Abstract
We examined how variation in working memory (WM) capacity due to aging or individual differences among young adults is associated with intrinsic or resting-state anticorrelations, particularly between (1) the medial prefrontal cortex (MPFC), a component of the default-mode network (DMN) that typically decreases in activation during external, attention-demanding tasks, and (2) the dorsolateral prefrontal cortex (DLPFC), a component of the fronto-parietal control network that supports executive functions and WM and typically increases in activation during attention-demanding tasks. We compared the magnitudes of MPFC-DLPFC anticorrelations between healthy younger and older participants (Experiment 1) and related the magnitudes of these anticorrelations to individual differences on two behavioral measures of working memory capacity in two independent groups of young adults (Experiments 1 and 2). Relative to younger adults, older adults exhibited reductions in working memory capacity and in MPFC-DLPFC anticorrelations. Within younger adults, greater MPFC-DLPFC anticorrelation at rest correlated with greater working memory capacity. These findings show that variation in MPFC-DLPFC anticorrelations, whether related to aging or to individual differences, may reflect an intrinsic functional brain architecture supportive of working memory capacity.
Keywords: default mode network, working memory, resting-state fMRI, aging, functional connectivity
1. Introduction
Working memory (WM) capacity, defined as the amount of goal-relevant information that can be both maintained and manipulated, declines with age (Craik et al. 1990) and varies considerably among individuals (Engle, 2002). In contrast to measures of simple short-term maintenance of information (e.g. digit span), greater WM capacity is associated with superior performance in a broad range of high-level cognitive domains, including reading comprehension, problem solving, and inhibitory control (Conway et al., 2003). WM capacity is thought to reflect central executive capability (Baddeley, 1992; Engle, 2002), and to depend on dorsolateral prefrontal cortex (DLPFC), parietal cortex, anterior cingulate cortex, and the basal ganglia (D’Esposito et al., 1999; D’Esposito et al., 2007; Frank et al., 2001; Levy and Goldman-Rakic, 2000). Here, we asked whether a relationship exists between variation in WM capacity, due to aging or across younger individuals, and the intrinsic functional architecture of the human brain as measured by resting-state functional connectivity.
Spontaneous fluctuations in functionally related brain regions are correlated with each other in the absence of external stimuli, and the patterns of these correlations have been thought to reveal intrinsic relations of brain regions (Beckmann et al., 2005; Biswal et al., 1995; Greicius et al., 2003). During rest, in young adults, there are strong correlations between components of the default-mode network (DMN), brain regions that are commonly deactivated during external or attention-demanding tasks involving mental control (Fox et al., 2005, Fransson, 2005, Greicius et al., 2003; Raichle et al., 2001). Anatomically, the DMN includes medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), left and right lateral parietal (LLP and RLP) cortices, and bilateral medial temporal lobe (MTL) regions (Buckner et al., 2008).
Resting-state correlations among the components of the DMN appear to be significantly reduced in age-associated pathologies (Greicius et al., 2004; Hedden et al., 2009) and in typically aging older adults (Andrews-Hanna et al., 2007; Balsters et al., 2013; Damoiseaux et al., 2008; Grady et al., 2010; Mowinckel et al., 2012; Sala-Llonch et al., 2012; Sambataro et al., 2010). This may be due, in part, to the particular vulnerability of long-range DMN functional connections to the effects of normal aging (Allen et al., 2011; Andrews-Hanna et al., 2007; Hafkemeijer et al., 2012; Fillippini et al., 2012; Tomasi & Volkow, 2012) or a consequence of more motion-related artifactual time points in elderly participants (Power et al., 2012; Van Dijk et al., 2012). Although widespread reductions in resting functional connectivity are observed with advancing age, some studies also report localized increases in resting-state functional connectivity. Older adults, relative to younger adults, have shown increased frontal-lobe coherence (Filippini et al., 2012) and increased functional connectivity within fronto-parietal cortical regions (Mowinckel et al., 2012).
Networks in the brain appear to have an intrinsic organization such that different networks may exhibit negative functional connectivity, or are anticorrelated with one another at rest. In young adults, components of the DMN are negatively correlated with brain networks comprised of regions commonly activated for external tasks that demand attention and mental control, including the DLPFC (Fox et al., 2005; Fransson, 2005). Evaluation of negatively correlated networks has proven controversial due to global signal regression, a method used commonly to mitigate physiological noise in resting-state functional imaging studies. Global signal regression is known to mathematically generate anticorrelations (Murphy et al., 2009; Saad et al., 2012). Given these issues, valid analysis of negatively correlated networks has developed into a topic of particular interest in the field (Chang and Glover, 2009; Fox et al., 2009; Hampson et al., 2010; Saad et al., 2012; Van Dijk et al., 2010; Weissenbacher et al., 2009). With the caveat that prior studies of the influence of age on anticorrelations have employed global signal regression, there is evidence that healthy aging is also characterized by reduced negative correlations at rest between the DMN and cortical regions commonly recruited during attention-demanding tasks (Wu et al., 2011).
Variation in DMN connectivity has been associated with variation in executive functions and WM capacity. Among older adults, reduced MPFC-PCC connectivity correlated with worse performance on executive-function and other cognitive measures (Andrews-Hanna et al., 2007) and reduced connectivity in a DMN network dominated by the MPFC correlated with worse performance on a trail-making test (Damoiseaux et al., 2008). Neither study reported a correlation between these brain measures and variation among young adults, because that was either not examined (Andrews-Hanna et al., 2007) or was not significant in 10 participants (Damoiseaux et al., 2008). For young adults, there is a report of a positive correlation between magnitude of MPFC-DLPFC anticorrelation and WM capacity, as measured by an n-back task (Hampson et al., 2010). The relation between reduced MPFC-DLPFC resting-state anticorrelation and reduced WM capacity is consistent with findings from patients with schizophrenia (Whitfield-Gabrieli et al., 2009).
Although variability in resting-state functional connectivity has been associated with variation in WM in relation to aging and to individual differences among young adults, there are two major gaps in the current understanding of that association. First, studies of aging have implicated positive correlations with the MPFC as being related to age-associated reduction in WM capacity, whereas the one study of variation among young adults has, instead, implicated negative correlations with MPFC. This leaves open the question about whether age-related changes in WM and individual differences among young adults in WM capacity are associated with shared or distinct variations in intrinsic functional connectivity (no one study has discovered such common variation in both younger and older adults). Second, the above functional connectivity findings were reported before it was well understood that greater movement in older than younger adults can produce artifactual results (Power et al., 2012; Van Dijk et al., 2012) or that global signal regression can mathematically generate anticorrelations (Murphy et al., 2009; Saad et al., 2012). Therefore, it is unknown whether the prior findings would hold when methodological improvements were implemented.
Here, we explored whether there exists shared or distinct characteristics of intrinsic brain function for age-related declines in WM capacity and for individual differences among young adults in WM capacity. We focused on MPFC positive and negative functional connectivity because bi-directional correlations of the MPFC with different regions have been implicated across studies of aging or of individual differences among young adults in relation to executive functions and WM capacity (Andrews-Hanna et al., 2007; Damoiseaux et al., 2007; Hampson et al., 2010). We examined the relation of MPFC-DLPFC anticorrelations and MPFC-PCC positive correlations to WM capacity (Experiment 1) in 27 younger and 27 older healthy adults with capacity measured by the Letter-Number Sequencing subtest from the Wechsler Adult Intelligence Scale (WAIS-III), and in 70 younger adults (Experiment 2) with a composite measure of Operation and Reading Span tests (Turner & Engle, 1989; Unsworth et al., 2005). In both experiments, we implemented methods that minimize the influence of motion artifacts and physiological noise and allow for valid interpretations of negative correlations (Behzadi et al., 2007; Chai et al., 2012; Whitfield-Gabrieli and Nieto Castanon, 2012).
2. Materials and Methods
2.1 Experiment 1
2.1.1 Participants
Participants were 27 older adults (15 women) between 65 and 89 years of age (M = 75.7 years, SD = 6.7) and 27 younger adults (15 women) between 20 and 33 years of age (M = 24.8, SD = 3.4). Written informed consent for participation in the study was obtained from all participants and approved by the MIT Institutional Review Board. All participants were healthy, right-handed individuals (Oldfield, 1971) from the Boston metropolitan area who satisfied the following criteria: native English speakers; no contraindications to MRI; and absence of neurological or psychiatric impairments or associated medications. All participants had normal or corrected-to-normal vision. No participant exhibited evidence of mild cognitive impairment or dementia; participants were excluded if they scored <27 on the Mini-Mental State Examination (Folstein & Folstein, 1975).
2.1.2 Neuropsychological and Demographic Measures
The Letter-Number Sequencing subtest from the Wechsler Adult Intelligence Scale (WAIS-III) was used as the measure of WM capacity. Participants were read a combination of numbers and letters, and then asked to recall first the numbers in ascending order and then the letters in alphabetical order. The score was the maximum number of items reordered and recalled correctly from WM (Wechsler, 2002). Two measures were used to assess comparability of the age groups. The American version of the National Reading Test (AMNART) (Grober & Sliwinksi, 1991) was used to estimate crystallized IQ. Socioeconomic status (SES) was measured with the Hollingshead SES scale, which separately ranks an individual’s educational and occupational attainment on scales ranging from 1–7. A weighted score was computed by multiplying the educational score by 4 and the occupational score by 7 and summing the 2 scores (Hollingshead, 1957). Lower scores indicate higher SES. Because the majority of younger participants had not yet completed their educations, we compared the older group to the SES scores for the parents of the younger group.
2.1.3 MRI Data Acquisition
Functional magnetic resonance imaging (fMRI) data were acquired using a 3-Tesla Siemens Tim Trio scanner (Siemens, Erlangen, Germany) paired with a 12-channel phased-array whole-head coil. Head motion was restrained with foam pillows and extendable padded head clamps--3D T1-weighted magnetization prepared rapid acquisition gradient echo (MP-RAGE) anatomical images were collected with the following parameters: time repetition (TR) = 2530ms, time echo (TE) = 3.39ms, flip angle (FA) = 7°, 1.33 x 1.0 x 1.33 mm resolution, 2x acceleration. Functional T2*-weighted images were acquired using a gradient-echo echo-planar pulse sequence sensitive to bold oxygenation level-dependent (BOLD) contrast (Kwong et al., 1992; Ogawa et al., 1992) with the following parameters: TR = 2000ms, TE = 30ms, FA = 90°, 3.0mm isotropic resolution. Thirty-six transverse slices covered the whole brain and were acquired in an interleaved fashion. Functional data were acquired while the participant was instructed to rest with eyes open for a period of 5 minutes consisting of 150 volumes. To allow for T1-equilibration effects, 4 dummy volumes were discarded prior to acquisition. Online prospective acquisition correction (PACE) was applied to the EPI sequence.
2.1.4 Resting State Preprocessing
Resting-state fMRI data were first preprocessed in SPM5 (Wellcome Department of Imaging Neuroscience, London, UK; (http://www.fil.ion.ucl.ac.uk/spm/spm5.html). Images were realigned (motion corrected), spatially normalized to the Montreal Neurological Institute (MNI) stereotactic space, and smoothed with a six mm kernel. Quality assurance was performed on the functional time series in order to detect outliers in the motion and global signal intensity using the in-house software art (http://www.nitrc.org/projects/artifact_detect). From each participant, an image was identified as an outlier if composite movement from a preceding image exceeded 0.5mm, or if the global mean intensity was greater than 3 standard deviations from the mean image intensity for the run in which it was collected. This composite motion measure was defined by the art tool. By default, art converts the 6 rotation/translation head motion parameters into another set of 6 parameters characterizing the trajectories of 6 points located on the center of each of the faces of a bounding box around the brain. It then computes the maximum scan-to-scan movement of any of these points as the single ‘composite’ scan-to-scan movement measure, which is thresholded to determine outliers. Identified outliers were included as nuisance parameters, as one regressor per outlier, within the first level general linear models.
2.1.5 Functional Connectivity Analysis
Functional connectivity analysis was performed with a seed-driven approach using the in-house, custom software Conn (http://www.nitrc.org/projects/conn; Whitfield-Gabrieli & Nieto-Castanon, 2012). The MPFC seed was defined a priori from the literature (Fox et al., 2005; Whitfield-Gabrieli et al., 2009) as 10mm spheres around the coordinates for the MPFC (−1, 47, −4) in MNI space. Physiological and other spurious sources of noise were estimated using the aCompcor method (Behzadi et al., 2007; Chai et al. 2012; Whitfield-Gabrieli et al., 2012), and removed together with movement-related and artifactual covariates. The residual BOLD time-series was band-pass filtered (0.009Hz to 0.08Hz). Each participant’s structural image was segmented into white matter (WM), gray matter (GM), and cerebral spinal fluid (CSF) using SPM8. WM and CSF masks were eroded by one voxel to avoid partial volume effects with adjacent gray matter. The first 3 principal components of the signals from the eroded WM and CSF noise ROIs were removed with regression.
First-level correlation maps were produced by extracting the residual BOLD time course from the MPFC seed and computing Pearson’s correlation coefficients between that time course and the time courses of all other voxels in the brain. Correlation coefficients were Fisher transformed into ‘Z’ scores, which increases normality and allows for improved second-level General Linear Model analyses. All reported clusters were significant at an FDR cluster-corrected threshold of p < .05.
Within Group Analyses
MPFC seed-to-voxel group analyses were separately performed using one-sample t-tests for within the young cohort (n = 27), and the older cohort (n = 27).
Between Group Analyses (Older vs. Younger)
MPFC seed-to-voxel between group connectivity analyses were performed using two sample t-tests.
MPFC functional connectivity correlation with WM
We investigated the relationship of (a) the magnitude of resting-state correlations from the MPFC that were either positively correlated with the PCC or negatively correlated with the bilateral DLPFC regions and (b) the measure of WM performance assessed outside of the scanner (Letter-Number Sequencing task). We performed a one-sample t-test for the entire group (n = 54) and functionally defined (a) the left and right DLPFC clusters (within BA 46/9) that were significantly anticorrelated with the MPFC, and (b) the PCC cluster (BA30/31) that was significantly positively correlated with the MPFC seed. We then extracted the mean Z-values from the bilateral DLPFC and PCC clusters for each participant of both cohorts and correlated those values with their WM capacities (as defined by the letter-number sequencing task) within both groups. Thus, the ROIs were unbiased because they were derived from all participants and independently from any behavioral measures.
2.1.6 Matched Groups on Motion Artifacts
To ensure that between group results were not driven by age-related differences in motion artifacts, we performed additional between-group analyses of subgroups of older and younger adults who did not differ significantly on movement and other artifacts. Within each cohort, 5 participants were removed (n = 44) to create groups equated for artifacts. Two-sample t-tests were performed to directly compare the connectivity maps between older and younger adults in the movement and artifact-matched groups.
2.2 Experiment 2
2.2.1 Participants
Participants were 70 younger adults (39 women) between the ages of 18 and 29 years of age (M = 21.6 years, SD = 2.6). Written informed consent was approved by the MIT Institutional Review Board. Participants were required to be adults between the ages of 18 and 45, right-handed, in good health, and not taking any drugs. They were recruited through web advertisements, physical flyers, and e-mail to the Northeastern and Tufts college mailing lists.
2.2.2 Neuropsychological and Demographic Measures
The automated Operation Span and Reading Span tasks were used as measures of complex WM capacity (Unsworth et al., 2005). For the Operation Span task, participants were presented with alternating letters and math equations, and asked to remember the letters while assessing whether each equation was valid. Set sizes ranged from 3-letters to 7-letters, with each set size presented for 3 trials over the course of the task, in a random order. At the end of each trial, participants reported the letters in the order they were presented. The dependent measure was the sum of all perfectly remembered letter sets. For the Reading Span task, participants were presented with alternating letters and sentences, and asked to remember the letters while assessing whether each sentence was sensical. Set sizes and scoring were identical to the automated Operation Span. Finally, Operation Span and Reading Span scores were summed to create a single measure estimating a participant’s complex WM capacity (composite score).
2.2.3 MRI Data Acquisition
Data were acquired on a 3T Tim Trio Siemens scanner using a 32-channel head coil. T1-weighted whole brain anatomical images (MPRAGE sequence, 256x256 voxels, 1x1.3-mm in-plane resolution, 1.3-mm slice thickness) were acquired. All participants underwent a resting functional MRI scan of 6 min with the instructions “keep your eyes closed and think of nothing in particular”. Resting scan images were obtained in 62 2-mm thick transverse slices, covering the entire brain (interleaved EPI sequence, T2*-weighted images; repetition time = 6 s, echo time = 30 ms, flip angle = 90, 67 slices with 2x2x2 mm voxels). PACE was applied to the EPI sequence.
2.2.4 Data Analysis
Resting-state fMRI data for Experiment 2 were first preprocessed in SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm), using standard spatial preprocessing steps. Images were slice-time corrected, realigned and resliced, normalized in MNI space and smoothed with a 4-mm kernel.
2.2.5 Functional Connectivity Analysis
The first four scans were excluded from analysis, as there were no dummy scans during initial acquisition. The preprocessing, artifact detection and rejection, aCompCor denoising to address physiological aliasing and subsequent seed driven functional connectivity analyses were identical to those in Experiment 1. Importantly, the left and right DLPFC clusters were defined from the entire group’s anticorrelation with the MPFC (independent of WM) and the mean DLPFC Z-values from the clusters were then correlated with the complex WM scores.
2.3 Results
2.3.1 Neuropsychological Measures for Experiment 1
There was no significant difference between younger (M = 119.74, SD = 5.8) and older (M = 120.37, SD = 7.6) groups for AMNART scores (t(52) = 0.36, p = 0.73) or for the Hollingshead SES scale (younger: M = 28.37, SD = 10.0; older: M = 31.96, SD = 11.6; (t(52) = 1.22, p = 0.22). The younger group (M = 15.15, SD = 3.4) performed significantly better than the older group (M = 10.70, SD = 2.5) on the Letter-Number Sequencing Test (t(52) = 5.44, p < 0.001)). Analyses of behavioral measures were performed with two-tailed t tests.
2.3.2 Artifact detection
Relative to younger adults (M = 1.1% of 150 time points, SD = 1.6%), older adults (M = 2.4% of 150 time points, SD = 3.0%) had significantly more artifacts (the union of motion and intensity outliers) (t(52) = 2.05, p < 0.05). MPFC correlations and anticorrelations increased post artifact detection and rejection, most noticeably in the older adults.
In order to make certain that group differences between younger and older adults were not were not driven by age-related differences in motion artifacts, we performed between group analyses on the groups who were matched on motion artifacts (after eliminating five participants from each cohort (n = 44)). For these matched groups, there was no significant difference in motion and other artifacts (t(42) = 0.00, p = 1.00).
2.3.3 Group Differences in Intrinsic Functional Organization
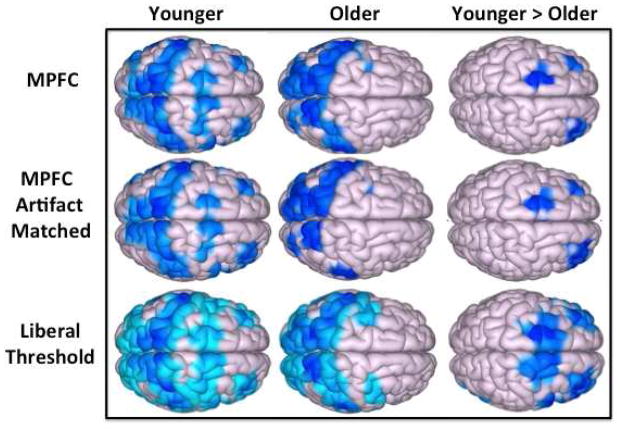
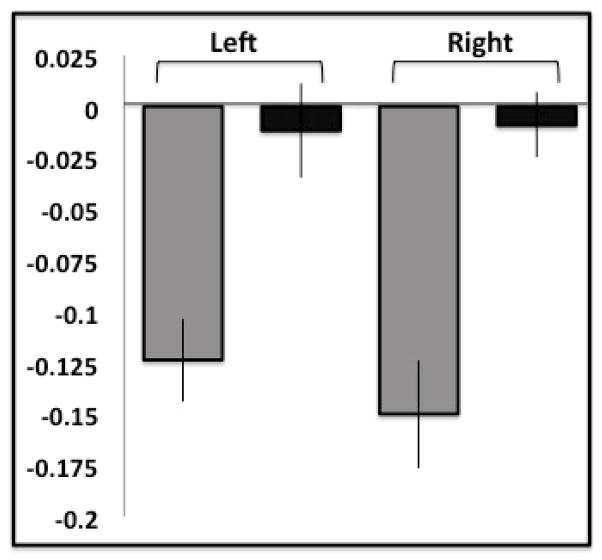
Using a seed-to-voxel analysis, the positive correlation between the MPFC seed and the PCC was significantly reduced in older relative to younger adults, (p < 0.05, cluster-level, FDR corrected). With the MPFC seed, there was a significant reduction in bilateral DLPFC anticorrelations in older relative to younger adults (Figure 1.A, top row; Table 1). There was a significant reduction in the magnitude of bilateral DLPFC anticorrelations with the MPFC seed in the artifact-matched groups (Figure 1.A, second row). The magnitude of the bilateral DLPFC anticorrelations defined by the entire (n = 54) group with respect to the MFPC seed was not significantly different from zero in older participants (right: t(52) = 0.61, p = 0.53, left: t(52) = 0.52, p = 0.61) (Figure 1.B). In fact, there were no observed MPFC-DLPFC anticorrelations in the elderly cohort, even when evaluated at a liberal threshold of p = 0.05 uncorrected (Figure 1.A, third row).
Figure 1.
(A) Resting-state anticorrelations for the medial prefrontal cortex (MPFC) seed are reduced in older adults; (top row) for younger adults (left column), older adults (middle column), and younger > older adults (right column). Top row depicts results from all participants. Second row depicts results from groups matched for motion artifacts. Results thresholded at p < 0.05, FDR cluster corrected in top two rows. The third row shows the same analyses at a more liberal threshold (p = .05, unc), and reveals that age-related differences occur only in frontal regions even at this threshold. (B) Resting-state anticorrelation for younger (gray) and older (black) adults between MPFC and bilateral DLPFCs; only younger adults exhibited significant anticorrelations in left and right DLPFC.
Table 1.
MPFC Anticorrelations Young > Old, FDR cluster corrected (p < 0.05)
| Clusters (x,y,z) (MNI) | Clusters | BA | k |
|---|---|---|---|
| (38, 34, 28) | right DLPFC | 9 | 166 |
| (30, 6, 8) | right Insular Cortex | 13 | 131 |
| (48, 38, 4) | right DLPFC | 46 | 130 |
| (−40, 40, 30) | left DLPFC | 9 | 113 |
| (−18, 2, 60) | left Premotor Cortex | 6 | 185 |
| (18, 22, 6) | right Anterior Cingulate | 33 | 100 |
| (20, 52, −6) | right Anterior PFC | 10 | 98 |
| (32, 38, 0) | right Inferior PFG | 47 | 102 |
| (30, 6, 8) | right Insular Cortex | 13 | 229 |
| (38, 34, 28) | right DLPFC | 9 | 61 |
| (48, 38, 4) | right DLPFC | 46 | 179 |
BA=Brodmann Area, k=spatial extent(voxel), FDR=false discovery rate
2.3.4 Correlations with WM performance
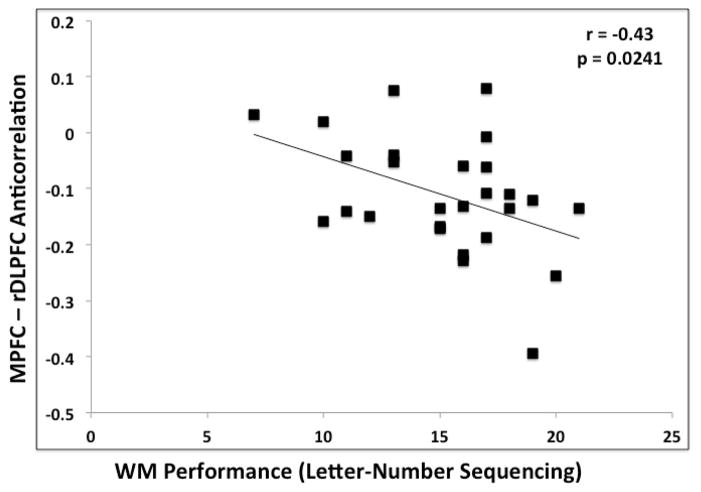
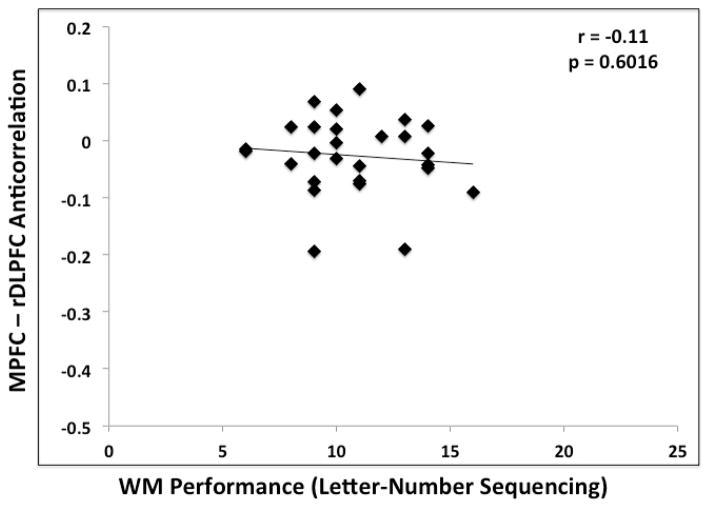
There was not a significant correlation between greater MPFC-PCC correlation and superior performance on the Letter-Number Sequencing task within either the older (r = 0.01, p = 0.95) or the younger group (r = 0.18, p = 0.38). Greater anticorrelation between the MPFC and the right DLPFC was significantly associated with better performance within the younger group (r = −0.43, p < 0.05), but not within the older group (r = −0.11, p = 0.51) (Figure 2). The lack of association within the older group may be attributable to a restricted range of variance in the greatly reduced anticorrelation between MPFC and right DLPFC. Correlations in the younger adults remained significant after removing a young adult with an apparent outlier value in the MPFC/DLPFC anticorrelations. The left DLPFC was not significantly correlated with WM performance within either group.
Figure 2.
Correlation between the magnitudes of MPFC-right DLPFC anticorrelation and WM performance (Letter-Number Sequencing) for younger (A) and older (B) adults for Experiment 1.
2.3.5 Results for Experiment 2
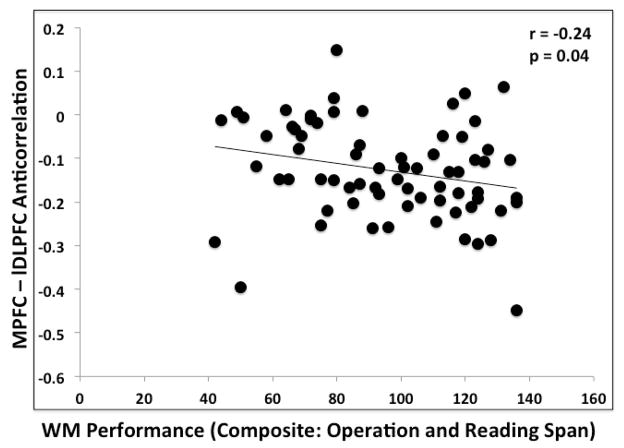
We replicated and extended our findings in Experiment 1 (that greater resting-state MPFC-DLPFC anticorrelation was associated with greater WM capacity among young adults) with a new independent group with a larger sample size (n=70). The primary analysis was performed with the composite score from two complex WM measures (i.e. Operation Span and Reading Span). Greater MPFC-left DLPFC anticorrelations were significantly correlated with composite WM scores (r = −0.24, p = 0.04) (Figure 3).
Figure 3.
Correlation between the magnitudes of MPFC–left DLPFC anticorrelation and WM performance (composite of Operation and Reading Span) for in younger adults for Experiment 2.
3. Discussion
We found convergent evidence from aging and from individual differences among young adults of a relation between greater WM capacity and greater magnitude of MPFC-DLPFC anticorrelation. Older adults exhibited both reduced WM and reduced MPFC-DLPFC anticorrelation relative to younger adults. Furthermore, greater WM capacity was associated with greater MPFC-DLPFC anticorrelation in two independent cohorts of young adults (total n = 97) with two different WM measures.
3.1 Age-Related Differences in WM Capacity and MPFC-DLPFC Anticorrelation
The behavioral findings were consistent with those generally observed in healthy aging (Hedden & Gabrieli, 2004, 2005). Younger and older participants scored similarly on the AMNART, a measure of vocabulary knowledge, consistent with evidence that crystallized knowledge or intelligence remains relatively intact during healthy aging (Park et al., 2002; Schaie, 1996). In contrast, older participants scored significantly less well on the measure of WM capacity, consistent with evidence that WM or fluid intelligence abilities decline in healthy aging (Park et al., 2002; Schaie, 1996). The validity of comparing these two groups of younger and older participants was supported by similar AMNART scores and similar SES status (with the use of parental SES for the younger adults who have often not completed education or reached final career and economic status).
There was also a significant age-related reduction in MPFC-DLPFC resting-state anticorrelation. This finding is consistent with a prior study that employed a potentially problematic method of global signal regression to examine anticorrelations (Wu et al., 2011). In addition, older adults, relative to younger adults, exhibited significant reductions in MPFC-PCC positive correlations. Reduced positive MPFC-PCC correlations are consistent with prior reports of reduced functional connectivity in normal aging (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008). Aging appeared to have a more severe impact on the MPFC-DLPFC anticorrelation because that anticorrelation was statistically absent, whereas the reduced MPFC-PCC positive correlation remained significantly above zero in the older adults.
Unlike prior studies reporting associations between MPFC-PCC positive correlations and measures of WM or other executive functions (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008), we did not observe such a correlation within either younger or older adults in Experiment 1. This difference across studies may be due to variations in analytic approaches, sample characteristics, choice of neuropsychological tests, or other factors. For example, some studies do not measure resting-state correlations as in the present study, but perform correlations on the residuals of an event-related activation design (e.g., Andrews-Hanna et al., 2007). Another study found a significant correlation between DMN anticorrelations during a 3-back task and WM performance on the 3-back task (Sala-Llonch et al, 2012). This relationship was not found in any of the less demanding n-back levels, but the anticorrelation during the interleaved fixation periods also correlated with WM performance during the 3-back condition (there was no report of the DMN anticorrelations during the pure rest condition and WM performance on the 3-back task). However, there is evidence that an active n-back task influences the rest periods within the task design, and therefore may be different from calculating the anticorrelations from pure rest (Pyka et al. 2009). In the present study, all resting-state measures were taken from pure resting-state scans, and are therefore not confounded with task performance.
The age-associated alteration in functional connectivity may be associated with age-related alteration in structural connectivity of white matter pathways as measured by diffusion tensor imaging (DTI). Older adults exhibit reduced integrity relative to younger people, especially in anterior regions near the DLPFC (e.g., Head et al., 2004; Pfefferbaum et al., 2005; Salat et al., 2005). Altered integrity of anterior white-matter pathways has been associated with age-related reductions in cognitive control and executive functions (Charlton et al., 2006; Deary et al., 2006; Grieve et al., 2007; O’Sullivan et al., 2001, Penke et al., 2010). Indeed, such region-specific alterations of white matter in typical aging have been related to individual differences in cognitive control among older adults, whereas cortical thickness and age-related alterations of temporal and parietal lobe white matter were unrelated (Ziegler et al., 2010).
Because many neural and psychological changes occur in concert with aging, it is difficult to make strong causal assertions between specific neural and specific psychological changes (Salthouse, 2010). Nevertheless, it is noteworthy that the MPFC is particularly vulnerable to aging functionally as the MPFC-DLPFC anticorrelation was statistically eliminated (also Wu et al., 2011).
3.2 Individual Differences in WM Capacity and MPFC-DLPFC
Greater MPFC-DLPFC anticorrelation at rest was associated with greater WM capacity across two independent samples totaling 97 young adults and using two different measures of WM capacity. In Experiment 1, greater WM capacity among young adults, as measured by a Letter-Number Sequencing task, was associated with greater right-lateralized MPFC-DLPFC anticorrelation. In Experiment 2, greater WM capacity among young adults, as measured by a composite score from the Operation-Span and Reading-Span tasks, was associated with greater left-lateralized MPFC-DLPFC anticorrelation. Importantly, all of these correlations were observed in an analysis in which MPFC-DLPFC anticorrelations were defined consistently and independently from the WM capacity measures. The observation that greater MPFCDLPFC anticorrelation was associated with greater WM capacity regardless of the specific measure of WM capacity indicates that this brain-behavior relation is generalizable.
Among young adults, the laterality of the MPFC-DLPFC anticorrelation shifted across different measures of WM capacity, and it is unclear what factor explains this difference. All three WM capacity measures involved simultaneous maintenance and manipulation of information in WM, with variation in the amount of that information. An advantage of such complex tasks is that they tax WM capacity, but a disadvantage is all the tasks involve many kinds of mental operations. In Experiment 2, the primary WM measure was a composite of the Operation-Span and Reading-Span tasks. Such a composite has the virtue that it provides a task-independent latent measure of the underlying construct of WM capacity that is not overly sensitive to the measurement properties of a single task. It is also possible that different WM measures are related to different neural circuits. Overall, however, the two experiments converged in showing that greater magnitudes of MPFC-DLPFC resting-state anticorrelations were associated with greater WM capacity among young adults.
3.3 Methodological Considerations
Compared to previous resting-state fMRI studies examining aging and individual differences, the present study had a number of potential methodological advantages. First, we used the aCompCor method of noise reduction (Behzadi et al., 2007) as implemented in Conn (Whitfield-Gabrieli and Nieto Castanon, 2012). This method avoids explicit global signal regression, a widely used preprocessing technique known to mathematically generate anticorrelations (Murphy et al., 2009; Saad et al., 2012; Van Dijk et al, 2010; Wong et al., 2012) which as a result renders anticorrelations uninterpretable (e.g., Chang & Glover, 2009) and may compromise the interpretability of positive correlations (Saad et al., 2012). The approach used in the present study is more likely to yield interpretable negative correlations and provides higher sensitivity and specificity for positive correlations (Chai et al., 2012). The differences in anticorrelations observed across age groups and individual differences within age groups observed in the present study are therefore less likely attributable to artifacts from data processing methods and may reflect biological processes. Second, we employed a method of artifact rejection above and beyond motion regression in order to reduce motion-related artifacts common in aging. As expected, the older adults had significantly more artifactual time points removed from analysis (although the percentage of time points removed was small for both groups). After artifact rejection, there was an apparent increase of posterior anticorrelations in the older adults, whereas frontal anticorrelations remained eliminated even at a liberal threshold of p = 0.05 uncorrected. These findings suggest that aging disproportionately degrades MPFC-DLPFC anticorrelations.
Greater movement in older relative to younger adults raised the possibility that differential connectivity findings could reflect differential movement. This appears unlikely, however, because age-related differences were also found when comparing younger and older groups after they were matched for movement and artifact outliers. Thus, the age-related differences are more likely to reflect actual differences in intrinsic brain organization.
Finally, the major findings regarding individual differences in intrinsic functional brain organization in relation to WM capacity were based on brain imaging correlations independent of behavioral measures. Specifically, the DLPFC regions were defined without reference to the WM behavior. Thus, the relations between the MPFC-DLPFC anticorrelations and WM performance were not contingent upon a whole-brain search for correlations with performance.
4. Limitations
One limitation to this study is that the older adult population may have substantially more cortical atrophy and more CSF, which may have differentially affected the normalization procedure. Another limitation is that although the use of different WM measures in Experiments 1 and 2 promote the generalizability of the findings, the different measures precluded a direct replication. Finally, anticorrelations are consistently found in young adults between specific neuroanatomical systems (e.g., the default mode network and the frontal-parietal network) and the magnitude of these anticorrelations relate to individual differences in behavior (e.g., WM performance), but the neural mechanisms underlying these anticorrelations remain unknown.
5. Conclusion
In older adults, there was reduced WM capacity and the apparent elimination of MPFC-DLPFC anticorrelation. In younger adults, there were associations between greater magnitudes of WM capacity and greater MPFC-DLPFC anticorrelations. These results suggest that intrinsic anticorrelations between the MPFC, a node in the DMN, and DLPFC, a cortical region involved in cognitive control, may serve as a shared indicator of WM capacity both in aging and in individual differences among young adults. Also, just as WM capacity declines in older adulthood, WM capacity grows markedly in development from childhood to young adulthood, and so do MPFC-DLPFC anticorrelations (Chai et al., 2014).
Differences in intrinsic functional organization in the resting state may reflect the ongoing history of interactions among brain regions during active cognitive performance in everyday life. Ultimately, it will be valuable to relate directly such resting-state and active-performance network dynamics. Resting-state studies are limited in interpretation by the absence of ongoing behavioral measures. Conversely, active performance studies are limited by the interpretation of brain activations occurring at different levels of performance in younger and older adults or among younger adults. Thus, one study reported that age-related reductions in activation during a WM task can be understood essentially in terms of variation in WM capacity (Schneider-Garces et al., 2009). Conversely, another study reported that age-related differences activation cannot be explained solely by variation in WM capacity (Bennett et al., 2013). Future studies that integrate resting-state and active-performance measures in younger and older adults with varying WM capacities in both age groups may clarify the extent to which individual differences among younger adults and age-related declines in older adults reflect shared and unique brain differences.
Acknowledgments
We thank Nina Wickens for her help in recruiting and testing participants, and the staff of the Martinos Imaging Center at the McGovern Institute at MIT for help in neuroimaging. This study was supported by NIH/NIA grant R21 AG030770. Funding support for J.B.K was provided by NIH grant T32 GM007484 and the Barbara J. Weedon Fund Fellowship at MIT; support for T.H. was provided by grant K01 AG040197.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD. A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. The Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder A, Vincent J, Lustig C, Head D, Raichle M, Buckner R. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;36:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Balsters JH, O’Connell RG, Galli A, Nolan H, Greco E, Kilcullen SM, Bokde ALW, Lai R, Upton N, Robertson IH. Changes in resting connectivity with age: a simultaneous electroencephalogram and functional magnetic resonance imaging investigation. Neurobiology of Aging. 2013;34:2194–2207. doi: 10.1016/j.neurobiolaging.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Beckmann C, DeLuca M, Devlin J, Smith S. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Rivera HG, Rypma B. Isolating age-group differences in working memory load-related neural activity: Assessing the contribution of working memory capacity using a partial-trial fMRI method. NeuroImage. 2013;72:20–32. doi: 10.1016/j.neuroimage.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin F, Haughton V, Hyde J. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self projection and the brain. Trends in Cognitive Science. 2007;11:50–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. Journal of Cognitive Neuroscience. 2014;26:501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. NeuroImage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Morris RG, Gick M. Adult age differences in working memory. In: Vallar G, Shallice T, editors. Neuropsychological impairments of short-term memory. Cambridge: Cambridge University Press; 1990. pp. 247–267. [Google Scholar]
- Christoff K, Cosmelli D, Legrand D, Thompson E. Specifying the self for cognitive neuroscience. Trends in Cognitive Science. 2011;15:104–112. doi: 10.1016/j.tics.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends in Cognitive Science. 2003;7:547–552. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the "default network" in normal aging. Cerebral Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, Wardlaw JM. White matter integrity and cognition in childhood and old age. Neurology. 2006;66:505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cognition. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- D’Esposito M. From cognitive to neural models of working memory. Philosophical Transactions of the Royal Society B. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002;11:19–23. [Google Scholar]
- Fillippini N, Nickerson LD, Beckmann CF, Ebmeier KP, Frisoni GB, Matthews PM, Smith SM, Mackay CE. Age-related adaptations of brain functions during a memory task are also present at rest. NeuroImage. 2012;59:3821–3828. doi: 10.1016/j.neuroimage.2011.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB. Brain changes in older adults at very low risk for Alzheimer’s Disease. Journal of Neuroscience. 2013;33:8237–8242. doi: 10.1523/JNEUROSCI.5506-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein‚ SE. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox M, Snyder A, Vincent J, Corbetta M, Van Essen D, Raichle M. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Procceedings of the National Academy of Sciences. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC. Interactions between the frontal cortex and basal ganglia in working memory: a computational model. Cognitive, Affective, & Behavioral Neuroscience. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Human Brain Mapping. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W. Frontal parietal control network regulates the anticorrelated default and dorsal attention networks. Human Brain Mapping. 2012;33:192–202. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JAE, Churchill N, McIntosh RA. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cerebral Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Science. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings National Academy of Science. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: A diffusion tensor MR imaging study. American Journal of Neuroradiology. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Gusnard D, Raichle M. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Science. 2001;98:4295–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafkemeijer A, van der Grond J, Rombouts SARB. Imaging the default mode network in aging and dementia. Biochimica et Biophysica Acta. 2012;1822:431–441. doi: 10.1016/j.bbadis.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magnetic Resonance Imaging. 2010;28:1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. Journal of Neuroscience. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, López-Solà M, Hernández-Ribas R, Deus J, Ortiz H, Soriano-Mas C, Yücel M, Pantelis C, Cardoner N. Consistency and functional specialization in the default mode brain network. Proceedings of the National Academy of Science. 2008;105:9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, William LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14:410– 423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Healthy and pathological processes in adult development: new evidence from neuroimaging of the aging brain. Current Opinion in Neurology. 2005;18:740–747. doi: 10.1097/01.wco.0000189875.29852.48. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. Journal of Neuroscience. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Mimeo. New Haven (CT): Yale University; 1957. Two factor index of social position. [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Science. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Experimental Brain Research. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Natural Neuroscience. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckiernan K, Kaufman J, Kucera-Thompson J, Binder J. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mowinckel AM, Espeseth T, Westlye LT. Network-specific effects of age and in-scanner subject motion: A resting-state fMRI study of 238 healthy adults. NeuroImage. 2012;63:1364–1373. doi: 10.1016/j.neuroimage.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Science. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones D, Summers P, Morris R, Williams S, Markus H. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology of Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Penke L, Maniega SM, Murray C, Gow AJ, Hernández MCV, Clayden JD, Starr JM, Wardlaw JM, Bastin ME, Deary IJ. A general factor of brain white matter integrity predicts information processing speed in healthy older people. Journal of Neuroscience. 2010;30:7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. NeuroImage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyka M, Beckmann CF, Schöning S, Hauke S, Heider D, Kugel H, Arolt V, Konrad C. Impact of working memory load on fMRI resting state pattern in subsequent resting phases. PLoS One. 2009;4(9):e7198. doi: 10.1371/journal.pone.0007198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Science. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connectivity. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan H-Y, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: impact on working memory performance. Neurobiology of Aging. 2010;31:839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Llonch R, Peña-Gómez C, Arenaza-Urquijo EM, Vidal-Piñeiro D, Bargalló N, Junqué C, Bartrés-Faz D. Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex. 2012;48:1187–1196. doi: 10.1016/j.cortex.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Salat D, Tuch D, Greve D, van der Kouwe A, Hevelone N, Zaleta A, Rosen B, Fischl B, Corkin S, Rosas H. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salthouse T. Neuroanatomical substrates of age-related cognitive decline. Psychological Bulletin. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW. Intellectual Development in Adulthood: The Seattle Longitudinal Study. Cambridge Univ. Press; Cambridge: 1996. [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee E, Sutton BP, Maclin EL, Gratton G, Fabiani M. Span, CRUNCH, and Beyond: Working memory capacity and the aging brain. Journal of Cognitive Neuroscience. 2012;22:655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Aging and functional brain networks. Molecular Psychiatry. 2012;17:549–558. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner ML, Engle RW. Is working memory capacity task dependent? Journal of Memory and Language. 1989;28:127–154. [Google Scholar]
- Unsworth N, Heitz RP, Schrock JC, Engle RW. An automated version of the operation span task. Behavior Research Methods. 2005;37:498–505. doi: 10.3758/bf03192720. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of Neurophysiology. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. WAIS-III/WMS-III technical manual: updated. San Antonio (TX): Psychological Corporation; 2002. [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. NeuroImage. 2009;47:1408– 1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- West R, Schwarb H. The influence of aging and frontal function on the neural correlations of regulative and evaluative aspects of cognitive control. Neuropsychology. 2006;20:468–481. doi: 10.1037/0894-4105.20.4.468. [DOI] [PubMed] [Google Scholar]
- Wicker B, Ruby P, Royet J, Fonlupt P. A relation between rest and self in the brain? Brain Research Reviews. 2003;43:224–230. doi: 10.1016/j.brainresrev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: A functional connectivity toolbox for correlated and anticorrelated networks. Brain Connectivity. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos H, Milanovic S, Tsuang M, Faraone S, McCarley R, Shenton M, Green A, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JDE, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, Olafsson V, Tal O, Liu TT. Anti-correlated networks, global signal regression, and the effects of caffeine in resting-state functional MRI. NeuroImage. 2012;63:356–364. doi: 10.1016/j.neuroimage.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JT, Wu HZ, Yan CG, Chen WX, Zhang HY, He Y, Yang HS. Agingrelated changes in the default mode network and its anti-correlated networks: A resting-state fMRI study. Neuroscience Letters. 2011;504:62–67. doi: 10.1016/j.neulet.2011.08.059. [DOI] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiology of Aging. 2010;31:1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]