Abstract
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels (h channels) form the molecular basis for the hyperpolarization-activated current, Ih and modulation of h channels contributes to changes in cellular properties critical for normal functions in the mammalian brain and heart. Numerous mechanisms underlie h channel modulation during both physiological and pathological conditions, leading to distinct changes in gating, kinetics, surface expression, channel conductance or subunit composition of h channels. Here we provide a focused review examining contemporary mechanisms of h channel regulation, with an emphasis on recent findings regarding interacting proteins such as TRIP8b. This review is intended to serve as a comprehensive resource for physiologists to provide potential molecular mechanisms underlying functionally important changes in Ih in different biological models, as well as for molecular biologists to delineate the predicted h channel changes associated with complex regulatory mechanisms in both normal function and in disease states.
Keywords: HCN channels, channel trafficking, TRIP8b, Ih, If, epilepsy, long QT syndrome, voltage-gated ion channel
Introduction
Voltage-gated ion channels (VGICs) are expressed by many cell types, but are of critical importance in excitable cells such as neurons or cells of the sinoatrial node (SAN) in the heart. Most VGICs are activated upon membrane depolarization, yet the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel (h channel) is activated by hyperpolarization1–5 and underlies the hyperpolarization-activated current, Ih (If or Iq in the heart, for funny or queer current, respectively; when discussing the neuronal or cardiac current Ih or If will be used, respectively), observed in many types of neuronal4,6 and cardiac3, 7, 8 cells. Four HCN pore-forming α-subunits have been identified, numbered HCN1–4,9–11 and their expression profiles are cell type-specific. h channels are permeable primarily to Na+ and K+ ions, and have roughly four times greater permeability for K+ compared to Na+, yet upon activation h channels conduct a net inward current.1 Because h channels are activated near typical resting membrane potential (RMP), Ih significantly contributes to the determination and stabilization of RMP as well as input resistance. In neurons, the presence of h channels in dendrites endows specific functional significance and helps regulate cellular input-output properties. Importantly, dendritic h channels regulate dendritic integration of excitatory post-synaptic potentials (EPSPs) and reduce temporal summation of distal inputs.12–15 Furthermore, dendritic Ih influences additional ionic conductances, such as T-type and N-type voltage-gated Ca2+ channels16 and the delayed rectifier M-type K+ channel.17 Such interactions have important functional consequences; for instance, HCN1 knockout mice demonstrate enhanced spatial learning and long-term potentiation (LTP), implicating HCN1 and Ih as an inhibitory constraint on these properties.18 These findings are partially explained by increased resting inactivation of Ca2+ channels of hippocampal area CA1 by HCN1-mediated Ih.16
h channels play important functional roles in both the central and peripheral nervous systems as well as in the heart in healthy and disease states. Because they are activated at hyperpolarized potentials, h channels can play a role in rhythmogenesis, which has been studied extensively in thalamocortical (TC) neurons and cells of the SAN.6 The contribution of Ih to rhythmicity may be exemplified in TC neurons, whereby membrane depolarization from activation of Ih activates a low-threshold t-type Ca2+ current It, which ultimately triggers a fast burst of Na+/K+ spikes. Deactivation of Ih by this depolarization as well as inactivation of It leads to a hyperpolarizing overshoot, and ultimately reactivation of Ih to renew the cycle.6 This intrinsic oscillatory mechanism of TC neurons is modified by reciprocally connected inhibitory GABAergic input from neighboring neurons of the reticular thalamic nucleus (RTN), which ultimately can be observed as delta and spindle waves in the electroencephalogram (EEG) of mammals in non-rapid eye movement sleep.19 If is also believed to contribute to rhythmicity and regularity in cardiac pacing, yet its role has not proven as straightforward as in neurons.5,8,20
Just as it is clear that h channels and Ih play rhythmogenic roles in homeostatic functions such as sleep and cardiac rhythm, research over the past decade has implicated h channel dysfunction in the pathophysiology of neurological and cardiac disorders, not surprisingly many involving dysrhythmia and altered cellular excitability. Changes in Ih have been demonstrated in multiple rodent models of epilepsy, including absence epilepsy and temporal lobe epilepsy (TLE).21 Mice lacking the HCN2 subunit demonstrate severe absence epilepsy, with significant changes in firing behavior of TC neurons and a shift from tonic to burst firing modes,22,23 and rat models of absence epilepsy have also demonstrated altered regulation of h channels in layer V cortical neurons.24 Numerous studies have found h channel misregulation in TLE, although the form of h channel dysfunction differs in different studies and includes changes inkinetics, protein expression, channel heteromerization and channel localization.25–30 Additionally, h channels have been implicated in neuropathic pain, whereby blockade of Ih with the selective antagonist ZD7288 significantly reduced allodynia following spinal nerve ligation.31
As summarized above, despite their relatively recent molecular discovery at the close of the 20th century, h channels are now known to play a diverse yet critical role in both normal function and may contribute to pathological conditions. Electrophysiological studies of h channels in neuronal networks have demonstrated that small changes in Ih current density, voltage gating and kinetics can have dramatic macroscopic consequences due to interaction with additional currents in complex systems.16,17 Thus, a comprehensive understanding of how h channels are situated within a dynamic environment of molecular modifiers will permit a more mechanistic-based understanding of observed changes in Ih. Here we provide a tool for the research community studying h channels or neuronal networks involving Ih and offer a straightforward, reductionist view of distinct h channel properties and the mechanisms that affect them. This review is thus organized by observed phenomena, i.e., altered V50, kinetics, etc., rather than by individual regulator of Ih, i.e., cAMP, intracellular pH, etc. Our goal is to aid researchers in translating an observed change in Ih or h channels in a given biological system to a mechanistic discovery, with the ultimate goal of identifying potential therapies for clinically significant disorders such as epilepsy, neuropathic pain and heart failure.
Mechanisms Regulating Distinct Properties of Ih and HCN Channels
Channel gating
(see Tables 1 and 2). As previously mentioned, the voltage gating of Ih is markedly different from most VGICs in that it is activated by hyperpolarization to potentials negative of −50 to −60 mV, rather than by depolarization. The mechanism underlying the activation of h channels by hyper-polarization involves the positively charged S4 voltage sensor similar to other VGICs, although how this region is coupled to channel opening by hyperpolarization rather than the more common depolarization is not yet completely understood.1,4 HCN channels are not the only VGICs activated by hyperpolarization. The Arabidopsis thaliana inwardly rectifying K+ channel KAT1 also demonstrates activation upon hyperpolarization, again with an activation mechanism reliant upon the S4 voltage sensor.32 In contrast, the inwardly rectifying K+ (Kir) channels, a family of tetrameric two-transmembrane ion channels that differ from the six-transmembrane voltage-gated K+ (Kv) channels, do not contain a voltage sensing S4 helix but rather activate upon hyperpolarization by removal of Mg2+ ions and polyamines that physically block the channel pore.33 The rate of unblocking by Mg2+ and polyamines differ, thus hyperpolarization of K(ir) channels leads to an initial time-independent current followed by a time-dependent current. Thus, multiple families of ion channels are activated by hyperpolarization, yet the mechanism by which they are activated by voltage may differ.
Table 1.
Regulatory mechanisms hyperpolarizing Ih/If voltage gating
| Modulatory mechanism or treatment |
Experimental system | Approximate magnitude of V50 shift (mV) |
Ref. |
|---|---|---|---|
| ↓[pH]i | Rat TC neurons | −2 to −3 (with pH decrease from 7.1 to 6.7) |
49 |
| HEK293 cells expressing HCN2 | −10 (with pH decrease from 7.4 to 6.0) |
50 | |
| Genistein (tyrosine kinase Inhibition) |
Rat ventricular myocytes | −14 | 57 |
| Orexin A | Mouse prelimbic cortex layer V pyramidal neurons | −8 | 67 |
| KCNE2 | Coexpression with HCN4 in oocytes | −23 | 90 |
| Coexpression with HCN4 in CHO cells | −14 | ||
| KCR1 | Coexpression with HCN2 in CHO cells | −8 | 93 |
| Viral overexpression in neonatal and adult ventriculocytes | −30 (single channel patch) | ||
| Filamin A | Coexpression with HCN1 in melanoma cell line | −8 | 94 |
| TRIP8b | Coexpression with HCN1 in HEK293T cells or oocytes | −7 to −10 | 106, 107 |
| Coexpresion with HCN2 in oocytes (inside-out patch + cAMP) | −10 | 114 | |
| Viral overexpression of TRIP8b in 6 DIV organotypic hippocampal slice culture |
−7 | 114 |
Table 2.
Regulatory mechanisms depolarizing Ih/If voltage gating
| Modulatory mechanism or treatment |
Experimental system | Approximate magnitude of V50 shift (mV) |
Ref. |
|---|---|---|---|
| cAMP | Numerous | Dependent on HCN channel subunit | Reviewed in 1–8 |
| PIP2 (or analogues) | Oocytes expressing various HCN channel subunits (Excised inside-out patches) |
>+20 | 41, 45–47 |
| Wortmannin treatment of dopaminergic midbrain neurons or embryonic cardiomyoctes |
+12 (neurons) +11 (cardiomyocytes) [inferred from wortmannin inhibition] |
46 | |
| PA, AA | Oocytes expressing HCN2 (excised inside-out patches) |
+6 | 48 |
| ↑[pH]i | Rat TC neurons | +4 to +5 (with pH increase from 7.1 to 7.5) | 49, 54 |
| HEK293 cells expressing HCN2 | +10 (with pH increase from 7.4 to 9.0) | 50 | |
| ↓[pH]o | FLP-IN-293 cells expressing HCN1 | +9 (with pH decrease from 7.4 to 4.9) | 51 |
| Tyrosine phosphorylation | Coexpression of constitutively-active Src with HCN4 in HEK293 cells |
+13 | 56, 61 |
| Phenylarsine oxide or sodium orthovanadate (non- specific tyrosine phosphatase inhibition) in adult rat ventricular myocytes |
Large positive shift in If activation threshold |
62 | |
| P38 MAPK | Treatment of hippocampal neurons with anisomycin (p38 MAPK activator) |
+11 | 63 |
| Proteolysis of HCN channel subunits |
Coexpression of proteolyzed HCN2 (HCN2ΔC) with HCN4 in HEK293 cells |
+4 compared to HCN4 alone | 66 |
When expressed individually in heterologous systems, HCN1–4 exhibit markedly different characteristics of voltage gating, with half maximal voltage of activation (V50) ranging from −70 mV for HCN1 to −100 mV for HCN4.1 Another important difference between h channel subunits is in sensitivity to the ubiquitous second messenger cAMP. HCN1 is weakly sensitive to cAMP, while HCN4 is extremely cAMP sensitive and HCN2 and HCN3 display intermediate sensitivities. cAMP shifts the voltage gating of h channels in a depolarizing direction, allowing more channels to open at less hyperpolarized membrane potentials.4 The structural basis of this modulation has been studied in HCN2 channels by Zagotta and colleagues, who examined the interaction of cAMP and cGMP with two different C-terminal fragments containing the cyclic nucleotide binding domain (CNBD) and the region connecting the CNBD to the channel pore (C-linker).34 X-ray crystallographic structures demonstrated that the C-linker consists of 6 α-helices and is almost solely responsible for tetramerization of channel subunits, while the CNBD binds cyclic nucleotides and consists of four α-helices with a β-roll between two helices that encompasses eight β-strands and is similar to the CNBDs of cAMP-binding proteins in Escherichia coli and cAMP-dependent protein kinase (PKA).34 Experiments performed by Wainger et al. involving deletion of specific regions of HCN1 and HCN2 followed by assessment of cAMP-induced changes in voltage gating have synergized with these structural data to provide a model for the mechanism by which cAMP alters h channel voltage gating.35 Deletion of the HCN2 CNBD C helix eliminated sensitivity to cAMP, yet did not change the V50 in the absence of cAMP.35 However, deletion of the entire HCN2 CNBD significantly depolarized the V50 in the absence of cAMP to a level near that of wild type channels in the presence of cAMP.35 Taken together with the structural data, the CNBD appears to exert an inhibitory effect on channel gating that is removed by cAMP binding to the CNBD C helix, which is transmitted to the pore region by the C-linker. Indeed, chimera studies have shown the C-linker is important in determining the different depolarizing effects of cAMP between h channel subunits.36
While regulation of h channels by cAMP is an important factor in determining Ih gating both in vitro and in vivo, it is far from the only factor. Expression of individual h channel subunits in heterologous cells yields expression of functional Ih, yet these currents do not completely recapitulate native Ih gating parameters that are highly variable in many cell types.4 One explanation is the formation of heteromeric channels by different h channel subunits. h channels are tetramers, and can exist as homotetramers or heterotetramers, which have been described in both in vitro and in vivo systems.30,37–39 An example of this phenomenon is the comparison of Ih recorded in oocytes expressing HCN1 or HCN2 in comparison to hippocampal area CA1 pyramidal neurons, which are known to express both HCN1 and HCN2.37,40 Oocytes coexpressing HCN1 and HCN2 subunits demonstrate Ih with voltage activation and cAMP sensitivity intermediate to that of these channel subunits expressed alone.37 These properties however were not recapitulated by merely summing the properties of HCN1 and HCN2 homotetramers, arguing against an Ih composed of distinct populations of HCN1 and HCN2 homomers. Rather, the properties of these presumed HCN1/HCN2 heteromeric channels resemble Ih recorded from CA1 pyramidal neurons, implying that functional heteromerization occurs in vivo. Biochemical and functional heteromerization appears to be possible between all h channel subunits with the exception of HCN2 and HCN3.39 Thus, heteromerization represents a distinct mechanism by which channels can alter their biophysical properties, with almost endless diversity depending on differential expression levels and presumably altered stoichiometries of heteromeric channels.
Joining cAMP, additional small molecules and ions can have distinct effects on h channel gating, including phosphoinositides and protons. As described earlier, much research in the h channel field has been devoted to reconciling differences in heterologously expressed Ih from Ih in vivo. Excised patches or long-term dialyzed whole-cell recordings from transfected heterologous cells or cells naturally expressing h channels demonstrate a “run-down” phenomenon, whereby voltage activation is shifted significantly in the hyperpolarizing direction over time, a difference that cannot be wholly accounted for by changes in cAMP levels.41 One mechanism attributed to run-down of other types of channels, including Kv,42 Kir,43 and P/Q-type voltage-gated Ca2+ channels44,45 is the loss of phosphatidylinositol 4,5-bisphosphate (PIP2), a membrane phospholipid. Experiments in oocytes expressing h channel subunits (as well as dopaminergic midbrain neurons and embryonic cardiomyoctes) have demonstrated that PIP2 and other phospholipids with a negatively charged head-group shift the V50 of Ih produced by HCN1, HCN2 and HCN4 by ~20 mV in the depolarizing direction in a quasi-cAMP-independent mechanism.41,46,47 Specifically, while PIP2 shifts V50 in the depolarizing direction even in the presence of saturating levels of cAMP, the overall magnitude of the effect is less than when observed in the absence of cAMP.41 It is thought that PIP2 is an allosteric regulator of h channels at a site distinct from the CNBD, because its depolarizing effect remains in HCN subunit constructs lacking the CNBD, and that there may be downstream convergence of the biophysical mechanisms altering the gating of Ih.
Additional research however has found that PIP2 is not the only molecule from the large group of lipid signaling molecules that regulates h channel gating. This possibility was suggested by the evidence that multiple receptors positively coupled to phospholipase C (PLC) enhanced gating of Ih, a finding opposite to that predicted if PIP2 was the only molecule of importance within this cascade.41,46–48 Fogle et al. sought to pharmacologically isolate the role of diacylglycerol (DAG) signaling on h channel function by using the DAG mimetic 4β-phorbol 12-myristate 13-acetate (4βPMA), an agonist of the C-1 DAG/4β-phorbol-binding site.48 Incubation of Xenopus oocytes expressing HCN1 and HCN2 cDNA with 4βPMA led to both a depolarizing shift in V50 as well as a dose-dependent 3–5-fold decline in maximal current amplitude. Multiple proteins contain C-1 binding sites, with the classical examples being the protein kinase C (PKC) conventional and novel isoforms, cPKC and nPKC, respectively. PKC activation can potentially alter intracellular cAMP and proton concentrations, both second messengers known to affect h channel biophysics.48 However, the actions of 4βPMA were separated from effects on cAMP/H+ concentration by the demonstration that 4βPMA evoked the same current modulation on h channels rendered insensitive to cAMP and proton concentration by point mutations, implying that activation of C-1 site-containing proteins operates independently of cAMP/H+ concentration. Experiments with cell-free excised inside-out patches revealed that 4βPMA does not interact directly with h channels but rather requires an intact intracellular environment, thus implying a signal transduction cascade downstream of the actions of 4βPMA. Further experimentation revealed that the actions of 4βPMA on h channel gating are directly manifest through phosphatidic acid (PA) and arachidonic acid (AA) generated specifically through two PKC-signaling pathways.48 Interestingly, while the voltage gating changes by 4βPMA are specific to h channels, studies using KAT1 demonstrate that the observed maximal current decrease by 4βPMA is not specific for h channels. The mechanism of action of PA and AA on h channels was demonstrated to most likely be due to specific channel-lipid interactions at the conserved core region.48
Similar to some other VGICs, h channels are sensitive to both intracellular and extracellular proton concentrations. In TC neurons of the rat ventrobasal complex, compared with control recordings performed at pH = 7.1 alkaline pipette solutions (pH = 7.5) elicited a 4–5 mV shift in V50 in the depolarizing direction while more acidic pipette solutions (pH = 6.7) brought about a small but Significant 2–3 mV hyperpolarizing shift in V50.49 These changes were also independent of cAMP concentration. Such modification of voltage gating of h channels by protons is controlled by a solitary histidine residue located at the boundary between the S4 helix and S4-S5 linker (H321 in HCN2) that is completely conserved amongst all four h channels sub-units.50 Although TC neuron V50 does not appear to be sensitive to changes in extracellular pH, Ih in cells of the vallate papilla was modified by extracellular pH, suggesting these currents are important in the signal transduction of sour taste.51 specifically, decreased extracellular pH induced the opposite effect on Ih as decreased intracellular pH, namely a shift of V50 to more depolarized potentials. The ability of protons to modify the voltage gating of h channels is similarly observed with other ion channels, including N-type Ca2+ channels and TRPV1.52,53 Repetitive firing in TC neurons leads to intracellular acidification as measured by an intracellular pH-sensitive indicator, which alters the properties of Ih from before firing to after, thus representing a dynamic, activity-dependent mechanism of Ih regulation.49 In addition, modification of intracellular pH may play a role in the mechanism of action of the anti-epileptic drug acetazolamide, a carbonic anhydrase inhibitor used for treatment of absence epilepsy. Extracellular acetazolamide induced an intracellular alkalinization of ~ 0.19 pH units, which led to a 5–7 mV depolarization of the Ih V50.54 Such enhancement of Ih most likely inhibits firing of rebound Ca2+ bursts and helps bring about the termination of oscillatory activity seen in absence epilepsy.
Phosphorylation is a central mechanism for modifying both the function and trafficking of ligand-gated and voltage gated ion channels, and a growing amount of evidence exists to support modification of h channel voltage gating by phosphorylation via different kinases. A hint of the importance of the tyrosine kinase Src for modulation of h channels stemmed from the use of the SH3 domain as bait in a yeast two-hybrid screen that ultimately identified HCN1.55 Multiple subsequent studies went on to find that tyrosine phosphorylation by Src alters channel properties of Ih in rabbit SAN, typically by increasing If conductance rather than altering voltage gating (see next section) .56–60 However, when expressed in Xenopus oocytes, the V50 of HCN2 was negatively shifted by genistein, a tyrosine kinase inhibitor, implying that tyrosine kinase activity leads to a depolarization of HCN2 gating. Similarly, in rat ventricular myocytes, which express mostly HCN2 subunits, application of genistein also shifted the midpoint of activation by 14 mV in the hyperpolarizing direction.57 Furthermore, coexpression of HCN4 with a constitutively active form of Src in HEK293 cells depolarized the half-maximal voltage of activation by ~13 mV, an affect likely mediated by phosphorylation at tyrosine 531.56,61 In addition to regulation of Ih by tyrosine kinase activity, Huang et al. recently hypothesized that altered activity of tyrosine phosphatases may also modify Ih . They found that the receptor-like protein-tyrosine phosphatase-a (RPTPα) dephosphorylates HCN2 tyrosines in HEK293 cells, which correlates with decreased surface expression of HCN2 and decreased Ih .62 From there, they went on to show that non-specific phosphatase inhibition using phenylarsine oxide or sodium orthovanadate shifted the threshold of activation of Ih in adult rat ventricular myocytes to significantly depolarized potentials.
Serine/threonine pathways have also been implicated in h channel modulation. A study by Poolos et al. demonstrated that p38 mitogen-activated protein kinase (p38 MAPK), but not other members of the MAPK family, can also regulate the voltage gating of Ih in hippocampal neurons.63 Pharmacological inhibition of p38 MAPK leads to a ~25 mV hyperpolarizing shift of V50, while anisomycin, a p38 MAPK activator, depolarizes Ih V50 by ~11 mV. Whether p38 acts through direct channel subunit phosphorylation or other mechanisms is as yet unknown.
A curious mechanism for regulation of the voltage dependence of Ih may be proteolysis of channel subunits. Knockout studies have identified important roles for both the HCN2 and HCN4 subunits in mouse heart, with If being significantly reduced in myocardium upon knockout of either of these subunits.22,64,65 Ye et al. performed immunoprecipitation studies in order to determine whether HCN2 and HCN4 form heteromeric channels in adult mouse heart and found that although the size of HCN4 protein was as expected, only a truncated, ~60 kDa form of HCN2 (HCN2ΔC) was expressed.66 HCN2ΔC lacks the C-terminal CNBD, and associates with HCN4 in both heart and heterologous cells upon coexpression. Interestingly, when expressed alone, HCN2ΔC does not express functional Ih, however, when coexpressed with HCN4, HCN2ΔC shifts the V50 of the resulting current slightly in the depolarized direction when compared to HCN4 V50 alone.
New data is also emerging to suggest that h channels are modulated by peptides. Orexin A, a peptide that promotes wakefulness, has been implicated in the regulation of h channels in layer V pyramidal neurons of the mouse prelimbic (PL) cortex.67 In PL cortical slices, application of orexin A increases cellular excitability. In exploring mechanisms for enhanced excitability, orexin A was found to significantly inhibit Ih amplitude in these neurons at almost all hyperpolarized voltages tested, and the V50 was shifted in the hyperpolarized direction by ~8 mV. PKC inhibitors attenuated the action of orexin A on Ih amplitude, suggesting that orexin A may act through a PKC-mediated mechanism to inhibit Ih, similar to the peptide neurotensin68 and the neurotransmitter serotonin.69 Thus, likely through second messengers, orexin A may contribute to arousal by modulation of h channels in the prefrontal cortex.67 Additional peptides, such as cortistatin, also influence Ih, and will be discussed at a later time.
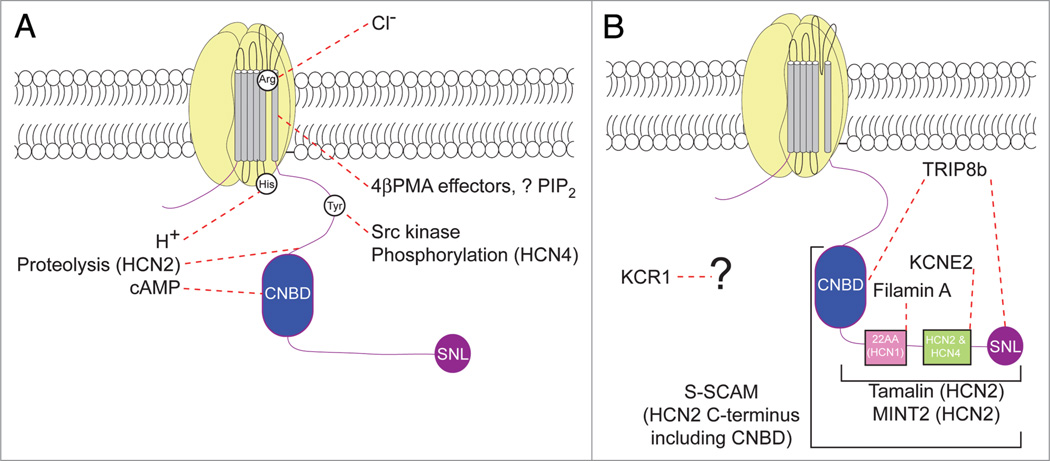
Finally, interacting proteins of h channels have emerged as an important group of modulators of h channel voltage gating. While the most basic characteristics of VGICs are intrinsically governed by their pore-forming α-subunits, association with additional proteins can have a profound effect on almost all of the most important properties of VGICs, including voltage gating, kinetics, surface expression, channel conductance and localization. Binding partners for VGICs are most often classified as scaffolding proteins, adaptor proteins, chaperones or auxiliary subunits, with some proteins occupying more than one category. Scaffolding proteins provide a single site for aggregation of multiple proteins that bind to the scaffold through a variety of interaction motifs and create a functional complex.70 Postsynaptic density protein-95 (PSD-95) serves as an example of a classical scaffolding protein at the postsynaptic density, where it links N-methyl D-aspartate (NMDA) receptors with downstream signal transduction molecules. Adaptor proteins can be thought of as a large subset of scaffolding proteins that bind proteins through diverse protein-protein interaction domains, linking together two or more proteins that would not have ordinarily bound to each other yet oftentimes participate in a signal transduction cascade.70 Chaperone proteins are highly conserved between species and temporarily interact with their binding partners to promote proper protein folding or trafficking, but are not part of the functional protein complex. Common examples include the heat shock proteins, which may be upregulated during periods of cellular stress and in disease.71 Finally, auxiliary subunits (also referred to as β-subunits) bind to their partner OC-subunit in a stable complex. Auxiliary subunits may be either cytoplasmic or transmembrane, and oftentimes their expression leads to profound changes in channel biophysics, surface trafficking or protein stability. For example, the Kv channel-interacting protein (KChIP) family binds to KV4 channels, promotes surface expression, and alters the kinetics of associated α-subunits.72 Auxiliary subunits have been identified for almost all VGICs,72–75 and mutations in many of these subunits lead to hereditary disorders in excitable cells of both the brain and heart. Examples include familial epilepsy (NaVβ1,76), periodic paralysis (KCNE3/ MiRP2,77), and familial long QT syndromes (LQTS) (KCNE1/ MinK78,79 and KCNE2/MiRP1,80,81).
Although VGICs differ markedly in their ion selectivity and voltage gating, the general structure of the pore-forming a-subunits follows a small number of common rules.75 Thus, although h channels differ in some respects from the voltage-gated potassium (KV) channels, cyclic nucleotide-gated (CNG) channels, and transient receptor potential (TRP) channels, their overall structure is similar in that they are each tetramers of subunits. Indeed, the similarity between h channels and CNG channels, which contain a cyclic nucleotide binding (CNB) site, was exploited during initial efforts to clone the channel genes underlying Ih.9–11, 82 Phylogenetic analysis of the four cloned HCN genes revealed that they form a new subfamily within the potassium channel superfamily, but that they are closely related to the ether-à-go-go (EAG)-related channels, CNG channels and KAT1 channels, all of which contain a CNBD.82
Because of shared structural motifs, it is not altogether surprising that an auxiliary subunit or group of auxiliary subunits interacts with more than one member of the K+ channel superfamily, which is indeed the case for the MinK-related peptide (MiRP) family. MiRPs are encoded by KCNE genes, of which there are five known members, KCNE1–5, with KCNE1 also known as MinK (minimal K+ channel protein). The gene products of KCNE1–5 are single transmembrane proteins with small intra- and extracellular domains, and MiRP family members have been reported to interact with KV1–4, human ether-à-go-go related gene (hERG) channels, KCNQ, and HCN channels.75,83,84 KCNE2 (MiRP1) has been demonstrated to interact with HCN1, HCN2 and HCN4 subunits, but its role in h channel voltage gating is controversial. Coexpression of HCN1, HCN2 or HCN4 with KCNE2 and subsequent evaluation of V50 in oocytes, HEK293 cells, or Chinese hamster ovary (CHO) cells failed to demonstrate any significant change in voltage gating.85–88 Furthermore, no change in V50 was found after adenoviral overexpression of HCN2 and KCNE2 in cultured neonatal rat ventricular myocytes.89 However, Decher and colleagues found that coexpression of HCN4 with KCNE2, but not KCNE1, 3 or 4, could hyperpolarize Ih V50 on the order of ~24 to ~14 mV for oocytes and CHO cells, respectively.90 The underlying reason for these discrepancies is unknown.
In an attempt to identify proteins required for functional expression of the channel underlying the low-threshold non-inactivating K+ current IK(ni), KCR1 was isolated by suppression cloning.91 KCR1 contains 12 transmembrane domains and is expressed in both brain (cerebrum and cerebellum) and a variety of peripheral tissues. Antisense experiments in oocytes injected with cerebellar poly(A)+ RNA demonstrated that KCR1 is required for fully functional IK(ni), and coexpression of KCR1 with a rat homologue of ether-à-go-go (r-EAG) in both oocytes and COS-7 showed an increase in channel activation that appears to be mediated by a direct protein-protein interaction.91 KCR1 also interacts with the human ether-à-go-go-related gene (HERG).92 KCR1, like KCNE2, binds to multiple members of the voltage gated K+ channel superfamily and has recently been found to interact with HCN2.93 Coexpression of KCR1 with HCN2 in CHO cells hyperpolarized the V50 by ~8 mV.93 KCR1 is expressed in multiple regions of rat and pig heart, and adenoviral infection of both neonatal and adult ventriculocytes with KCR1 also significantly hyperpolarized V50 of endogenous If. The opposite effects were found when siRNA was used to knockdown levels of KCR1 in neonatal myocytes.93 The role of KCR1 in vivo with respect to h channels is difficult to answer since it is also expressed in many tissues not known to express h channels, and therefore may interact with a multitude of additional proteins.
Scaffolding proteins can also influence voltage gating of h channels in vitro. For example, in heterologous cells, expression of filamin A with HCN1 significantly hyperpolarized the V50 of Ih and slowed both activation and deactivation kinetics.94 The filamin gene family in mammals consists of FLNA, FLNB and FLNC, which code for the three filamin proteins, filamin A, B and C, respectively. Filamin A is a large cytoskeletal protein capable of binding actin, and contains 24 immunoglobin-like repeats and an actin-binding site at the N-terminus.95,96 It has been reported to interact with multiple ligand- and voltage-gated ion channels, including the dopamine receptors D2 and D3,97 KV4.2,98 and Kir2.1.99 Similarly to KCNE2, in addition to binding to voltage-gated potassium channels, filamin A also binds the HCN1 C-terminus via its final two Ig-like repeats.94 Filamin A did not interact with HCN2 or HCN4, and bound a 22 amino acid (amino acids 694–715) region distal to the HCN1 CNBD.94
A number of recent papers have elevated the tetratricopeptide-containing Rab8b–interacting protein (TRIP8b) toward the top of the list for researchers interested in how auxiliary subunits and binding proteins may affect the surface expression, subcellular localization, gating and kinetics of h channels in neurons. Two independent groups seeking answers to different questions are credited with identifying the TRIP8b protein. Chen et al. were interested in further understanding the function of Rab8b, a small GTP binding protein of the large Rab family of proteins involved in vesicle trafficking and identified TRIP8b as a Rab8b binding partner through a yeast two-hybrid (Y2 h) library screen of rat brain cDNA.100 TRIP8b is ~70 kDa in size, and contains six TPR motifs to form a TPR domain, a common protein-protein interaction domain. Specific antisera revealed that TRIP8b was highly enriched in the brain and overexpression of TRIP8b, similarly to overexpression of Rab8b, was found to increase cAMP-induced secretion of ACTH in ATt20 cells, implicating TRIP8b and Rab8b in a secretory pathway for forward vesicle trafficking.100
TRIP8b is closely related to the peroxisomal import receptor protein Pex5p, exhibiting significant identity within the C-terminal TPR domains. Pex5p binds to proteins containing a peroxisomal-targeting signal 1 (PTS1), a tripeptide located at the C-terminus of proteins destined for peroxisomal import.101 In a screen for proteins related to Pex5p Amery et al. discovered Pex5p–related protein (Pex5Rp, their nomenclature for TRIP8b) via in silico and in vitro approaches.102 Although Pex5Rp bound PTS1 in in vitro assays, it did not appear to act as a peroxisomal import protein, as it was unable to bind to Pex14p or Pex12p, and expression of Pex5Rp in Pex5p–deficient mouse fibroblasts did not rescue the peroxisomal import defect. In agreement with the findings of Chen et al. in rodent brain, Pex5Rp was also highly enriched in human brain, but mRNA was also found in much lower abundance in the pancreas, testis and pituitary gland.100,102 Taken together, these two initial reports on TRIP8b/Pex5Rp demonstrated the protein to be a brain-enriched cytoplasmic protein able to bind PTS1 and function in vesicle trafficking, but that TRIP8b/Pex5Rp is not sufficient for peroxisomal protein import.
A Y2 h screen of a mouse brain cDNA library aimed at the identification of new h channel interacting proteins identified TRIP8b as a binding partner of all four h channel isoforms.103 Similar to the binding mechanism with PTS1-containing peptides, the binding of TRIP8b to the h channel subunits required the presence of the C-terminal tripeptide, which in the case of HCN1, 2 and 4 ends -SNL, and for HCN3, -ANM. Immunohistochemistry for TRIP8b and HCN1 demonstrated exquisite costaining in distal dendrites of CA1 and layer V neocortical pyramidal neurons, implying that TRIP8b’s association with at least HCN1 may play a functional role in vivo, an implication strengthened by the finding that TRIP8b enrichment in the distal dendrites of layer V neurons in the cortex was abolished in the HCN1−/− mouse. Given the association between TRIP8b and HCN1 in regions of the mouse brain known to possess high levels of h channels and Ih,15,40,104,105 it was surprising that Santoro et al. found coexpression of HCN1 or HCN2 with TRIP8b led to dramatic downregulation of Ih and surface-associated h channel protein in oocytes as well as in cultured hippocampal neurons, with the decrease in Ih dependent upon the presence of the h channel C-terminal tripeptide.103 These effects on Ih and h channel protein seemed to be mediated at least in part by trafficking to an EEA1+ endosomal compartment.
The functional findings of TRIP8b on Ih in vitro were difficult to rectify given the in vivo immunohistochemical and biochemical findings. Recently, our group and others have proposed alternative splicing of the TRIP8b N-terminus as a potential explanation for this paradox, and have found at least nine different isoforms in vivo,106,107 which encompass isoforms identified in the Santoro et al. 2004 study as well as those found by Chen et al.100,103 Alternative splicing in nervous tissue is a common mechanism to generate diversity,108 and has been described before in both ion channels109–111 and ion channel binding and scaffolding proteins.112, 113 TRIP8b splicing appears to be regulated on a developmental basis,106 and different isoforms increase or decrease Ih and h channel surface expression in oocytes, HEK cells and cultured hippocampal neurons,106,107,114 discussed later in this review. Furthermore, via independent methodologies, Lewis et al. and Santoro et al. arrived at similar conclusions regarding the major two TRIP8b splice isoforms expressed in the brain. All splice isoforms of TRIP8b similarly hyperpolarized the V50 of Ih, indicating this action is independent of the variable N-terminus. The shift in voltage gating of Ih induced by TRIP8b appears to be mediated by antagonism of cAMP’s effects on Ih,107,114 thereby hyperpolarizing V50. These voltage gating changes may be mediated through a novel interaction between TRIP8b and h channels different from the previously described PTS1-like interaction. Specifically, the gating effect of TRIP8b on Ih requires the TRIP8b N-terminal core114 which was found to be required for TRIP8b binding to the HCN1 CNBD and represents a likely mechanism by which TRIP8b alters the voltage gating of Ih.106
Channel kinetics
(see Table 3) Just as different h channel subunits have distinctive voltage gating properties, so do they have differing kinetic properties. HCN1 has the fastest channel activation, with HCN4 displaying the slowest activation, while HCN2 and HCN3 lie somewhere in between. In vivo, kinetics of activation and deactivation vary markedly by cell type, likely a function of the exact h channel subunits expressed as well as regulatory factors, some of which are discussed herein.1,4,82 The specific time constant for kinetics of activation of HCN1 and HCN2 are a function of test potential, and HCN2 activates roughly 15-times slower than HCN1. While application of cAMP does speed kinetics, this is attributable to changes in V50, with HCN1 still activating faster than HCN2 in the presence of cAMP, even though HCN2 V50 is more significantly modified by cAMP than HCN1.35 Indeed, as discussed above for V50, deletion of the CNBD of HCN1 and HCN2, thought to relieve inhibition on channel opening, altered channel kinetics to a degree qualitatively similarly as when the channel is exposed to cAMP. Channel deactivation is also slowed by the presence of cAMP. Therefore, cAMP serves to promote both quicker activation and slower deactivation of Ih.
Table 3.
Regulatory mechanisms altering Ih/If activation kinetics
| Modulatory mechanism or treatment |
Experimental system | Increased or decreased rate of activation |
Ref. |
|---|---|---|---|
| cAMP | Numerous | Increased | 1–8 |
| PIP2 | Oocytes expressing HCN2 (inside-out patch) | Decreased at some but not all tested potentials |
41, 46, 47 |
| [pH]i | Rat TC neurons or HEK293 cells expressing HCN2 | Increased with ↑pH; decreased with ↓pH |
49, 50, 54 |
| [pH]o | FLP-IN-293 cells expressing HCN1 or HCN4 | Increased with ↓pH | 51 |
| Genistein (tyrosine kinase inhibition) |
Oocytes expressing HCN1, 2 or 4 | Decreased for HCN2 and 4, no effect for HCN1 |
57 |
| Rat ventricular myocytes | Decreased | ||
| Src kinase | HEK293 cells expressing HCN2 treated with PP2 (Src inhibitor) | Decreased with PP2 treatment | 60 |
| Proteolysis of HCN channel subunits |
Coexpression of proteolyzed HCN2 (HCN2ΔC) with HCN4 in HEK293 cells |
Increased compared to HCN4 alone |
66 |
| KCNE2 | Oocytes expressing HCN1 and HCN2 | Increased | 85 |
| Oocytes or CHO cells expressing HCN4 | Decreased | 90 | |
| Adenoviral overexpression in cultured neonatal rat ventricular myocytes |
Increased | 89 | |
| CHO cells expressing HCN1, 2, 4 | Increased | 88 | |
| TRIP8b | Oocytes expressing HCN1 | Decreased | 107 |
| Filamin A | Coexpression with HCN1 in melanoma cell line | Decreased | 94 |
Magnitude is not included here as it is typically dependent upon voltage step.
Similarly to properties of channel gating, kinetics of Ih differ in a cell-specific manner, and are rarely recapitulated by expression of a single h channel subunit, intimating a role for heterotetrameric channels in vivo. When coexpressed in oocytes, the activation kinetics of Ih from HCN1 and HCN2 lie somewhere intermediate between those produced by either subunit alone and could not be reproduced by an algebraic sum of two hypothetical populations of homotetramers, demonstrating that heteromerization is a mechanism by which Ih kinetics may be regulated.37
Although PIP2 can significantly affect channel gating, its effects on activation kinetics are less clear-cut. Exposure to PIP2 or analogues significantly slowed opening of HCN2 in oocytes at −135 mV, but not at −125 or −110 mV, and slowed the speed of deactivation at −40 mV.41 PIP2-induced changes in kinetics occur via mechanisms that are both convergent and unique to kinetic changes by cAMP.
In addition to investigating modulation of voltage dependence by both intracellular and extracellular pH, Munsch and Pape, as well as Zong and colleagues investigated the effect of pH on kinetics of Ih activation.49,50,54 Increases in pipette pH evoked decreased time constants of activation. This change was substantial; for example, the τ of activation when tested at −93 mV for a pipette pH of 7.5 is half that at a pH of 6.7. As expected, the regulation of Ih activation kinetics, like V50, is dependent on histidine residue 321, and although not explicitly tested, it is presumed that the modulation of activation kinetics by intracellular protons is independent of cAMP-induced changes in activation kinetics.50 Interestingly, the effect of extracellular protons on activation kinetics is opposite to that of intracellular protons. Examination of the τ of activation of Ih from heterologously expressed HCN4 revealed a drastic increase in speed of activation with decreased extracellular pH. This response was much more exaggerated for HCN4 than for HCN1, and the dramatic change in HCN4 activation kinetics at low pH may explain this channel’s role in transduction of sour stimuli.51
Studies directed toward understanding the role of Src kinase in modulation of Ih have also found that tyrosine phosphorylation may alter kinetics in addition to voltage gating. Using genistein, Yu et al. found that this non-specific tyrosine kinase inhibitor differentially alters activation kinetics in heterologously expressed h channels whereby it slows the τ of HCN2 and HCN4 at some membrane potentials, but not HCN1. Furthermore, genistein slowed the τ of activation of cultured ventricular myocytes.57 Such studies however were unable to demarcate between a direct effect on h channels or a process upstream of activation kinetics. One explanation for the actions of genistein may be by inhibition of Src kinase, which was later found to directly modulate both the activation and deactivation kinetics of Ih as well as bind to the C-terminus of h channels.56,60 Specifically, inhibition of Src kinase slowed both activation and deactivation of heterologously expressed HCN2 in HEK293 cells, and this effect on activation kinetics persisted qualitatively in the presence of 1 mM cAMP.60 These effects on both activation and deactivation kinetics appear to be mediated by direct phosphorylation of tyrosine 476 (Y476).
Furthermore, a Y476 mutant was no longer sensitive to kinetics changes by genistein. Similarly, mutation of the corresponding tyrosine of HCN4, Y554, also led to insensitivity of kinetics by inhibition of Src. Finally, inhibition of Src in cultured cells from heart or dorsal root ganglion (DRG) caused significant slowing of activation kinetics, solidifying Src as a physiologically important regulator of h channel kinetics.60 A recent report has demonstrated that inhibition of tyrosine phosphatase activity speeds activation kinetics of Ih in adult ventricular myocytes, providing further evidence for tyrosine phosphorylation as a modifier of h channel kinetics.62 Finally, it is worth noting that not all phosphorylation mechanisms that induce changes in channel voltage gating produce significant alterations in channel kinetics, as blockade of p38 MAPK did not significantly change kinetics of Ih activation in pyramidal neurons despite its potent ability to shift V50.63
The addition of truncated HCN2, HCN2ΔC, along with full length HCN4, generates an Ih with activation kinetics different from those observed with expression of HCN2 or HCN4 alone.66 Interestingly, HCN2ΔC significantly speeds the π of activation to values very similar to that observed in endogenous heart, providing further evidence that this post-translational processing of HCN2 may be important for processes in vivo.66
Finally, just as seen with voltage gating, interacting proteins may also alter the kinetics of Ih, although oftentimes by poorly understood mechanisms. KCNE2 has been found to have an effect on kinetics of Ih in multiple studies, but similar to its effects on gating, results from different groups do not always agree, implying sensitivity to experimental conditions. In oocytes, cooexpression of KCNE2 with HCN1 or HCN2 increased the speed of activation with no change in the time constant of deactivation.85 This study reaches different conclusions than multiple other reports, including that of Altomare et al. which found no effect of KCNE2 on HCN4 or an HCN4-1 concatameric construct in HEK293 cells,87 or of Decher et al. who found that rather than speed activation, coexpression of KCNE2 with HCN4 in CHO cells and oocytes significantly slowed the time constant of activation.90 Furthermore, adenoviral expression of KCNE2 with HCN2 in cultured neonatal rat ventricular myocytes demonstrated increased activation kinetics of Ih.89
The effects of TRIP8b on Ih kinetics have been recently elucidated.107 All isoforms inhibit h channel opening by both slowing the kinetics of activation and speeding the kinetics of deactivation, an effect most likely mediated by the central core of TRIP8b, similar to the mechanism affecting activation gating. These findings were observed in both oocytes and cultured hippocampal neurons.
Finally, the scaffolding protein filamin A, like TRIP8b, slowed Ih activation kinetics when overexpressed with HCN1, but also slowed the deactivation kinetics.94 Yeast two-hybrid results indicated that HCN1, but not HCN2 or HCN4, interacted with filamin A. As expected, coexpression of HCN4 with filamin A did not change channel kinetics, implying that the kinetic action of filamin A is specific for HCN1. Much work remains to be done in order to elucidate what role filamin A may play in vivo with respect to HCN1. In vitro evidence demonstrates effects of filamin A on both HCN1 membrane clustering as well is its biophysical properties,94 but whether this effect occurs in neurons is yet unknown.
Ih amplitude via changes in channel conductance and surface expression
(see Table 4) The overall effect of a VGIC is determined not only by voltage activation, whose action is in turn governed by voltage gating as well as kinetics, but also by the number of channels expressed on the membrane surface their conductance. Thus, even under voltage conditions highly favorable for channel activation, if few channels are properly expressed on a cell surface or their conductance is very low, minimal current and little effect on membrane potential may be observed. We have previously described how a diverse range of molecules can affect essentially all voltage related characteristics of h channels and Ih. Here, we shift focus to concentrate on how this group of regulators alters h channel conductance and surface expression, properties critical for completing the picture of h channel regulation.
Table 4.
Regulatory mechanisms altering Ih/If amplitude
| Modulatory mechanism or treatment |
Experimental system | Effect on Ih amplitude | Ref. |
|---|---|---|---|
| cAMP | Rat CA1 pyramidal neurons | Increased conductance | 115 |
| Orexin A | Mouse prelimbic cortical layer V pyramidal neurons | Decreased | 67 |
| Thyroid hormone (T3) | Neonatal atrial myocytes Rabbit SAN cells | Increased current density and conductance |
118–120 |
| [Cl−]o | Rabbit SAN myocytes (whole cell and isolated patch) | Decreased with ↓[Cl−]o (less effect on HCN1 than HCN2, 4) |
121–124 |
| [Cl−]i | HEK293 cells expressing HCN4 | Decreased instantaneous current with ↑[Cl−]I, no effect on steady state current |
125 |
| Genistein (tyrosine kinase inhibition) |
Rabbit SAN myocytes | Decreased If conductance | 59 |
| Oocytes expressing HCN1, HCN2 or HCN4 | Decreased for HCN2 and HCN4, no effect for HCN1 |
57 | |
| Rat ventricular myoctes | Decreased | ||
| RPTPα | HEK293 cells expressing HCN2 | Decreased surface expression and current density |
62 |
| KCR1 | CHO cells expressing HCN2 | Decreased | 93 |
| Adenoviral overexpression of KCR1 in neonatal rat cardiomyocytes |
Decreased | ||
| siRNA knockdown of endogenous KCR1 in neonatal rat cardiomyocytes |
Increased | ||
| KCNE2 | Oocytes expressing HCN1 or HCN2 | Increased Ih conductance | 85 |
| CHO cells expressing HCN2 | Decreased steady state Ih, but Increased instantaneous Ih |
86 | |
| Oocytes and CHO cells expressing HCN4 | Increased | 90 | |
| Adenoviral expression in neonatal rat ventricular myocytes |
Increased conductance | 89 | |
| CHO cells expressing HCN1, 2, 4 | Increased | 88 | |
| TRIP8b | Oocytes or HEK293 cells expressing HCN1 | Increased or Decreased surface expression depending on TRIP8b isoform |
103, 106, 107, 114 |
| siRNA knockdown of all TRIP8b isoforms in cultured rat hippocampal neurons |
Decreased | 106 | |
| Filamin A | Coexpression with HCN1 in melanoma cell line | Decreased Ih conductance | 94 |
cAMP has been discussed previously and has effects on Ih voltage gating and kinetics. Interestingly, cAMP has been reported to have a small effect on the maximal conductance of Ih in CA1 pyramidal neurons, as addition of 8-bromoadenosine 3'5'-cyclic monophosphate (8-Br-cAMP) led to a 6% increase of Ih recorded at −140 mV.115
The hyperpolarizing action on Ih V50 by the peptide orexin A was previously discussed. In addition, orexin A suppressed Ih inward currents in PL pyramidal neurons as well as reduced voltage sag in response to hyperpolarizing current injection. These effects were at least partially mediated by orexin receptor 1 (OXR1) as blockade with the specific OXR1 antagonist SB334867 partly eliminated the effects of orexin A on Ih currents.67 Orexin A is not the only peptide found to modify Ih. Cortistatin (CST) is a brain-specific peptide similar to somatostatin (SST), and has sleep modifying properties. CST and SST bind to similar receptors, and therefore produce similar effects, but CST can also produce physiological effects distinct from those of SST, implying divergent actions.116 For example, CST induces slow-wave sleep while SST promotes rapid eye movement sleep. Because sleep is intimately associated with rhythmic network activity, Schweitzer et al. sought to determine postsynaptic actions of CST and SST on CA1 hippocampal neurons, and examine where the two peptides diverged in attempt to uncover CST-specific effects.117 Both peptides enhanced IM and IK(L); however, CST but not SST enhanced Ih conductance by ~35% in a CST concentration-de-pendent manner that was independent of cAMP. The Ih blocker ZD7288 blocked this effect, and CST did not shift the Ih voltage activation curve. CST joins muscarinic receptor agonists, gabapentin, serotonin, and a κ-opioid receptor agonist as modulators of hippocampal Ih independent of cAMP, an interesting finding given that many modulators of Ih act by altering cAMP levels.6,117 Schweitzer et al. hypothesized that CST may act by intracellular calcium release, although this hypothesis awaits substantiation with additional evidence.
Thyroid hormone (TH) has important cardiac chronotropic effects, yet the mechanism of action of TH on heart rhythmicity is still not completely understood. Hyperthyroidism leads to tachycardia and a tendency to develop arrhythmias, while a hypothyroid state oftentimes leads to bradycardia and prolonged cardiac repolarization. If is one of multiple currents that contributes to pacemaker activity in many regions of the heart, including the SAN and Purkinje fibers. In neonatal atrial myocytes, 3,3’,5-triiodo-L-thyronine (T3) Significantly increased the beating rate in a time- and dose-dependent manner.118 Interestingly however, in experiments designed to test whether T3 enhanced If, only about half of the cells tested exhibited If, regardless of treatment with T3 When only the cells with discernible If were examined, treatment with T3, led to an ~4-fold increase in If current density. These data agree with studies conducted in adult rabbit SAN that found ~2-fold increases in If maximal conductance after treatment with T3 without changes in voltage activation.119 Interestingly however, application of 1 mM Cs+, which blocks If, did not change the basal rate of beating, both with and without T3 treatment, suggesting that If is not important for pacemaker activity in neonatal atrium. T3 acts via two sets of nuclear thyroid hormone receptors (TRs), TRα1, TRβ1, TRβ2 and TRβ3, which are encoded by two genes. In heart, TRα1 is the major expressed isoform, with TRβ1 being the other expressed iso-form.120 Gassanov et al. explored the role of these two TR’s on If activity by adenoviral-mediated gene delivery in spontaneously beating neonatal rat cardiomyoctes.120 Overexpression of TRα1 led to increased spontaneous beating rate compared to control, and TRα1+ cells treated with T3 demonstrated increased beating rate compared to control cells treated with T3. Interestingly, TRα1-overexpression significantly sped up the diastolic depolarization velocity. Conversely, cells overexpressing TRβ1 exhibited decreased spontaneous beating rate that was unaffected by T3 treatment. Additionally, TRβ1-expressing cells demonstrated decreased diastolic depolarization velocity. If current density was significantly increased in cells overexpressing TRα1 with or without treatment with T3, with no change in V50 or kinetics. TRβ1 cells trended toward decreased If current density and no change with T3 treatment. Mechanistically, these differential changes in If with TRα1 or TRβ1 overexpression are potentially due to changes in HCN2 and/or HCN4 gene transcription and protein expression.120
If/Ih amplitude is sensitive to extracellular chloride concentration.121,122 In myocytes from rabbit SAN, replacement of extracellular chloride with larger anions such as aspartate decreased If amplitude, and was found to be a function of extracellular chloride concentration.123 This change in amplitude was independent of a change in voltage gating, implying that extracellular chloride regulates If conductance, and extracellular chloride concentration also did not affect channel kinetics. The effects of chloride substitution were still observed in excised patch conformation. The intriguing feature of this system is that although If amplitude is highly sensitive to extracellular chloride concentration, If is completely impermeable to chloride anions. Frace et al. suggest that If sensitivity to external chloride concentration is due to chloride acting as a “positive charge screen” that is required for cation permeability.123 Wahl-Schott et al. sought to further understand the mechanism behind this phenomenon and found that cloned HCN1, HCN2 and HCN4 channels had differing sensitivities to extracellular chloride, with HCN1 channels still passing ~50% of normal current even in the absence of chloride, while HCN2 and HCN4 current was almost entirely eliminated under the same conditions.124 By exploiting the different chloride sensitivities of the three h channels and construction of multiple chimeras, Wahl-Schott et al. determined that chloride sensitivity is determined by a single arginine residue in the channel pore region that is present in HCN2 and HCN4 but is replaced by an alanine residue in HCN1.124 This finding sheds further light on chloride’s mechanism of action, and the authors hypothesized that chloride binding to this arginine, which is located very close to the pore opening, may induce a conformational change permitting passage of cations. A regulatory role for intracellular chloride has also been found. If is comprised of a fast component that activates immediately upon hyperpolarization as well as the more commonly described slow component that reaches steady state.86 This instantaneous current is suppressed by high intracellular chloride, but the steady state current is insensitive to intracellular chloride concentration.125
We have previously discussed the major role phosphorylation plays in regulating the biophysical properties of Ih. In addition to altering voltage gating and kinetics, phosphorylation can also alter channel conductance. Inhibition of endogenous tyrosine kinases with either genistein or herbimycin A in rabbit SAN myocytes significantly reduced If conductance.59 This inhibition was via a cAMP-independent signaling pathway. To support this finding, treatment of rabbit SAN myocytes with epidermal growth factor led to increased If that was blocked by genistein.58 This effect was presumed to be via tyrosine phosphorylation by the EGF receptor tyrosine kinase. Interestingly, in Xenopus oocytes, inhibition of tyrosine kinases by genistein selectively inhibited If for HCN2 and HCN4 but not for HCN1.57 In rat ventricular myocytes, HCN2 is the most highly expressed h channel isoform. Accordingly, Yu et al. found that treatment of isolated rat ventricular myoctes with genistein significantly reduced If current density.57 Evidence for phosphorylation of h channels was reported recently by Huang et al. who found that RPTPα-enhanced tyrosine dephosphorylation led to a decrease or abolition of HCN2-mediated Ih in HEK293 cells that correlated with dephosphorylation of HCN2 protein.62 Furthermore, dephoshorylation of HCN2 by RPTPα led to a significant decrease in surface expression of channel protein as evidenced by immunocytochemistry.
As reviewed earlier, protein binding partners for VGICs are typically classified as scaffolds, adaptors, chaperones or auxiliary subunits. While these proteins can influence biophysical properties, they are also often involved in regulating the trafficking of their VGIC binding partners. h channels have numerous interacting proteins, some of which have been found to affect channel trafficking to and from the cell surface. KCR1 when coexpressed with HCN2 in CHO cells dramatically decreases Ih current density. Consistent with these in vitro findings, native Ih amplitude was either down or upregulated when native KCR1 was adenovirally overexpressed or knocked down by siRNA in neonatal rat cardiomyocytes, respectively.93 KCR1 may be an important regulatory molecule of If in vivo, especially given the finding that overexpression or decreased expression has the functional consequences of altering spontaneous beating of neonatal cardiomyoctes.
KCNE2 has previously been discussed as an h channel interacting molecule that appears to have differing biophysical effects depending on experimental system. In Xenopus oocytes, coexpression of KCNE2 with HCN1 or HCN2 led to a 3- or 5-fold increase in Ih conductance, respectively, while coexpression with KCNE1 did not enhance Ih.85 Additionally, protein expression of KCNE2 and HCN1 was increased when coexpressed versus individual expression. In contrast, CHO cells coexpressing KCNE2 and HCN2 exhibited decreased rather than increased Ih.86 This study also identified an instantaneous inward current, Iinst(HCN2) upon HCN2 overexpression that is modulated by cAMP, requires surface expression of HCN2, and whose amplitude correlates with Ih. KCNE2 expression increased Iinst(HCN2), demonstrating that KCNE2 has opposite effects on two different currents presumably provided by the same a-subunit. In HEK293 cells, KCNE2 did not affect the current amplitude, kinetics, or voltage gating of HCN4 Ih, or of Ih resultant from a concatameric HCN4-1 con-struct.87 In rabbit SAN, HCN1, HCN4 and KCNE2 are highly expressed,85,126–128 thus these results suggest the physiological role of KCNE2 at least in rabbit SAN is limited. However, another study similar in design to that of Altomare et al. found that in Xenopus oocytes and CHO cells KCNE2 had a profound effect on human HCN4, enhancing current amplitude, hyperpolarizing the V50, and slowing the activation kinetics.90 This study also found direct biochemical interaction between h channels and KCNE2. Finally, a recent study reexamined the effects of KCNE2 on the h channel subunits most highly expressed in the heart, HCN1, HCN2 and HCN4, and found that KCNE2 significantly increased current densities and sped activation kinetics of all subunits without an effect on channel voltage gating in CHO cells, in partial agreement with previous studies.88 However, a novel finding of the study was that KCNE2 differentially regulated the mean open time of single h channels depending on subunit identity while at the same time increasing single channel amplitude and conductance. The evidence for functional in vitro interaction of KCNE2 with h channels is thus highly variable and greatly dependent upon both the h channel isoform studied and the system in which it is studied. While the heterologous data is confusing, there is even less information concerning the role of KCNE2 as an h channel β-subunits in vivo. To address this, Qu and colleagues used adenoviral overexpression of HCN2 and KCNE2 in cultured neonatal rat ventricular myocytes and found that KCNE2 coexpression led to a significant increase in maximal Ih conductance and activation kinetics with no change in V50.89 Furthermore, coimmunoprecipitation experiments provided some evidence for a protein complex between HCN2 and KCNE2. Taken together, this is the first and to our knowledge only series of experiments that provide evidence for the role of KCNE2 as an h channel β-subunit in primary cells.
A growing body of evidence implicates If dysfunction in the pathophysiology of heart disease,20,129,130 and misregulation may stem from auxiliary subunit derangement rather than from the pore-forming subunit itself. Numerous mutations in the KCNE gene family have been reported to lead to LQTS resulting from impaired repolarization, and mutations in the KCNE2 gene specifically have also been found to cause LQTS.80,84 However, whether pathological mutations or altered expression of KCNE2 might effect If and lead to cardiac disorders remains unknown. Ventricular and atrial HCN2 and HCN4 mRNA and protein is upregulated in patients with heart failure. However, this remodeling of If by altered h channel expression is not accompanied by changes in either KCNE2 mRNA or protein level.131 Furthermore, an experimental canine model of atrial tachyarrhythmia demonstrated that 7-day atrial tachypacing significantly decreased SAN HCN2 and HCN4 mRNA levels and decreased If current densities, yet again no change in KCNE2 mRNA was observed.132 Interestingly, mRNA for MinK was also significantly reduced. Thus, direct evidence for KCNE2 alterations causing human disease specifically through disruption of If is lacking. The high promiscuity of KCNE2 in vitro clouds a clear understanding of its role in vivo. Indeed, KCNE2 has been shown to interact with hERG, KCNQ1–3, KV3.1, KV3.2, KV4.2, KV4.3, and as discussed above, h channels.84 Mice with a targeted deletion of kcne2 have been useful to understand the protein’s function in processes as diverse as gastric acid secretion,133 ventricular repolarization,134 and synthesis of thyroid hormone.135 Future research will need to examine Ih/If in these knockout animals to determine if kcne2 is indeed required for proper Ih expression in vivo.
As previously mentioned, TRIP8b is subject to extensive alternative splicing at the molecule’s N-terminus that does not participate in either of the channels presumed multiple interaction domains with h channels, and the N-terminus is also not required to elicit the V50 hyperpolarization in Ih, nor is it likely to play a role in kinetic changes brought about by TRIP8b.106,107,114 However, the identity of the TRIP8b N-terminus appears to be critically important for regulating h channel surface trafficking. Our group, as well as Santoro et al. identified splice isoforms that either enhanced or decreased Ih current densities in either HEK293 cells or Xenopus oocytes when coexpressed with h channels.103,106,107 Additional experiments using flow cytometry, biochemistry, and confocal microscopy suggested that this change in current density is due to a change in the number of h channels present on the membrane surface and not in channel conductance. This is consistent with findings both in cultured hippocampal neurons and in vivo in hippocampus that different isoforms of TRIP8b can dramatically rearrange the subcellular localization of h channels.106,107 Furthermore, in hippocampal neurons, overexpression of a TRIP8b isoform predicted to enhance h channel surface expression, or siRNA knockdown of TRIP8b, led to an increase or decrease of native Ih, respectively, demonstrating a role for TRIP8b in the trafficking of in vivo h channels.106,107 How exactly does the TRIP8b N-terminus carry out this trafficking function? From research reports so far, the answer is not straightforward. Santoro et al. explored the role of consensus protein trafficking motifs variably present in different exons of the TRIP8b N-terminus.107 Exon 2 contains a tyrosine-based trafficking motif (YXXØ), while exons 4, 5 and 6 contain dileucine-based ([DE]XXXL[LI]) trafficking motifs, both of which can interact with cellular protein sorting systems. Mutation of the YXXØ motif in exon 2 of TRIP8b(1b-2) eliminated its strong downregulating effects, suggesting that this motif is required for Ih downregulation. However, TRIP8b(1a) does not have such a motif, yet was still found in this study (but not in Lewis et al.106) to be a downregulating isoform. Mutation of the dileucine-based trafficking motif of TRIP8b(1a) in exon 5, but not in exon 6, abolished TRIP8b(1a) downregulation, which suggests that this dileucine motif is another motif important for downregulation. The story is not finished there however, since TRIP8b(1a-4), which contains dileucine motifs in exons 4, 5 and 6, is strongly upregulating for Ih. Thus, it remains to be seen whether the actions of individual TRIP8b isoforms can be distilled to the presence of specific sequences or if combinatorial effects of trafficking motifs are important. Interestingly, in vivo TRIP8b forms a molecular complex with HCN1 and AP-2, suggesting a potential mechanism for h channel endocytosis by some isoforms of TRIP8b.107
Filamin A has also been found to regulate h channel surface expression and Ih in vitro. The interaction of HCN1 with filamin A appears to cluster HCN1 protein on cell membranes and decrease Ih conductance in a melanoma cell line.94 As noted above in discussing effects on gating and kinetics, the role of filamin A in h channel surface expression and clustering in neurons is yet to be explored.
h Channel Interacting Proteins with as Yet Unknown Functions on Ih
Tamalin/GRP1-associated scaffold protein (GRASP)
Tamalin, or GRP1-associated scaffold protein (GRASP), was identified by a yeast two-hybrid screen of a rat brain cDNA library using the class I PDZ-binding motif-containing C-terminal sequences of group 1 and group 2 metabotropic glutamate receptors (mGluRs) as bait.136 It is the same gene product as GRASP, first reported as a retinoic acid-induced gene137 isolated from P19 embryonal carcinoma cells. Tamalin contains multiple distinct protein regions, including a PDZ-domain, alanine-rich, proline-rich and glycine-rich regions, a leucine zipper sequence, four YXXL motifs, and a C-terminal PDZ-binding motif.136 Ultimately it was found that tamalin increases intracellular trafficking and surface expression of group 1 mGluRs in COS-7 cells and neurites of cultured hippocampal neurons, and forms an in vivo complex with mGluR1a and cytohesin-2.136
Like group I and group II mGluRs, HCN1–4 contain potential PDZ-binding motifs at their C-termini,138 prompting Kimura et al. to ask whether tamalin also binds to these h channel subunits. A yeast two-hybrid assay surprisingly demonstrated that tamalin binds to the final seven amino acids of HCN2, but not HCN1, 3 or 4, an interaction confirmed by coimmunoprecipitation experiments with tagged full length HCN2 and tamalin in COS-7 cells.139 Protein interaction studies revealed not surprisingly that HCN2 interacted with the N-terminal PDZ-domain of tamalin. However, this interaction is not completely straightforward, as deletion of the C-terminal 10 amino acids from HCN2 reduced, but did not completely eliminate HCN2 binding to tamalin. Binding was eliminated only after deletion of all HCN2 amino acids distal to the CNBD.139 These data therefore implicate not only the PDZ-binding motif of HCN2 but also a region within the intracellular C-terminal region between the CNBD and the PDZ-binding motif as important for this protein-protein interaction.
The functional consequences of the interaction of tamalin with HCN2 remain to be elucidated. specifically, it is unknown whether tamalin binds directly to HCN2 in vivo, although tamalin binds to proteins known to bind HCN2 in vivo (see below). Recently a tamalin knockout mouse has been reported, demonstrating no differences in protein levels or localization of mGluR1a, mGluR2, mGluR5 and GABAB2.140 It is still unknown whether these mice have alterations in Ih in any region in which tamalin and HCN2 are expressed. Such experiments will be critical for tamalin to be established as a physiologically important interacting protein with HCN2.
Synaptic scaffolding molecule (S-SCAM) and Mint2 (X11-like)
In addition to binding HCN2, tamalin is known to form complexes with multiple other proteins, including synaptic scaffolding molecule (S-SCAM), Mint2 and PSD-95.141 S-SCAM is a brain specific protein that contains a number of protein interaction motifs, specifically a guanylate kinase-like domain, two WW and five PDZ domains, and binds to a large number of functionally important yet diverse proteins, including HCN2.139,142 Mint2 is also a brain specific protein that contains two PDZ-domains and a phosphotyrosine domain and interacts with amyloid precursor protein (APP).143,144 Mint2 plays a role in APP processing, and mice with a genetic deletion of Mint2 demonstrate increased levels of β-secretase catalyzed C-terminal fragments of APP.145 Recently, Mint2 has been found to play a role in the behavioral neurobiology underlying murine conflict resolution.146 Mint2 also interacts with a number of other proteins involved with governing cellular properties including Munc-18 and HCN2.139,144
Thus, both S-SCAM and Mint2 have been demonstrated to bind to a large number of different proteins including voltageand ligand-gated ion channels and cell adhesion molecules, and both bind HCN2 from rat brain extract.139 To rule out a possible indirect interaction of S-SCAM or Mint2 with HCN2 through protein complexes, co-immunoprecipitation experiments in transfected COS-7 cells were performed demonstrating that indeed HCN2 interacts directly with both S-SCAM and Mint2. S-SCAM, like tamalin, binds to HCN2 via a PDZ-domain, and binding to HCN2 is reduced by deletion of an HCN2 portion distal to the CNBD with abolition of binding following deletion of HCN2 CNBD and beyond.139 Mint2 interacts with the region distal to the HCN2 CNBD via its munc18-interacting domain. Interestingly, Mint2 appeared to exert a protein stabilizing effect on HCN2, as coexpression of Mint2 with HCN2 increased HCN2 expression compared to HCN2 expressed alone or HCN2 coexpressed with a Mint2 mutant unable to bind HCN2.139
What is the physiological importance of the binding of S-SCAM and Mint2 to HCN2? First, it remains to be seen whether S-SCAM and Mint2 bind with any other HCN subunits. From a more hyperopic point of view, S-SCAM appears to be an important synaptic scaffolding molecule for many classes of proteins, including ion channels, but also neuronal cell adhesion molecules which may play important roles in poorly understood diseases such as autism. In neocortex and hippocampus, the predominant expression of HCN1 and HCN2 is along the plasma membrane of dendritic shafts, with less h channel immunoreactivity in the spine and none observed at asymmetric synapses.40,105 S-SCAM is highly expressed in cortex and interacts strongly with various transmembrane AMPA receptor regulatory protein (TARP) isoforms.147 Yet since HCN2 is not highly expressed in dendritic spines and absent at synapses of cortical pyramidal neurons, it is possible that the interaction of S-SCAM with HCN2 plays another unknown role. Knockout mice with genetic deletion of S-SCAMα (the longest of three alternatively spliced forms of S-SCAM) have been generated, and although they develop and are born live, die within 24 hours. These mice have profound abnormalities in neuronal spine morphology.148 A more viable conditional knockout mouse model of S-SCAM might help explore the physiological importance of S-SCAM’s association with HCN2.
Mint2 is highly expressed in excitatory neurons of the hippocampus, although it is expressed throughout the brain.149,150 In the hippocampus, Mint2 staining appears most strongly in cell bodies with weaker staining in dendrites. Mint2 does not appear to be expressed in presynaptic compartments or in the endoplasmic reticulum in cultured hippocampal neurons, but strongly colocalizes with TGN38, a marker of the trans-golgi network, a localization similar to that of APP. In HEK293 cells, expression of Mint2 increased the expression levels of cotransfected APP,150 similarly to what was described for Mint2 and HCN2.139 Thus, unlike S-SCAM, Mint2 may play a role in the early processing of h channels before trafficking from the cell body to dendrites. Analysis of Mint2-deficient mice may help narrow down the role of Mint2 on h channel function and/or localization.
Conclusions
In this review we have attempted to provide an overview of the major modulatory factors and interacting proteins of h channel subunits that affect h channel biophysical properties and surface expression (Table 5). These channels are members of a larger superfamily of voltage-gated ion channels, and many of the processes that influence HCN channels also influence other members of this superfamily. When evaluating studies on this topic, two questions should be kept in the mind of the reader. First, where in the signaling pathway are the regulatory mechanism(s) and h channel located? For example, does the modulator acts directly on the h channel, such as with cAMP or TRIP8b? Or is the h channel downstream of the effector, such as occurs with 4βPMA? This will help reveal how global signaling changes may affect h channels. Second, especially when evaluating new h channel interacting proteins, one must ask which of these interactions are of relevance in vivo? KCNE2 provides an excellent example for the requirement of in vivo work. Studies attempting to elucidate the role of KCNE2 in regulating Ih via interaction with HCN channel subunits have been hampered by great variability between model systems. On the other hand, a dearth of work has been published examining what role, if any, KCNE2 plays in regulating Ih in the living animal, whether through pantissue or tissue-specific knockout animals, virally-expressed siRNA knockdown, or at the very least, cultured primary cells such as cardiomyocytes or neurons. Additional proteomic work such as performed by Zolles and colleagues for determination of TRIP8b interaction with h channels is also of great utility in exploring the role of a particular interacting partner in vivo. This type of work demonstrated that the overwhelming majority of h channels in the brain are associated with TRIP8b, contributing to the hypothesis that TRIP8b serves as a critically important auxiliary subunit in rodent brain.114 As researchers continue to elucidate roles for h channels in both normal and pathological states of the nervous system and the heart, interest in the regulatory molecules and proteins of h channels will undoubtedly grow. Furthermore, just as mutations in regulatory subunits rather than pore-forming alpha subunits of K+ channels have been found to cause pathologies in humans such as LQTS, there exists the chance that mutations in h channel regulatory subunits may exert a pathological function through either overt or subtle changes in Ih in multiple tissues. Because Ih is a subthreshold conductance in which small changes in amplitude, kinetics, or gating may be amplified to have large effects on overall circuits, a greater understanding of the mechanisms by which Ih is regulated through interacting ions, molecules and proteins is required to begin to design therapeutics to target Ih dysfunction.
Table 5.
Holistic overview of Ih/If regulatory mechanisms
| Modulatory mechanism or treatment |
V50 | Rate of activation | Current amplitude | Refs. |
|---|---|---|---|---|
| cAMP | ↑ | ↑ | ↑, NE | 1–8, 115 |
| PIP2 | ↑ | ↓, NE (dependent on test-potential) |
NE | 41, 45–47 |
| PA, AA | ↑ | NDI | Effect not specific for Ih | 48 |
| [pH]i | ↑ (↑[pH]i) ↓ (↓[pH]i) | ↑ (↑[pH]i) ↓ (↓[pH]i) | NE | 49, 50, 54 |
| [pH]o | ↓ (↑[pH]o) ↑ (↓[pH]o) | ↓ (↑[pH]o) ↑ (↓[pH]o) | NE | 51, but see 50 |
| Tyrosine phosphorylation | ↑ | ↑ at some potentials | ↑ | 56, 58, 61, 62 |
| p38 MAPK | ↑ | NE | NE | 63 |
| HCN channel proteolysis | ↑ | ↑ | NDI | 66 |
| Genistein (tyrosine kinase inhibition) |
↓ or NE (depends on HCN isoform) |
↓ or NE | ↓ or NE | 57, 59 |
| Orexin A | ↓ | NDI | ↓ | 67 |
| KCNE2 | ↓ | ↑↓ | ↑↓ | 85–90 |
| KCR1 | ↓ | ↓ | ↓ | 93 |
| Filamin A | ↓ | ↓ | ↓ | 94 |
| TRIP8b | ↓ | ↓ | ↑↓ | 103, 106, 107, 114 |
| Thyroid hormone (T3) | NE | NE | ↑ | 118–120 |
| [Cl−]i | ↑ with ↑ [Cl−]i | NE | ↑ Ih(inst) w/↑ [Cl−]i | 125 |
| [Cl−]o | NE | NE | ↓ w/↓ [Cl−]o | 121–124 |
| RPTPα | NDI | NDI | ↓ | 62 |
↑, increased/depolarized; ↓, decreased/hyperpolarized; ↑↓, both reported; NE, no effect; NDI, not directly investigated.
Figure 1.
Directly interacting small molecule and protein regulatory mechanisms of of Ih function. See text for details on binding sites. (A) Schematic depicting HCN channel tetrameric structure. One HCN channel subunit is shown in detail. Dashed red lines indicate known sites of interaction of small molecules. (B) Schematic depicting HCN channel and known interaction sites of HCN channel interacting proteins. CNBD, cyclic nucleotide binding domain; SNL-C-terminal tripeptide of HCN1, HCN2 and HCN4.
Acknowledgements
This work was supported by National Institutes of Health grants 1F30NS064757 (A.S.L.) and 1K02NS05595 and 1R01NS059934 (D.M.C.).
Abbreviations
- HCN
hyperpolarization-activated cyclic nucleotide-gated
- Ih
hyperpolarization-activated current
- If
funny current
- TRIP8b
tetratricopeptide repeat-containing Rab8b–interacting protein
- VGIC
voltage-gated ion channel
- SAN
sinoatrial node
- Iq
queer current
- RMP
resting membrane potential
- EPSP
excitatory post-synaptic potential
- TC
thalamocortical
- It
low-threshold Ca2+ current
- RTN
reticular thalamic nucleus
- IPSP
inhibitory post-synaptic potential
- TLE
temporal lobe epilepsy
- cAMP
3'-5'-cyclic adenosine monophosphate
- CNBD
cyclic nucleotide binding domain
- cGMP
3'-5'-cyclic guanosine monophosphate
- PKA
cAMP-dependent protein kinase (protein kinase A)
- PIP2
phosphatidylinositol 4,5-bisphosphate
- DAG
diacylglycerol
- 4βPMA
4β-phorbol 12-myristate 13-acetate
- PKC
protein kinase C
- PA
phosphatidic acid
- AA
arachidonic acid
- TRPV1
transient receptor potential cation channel, subfamily V, member 1
- RPTPα
receptor-like protein-tyrosine phosphatase-α
- MAPK
mitogen-activated protein kinase
- PL
prelimbic
- PSD-95
postsynaptic density-95
- NMDA
N-methyl D-aspartate
- KChIP
Kv channel-interacting protein
- CNG
cyclic nucleotide-gated
- EAG
ether-á-go-go
- MiRP
MinK-related peptide
- MinK
minimal K+ channel protein
- hERG
human ether-à-go-go related gene
- CHO
chinese hamster ovary
- HEK
human embryonic kidney
- KCR1
K+-channel regulator protein 1
- Y2 h
yeast two-hybrid
- Pex5p
peroxin protein 5
- OXR1
orexin receptor 1
- CST
cortistatin
- SST
somatostatin
- TH
thyroid hormone
- T3
3,3',5-triiodo-L-thyronine
- TR
thyroid hormone receptor
- LQTS
long QT syndrome
- mGluR
metabotropic glutamate receptor
- PDZ
PSD-95/disc large/zonula occludens 1
- S-SCAM
synaptic scaffolding molecule
- TARP
transmembrane AMPA receptor regulatory protein
- DIV
day in vitro
- PTS1
peroxisomal targeting sequence 1
- PLC
phospholipase C
References
- 1.Wahl-Schott C, Biel M. HCN channels: Structure, cellular regulation and physiological function. Cell Mol Life Sci. 2008 doi: 10.1007/s00018-008-8525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu Rev Physiol. 2006;68:375–401. doi: 10.1146/annurev.physiol.68.040104.134728. [DOI] [PubMed] [Google Scholar]
- 3.Baruscotti M, Bucchi A, Difrancesco D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol Ther. 2005;107:59–79. doi: 10.1016/j.pharmthera.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 5.Biel M, Schneider A, Wahl C. Cardiac HCN channels: structure, function and modulation. Trends Cardiovasc Med. 2002;12:206–212. doi: 10.1016/s1050-1738(02)00162-7. [DOI] [PubMed] [Google Scholar]
- 6.Pape HC. Queer current and pacemaker: the hyper-polarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- 7.Accili EA, Proenza C, Baruscotti M, DiFrancesco D. From funny current to HCN channels: 20 years of excitation. News Physiol Sci. 2002;17:32–37. doi: 10.1152/physiologyonline.2002.17.1.32. [DOI] [PubMed] [Google Scholar]
- 8.DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- 9.Gauss R, Seifert R, Kaupp UB. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature. 1998;393:583–587. doi: 10.1038/31248. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- 11.Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, et al. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- 12.Angelo K, London M, Christensen SR, Hausser M. Local and global effects of I(h) distribution in dendrites of mammalian neurons. J Neurosci. 2007;27:8643–8653. doi: 10.1523/JNEUROSCI.5284-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuart G, Spruston N. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J Neurosci. 1998;18:3501–3510. doi: 10.1523/JNEUROSCI.18-10-03501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- 15.Williams SR, Stuart GJ. Site independence of EPSP time course is mediated by dendritic I(h) in neocortical pyramidal neurons. J Neurophysiol. 2000;83:3177–3182. doi: 10.1152/jn.2000.83.5.3177. [DOI] [PubMed] [Google Scholar]
- 16.Tsay D, Dudman JT, Siegelbaum SA. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron. 2007;56:1076–1089. doi: 10.1016/j.neuron.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George MS, Abbott LF, Siegelbaum SA. HCN hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K(+) channels. Nat Neurosci. 2009;12:577–584. doi: 10.1038/nn.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, et al. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Luthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron. 1998;21:9–12. doi: 10.1016/s0896-6273(00)80509-7. [DOI] [PubMed] [Google Scholar]
- 20.Barbuti A, DiFrancesco D. Control of cardiac rate by “funny” channels in health and disease. Ann N Y Acad Sci. 2008;1123:213–223. doi: 10.1196/annals.1420.024. [DOI] [PubMed] [Google Scholar]
- 21.Poolos NP. The h-channel: a potential channelopathy in epilepsy? Epilepsy Behav. 2005;7:51–56. doi: 10.1016/j.yebeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 2003;22:216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung WK, Shin M, Jaramillo TC, Leibel RL, LeDuc CA, Fischer SG, et al. Absence epilepsy in apathetic, a spontaneous mutant mouse lacking the h channel subunit, HCN2. Neurobiol Dis. 2009;33:499–508. doi: 10.1016/j.nbd.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kole MH, Brauer AU, Stuart GJ. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J Physiol. 2007;578:507–525. doi: 10.1113/jphysiol.2006.122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin M, Brager D, Jaramillo TC, Johnston D, Chetkovich DM. Mislocalization of h channel subunits underlies h channelopathy in temporal lobe epilepsy. Neurobiol Dis. 2008;32:26–36. doi: 10.1016/j.nbd.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung S, Jones TD, Lugo JN, Jr, Sheerin AH, Miller JW, D’Ambrosio R, et al. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah MM, Anderson AE, Leung V, Lin X, Johnston D. Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons. Neuron. 2004;44:495–508. doi: 10.1016/j.neuron.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Z, Walker MC, Shah MM. Loss of dendritic HCN1 subunits enhances cortical excitability and epileptogenesis. J Neurosci. 2009;29:10979–10988. doi: 10.1523/JNEUROSCI.1531-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brewster AL, Bernard JA, Gall CM, Baram TZ. Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis. 2005;19:200–207. doi: 10.1016/j.nbd.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunlop J, Vasilyev D, Lu P, Cummons T, Bowlby MR. Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels and pain. Curr Pharm Des. 2009;15:1767–1772. doi: 10.2174/138161209788186281. [DOI] [PubMed] [Google Scholar]
- 32.Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- 33.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function and physiological roles. Physiol Rev. 90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 34.Zagotta WN, Olivier NB, Black KD, Young EC, Olson R, Gouaux E. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature. 2003;425:200–205. doi: 10.1038/nature01922. [DOI] [PubMed] [Google Scholar]
- 35.Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 2001;411:805–810. doi: 10.1038/35081088. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Chen S, Siegelbaum SA. Regulation of hyperpolarization-activated HCN channel gating and cAMP modulation due to interactions of COOH terminus and core transmembrane regions. J Gen Physiol. 2001;118:237–250. doi: 10.1085/jgp.118.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Er F, Larbig R, Ludwig A, Biel M, Hofmann F, Beuckelmann DJ, et al. Dominant-negative suppression of HCN channels markedly reduces the native pacemaker current I(f) and undermines spontaneous beating of neonatal cardiomyocytes. Circulation. 2003;107:485–489. doi: 10.1161/01.cir.0000045672.32920.cb. [DOI] [PubMed] [Google Scholar]
- 39.Much B, Wahl-Schott C, Zong X, Schneider A, Baumann L, Moosmang S, et al. Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem. 2003;278:43781–43786. doi: 10.1074/jbc.M306958200. [DOI] [PubMed] [Google Scholar]
- 40.Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- 41.Pian P, Bucchi A, Robinson RB, Siegelbaum SA. Regulation of gating and rundown of HCN hyperpolarization-activated channels by exogenous and endogenous PIP2 . J Gen Physiol. 2006;128:593–604. doi: 10.1085/jgp.200609648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliver D, Lien CC, Soom M, Baukrowitz T, Jonas P, Fakler B. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004;304:265–270. doi: 10.1126/science.1094113. [DOI] [PubMed] [Google Scholar]
- 43.Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, et al. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- 44.Wu L, Bauer CS, Zhen XG, Xie C, Yang J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5) P2. Nature. 2002;419:947–952. doi: 10.1038/nature01118. [DOI] [PubMed] [Google Scholar]
- 45.Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Zolles G, Klocker N, Wenzel D, Weisser-Thomas J, Fleischmann BK, Roeper J, et al. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron. 2006;52:1027–1036. doi: 10.1016/j.neuron.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Pian P, Bucchi A, Decostanzo A, Robinson RB, Siegelbaum SA. Modulation of cyclic nucleotide-regulated HCN channels by PIP(2) and receptors coupled to phospholipase C. Pflugers Arch. 2007;455:125–145. doi: 10.1007/s00424-007-0295-2. [DOI] [PubMed] [Google Scholar]
- 48.Fogle KJ, Lyashchenko AK, Turbendian HK, Tibbs GR. HCN pacemaker channel activation is controlled by acidic lipids downstream of diacylglycerol kinase and phospholipase A2. J Neurosci. 2007;27:2802–2814. doi: 10.1523/JNEUROSCI.4376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munsch T, Pape HC. Modulation of the hyperpolarization-activated cation current of rat thalamic relay neurones by intracellular pH. J Physiol. 1999;519:493–504. doi: 10.1111/j.1469-7793.1999.0493m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zong X, Stieber J, Ludwig A, Hofmann F, Biel M. A single histidine residue determines the pH sensitivity of the pacemaker channel HCN2. J Biol Chem. 2001;276:6313–6319. doi: 10.1074/jbc.M010326200. [DOI] [PubMed] [Google Scholar]
- 51.Stevens DR, Seifert R, Bufe B, Muller F, Kremmer E, Gauss R, et al. Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature. 2001;413:631–635. doi: 10.1038/35098087. [DOI] [PubMed] [Google Scholar]
- 52.Kiss L, Korn SJ. Modulation of N-type Ca2+ channels by intracellular pH in chick sensory neurons. J Neurophysiol. 1999;81:1839–1847. doi: 10.1152/jn.1999.81.4.1839. [DOI] [PubMed] [Google Scholar]
- 53.Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, et al. TRPV1 is activated by both acidic and basic pH. J Neurosci. 2009;29:153–158. doi: 10.1523/JNEUROSCI.4901-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Munsch T, Pape HC. Upregulation of the hyperpolarization-activated cation current in rat thalamic relay neurones by acetazolamide. J Physiol. 1999;519:505–514. doi: 10.1111/j.1469-7793.1999.0505m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santoro B, Grant SG, Bartsch D, Kandel ER. Interactive cloning with the SH3 domain of N-src identifies a new brain specific ion channel protein, with homology to eag and cyclic nucleotide-gated channels. Proc Natl Acad Sci USA. 1997;94:14815–14820. doi: 10.1073/pnas.94.26.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arinsburg SS, Cohen IS, Yu HG. Constitutively active Src tyrosine kinase changes gating of HCN4 channels through direct binding to the channel proteins. J Cardiovasc Pharmacol. 2006;47:578–586. doi: 10.1097/01.fjc.0000211740.47960.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu HG, Lu Z, Pan Z, Cohen IS. Tyrosine kinase inhibition differentially regulates heterologously expressed HCN channels. Pflugers Arch. 2004;447:392–400. doi: 10.1007/s00424-003-1204-y. [DOI] [PubMed] [Google Scholar]
- 58.Wu JY, Yu H, Cohen IS. Epidermal growth factor increases i(f ) in rabbit SA node cells by activating a tyrosine kinase. Biochim Biophys Acta. 2000;1463:15–19. doi: 10.1016/s0005-2736(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 59.Wu JY, Cohen IS. Tyrosine kinase inhibition reduces i(f) in rabbit sinoatrial node myocytes. Pflugers Arch. 1997;434:509–514. doi: 10.1007/s004240050430. [DOI] [PubMed] [Google Scholar]
- 60.Zong X, Eckert C, Yuan H, Wahl-Schott C, Abicht H, Fang L, et al. A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotide-gated channels by Src kinase. J Biol Chem. 2005;280:34224–34232. doi: 10.1074/jbc.M506544200. [DOI] [PubMed] [Google Scholar]
- 61.Li CH, Zhang Q, Teng B, Mustafa SJ, Huang JY, Yu HG. Src tyrosine kinase alters gating of hyperpolarization-activated HCN4 pacemaker channel through Tyr531. Am J Physiol Cell Physiol. 2008;294:355–362. doi: 10.1152/ajpcell.00236.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang J, Huang A, Zhang Q, Lin YC, Yu HG. Novel mechanism for suppression of hyperpolarization-activated cyclic nucleotide-gated pacemaker channels by receptor-like tyrosine phosphatase-alpha. J Biol Chem. 2008;283:29912–29919. doi: 10.1074/jbc.M804205200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poolos NP, Bullis JB, Roth MK. Modulation of h-channels in hippocampal pyramidal neurons by p38 mitogen-activated protein kinase. Journal of Neuroscience. 2006;26:7995–8003. doi: 10.1523/JNEUROSCI.2069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stieber J, Herrmann S, Feil S, Loster J, Feil R, Biel M, et al. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci USA. 2003;100:15235–15240. doi: 10.1073/pnas.2434235100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herrmann S, Stieber J, Stockl G, Hofmann F, Ludwig A. HCN4 provides a ‘depolarization reserve’ and is not required for heart rate acceleration in mice. EMBO J. 2007;26:4423–4432. doi: 10.1038/sj.emboj.7601868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye B, Nerbonne JM. Proteolytic processing of HCN2 and co-assembly with HCN4 in the generation of cardiac pacemaker channels. J Biol Chem. 2009;284:25553–25559. doi: 10.1074/jbc.M109.007583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li B, Chen F, Ye J, Chen X, Yan J, Li Y, et al. The Modulation of Orexin A on HCN Currents of Pyramidal Neurons in Mouse Prelimbic Cortex. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp241. [DOI] [PubMed] [Google Scholar]
- 68.Cathala L, Paupardin-Tritsch D. Neurotensin inhibition of the hyperpolarization-activated cation current (Ih) in the rat substantia nigra pars compacta implicates the protein kinase C pathway. J Physiol. 1997;503:87–97. doi: 10.1111/j.1469-7793.1997.087bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Z, Bunney EB, Appel SB, Brodie MS. Serotonin reduces the hyperpolarization-activated current (Ih) in ventral tegmental area dopamine neurons: involvement of 5-HT2 receptors and protein kinase C. J Neurophysiol. 2003;90:3201–3212. doi: 10.1152/jn.00281.2003. [DOI] [PubMed] [Google Scholar]
- 70.Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- 71.Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- 72.Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Isom LL, De Jongh KS, Catterall WA. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994;12:1183–1194. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 74.Trimmer JS. Regulation of ion channel expression by cytoplasmic subunits. Curr Opin Neurobiol. 1998;8:370–374. doi: 10.1016/s0959-4388(98)80063-9. [DOI] [PubMed] [Google Scholar]
- 75.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 76.Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Jr, Phillips HA, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19:366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- 77.Abbott GW, Butler MH, Bendahhou S, Dalakas MC, Ptacek LJ, Goldstein SA. MiRP2 forms potassium channels in skeletal muscle with KV3.4 and is associated with periodic paralysis. Cell. 2001;104:217–231. doi: 10.1016/s0092-8674(01)00207-0. [DOI] [PubMed] [Google Scholar]
- 78.Splawski I, Tristani-Firouzi M, Lehmann MH, Sanguinetti MC, Keating MT. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 79.Sesti F, Goldstein SA. Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome. J Gen Physiol. 1998;112:651–663. doi: 10.1085/jgp.112.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, et al. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 81.Sesti F, Abbott GW, Wei J, Murray KT, Saksena S, Schwartz PJ, et al. A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proc Natl Acad Sci USA. 2000;97:10613–10618. doi: 10.1073/pnas.180223197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santoro B, Tibbs GR. The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann N Y Acad Sci. 1999;868:741–764. doi: 10.1111/j.1749-6632.1999.tb11353.x. [DOI] [PubMed] [Google Scholar]
- 83.Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988;242:1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- 84.McCrossan ZA, Abbott GW. The MinK-related pep-tides. Neuropharmacology. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 85.Yu H, Wu J, Potapova I, Wymore RT, Holmes B, Zuckerman J, et al. MinK-related peptide 1: A beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ Res. 2001;88:84–87. doi: 10.1161/hh1201.093511. [DOI] [PubMed] [Google Scholar]
- 86.Proenza C, Angoli D, Agranovich E, Macri V, Accili EA. Pacemaker channels produce an instantaneous current. J Biol Chem. 2002;277:5101–5109. doi: 10.1074/jbc.M106974200. [DOI] [PubMed] [Google Scholar]
- 87.Altomare C, Terragni B, Brioschi C, Milanesi R, Pagliuca C, Viscomi C, et al. Heteromeric HCN1-HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J Physiol. 2003;549:347–359. doi: 10.1113/jphysiol.2002.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brandt MC, Endres-Becker J, Zagidullin N, Motloch LJ, Er F, Rottlaender D, et al. Effects of KCNE2 on HCN isoforms: distinct modulation of membrane expression and single channel properties. Am J Physiol Heart Circ Physiol. 2009;297:355–363. doi: 10.1152/ajpheart.00154.2009. [DOI] [PubMed] [Google Scholar]
- 89.Qu J, Kryukova Y, Potapova IA, Doronin SV, Larsen M, Krishnamurthy G, et al. MiRP1 modulates HCN2 channel expression and gating in cardiac myocytes. J Biol Chem. 2004;279:43497–43502. doi: 10.1074/jbc.M405018200. [DOI] [PubMed] [Google Scholar]
- 90.Decher N, Bundis F, Vajna R, Steinmeyer K. KCNE2 modulates current amplitudes and activation kinetics of HCN4: influence of KCNE family members on HCN4 currents. Pflugers Arch. 2003;446:633–640. doi: 10.1007/s00424-003-1127-7. [DOI] [PubMed] [Google Scholar]
- 91.Hoshi N, Takahashi H, Shahidullah M, Yokoyama S, Higashida H. KCR1, a membrane protein that facilitates functional expression of non-inactivating K+ currents associates with rat EAG voltage-dependent K+ channels. J Biol Chem. 1998;273:23080–23085. doi: 10.1074/jbc.273.36.23080. [DOI] [PubMed] [Google Scholar]
- 92.Kupershmidt S, Yang IC, Hayashi K, Wei J, Chanthaphaychith S, Petersen CI, et al. The IKr drug response is modulated by KCR1 in transfected cardiac and noncardiac cell lines. FASEB J. 2003;17:2263–2265. doi: 10.1096/fj.02-1057fje. [DOI] [PubMed] [Google Scholar]
- 93.Michels G, Er F, Khan IF, Endres-Becker J, Brandt MC, Gassanov N, et al. K+ channel regulator KCR1 suppresses heart rhythm by modulating the pacemaker current If. PLoS One. 2008;3:1511. doi: 10.1371/journal.pone.0001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gravante B, Barbuti A, Milanesi R, Zappi I, Viscomi C, DiFrancesco D. Interaction of the pacemaker channel HCN1 with filamin A. J Biol Chem. 2004;279:43847–43853. doi: 10.1074/jbc.M401598200. [DOI] [PubMed] [Google Scholar]
- 95.van der Flier A, Sonnenberg A. Structural and functional aspects of filamins. Biochim Biophys Acta. 2001;1538:99–117. doi: 10.1016/s0167-4889(01)00072-6. [DOI] [PubMed] [Google Scholar]
- 96.Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol. 2004;6:1034–1038. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- 97.Lin R, Karpa K, Kabbani N, Goldman-Rakic P, Levenson R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc Natl Acad Sci USA. 2001;98:5258–5263. doi: 10.1073/pnas.011538198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petrecca K, Miller DM, Shrier A. Localization and enhanced current density of the KV4.2 potassium channel by interaction with the actin-binding protein filamin. J Neurosci. 2000;20:8736–8744. doi: 10.1523/JNEUROSCI.20-23-08736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sampson LJ, Leyland ML, Dart C. Direct interaction between the actin-binding protein filamin-A and the inwardly rectifying potassium channel, Kir2.1. J Biol Chem. 2003;278:41988–41997. doi: 10.1074/jbc.M307479200. [DOI] [PubMed] [Google Scholar]
- 100.Chen S, Liang MC, Chia JN, Ngsee JK, Ting AE. Rab8b and its interacting partner TRIP8b are involved in regulated secretion in AtT20 cells. J Biol Chem. 2001;276:13209–13216. doi: 10.1074/jbc.M010798200. [DOI] [PubMed] [Google Scholar]
- 101.Subramani S. Components involved in peroxisome import, biogenesis, proliferation, turnover and movement. Physiol Rev. 1998;78:171–188. doi: 10.1152/physrev.1998.78.1.171. [DOI] [PubMed] [Google Scholar]
- 102.Amery L, Sano H, Mannaerts GP, Snider J, Van Looy J, Fransen M, et al. Identification of PEX5p–related novel peroxisome-targeting signal 1 (PTS1)-binding proteins in mammals. Biochem J. 2001;357:635–646. doi: 10.1042/0264-6021:3570635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santoro B, Wainger BJ, Siegelbaum SA. Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J Neurosci. 2004;24:10750–10762. doi: 10.1523/JNEUROSCI.3300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- 106.Lewis AS, Schwartz E, Chan CS, Noam Y, Shin M, Wadman WJ, et al. Alternatively spliced isoforms of TRIP8b differentially control h channel trafficking and function. J Neurosci. 2009;29:6250–6265. doi: 10.1523/JNEUROSCI.0856-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Santoro B, Piskorowski RA, Pian P, Hu L, Liu H, Siegelbaum SA. TRIP8b splice variants form a family of auxiliary subunits that regulate gating and trafficking of HCN channels in the brain. Neuron. 2009;62:802–813. doi: 10.1016/j.neuron.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Q, Lee JA, Black DL. Neuronal regulation of alternative pre-mRNA splicing. Nat Rev Neurosci. 2007;8:819–831. doi: 10.1038/nrn2237. [DOI] [PubMed] [Google Scholar]
- 109.Francesconi A, Duvoisin RM. Alternative splicing unmasks dendritic and axonal targeting signals in metabotropic glutamate receptor 1. J Neurosci. 2002;22:2196–2205. doi: 10.1523/JNEUROSCI.22-06-02196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron. 2003;40:581–594. doi: 10.1016/s0896-6273(03)00676-7. [DOI] [PubMed] [Google Scholar]
- 111.Jaskolski F, Coussen F, Nagarajan N, Normand E, Rosenmund C, Mulle C. Subunit composition and alternative splicing regulate membrane delivery of kain-ate receptors. J Neurosci. 2004;24:2506–2515. doi: 10.1523/JNEUROSCI.5116-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chetkovich DM, Bunn RC, Kuo SH, Kawasaki Y, Kohwi M, Bredt DS. Postsynaptic targeting of alternative postsynaptic density-95 isoforms by distinct mechanisms. J Neurosci. 2002;22:6415–6425. doi: 10.1523/JNEUROSCI.22-15-06415.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.DeSouza S, Fu J, States BA, Ziff EB. Differential palmitoylation directs the AMPA receptor-binding protein ABP to spines or to intracellular clusters. J Neurosci. 2002;22:3493–3503. doi: 10.1523/JNEUROSCI.22-09-03493.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zolles G, Wenzel D, Bildl W, Schulte U, Hofmann A, Muller CS, et al. Association with the auxiliary subunit PEX5R/Trip8b controls responsiveness of HCN channels to cAMP and adrenergic stimulation. Neuron. 2009;62:814–825. doi: 10.1016/j.neuron.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 115.Gasparini S, DiFrancesco D. Action of serotonin on the hyperpolarization-activated cation current (Ih) in rat CA1 hippocampal neurons. Eur J Neurosci. 1999;11:3093–3100. doi: 10.1046/j.1460-9568.1999.00728.x. [DOI] [PubMed] [Google Scholar]
- 116.de Lecea L, Criado JR, Prospero-Garcia O, Gautvik KM, Schweitzer P, Danielson PE, et al. A cortical neuropeptide with neuronal depressant and sleep-modulating properties. Nature. 1996;381:242–245. doi: 10.1038/381242a0. [DOI] [PubMed] [Google Scholar]
- 117.Schweitzer P, Madamba SG, Siggins GR. The sleep-modulating peptide cortistatin augments the h-current in hippocampal neurons. J Neurosci. 2003;23:10884–10891. doi: 10.1523/JNEUROSCI.23-34-10884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun ZQ, Ojamaa K, Nakamura TY, Artman M, Klein I, Coetzee WA. Thyroid hormone increases pacemaker activity in rat neonatal atrial myocytes. J Mol Cell Cardiol. 2001;33:811–824. doi: 10.1006/jmcc.2001.1353. [DOI] [PubMed] [Google Scholar]
- 119.Renaudon B, Lenfant J, Decressac S, Bois P. Thyroid hormone increases the conductance density of f-channels in rabbit sino-atrial node cells. Receptors Channels. 2000;7:1–8. [PubMed] [Google Scholar]
- 120.Gassanov N, Er F, Endres-Becker J, Wolny M, Schramm C, Hoppe UC. Distinct regulation of cardiac I(f) current via thyroid receptors alpha1 and beta1. Pflugers Arch. 2009;458:1061–1068. doi: 10.1007/s00424-009-0691-x. [DOI] [PubMed] [Google Scholar]
- 121.Seyama I. Characteristics of the anion channel in the sino-atrial node cell of the rabbit. J Physiol. 1979;294:447–460. doi: 10.1113/jphysiol.1979.sp012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yanagihara K, Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980;385:11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]
- 123.Frace AM, Maruoka F, Noma A. Control of the hyperpolarization-activated cation current by external anions in rabbit sino-atrial node cells. J Physiol. 1992;453:307–318. doi: 10.1113/jphysiol.1992.sp019230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wahl-Schott C, Baumann L, Zong X, Biel M. An arginine residue in the pore region is a key determinant of chloride dependence in cardiac pacemaker channels. J Biol Chem. 2005;280:13694–13700. doi: 10.1074/jbc.M413197200. [DOI] [PubMed] [Google Scholar]
- 125.Mistrik P, Pfeifer A, Biel M. The enhancement of HCN channel instantaneous current facilitated by slow deactivation is regulated by intracellular chloride concentration. Pflugers Arch. 2006;452:718–727. doi: 10.1007/s00424-006-0095-0. [DOI] [PubMed] [Google Scholar]
- 126.Ishii TM, Takano M, Xie LH, Noma A, Ohmori H. Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J Biol Chem. 1999;274:12835–12839. doi: 10.1074/jbc.274.18.12835. [DOI] [PubMed] [Google Scholar]
- 127.Shi W, Wymore R, Yu H, Wu J, Wymore RT, Pan Z, et al. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ Res. 1999;85:1–6. doi: 10.1161/01.res.85.1.e1. [DOI] [PubMed] [Google Scholar]
- 128.Moroni A, Gorza L, Beltrame M, Gravante B, Vaccari T, Bianchi ME, et al. Hyperpolarization-activated cyclic nucleotide-gated channel 1 is a molecular determinant of the cardiac pacemaker current I(f) J Biol Chem. 2001;276:29233–29241. doi: 10.1074/jbc.M100830200. [DOI] [PubMed] [Google Scholar]
- 129.Ludwig A, Herrmann S, Hoesl E, Stieber J. Mouse models for studying pacemaker channel function and sinus node arrhythmia. Prog Biophys Mol Biol. 2008;98:179–185. doi: 10.1016/j.pbiomolbio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 130.DiFrancesco D, Borer JS. The funny current: cellular basis for the control of heart rate. Drugs. 2007;67:15–24. doi: 10.2165/00003495-200767002-00003. [DOI] [PubMed] [Google Scholar]
- 131.Stillitano F, Lonardo G, Zicha S, Varro A, Cerbai E, Mugelli A, et al. Molecular basis of funny current (If) in normal and failing human heart. J Mol Cell Cardiol. 2008;45:289–299. doi: 10.1016/j.yjmcc.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 132.Yeh YH, Burstein B, Qi XY, Sakabe M, Chartier D, Comtois P, et al. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia: a molecular basis for tachycardia-bradycardia syndrome. Circulation. 2009;119:1576–1585. doi: 10.1161/CIRCULATIONAHA.108.789677. [DOI] [PubMed] [Google Scholar]
- 133.Roepke TK, Anantharam A, Kirchhoff P, Busque SM, Young JB, Geibel JP, et al. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem. 2006;281:23740–23747. doi: 10.1074/jbc.M604155200. [DOI] [PubMed] [Google Scholar]
- 134.Roepke TK, Kontogeorgis A, Ovanez C, Xu X, Young JB, Purtell K, et al. Targeted deletion of kcne2 impairs ventricular repolarization via disruption of I(K,slow1) and I(to,f) FASEB J. 2008;22:3648–3660. doi: 10.1096/fj.08-110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roepke TK, King EC, Reyna-Neyra A, Paroder M, Purtell K, Koba W, et al. Kcne2 deletion uncovers its crucial role in thyroid hormone biosynthesis. Nat Med. 2009;15:1186–1194. doi: 10.1038/nm.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kitano J, Kimura K, Yamazaki Y, Soda T, Shigemoto R, Nakajima Y, et al. Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins. J Neurosci. 2002;22:1280–1289. doi: 10.1523/JNEUROSCI.22-04-01280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nevrivy DJ, Peterson VJ, Avram D, Ishmael JE, Hansen SG, Dowell P, et al. Interaction of GRASP, a protein encoded by a novel retinoic acid-induced gene, with members of the cytohesin family of guanine nucleotide exchange factors. J Biol Chem. 2000;275:16827–16836. doi: 10.1074/jbc.275.22.16827. [DOI] [PubMed] [Google Scholar]
- 138.Monteggia LM, Eisch AJ, Tang MD, Kaczmarek LK, Nestler EJ. Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain. Brain Res Mol Brain Res. 2000;81:129–139. doi: 10.1016/s0169-328x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- 139.Kimura K, Kitano J, Nakajima Y, Nakanishi S. Hyperpolarization-activated, cyclic nucleotide-gated HCN2 cation channel forms a protein assembly with multiple neuronal scaffold proteins in distinct modes of protein-protein interaction. Genes Cells. 2004;9:631–640. doi: 10.1111/j.1356-9597.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 140.Ogawa M, Miyakawa T, Nakamura K, Kitano J, Furushima K, Kiyonari H, et al. Altered sensitivities to morphine and cocaine in scaffold protein tamalin knockout mice. Proc Natl Acad Sci USA. 2007;104:14789–14794. doi: 10.1073/pnas.0706945104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kitano J, Yamazaki Y, Kimura K, Masukado T, Nakajima Y, Nakanishi S. Tamalin is a scaffold protein that interacts with multiple neuronal proteins in distinct modes of protein-protein association. J Biol Chem. 2003;278:14762–14768. doi: 10.1074/jbc.M300184200. [DOI] [PubMed] [Google Scholar]
- 142.Hirao K, Hata Y, Ide N, Takeuchi M, Irie M, Yao I, et al. A novel multiple PDZ domain-containing molecule interacting with N-methyl-D-aspartate receptors and neuronal cell adhesion proteins. J Biol Chem. 1998;273:21105–21110. doi: 10.1074/jbc.273.33.21105. [DOI] [PubMed] [Google Scholar]
- 143.Tomita S, Ozaki T, Taru H, Oguchi S, Takeda S, Yagi Y, et al. Interaction of a neuron-specific protein containing PDZ domains with Alzheimer’s amyloid precursor protein. J Biol Chem. 1999;274:2243–2254. doi: 10.1074/jbc.274.4.2243. [DOI] [PubMed] [Google Scholar]
- 144.Okamoto M, Sudhof TC. Mints, Munc18-interacting proteins in synaptic vesicle exocytosis. J Biol Chem. 1997;272:31459–31464. doi: 10.1074/jbc.272.50.31459. [DOI] [PubMed] [Google Scholar]
- 145.Sano Y, Syuzo-Takabatake A, Nakaya T, Saito Y, Tomita S, Itohara S, et al. Enhanced amyloidogenic metabolism of the amyloid beta-protein precursor in the X11L–deficient mouse brain. J Biol Chem. 2006;281:37853–37860. doi: 10.1074/jbc.M609312200. [DOI] [PubMed] [Google Scholar]
- 146.Sano Y, Ornthanalai VG, Yamada K, Homma C, Suzuki H, Suzuki T, et al. X11-like protein deficiency is associated with impaired conflict resolution in mice. J Neurosci. 2009;29:5884–5896. doi: 10.1523/JNEUROSCI.5756-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Deng F, Price MG, Davis CF, Mori M, Burgess DL. Stargazin and other transmembrane AMPA receptor regulating proteins interact with synaptic scaffolding protein MAGI-2 in brain. J Neurosci. 2006;26:7875–7884. doi: 10.1523/JNEUROSCI.1851-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iida J, Ishizaki H, Okamoto-Tanaka M, Kawata A, Sumita K, Ohgake S, et al. Synaptic scaffolding molecule alpha is a scaffold to mediate N-methyl-D-aspartate receptor-dependent RhoA activation in dendrites. Mol Cell Biol. 2007;27:4388–4405. doi: 10.1128/MCB.01901-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee JH, Lau KF, Perkinton MS, Standen CL, Rogelj B, Falinska A, et al. The neuronal adaptor protein X11beta reduces amyloid beta-protein levels and amyloid plaque formation in the brains of transgenic mice. J Biol Chem. 2004;279:49099–49104. doi: 10.1074/jbc.M405602200. [DOI] [PubMed] [Google Scholar]
- 150.Biederer T, Cao X, Sudhof TC, Liu X. Regulation of APP-dependent transcription complexes by Mint/X11s: differential functions of Mint isoforms. J Neurosci. 2002;22:7340–7351. doi: 10.1523/JNEUROSCI.22-17-07340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]