Abstract
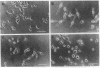
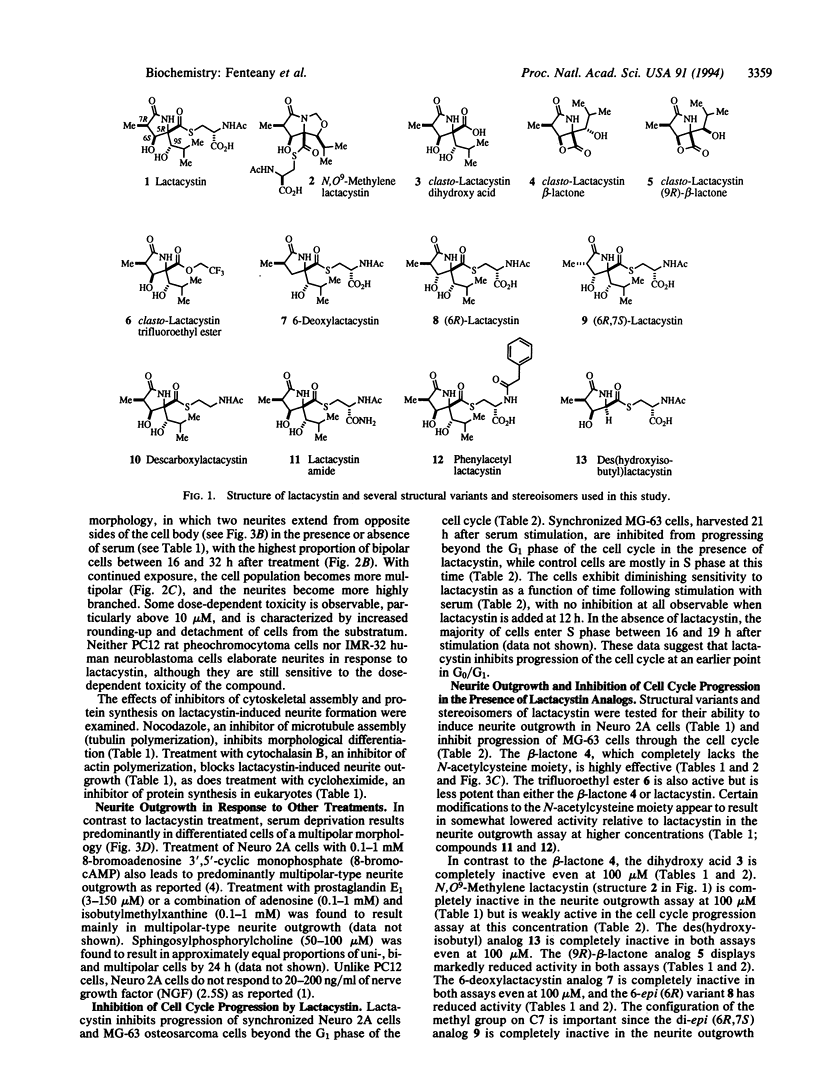
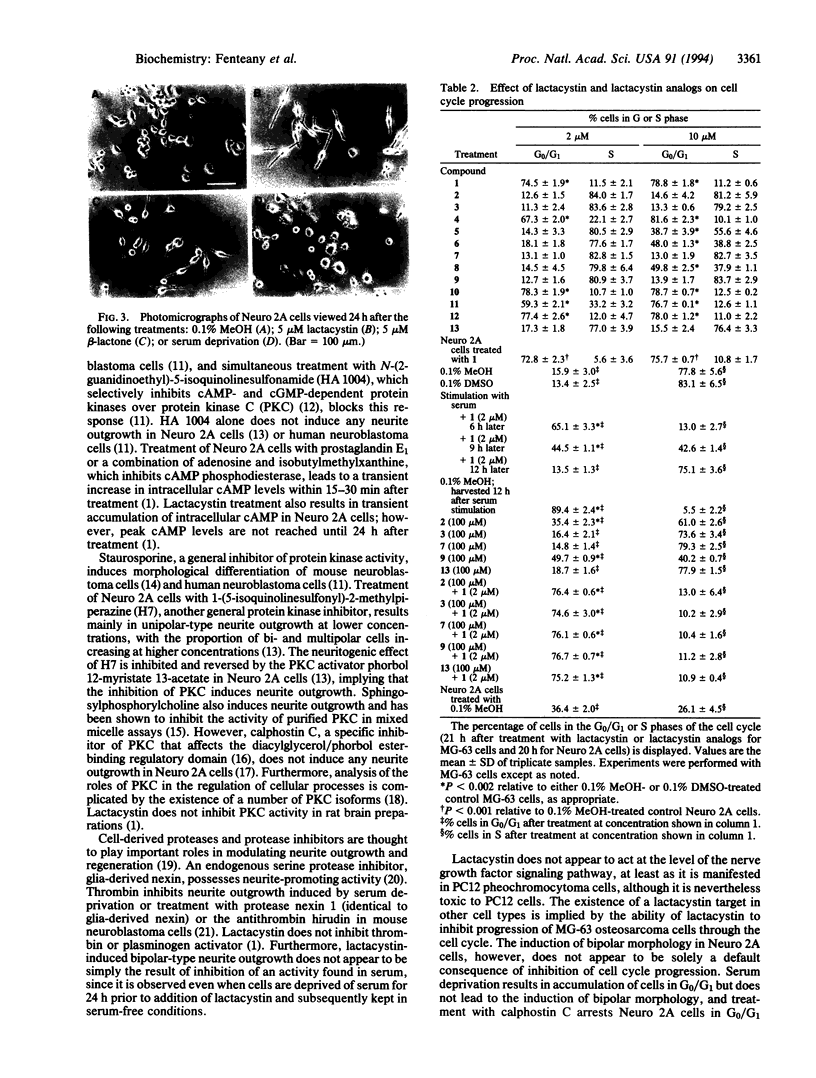
Lactacystin, a microbial natural product, induces neurite outgrowth in Neuro 2A mouse neuroblastoma cells and inhibits progression of synchronized Neuro 2A cells and MG-63 human osteosarcoma cells beyond the G1 phase of the cell cycle. A related beta-lactone, clasto-lactacystin beta-lactone, formally the product of elimination of N-acetylcysteine from lactacystin, is also active, whereas the corresponding clastolactacystin dihydroxy acid is completely inactive. Structural analogs of lactacystin altered only in the N-acetylcysteine moiety are active, while structural or stereochemical modifications of the gamma-lactam ring or the hydroxyisobutyl group lead to partial or complete loss of activity. The inactive compounds do not antagonize the effects of lactacystin in either neurite outgrowth or cell cycle progression assays. The response to lactacystin involves induction of a predominantly bipolar morphology that is maximal 16-32 h after treatment and is distinct from the response to several other treatments that result in morphological differentiation. Neurite outgrowth in response to lactacystin appears to be dependent upon microtubule assembly, actin polymerization, and de novo protein synthesis. The observed structure-activity relationships suggest that lactacystin and its related beta-lactone may act via acylation of one or more relevant target molecule(s) in the cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgström B. Mode of action of tetrahydrolipstatin: a derivative of the naturally occurring lipase inhibitor lipstatin. Biochim Biophys Acta. 1988 Oct 14;962(3):308–316. doi: 10.1016/0005-2760(88)90260-3. [DOI] [PubMed] [Google Scholar]
- Chiron M., Darbon J. M., Roubinet F., Cassar G., Jaffrezou J. P., Bordier C., Laurent G. Quantitative analysis of CD5 antigen modulation by 12-O-tetradecanoylphorbol-13-acetate in T-lymphoblastic leukemia cells: individual response patterns and their relationships with both maturation and protein kinase C content. Cell Immunol. 1990 Oct 15;130(2):339–351. doi: 10.1016/0008-8749(90)90277-x. [DOI] [PubMed] [Google Scholar]
- Choi GM, Tuller HL, Goldschmidt D. Electronic-transport behavior in single-crystalline Ba0.03Sr0.97TiO3. Phys Rev B Condens Matter. 1986 Nov 15;34(10):6972–6979. doi: 10.1103/physrevb.34.6972. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Trayner I., Menaya J. The role of protein kinase C isoenzymes in the regulation of cell proliferation and differentiation. J Cell Sci. 1992 Dec;103(Pt 4):881–887. doi: 10.1242/jcs.103.4.881. [DOI] [PubMed] [Google Scholar]
- Gloor S., Odink K., Guenther J., Nick H., Monard D. A glia-derived neurite promoting factor with protease inhibitory activity belongs to the protease nexins. Cell. 1986 Dec 5;47(5):687–693. doi: 10.1016/0092-8674(86)90511-8. [DOI] [PubMed] [Google Scholar]
- Greenspan M. D., Bull H. G., Yudkovitz J. B., Hanf D. P., Alberts A. W. Inhibition of 3-hydroxy-3-methylglutaryl-CoA synthase and cholesterol biosynthesis by beta-lactone inhibitors and binding of these inhibitors to the enzyme. Biochem J. 1993 Feb 1;289(Pt 3):889–895. doi: 10.1042/bj2890889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D., Cunningham D. D. Thrombin modulates and reverses neuroblastoma neurite outgrowth. Proc Natl Acad Sci U S A. 1988 May;85(10):3440–3444. doi: 10.1073/pnas.85.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science. 1987 Feb 6;235(4789):670–674. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Mayer R. J., Louis-Flamberg P., Elliott J. D., Fisher M., Leber J. Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A synthase by antibiotic 1233A and other beta-lactones. Biochem Biophys Res Commun. 1990 Jun 15;169(2):610–616. doi: 10.1016/0006-291x(90)90374-v. [DOI] [PubMed] [Google Scholar]
- Mitsui K., Tsuji S., Yamazaki M., Nagai Y. Multiple neurite formation in neuroblastoma cell lines by griseolic acid, a potent inhibitor of cyclic nucleotide phosphodiesterases. J Neurochem. 1991 Aug;57(2):556–561. doi: 10.1111/j.1471-4159.1991.tb03786.x. [DOI] [PubMed] [Google Scholar]
- Miñana M. D., Felipo V., Grisolía S. Differential effects of the protein kinase C inhibitors H7 and calphostin C on the cell cycle of neuroblastoma cells. Brain Res. 1992 Nov 20;596(1-2):157–162. doi: 10.1016/0006-8993(92)91543-n. [DOI] [PubMed] [Google Scholar]
- Miñana M. D., Felipo V., Grisolía S. Inhibition of protein kinase C induces differentiation in Neuro-2a cells. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4335–4339. doi: 10.1073/pnas.87.11.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monard D. Cell-derived proteases and protease inhibitors as regulators of neurite outgrowth. Trends Neurosci. 1988 Dec;11(12):541–544. doi: 10.1016/0166-2236(88)90182-8. [DOI] [PubMed] [Google Scholar]
- Omura S., Fujimoto T., Otoguro K., Matsuzaki K., Moriguchi R., Tanaka H., Sasaki Y. Lactacystin, a novel microbial metabolite, induces neuritogenesis of neuroblastoma cells. J Antibiot (Tokyo) 1991 Jan;44(1):113–116. doi: 10.7164/antibiotics.44.113. [DOI] [PubMed] [Google Scholar]
- Omura S., Matsuzaki K., Fujimoto T., Kosuge K., Furuya T., Fujita S., Nakagawa A. Structure of lactacystin, a new microbial metabolite which induces differentiation of neuroblastoma cells. J Antibiot (Tokyo) 1991 Jan;44(1):117–118. doi: 10.7164/antibiotics.44.117. [DOI] [PubMed] [Google Scholar]
- Shea T. B., Beermann M. L., Leli U., Nixon R. A. Opposing influences of protein kinase activities on neurite outgrowth in human neuroblastoma cells: initiation by kinase A and restriction by kinase C. J Neurosci Res. 1992 Nov;33(3):398–407. doi: 10.1002/jnr.490330306. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Ono K., Katayama N., Yamagata Y., Kikuchi K., Tsuchiya T. Neurite outgrowth from mouse neuroblastoma and cerebellar cells induced by the protein kinase inhibitor H-7. Neurosci Lett. 1989 Nov 6;105(3):241–245. doi: 10.1016/0304-3940(89)90627-7. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Yamashita T., Tanaka M., Nagai Y. Synthetic sialyl compounds as well as natural gangliosides induce neuritogenesis in a mouse neuroblastoma cell line (Neuro2a). J Neurochem. 1988 Feb;50(2):414–423. doi: 10.1111/j.1471-4159.1988.tb02928.x. [DOI] [PubMed] [Google Scholar]
- Weibel E. K., Hadvary P., Hochuli E., Kupfer E., Lengsfeld H. Lipstatin, an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. I. Producing organism, fermentation, isolation and biological activity. J Antibiot (Tokyo) 1987 Aug;40(8):1081–1085. doi: 10.7164/antibiotics.40.1081. [DOI] [PubMed] [Google Scholar]