M, Kampe J, Oldfield BJ. The trajectory of sensory pathways from the lamina terminal is to the insular and cingulate cortex: A neuroanatomical framework for the generation of thirst. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2008;294:R1390–R1401. doi: 10.1152/ajpregu.00869.2007. [DOI] [PubMed] [Google Scholar]
- Holzer P. The role of the vagus nerve in afferent signaling and homeostasis during visceral inflammation. NeuroImmune Biology. 2009;8:321–338. [Google Scholar]
- Hölzl R, Erasmus LP, Moltner A. Detection, discrimination and sensation of visceral stimuli. Biological Psychology. 1996;42:199–214. doi: 10.1016/0301-0511(95)05155-4. [DOI] [PubMed] [Google Scholar]
- Hopkins J, Marcus M, Campbell SB. Postpartum depression: A critical review. Psychological Bulletin. 1984;95:498–515. [PubMed] [Google Scholar]
- Hong JY, Kilpatrick LA, Labus J, Gupta A, Jiang Z, Ashe-McNalley C, Mayer EA. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. The Journal of Neuroscience. 2013;33:11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp H, Shallcross AJ, Ford BQ, Troy AS, Wilhelm FH, Mauss IB. High cardiac vagal control protects against future depressive symptoms under conditions of high social support. Biological Psychology. 2013;93:143–149. doi: 10.1016/j.biopsycho.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, Walter M. Glutamatergic and resting-state functional connectivity correlates of severity in major depression–the role of pregenual anterior cingulate cortex and anterior insula. Frontiers in Systems Neuroscience. 2010;4:33. doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath G, Kekesi G. Interaction of endogenous ligands mediating antinociception. Brain Research Reviews. 2006;52:69–92. doi: 10.1016/j.brainresrev.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebro ventricular lipopolysaccharide. Neurobiology of Aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MM, Dennett DC, Adams RB., Jr . Inside jokes: Using humor to reverse-engineer the mind. Boston, MA: MIT Press; 2011. [Google Scholar]
- Ibañez A, Gleichgerrcht E, Manes F. Clinical effects of insular damage in humans. Brain Structure and Function. 2010;214:397–410. doi: 10.1007/s00429-010-0256-y. [DOI] [PubMed] [Google Scholar]
- Ijzerman H, Semin GR. The thermometer of social relations: Mapping social proximity on temperature. Psychological Science. 2009;20:1214–1220. doi: 10.1111/j.1467-9280.2009.02434.x. [DOI] [PubMed] [Google Scholar]
- Indo Y. Nerve growth factor, interoception, and sympathetic neuron: lesson from congenital insensitivity to pain with anhidrosis. Autonomic Neuroscience. 2009;147:3–8. doi: 10.1016/j.autneu.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Swartz M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. NeuroImage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jain R. The epidemiology and recognition of pain and physical symptoms in depression. Journal of Clinical Psychiatry. 2009;70:e04. doi: 10.4088/JCP.8001tx1c.e04. [DOI] [PubMed] [Google Scholar]
- Jang D-P, Lee S-H, Park C-W, Lee S-Y, Kim Y-B, Cho Z-H. Effects of fluoxetine on the rat brain in the forced swimming test: A [F-18]FDG micro-PET imaging study. Neuroscience Letters. 2009;451:60–64. doi: 10.1016/j.neulet.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. The hippocampus and motivation. Psychological Bulletin. 1973;79:1–12. doi: 10.1037/h0033792. [DOI] [PubMed] [Google Scholar]
- James W. The principles of psychology. New York: Henry Holt; 1890. [Google Scholar]
- Joffe H, Soares CN, Thurston RC, White DP, Cohen LS, Hall JE. Depression is associated with worse objectively and subjectively measured sleep, but not more frequent awakenings, in women with vasomotor symptoms. Menopause. 2009;16:671–679. doi: 10.1097/gme.0b013e3181957377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe H, Deckersbach T, Lin NU, Makris N, Skaar TC, Rauch SL, Hall JE. Metabolic activity in the insular cortex and hypothalamus predicts hot flashes: An FDG-PET study. Journal of Clinical Endocrinology & Metabolism. 2012;97:3207–3215. doi: 10.1210/jc.2012-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, Chilcoat HD, Breslau N. Trouble sleeping and anxiety/depression in childhood. Psychiatry Research. 2000;94:93–102. doi: 10.1016/s0165-1781(00)00145-1. [DOI] [PubMed] [Google Scholar]
- Johnson JI, Buchanan KJ, Morris JA, Fobbs AJ., Jr . Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. Interrelation of gyral formation, cytoarchitectural variations, and sensory regions in human insular cortex. Program No 464.9/DD1, 2009, (Online) [Google Scholar]
- Joiner TE. Contagious depression: Existence, specificity to depressed symptoms, and the role of reassurance seeking. Journal of Personality and Social Psychology. 1994;67:287–296. doi: 10.1037//0022-3514.67.2.287. [DOI] [PubMed] [Google Scholar]
- Joiner TE. Depression in its interpersonal context. In: Gotlib IH, Hammen CL, editors. Handbook of depression. New York: Guilford Press; 2002. pp. 295–314. [Google Scholar]
- Joiner TE, Katz J. Contagion of depressive symptoms and mood: Meta-analytic review and explanations from cognitive, behavioral, and interpersonal viewpoints. Clinical Psychology: Science and Practice. 1999;6:149–164. [Google Scholar]
- Jones GE. Perception of visceral sensations: A review of recent findings, methodologies, and future directions. In: Jennings JR, Ackles PK, Coles MGH, editors. Advances in psychophysiology. Vol. 5. London: Jessica Kingsley Publishers; 1994. pp. 55–192. [Google Scholar]
- Jones GE, Jones KR, Rouse CH, Scott DM, Caldwell JA. The effects of body position on the perception of cardiac sensations: An experiment and theoretical implications. Psychophysiology. 1987;24:300–311. doi: 10.1111/j.1469-8986.1987.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Jorm AF. Does old age reduce the risk of anxiety and depression? A review of epidemiological studies across the adult life span. Psychological Medicine. 2000;30:11–22. doi: 10.1017/s0033291799001452. [DOI] [PubMed] [Google Scholar]
- Kamysheva E, Skouteris H, Wertheim EH, Paxton SJ, Milgrom J. A prospective investigation of the relationships among sleep quality, physical symptoms, and depressive symptoms during pregnancy. Journal of Affective Disorders. 2010;123:317–320. doi: 10.1016/j.jad.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Kang Y, Williams LE, Clark M, Gray JR, Bargh JA. Physical temperature effects on trust behavior: The role of insula. Social Cognitive and Affective Neuroscience. 2011;6:507–515. doi: 10.1093/scan/nsq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Hamaguchi T, Itoh M, Yanai K, Fukudo S. Correlation between alexithymia and hypersensitivity to visceral stimulation in human. Pain. 2007;132:252–263. doi: 10.1016/j.pain.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Kano M, Farmer AD, Aziz Q, Giampietro V, Brammer MJ, Williams SC, Coen SJ. Sex differences in brain response to anticipated and experienced visceral pain in healthy subjects. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2013;304:G687–G699. doi: 10.1152/ajpgi.00385.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfhammer HP. Somatic symptoms in depression. Dialogues in Clinical Neuroscience. 2006;8:227–239. doi: 10.31887/DCNS.2006.8.2/hpkapfhammer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavidas MK, Lehrer PM, Vaschillo E, Vaschillo B, Marin H, Buyske S, Hassett A. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback. 2007;32:19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Baier B. Right insula for our sense of limb ownership and self-awareness of actions. Brain Structure and Function. 2010;214:411–417. doi: 10.1007/s00429-010-0250-4. [DOI] [PubMed] [Google Scholar]
- Kaschak MP, Maner JK. Embodiment, evolution, and social cognition: An integrative framework. European Journal of Social Psychology. 2009;39:1236–1244. [Google Scholar]
- Katkin ES. Blood, sweat, and tears: Individual differences in autonomic self-perception. Psychophysiology. 1985;22:125–137. doi: 10.1111/j.1469-8986.1985.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Katkin ES, Morell MA, Goldband S, Bernstein GI, Wise JA. Individual differences in heartbeat discrimination. Psychophysiology. 1982;19:160–166. doi: 10.1111/j.1469-8986.1982.tb02538.x. [DOI] [PubMed] [Google Scholar]
- Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biological Psychiatry. 2003;54:216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- Katz J, Beach SRH, Joiner TE. Contagious depression in dating couples. Journal of Social and Clinical Psychology. 1999;18:1–13. [Google Scholar]
- Kelley KW, O’Connor JC, Lawson MA, Dantzer R, Rodriguez-Zas SL, Cusker RHM. Aging leads to prolonged duration of inflammation-induced depression-like behavior caused by Bacillus Calmette-Guérin. Brain, Behavior, and Immunity. 2013;32:63–69. doi: 10.1016/j.bbi.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biological Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Sex differences in the pathways to major depression: A study of opposite-sex twin pairs. American Journal of Psychiatry. 2014;171:426–435. doi: 10.1176/appi.ajp.2013.13101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Sex differences in the relationship between social support and risk for major depression: A longitudinal study of opposite-sex twin pairs. American Journal of Psychiatry. 2005;162:250–256. doi: 10.1176/appi.ajp.162.2.250. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Krüger S, Mayberg H, Meyer J, McCann S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. The Journal of Neuroscience. 2004;24:6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proceedings of the National Academy of Sciences. 2009;106:10805–10810. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Archives of General Psychiatry. 2006;63:1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Kern MK, Jaradeh S, Arndorfer RC, Jesmanowicz A, Hyde J, Shaker R. Gender differences in cortical representation of rectal distension in healthy humans. American Journal of Physiology: Gastrointestinal & Liver Physiology. 2001;281:G1512–G1523. doi: 10.1152/ajpgi.2001.281.6.G1512. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Towards a unifying neural theory of social cognition. Progress in Brain Research. 2006;156:379–401. doi: 10.1016/S0079-6123(06)56021-2. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nature Reviews Neuroscience. 2010;11:417–428. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Tranel D. Interoceptive awareness declines with age. Psychophysiology. 2009;46:1130–1136. doi: 10.1111/j.1469-8986.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. Measuring depression in a multicultural society: Conceptual issues and research recommendations. Hallym International Journal of Aging. 2010;12:27–46. [Google Scholar]
- King AB, Menon RS, Hachinski V, Cechetto DF. Human forebrain activation by visceral stimuli. Journal of Comparative Neurology. 1999;413:572–582. [PubMed] [Google Scholar]
- Kirchhoff MD, Newsome W. Distributed cognitive agency in virtue epistemology. Philosophical Explorations. 2012;15:165–180. [Google Scholar]
- Kirmayer LJ. Cultural variations in the clinical presentation of depression and anxiety: Implications for diagnosis and treatment. Journal of Clinical Psychiatry. 2001;62:22–28. [PubMed] [Google Scholar]
- Kirmayer LJ, Young A. Culture and somatization: Clinical, epidemiological, and ethnographic perspectives. Psychosomatic Medicine. 1998;60:420–430. doi: 10.1097/00006842-199807000-00006. [DOI] [PubMed] [Google Scholar]
- Kirmil-Gray K, Eaglston JR, Gibson E, Thoresen CE. Sleep disturbance in adolescents: Sleep quality, sleep habits, beliefs about sleep, and daytime functioning. Journal of Youth and Adolescence. 1984;13:375–384. doi: 10.1007/BF02088636. [DOI] [PubMed] [Google Scholar]
- Kleinman A. Culture and depression. New England Journal of Medicine. 2004;351:951–953. doi: 10.1056/NEJMp048078. [DOI] [PubMed] [Google Scholar]
- Kleinman A, Becker AE. “Sociosomatics”: The contributions of anthropology to psychosomatic medicine. Psychosomatic Medicine. 1998;60:389–393. doi: 10.1097/00006842-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Knapp-Kline K, Kline JP. Heart rate, heart rate variability, and heartbeat detection with the method of constant stimuli: Slow and steady wins the race. Biological Psychology. 2005;69:387–396. doi: 10.1016/j.biopsycho.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Knoll JF, Hodapp V. A comparison between two methods for assessing heartbeat perception. Psychophysiology. 1992;29:218–222. doi: 10.1111/j.1469-8986.1992.tb01689.x. [DOI] [PubMed] [Google Scholar]
- Koch A, Pollatos O. Cardiac sensitivity in children: Sex differences and its relationship to parameters of emotional processing. Psychophysiology. 2014;51:932–941. doi: 10.1111/psyp.12233. [DOI] [PubMed] [Google Scholar]
- Kofman O. The role of prenatal stress in the etiology of developmental behavioural disorders. Neuroscience and Biobehavioral Reviews. 2002;26:457–470. doi: 10.1016/s0149-7634(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Kong L, Wu F, Tang Y, Ren L, Kong D, Liu Y, Wang F. Frontal-subcortical volumetric deficits in single episode, medication-naïve depressed patients and the effects of 8 weeks fluoxetine treatment: A VBM-DARTEL Study. PloS ONE. 2014;9:e79055. doi: 10.1371/journal.pone.0079055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp KJ. Mirror, mirror on the wall…Who’s the saddest of them all? Exploring the link between self-objectification and depression in mothers. Dissertation Abstracts International. 2009;70:1947B. (UMI No. 3350608). [Google Scholar]
- Kosel M, Brockmann H, Frick C, Zobel A, Schlaepfer TE. Chronic vagus nerve stimulation for treatment-resistant depression increases regional cerebral blood flow in the dorsolateral prefrontal cortex. Psychiatry Research: Neuroimaging. 2011;191:153–159. doi: 10.1016/j.pscychresns.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: A brain-mapping meta-analysis. Experimental Brain Research. 133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- Kouros CD, Cummings EM. Longitudinal associations between husbands’and wives’ depressive symptoms. Journal of Marriage and Family. 2010;72:135–147. doi: 10.1111/j.1741-3737.2009.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahl SE, Senanayake SS, Pekary AE, Sattin A. Vagus nerve stimulation (VNS) is effective in a rat model of antidepressant action. Journal of Psychiatric Research. 2004;38:237–240. doi: 10.1016/j.jpsychires.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Kraus C, Ganger S, Losak J, Hahn A, Savli M, Kranz GS, Lanzenberger R. Gray matter and intrinsic network changes in the posterior cingulate cortex after selective serotonin reuptake inhibitor intake. NeuroImage. 2014;84:236–244. doi: 10.1016/j.neuroimage.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Kraus T, Hösl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. Journal of Neural Transmission. 2007;114:1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Stein A, van Hartevelt TJ. The functional human neuroanatomy of food pleasure cycles. Physiology & Behavior. 2012;106:307–316. doi: 10.1016/j.physbeh.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Linking molecules to mood: New insight into the biology of depression. American Journal of Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL. Gender differences in the reporting of physical and somatoform symptoms. Psychosomatic Medicine. 1998;60:150–155. doi: 10.1097/00006842-199803000-00006. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kuo Z-Y. The dynamics of behavior development: An epigenetic view. New York: Random House; 1967. [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioral Neuroscience. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplaisir J. Development of sleep patterns in early adolescence. Journal of Sleep Research. 2001;10:59–67. doi: 10.1046/j.1365-2869.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- Lacerda ALT, Keshavan MS, Hardan AY, Yorbik O, Brambilla P, Sassi RB, Soares JC. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biological Psychiatry. 2004;55:353–358. doi: 10.1016/j.biopsych.2003.08.021. [DOI] [PubMed] [Google Scholar]
- LaGory M, Fitzpatrick K. The effects of environmental context on elderly depression. Journal of Aging and Health. 1992;4:459–479. [Google Scholar]
- Lai CH, Wu YT. Frontal-insula gray matter deficits in first-episode medication-naïve patients with major depressive disorder. Journal of Affective Disorders. 2014;160:74–79. doi: 10.1016/j.jad.2013.12.036. [DOI] [PubMed] [Google Scholar]
- Lakin JL, Jefferis VE, Cheng CM, Chartrand TL. The chameleon effect as social glue: Evidence for the evolutionary significance of nonconscious mimicry. Journal of Nonverbal Behavior. 2003;27:145–162. [Google Scholar]
- Lakoff G, Johnson M. Philosophy in the flesh: The embodied mind and its challenge to western thought. Basic Books; 1999. [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Structure and Function. 2010;214:579–591. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Lane RD, Weidenbacher H, Smith R, Fort C, Thayer JF, Allen JJ. Subgenual anterior cingulate cortex activity covariation with cardiac vagal control is altered in depression. Journal of Affective Disorders. 2013;150:565–570. doi: 10.1016/j.jad.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain, Behavior, and Immunity. 2001;15:371–387. doi: 10.1006/brbi.2001.0643. [DOI] [PubMed] [Google Scholar]
- Lathe R. Hormones and the hippocampus. Journal of Endocrinology. 2001;169:205–231. doi: 10.1677/joe.0.1690205. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Yousem H, Arenberg D. Hunger and perception. Journal of Personality. 1953;21:312–328. doi: 10.1111/j.1467-6494.1953.tb01774.x. [DOI] [PubMed] [Google Scholar]
- Le Magnen J. Advances in studies on the physiological control and regulation of food intake. In: Stellar E, Sprague JM, editors. Progress in physiological psychology, Vol. 4. New York: Academic Press; 1971. pp. 203–261. [Google Scholar]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: Evidence from functional neuroimaging. Neuron. 2009;64:431–439. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Lebreton M, Kawa S, d'Arc BF, Daunizeau J, Pessiglione M. Your goal is mine: Unraveling mimetic desires in the human brain. Journal of Neuroscience. 2012;32:7146–7157. doi: 10.1523/JNEUROSCI.4821-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF. Brain peptides and obesity: Pharmacologic treatment. Obesity Research. 1995;3:573S–589S. doi: 10.1002/j.1550-8528.1995.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR. Relationship of spontaneous vagal activity to wakefulness and sleep in the cat. Experimental Neurology. 1972;35:194–210. doi: 10.1016/0014-4886(72)90069-6. [DOI] [PubMed] [Google Scholar]
- Lesser IM. A review of the alexithymia concept. Psychosomatic Medicine. 1981;43:531–543. doi: 10.1097/00006842-198112000-00009. [DOI] [PubMed] [Google Scholar]
- Levine MP, Smolak L. Toward a model of the developmental psychopathology of eating disorders: The example of early adolescence. In: Crowther JH, Tennenbaum DL, Hobfoll SE, Stephens MAP, editors. The etiology of bulimia nervosa: The individual and familial context. Washington, DC: Hemisphere Publishing; 1992. pp. 59–80. [Google Scholar]
- Levine R, Chein I, Murphy G. The relation of the intensity of a need to the amount of perceptual distortion: A preliminary report. The Journal of Psychology. 1942;13:283–293. [Google Scholar]
- Lewinsohn PM, Rohde P, Seeley JR, Fischer SA. Age and depression: Unique and shared effects. Psychology and Aging. 1991;6:247–260. doi: 10.1037//0882-7974.6.2.247. [DOI] [PubMed] [Google Scholar]
- Lewis-Fernández R, Kleinman A. Culture, personality, and psychopathology. Journal of Abnormal Psychology. 1994;103:67–71. doi: 10.1037//0021-843x.103.1.67. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu XY, Owyang C. Serotonin and cholecystokinin synergistically stimulate rat vagal primary afferent neurons. Journal of Physiology. 2004;559:651–662. doi: 10.1113/jphysiol.2004.064816. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lighthall NR, Sakaki M, Vasunilashorn S, Nga L, Somayajula S, Chen EY, Mather M. Gender differences in reward-related decision processing under stress. Social Cognitive and Affective Neuroscience. 2012;7:476–484. doi: 10.1093/scan/nsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ATW, Oei TP, Funder JW. Prolonged foot-shock induced analgesia: Glucocorticoids and non-pituitary opioids are involved. Neuroendocrinology. 1983;37:48–51. doi: 10.1159/000123514. [DOI] [PubMed] [Google Scholar]
- Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, Denton D. Brain responses associated with consciousness of breathlessness (air hunger) Proceedings of the National Academy of Sciences. 2001;98:2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowski ZJ. Somatization: The concept and its clinical application. American Journal of Psychiatry. 1988;145:1358–1368. doi: 10.1176/ajp.145.11.1358. [DOI] [PubMed] [Google Scholar]
- Liu CH, Jing B, Ma X, Xu PF, Zhang Y, Li F, Wang CY. Voxel-based morphometry study of the insular cortex in female patients with current and remitted depression. Neuroscience. 2014;262:190–199. doi: 10.1016/j.neuroscience.2013.12.058. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xu C, Xu Y, Wang Y, Zhao B, Lv Y, Du C. Decreased regional homogeneity in insula and cerebellum: a resting-state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Research: Neuroimaging. 2010;182:211–215. doi: 10.1016/j.pscychresns.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock M. Menopause in cultural context. Experimental Gerontology. 1994;29:307–317. doi: 10.1016/0531-5565(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Loewenstein G. Out of control: Visceral influences on behavior. Organizational Behavior and Human Decision Processes. 1996;65:272–292. [Google Scholar]
- Loewenstein G. Hot-cold empathy gaps in medical decision-making. Health Psychology. 2005;24:S49–S56. doi: 10.1037/0278-6133.24.4.S49. [DOI] [PubMed] [Google Scholar]
- Lomarev M, Denslow S, Nahas Z, Chae JH, George MS, Bohning DE. Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. Journal of Psychiatric Research. 2002;36:219–227. doi: 10.1016/s0022-3956(02)00013-4. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lyte M, Li M, Opitz N, Gaykema RPA, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiology & Behavior. 2006;89:350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Tkach C. The consequences of dysphoric rumination. In: Papageorgiou C, Wells A, editors. Depressive rumination: Nature, theory, and treatment. Chichester, UK: Wiley; 2003. [Google Scholar]
- Ma-Kellams C, Blascovich J, McCall C. Culture and the body: East–West differences in visceral perception. Journal of Personality and Social Psychology. 2012;102:718–728. doi: 10.1037/a0027010. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maister L, Tsakiris M. My face, my heart: Cultural differences in integrated bodily self-awareness. Cognitive Neuroscience. 2014;5:10–16. doi: 10.1080/17588928.2013.808613. [DOI] [PubMed] [Google Scholar]
- Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, Sorg C. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Frontiers in Human Neuroscience. 2014;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Marks PA, Monroe LJ. Correlates of adolescent poor sleepers. Journal of Abnormal Psychology. 1976;85:243–246. doi: 10.1037//0021-843x.85.2.243. [DOI] [PubMed] [Google Scholar]
- Marshall GD, Zimbardo PG. Affective consequences of inadequately explained physiological arousal. Journal of Personality and Social Psychology. 1979;37:970–988. [Google Scholar]
- Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosomatic Medicine. 2007;69:709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- Martin LA, Neighbors HW, Griffith DM. The experience of symptoms of depression in men vs women: Analysis of the National Comorbidity Survey Replication. JAMA psychiatry. 2013;70:1100–1106. doi: 10.1001/jamapsychiatry.2013.1985. [DOI] [PubMed] [Google Scholar]
- Marvel FA, Chen CC, Badr N, Gaykema R, Goehler LE. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain, Behavior, and Immunity. 2004;18:123–134. doi: 10.1016/j.bbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Mather M. The emotion paradox in the aging brain. Annals of the New York Academy of Sciences. 2012;1251:33–49. doi: 10.1111/j.1749-6632.2012.06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias E, Schandry R, Duschek S, Pollatos O. On the relationship between interoceptive awareness and the attentional processing of visual stimuli. International Journal of Psychophysiology. 2009;72:154–159. doi: 10.1016/j.ijpsycho.2008.12.001. [DOI] [PubMed] [Google Scholar]
- May AC, Stewart JL, Tapert SF, Paulus MP. The effect of age on neural processing of pleasant soft touch stimuli. Frontiers in Behavioral Neuroscience. 2014;8:52. doi: 10.3389/fnbeh.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Fox PT. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. The Journal of Clinical Investigation. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Arturo SJ, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, Jerabek PA. The functional neuroanatomy of the placebo effect. American Journal of Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biological Psychiatry. 2009;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayer EA. Gut feelings: The emerging biology of gut–brain communication. Nature Reviews Neuroscience. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annual Review of Medicine. 2011;62:381–396. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Naliboff B, Munakata J. The evolving neurobiology of gut feelings. Progress in Brain Research. 1999;122:195–206. doi: 10.1016/s0079-6123(08)62139-1. [DOI] [PubMed] [Google Scholar]
- Mazzola V, Latorre V, Petito A, Gentili N, Fazio L, Popolizio T, Bondolfi G. Affective response to a loved one’s pain: Insula activity as a function of individual differences. PLoS One. 2010;5:e15268. doi: 10.1371/journal.pone.0015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanic D. Social psychological factors affecting the presentation of bodily complaints. New England Journal of Medicine. 1972;286:1132–1139. doi: 10.1056/NEJM197205252862105. [DOI] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response. Brain Structure and Function. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina A, Beadle JN, Paradiso S. Towards a classification of alexithymia: Primary, secondary and organic. Journal of Psychopathology. 2014;20:38–49. [Google Scholar]
- McCauley E, Carlson GA, Calderon R. The role of somatic complaints in the diagnosis of depression in children and adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1991;30:631–635. doi: 10.1097/00004583-199107000-00016. [DOI] [PubMed] [Google Scholar]
- McFarland RA. Heart rate perception and heart rate control. Psychophysiology. 1975;12:402–405. doi: 10.1111/j.1469-8986.1975.tb00011.x. [DOI] [PubMed] [Google Scholar]
- McIntosh DN, Reichmann-Decker A, Winkielman P, Wilbarger JL. When the social mirror breaks: Deficits in automatic, but not voluntary, mimicry of emotional facial expressions in autism. Developmental Science. 2006;9:295–302. doi: 10.1111/j.1467-7687.2006.00492.x. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Stenstrom RJ, Taylor D, Stokes KA, Edson N. State-dependent learning following electrical stimulation of the hippocampus: Intact and split-brain rats. Physiology & Behavior. 1985;34:133–139. doi: 10.1016/0031-9384(85)90091-5. [DOI] [PubMed] [Google Scholar]
- McKernan DP, Fitzgerald P, Dinan TG, Cryan JF. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterology & Motility. 2010;22:1029-e268. doi: 10.1111/j.1365-2982.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- Mehling WE, Gopisetty V, Daubenmier J, Price CJ, Hecht FM, Stewart A. Body awareness: Construct and self-report measures. PLoS ONE. 2009;4:e5614. doi: 10.1371/journal.pone.0005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB. Weakened links between mind and body in older age: The case for maturational dualism in the experience of emotion. Emotion Review. 2010;2:240–244. [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. British Journal of Nutrition. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Micali S. The transformation of intercorporeality in melancholia. Phenomenology and the Cognitive Sciences. 2011:1–20. [Google Scholar]
- Michalak J, Burg J, Heidenreich T. Mindfulness, embodiment, and depression. In: Koch SC, Fuchs T, Summa M, Müller C, editors. Body Memory, Metaphor and Movement. Amsterdam, The Netherlands: John Benjamins Publication Company; 2012. pp. 393–416. [Google Scholar]
- Miller LC, Murphy R, Buss AH. Consciousness of body: Private and public. Journal of Personality and Social Psychology. 1981;41:397–406. [Google Scholar]
- Mistlberger RE, Skene DJ. Social influences on mammalian circadianrhythms: Animal and human studies. Biological Reviews. 2004;79:533–556. doi: 10.1017/s1464793103006353. [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Mantonakis L, Chalarakis N. Nocebo in clinical trials for depression: A meta-analysis. Psychiatry Research. 2014;215:82–86. doi: 10.1016/j.psychres.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Kumari V, Malhi GS, Brown RG, Giampietro VP, Brammer MJ, Sharma T. Neural response to pleasant stimuli in anhedonia: An fMRI study. NeuroReport. 2003;14:177–182. doi: 10.1097/00001756-200302100-00003. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Hyare H, Lee S. Selective attention to food-related stimuli in hunger: Are attentional biases specific to emotional and psychopathological states, or are they also found in normal drive states? Behaviour Research and Therapy. 1998;36:227–237. doi: 10.1016/s0005-7967(97)00062-4. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neuroscience & Biobehavioral Reviews. 2012;36:341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Negreira A, Weierich M, Dautoff R, Dickerson BC, Wright CI, Barrett LF. Differential hemodynamic response in affective circuitry with aging: An FMRI study of novelty, valence, and arousal. Journal of Cognitive Neuroscience. 2011;23:1027–1041. doi: 10.1162/jocn.2010.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Dolan RJ. Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. Journal of Neuroscience. 2001;21:5304–5310. doi: 10.1523/JNEUROSCI.21-14-05304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DN, McGee R, Stanton WR. Sleep problems in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:94–99. doi: 10.1097/00004583-199201000-00014. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2006;291:R257–R267. doi: 10.1152/ajpregu.00084.2006. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, Ernst M. Early-life stress is associated with impairment in cognitive control in adolescence: An fMRI study. Neuropsychologia. 2010;48:3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6:1231–1248. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Mussgay L, Klinkenberg N, Rüddel H. Heart beat perception in patients with depressive, somatoform, and personality disorders. Journal of Psychophysiology. 1999;13:27–36. [Google Scholar]
- Must A, Szabo Z, Bodi N, Szasz A, Janka Z, Keri S. Sensitivity to reward and punishment and the prefrontal cortex in major depression. Journal of Affective Disorders. 2006;90:209–215. doi: 10.1016/j.jad.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Ball T, Wankerl J, Strigo IA. Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neuroscience Letters. 2012;520:204. doi: 10.1016/j.neulet.2012.03.095. [DOI] [PubMed] [Google Scholar]
- Nahab FB, Hattori N, Saad ZS, Hallett M. Contagious yawning and the frontal lobe: An fMRI study. Human Brain Mapping. 2009;30:1744–1751. doi: 10.1002/hbm.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahas Z, Burns C, Foust MJ, Short B, Herbsman T, George MS. Vagus nerve stimulation (VNS) for depression: What do we know now and what should be done next? Current Psychiatry Reports. 2006;8:445–451. doi: 10.1007/s11920-006-0049-4. [DOI] [PubMed] [Google Scholar]
- Nahas Z, Teneback C, Chae JH, Mu Q, Molnar C, Kozel FA, George MS. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology. 2007;32:1649–1660. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Näring GWB, Van der Staak CPF. Perception of heart rate and blood pressure: the role of alexithymia and anxiety. Psychotherapy and Psychosomatics. 1995;63:193–200. doi: 10.1159/000288959. [DOI] [PubMed] [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterology & Motility. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. The problem of the frontal lobe: A reinterpretation. Journal of Psychiatry Research. 1971;8:167–187. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology & Motility. 2011;23:255–265. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- Neumann R, Strack F. 'Mood contagion’: The automatic transfer of mood between persons. Journal of Personality and Social Psychology. 2000;79:211–223. doi: 10.1037//0022-3514.79.2.211. [DOI] [PubMed] [Google Scholar]
- Nirupa G, Workman JL, Lee TT, Innala L, Viau V. Sex differences in the HPA axis. Comprehensive Physiology. 2014 doi: 10.1002/cphy.c130054. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Emotion regulation and psychopathology: The role of gender. Annual Review of Clinical Psychology. 2012;8:161–187. doi: 10.1146/annurev-clinpsy-032511-143109. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Aldao A. Gender and age differences in emotion regulation strategies and their relationship to depressive symptoms. Personality and Individual Differences. 2011;51:704–708. [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychological Bulletin. 1994;115:424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Nordgren LF, van der Pligt J, van Harreveld F. Visceral drives in retrospect: Explanations about the inaccessible past. Psychological Science. 2006;17:635–640. doi: 10.1111/j.1467-9280.2006.01756.x. [DOI] [PubMed] [Google Scholar]
- Nordgren LF, van der Pligt J, van Harreveld F. Evaluating eve: Visceral states influence the evaluation of impulsive behavior. Journal of Personality and Social Psychology. 2007;93:75–84. doi: 10.1037/0022-3514.93.1.75. [DOI] [PubMed] [Google Scholar]
- Northoff G, Wiebking C, Feinberg T, &Panksepp J. The ‘resting-state hypothesis’ of major depressive disorder--A translational subcortical–cortical framework for a system disorder. Neuroscience & Biobehavioral Reviews. 2011;35:1929–1945. doi: 10.1016/j.neubiorev.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Nowakowski S, Meliska CJ, Martinez LF, Parry BL. Sleep and menopause. Current Neurology and Neuroscience Reports. 2009;9:165–172. doi: 10.1007/s11910-009-0025-6. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VS. The simulating social mind: The role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychological Bulletin. 2007;133:310–327. doi: 10.1037/0033-2909.133.2.310. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Winkielman P, Ramachandran VS. Slow echo: Facial EMG evidence for the delay of spontaneous, but not voluntary, emotional mimicry in children with autism spectrum disorders. Developmental Science. 2009;12:510–520. doi: 10.1111/j.1467-7687.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- Olausson H, Charron J, Marchand S, Villemure C, Strigo IA, Bushnell MC. Feelings of warmth correlate with neural activity in right anterior insular cortex. Neuroscience Letters. 2005;389:1–5. doi: 10.1016/j.neulet.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Olin B, Jayewardene AK, Bunker M, Moreno F. Mortality and suicide risk in treatment-resistant depression: An observational study of the long-term impact of intervention. PloS ONE. 2012;7:e48002. doi: 10.1371/journal.pone.0048002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onrust SA, Cuijpers P. Mood and anxiety disorders in widowhood: A systematic review. Aging & Mental Health. 2006;10:327–334. doi: 10.1080/13607860600638529. [DOI] [PubMed] [Google Scholar]
- Owen L, Reinders M, Narramore R, Marsh AM, Gar Lui F, Baron R, Corfe BM. A double blind, placebo controlled, randomised pilot trial examining the effects of probiotic administration on mood and cognitive function. Proceedings of the Nutrition Society. 2014;73:E29. [Google Scholar]
- Padoa-Schioppa C, Cai X. The orbitofrontal cortex and the computation of subjective value: Consolidated concepts and new perspectives. Annals of the New York Academy of Sciences. 2011;1239:130–137. doi: 10.1111/j.1749-6632.2011.06262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Sheikh SA, Schwindt GC, Lee JT, Kuskowski MA, Surerus C, Rittberg BR. Chronic vagus nerve stimulation for treatment-resistant depression decreases resting ventromedial prefrontal glucose metabolism. NeuroImage. 2008;42:879–889. doi: 10.1016/j.neuroimage.2008.04.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G, Van Bavel JJ, Vasey MW, Egan E, Thayer JF. From the heart to the mind's eye: Cardiac vagal tone is related to visual perception of fearful faces at high spatial frequency. Biological Psychology. 2012;90:171–178. doi: 10.1016/j.biopsycho.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Park G, Van Bavel JJ, Vasey MW, Thayer JF. Cardiac vagal tone predicts attentional engagement to and disengagement from fearful faces. Emotion. 2013;13:645–656. doi: 10.1037/a0032971. [DOI] [PubMed] [Google Scholar]
- Patriquin MA, Lorenzi J, Scarpa A, Bell MA. Developmental trajectories of respiratory sinus arrhythmia: Associations with social responsiveness. Developmental Psychobiology. 2014;56:317–326. doi: 10.1002/dev.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC. The spectrum of eating disorder in adolescence. Journal of Psychosomatic Research. 1988;32:579–584. doi: 10.1016/0022-3999(88)90006-2. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Decision making dysfunctions in psychiatry—altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure and Function. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacology Biochemistry and Behavior. 2009;94:1–7. doi: 10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Otaky N, Caramanos Z, Macdonald D, Zijdenbos A, d'Avirro D, Evans AC. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: Hemispheric asymmetries, gender differences and probability maps. Journal of Comparative Neurology. 1996;376:664–673. doi: 10.1002/(SICI)1096-9861(19961223)376:4<664::AID-CNE12>3.0.CO;2-M. doi:10.1002/(SICI)1096-9861(19961223)376: 4<664::AID-CNE12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Paykel ES. Depression and appetite. Journal of Psychosomatic Research. 1977;21:401–407. doi: 10.1016/0022-3999(77)90049-6. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Emms EM, Fletcher J, Rassaby ES. Life events and social support in puerperal depression. British Journal of Psychiatry. 1980;136:339–346. doi: 10.1192/bjp.136.4.339. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: An important outcome in depression. Psychological Medicine. 1995;25:1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Hamilton R. The metamodal organization of the brain. Progress in Brain Research. 2001;134:427–445. doi: 10.1016/s0079-6123(01)34028-1. [DOI] [PubMed] [Google Scholar]
- Peñaloza-Rojas J, Barrera-Mera B, Kubli-Garfias C. Behavioral and brain electrical changes after vagal stimulation. Experimental Neurology. 1969;23:378–383. doi: 10.1016/0014-4886(69)90085-5. [DOI] [PubMed] [Google Scholar]
- Penfield W, Faulk ME., Jr The insula further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- Peng J, Liu J, Nie B, Li Y, Shan B, Wang G, Li K. Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. European Journal of Radiology. 2011;80:395–399. doi: 10.1016/j.ejrad.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW. The psychology of physical symptoms. New York: Springer-Verlag; 1982. [Google Scholar]
- Pennebaker JW. Beyond laboratory-based cardiac perception: Ecological interoception. In: Vaitl D, Schandry R, editors. From the Heart to the Brain: Psychophysiology of Circulation-Brain Interaction. New York: Peter Lang; 1995. pp. 389–406. [Google Scholar]
- Pennebaker JW, Lightner JM. Competition of internal and external information in an exercise setting. Journal of Personality and Social Psychology. 1980;39:165–174. doi: 10.1037//0022-3514.39.1.165. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Hoover CW. Visceral perception versus visceral detection: Disentangling methods and assumptions. Biofeedback and Self-Regulation. 1984;9:339–352. doi: 10.1007/BF00998977. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Roberts T-A. Toward a his and hers theory of emotion: Gender differences in visceral perception. Journal of Social and Clinical Psychology. 1992;11:199–212. [Google Scholar]
- Pennebaker JW, Watson D. Blood pressure estimation and beliefs among normotensives andhypertensives. Health Psychology. 1988;7:309–328. doi: 10.1037//0278-6133.7.4.309. [DOI] [PubMed] [Google Scholar]
- Perez-Burgos A, Wang B, Mao YK, Mistry B, Neufeld KAM, Bienenstock J, Kunze W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2013;304:G211–G220. doi: 10.1152/ajpgi.00128.2012. [DOI] [PubMed] [Google Scholar]
- Persinger MA. Shifting gustatory thresholds and food cravings during pregnancy as expanding uterine-induced steady potential shifts within the insula: An hypothesis. Perceptual and Motor Skills. 2001;92:50–52. doi: 10.2466/pms.2001.92.1.50. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. Neural representations of subjective reward value. Behavioural Brain Research. 2010;213:135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Pettit JW, Joiner TE. Chronic depression: Interpersonal sources, therapeutic solutions. Washington, D.C.: American Psychological Association; 2006. [Google Scholar]
- Piech RM, Pastorino MT, Zald DH. All I saw was the cake. Hunger effects on attentional capture by visual food cues. Appetite. 2010;54:579–582. doi: 10.1016/j.appet.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PS, Rawlins JNP. Dissociating pain from itsanticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R. Emotional processing and emotional memory are modulated by interoceptive awareness. Cognition and Emotion. 2008;22:272–287. [Google Scholar]
- Pollatos O, Kirsch W, Schandry R. On the relationship between interoceptive awareness, emotional experience, and brain processes. Cognitive Brain Research. 2005;25:948–962. doi: 10.1016/j.cogbrainres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping. 2007;28:9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Traut-Mattausch E, Schandry R. Differential effects of anxiety and depression on interoceptive accuracy. Depression and Anxiety. 2009;26:167–173. doi: 10.1002/da.20504. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Research. 2007;1141:178–187. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schübo A, Herbert BM, Matthias E, Schandry R. Deficits in early emotional reactivity in alexithymia. Psychophysiology. 2008;45:839–846. doi: 10.1111/j.1469-8986.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- Pollitt JD. Suggestions for a physiological classification of depression. British Journal of Psychiatry. 1965;111:489–495. [Google Scholar]
- Porges SW. Body perception questionnaire. Laboratory of Developmental Assessment, University of Maryland. 1993 [Google Scholar]
- Porges SW. Cardiac vagal tone: A physiological index of stress. Neuroscience and Biobehavioral Reviews. 1995;19:225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Porges SW. Emotion: An evolutionary by-product of the neural regulation of the autonomic nervous system. Annals of the New York Academy of Sciences. 1997;807:62–77. doi: 10.1111/j.1749-6632.1997.tb51913.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: New insights into adaptive reactions of the autonomic nervous system. Cleveland Clinic Journal of Medicine. 2009;76:S86–S90. doi: 10.3949/ccjm.76.s2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication, and self-regulation. W. W. Norton & Company; 2011. [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development. 1994;59:167–186. [PubMed] [Google Scholar]
- Portella MJ, de Diego-Adeliño J, Gómez-Ansón B, Morgan-Ferrando R, Vives Y, Puigdemont D, Pérez V. Ventromedial prefrontal spectroscopic abnormalities over the course of depression: A comparison among first episode, remitted recurrent and chronic patients. Journal of Psychiatric Research. 2011;45:427–434. doi: 10.1016/j.jpsychires.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Musings on the wanderer: What’s new in our understanding of vago-vagal reflexes? I. Morphology and topography of vagal afferents innervating the GI tract. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2002;283:G1217–G1225. doi: 10.1152/ajpgi.00249.2002. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Lupien S. Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Pujol J, López A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T. Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. NeuroImage. 2002;15:847–855. doi: 10.1006/nimg.2001.1004. [DOI] [PubMed] [Google Scholar]
- Radel R, Clément-Guillotin C. Evidence of motivational influences in early visual perception hunger modulates conscious access. Psychological science. 2012;23:232–234. doi: 10.1177/0956797611427920. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Malaise, melancholia and madness: The evolutionary legacy of an inflammatory bias. Brain, Behavior, and Immunity. 2013;31:1–8. doi: 10.1016/j.bbi.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola I, Goodyear B. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Frontiers in Psychiatry. 2014;5:17. doi: 10.3389/fpsyt.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, Logan AC. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathogens. 2009;1:1–6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JP, Price JL. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1993;337:1–31. doi: 10.1002/cne.903370102. [DOI] [PubMed] [Google Scholar]
- Reekie YL, Braesicke K, Man MS, Roberts AC. Uncoupling of behavioral and autonomic responses after lesions of the primate orbitofrontal cortex. Proceedings of the National Academy of Sciences. 2008;105:9787–9792. doi: 10.1073/pnas.0800417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Reisenzein R. The Schachter theory of emotion: Two decades later. Psychological Bulletin. 1983;94:239–264. [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depression and anxiety. 2000;12:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. doi:10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Reynard A, Gevirtz R, Berlow R, Brown M, Boutelle K. Heart rate variability as a marker of self-regulation. Applied Psychophysiology and Biofeedback. 2011;36:209–215. doi: 10.1007/s10484-011-9162-1. [DOI] [PubMed] [Google Scholar]
- Rice SM, Fallon BJ, Aucote HM, Möller-Leimkühler A, Treeby MS, Amminger GP. Longitudinal sex differences of externalising and internalising depression symptom trajectories: Implications for assessment of depression in men from an online study. International Journal of Social Psychiatry. doi: 10.1177/0020764014540149. in press. [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Donovan M, Giacobbe P, Placenza F, Rotzinger S, Kennedy SH. Neurostimulation therapies for treatment resistant depression: A focus on vagus nerve stimulation and deep brain stimulation. International Review of Psychiatry. 2011;23:424–436. doi: 10.3109/09540261.2011.630993. [DOI] [PubMed] [Google Scholar]
- Roberts AC. Primate orbitofrontal cortex and adaptive behaviour. Trends in Cognitive Sciences. 2006;10:83–90. doi: 10.1016/j.tics.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ. Does growing old increase the risk for depression? American Journal of Psychiatry. 1997;154:1384–1390. doi: 10.1176/ajp.154.10.1384. [DOI] [PubMed] [Google Scholar]
- Roberts TA, Pennebaker JW. Gender differences in perceiving internal state: Toward a his-and-hers model of perceptual cue use. Advances in Experimental Social Psychology. 1994;27:143–175. [Google Scholar]
- Rocca J. Galen on the brain: Anatomical knowledge and physiological speculation in the second century. A. D. Leiden/Boston: Brill Academic Publishers; 2003. [PubMed] [Google Scholar]
- Rogers PJ, Hill AJ. Breakdown of dietary restraint following mere exposure to food stimuli: Interrelationships between restraint, hunger, salivation, and food intake. Addictive Behaviors. 1989;14:387–397. doi: 10.1016/0306-4603(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Sienkiewicz ZJ, Yaxley S. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. European Journal of Neuroscience. 1989;1:53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rosow I. The social context of the aging self. The Gerontologist. 1973;13:82–87. [Google Scholar]
- Ross LE, Murray BJ, Steiner M. Sleep and perinatal mood disorders: A critical review. Journal of Psychiatry and Neuroscience. 2005;30:247–256. [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44:450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Rouse CH, Jones GE, Jones KR. The effect of body composition and gender on cardiac awareness. Psychophysiology. 1988;25:400–407. doi: 10.1111/j.1469-8986.1988.tb01876.x. [DOI] [PubMed] [Google Scholar]
- Rush AJ, George MS, Sackeim HM, Marangell LB, Husain MM, Giller C, Goodman R. Vagus nerve stimulation (VNS) for treatment-resistant depressions: A multicenter study. Biological Psychiatry. 2000;47:276–286. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, Cooke RG. Vagus nerve stimulation for treatment-resistant depression: A randomized, controlled acute phase trial. Biological Psychiatry. 2005;58:347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychological Review. 2003;110:145–172. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- Ryan ND, Puig-Antich J, Ambrosini P, Rabinovich H, Robinson D, Nelson B, Twomey J. The clinical picture of major depression in children and adolescents. Archives of General Psychiatry. 1987;44:854–861. doi: 10.1001/archpsyc.1987.01800220016003. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Keilp JG, Rush AJ, George MS, Marangell LB, Dormer JS, Zboyan H. The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2001;14:53–62. [PubMed] [Google Scholar]
- Sala M, Perez J, Soloff P, Ucelli di Nemi S, Caverzasi E, Soares JC, Brambilla P. Stress and hippocampal abnormalities in psychiatric disorders. European Neuropsychopharmacology. 2004;14:393–405. doi: 10.1016/j.euroneuro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Salmaso N, Cossette MP, Woodside B. Pregnancy and maternal behavior induce changes in glia, glutamate and its metabolism within the cingulate cortex. PLoS ONE. 2011;6:e23529. doi: 10.1371/journal.pone.0023529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford RN. The effects of abstinence from food upon imaginal processes: A preliminary experiment. The Journal of Psychology. 1936;2:129–136. [Google Scholar]
- Sanford RN. The effects of abstinence from food upon imaginal processes: A further experiment. The Journal of Psychology. 1937;3:145–159. [Google Scholar]
- Sapolsky RM. Neuroendocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D, editors. Behavioral endocrinology. Cambridge, Massachusetts: The MIT Press; 1992. pp. 287–324. [Google Scholar]
- Sapolsky RM. The physiology and pathophysiology of unhappiness. In: Kahneman D, Diener E, Schwartz N, editors. Well-being: the foundations of hedonic psychology. New York: Russell Sage Foundation; 1999. pp. 453–469. [Google Scholar]
- Schachter S. The psychology of affiliation. Stanford, CA: Stanford University Press; 1959. [Google Scholar]
- Schachter S, Singer JE. Cognitive, social, and physiological determinants of emotional state. Psychological Review. 1962;69:379–399. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Schachter S, Goldman R, Gordon A. Effects of fear, food deprivation, and obesity on eating. Journal of Personality and Social Psychology. 1968;10:91–97. doi: 10.1037/h0026284. [DOI] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18:483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Schandry R, Sparrer R, Weitkunat B. From the heart to the brain: A study of heartbeat contingent scalp potentials. International Journal of Neuroscience. 1986;30:261–275. doi: 10.3109/00207458608985677. [DOI] [PubMed] [Google Scholar]
- Schedlowski M, Engler H, Grigoleit JS. Endotoxin-induced experimental systemic inflammation in humans: A model to disentangle immune-to-brain communication. Brain, Behavior, and Immunity. 2014;35:1–8. doi: 10.1016/j.bbi.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Frick C, Zobel A, Maier W, Heuser I, Bajbouj M, Hasdemir M. Vagus nerve stimulation for depression: Efficacy and safety in a European study. Psychological Medicine. 2008;38:651–662. doi: 10.1017/S0033291707001924. [DOI] [PubMed] [Google Scholar]
- Schulz A, Lass-Hennemann J, Sütterlin S, Schächinger H, Vögele C. Cold pressor stress induces opposite effects on cardioceptive accuracy dependent on assessment paradigm. Biological Psychology. 2013;93:167–174. doi: 10.1016/j.biopsycho.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Schuyler D. The depressive spectrum. New York: Aronson; 1974. [Google Scholar]
- Scott KM, Von Korff M, Alonso J, Angermeyer M, Bromet EJ, Bruffaerts R, Williams D. Age patterns in the prevalence of DSM-IV depressive/anxiety disorders with and without physical co-morbidity. Psychological Medicine. 2008;38:1659–1669. doi: 10.1017/S0033291708003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeño L, Couto B, Melloni M, Canales-Johnson A, Yoris A, Baez S, Ibanez A. How do you feel when you can't feel your body? Interoception, functional connectivity and emotional processing in depersonalization-derealization disorder. PloS ONE. 2014;9:e98769. doi: 10.1371/journal.pone.0098769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Singer B, Wilkinson CW, McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26:225–240. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Segrin C. Social skills deficits associatedwith depression. Clinical Psychology Review. 2000;20:379–403. doi: 10.1016/s0272-7358(98)00104-4. [DOI] [PubMed] [Google Scholar]
- Seth AK. Interoceptive inference, emotion, and the embodied self. Trends in Cognitive Sciences. 2013;17:565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Frontiers in Psychology. 2012;2:1–16. doi: 10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cognitive and Behavioral Neurology. 2005;18:55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- Shaw ME. Adolescent breakfast skipping: An Australian study. Adolescence. 1998;33:851–861. [PubMed] [Google Scholar]
- Sheline Y, Wang P, Gado M, Csernansky J, Vannier M. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SA, Mallory ME, Simon A. The body awareness questionnaire: Reliability and validity. Journal of Personality Assessment. 1989;53:802–815. [Google Scholar]
- Sidis B, Goodhart SP. Multiple personality. D. Appleton; 1905. [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: An fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behavioral Brain Research. 2009;198:149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Siepmann M, Aykac V, Unterdörfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Applied Psychophysiology and Biofeedback. 2008;33:195–201. doi: 10.1007/s10484-008-9064-z. [DOI] [PubMed] [Google Scholar]
- Sierra M, Berrios GE. Depersonalization: A conceptual history. History of Psychiatry. 1997;8:213–229. doi: 10.1177/0957154X9700803002. [DOI] [PubMed] [Google Scholar]
- Silk DBA, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Alimentary Pharmacology & Therapeutics. 2008;29:508–518. doi: 10.1111/j.1365-2036.2008.03911.x. [DOI] [PubMed] [Google Scholar]
- Silverstein B. Gender differences in the prevalence of somatic versus pure depression: A replication. American Journal of Psychiatry. 2002;159:1051–1052. doi: 10.1176/appi.ajp.159.6.1051. [DOI] [PubMed] [Google Scholar]
- Silverstein B, Edwards T, Gamma A, Ajdacic-Gross V, Rossler W, Angst J. The role played by depression associated with somatic symptomatology in accounting for the gender difference in the prevalence of depression. Social Psychiatry and Psychiatric Epidemiology. 2013;48:257–263. doi: 10.1007/s00127-012-0540-7. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Arce E, Lovero KL, Stein MB, Paulus MP. Subchronic SSRI administration reduces insula response during affective anticipation in healthy volunteers. International Journal of Neuropsychopharmacology. 2009;12:1009–1020. doi: 10.1017/S1461145709990149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, VonKorff M, Piccinelli M, Fullerton C, Ormel J. An international study of the relation between somatic symptoms and depression. New England Journal of Medicine. 1999;341:1329–1335. doi: 10.1056/NEJM199910283411801. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Skaf CR, Yamada A, Garrido GEJ, Buchpiguel CA, Akamine S, Castro CC, Busatto JF. Psychotic symptoms in major depressive disorder are associated with reduced regional cerebral blood flow in the subgenual anterior cingulate cortex: A voxel-based single photon emission computed tomography (SPECT) study. Journal of Affective Disorders. 2002;68:295–305. doi: 10.1016/s0165-0327(00)00365-7. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: A psychobiological model of social rejection and depression. Neuroscience & Biobehavioral Reviews. 2010;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliz D, Hayley S. Major depressive disorder and alterations in insular cortical activity: A review of current functional magnetic imaging research. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM. Taste representation in the human insula. Brain Structure and Function. 2010;214:551–561. doi: 10.1007/s00429-010-0266-9. [DOI] [PubMed] [Google Scholar]
- Small DM, Bender G, Veldhuizen MG, Rudenga K, Nachtigal D, Felsted J. The role of the human orbitofrontal cortex in taste and flavor processing. Annals of the New York Academy of Sciences. 2007;1121:136–151. doi: 10.1196/annals.1401.002. [DOI] [PubMed] [Google Scholar]
- Smith ER. Social relationships and groups: New insights on embodied and distributed cognition. Cognitive Systems Research. 2008;9:24–32. [Google Scholar]
- Smith ER, Semin GR. Situated social cognition. Current Directions in Psychological Science. 2007;16:132–135. doi: 10.1111/j.1467-8721.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Steiner H. Psychopathology in anorexia nervosa and depression. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31:841–843. doi: 10.1097/00004583-199209000-00009. [DOI] [PubMed] [Google Scholar]
- Smith TW, Greenberg J. Depression and self-focused attention. Motivation and Emotion. 1981;5:323–331. [Google Scholar]
- Smith VC. Perception and estimation of blood pressure fluctuations in natural settings. Dallas, Texas: Southern Methodist University; 1986. (Unpublished Master’s thesis. [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Soriano-Mas C, Hernández-Ribas R, Pujol J, Urretavizcaya M, Deus J, Harrison BJ, Cardoner M. Cross-sectional and longitudinal assessment of structural brain alterations in melancholic depression. Biological Psychiatry. 2011;69:318–325. doi: 10.1016/j.biopsych.2010.07.029. [DOI] [PubMed] [Google Scholar]
- Speranza G, Verlato G, Albiero A. Autonomic changes during pregnancy: Assessment by spectral heart rate variability analysis. Journal of Electrocardiology. 1998;31:101–110. doi: 10.1016/s0022-0736(98)90040-1. [DOI] [PubMed] [Google Scholar]
- Sperling W, Biermann T, Spannenberger R, Clepce M, Padberg F, Reulbach U, Thuerauf N. Changes in gustatory perceptions of patients with major depression treated with vagus nerve stimulation (VNS) Pharmacopsychiatry. 2011;44:67–71. doi: 10.1055/s-0030-1268427. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Steele JD, Mwangi B, Kumar P, Christmas D, Milders M, Matthews K. The insular cortex and the neuroanatomy of major depression. Journal of Affective Disorders. 2011;133:120–127. doi: 10.1016/j.jad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation duringemotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: From menarche to menopause and beyond. Journal of Affective Disorders. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The “other” circadian system: Food as a zeitgeber. Journal of Biological Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behavior, and Immunity. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Stevens S, Gerlach AL, Cludius B, Silkens A, Craske MG, Hermann C. Heartbeat perception in social anxiety before and during speech anticipation. Behavior Research and Therapy. 2011;49:138–143. doi: 10.1016/j.brat.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Stice E, Bearman SK. Body-image and eating disturbances prospectively predict increases in depressive symptoms in adolescent girls: a growth curve analysis. Developmenta l Psychology. 2001;37:597–607. doi: 10.1037//0012-1649.37.5.597. [DOI] [PubMed] [Google Scholar]
- Stice E, Ragan J, Randall P. Prospective relations between social support and depression: Differential direction of effects for parent and peer support? Journal of Abnormal Psychology. 2004;113:155–159. doi: 10.1037/0021-843X.113.1.155. [DOI] [PubMed] [Google Scholar]
- Stice E, Hayward C, Cameron RP, Killen JD, Taylor CB. Body-image and eating disturbances predict onset of depression among female adolescents: a longitudinal study. Journal of Abnormal Psychology. 2000;109:438–444. [PubMed] [Google Scholar]
- Stokes D. Perceiving and desiring: A new look at the cognitive penetrability of experience. Philosophical Studies. 2012;158:477–492. [Google Scholar]
- Strack S, Coyne JC. Social confirmation of dysphoria: Shared and private reactions to depression. Journal of Personality and Social Psychology. 1983;44:798–806. doi: 10.1037//0022-3514.44.4.798. [DOI] [PubMed] [Google Scholar]
- Suschinsky KD, Lalumière ML. Is sexual concordance related to awareness of physiological states? Archives of Sexual Behavior. 2012;41:199–208. doi: 10.1007/s10508-012-9931-9. [DOI] [PubMed] [Google Scholar]
- Suschinsky KD, Lalumière ML. The relationship between sexual concordance and interoception in anxious and nonanxious women. The Journal of Sexual Medicine. 2014;11:942–955. doi: 10.1111/jsm.12250. [DOI] [PubMed] [Google Scholar]
- Tahmasian M, Knight DC, Manoliu A, Schwerthöffer D, Scherr M, Meng C, Sorg C. Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Frontiers in Human Neuroscience. 2013;7:639. doi: 10.3389/fnhum.2013.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak LM, Janssens KA, Dietrich A, Slaets JP, Rosmalen JG. Age-specific associations between cardiac vagal activity and functional somatic symptoms: A population-based study. Psychotherapy and Psychosomatics. 2010;79:179–187. doi: 10.1159/000296136. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tamaru T, Imai J, Washizuka S, Ozawa H, Harada Y, Amano N. Pathology in senile patients with abnormal body sensation. Psychogeriatrics. 2001;1:139–142. [Google Scholar]
- Takahashi T, Yücel M, Lorenzetti V, Tanino R, Whittle S, Suzuki M, Allen NB. Volumetric MRI study of the insular cortex in individuals with current and past major depression. Journal of Affective Disorders. 2010;121:231–238. doi: 10.1016/j.jad.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Ishii K, Kakigi T, Yokoyama K. Gender and age differences in normal adult human brain: Voxel-based morphometric study. Human Brain Mapping. 2011;32:1050–1058. doi: 10.1002/hbm.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proceedings of the National Academy of Sciences. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CB. Depression, heart rate related variables and cardiovascular disease. International Journal of Psychophysiology. 2010;78:80–88. doi: 10.1016/j.ijpsycho.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Teasdale JD. Emotional processing, three modes of mind and the prevention of relapse in depression. Behaviour Research and Therapy. 1999;37:S53–S77. doi: 10.1016/s0005-7967(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Terhaar J, Viola FC, Bär KJ, Debener S. Heartbeat evoked potentials mirror altered body perception in depressed patients. Clinical Neurophysiology. 2012;123:1950–1957. doi: 10.1016/j.clinph.2012.02.086. [DOI] [PubMed] [Google Scholar]
- Terry R, Tarver WB, Zabara J. An implantable neurocybernetic prosthesis system. Epilepsia. 1990;31:S33–S37. doi: 10.1111/j.1528-1157.1990.tb05846.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Brosschot JF. Psychosomatics and psychopathology: Looking up and down from the brain. Psychoneuroendocrinology. 2005;30:1050–1058. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thelen E, Smith L. A dynamic systems approach to the development of cognition and action. Massachusetts: MIT Press; 1994. [Google Scholar]
- Thomas BC, Croft KE, Tranel D. Harming kin to save strangers: Further evidence for abnormally utilitarian moral judgments after ventromedial prefrontal damage. Journal of Cognitive Neuroscience. 2011;23:2186–2196. doi: 10.1162/jocn.2010.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RA, Lewis MD, Calkins SD. Reassessing emotion regulation. Child Development Perspectives. 2008;2:124–131. [Google Scholar]
- Timlin MT, Pereira MA, Story M, Neumark-Sztainer D. Breakfast eating and weight change in a 5-year prospective analysis of adolescents: Project EAT (Eating Among Teens) Pediatrics. 2008;121:e638–e645. doi: 10.1542/peds.2007-1035. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cerebral Cortex. 2004;14:966–973. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Tracy AL, Jarrard LE, Davidson TL. The hippocampus and motivation revisited: Appetite and activity. Behavioural Brain Research. 2001;127:13–23. doi: 10.1016/s0166-4328(01)00364-3. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Tajadura-Jiménez A, Costantini C. Just a heartbeat away from one’s body: Interoceptive sensitivity predicts malleability of body-representations. Proceedings of the Royal Society B. 2011;278:2470–2476. doi: 10.1098/rspb.2010.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey M. Ecological foundations of cognition: Invariants of perception and action. In: Pick HL Jr, van den Broek P, Knill DC, editors. Cognition: Conceptual and methodological issues. Washington, DC: American Psychological Association; 1992. pp. 85–117. [Google Scholar]
- Tylee A, Gandhi P. The importance of somatic symptoms in depression in primary care. Primary Care Companion to the Journal of Clinical Psychiatry. 2005;7:167–176. doi: 10.4088/pcc.v07n0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. The anterior insula in autism: Underconnected and under-examined. Neuroscience & Biobehavioral Reviews. 2009;33:1198–1203. doi: 10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungredda T, Gluck ME, Geliebter A. Pathophysiological and neuroendocrine aspects of night eating syndrome. In: Lundgren JD, Allison KC, Stunkard AJ, editors. Night Eating Syndrome: Research, Assessment, and Treatment. New York: The Guilford Press; 2012. pp. 27–39. [Google Scholar]
- Vaccarino V, Lampert R, Bremner JD, Lee F, Su S, Maisano C, Goldberg J. Depressive symptoms and heart rate variability: Evidence for a shared genetic substrate in a study of twins. Psychosomatic Medicine. 2008;70:628–636. doi: 10.1097/PSY.0b013e31817bcc9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya JG, Paradiso S, Boles Ponto LL, McCormick LM, Robinson RG. Aging, grey matter, and blood flow in the anterior cingulate cortex. NeuroImage. 2007;37:1346–1353. doi: 10.1016/j.neuroimage.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Vaitl D. Interoception. Biological Psychology. 1996;42:1–27. doi: 10.1016/0301-0511(95)05144-9. [DOI] [PubMed] [Google Scholar]
- Vallido T, Jackson D, O’Brien L. Mad, sad and hormonal: The gendered nature of adolescent sleep disturbance. Journal of Child Health Care. 2009;13:7–18. doi: 10.1177/1367493508098377. [DOI] [PubMed] [Google Scholar]
- van Baaren RB, Holland RW, Kawakami K, Van Knippenberg A. Mimicry and prosocial behavior. Psychological Science. 2004;15:71–74. doi: 10.1111/j.0963-7214.2004.01501012.x. [DOI] [PubMed] [Google Scholar]
- van Baaren RB, Fockenberg DA, Holland RW, Janssen L, van Knippenberg A. The moody chameleon: The effect of mood on non-conscious mimicry. Social Cognition. 2006;24:426–437. [Google Scholar]
- Van Boven L, Loewenstein G. Social projection of transient drive states. Personality and Social Psychology Bulletin. 2003;29:1159–1168. doi: 10.1177/0146167203254597. [DOI] [PubMed] [Google Scholar]
- Van der Does AJW, Van Dyck R, Spinhoven P. Accurate heartbeat perception in panic disorder: Fact and artefact. Journal of Affective Disorders. 1997;43:121–130. doi: 10.1016/s0165-0327(96)01414-0. [DOI] [PubMed] [Google Scholar]
- van Tol MJ, Li M, Metzger CD, Hailla N, Horn DI, Li W, Walter M. Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychological Medicine. 2014;44:2053–2065. doi: 10.1017/S0033291713002742. [DOI] [PubMed] [Google Scholar]
- van't Veer-Tazelaar PJN, van Marwijk HW, Jansen AP, Rijmen F, Kostense PJ, van Oppen P, Beekman AT. Depression in old age (75+), the PIKO study. Journal of Affective Disorders. 2008;106:295–299. doi: 10.1016/j.jad.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Varcin KJ, Bailey PE, Henry JD. Empathic deficits in schizophrenia: The potential role of rapid facial mimicry. Journal of the International Neuropsychological Society. 2010;16:621–629. doi: 10.1017/S1355617710000329. [DOI] [PubMed] [Google Scholar]
- Veer IM, Beckmann C, Van Tol MJ, Ferrarini L, Milles J, Veltman D, Rombouts SA. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Frontiers in Systems Neuroscience. 2010;4:41. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neuroscience & Biobehavioral Reviews. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Vianna EPM, Weinstock J, Elliott D, Summers R, Tranel D. Increased feelings with increased body signals. Social Cognitive & Affective Neuroscience. 2006;1:37–48. doi: 10.1093/scan/nsl005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen M, Panzer A. Non-termination of sickness behavior as precipitating factor for mental disorders. Medical Hypotheses. 2005;65:316–329. doi: 10.1016/j.mehy.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Vink D, Aartsen MJ, Schoevers RA. Risk factors for anxiety and depression in the elderly: A review. Journal of Affective Disorders. 2008;106:29–44. doi: 10.1016/j.jad.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Vrijsen JN, Lange WG, Becker ES, Rinck M. Socially anxious individuals lack unintentional mimicry. Behaviour Research and Therapy. 2010;48:561–564. doi: 10.1016/j.brat.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Wade TJ, Cairney J, Pevalin DJ. Emergence of gender differences in depression during adolescence: National panel results from three countries. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:190–198. doi: 10.1097/00004583-200202000-00013. [DOI] [PubMed] [Google Scholar]
- Wan W, Wetmore L, Sorensen CM, Greenberg AH, Nance DM. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Research Bulletin. 1994;34:7–14. doi: 10.1016/0361-9230(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Korczykowski R, Rao H, Fan Y, Pluta J, Gur RC, Detre JA. Gender difference in neural response to psychological stress. Social Cognitive and Affective Neuroscience. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-G, Tomasi D, Backus W, Wang R, Telang F, Geliebter R, Volkow ND. Gastric distention activates satiety circuitry in the human brain. NeuroImage. 2008;39:1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Wang L, Hermens DF, Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. Journal of Affective Disorders. 2012;142:6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Wang L, Dai Z, Peng H, Tan L, Ding Y, He Z, Li L. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Human Brain Mapping. 2014;35:1154–1166. doi: 10.1002/hbm.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang BR, Zhang XJ, Xu Z, Ding YQ, Ju G. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World Journal of Gastroenterology. 2002;8:540–545. doi: 10.3748/wjg.v8.i3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Newport R, Hamilton AFDC. Eye contact enhances mimicry of intransitive hand movements. Biology Letters. 2011;7:7–10. doi: 10.1098/rsbl.2010.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WH. The dynamics of perception and action. Psychological Review. 2006;113:358–389. doi: 10.1037/0033-295X.113.2.358. [DOI] [PubMed] [Google Scholar]
- Webb TL, Miles E, Sheeran P. Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychological Bulletin. 2012;138:775–808. doi: 10.1037/a0027600. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Grundriss der Psychiatrie in klinischen Vorlesungen. Leipzig: Georg Thieme; 1906. [Google Scholar]
- West MJ, King AP. Settling nature and nurture into an ontogenetic niche. Developmental Psychobiology. 2004;20:549–562. doi: 10.1002/dev.420200508. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Drescher VM. Perception of gastric contractions and self-control of gastric motility. Psychophysiology. 1980;17:552–558. doi: 10.1111/j.1469-8986.1980.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Drescher VM, Heiman P, Blackwell B. Relation of heart rate control to heartbeat perception. Biofeedback and Self Regulation. 1977;2:371–392. [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet J-P, Gallese V, Rizzolatti G. Both of us disgusted in my insula: The common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wiebking C, Bauer A, De Greck M, Duncan NW, Tempelmann C, Northoff G. Abnormal body perception and neural activity in the insula in depression: An fMRI study of the depressed “material me”. World Journal of Biological Psychiatry. 2010;11:538–549. doi: 10.3109/15622970903563794. [DOI] [PubMed] [Google Scholar]
- Wiebking C, Duncan NW, Tiret B, Hayes DJ, Marjaǹska M, Doyon J, Northoff G. GABA in the insula—A predictor of the neural response to interoceptive awareness. Neuroimage. 2014;86:10–18. doi: 10.1016/j.neuroimage.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens S. Interoception in emotional experience. Current Opinions in Neurology. 2005;18:442–447. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- Wiens S, Palmer SN. Quadratic trend analysis and heartbeat detection. Biological Psychology. 2001;58:159–175. doi: 10.1016/s0301-0511(01)00110-7. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen I. Brain-gut axis as an example of the bio-psycho-social model. Gut. 2000;47(Suppl. 4):5–7. doi: 10.1136/gut.47.suppl_4.iv5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie G, Shapiro CM. Sleep deprivation and the postnatal blues. Journal of Psychosomatic Research. 1992;36:309–316. doi: 10.1016/0022-3999(92)90067-c. [DOI] [PubMed] [Google Scholar]
- Williams EL, Bargh JA. Experiencing physical warmth promotes interpersonal warmth. Science. 2008;322:606–607. doi: 10.1126/science.1162548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, van Wassenhove V, Craig AD, Paulus MP. The neural substrates of subjective time dilation. Frontiers in Human Neuroscience. 2010;4:1–9. doi: 10.3389/neuro.09.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson AR, Crowley SJ, Anwer U, Bassett JL. Changes in sleep patterns and depressive symptoms in first-time mothers: Last trimester to 1-year postpartum. Behavioral Sleep Medicine. 2003;1:54–67. doi: 10.1207/S15402010BSM0101_6. [DOI] [PubMed] [Google Scholar]
- Wool CA, Barsky AJ. Do women somatize more than men?: Gender differences in somatization. Psychosomatics. 1994;35:445–452. doi: 10.1016/S0033-3182(94)71738-2. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: Mediation by cytokine activation. Brain, Behavior, and Immunity. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophrenia Research. 2010;123:93–104. doi: 10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Bai F, Yu H, Shi Y, Yuan Y, Chen G, Li SJ. Abnormal insula functional network is associated with episodic memory decline in amnestic mild cognitive impairment. NeuroImage. 2012;63:320–327. doi: 10.1016/j.neuroimage.2012.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara TK. The dissociation of valence and intensity using alliesthesia and thermal stimulation. New York: Columbia University; 2012. (Unpublished doctoral dissertation) [Google Scholar]
- Yao Z, Wang L, Lu Q, Liu H, Teng G. Regional homogeneity in depression and its relationship with separate depressive symptom clusters: A resting-state fMRI study. Journal of Affective Disorders. 2009;115:430–438. doi: 10.1016/j.jad.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Yarcheski A, Mahon NE. A study of sleep during adolescence. Journal of Pediatric Nursing. 1994;9:357–367. [PubMed] [Google Scholar]
- Yates AJ, Jones KE, Marie GV, Hogben JH. Detection of the heartbeat and events in the cardiac cycle. Psychophysiology. 1985;22:561–567. doi: 10.1111/j.1469-8986.1985.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Yilmaz P, Diers M, Dienera S, Rancea M, Wessaa M, Flor H. Brain correlates of stress-induced analgesia. Pain. 2010;151:522–529. doi: 10.1016/j.pain.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Young L, Bechara A, Tranel D, Damasio H, Hauser M, Damasio A. Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron. 2010;65:845–851. doi: 10.1016/j.neuron.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusim A, Anbarasan D, Hall B, Goetz R, Neugebauer R, Stewart T, Ruiz P. Sociocultural domains of depression among indigenous populations in Latin America. International Review of Psychiatry. 2010;22:370–377. doi: 10.3109/09540261.2010.500870. [DOI] [PubMed] [Google Scholar]
- Zagon A. Does the vagus nerve mediate the sixth sense? TRENDS in Neurosciences. 2001;24:671–673. doi: 10.1016/s0166-2236(00)01929-9. [DOI] [PubMed] [Google Scholar]
- Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behavioural Brain Research. 2006;175:323–328. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage. 2012;62:493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proceedings of the National Academy of Sciences. 2009;106:11382–11387. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhu X, Wang X, Zhu X, Zhong M, Yi J, Yao S. First-episode medication-naive major depressive disorder is associated with altered resting brain function in the affective network. PloS ONE. 2014;9:e85241. doi: 10.1371/journal.pone.0085241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillmann D. Cognition-excitation interdependencies in aggressive behavior. Aggressive Behavior. 1988;14:51–64. doi:10.1002/1098-2337(1988)14:1<51::AID-AB2480140107>3.0.CO;2-C. [Google Scholar]
- Zillmann D. Sequential dependencies in emotional experience and behavior. In: Kavanaugh RD, Zimmerberg B, Fein S, editors. Emotion: Interdisciplinary perspectives. New Jersey: Lawrence Erlbaum Associates; 1996. pp. 243–272. [Google Scholar]
- Zucker N, Harshaw C. Emotion, attention, and relationships: A developmental model of self-regulation in anorexia nervosa and related disordered eating behaviors. In: Lock J, editor. The Oxford handbook of child and adolescent eating disorders: Developmental perspectives. Oxford University Press; 2012. pp. 67–87. [Google Scholar]