Abstract
Prostate cancer is the second leading cause of cancer-related deaths in men in the U.S. At present, no single or combination therapy has shown efficacy in decreasing disease progression in patients with metastatic disease. A potentially viable approach for treating late-stage prostate cancer is gene therapy. Adenoviruses (Ad) are the most commonly used mode of gene delivery, but progress using this vector has been hampered by concerns over the safety and practicality of viruses including conditionally replicating Ads (CRAds), particularly for intravenous delivery, and the inefficiency of non-viral transfection techniques. Major challenges for effective gene therapy using Ads are the limited infectivity of regular Ad serotype 5 (Ad5) and the inability to specifically deliver the therapeutic directly into diseased tissue without trapping in the liver or elimination by the immune system. The shortcoming in using Ad5 is mostly attributed to a reduction in Coxsackie-adenovirus receptors (CAR) on the surface of cancer cells, which can be mitigated by generating tropism-modified Ads permitting CAR-independent infection of tumor cells. The limitations of systemic gene delivery can now be overcome by using a novel targeted-delivery approach such as ultrasound (US) contrast agents (microbubbles) to deliver effective therapeutic reagents, Ads, or recombinant proteins, combined with ultrasound-targeted microbubble destruction (UTMD), to develop a site-specific therapy in immune competent transgenic mouse models. These unique strategies for enhancing the efficacy of gene therapy provide a direct path to translation from the laboratory into the clinic for developing an effective gene therapy of prostate cancer.
Introduction
Prostate cancer is the most common cancer and the second leading cause of cancer-related deaths in men in the US (Damber and Aus, 2008). It is estimated that 217,730 new cases of prostate cancer will have been diagnosed in 2010 alone. The therapeutic options for patients with prostate cancer include radiotherapy and treatment with cytotoxic chemotherapeutic agents. Despite a palliative benefit, these approaches do not engender a long-term beneficial effect on the overall survival of patients. Late stage prostate cancer patients may benefit from hormone therapy, which removes a primary factor mediating tumor growth, male hormones (androgens) (Di Lorenzo and De Placido, 2006; Sternberg, 2002). Unfortunately, after a few years most patients become non-responsive to this treatment, resulting in uncontrolled disease and patient death. In these contexts, there is a pressing need to develop more effective therapeutic approaches for end-stage prostate cancer patients and genetic therapies represent promising approaches for the treatment of this neoplasm.
The prostate gland is not vital for survival and is accessible by ultrasound, potential therapeutic genes can be injected directly into the primary tumor, tissue-specific promoters exist that can target therapeutic gene expression or viral replication uniquely in the prostate gland, and disease progression can be monitored by measuring prostate-specific antigen (PSA) (Anderson, 1998; Gopalkrishnan et al., 2001; Mabjeesh et al., 2002). Since prostate cancer is commonly a relatively slow-growing disease, it may be necessary to use repeated gene therapy approaches, with single or multiple genes, over the lifespan of the patient. Adenoviruses (Ads) are the most commonly used vehicle for gene therapy approaches because the technology for virus production at high titers is established and Ad structure, genome, and replication cycle are well characterized thus facilitating the engineering of these viruses for therapeutic purposes. Although promising in vitro and in vivo results have been achieved using Ad vectors, administering unmodified serotype 5 Ads (Ad5) for gene delivery faces a number of clinical limitations. These include down-regulation of Coxsackie-adenovirus (CAR) receptors in cancer cells resulting in failure to transduce the majority of tumor cells by Ad5 (Anderson, 1998; Haviv et al., 2002; Mabjeesh et al., 2002).
An effective systemic gene delivery method is required to ensure safe and targeted delivery as administration of Ad-based gene therapy results in hepatic sequestration of the Ad so that very little reaches the target tumor tissue and clearance of the Ad from the circulation by the immune system. To develop safe and more efficient systemic delivery systems, we are focusing on ultrasound (US) contrast agents (microbubbles) (UTMD: ultrasound-targeted microbubble destruction) to enhance delivery of molecules in vivo. This review will summarize unique and novel aspects of effective gene therapy for prostate cancer that offers significant promise for moving basic science studies in the laboratory into the clinic to hopefully develop a ‘cure’ for advanced prostate cancer.
Gene Therapy for Prostate Cancer
Using subtraction hybridization combined with induction of cancer cell terminal differentiation, our laboratory cloned mda-7/IL-24 (Jiang et al., 1995), a novel member of the IL-10-related cytokine gene family (Dash et al., 2010a; Gupta et al., 2006; Pestka et al., 2004; Sauane et al., 2003). Subsequent experiments documented that mda-7/IL-24 had nearly ubiquitous antitumor properties in vitro and in vivo, which led to its successful entry into the clinic in an unprecendented 5 years after discovery where acceptable safety and clinical efficacy, when administered by Ad (Ad.mda-7; INGN 241), has been demonstrated in Phase I clinical trials in humans with advanced carcinomas and melanomas (Cunningham et al., 2005; Eager et al., 2008; Emdad et al., 2009; Fisher, 2005; Fisher et al., 2003; Fisher et al., 2007; Lebedeva et al., 2007; Lebedeva et al., 2005; Tong et al., 2005). Its mechanism of action involves preferential induction of autophagy and apoptosis in prostate cancer without exerting harmful effects to normal cells (Bhutia et al., 2010; Dash et al., 2010b; 2010c; Gao et al., 2008; Miyahara et al., 2006; Sarkar et al., 2007a; Sauane et al., 2003; Su et al., 2005a; Su et al., 2001a; Su et al., 2006; Yacoub et al., 2008). Additional targets of mda-7/IL-24 action have also been investigated supporting its considerable potential as a gene therapeutic for cancer. Forced mda-7/IL-24 expression in cancer cells inhibits angiogenesis, stimulates an anti-tumor immune response (Gao et al., 2008; Miyahara et al., 2006), sensitizes cancer cells to radiation-, chemotherapy-, and antibody-induced killing (McKenzie et al., 2004; Su et al., 2005a; Su et al., 2001a; Su et al., 2006), and elicits potent “antitumor bystander activity” (Chada et al., 2004; Sauane et al., 2008; Su et al., 2001; Su et al., 2005a). MDA-7/IL-24 protein induces a sustained ER stress response as evidenced by expression of ER stress markers (BiP/GRP78, GADD153, GRP94, and phospho-eIF2α) and production of reactive oxygen species (ROS), indicating that both intracellular and secreted proteins activate similar signaling pathways to induce apoptosis (Sauane et al., 2008).
As prostate cancer requires repeated gene therapy approaches, the use of replication defective Ads to administer therapeutic and cytotoxic genes and conditionally replication competent Ads (CRAds) to selectively induce cytolysis in prostate tumor cells represent feasible treatment options (Anderson, 1998; Mabjeesh et al., 2002). Using subtraction hybridization we cloned a novel rodent gene, progression elevated gene-3 (PEG-3), in the context of tumor progression in transformed rat embryo cells (Su et al., 1997). PEG-3 (i) displays elevated expression as a function of oncogenic transformation (by diverse oncogenes) (Park et al., 2001; Su et al., 1997), (ii) induces an aggressive cancer phenotype without promoting transformation when expressed in normal cells (Emdad et al., 2005a; Emdad et al., 2005b; Park et al., 2001; Su et al., 1999; Su et al., 2002; Su et al., 1997), and (iii) is regulated by a gene promoter (PEG-Prom) shown to display elevated expression in both rodent and human tumors (including prostate carcinomas) with negligible expression in normal cells (including human prostate epithelium) (Park et al., 2001; Su et al., 2000; Su et al., 2001b; Su et al., 2005b).
To test the cancer specificity of the PEG-Prom for tumor imaging in vivo, we used a firefly luciferase reporter PGL3-PEG-prom-Luc (pPEG-Luc) (Bhang et al., 2011; Su et al., 2005b). After confirmation of the presence of metastatic nodules in the lung by computed tomography (CT) at 4-6 weeks after intravenous administration of the human malignant breast cancer cell line (MDA-MB-231) or melanoma (MeWo), animals (athymic nude mice) received an intravenous dose of pPEG-Luc/PEI polyplex (PolyplusTransfection). Forty-eight hours after plasmid DNA (pDNA) delivery, PEG-Prom-driven gene expression was assessed by bioluminescence imaging (BLI). Quantification of the BLI signal intensity from the thoracic cavity, which represents Luc expression mainly in lung, showed significantly higher PEG-Prom activity in the model of melanoma or breast cancer metastasis as compared to controls, which did not show a detectable signal (Bhang et al., 2011). Additionally, it was possible to use repeat administrations of pPEG-Luc/PEI in tumor-bearing animals, which permitted us to follow growth and development of new metastatic lesions over time (Bhang et al., 2011).
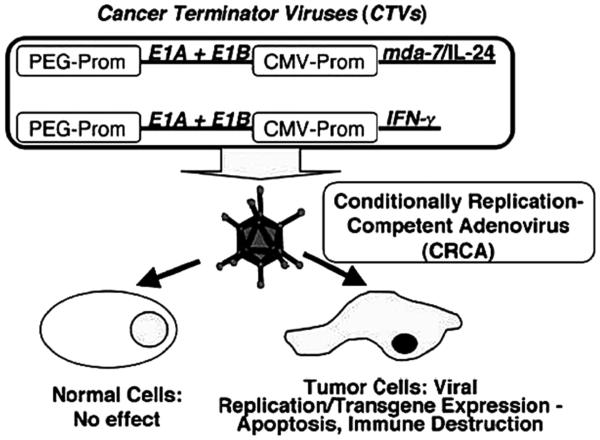
Considering the cancer-specific expression property of the PEG-Prom, we constructed a bipartite CRAd [called a Cancer Terminator Virus (CTV)] in which the expression of E1A and E1B genes of Ad, necessary for replication, is regulated by the PEG-Prom (Figure 1) (Sarkar et al., 2005). This novel biCRAd (CTV) also expressed mda-7/IL-24 in the E3 region (Ad.PEG-E1A-mda-7). To test our hypothesis of cancer-specific activity and therapeutic effectiveness of the Ad.PEG-E1A-mda-7, experiments were done in three prostate cancer cell lines, androgen-nonresponsive DU-145 and PC-3 cells, and androgen-responsive LNCaP cells and their Ad.mda-7-resistant variants (i.e., DU-145-Bcl-2, DU-145-Bcl-xL, PC3-Bcl-2 and PC-3-Bcl-xL, DU-145, and PC3 that stably expresses Bcl-2 and Bcl-xL). As a control, P69 normal prostate epithelial cells immortalized by SV40 T/t antigen were used (Sarkar et al., 2007b). From Western blot analysis it was evident that infection of normal immortal human P69 prostate epithelial cells with Ad.CMV-E1A (CRAd where E1A is driven under CMV promoter control) or Ad.CMV-E1A-mda-7 (bipartite CRAd where both E1A and mda-7/IL-24 are driven by the CMV promoter) but not with Ad.PEG-E1A (CRAd where E1A is driven under CMV promoter control) or Ad.PEG-E1A-mda-7 (CTV) resulted in production of E1A proteins; whereas in prostate cancer cells, infection with all four replication-competent Ads generated E1A proteins. In P69 cells, infection with Ad.CMV-E1A-mda-7 or Ad.CMV-mda-7 resulted in MDA-7/IL-24 protein production, whereas infection with Ad.PEG-mda-7 or Ad.PEG-E1A-mda-7 (CTV) resulted in barely detectable levels of MDA-7/IL-24 protein production. In prostate cancer cells, infection with Ad.CMV-mda-7, Ad.PEG-mda-7, Ad.CMV-E1A-mda-7, or Ad.PEG-E1A-mda-7 (CTV) generated significant MDA-7/IL-24 production. No MDA-7/IL-24 protein production could be detected in uninfected control cells or following infection with Ad.vec, Ad.CMV-E1A, or Ad.PEG-E1A (Sarkar et al., 2007b). These findings document that the PEG-Prom facilitates cancer cell-selective replication of Ads and concomitant mda-7/IL-24 expression. The effects of the engineered Ads on cell viability and apoptosis were evaluated in the various prostate cell lines. In P69 cells, infection with only Ad.CMV-E1A or Ad.CMV-E1A-mda-7, but not with Ad.PEG-E1A, Ad.CMV-mda-7, Ad.PEG-mda-7, or Ad.PEG-E1A-mda-7 (CTV), induced profound growth inhibition (Sarkar et al., 2007b). In contrast, in all prostate cancer cells, both parental and mda-7/IL-24-resistant, Ad.CMV-E1A-mda-7, Ad.PEG-E1A-mda-7 (CTV), Ad.CMV-E1A, and Ad.PEG-E1A infection resulted in significant growth inhibition, indicating potential therapeutic applications of the CTV in prostate cancer patients frequently showing Bcl-2 and Bcl-xL over-expression. Replication of Ad.PEG-E1A-mda-7 results in robust amounts of mda-7/IL-24 production resulting in a potent antitumor immune response. Moreover, in vivo assays in established melanoma, breast cancer, and therapy-resistant prostate cancer xenografts in athymic nude (immunocompromized) mice showed that injection of Ad.PEG-E1A-mda-7 completely eradicated not only the primary tumors but also distant tumors (Sarkar et al., 2007b; Sarkar et al., 2008; Sarkar et al., 2005).
Figure 1.
Schematic representation of cancer terminator viruses (CTVs). In the CTVs the PEG-Prom drives the expression of E1A and E1B genes thus ensuring cancer-specific replication while the CMV-Prom regulates the expression of either mda-7/IL-24 or IFNγ in the E3 region of the Ad. These conditionally replication competent adenoviruses (CRCA) do not harm normal cells but induce oncolysis by Ad replication and diverse tumor-suppressor effects of the expressed transgene. (Reproduced with permission of the publisher, from Sarkar et al., 2005).
Construction of tropism modified Ads for enhanced therapeutic efficacy for prostate cancer cells
The most frequently used serotype of Ad for gene therapy has been recombinant forms of the type 5 Ad (Ad.5). Ad.5 utilizes CAR for infective entry into cells (Glasgow et al., 2004). In many tumor types, for example malignant glioma, ovarian cancer, renal cancer, and prostate cancer, and particularly in primary tumor specimens, it has been noted that the expression of CAR is reduced or absent in tumor cells compared to surrounding non-tumor tissue. Reduced CAR expression precludes efficient transduction of cancer cells by Ad.5 (Paul et al., 2008; Tsuruta et al., 2007). This finding may in part explain why gene therapy approaches using Ad.5 have not been as successful as the studies performed in vitro using established cell lines.
CAR is expressed in established cell lines at a higher level than that observed in primary tumors in patients. An approach to circumventing the low efficiency of Ad.5 infection of tumor cells involves “tropism modification” in which the virus capsid proteins that normally associate with CAR are modified, permitting CAR-independent infectivity of tumor cells. To achieve enhanced infectivity, the infective viral capsid “knob” has been modified to bind to surface integrin proteins whose expression is enhanced upon transformation (RGD/DRGD modification) (Glasgow et al., 2004; Paul et al., 2008; Tsuruta et al., 2007). Additionally, insertion into the knob of multiple lysine residues (pK7/PK) which will increase viral interaction with cells by electrostatic effects, or by including portions of type 3 Ad in the viral capsid knob (Ad.5/3), has been engineered (Tsuruta et al., 2007).
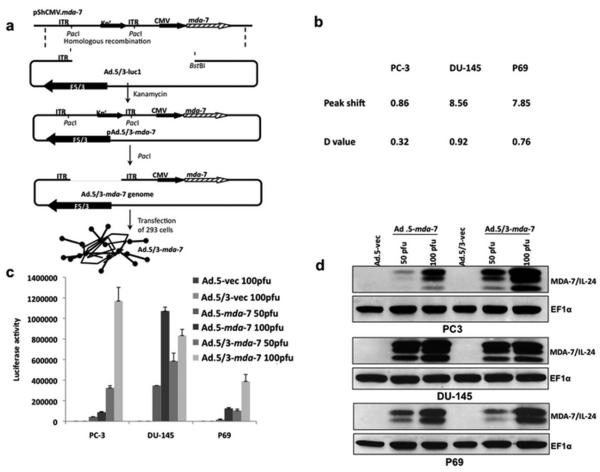
Experiments were performed to directly compare the activity of Ad.5/3 chimeric viruses (expressing luciferase or mda-7/IL-24) vs. Ad.5 viruses (expressing luciferase or mda-7/IL-24) in the context of prostate carcinoma cells that contain reduced levels of CAR on their surface (Figure 2, Figure 2A). For this purpose, we chose PC-3 cells, which have a reduced level of CAR in comparison with DU-145 or LNCaP prostate carcinoma cells (Figure 2B). As a first test of comparative transduction efficiency we constructed Ads that express luciferase and evaluated relative expression as a function of infection of PC-3 cells. When PC-3 cells were infected with Ad.5/3-Luc, the level of luciferase activity with lower p.f.u. was significantly greater than that observed with Ad.5-Luc (Figure 2C). On the other hand, the level of luciferase activities in DU-145 with Ad.5/3-Luc and Ad.5-Luc was comparable. Studies were next performed using two Ads genetically engineered to express mda-7/IL-24, including Ad.mda-7 (mda-7/IL-24 cloned into an Ad.5 virus) and Ad.5/3-mda-7 (mda-7/IL-24 cloned into an Ad.5/3 virus) (Dash et al., 2010b). Infection of PC-3 cells with Ad.5-mda-7 was significantly less effective in reducing cell proliferation and viability than Ad.5/3-mda-7. This differential effect correlated with a reduced level of MDA-7/IL-24 protein being produced in PC-3 cells infected with Ad.mda-7 vs. Ad.5/3-mda-7 (Figure 2D). Correlating with the in vitro data, Ad.5/3-mda-7 also showed profound enhanced antitumor activity (Dash et al., 2010b) as compared to Ad.5-mda-7 in PC-3 xenograft models using nude mice. These findings provide definitive evidence for enhanced therapeutic efficacy of the Ad.5/3-mda-7 virus vs. Ad.5-mda-7 in prostate cancer cells with reduced CAR. Further results provide definitive evidence for enhanced therapeutic efficacy of the Ad.5/3-mda-7 virus vs. Ad.5-mda-7 in ovarian carcinoma, malignant glioma, and renal carcinomas with reduced CAR (Hamed et al., 2010; Park et al., 2010; Yacoub et al. 2010).
Figure 2.
Tropism-modified adenovirus (Ad.5/3) shows enhanced infectivity in PC-3 low Coxsackie-adenovirus receptor (CAR) prostate cancer cells. (a) Schematic representation showing the construction of tropism-modified Ad for delivery of mda-7/IL-24. (b) Expression of CARs on the surface of DU-145 and PC-3 prostate carcinoma cells and P69 SV40 immortalized normal prostate epithelial cells. (c) P69, DU-145 and PC-3 cells were infected with the indicated p.f.u. per cell of Ad.5-Luc or Ad.5/3-Luc; and luciferase activity was determined 48 hours later. (d) P69, DU-145 and PC-3 cells were infected with the indicated p.f.u. per cell of Ad for 48 hours and total proteins were isolated. The expressions of MDA-7/IL-24 and EF-1a (as a loading control) proteins were analyzed by Western blotting analyses. (Reproduced with permission of the publisher, from Dash et al., 2010a).
Microbubble-assisted gene therapy
The quest for novel, safe, and more efficient systemic gene delivery systems has recently highlighted ultrasound (US) contrast agents (microbubbles) as a potential candidate for enhancing delivery of molecules to target tissue (Goldberg et al., 1994; Larina et al., 2005; Lawrie et al., 2000; Ng and Liu, 2002). Currently used US contrast agents (microbubbles) contain high-molecular weight gases with less solubility and diffusivity, which improves microbubble persistence and allows passage through the microcirculation. Microbubbles can be injected in peripheral veins, as more robust bubbles can re-circulate through the systemic circulation numerous times, surviving for several minutes within the bloodstream (Goldberg et al., 1994). The ideal microbubble diameter is likely 2.5 to 4 μm. This is small enough to prevent entrapment within the pulmonary capillary bed (ranging from 5 to 8 μm in diameter), but big enough to entrap and protect viral vectors such as Ad from the environment. The gas-filled microspheres effectively lower the energy threshold for non-thermal cavitation. Ultrasound-targeted microbubble destruction (UTMD) enables focal release of entrapped materials as well as the creation of small shock waves that increase cellular permeability (Pitt et al., 2004). In addition, the microbubbles protect the viruses from rapid degradation by the immune system, thus allowing for intravenous injection rather than direct target organ delivery by catheter-based approaches or operative bed injection (Howard et al., 2006). This is particularly important in cancer gene therapy of potentially inaccessible tumors because the microbubbles may also limit the amount of inflammatory response to the viruses and may allow repeated injections.
Targeting ligands on the surface of microbubbles permit the selective accumulation of these particles in the areas of interest, such as up-regulated levels of receptor/prognostic marker molecules on vascular endothelium or tumor cells. Decorated microbubbles coupled (covalently or non-covalently) with small targeting ligands/peptides have been designed to achieve maximum tissue-specific accumulation for enhanced US-based imaging (Weller et al., 2005). Studies involving Phase I to III clinical trials have demonstrated that US contrast agent (microbubbles) are safe and well tolerated even at higher doses (Bhatia and Senior, 2008).
Proof-of-principle for site-specific delivery of Ad.PEG-E1A-mda-7 (CTV) in vivo
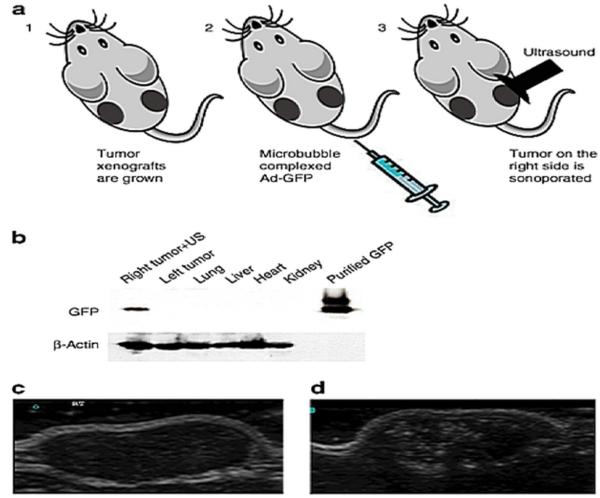
To confirm the ability of microbubble (Targestar-P) to deliver viruses efficiently and specifically, we performed a pilot study in which tumor xenografts were established in both flanks of athymic nude mice by injecting each site with 2 × 106 DU-145 human prostate carcinoma cells (Figure 3). The DU-145 tumor-bearing nude mice (n = 5) were then injected in their tail vein with 100 mL of Targestar-P contrast agent that was reconstituted with Ad-GFP or water as control as previously reported, and a portable SonoSite Micro-Maxx ultrasound platform (SonoSite, Inc., Bothell, WA) equipped with an L25 linear array transducer set at 0.7 Mechanical Index (MI), 1.8 MPa for 10 minutes, was used to sonoporate only the tumor implanted on the right side (Figure 3A). Tissues from different sites were then harvested and snap frozen. Figure 3B shows specific delivery to the right tumor as evidenced by expression of the green fluorescence protein (GFP) in an immunoblot in which total protein extracts were run on a 10% SDS-PAGE gel. As a GFP control, GST-GFP fusion protein was loaded (Figure 3B). Protein gel loading was normalized using β-actin as a control. These results support the application of microtubules for systemic delivery of viruses. We next explored the systemic delivery of the antitumor gene mda-7/IL-24 by microbubbles. For this proof-of-principle study, we used DU-145-Bcl-xL (DU-145 ectopically express Bcl-XL), an Ad.mda-7-resistant variant of DU-145 human prostate cancer cells (Greco et al., 2010). The therapeutic arm of this work included the CTV (Ad.PEG-E1A-mda-7) (Sarkar et al., 2006; Sarkar et al., 2002; Sarkar et al., 2008; Sarkar et al., 2005). DU-Bcl-xL tumor xenografts were established on both flanks of nude mice by injecting 2 × 106 cells in each side of the animal. Treatment was initiated when the tumor reached a size of 250-350 mm3. Four injections of the various Ad/microbubble complexes into the tail vein once per week (total of 4 weeks) were administered followed by US for 10 minutes on the tumor on the right side only. No treatment was performed on the tumor xenografted on the left flank. Animals receiving the Ad-GFP-microbubble complexes plus US treatment showed no statistically significant effect on the growth of DU-145-Bcl-xL tumors. CTV/Microbubble elicited a sustained growth inhibition of the therapy resistant DU-Bcl-xL tumor xenografts in both primary and distant tumors (Greco et al., 2010). A Western blotting analysis of total protein extracts from the harvested tumors showed expression of MDA-7/IL-24 protein in both the tumor samples implanted on the right and left flank validating the “bystander” effects of MDA-7/IL-24 previously reported (Sauane et al., 2008; Su et al., 2005a). The amplified expression of MDA-7/IL-24 in the non-injected left tumor may also reflect secondary viral infection by the CRAd (Sarkar et al., 2007b). Control tumors treated with Ad-GFP-microbubble complexes were mostly TUNEL negative. Treatments with the CTV-microbubble complexes plus US disrupted the tumor cyto-architecture, which correlated with an increase in the number of TUNEL positive tumor cells in both sonoporated right and untreated left tumors. Control tumors treated with Ad-GFP-microbubble complexes were TUNEL negative. B-mode ultrasound-scan of DU-Bcl-xL tumors showed dramatic volume reductions in the tumors after 2 and 4 weeks of treatments with CTV-microbubble complexes and US leading to the eradication of the tumor xenograft. Additionally, no tumor regrowth in the primary or distant sites was evident in CTV-microbubble complex and US-treated DU-Bcl-xL animals after an additional three weeks post-treatment (Greco et al., 2010). To investigate if the tumor would recur after a longer period of time following the last treatment, three out of ten animals initially treated with CTV-microbubble complexes were not sacrificed at the endpoint of the study and were maintained for an additional 3 months. The mice were then sacrificed and dissected to look for potential tumor recurrence and/or eventual tumor spread. We did not observe any local tumor recurrence or distant metastasis in the lungs or liver in these mice that were treated with CTV-microbubble complexes.
Figure 3.
Microbubble-assisted gene delivery. (a) Schematic representation of the microbubble delivery of Ad-GFP complexes and ultrasound (US) release in a tumor target site of the mouse. (b) Western blotting analysis of Ad-GFP/microbubble–transduced DU-145 tumor xenografts. Immunoblot showing the expression levels of green fluorescent protein (GFP) in DU-145 cells following ultrasound-targeted microbubble/Ad transduction of GFP at 96 hours. Only the tumor on the right flank was sonoporated for 10 min resulting in the delivery and expression of GFP. The left tumor, heart, lung, liver, and kidney were negative for GFP expression. GST–GFP was used as a positive control. Protein gel loading was normalized using β-actin as a control. (c) Ultrasound imaging and US contrast enhancement of in vivo transduced DU-145 tumor xenografts. B-mode US imaging of a tumor before MB contrast agent injection. (d) B-mode US imaging of the same tumor depicted in c following injection of microbubbles/Ad-GFP complexes. MBs cavitation within the targeted tumor dramatically enhances the tumor image within the US field of view. Ad, adenovirus; MB, microbubbles. (Reproduced with permission of the publisher, from Greco et al., 2010.)
Microbubble Encapsulated Ads Display Reduced Immunogenicity
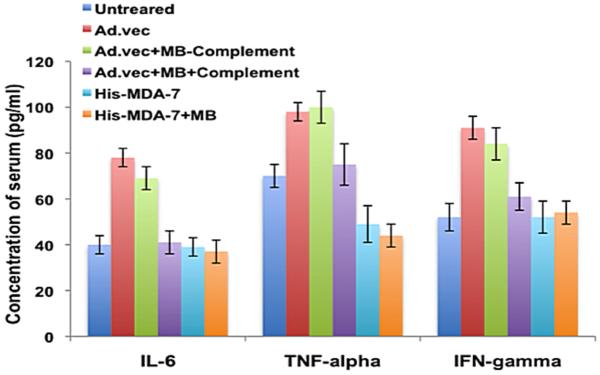
Although Ads are the most commonly used vector for gene therapy, major disadvantages of this vehicle are its ability to be sequestered in the liver and to elicit robust innate immune and inflammatory responses when injected systemically (Koizumi et al., 2007). Improvement of therapeutic index of Ad-based gene therapy requires a method to shield viruses from exposure to the immune system. We hypothesized that microbubble (MB) encapsulated Ads (at a dose of 1 × 1012 p.f.u.) or recombinant protein (10 nM) will not be exposed to the immune system following systemic injection. As predicted, microbubble-encapsulated Ad.vec (non-replicative Ad which do not express any transgene) treated with complement (Greco et al., 2010) did not elicit an innate immune response (i.e., activation of IL-6, TNF-α and IFN-γ) at 12 hours following intravenous tail vein injection into the C57B6 mice, whereas the Ad.vec alone or microbubble-encapsulated Ad.vec without complement treatment were immunogenic (Figure 4). We also observed a similar inflammatory cytokine profile after 24 hours of intravenous injection into mice (data not shown). Interestingly, neither the His-MDA-7 (Histidine tagged recombinant MDA-7/IL-24) nor the microbubble-encapsulated His-MDA-7 induced an immune response following intravenous injection into immunocompetent C57B6 mice. These results suggest that the microbubble can protect the viruses from exposure to the immune system and His-MDA-7 may not be immunogenic (Figure 4).
Figure 4.
Microbubble Encapsulated Ads Display Reduced Immunogenicity. The indicated vectors were injected systemically (i.v.) into the tail veins of C57B6 mice (n = 5). Complement (Sigma) was added to one set of the microbubble/Ad complex, whereas another microbubble/Ad complex was not treated with complement. 12 hours after the injections serum was collected from the mice. The indicated cytokines were tested using the Bio-Plex mouse cytokine 23-plexpanel kit with mouse serum samples as described by Bio-Rad Laboratories.
Conclusion
Therapy of cancer using Ads has been restricted for a number of reasons, particularly when utilizing systemic administration routes. These include: limitations in tumor transduction efficiency that are frequently mediated by a reduction in the number of CAR that regulate Ad entry into cancer cells (Paul et al., 2008; Tsuruta et al., 2007); sequestering of Ads in the liver limiting virus delivery to disseminated tumors (Koizumi et al., 2007); neutralization of viruses by the immune system (Koizumi et al., 2007); and absence of broad-spectrum anti-tumor agents capable of selectively killing cancer cells and provoking elimination of disseminated metastatic tumors through potent “bystander’“anti-tumor activity (Fisher, 2005; Sarkar et al., 2007b; Sarkar et al., 2008; Sarkar et al., 2005). We have attempted to overcome these barriers to achieve effective systemic therapy of cancer using a number of innovative approaches. We have modified the infectivity tropism of Ad by producing chimeric viruses containing regions of both Ad type 5 and Ad type 3, Ad.5/3, which allow CAR-independent transduction of tumor cells. Ad.5/3 shows superiority in transducing genes in a CAR-independent manner in prostate cancer and is effective in cells with both low and high CAR receptors (Dash et al., 2010b; Hamed et al., 2010; Park et al., 2010; Yacoub et al., 2010). To prevent trapping of Ads in the liver and elimination of viruses by the immune system, we have developed a novel approach in which Ads, both replication incompetent and conditionally replication competent, are incorporated in a perfluorocarbon microbubble that is treated with complement (to inactivate and mask viruses on the surface of the microbubble from the immune system) and then administered systemically and released in the tumor microenvironment through ultrasound, i.e., the UTMD approach (Greco et al., 2010). Early phase clinical studies suggest that mda-7/IL-24 may be an effective agent for gene therapy of primary and metastatic cancers (Cunningham et al., 2005; Tong et al., 2005). This novel IL-10-family member cytokine selectively kills cancer cells without harming normal cells, displays potent systemic “bystander“ antitumor effects, inhibits tumor angiogenesis, stimulates the immune system resulting in long term antitumor effects, and potentiates the therapeutic activity of currently used modalities of therapy, including radiation, chemotherapy, and monoclonal antibodies (Emdad et al., 2007; Gupta et al., 2008; Lebedeva et al., 2007; Su et al., 2006; Dash et al., 2010b). We have now generated Ad.5/3-CTV (tropism modified CTV), which we intend to evaluate this virus for delivery into the prostate of immunocompetent prostate cancer transgenic mice (Hi-Myc) by the UTMD technique. Successful completion of our proposed studies using tropism-modified viruses, including the CTV, and the UTMD technology will provide a direct path for translation into the clinic for potentially improving the therapy for advanced prostate cancer and other difficult to treat neoplastic diseases.
Acknowledgment
The studies described in this review were supported by NIH grants R01 CA097318, R01 CA127641, and P01 CA104177, and the National Foundation for Cancer Research (NFCR). D. Sarkar is a Harrison Scholar in Cancer Research, and P.B. Fisher holds the Thelma Newmeyer Corman Chair in Cancer Research in the Massey Cancer Center. R. Dash has been selected to receive a Postdoctoral Prostate Cancer Training Award from the U.S. Army Medical Research and Materiel Command Congressionally Directed Medical Research Program’s 2010 Prostate Cancer Research Program.
Footnotes
Disclosure
The authors disclose no conflicts of interest relative to the studies described in this review article.
References
- Anderson WF. Human gene therapy. Nature. 1998;392(6679 Suppl):25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- Bhang HE, Gabrielson KL, Laterra J, Fisher PB, Pomper MG. Tumor-specific imaging through progression elevated gene-3 promoter-driven gene expression. Nature Med. 2011;17(1):123–129. doi: 10.1038/nm.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia VK, Senior R. Contrast echocardiography: evidence for clinical use. J Am Soc Echocardiogr. 2008;21(5):409–416. doi: 10.1016/j.echo.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, Grant S, Yacoub A, Dent P, Curiel DT, Sarkar D, Fisher PB. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res. 2010;70(9):3667–3676. doi: 10.1158/0008-5472.CAN-09-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada S, Mhashilkar AM, Ramesh R, Mumm Jb, Sutton Rb, Bocangel D, Zheng M, Grimm Ea, Ekmekcioglu S. Bystander activity of Ad-mda7: human MDA-7 protein kills melanoma cells via an IL-20 receptor-dependent but STAT3-independent mechanism. Mol Ther. 2004;10(6):1085–1095. doi: 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, Hood J, Coffee K, Nemunaitis J. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11(1):149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Damber JE, Aus G. Prostate cancer. Lancet. 2008;371:1710–1721. doi: 10.1016/S0140-6736(08)60729-1. [DOI] [PubMed] [Google Scholar]
- Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelman TP, Das SK, Kim K, Lee SG, Park MA, Yacoub A, Rahmani M, Emdad L, Dmitriev IP, Wang XY, Sarkar D, Grant S, Dent P, Curiel DT, Fisher PB. mda-7/IL-24: a unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev. 2010a;21(5):381–391. doi: 10.1016/j.cytogfr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Dmitriev I, Su ZZ, Bhutia SK, Azab B, Vozhilla N, Yacoub A, Dent P, Curiel DT, Sarkar D, Fisher PB. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) improves therapeutic efficacy in low CAR prostate cancer cells. Cancer Gene Ther. 2010b;17(7):447–456. doi: 10.1038/cgt.2009.91. [DOI] [PubMed] [Google Scholar]
- Dash R, Richards JE, Su ZZ, Bhutia SK, Azab B, Rahmani M, Dasmahapatra G, Yacoub A, Dent P, Dmitriev IP, Curiel DT, Grant S, Pellecchia M, Reed JC, Sarkar D, Fisher PB. Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine. Cancer Res. 2010c;70(12):5034–5045. doi: 10.1158/0008-5472.CAN-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLorenzo G, DePlacido S. Hormone refractory prostate cancer (HRPC): present and future approaches of therapy. Int J Immunopathol Pharmacol. 2006;19(1):11–34. [PubMed] [Google Scholar]
- Eager R, Harle L, Nemunaitis J. Ad-MDA-7; INGN 241: a review of preclinical and clinical experience. Expert Opin Biol Ther. 2008;8(10):1633–1643. doi: 10.1517/14712598.8.10.1633. [DOI] [PubMed] [Google Scholar]
- Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sarkar D, Settleman J, Fisher PB. Combinatorial treatment of non-small-cell lung cancers with gefitinib and Ad.mda-7 enhances apoptosis-induction and reverses resistance to a single therapy. J Cell Physiol. 2007;210(2):549–559. doi: 10.1002/jcp.20906. [DOI] [PubMed] [Google Scholar]
- Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sauane M, Dash R, Grant S, Dent P, Curiel DT, Sarkar D, Fisher PB. Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24. Cancer Biol Ther. 2009;8(5):391–400. doi: 10.4161/cbt.8.5.7581. [DOI] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Su ZZ, Boukerche H, Bar-Eli M, Fisher PB. Progression elevated gene-3 (PEG-3) induces pleiotropic effects on tumor progression: modulation of genomic stability and invasion. J Cell Physiol. 2005a;202(1):135–146. doi: 10.1002/jcp.20097. [DOI] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Su ZZ, Fisher PB. Emerging roles of centrosomal amplification and genomic instability in cancer. Front Biosci. 2005b;10:728–742. doi: 10.2741/1567. [DOI] [PubMed] [Google Scholar]
- Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65(22):10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2(4 Suppl 1):S23–37. [PubMed] [Google Scholar]
- Fisher PB, Sarkar D, Lebedeva IV, Emdad L, Gupta P, Sauane M, Su ZZ, Grant S, Dent P, Curiel DT, Senzer N, Nemunaitis J. Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): novel gene therapeutic for metastatic melanoma. Toxicol Appl Pharmacol. 2007;224(3):300–307. doi: 10.1016/j.taap.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Sun X, Chen X, Wang Y, Foster BA, Subjeck J, Fisher PB, Wang XY. Secretable chaperone Grp170 enhances therapeutic activity of a novel tumor suppressor, mda-7/IL-24. Cancer Res. 2008;68(10):3890–3898. doi: 10.1158/0008-5472.CAN-08-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow JN, Bauerschmitz GJ, Curiel DT, Hemminki A. Transductional and transcriptional targeting of adenovirus for clinical applications. Curr Gene Ther. 2004;4(1):1–14. doi: 10.2174/1566523044577997. [DOI] [PubMed] [Google Scholar]
- Goldberg BB, Liu JB, Forsberg F. Ultrasound contrast agents: a review. Ultrasound Med Biol. 1994;20(4):319–333. doi: 10.1016/0301-5629(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Gopalkrishnan RV, Kang DC, Fisher PB. Molecular markers and determinants of prostate cancer metastasis. J Cell Physiol. 2001;189(3):245–256. doi: 10.1002/jcp.10023. [DOI] [PubMed] [Google Scholar]
- Greco A, DI Benedetto A, Howard CM, Kelly S, Nande R, Dementieva Y, Miranda M, Brunetti A, Salvatore M, Claudio L, Sarkar D, Dent P, Curiel DT, Fisher PB, Claudio PP. Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Mol Ther. 2010;18(2):295–306. doi: 10.1038/mt.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Emdad L, Lebedeva IV, Sarkar D, Dent P, Curiel DT, Settleman J, Fisher PB. Targeted combinatorial therapy of non-small cell lung carcinoma using a GST-fusion protein of full-length or truncated MDA-7/IL-24 with Tarceva. J Cell Physiol. 2008;215(3):827–836. doi: 10.1002/jcp.21369. [DOI] [PubMed] [Google Scholar]
- Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L, Bachelor MA, Grant S, Curiel DT, Dent P, Fisher PB. mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther. 2006;111(3):596–628. doi: 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed HA, Yacoub A, Park MA, Eulitt PJ, Dash R, Sarkar D, Dmitriev IP, Lesniak MS, Shah K, Grant S, Curiel DT, Fisher PB, Dent P. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol Ther. 2010;18(6):1130–1142. doi: 10.1038/mt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Haviv YS, Blackwell JL, Kanerva A, Nagi P, Krasnykh V, Dmitriev I, Wang M, Naito S, Lei X, Hemminki A, Carey D, Curiel DT. Adenoviral gene therapy for renal cancer requires retargeting to alternative cellular receptors. Cancer Res. 2002;62(15):4273–4281. [PubMed] [Google Scholar]
- Howard CM, Forsberg F, Minimo C, Liu JB, Merton DA, Claudio PP. Ultrasound guided site specific gene delivery system using adenoviral vectors and commercial ultrasound contrast agents. J Cell Physiol. 2006;209(2):413–421. doi: 10.1002/jcp.20736. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11(12):2477–2486. [PubMed] [Google Scholar]
- Koizumi N, Yamaguchi T, Kawabata K, Sakurai F, Sasaki T, Watanabe Y, Hayakawa T, Mizuguchi H. Fiber-modified adenovirus vectors decrease liver toxicity through reduced IL-6 production. J Immunol. 2007;178(3):1767–1773. doi: 10.4049/jimmunol.178.3.1767. [DOI] [PubMed] [Google Scholar]
- Larina IV, Evers BM, Esenaliev RO. Optimal drug and gene delivery in cancer cells by ultrasound-induced cavitation. Anticancer Res. 2005;25(1A):149–156. [PubMed] [Google Scholar]
- Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther. 2000;7(23):2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, Staudt MR, Liu SJ, Taher MM, Xiao R, Barral P, Lee SG, Wang D, Vozhilla N, Park ES, Chatman L, Boukerche H, Ramesh R, Inoue S, Chada S, et al. mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (Review) Int J Oncol. 2007;31(5):985–1007. [PubMed] [Google Scholar]
- Lebedeva IV, Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Gupta P, Nemunaitis J, Cunningham C, Yacoub A, Dent P, Fisher PB. mda-7/IL-24: exploiting cancer’s Achilles’ heel. Mol Ther. 2005;11(1):4–18. doi: 10.1016/j.ymthe.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Mabjeesh NJ, Zhong H, Simons JW. Gene therapy of prostate cancer: current and future directions. Endocr Relat Cancer. 2002;9(2):115–139. doi: 10.1677/erc.0.0090115. [DOI] [PubMed] [Google Scholar]
- Mckenzie T, Liu Y, Fanale M, Swisher SG, Chada S, Hunt KK. Combination therapy of Ad-mda7 and trastuzumab increases cell death in Her-2/neu-overexpressing breast cancer cells. Surgery. 2004;136(2):437–442. doi: 10.1016/j.surg.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Miyahara R, Banerjee S, Kawano K, Efferson C, Tsuda N, Miyahara Y, Ioannides CG, Chada S, Ramesh R. Melanoma differentiation-associated gene-7 (mda-7)/interleukin (IL)-24 induces anticancer immunity in a syngeneic murine model. Cancer Gene Ther. 2006;13(8):753–761. doi: 10.1038/sj.cgt.7700954. [DOI] [PubMed] [Google Scholar]
- Ng KY, Liu Y. Therapeutic ultrasound: its application in drug delivery. Med Res Rev. 2002;22(2):204–223. doi: 10.1002/med.10004. [DOI] [PubMed] [Google Scholar]
- Park JS, Qiao L, Su ZZ, Hinman D, Willoughby K, Mckinstry R, Yacoub A, Duigou GJ, Young CS, Grant S, Hagan MP, Ellis E, Fisher PB, Dent P. Ionizing radiation modulates vascular endothelial growth factor (VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene. 2001;20(25):3266–3280. doi: 10.1038/sj.onc.1204258. [DOI] [PubMed] [Google Scholar]
- Park M, Mitchell C, Hamed HA, Cruickshanks N, Dash R, Allegood J, Dmitriev IP, Tye G, Ogretmen B, Spiegel S, Yacoub A, Grant S, Curiel DT, Fisher PB, Dent P. A serotype 5/3 adenovirus expressing MDA-7/IL-24 infects renal carcinoma cells and promotes toxicity of agents that increase ROS and ceramide levels. Mol Pharmacol. 2010 doi: 10.1124/mol.110.069484. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul CP, Everts M, Glasgow JN, Dent P, Fisher PB, Ulasov IV, Lesniak MS, Stoff-Khalili MA, Roth JC, Preuss MA, Dirven CM, Lamfers ML, Siegal GP, Zhu ZB, Curiel DT. Characterization of infectivity of knob-modified adenoviral vectors in glioma. Cancer Biol Ther. 2008;7(5):786–793. doi: 10.4161/cbt.7.5.5421. [DOI] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery — a general review. Expert Opin Drug Deliv. 2004;1(1):37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, Curiel DT, Fisher PB. Melanoma differentiation associated gene-7 (mda-7)/IL-24: a ‘magic bullet’ for cancer therapy? Expert Opin Biol Ther. 2007a;7(5):577–586. doi: 10.1517/14712598.7.5.577. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Su ZZ, Park ES, Chatman L, Vozhilla N, Dent P, Curiel DT, Fisher PB. Eradication of therapy-resistant human prostate tumors using a cancer terminator virus. Cancer Res. 2007b;67(11):5434–5442. doi: 10.1158/0008-5472.CAN-07-0195. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Fisher PB. Unique conditionally replication competent bipartite adenoviruses-cancer terminator viruses (CTV): efficacious reagents for cancer gene therapy. Cell Cycle. 2006;5(14):1531–1536. doi: 10.4161/cc.5.14.3095. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, Dent P, Fisher PB. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci U S A. 2002;99(15):10054–10059. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Park ES, Vozhilla N, Dent P, Curiel DT, Fisher PB. A cancer terminator virus eradicates both primary and distant human melanomas. Cancer Gene Ther. 2008;15(5):293–302. doi: 10.1038/cgt.2008.14. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci U S A. 2005;102(39):14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Lebedeva IV, Dent P, Pestka S, Fisher PB. MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 2003;14(1):35–51. doi: 10.1016/s1359-6101(02)00074-6. [DOI] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, Fisher PB. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc Natl Acad Sci U S A. 2008;105(28):9763–9768. doi: 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg CN. Highlights of contemporary issues in the medical management of prostate cancer. Crit Rev Oncol Hematol. 2002;43(2):105–121. doi: 10.1016/s1040-8428(02)00023-9. [DOI] [PubMed] [Google Scholar]
- Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, James CD, Randolph A, Valerie K, Walter MR, Dent P, Fisher PB. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005a;24(51):7552–7566. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- Su Z, Lebedeva IV, Gopalkrishnan RV, Goldstein NI, Stein CA, Reed JC, Dent P, Fisher PB. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci U S A. 2001a;98(18):10332–10337. doi: 10.1073/pnas.171315198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Shi Y, Fisher PB. Cooperation between AP1 and PEA3 sites within the progression elevated gene-3 (PEG-3) promoter regulate basal and differential expression of PEG-3 during progression of the oncogenic phenotype in transformed rat embryo cells. Oncogene. 2000;19(30):3411–3421. doi: 10.1038/sj.onc.1203666. [DOI] [PubMed] [Google Scholar]
- Su Z, Shi Y, Friedman R, Qiao L, Mckinstry R, Hinman D, Dent P, Fisher PB. PEA3 sites within the progression elevated gene-3 (PEG-3) promoter and mitogen-activated protein kinase contribute to differential PEG-3 expression in Ha-ras and v-raf oncogene transformed rat embryo cells. Nucleic Acids Res. 2001b;29(8):1661–1671. doi: 10.1093/nar/29.8.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Goldstein NI, Jiang H, Wang MN, Duigou GJ, Young CS, Fisher PB. PEG-3, a nontransforming cancer progression gene, is a positive regulator of cancer aggressiveness and angiogenesis. Proc Natl Acad Sci U S A. 1999;96(26):15115–15120. doi: 10.1073/pnas.96.26.15115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Gopalkrishnan RV, Narayan G, Dent P, Fisher PB. Progression elevated gene-3, PEG-3, induces genomic instability in rodent and human tumor cells. J Cell Physiol. 2002;192(1):34–44. doi: 10.1002/jcp.10114. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Lebedeva IV, Sarkar D, Emdad L, Gupta P, Kitada S, Dent P, Reed JC, Fisher PB. Ionizing radiation enhances therapeutic activity of mda-7/IL-24: overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells overexpressing the antiapoptotic proteins bcl-xL or bcl-2. Oncogene. 2006;25(16):2339–2348. doi: 10.1038/sj.onc.1209271. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Sarkar D, Emdad L, Duigou GJ, Young CS, Ware J, Randolph A, Valerie K, Fisher PB. Targeting gene expression selectively in cancer cells by using the progression-elevated gene-3 promoter. Proc Natl Acad Sci U S A. 2005b;102(4):1059–1064. doi: 10.1073/pnas.0409141102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Shi Y, Fisher PB. Subtraction hybridization identifies a transformation progression-associated gene PEG-3 with sequence homology to a growth arrest and DNA damage-inducible gene. Proc Natl Acad Sci U S A. 1997;94(17):9125–9130. doi: 10.1073/pnas.94.17.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, Coffee K, Ramesh R, Ekmekcioglu S, Grimm EA, Van Wart Hood J, Merritt J, Chada S. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11(1):160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Tsuruta Y, Pereboeva L, Glasgow JN, Rein DT, Kawakami Y, Alvarez RD, Rocconi RP, Siegal GP, Dent P, Fisher PB, Curiel DT. A mosaic fiber adenovirus serotype 5 vector containing reovirus sigma 1 and adenovirus serotype 3 knob fibers increases transduction in an ovarian cancer ex vivo system via a coxsackie and adenovirus receptor-independent pathway. Clin Cancer Res. 2007;13(9):2777–2783. doi: 10.1158/1078-0432.CCR-06-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller GE, Wong MK, Modzelewski RA, Lu E, Klibanov AL, Wagner WR, Villanueva FS. Ultrasonic imaging of tumor angiogenesis using contrast microbubbles targeted via the tumor-binding peptide arginine-arginine-leucine. Cancer Res. 2005;65(2):533–539. [PubMed] [Google Scholar]
- Yacoub A, Hamed H, Emdad L, Dos Santos W, Gupta P, Broaddus WC, Ramakrishnan V, Sarkar D, Shah K, Curiel DT, Grant S, Fisher PB, Dent P. MDA-7/IL-24 plus radiation enhance survival in animals with intracranial primary human GBM tumors. Cancer Biol Ther. 2008;7(6):917–933. doi: 10.4161/cbt.7.6.5928. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Liu R, Park MA, Hamed HA, Dash R, Schramm DN, Sarkar D, Dimitriev IP, Bell JK, Grant S, Farrell NP, Curiel DT, Fisher PB, Dent P. Cisplatin enhances protein kinase R-like endoplasmic reticulum kinase- and CD95-dependent melanoma differentiation-associated gene-7/interleukin-24-induced killing in ovarian carcinoma cells. Mol Pharmacol. 2010;77(2):298–310. doi: 10.1124/mol.109.061820. [DOI] [PMC free article] [PubMed] [Google Scholar]