Abstract
Although the thalamus is an important module in “pain networks,” there are few studies of the effect of experimental pain upon thalamic oscillations. We have now examined the hypothesis that, during a series of painful cutaneous laser stimuli, thalamic signals will show stimulus-related gamma-band spectral activity, which is modulated by attention to vs. distraction from the painful stimulus. When the series of laser stimuli was presented, attention was focused by counting the laser stimuli (count laser task), while distraction was produced by counting backward (count back plus laser task). We have studied the effect of a cutaneous laser on thalamic local field potentials and EEG activity during awake procedures (deep brain stimulation implants) for the treatment of essential tremor. At different delays after the stimulus, three low gamma- (30–50 Hz) and two high gamma-band (70–90 Hz) activations were observed during the two tasks. Greater high-gamma activation was found during the count laser task for the earlier window, while greater high-gamma activation was found during the count back plus laser task for the later window. Thalamic signals were coherent with EEG signals in the beta band, which indicated significant synchrony. Thalamic cross-frequency coupling analysis indicated that the phase of the lower frequency activity (theta to beta) modulated the amplitude of the higher frequency activity (low and high gamma) more strongly during the count laser task than during the count back plus laser task. This modulation might result in multiplexed signals each encoding a different aspect of pain.
Keywords: attention, thalamus, gamma band oscillations, EEG, human, pain
a broad range of evidence demonstrates the involvement of both the thalamus and the cortex in nociceptive processing (Apkarian et al. 2005; Lenz et al. 2010; Peyron et al. 2000). Our laboratory's recent studies have demonstrated that activity related to a painful cutaneous laser stimulus shows synchrony between modules, including the primary somatic sensory cortex (S1), the parasylvian cortex (PS), and the medial frontal cortex (MF) (Ohara et al. 2006). This synchrony may indicate the presence of cortical functional connectivity, which is dependent upon attention to vs. distraction from the painful laser. These studies demonstrate that pain networks are dynamic and task specific and may switch rapidly between different tasks.
Thalamic structures are likely modules in pain networks based upon their involvement in densely interconnected thalamocortical assemblies (Destexhe and Sejnowski 2001; Steriade et al. 1997). Cortico-cortical synchrony and directed functional interactions may be related to thalamic modules by mechanisms, including the following: afferent volleys traversing the thalamus, intrinsic thalamic oscillations, and divergent pathways from the thalamus to the cortex (Apkarian and Shi 1994; Burton 1975, 1984).
Previous studies have examined local field potentials (LFPs) in thalamic nuclei, including ventral caudal (Vc), ventral intermediate (Vim), central median, and the reticular nucleus, as mapped in Fig. 1. These studies were focused on the theta to alpha bands (see first section of methods) in patients with Parkinson's disease, epilepsy, tremor, and chronic pain (Kane et al. 2009 Kempf et al. 2009; Marsden et al. 2000; Sarnthein et al. 2003); gamma-band activity was reported in one study (Marsden et al. 2000).
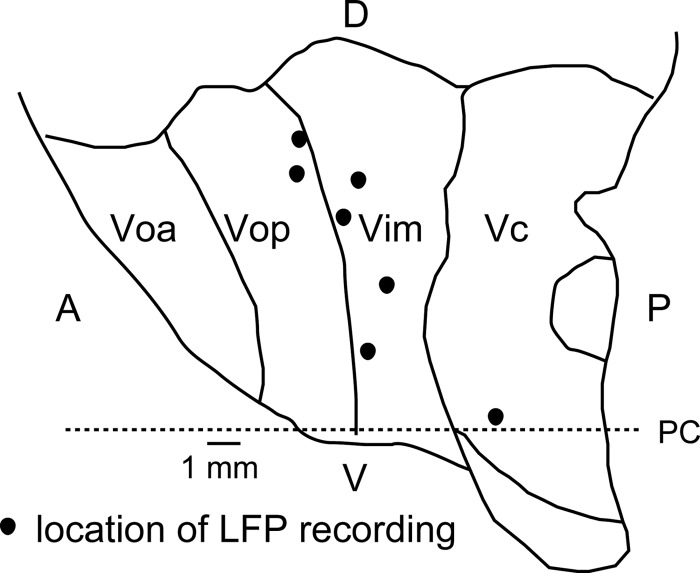
Fig. 1.
Schematic map of the thalamus showing recording locations for local field potentials (LFPs) [sagittal atlas map at lateral 13.5 mm (Schaltenbrand and Walker 1982)]. Each location was estimated by a physiological mapping technique which located neurons with deep and cutaneous receptive fields, as previously described (Kobayashi et al. 2009b). The anterior border of the ventral caudal (Vc) nucleus is identified by most anterior neuron in the region, where most neurons had deep or cutaneous receptive fields. Recording locations relative to nuclear boundaries were then shown relative to the anterior border of Vc and the anterior commissure-posterior commissure (AC-PC) line, indicated by the horizontal dashed line with PC as labeled. Voa, ventral oral anterior nucleus; Vop, ventral oral posterior nucleus; Vim, ventral intermediate nucleus; PC, posterior commissure; A, anterior; P, posterior; D, dorsal; V, ventral.
Another study found peaks of thalamic LFP power and thalamic LFP × EEG coherence in patients with chronic severe neurogenic pain (Sarnthein and Jeanmonod 2008). As reviewed above, human thalamic gamma-band activity has rarely been studied. To our knowledge, there has been no study of the effect of experimental painful somatic stimuli upon thalamic oscillations overall, or upon high-gamma-band oscillations specifically.
Gamma activity and synchrony (Engel and Singer 2001; Tallon-Baudry et al. 1996) induced by painful stimulation would be of interest because they may bind activity representing the different dimensions of pain into a unified sensation (Melloni et al. 2007; Singer 2011). Cross-frequency coupling of low-frequency and gamma activity may multiplex signals representing the different dimensions of pain (Canolty and Knight 2010; Lisman and Jensen 2013). Finally, gamma-band synchrony may be related to local functional interactions more than to long distance functional interactions (Ermentrout and Kopell 1998; Kopell et al. 2000).
We now address the hypothesis that, during a series of painful cutaneous laser stimuli, thalamic signals will show stimulus-related gamma-band spectral activity, which is modulated by attention to vs. distraction from the painful stimulus. We have studied the effect of a painful cutaneous laser upon thalamic LFPs and EEG activity, which were recorded during awake thalamic procedures (deep brain stimulation electrode implants) for the treatment of essential tremor (Kobayashi et al. 2009a). The results show event-related thalamic LFP activation across frequencies extending into the high gamma band.
METHODS
These studies were carried out in six subjects (7 thalami) at the Johns Hopkins Hospital (2013–2014) during the physiological exploration of the thalamus for the deep brain stimulation surgery for the treatment of essential tremor. A neurologist specializing in movement disorders confirmed the diagnosis of essential tremor. The protocol for these studies was reviewed and approved annually by the Institutional Review Board of the Johns Hopkins University. All patients signed an informed consent for these studies.
We used the following classification of frequency bands of oscillatory activities: theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), low gamma (30–50 Hz) and high gamma (70–90 Hz). For the purposes of this analysis, theta and alpha bands were analyzed together in the case of coherence because peak coherences were often found at the border between theta and alpha bands.
Intraoperative procedures.
The intraoperative procedures used here are similar to those which have been described previously (Kobayashi et al. 2009a). Briefly, the thalamic exploration was performed as a stereotactic procedure using the Leksell frame (Elekta Instruments, Stockholm, Sweden). The frame coordinates of the anterior and posterior commissures (Fig. 1) were determined by magnetic resonance imaging (MRI), which was imported into stereotactic planning software (Framelink, Medtronic, Minneapolis, MN).
The coordinates for target nuclei were estimated with microelectrode recordings referenced to a guide tube located 2 cm dorsal to the microelectrode (Kim et al. 2009). The microelectrode was advanced through a burr hole 2.5 cm off the midline anterior to the coronal suture. The initial trajectories were focused on Vc to use the response of neurons in this area to somatosensory stimulation as indicators of the borders of Vim (Kim et al. 2009). LFPs were recorded from a 1-mm cylindrical contact located along the cannula for the microelectrode at 3 mm above the tip of the microelectrode (Neuroprobe, Alpha Omega). Simultaneous EEG monitoring was applied at Cz (10–20 system) with disposable gold cup electrodes (Grass) referenced to bilateral ear lobe electrodes (Jasper 1958).
During the laser studies, subjects were in a supine position without movement or tremor, as confirmed by EMG recording, but with their eyes open, carrying out the tasks described below. Cutaneous heat stimulation was delivered by thulium YAG laser (duration 1 ms, beam diameter 0.6 cm, Neurotest, Wavelight, Starnberg, Germany). Prior to the experiment, a series of laser pulses were delivered (400 to 900 mJ) to each subject to select the laser energy level producing pain of around 5 out of 10 on the visual analog scale of pain intensity. After we decided on a location of interest within the thalamus, the laser was applied on the dorsum of the hand on the side contralateral to the recordings. The laser beam was moved at random to a slightly different position for each stimulus to avoid fatigue or sensitization of the nociceptors.
The mean selected energy level used for the experiment was 811 mJ. During the laser stimulation, the subject was asked to do one of the following tasks: 1) count laser task: the subject counted the painful laser stimuli; or 2) count back plus laser task: the subject counted back serially from 100 by 7’s. The interstimulus interval was about 4 s, and 40 stimuli were delivered in each block; the protocol was composed of two blocks for each of the two tasks. Each task was presented in each subject for paired comparison. The order of the blocks of different tasks was randomized by patient and counterbalanced across the population.
Data collection.
All signals were recorded digitally (MicroGuide, Alpha Omega) and stored to a personal computer for off-line analysis. The setting parameters of LFP and EEG signals were as follows: gain × 10,000 band-pass filter 1.5–250 Hz, sampling rate 1,500 Hz. Each signal was imported into MATLAB 7.1 (The MathWorks, Natick, MA) and down sampled to a common 500 Hz for further analysis. LFPs and EEGs were band-pass filtered at 1–100 Hz and notch filtered at 58–62 Hz before analysis. Recordings with artifact were excluded from analysis e.g., cautery. Laser evoked potentials (LEPs) were calculated for LFP signals as previously described for intracranial recordings (Lenz et al. 1998a).
Power spectral density analysis.
Power spectral density (PSD) describes the power distribution of the signal over frequency (Oppenheim and Schafer 1975). The spectral power of continuous signals was calculated and plotted using the spectopo function in EEGLAB toolbox (Delorme and Makeig 2004), which utilizes the pwelch function from MATLAB Signal Processing Toolbox (MATLAB R 2011b, The MathWorks). We adopted a classification of oscillatory activities by frequency bands as listed in the first section of the methods. The frequency ranges below 3 Hz and between 50 and 70 Hz were not used for statistical analysis to avoid possible artifacts. The PSD value was log transformed and plotted as in Fig. 2. For statistical analysis, the sum of the PSD values in each frequency band between delta and gamma were calculated and compared pairwise between the two tasks.
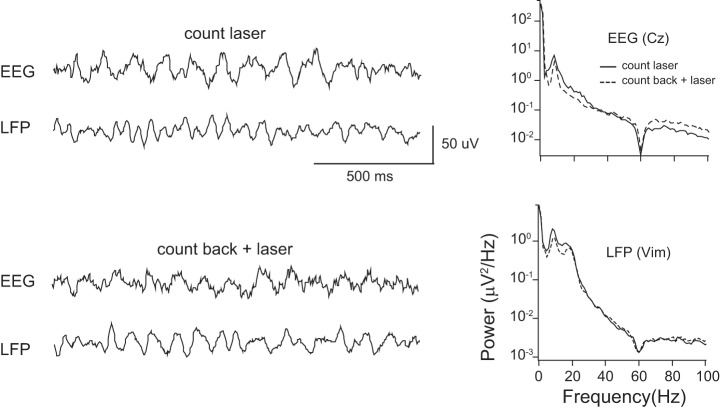
Fig. 2.
Example of intraoperative simultaneous recording of EEG (Cz) and thalamic LFP in two different tasks. Recordings were performed while the subject carried out the count laser task (top left), or count back task plus laser task (bottom left). Right: plots of power spectral density (PSD) of EEG and LFP during both tasks in the same subject. Results of both tasks were plotted on the same panels for comparison. EEG shows prominent theta power in both tasks. LFP shows prominent theta and beta power in both tasks, although less during the count back plus laser task.
Event-related spectral power analysis.
The event-related spectral power (ERSP) was used to compare the event-related non-phase-locked responses after painful laser stimulation vs. before in the two different tasks. This technique measured event-related changes in the power spectrum across different frequency bands in the poststimulus period and was performed partly by using the newtimef.m function of EEGLAB (Delorme and Makeig 2004). This function utilized the windowed fast Fourier transform (FFT) on data epochs that were extracted from every trial with a fixed interval of 1.024 s before and 1.024 s after the laser stimulus.
The minimum and maximum frequencies for the FFT time-frequency analysis were set from 2 Hz to 150 Hz, which covered the theta through high-gamma bands in LFPs. The width of the FFT window was 256 ms, and the analysis was done by sliding this FFT window across the entire epoch. The resulting time-frequency estimates were set to have 150 linear-spaced frequency bins ranging from ∼2 Hz to ∼150 Hz, and 400-ms time bins. In this study, the values of ERSP represented a ratio of pre- and post- to mean prestimulus frequency power. The prestimulus frequency power was computed by averaging the spectral estimates from −0.2 s to 0 s. As a result, if the ERSP was larger than 1, it was considered as event-related synchronization (ERS); if smaller than 1, it was considered as event-related desynchronization. For each window of interest, the Wilcoxon signed-rank test was applied to test whether the ERSP was significantly different between tasks (see results).
Coherence analysis.
Coherence analyses were used to estimate synchrony between signals recorded from the scalp EEG (midline Cz channel) and the thalamic LFP. The magnitude of the coherence function provides estimates of the strength of coupling between two signals x(t) and y(t) (Halliday et al. 1995), defined as a normalized cross-spectrum Cxy(f) = |Pxy(f)|2/[Pxx(f) × Pyy(f)], where Pxy denotes a cross-spectrum, and Pxx and Pyy refer to auto-spectra. The magnitude of coherence ranges between 0 (independence) and 1 (complete linear relationship). To test whether the coherence was nonzero, the assumption of independence was used to estimate the upper 95% of confidence limit (Amjad et al. 1997; Halliday et al. 1995). To evaluate the changes of coherence after laser stimulation, we evaluated the time-dependent changes of coherence. The above computations were performed using the sp2a2_m1 function of the Neurospec 2.0 toolbox (www.neurospec.org) implemented in MATLAB (Halliday et al. 1995; Amjad et al. 1997). This function computed the coherence values and estimated the upper 95% confidence limit using all data epochs that were extracted from every trial with a fixed interval of 0.5 s before and 1.5 s after the onset of the laser stimuli. The procedure for estimating the upper 95% confidence limit involved two steps. First, the overall standard deviation of the coherence values was computed, and, second, under the assumption of asymptotic normality, the upper 95% the upper confidence limit was obtained by 1.96 multiplied times the obtained overall standard deviation plus the overall mean coherence value. After the raw data were down-sampled to 300 Hz and high-pass filtered at 1 Hz, both the EEG and LFP were normalized, and the coherence between signals was calculated in 233-ms windows with a sliding step of 17 ms. We used 256 data points to perform the Fourier transformation and to calculate the coherence; the final frequency resolution of coherence was 1.17 Hz. Individual coherence was averaged across all trials, and the upper 95% confidence limit for the coherence estimate was calculated. These coherence estimates were averaged across subjects and plotted for each task.
Cross-frequency coupling.
Many studies have suggested that the cross-frequency couplings between oscillators in LFP reflect specific brain functions. For example, the coupling between oscillators of low-frequency theta-alpha and high-frequency gamma (>30 Hz) have been extensively studied in relation to a wide range of cognitive behaviors and tasks (Canolty and Knight 2010; Kahana et al. 1999; Louie and Wilson 2001).
In the present study, coupling between low and high frequencies was examined by computing a measure called the modulation index (MI) (Canolty et al. 2006). In brief, the raw LFP signal was first band-pass filtered into low and high frequencies of interest using a two-way least squares error minimization finite impulse response filter (eegfilt.m from the EEGLAB toolbox) (Delorme and Makeig 2004). The order for this filter equals the closest integer to three times the ratio between sampling rate and low-cutoff frequency (Canolty et al. 2006; Tort et al. 2009). A Hilbert transform was then applied in the second step to obtain the complex-valued analytic signal. The power and the phase series of the filtered signals were then extracted from the analytic signal for both low and high frequency of interest. Next, the low-frequency phase series was divided into 18 equally spaced intervals within the (−π, π) radian interval, where π radians corresponds to the troughs, while 0 radians corresponds to the peaks in the phase series. For each of these divided phase intervals, the corresponding high-frequency power amplitude was computed by summing the amplitude estimates, which had their phase values falling inside the corresponding phase interval.
Assuming no coupling between the low-frequency phase and the high-frequency power, a uniform power is expected in all 18 phase intervals. On the other hand, if a coupling relationship indeed existed, then a nonuniform distributed power is expected. Therefore, the MI value was intended to quantify how far the power distribution of these 18 phase intervals deviated from the uniform distribution. It should be clear that a MI value of 0 indicates low phase amplitude modulation, and a large MI value suggests the opposite (Tort et al. 2008).
To assess the statistical significance of the MI values for each subject, the MI values of the poststimulus interval were normalized (or z-transformed) by subtracting the mean and dividing by the standard deviation of the MI values using all trials during the prestimulus interval (Tort et al. 2008). This normalization was used to establish a statistical comparison between stimulus-relevant and stimulus-irrelevant brain oscillatory activities in this study. A significance threshold was set corresponding to P < 0.01.
RESULTS
Simultaneous thalamic LFP and EEG recordings were performed in seven thalami (left recordings 3, right 4) from six subjects with essential tremor and normal somatosensory examinations (Lenz et al. 1993). Subject S5 was operated on the second side 4 mo after the first. The LFP recording electrode was located 3 mm above from the tip of the microelectrode. The actual recording region of LFP was estimated from the physiological mapping of neuronal receptive fields, as previously described (Kobayashi et al. 2009a). Neurons closest to the radiological estimates of the nuclear boundaries all had receptive fields in the upper extremity. The estimated nuclear location of the recording sites is shown in Fig. 1.
LEPs.
Figure 3 shows the negative results of laser stimulus triggered averaging for LEPs from recordings of LFPs within the lateral nuclear group. The absence of LEP responses in these LFP recordings is consistent with the nonlaminar structure of the thalamic nuclei (Andersen et al. 1964; Baumgartner et al. 2011).
Fig. 3.
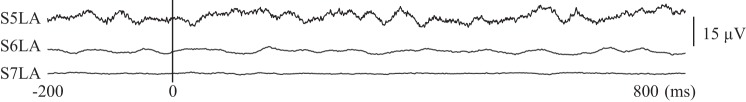
LFP activity processed to demonstrate event-related potentials in response to laser stimuli in three thalami, as labeled. The horizontal scale is time relative to the laser.
PSD analysis.
PSD of both EEG and LFP during each task was calculated for each subject, as shown in Fig. 2 for subject S6. In individual subjects, we observed two prominent peaks in low-frequency bands at theta to beta in thalamic LFP recordings. There was a difference in the power of frequency between tasks in individual recordings (Fig. 2). The mean PSD of the LFP signal showed two peaks in the low-frequency range in both tasks, which were located in the theta-alpha and beta bands.
The PSD results of EEG recordings were similar to those of LFP in each individual, but less power was found in the beta range. When we compared the PSD across both tasks in each frequency band, the result did not show significant differences in either the LFP and EEG recordings (Wilcoxon signed-ranked test, P > 0.05).
Nonphase-locked induced responses.
Time frequency plots relative to the laser stimulation were constructed for the count laser task and the count back plus laser task (Fig. 4, middle and bottom). The grand average of laser stimuli for both tasks showed prominent ERS of the low- and high-gamma bands (Fig. 4, top). The average ERSP plot of each different task showed a different pattern of ERS. The count back plus laser task showed ERS in low gamma (windows 1–3) but relatively more ERS in later high gamma (windows 4 and 5) than that in the count laser task. In addition, there was a beta ERS (no window assigned) in the count laser task but not the count back plus laser task.
Fig. 4.
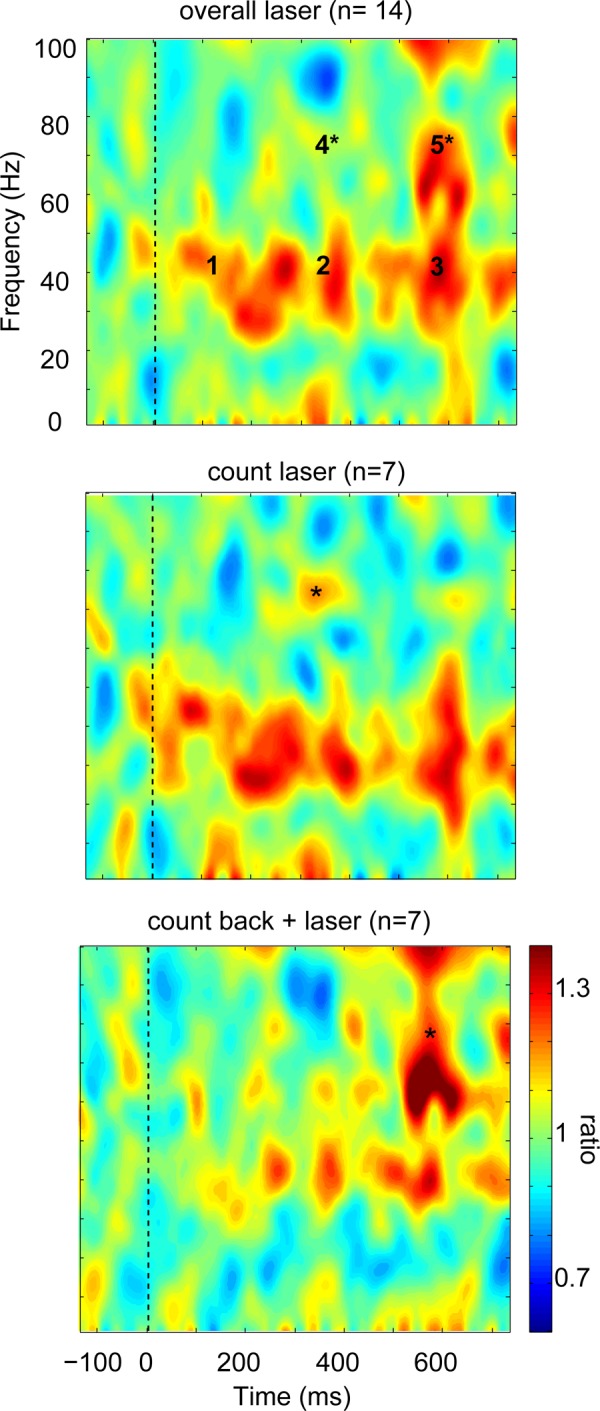
Thalamic LFP event-related spectral power (ERSP) shown as a time frequency plot. Top: grand average of all laser stimuli shows prominent event-related synchronization (ERS) mainly in the low gamma band. Five windows indicated by numbers are used for the statistical testing, as listed in Table 1. Middle: count laser task shows sustained ERS in gamma and beta band with early ERS in lower frequency bands. Bottom: count back plus laser task shows later low-gamma ERS without beta ERS. The color scale is the time frequency spectral power (pre- and poststimulus) divided by the mean prestimulus spectral power.
Gamma bands were tested statistically in each of the five time-frequency windows (Table 1). We carried out paired comparisons of the median value of ERSP in each window by task with a Wilcoxon signed-rank test and Bonferroni correction. The results showed that there was no statistical difference between the count laser task vs. the count back plus laser task in the low-gamma region (windows 1–3), which suggests that low-gamma activity is commonly induced after the laser stimulation in both tasks (Table 1).
Table 1.
Time and frequency range of windows for event-related spectral power analysis
| Window 1 | Window 2 | Window 3 | Window 4 | Window 5 | |
|---|---|---|---|---|---|
| Time range, ms | 0–300 | 300–400 | 500–600 | 300–400 | 500–600 |
| Frequency range, Hz | 30–50 | 30–50 | 30–50 | 70–90 | 70–90 |
| P value | 0.078 | 0.69 | 0.69 | 0.031 | 0.031 |
Significance of the difference between tasks for each window are indicated in the bottom row.
The high-gamma windows (windows 4 and 5) showed significant differences between the two tasks, as indicated in Fig. 4 by the asterisk for the window and task with the higher ERSP. Specifically, the count laser task showed more ERS in the 300- to 400-ms period (window 4; P = 0.0313), as indicated by the asterisk in the middle panel. On the contrary, the count back plus laser task showed more ERS in the 500- to 600-ms period (window 5; P = 0.0313), as indicated by the asterisk in the bottom panel. High-gamma ERSP was found over window 4 during the count laser task in six of seven thalami and over window 5 for five of seven thalami during count back plus laser task. Window 4 has significant differences between the two tasks and is in the range of LEPs latencies, as measured by intracranial recordings (Lenz et al. 1998a). We next compared results of the analyses of thalamic LFP with those of EEG.
The thalamic LFP data can be compared with EEG data for the PSD (see above and Fig. 2) and the ERSP. The PSD of both thalamic LFP and EEG showed a consistent pattern of overall peaks in theta-alpha and beta ranges. The overall EEG (Cz) ERSP is shown in Fig. 5. This plot shows a component of low and high gamma, which does not overlap with the windows of thalamic LFP ERSP (Fig. 4, top), and which has a different pattern of ERSP than thalamic LFP.
Fig. 5.
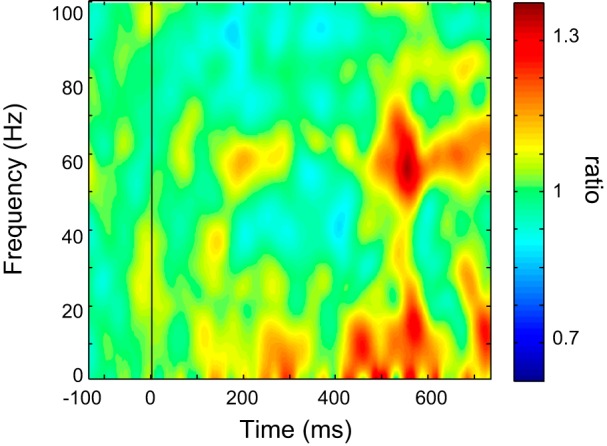
Cz EEG ERSP shown as a time frequency plot. All conventions are the same as in the legend for Fig. 4.
Thalamocortical coherence.
We next calculated coherence between the thalamic LFP and the EEG signals (Cz contact). First, the coherence during the whole period of a task was evaluated. We observed two peaks of significantly increased coherence in the theta-alpha and beta bands (Fig. 6A, subject S4). The number of time frequency peaks, mean peak and mean coherence value were counted and calculated in each task (Table 2). The mean coherence value in the low-frequency region (theta-alpha and beta) was not significantly different during the count back plus laser task than during the count laser task (P > 0.05, Mann-Whitney test) (Table 2). The mean coherence value of the low gamma was significantly less in the count laser task (P = 0.041, Mann-Whitney test), although each value was small (Table 2).
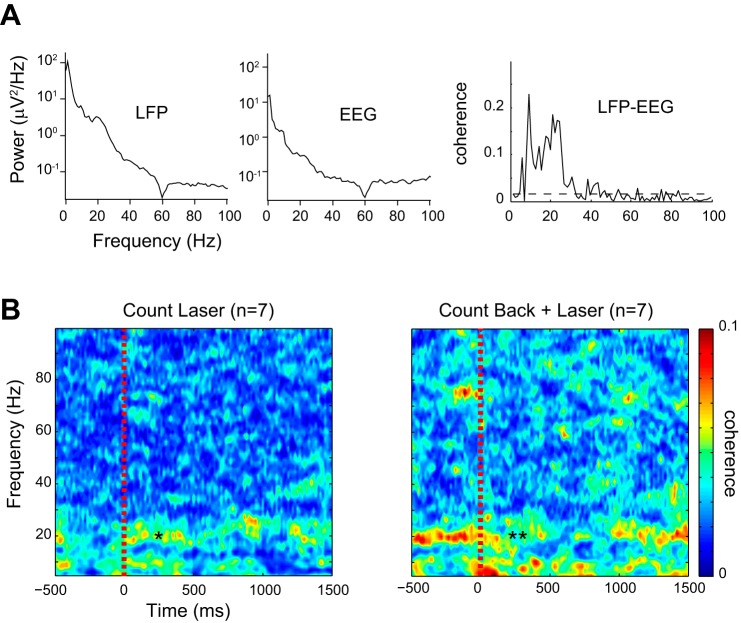
Fig. 6.
Thalamic LFP × EEG coherence results. A: a representative example of the LFP × EEG coherence during overall task periods. LFP from the thalamus and EEG from Cz showed significant increase of coherence in the theta-alpha and the beta bands with peak frequencies of 9.4 Hz and 21.1 Hz. The horizontal dashed gray line in the coherence estimate is the upper 95% confidence limit based on the assumption of independence. B: time- and frequency-dependent changes of coherence during peristimulus period. In the count laser task, beta and theta thalamic cortical coherence was increased after the stimulation. In the count back and laser task, beta coherence was decreased after the stimulus. The color scale indicates the magnitude of the coherence, where blue is the lower coherence.
Table 2.
Number and mean peak frequency of coherence during the whole task period
| Count Laser (n = 7) |
Count Back + Laser (n = 7) |
|||||
|---|---|---|---|---|---|---|
| n of Significant coherence | Mean peak, Hz | Mean coherence | n of Significant coherence | Mean peak, Hz | Mean coherence | |
| Theta-alpha | 4 | 7.3 | 0.052 | 3 | 6.5 | 0.12 |
| Beta | 5 | 21 | 0.060 | 5 | 20 | 0.12 |
| Low gamma | 3 | 38 | 0.027* | 3 | 40 | 0.070* |
| 2 | 78 | 0.027 | 3 | 79 | 0.10 | |
n, Number.
Significant difference in coherence between tasks (P = 0.0409; Mann-Whitney test).
Because these results represent overall coherence between the thalamus and midline EEG (Cz), we used a time-dependent coherence plot to measure changes of coherence after the laser stimulation in each task. Time-dependent changes of coherence were calculated across all trials and averaged within each task. The data epochs between 500 ms before and 1,500 ms after the laser stimulus were extracted for the analysis (Fig. 6A). The time-resulting averages were further averaged across subjects to make a grand average plot in each task (Fig. 6B).
The values of the coherence were averaged across subjects and color coded so that hot colors have high coherence in Fig. 6B. These plots showed greater coherence around and below 10 Hz and around 20 Hz in both tasks (Fig. 6B). High coherence values were not prominent within low- or high-gamma bands for either task. Low-frequency coherence was stronger after the stimulus (indicated by asterisk in Fig. 6B, left) than before the stimulus in the count laser task, but weaker after the stimulus (indicated by double asterisk in Fig. 6B, right) than before the stimulus in the count back plus laser task. The coherence magnitude was stronger in the latter task. The decrease of low-frequency coherence during the count back plus laser task recovered to baseline about 1 s after the stimuli. The high levels of prestimulus coherence can be attributed to the effect of the task, which influences both pre- and poststimulus activity (Liu et al. 2011c; Ohara et al. 2006).
Cross-frequency coupling in the thalamus.
These results showed strong low-frequency (theta to beta) thalamic PSD (Fig. 3), prominent thalamic high-frequency ERS (gamma) power (Fig. 4), and thalamocortical coherence in the beta frequency band (Fig. 6). These results suggest the possibility of a relationship between the thalamic low- and high-frequency bands. Therefore, we studied the phase-amplitude coupling between these bands in the LFPs recorded in the thalamus during both tasks.
Figure 7A showed that the gamma (70–90 Hz) band power was modulated by the phase of the beta (13–30 Hz) band. The averaged low-frequency signal was indicated by a solid black waveform line and plotted on the top of the time-frequency map for the high-frequency signals (see Fig. 7A). Comodulograms averaged across all subjects were plotted separately for the count laser task and count back plus laser task in Fig. 7B (top and bottom, respectively). The strength of the statistically significant MIs was color coded, where significance is indicated by colors hotter than blue in Fig. 7B. These plots showed less coupling activities between the 2- and 15-Hz LFP phase and the 40- and 100-Hz LFP power in both tasks. On the other hand, the strong beta-gamma (beta: ∼15–30 Hz, gamma: 45–90 Hz) coupling was observed only in the count laser task (Fig. 7B, top), and less prominent during the count back plus laser task. Our coupling analysis indicated that the amplitude of the high-frequency band is modulated by the phase of the low-frequency band.
Fig. 7.
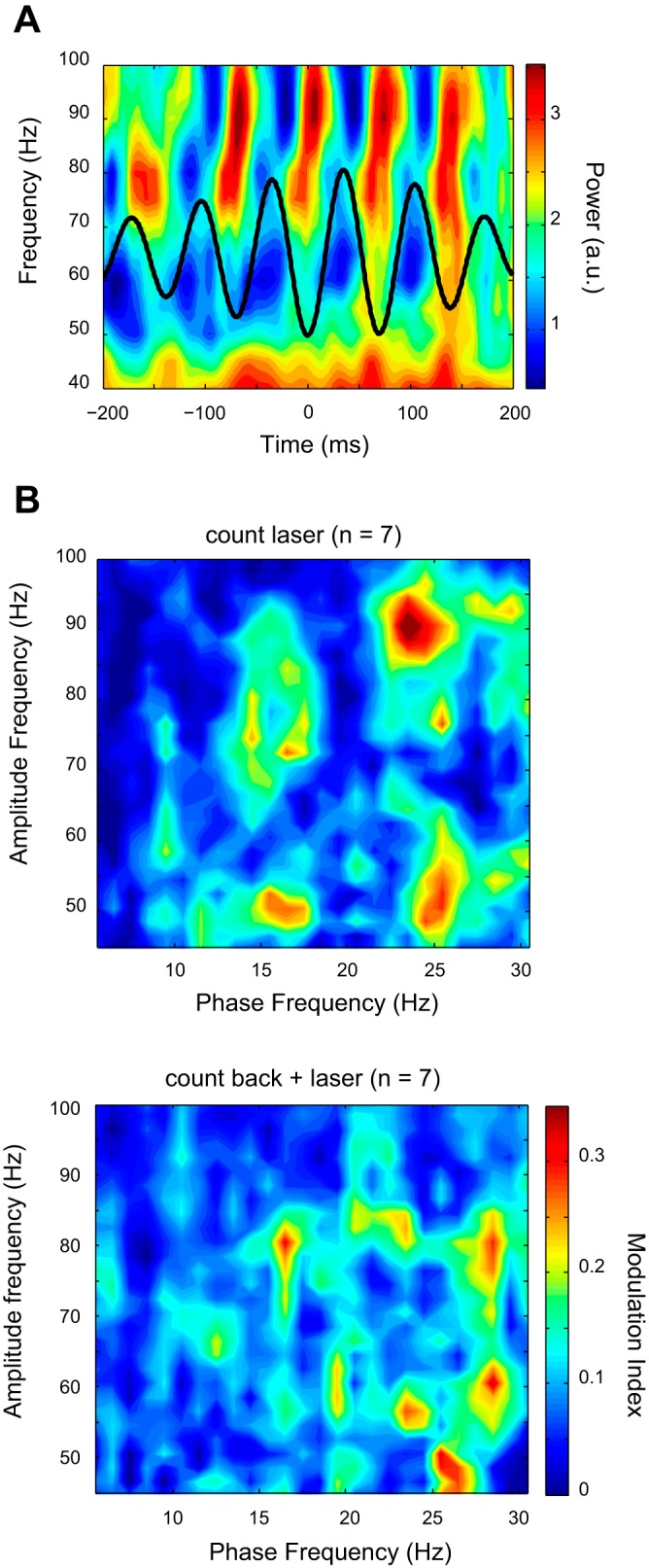
Cross-frequency coupling in the thalamus. A: example of phase-locked modulation of power in the LFP of the thalamus during the count laser task. Time-frequency plot of mean power modulation time-locked to the beta phase (13–15 Hz). B: example of the comodulogram shows the modulation index between low-frequency (theta-beta) and high-frequency (gamma) bands. Modulation indexes were averaged over 40 trials of laser stimulation. Red-colored region indicates significantly increased modulation index compared with mean prestimulus period. Most prominent cross-frequency coupling is located between beta and high-gamma frequency bands.
DISCUSSION
We now address the hypothesis that, during a series of painful cutaneous laser stimuli, thalamic signals will show stimulus-related gamma-band spectral activity, which is modulated by attention to vs. distraction from the painful stimulus. Both distraction from (count back plus laser task) and attention to the painful laser stimuli (count laser task) show gamma-band activation (Fig. 4). Significantly greater high-gamma activation was found during the count laser task for window 4, while greater high-gamma activation was found during the count back plus laser task for window 5 (Fig. 4).
Gamma-frequency activity has been recorded previously in the human thalamus (Kempf et al. 2009). That report described a narrow band (70–80 Hz) signal, which was recorded in the central median and VIM nuclei of patients with tremor, dystonia and epilepsy. These high-gamma signals were related to rapid eye movement sleep states, treated Parkinson's disease, and startle reactions. The signals recorded in Vim were coherent with those recorded in internal globus pallidus in a narrow band (70–80 Hz), but coherence of Vim with EEG signals was not studied.
High coherence of beta activity is evidence of synchronous thalamic and EEG activities, which may indicate functional interaction between thalamic activity and EEG activity. In particular, synchrony between cortical structures during attention to vs. distraction from the laser stimulus has also been demonstrated (Ohara et al. 2006). Synchrony and gamma-band activity (Engel and Singer 2001; Tallon-Baudry et al. 1996) may bind activity in different sensory modalities together and so produce an integrated sensation (Canolty and Knight 2010; Lisman and Jensen 2013). In the case of pain sensations, synchrony and gamma EEG activity induced by painful stimuli may bind together different dimensions of pain into an integrated sensation (Gross et al. 2007; Hauck et al. 2007; Zhang et al. 2012). Binding might occur as a result of the gamma-band oscillations themselves or as a result of nonspecific psychological phenomena related to gamma-band oscillations, e.g., attention (Fig. 4 and below).
Cross-frequency coupling may be another mechanism of interaction between different dimensions of pain. In the thalamus, we found that the phase of the low-frequency activity (theta to beta) modulated the amplitude of the higher frequency activity (low to high gamma) more strongly during the count laser task than during the count back plus laser task (Fig. 7B). This modulation may be a mechanism for the multiplexing of signals representing different components of a sensation (Canolty and Knight 2010; Lisman and Jensen 2013), such as different dimensions of pain, e.g., intensity, unpleasantness, cognitive, etc.
The different roles for gamma and beta bands have been suggested. The beta band is more related to long distance synchrony, and the gamma band is more related to local synchrony (Ermentrout and Kopell 1998; Kopell et al. 2000). Therefore, the high-gamma activity observed in the thalamus may be related to local synchrony, and beta activity may be related to thalamocortical connectivity, which is consistent with our analysis of coherence (Fig. 6). The beta band has the highest thalamic LFP × EEG coherence (Fig. 6), which is consistent with the potential involvement of beta-band activity in attentional processing (Buschman and Miller 2009) and in long-range communication between the thalamus and the cortex during attentional tasks (Engel and Fries 2010).
Mechanisms of synchrony between thalamus and cortex.
Synchrony between the thalamus and cortex may be related to the corticothalamic or thalamocortical connections. Corticothalamic fibers arise from layer 6 that terminate in the associated relay nucleus, such as S1 to Vc, while those arising from layer V are dispersed across several nuclei (Rausell et al. 1992a; Jones 2002). In turn, thalamocortical fibers from parvalbumen staining zones project to somatopically parcellated and layer-specific cortical zones, such as Vc to S1. In calbinden staining zones, adjacent neurons and divergent fibers can project to more than one cortical area, such as the nuclei located posterior to Vc which project to S1 and to cortical areas in and around PS (Rausell and Jones 1991; Rausell et al. 1992a). The present results may have been recorded from calbinden compartments of thalamic nuclei, which may be involved in mechanisms of acute and chronic pain (Rausell et al. 1992a, 1992b).
Direct physiological evidence of thalamocortical interactions has been found in reports of synchrony between feline thalamic neurons and lamina IV cortical neurons in response to innocuous cutaneous stimuli (Johnson and Alloway 1996). The synchrony between the thalamus and the cortex is greater during stimulus-evoked activity than during spontaneous activity (Alloway et al. 1995) and during synchronous firing between thalamic neurons (Roy and Alloway 2001). This firing was not found to be oscillatory either before or after the stimulus (Johnson and Alloway 1996).
Synchronous thalamocortical neuronal oscillatory activity may also be induced by somatic stimuli. These induced oscillatory activities have been described for thalamic beta- and gamma-band activity in the feline thalamus during behavioral activation, such as wakefulness or rapid eye movement sleep (Amzica et al. 1997; Steriade et al. 1996). Synchronized thalamic and cortical activity is also related to intrathalamic or thalamocortical connections and networks (Steriade et al. 1997). These mechanisms may explain the induced activity in the human thalamus and EEG activity during health and disease, such as chronic pain.
Patients with chronic pain show changes in ongoing thalamic activity (Sarnthein and Jeanmonod 2008). Specifically, the hypothesis of thalamocortical dysrhythmia has been proposed as the mechanism of a wide range of neurological and psychiatric diseases, including chronic pain (Llinas et al. 1999). Thalamocortical dysrhythmia is characterized by thalamic LFP with increased theta-band power, and by thalamic neurons with increased postinhibitory bursting. This hypothesis also suggests that beta- to gamma-band activity is found at the boundary (or edge) of the region where activity is characterized by low-frequency power.
Methodological considerations.
The terms “gamma band (>30 Hz)” or “40 Hz” have often been used to describe synchronous neuronal signals. However, the actual frequency ranges showing synchronization between neurons in different structures are variable and are mostly in the range of 20–60 Hz, which includes the activity below the gamma band (Engel and Singer 2001). Beta- and/or alpha-band synchrony (coherence) between separate brain regions have been reported in signals from EEG electrodes (Andres et al. 1999; Classen et al. 1998; Mima et al. 2001; von Stein et al. 1999), subdural electrodes (Ohara et al. 2001, 2004), and depth electrodes (Liu et al. 2011a; Tallon-Baudry et al. 2001). Therefore, synchronous oscillations are not necessarily limited to the gamma band or to particular cortical structures, especially in the case of large-scale synchrony.
The present studies are carried out in patients with essential tremor, which is often considered to be a monosymptomatic disorder with postural or kinetic tremor or both as the only symptoms (Elble 2006). Essential tremor leads to thalamic neuronal activity at the frequency of tremor in the theta range (Hua and Lenz 2005; Zakaria et al. 2013). To examine spontaneous activity in the absence of tremor, we have carried out all recordings in this study with the arm at rest, as confirmed by EMG analysis. This approach excludes tremor-related activity from the present analysis.
The present recordings were usually carried out in thalamic nuclei other than those primarily associated with somatic sensation e.g., Vc (Fig. 1). Neuronal pain-related activity is commonly found in functional imaging analysis or in single neuron analysis of thalamic and cortical structures, which do not primarily subserve somatic sensation (Apkarian et al. 2005; Lenz et al. 2010; Peyron et al. 2000). Therefore, it is not surprising that the thalamic LFP recording sites with pain-related oscillatory activity are not limited to primary somatic sensory nuclei, such as Vc (Fig. 1).
There are a number of controls that would be useful in the interpretation of the present results, such as the thalamic responses to ipsilateral or nonpainful somatic stimulation. However, the time constraints of these intraoperative controls obviate their being studied. Despite these constraints, the present study provides an important initial step in the study of pain-related gamma thalamic oscillations.
The presence of an evoked component in the ERSP can be detected by subtraction of the ERP from every epoch, which diminishes the phase-locked components of the ERSP (Bastiaansen and Hagoort 2003; Tallon-Baudry and Bertrand 1999). The difference between the ERSP with subtraction vs. without subtraction has been observed to be much smaller than the ongoing signal (Makeig 1993). We have been unable to record thalamic LEPs now (Fig. 3) or in the past (Kobayashi et al. 2009b), which is consistent with a prior report by another group (Valeriani et al. 2009). Therefore, we were unable to calculate non-phase-locked ERSP (with subtraction). However, we have reported laser peristimulus time histograms of thalamic single neuron activity (Kobayashi et al. 2009b) with peak latencies comparable with those of intracranial LEPs (Lenz et al. 1998a; 1998b). On this basis, we assume that the ERSP includes a component of evoked as well as induced activity.
Cognitive modulation of ongoing thalamic activity.
Our laboratory has previously examined poststimulus intervals while the subject attended to (count laser stimuli), or was distracted from, the painful laser stimulus by reading a story for comprehension (Ohara et al. 2006). The attention task was associated with more pairs of electrodes showing synchronous activity. Increased poststimulus vs. prestimulus synchrony was observed between MF and PS and between MF and S1 with attention (Ohara et al. 2006).
Directed functional interactions between these cortical areas may be due to interactions between the cortical areas (Liu et al. 2011b, 2011c) or due to interactions between cortical areas through unobserved modules, such as the thalamus. This latter suggestion is consistent with the present evidence of significant thalamocortical coherence during the laser-evoked pain (Fig. 6).
The interaction of cognition and laser evoked pain may be modeled by the activity of a “task-positive network,” which is increased both by the cognitive task and by pain. Therefore, the attentional resources may be assigned either to the cognitive task or to pain (Seminowicz and Davis 2007), which is consistent with the model that the two compete for attentional resources (Eccleston and Crombez 1999). Shifts in attention-related resources between the cognitive task and pain may result from changes in thalamic activity, or cortical activity, or their interaction (Kim et al. 2009; Kobayashi et al. 2009b). The present evidence of synchrony between the thalamus and the cortex raises the possibility of interactions related to common input from the thalamus to different cortical areas.
GRANTS
This work was supported by the National Institute of Neurological Disorders and Stroke (NS38493 to F. A. Lenz), by the Hopkins Neurosurgery Pain Research Institute (to F. A. Lenz), and by the Kil Chung-Hee Fellowship Fund, Korea University (to J. H. Kim).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.H.K., J.H.C., C.C.L., and F.A.L. performed experiments; J.H.K., J.H.C., and C.C.L. analyzed data; J.H.K., C.C.L., and F.A.L. interpreted results of experiments; J.H.K. prepared figures; J.H.K., J.H.C., C.C.L., and F.A.L. edited and revised manuscript; J.H.C., C.C.L., and F.A.L. approved final version of manuscript; C.C.L. and F.A.L. conception and design of research; F.A.L. drafted manuscript.
REFERENCES
- Alloway KD, Johnson MJ, Aaron GB. A comparative analysis of coordinated neuronal activity in the thalamic ventrobasal complex of rats and cats. Brain Res 691: 46–56, 1995. [DOI] [PubMed] [Google Scholar]
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Methods 73: 69–79, 1997. [DOI] [PubMed] [Google Scholar]
- Amzica F, Neckelmann D, Steriade M. Instrumental conditioning of fast (20- to 50-Hz) oscillations in corticothalamic networks. Proc Natl Acad Sci U S A 94: 1985–1989, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Brooks CM, Eccles JC, Sears TA. The ventro-basal nucleus of the thalamus: potential fields, synaptic transmission and excitability of both presynaptic and post-synaptic components. J Physiol 174: 348–369, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres FG, Mima T, Schulman AE, Dichgans J, Hallett M, Gerloff C. Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain 122: 855–870, 1999. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484, 2005. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Shi T. Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J Neurosci 14: 6779–6795, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Event-induced theta responses as a window on the dynamics of memory. Cortex 39: 967–992, 2003. [DOI] [PubMed] [Google Scholar]
- Baumgartner U, Vogel H, Ohara S, Treede RD, Lenz FA. Dipole source analyses of laser evoked potentials (LEP) obtained from subdural grid recordings from primary somatic sensory cortex. J Neurophysiol 106: 722–730, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H. Responses of spinal cord neurons to systematic changes in hindlimb skin temperatures in cats and primates. J Neurophysiol 38: 1060–1079, 1975. [DOI] [PubMed] [Google Scholar]
- Burton H. Corticothalamic connections from the second somatosensory area and neighboring regions in the lateral sulcus of macaque monkeys. Brain Res 309: 367–372, 1984. [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Serial, covert shifts of attention during visual search are reflected by the frontal eye fields and correlated with population oscillations. Neuron 63: 386–396, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science 313: 1626–1628, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci 14: 506–515, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Gerloff C, Honda M, Hallett M. Integrative visuomotor behavior is associated with interregionally coherent oscillations in the human brain. J Neurophysiol 79: 1567–1573, 1998. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Sejnowski TJ. Thalamcortical Assemblies. New York: Oxford University Press, 2001. [Google Scholar]
- Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull 125: 356–366, 1999. [DOI] [PubMed] [Google Scholar]
- Elble RJ. Report from a U.S. conference on essential tremor. Mov Disord 21: 2052–2061, 2006. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations–signalling the status quo? Curr Opin Neurobiol 20: 156–165, 2010. [DOI] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci 5: 16–25, 2001. [DOI] [PubMed] [Google Scholar]
- Ermentrout GB, Kopell N. Fine structure of neural spiking and synchronization in the presence of conduction delays. Proc Natl Acad Sci U S A 95: 1259–1264, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Schnitzler A, Timmermann L, Ploner M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol 5: e133, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data–theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64: 237–278, 1995. [DOI] [PubMed] [Google Scholar]
- Hauck M, Lorenz J, Engel AK. Attention to painful stimulation enhances gamma-band activity and synchronization in human sensorimotor cortex. J Neurosci 27: 9270–9277, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua SE, Lenz FA. Posture-related oscillations in human cerebellar thalamus in essential tremor are enabled by voluntary motor circuits. J Neurophysiol 93: 117–127, 2005. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the international federation. EEG Clin Neurophysiol 10: 371–375, 1958. [PubMed] [Google Scholar]
- Johnson MJ, Alloway KD. Cross-correlation analysis reveals laminar differences in thalamocortical interactions in the somatosensory system. J Neurophysiol 75: 1444–1457, 1996. [DOI] [PubMed] [Google Scholar]
- Jones EG. Thalamic circuitry and thalamocortical synchrony. Philos Trans R Soc Lond B Biol Sci 357: 1659–1673, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399: 781–784, 1999. [DOI] [PubMed] [Google Scholar]
- Kane A, Hutchison WD, Hodaie M, Lozano AM, Dostrovsky JO. Enhanced synchronization of thalamic theta band local field potentials in patients with essential tremor. Exp Neurol 217: 171–176, 2009. [DOI] [PubMed] [Google Scholar]
- Kempf F, Brucke C, Salih F, Trottenberg T, Kupsch A, Schneider GH, Doyle Gaynor LM, Hoffmann KT, Vesper J, Wohrle J, Altenmuller DM, Krauss JK, Mazzone P, Di L, V, Yelnik J, Kuhn AA, Brown P. Gamma activity and reactivity in human thalamic local field potentials. Eur J Neurosci 29: 943–953, 2009. [DOI] [PubMed] [Google Scholar]
- Kim JH, Ohara S, Lenz FA. Mental arithmetic leads to multiple discrete changes from baseline in the firing patterns of human thalamic neurons. J Neurophysiol 101: 2107–2119, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Kim JH, Anderson WS, Lenz FA. Neurosurgical treatment of tremor. In: Youman's Neurological Surgery, edited by Winn R. New York: Saunders, 2009a, p. 932–937. [Google Scholar]
- Kobayashi K, Winberry J, Liu CC, Treede RD, Lenz FA. A painful cutaneous laser stimulus evokes responses from single neurons in the human thalamic principal somatic sensory nucleus ventral caudal-Vc. J Neurophysiol 101: 2210–2217, 2009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A 97: 1867–1872, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz FA, Casey KL, Jones EG, Willis WD Jr. The Human Pain System: Experimental and Clinical Perspectives. New York: Cambridge University Press, 2010. [Google Scholar]
- Lenz FA, Rios M, Chau D, Krauss GL, Zirh TA, Lesser RP. Painful stimuli evoke potentials recorded from the parasylvian cortex in humans. J Neurophysiol 80: 2077–2088, 1998a. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Rios M, Zirh A, Chau D, Krauss G, Lesser RP. Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus. J Neurophysiol 79: 2231–2234, 1998b. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Seike M, Richardson RT, Lin YC, Baker FH, Khoja I, Jaeger CJ, Gracely RH. Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J Neurophysiol 70: 200–212, 1993. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Jensen O. The theta-gamma neural code. Neuron 77: 1002–1016, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Crone NE, Franaszczuk PJ, Cheng D, Schretlen DS, Lenz FA. Fear conditioning is associated with dynamic directed functional interactions between and within the human amygdala, hippocampus, and frontal lobe. Neuroscience 189: 359–369, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Ohara S, Franaszczuk PJ, Crone NE, Lenz FA. Attention to painful cutaneous laser stimuli evokes directed functional interactions between human sensory and modulatory pain-related cortical areas. Pain 152: 2781–2791, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Ohara S, Franaszczuk PJ, Lenz FA. Attention to painful cutaneous laser stimuli evokes directed functional connectivity between activity recorded directly from human pain-related cortical structures. Pain 152: 664–675, 2011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A 96: 15222–15227, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron 29: 145–156, 2001. [DOI] [PubMed] [Google Scholar]
- Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol 86: 283–293, 1993. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Ashby P, Limousin-Dowsey P, Rothwell JC, Brown P. Coherence between cerebellar thalamus, cortex and muscle in man: cerebellar thalamus interactions. Brain 123: 1459–1470, 2000. [DOI] [PubMed] [Google Scholar]
- Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci 27: 2858–2865, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima T, Oluwatimilehin T, Hiraoka T, Hallett M. Transient interhemispheric neuronal synchrony correlates with object recognition. J Neurosci 21: 3942–3948, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Attention to a painful cutaneous laser stimulus modulates electrocorticographic event-related desynchronization in humans. Clin Neurophysiol 115: 1641–1652, 2004. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Analysis of synchrony demonstrates “pain networks” defined by rapidly switching, task-specific, functional connectivity between pain-related cortical structures. Pain 123: 244–253, 2006. [DOI] [PubMed] [Google Scholar]
- Ohara S, Mima T, Baba K, Ikeda A, Kunieda T, Matsumoto R, Yamamoto J, Matsuhashi M, Nagamine T, Hirasawa K, Hori T, Mihara T, Hashimoto N, Salenius S, Shibasaki H. Increased synchronization of cortical oscillatory activities between human supplementary motor and primary sensorimotor areas during voluntary movements. J Neurosci 21: 9377–9386, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW. Digital Signal Processing. Englewood Heights, NJ: Prentice-Hall, 1975. [Google Scholar]
- Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin 30: 263–288, 2000. [DOI] [PubMed] [Google Scholar]
- Rausell E, Bae CS, Vinuela A, Huntley GW, Jones EG. Calbindin and parvalbumin cells in monkey VPL thalamic nucleus: distribution, laminar cortical projections, and relations to spinothalamic terminations. J Neurosci 12: 4088–4111, 1992a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausell E, Cusick CG, Taub E, Jones EG. Chronic deafferentation in monkeys differentially affects nociceptive and nonnociceptive pathways distinguished by specific calcium-binding proteins and down-regulates gamma-aminobutyric acid type A receptors at thalamic levels. Proc Natl Acad Sci U S A 89: 2571–2575, 1992b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausell E, Jones EG. Chemically distinct compartments of the thalamic VPM nucleus in monkeys relay principal and spinal trigeminal pathways to different layers of the somatosensory cortex. J Neurosci 11: 226–237, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SA, Alloway KD. Coincidence detection or temporal integration? What the neurons in somatosensory cortex are doing. J Neurosci 21: 2462–2473, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnthein J, Jeanmonod D. High thalamocortical theta coherence in patients with neurogenic pain. Neuroimage 39: 1910–1917, 2008. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Morel A, von Stein A, Jeanmonod D. Thalamic theta field potentials and EEG: high thalamocortical coherence in patients with neurogenic pain, epilepsy and movement disorders. Thalamus Relat Syst 2: 231–238, 2003. [Google Scholar]
- Schaltenbrand G, Walker AE. Stereotaxy of the Human Brain. New York: Thieme-Stratton, 1982. [Google Scholar]
- Seminowicz DA, Davis KD. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J Neurophysiol 97: 3651–3659, 2007. [DOI] [PubMed] [Google Scholar]
- Singer W. Dynamic formation of functional networks by synchronization. Neuron 69: 191–193, 2011. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Amzica F, Timofeev I. Synchronization of fast (30–40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J Neurosci 16: 2788–2808, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Jones EG, McCormick DA. Thalamus Organisation and Function. Amsterdam: Elsevier, 1997. [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3: 151–162, 1999. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci 16: 4240–4249, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Fischer C. Oscillatory synchrony between human extrastriate areas during visual short-term memory maintenance. J Neurosci 21: RC177, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci U S A 106: 20942–20947, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci U S A 105: 20517–20522, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeriani M, Truini A, Le PD, Insola A, Galeotti F, Petrachi C, Mazzone P, Cruccu G. Laser evoked potential recording from intracerebral deep electrodes. Clin Neurophysiol 120: 790–795, 2009. [DOI] [PubMed] [Google Scholar]
- von Stein A, Rappelsberger P, Sarnthein J, Petsche H. Synchronization between temporal and parietal cortex during multimodal object processing in man. Cereb Cortex 9: 137–150, 1999. [DOI] [PubMed] [Google Scholar]
- Zakaria R, Lenz FA, Hua S, Avin BH, Liu CC, Mari Z. Thalamic physiology of intentional essential tremor is more like cerebellar tremor than postural essential tremor. Brain Res 1529: 188–199, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Hu L, Hung YS, Mouraux A, Iannetti GD. Gamma-band oscillations in the primary somatosensory cortex–a direct and obligatory correlate of subjective pain intensity. J Neurosci 32: 7429–7438, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]