Significance
Patients with the autoimmune disease systemic lupus erythematosus (SLE) develop pathogenic antibodies against their own “self”-antigens. The development of such autoantibodies is thought in part to require help from CD4+ T cells that also recognize the same self-antigens and direct autoantibody production. In this study, antigen-specific CD4+ T cells in lupus are identified and tracked for the first time using tetramers containing peptides from the spliceosomal protein U1-70. The proportion of circulating, tetramer-positive CD4+ cells correlates with the presence of U1-70–specific autoantibodies, and antigen-specific T cells are directly implicated in disease activity by their production of the proinflammatory cytokine IL-17. The role of these cells in disease pathology makes them candidate targets for therapeutic intervention.
Keywords: tetramer, autoimmunity, lupus, SLE, IL-17
Abstract
Antigen-specific CD4+ T cells are implicated in the autoimmune disease systemic lupus erythematosus (SLE), but little is known about the peptide antigens that they recognize and their precise function in disease. We generated a series of MHC class II tetramers of I-Ek–containing peptides from the spliceosomal protein U1-70 that specifically stain distinct CD4+ T-cell populations in MRL/lpr mice. The T-cell populations recognize an epitope differing only by the presence or absence of a single phosphate residue at position serine140. The frequency of CD4+ T cells specific for U1-70(131-150):I-Ek (without phosphorylation) correlates with disease severity and anti–U1-70 autoantibody production. These T cells also express RORγt and produce IL-17A. Furthermore, the U1-70–specific CD4+ T cells that produce IL-17A are detected in a subset of patients with SLE and are significantly increased in patients with mixed connective tissue disease. These studies provide tools for studying antigen-specific CD4+ T cells in lupus, and demonstrate an antigen-specific source of IL-17A in autoimmune disease.
Systemic lupus erythematosus (SLE) is an autoimmune disease in which patients develop high-titer, highly specific, isotype-switched autoantibodies against DNA- and RNA- containing autoantigens (1). U1-70, U1-A, and U1-C, together with U1-RNA and the seven Smith proteins, compose the U1-small nuclear ribonucleoprotein (U1-snRNP) complex. This U1-snRNP complex is one component of the spliceosome (1, 2). A subset of patients with SLE, and all patients with mixed connective tissue disease (MCTD), develop autoantibodies against U1-snRNP, and U1-70 in particular (1, 3–5). Anti-snRNP autoantibodies are detectable before overt disease in SLE in what is termed a “pathogenic autoimmunity” phase (6).
The role of CD4+ T helper (Th) cells in SLE is a long-standing area of investigation, with evidence of both T-cell–dependent and –independent autoantibody production. In support of T-cell–dependent mechanisms, CD4+ T cells are required for disease in the MRL/lpr murine model of lupus (7, 8), a model in which mice deficient in fas develop spontaneous autoimmunity (9). MRL/lpr mice with a limited T-cell receptor (TCR) repertoire have increased survival and develop fewer autoantibodies (10), indicating that antigen-specific T-cell help may be required for disease. Furthermore, adoptive transfer of CD4+ T cells from MRL/lpr mice into nonautoimmune anti-snRNP B-cell receptor (BCR) transgenic mice is sufficient for autoantibody synthesis, indicating that cognate T- and B-cell interactions are important for the development of anti–U1-snRNP autoantibodies specifically (11).
Despite evidence that antigen-specific T-cell help is required for autoantibody production and full manifestation of disease, T-cell–independent autoantibody production has been observed in the pristane model of lupus (12), as well as in MRL/lpr mice expressing a transgenic BCR recognizing self-IgG2a (13). In these cases, Toll-like receptor 7 (TLR7) signaling and interferons were required for autoantibodies against RNA-containing antigens. In addition, autoantibodies were sufficient to induce disease in nonautoimmune mice following adoptive transfer of antibodies from the BXD2 murine model of lupus (14); however, in BXD2 mice, treatment with CTLA4Ig before disease onset resulted in long-term suppression of autoantibodies (15), indicating that CD4+ T cells may be important early on, before autoantibody production.
Various therapies that target T cells are being investigated in SLE patients (16), including antigen-specific tolerizing therapy using a peptide derived from U1-70 (17). The role of antigen-specific CD4+ T cells in disease remains unclear, however, in part because the field has lacked a reagent for use in studying these cells directly. Here we report the generation of the first MHC class II tetramers to detect autoreactive CD4+ T cells in Mrl/lpr mice. These tetramers were used to identify a population of CD4+ T cells that recognize the self-protein U1-70 and produce the proinflammatory cytokine IL-17A. Such cells appear to be present not only in the MRL/lpr mice, but also in patients with SLE and MCTD.
Results
U1-70 Tetramers Specifically Detect MRL/lpr CD4+ T Cells.
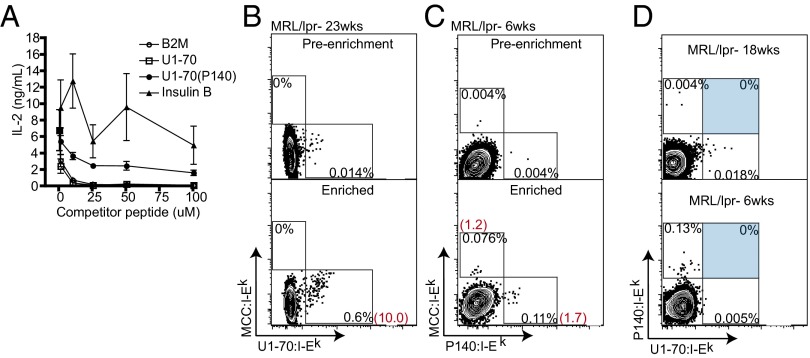
Our approach to generating stable, relevant tetramers to test in MRL/lpr mice was to identify peptides from known lupus autoantigens that (i) bind the class II MHC molecule I-Ek, which is expressed in MRL/lpr mice, and (ii) form stable recombinant peptide:I-Ek complexes. We evaluated a list of candidate peptides from lupus autoantigens that were previously identified in the literature (18–20) or identified as potential I-Ek binders using an epitope prediction program (21) (Table S1). The peptide U1-70 131–150, hereinafter designated “U1-70,” and the peptide U1-70 131–150 (P140), which is phosphorylated on serine residue 140, hereinafter “P140,” demonstrated binding to I-Ek in a competition assay with a moth cytochrome c (MCC) peptide (88–103), which binds I-Ek (22) (Fig. 1A). The P140 peptide has been administered as a tolerizing therapy in MRL/lpr mice and has entered pivotal Phase 3 clinical trials in human SLE patients, where it has produced a modest improvement in disease (17, 20).
Fig. 1.
U1-70:I-Ek and P140:I-Ek tetramers specifically detect and enrich MRL/lpr CD4+ T cells. (A) Competition assay for peptide binding to I-Ek. CH27 B cells were pulsed with a suboptimal concentration of MCC and increasing amounts of competitor peptide. IL-2 production from 2B4 T cells, cultured with peptide-pulsed CH27 B cells, was measured by ELISA. Data are representative of two individual experiments. Error bars are derived from triplicate conditions. (B and C) Tetramer staining of CD4+ T cells from MRL/lpr lymph nodes with U1-70:I-Ek (B) and P140:I-Ek (C) before enrichment (Upper) and after positive selection enrichment of tetramer-positive cells using anti-PE or anti-APC magnetic microbeads (Lower). Values in black are percentages in the gate shown; values in red are the calculated numbers of tetramer-positive cells among 106 CD4+ T cells. (D) U1-70:I-Ek and P140:I-Ek tetramers did not costain CD4+ T cells from MRL/lpr mice at 18 or 6 wk of age. Flow cytometry plots show the enrichment of CD4+ T cells from two individual MRL/lpr mice that were processed, stained with tetramers, and enriched within the same experiment. Results are representative of 10 mice tested. Cells are gated on MCC:I-Ek-negative CD4+ T cells.
We generated recombinant I-Ek monomers with a cleavable peptide that could be exchanged out of the I-Ek binding cleft for another peptide at low pH (23). Both U1-70 and P140 formed stable complexes with I-Ek, similar to MCC (Fig. S1), allowing us to generate recombinant U1-70:I-Ek and P140:I-Ek tetramers. We tested the panel of tetramers in MRL/lpr mice and observed a low frequency of tetramer-positive T cells, at or below the limit of reliable detection (Fig. 1 B and C).
To better evaluate the tetramer staining of these rare populations, we applied a sensitive enrichment method using magnetic beads to positively select tetramer-stained cells (24–26). Using this enrichment protocol, we detected CD4+ T cells staining positive for U1-70:I-Ek (Fig. 1B), P140:I-Ek, and MCC:I-Ek (Fig. 1C). All plots of tetramer staining shown in the subsequent figures were produced following enrichment. Given that the difference between the two peptides U1-70 and P140 is a single phosphorylation modification, depending on the contact residues, it is conceivable that one T-cell receptor (TCR) could recognize both peptides. Alternatively, distinct T-cell populations might recognize each peptide. We repeatedly observed that CD4+ T cells failed to costain with the two tetramers, indicating that the T-cell populations are distinct (Fig. 1D). Furthermore, we observed greater frequencies of U1-70:I-Ek–specific T cells at age 18 wk than at age 6 wk, demonstrating differences in the frequencies of T-cell specificities associated with the course and severity of disease.
U1-70:I-Ek–Specific T Cells Are Associated with Disease.
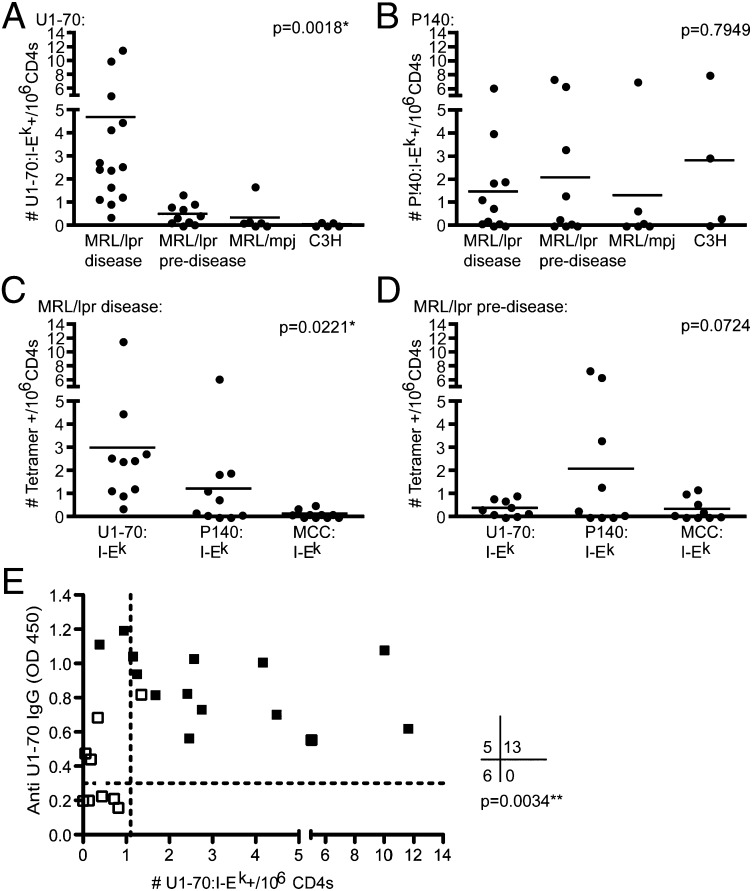
To evaluate differences in antigen-specific T-cell frequencies, we divided the MRl/lpr mice into predisease and disease groups. These groups were determined based on the age at which we observed elevated gamma globulin levels and proteinuria (Fig. S2A) and when we detected significant U1-70 specific autoantibody production in addition to other common autoantibody reactivities using autoantigen microarray technology (Fig. S2B). The frequency of U1-70:I-Ek–specific T cells was increased in MRL/lpr mice with disease compared with predisease MRl/lpr mice, MRL/mpj mice (which are fas-intact), and nonautoimmune C3H mice (*P = 0.0081) (Fig. 2A). In contrast, there were no significant differences in the frequency of P140:I-Ek–specific T cells among these groups of mice (P = 0.7949) (Fig. 2B). The average frequency of U1-70:I-Ek–specific T cells in MRL/lpr mice with disease was 4.7/106 CD4+ T cells, or roughly 1/200,000 CD4+ T cells, with frequency as high as 1/80,000 CD4+ T cells, which is within the range of previous reports for autoreactive CD4+ T-cell frequencies (27).
Fig. 2.
The frequency of U1-70:I-Ek–specific T cells is associated with disease and autoantibody production. (A and B) The frequencies of U1-70:I-Ek–specific (A) and P140:I-Ek–specific (B) CD4+ T cells were determined based on the number of tetramer-positive cells detected following enrichment, and were compared across experiments in MRL/lpr mice with disease (age >15 wk; n = 15), MRL/lpr mice predisease (age <14 wk; n = 10), MRL/mpj mice (age 13–31 wk; n = 6), and C3H mice (age 6–27 wk; n = 5). One-way ANOVA was performed, and Tukey’s posttest revealed significant differences (P < 0.05) in the following groups in A: MRL/lpr mice with disease and MRL/lpr mice predisease, MRL/lpr mice with disease and MRL/mpj, and MRL/lpr mice with disease and C3H. All other comparisons were not statistically different. (C and D) The frequencies of three peptide specificities, stained within the same samples, were compared across experiments in the MRL/lpr mice aged >15 wk (C; n = 10) and MRL/lpr mice aged <14 wk (D; n = 9). One-way ANOVA was performed to determine significant differences between group means; P values are shown, and Tukey’s posttest revealed that U1-70 and MCC specificities were significantly different in C. All other comparisons were not statistically different. (E) The levels of IgG autoantibodies against the whole protein U1-70 in sera from MRL/lpr mice were measured by ELISA and graphed alongside the frequency of U1-70:I-Ek–specific T cells. Solid black dots indicate MRL/lpr mice with disease, and open black dots indicate MRL/lpr mice predisease. Fisher’s exact analysis was performed (n = 24 mice). Dotted lines indicate the cutoffs used for determining categorical groupings, which were determined by calculating 4 SD above the mean levels of anti–U1-70 autoantibodies and mean frequencies of U1-70:I-Ek–specific T cells in control C3H mice.
To address the significance of the U1-70–specific T-cell expansion, we compared the frequencies of T-cell populations recognizing U1-70:I-Ek, P140:I-Ek, or MCC:I-Ek in MRL/lpr mice with disease that were stained simultaneously with all three tetramers. The frequency of U1-70:I-Ek–specific T cells was significantly greater compared with that of T cells recognizing the other specificities (*P = 0.0221) (Fig. 2C), suggesting that lymphadenopathy does not increase all T-cell specificities equally, and that starting with more CD4+ T cells does not increase nonspecific tetramer staining. There were no significant differences among the three specificities in MRL/lpr mice predisease (P = 0.0724) (Fig. 2D).
Furthermore, the presence of U1-70:I-Ek–specific CD4+ T cells correlated with anti–U1-70 IgG antibodies in the serum (**P = 0.0034) (Fig. 2E). We performed Fisher’s exact test, treating anti–U1-70 and U1-70:I-Ek T-cell frequency as categorical variables. A few mice with disease had no detectable U1-70:I-Ek–positive T cells but had measurable anti–U1-70, indicating either that autoantibody production occurred through T-cell–independent mechanisms or that CD4+ T cells recognizing epitopes other than U1-70 (131–150) provided help to anti–U1-70 B cells. In addition, T-cell help could have come from T cells recognizing epitopes from other proteins that are part of the U1-snRNP macromolecular complex (1, 28). Autoantibodies against U1-70 were IgG2a and IgG3 isotypes (Fig. S2C), suggesting that T-cell help was provided to U1-70–specific B cells. In addition, the U1-70:I-Ek–positive T cells expanded around the same time as the anti–U1-70 autoantibodies at the beginning of the disease process (Fig. S3).
U1-70–Specific T Cells Produce IFNγ and IL-17 in MRL/lpr Mice.
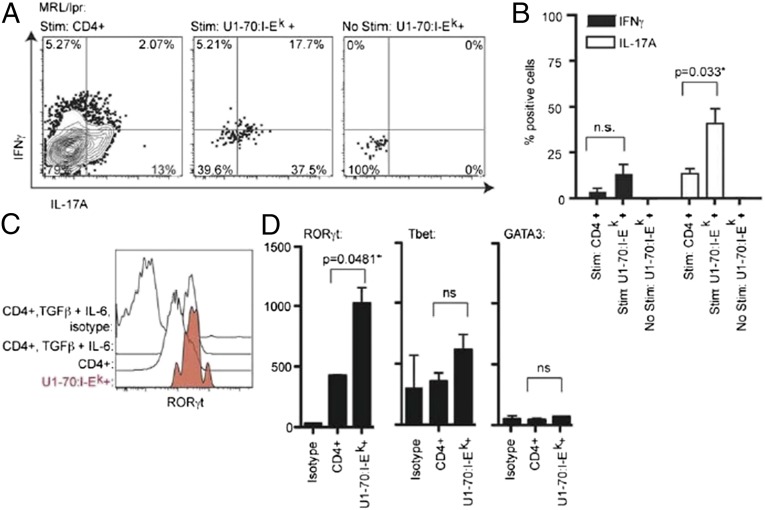
To elucidate the function of the disease-associated U1-70:I-Ek–specific T cells, we evaluated intracellular cytokine production. The majority of U1-70:I-Ek–specific CD4 T cells produced IL-17A, and some produced both IL-17A and IFNγ, as described previously for Th17 cells (29) (Fig. 3A). The percentage of U1-70–specific T cells that produced IL-17A compared with the whole CD4+ T-cell population was significantly greater (Fig. 3B), indicating that this population is enriched for Th17 cells.
Fig. 3.
U1-70:I-Ek–specific T cells are TH17 cells. (A) Flow cytometry plots showing intracellular IL-17 and IFNγ production by MRL/lpr CD4+ T cells, stimulated with PMA/ionomycin for 3 h at 37 °C. The plots show the enrichment fraction and are gated on CD4+ U1-70:I-Ek–negative cells (Left), CD4+ U1-70:I-Ek–positive cells (Middle), and CD4+ U1-70:I-Ek–positive cells that were not stimulated (Right). (B) Intracellular cytokine staining results summarized and depicted as a bar graph. The Student t test was performed (n = 4). (C) Histograms showing the frequency of RORγt expression levels as measured by intracellular staining. As a positive control, CD4+ T cells were purified by negative selection from MRL/lpr mice and cultured with plate-bound anti-CD3, anti-CD28, TGFβ, and IL-6 for 2 d to expand TH17- and RORγt-expressing cells. Results are representative of two experiments. (D) Transcription factor expression levels for RORγt, Tbet, and GATA3 were measured by intracellular staining and gated on tetramer-positive populations as indicated from MRL/lpr mice. The Student t test was performed to determine significant differences between CD4+ U1-70:I-Ek–positive cells and CD4+ U1-70:I-Ek–negative cells.
To confirm the Th17 phenotype, we analyzed the expression levels of the transcription factor RORγt by flow cytometry. The levels of RORγt expression in U1-70:I-Ek –specific T cells were similar in magnitude to those in CD4+ T cells cultured with TGFβ and IL-6, conditions that expand Th17 cells (30, 31) (Fig. 3C). The levels of RORγt expression in U1-70:I-Ek–specific T cells were significantly greater than those detected across the whole CD4 population, whereas the levels of Tbet and GATA3 were not significantly different (Fig. 3D). Similarly, P140:I-Ek–specific T cells expressed RORγt and Tbet (Fig. S4); however, we detected no significant cytokine production. It is possible that these cells are less responsive ex vivo. The P140:I-Ek T cells also did not express detectable levels of Foxp3 or IL-10, indicating that they do not appear to have a regulatory phenotype (Fig. S5). Therefore, although the P140:I-Ek–positive cells exhibit a phenotype similar to that of the U1-70:I-Ek–specific cells, the U1-70:I-Ek–specific CD4+ T cells are the more prominent population associated with disease and IL-17A production.
U1-70–Specific T Cells Produce IL-17A in Patients with MCTD and in Some Patients with SLE.
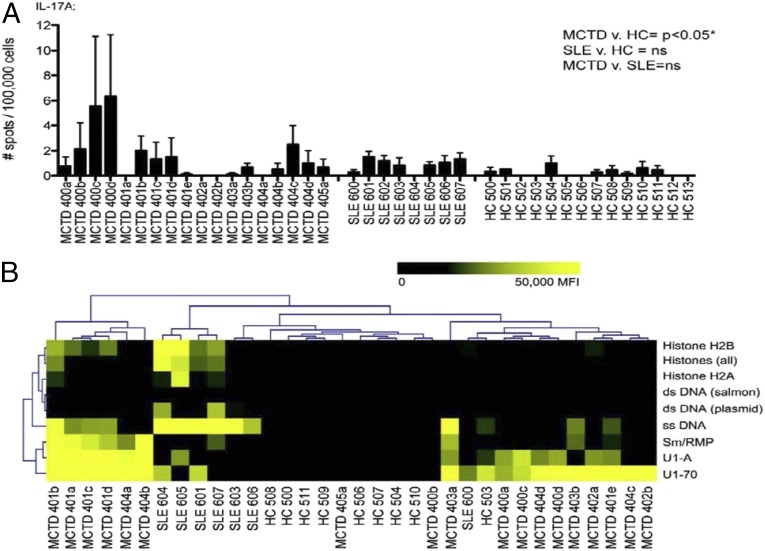
To evaluate the foregoing findings in patients with SLE and MCTD, we collected samples from a cohort of SLE patients (n = 8), a cohort of MCTD patients (n = 6; including 18 samples collected from 6 patients over multiple visits), and age- and sex- matched healthy controls. We evaluated the frequency of IL-17A–secreting U1-70–specific T cells in our cohorts of SLE and MCTD patients and in healthy controls using enzyme-linked immunospot (ELISPOT) assays on peripheral blood mononuclear cells (PBMCs) that were expanded and restimulated with the U1-70 peptide. Patients with MCTD had significantly higher levels of IL-17A–secreting U1-70–specific T cells compared with healthy controls (Fig. 4A). SLE patients had slightly higher levels of IL-17A–secreting U1-70–specific T cells compared with controls, but the difference did not reach statistical significance.
Fig. 4.
U1-70–specific T cells from SLE and MCTD patients produce IL-17. (A) PMBCs and serum were collected from cohorts of patients with MCTD (n = 6; a–e designate temporally ordered, sequential clinic visits for the same patient over time), patients with SLE (n = 8), and healthy controls (n = 14). Bar graphs depict IL-17A production from PBMCs in response to the U1-70 peptide, as detected by ELISPOT. Error bars reflect triplicate wells. One-way ANOVA with Tukey’s posttest was performed to determine statistically significant differences. *P = 0.0309. (B) Heat map of antigens identified as being significantly different in MTCD patients, SLE patients, and healthy controls by SAM. Data are derived from autoantigen microarrays probed with individual patient serum samples. The heat map represents a gradient from low (pseudocolored black) to high (pseudocolored yellow) IgG levels. Data for each MCTD patient visit are shown in A and B to demonstrate variation over time. For statistical analysis of ELISPOT and autoantigen microarray experiments, the average of all visits for each MCTD patient was used.
We evaluated the profiles of autoantibody reactivities in these patient cohorts, using autoantigen microarrays. Autoantibodies against U1-70, U1-A, and Sm/RNP were significantly increased in the MCTD patients, whereas dsDNA, ssDNA, and histones were significantly higher in the SLE patients (Fig. 4B). A hierarchical clustering based on significant reactivities showed clustering (and separation) of MCTD and SLE patients and healthy controls. A few patients with MCTD produced IFNγ and IL-17F in response to U1-70 stimulation (Fig. S6A). The profile of cytokine production varied among the MCTD patients, but remained similar in individual patients between visits. Furthermore, the levels of IL-17F, IFNγ, and IL-17A were significantly higher in the serum of patients with MCTD compared with controls when measured by a multiplexed bead-based assay (Fig. S6B), suggesting that antigen-specific T-cell responses may contribute in part to the increased proinflammatory cytokine environment observed in these patients.
Discussion
An obstacle to understanding the role of antigen-specific CD4+ T cells in lupus has been the lack of reagents for use in studying these cells directly. Tetramer studies have provided insight into CD4+ T-cell molecular interactions, function, frequency, location, and response to therapy in other autoimmune diseases, including multiple sclerosis (32, 33), type 1 diabetes (34, 35), rheumatoid arthritis (36), and celiac disease (37). Tetramers also have been used to detect IFNγ-secreting CD8+ T cells specific for myelin in neuropsychiatric SLE (38); however, the use of tetramers to identify and characterize self-reactive CD4+ T cells has not been reported in lupus. Here we describe MHC class II tetramers for use in studying CD4+ T cells in lupus-prone mice, and show that U1-70–specific CD4+ T cells produce IL-17A and express RORγt. Furthermore, levels of U1-70–specific CD4+ T cells that produce IL-17A are significantly higher in MCTD patients compared with healthy controls, and are detected in SLE patients.
The role of Th17 cells in immune responses to infection and autoimmune disease is an expanding area of investigation (39, 40). Th17 cells are being increasingly recognized as important in lupus pathology. In SLE, IL-17 levels are elevated in serum (41, 42), and the frequency of Th17 cells, which infiltrate the kidneys, is increased compared with controls (41–44). In IL-17R−/− BXD2 mice, antigen-specific autoantibodies of pathogenic isotypes are decreased (44). It is possible that Th17 cells contribute directly to inflammation and tissue injury through the secretion of soluble factors and/or provide signals to B cells instructing specific autoantibody production. Such a dual role for IL-17–producing CD4+ T cells was shown in the K/BxN model of spontaneous arthritis, where T cells enhance autoantibody-transferred arthritis and contribute to the IL-17 inflammatory cascade (45). T-cell help at extrafollicular sites in MRL/lpr mice also has been shown to occur through a mechanism involving IL-21 (46). IL-21 is critical for autoantibody responses in B cells (47), and Th17 cells produce IL-21 (48), suggesting that IL-21 may be a link between Th17 cells and antibody production; however, more work is needed to directly test this mechanism. Our findings support a dual role for antigen-specific Th17 cells in autoimmunity, where U1-70–specific T cells correlate with autoantibody production and secrete IL-17A.
The tetramer reagents in the present study provide a foundation for further study of antigen-specific CD4+ T cells in lupus and MCTD. Although MHC class II molecules are traditionally more difficult to develop than class I molecules (49), the peptide-exchange method allows for the production of many more tetramer specificities with just a single preparation of refolded monomer (23), and the protocol for tetramer enrichment allows for reliable detection of low-frequency autoreactive T cells (24, 25). Future studies are needed to determine whether other autoantigen-specific T cells produce IL-17 or whether this phenotype is unique to U1-70–specific T cells. Although lupus is traditionally considered a B-cell–mediated disease, evidence of antigen-specific T-cell help, the role of IL-17, and the involvement of Th17 cells may demonstrate a more central role for T cells than previously thought. Furthermore, these data regarding IL-17 and T-cell help may reveal new avenues of therapy for autoimmune disease, and may be particularly important for considering and evaluating antigen-specific therapies.
Methods
Peptides.
The following peptides, purchased from Biosynthesis, were used for the tetramer studies: MCC 88–103 (ANERADLIAYLKQATK), U1-70 131–150 (RIHMVYSKRSGKPSGYAFIE), and U1-70 131–150 (P140) [RIHMVYSKRS(phospho)GKPRGYAFIE]. The photocleavable MCC used for refolding exchangable I-Ek containing an N-terminal His 6 tag (HHHHHHGSGSANERADLIAYL[ANP]QATK) was synthesized at Stanford’s Protein and Nucleic Acid Facility, and the ANP linker was obtained from Advanced ChemTech.
Mice and Cell Lines.
All animal experiments were approved by and performed in compliance with the guidelines of the Institutional Animal Care and Use Committee of Stanford University. Breeding pairs and female MRL/Mpj-Faslpr and MRL/Mpj mice were purchased from The Jackson Laboratory and were housed and bred at the Stanford Animal Facility. C3H mice were obtained from Stanford in-house animal colonies. The 2B4 T-cell hybridoma and CH27 B-cell lymphoma cells were provided by Mark M. Davis.
Production of I-Ek Tetramers and Peptide Exchange.
The expression and purification of recombinant I-Ek monomers have been described previously (50). In brief, I-Ek α and β chains were expressed in Escherichia coli as inclusion bodies, refolded, and purified with a conditional peptide ligand (an N-terminal His6-tagged MCC peptide with K99ANP; see above). I-Ek monomers were stored in 50% glycerol at –20 °C. Peptides (100 μM) were exchanged into I-Ek monomer in sodium citrate buffer (pH 5.6; 50 μM) and incubated with protease inhibitors overnight at 4 °C. Then 50 mM Hepes (pH 7.4) was added to neutralize, and the exchanged I-Ek was subjected to nickel-NTA resin purification. Exchanged monomers were incubated with streptavidin conjugated to fluorophores PE, APC, PE-Cy5, and PE-Cy7 (eBioscience).
Flow Cytometry and Antibodies.
All flow cytometry experiments were conducted on a BD LSR II flow cytometer, housed in the Stanford Shared FACS Facility. The monoclonal antibodies used for surface staining were purchased from eBioscience, except for anti- I-Ek (14.4.4-S), purchased from BD Pharmingen. The following antibodies were used for intracellular staining: Foxp3-FITC (FJK-16s), RORγt-APC (AFKJS-9), Tbet-PerCPCy5.5 (eBio4B10), GATA3-Alexa488 (L50-823), IFNγ-FITC (XMG1.2), IL-17A-APC (eBio17B7), and IL-10-APC (JES5-16E3) (all purchased from eBioscience).
Tetramer Staining and Enrichment.
Lymph nodes (axial, brachial, inguinal, and mesenteric) were harvested and purified with CD3+ T-cell enrichment columns (R&D Systems). T cells were washed in FACS buffer (1× PBS, 2% FCS, 0.1% Na-azide, 1 mM EDTA), and incubated with Fc block 24G2 (BD Pharmingen) and tetramers at 150 nM each for 3 h on ice. Cells were stained for surface markers and with LIVE/DEAD Fixable Aqua (Life Technologies) following the manufacturer’s protocol. Tetramer enrichment has been described previously (25). In brief, anti-PE and/or anti-APC microbeads were added for 15 min at 4 °C, and cells were subjected to positive magnetic selection over LS columns (Miltenyi Biotec). The frequency of tetramer-positive cells was calculated based on the number of tetramer-positive cells detected in the enriched fraction divided by the total number of CD4+ cells isolated from the mouse.
Lupus Autoantigen Microarrays.
Each antigen was diluted to 0.2 mg/mL in PBS and printed in ordered arrays on nitrocellulose-coated FAST slides (Whatman Schleicher & Schuell BioScience) using a VersArray ChipWriter Pro Robotic Arrayer (Bio-Rad). Individual arrays were blocked with PBS containing 5% nonfat milk for 1 h at room temperature. Arrays were probed with 450 μL of mouse serum diluted 1:250 for 2 h at room temperature, followed by washing and incubation with a 1:2,000 dilution of Cy3-conjugated goat anti-mouse IgG secondary antibody (Jackson ImmunoResearch). For human patient samples, serum was diluted 1:100 for 2 h at room temperature, followed by washing and incubation with a 1:500 dilution of Cy3-labeled goat anti-human IgM/G secondary antibody (Jackson ImmunoResearch). Arrays were scanned using a GenePix 4000B scanner (Molecular Devices). Detailed protocols are available online in updated form at http://www.stanford.edu/group/antigenarrays. The median pixel intensities of each feature were determined using GenePix Pro version 6.0 software (Molecular Devices), and background values were subtracted. Unless indicated otherwise, the parametric, multigroup SAM (Significance Analysis of Microarrays) algorithm was performed (51). A hierarchical clustering algorithm was then applied to the dataset.
Intracellular Staining.
CD3-enriched T cells were stimulated for 3 h at 37 °C at 5 × 106 cells/mL with PMA/ionomycin (Sigma-Aldrich). Stimulated and unstimulated cells were subjected to tetramer and surface staining, followed by incubation with microbeads. Cells were fixed and permeabilized with a Foxp3 staining buffer kit (eBioscience). Samples were stained for intracellular cytokines or transcription factors for 30 min on ice, and then subjected to tetramer enrichment.
ELISPOT Assays.
PBMCs were thawed and cultured for 7 d in the presence of 10 μM U1-70 peptide and 50 U/mL IL-2 at 5 × 106 cells/mL in 24-well plates. On day 7, PBMCs were collected, washed, and restimulated in ELISPOT plates with 25 μM U1-70 for 48 h at 105 cells/well. ELISPOT assays were processed according to the manufacturer’s protocol (BD Biosciences), counted using an ImmunoSpot automated ELISPOT reader (CTL), and analyzed with ImmunoSpot software (CTL).
Antigen ELISAs.
Whole protein U1-70, also called U1-68 (Diarect), or peptides were coated on Nunc ELISA plates in carbonate buffer (pH 9.5) at 1 μg/mL overnight at 4 °C. Mouse serum was diluted 1:250 in PBST + 2% FCS and incubated for 2 h. Goat anti-mouse IgG-Hrp (Jackson ImmunoResearch) was used for detection.
Statistical Analysis.
The unpaired two-tailed Student t test was used to compare two groups. For mouse data, unpaired, two-tailed, one-way ANOVA with 95% confidence interval was used to compare more than two groups, with Tukey’s multiple-comparison test as a posttest (P < 0.05). Patient data were compared using one-way ANOVA with >95% confidence interval and Tukey’s posttest (P < 0.05). Fisher’s exact test was used to test for contingency. All statistical analyses were performed in GraphPad Prism.
Supplementary Material
Acknowledgments
We thank J. Haddon for commenting on the manuscript; R. Cheung, M. Kuhns, J. Price, L. Su, M. Wong, and Y. Wong for helpful discussions and technical advice; C. Crumpton and J. Van Dyke (Stanford FACS Facility) for technical assistance; R. Gupta for processing SLE and MCTD patient samples; and Y. Rosenberg-Hasson and the Stanford Human Immune Monitoring Core for performing the Luminex assay.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424796112/-/DCSupplemental.
References
- 1.Kattah NH, Kattah MG, Utz PJ. The U1-snRNP complex: Structural properties relating to autoimmune pathogenesis in rheumatic diseases. Immunol Rev. 2010;233(1):126–145. doi: 10.1111/j.0105-2896.2009.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pomeranz Krummel DA, Oubridge C, Leung AK, Li J, Nagai K. Crystal structure of human spliceosomal U1 snRNP at 5.5-A resolution. Nature. 2009;458(7237):475–480. doi: 10.1038/nature07851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerner MR, Steitz JA. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci USA. 1979;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharp GC, et al. Association of antibodies to ribonucleoprotein and Sm antigens with mixed connective-tissue disease, systematic lupus erythematosus and other rheumatic diseases. N Engl J Med. 1976;295(21):1149–1154. doi: 10.1056/NEJM197611182952101. [DOI] [PubMed] [Google Scholar]
- 5.Dieker J, et al. Apoptosis-linked changes in the phosphorylation status and subcellular localization of the spliceosomal autoantigen U1-70K. Cell Death Differ. 2008;15(4):793–804. doi: 10.1038/sj.cdd.4402312. [DOI] [PubMed] [Google Scholar]
- 6.Arbuckle MR, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 7.Koh DR, et al. Murine lupus in MRL/lpr mice lacking CD4 or CD8 T cells. Eur J Immunol. 1995;25(9):2558–2562. doi: 10.1002/eji.1830250923. [DOI] [PubMed] [Google Scholar]
- 8.Jevnikar AM, Grusby MJ, Glimcher LH. Prevention of nephritis in major histocompatibility complex class II-deficient MRL-lpr mice. J Exp Med. 1994;179(4):1137–1143. doi: 10.1084/jem.179.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen PL, Eisenberg RA. Lpr and gld: Single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 10.Perkins DL, et al. Restriction of the TCR repertoire inhibits the development of memory T cells and prevents autoimmunity in lpr mice. J Immunol. 1996;156(12):4961–4968. [PubMed] [Google Scholar]
- 11.Yan J, Mamula MJ. Autoreactive T cells revealed in the normal repertoire: Escape from negative selection and peripheral tolerance. J Immunol. 2002;168(7):3188–3194. doi: 10.4049/jimmunol.168.7.3188. [DOI] [PubMed] [Google Scholar]
- 12.Thibault DL, et al. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J Clin Invest. 2008;118(4):1417–1426. doi: 10.1172/JCI30065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25(3):417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Hsu HC, et al. Production of a novel class of polyreactive pathogenic autoantibodies in BXD2 mice causes glomerulonephritis and arthritis. Arthritis Rheum. 2006;54(1):343–355. doi: 10.1002/art.21550. [DOI] [PubMed] [Google Scholar]
- 15.Hsu HC, et al. Overexpression of activation-induced cytidine deaminase in B cells is associated with production of highly pathogenic autoantibodies. J Immunol. 2007;178(8):5357–5365. doi: 10.4049/jimmunol.178.8.5357. [DOI] [PubMed] [Google Scholar]
- 16.Schröder JO, Zeuner RA. Biologics as treatment for systemic lupus: Great efforts, sobering results, new challenges. Curr Drug Discov Technol. 2009;6(4):252–255. doi: 10.2174/157016309789869010. [DOI] [PubMed] [Google Scholar]
- 17.Muller S, et al. Spliceosomal peptide P140 for immunotherapy of systemic lupus erythematosus: Results of an early phase II clinical trial. Arthritis Rheum. 2008;58(12):3873–3883. doi: 10.1002/art.24027. [DOI] [PubMed] [Google Scholar]
- 18.Freed JH, Marrs A, VanderWall J, Cohen PL, Eisenberg RA. MHC class II-bound self-peptides from autoimmune MRL/lpr mice reveal potential T cell epitopes for autoantibody production in murine systemic lupus erythematosus. J Immunol. 2000;164(9):4697–4705. doi: 10.4049/jimmunol.164.9.4697. [DOI] [PubMed] [Google Scholar]
- 19.Monneaux F, Dumortier H, Steiner G, Briand JP, Muller S. Murine models of systemic lupus erythematosus: B and T cell responses to spliceosomal ribonucleoproteins in MRL/Fas(lpr) and (NZB x NZW)F(1) lupus mice. Int Immunol. 2001;13(9):1155–1163. doi: 10.1093/intimm/13.9.1155. [DOI] [PubMed] [Google Scholar]
- 20.Monneaux F, Lozano JM, Patarroyo ME, Briand JP, Muller S. T cell recognition and therapeutic effect of a phosphorylated synthetic peptide of the 70K snRNP protein administered in MRL/lpr mice. Eur J Immunol. 2003;33(2):287–296. doi: 10.1002/immu.200310002. [DOI] [PubMed] [Google Scholar]
- 21.Guan P, Doytchinova IA, Zygouri C, Flower DR. MHCPred: A server for quantitative prediction of peptide-MHC binding. Nucleic Acids Res. 2003;31(13):3621–3624. doi: 10.1093/nar/gkg510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis MM, et al. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 23.Toebes M, et al. Design and use of conditional MHC class I ligands. Nat Med. 2006;12(2):246–251. doi: 10.1038/nm1360. [DOI] [PubMed] [Google Scholar]
- 24.Day CL, et al. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J Clin Invest. 2003;112(6):831–842. doi: 10.1172/JCI18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27(2):203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pepper M, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11(1):83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nepom GT. Tetramer analysis of human autoreactive CD4-positive T cells. Adv Immunol. 2005;88:51–71. doi: 10.1016/S0065-2776(05)88002-2. [DOI] [PubMed] [Google Scholar]
- 28.Fatenejad S, Mamula MJ, Craft J. Role of intermolecular/intrastructural B- and T-cell determinants in the diversification of autoantibodies to ribonucleoprotein particles. Proc Natl Acad Sci USA. 1993;90(24):12010–12014. doi: 10.1073/pnas.90.24.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 30.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 31.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 32.Korn T, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13(4):423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy J, et al. Detection of autoreactive myelin proteolipid protein 139-151-specific T cells by using MHC II (IAs) tetramers. J Immunol. 2003;170(2):870–877. doi: 10.4049/jimmunol.170.2.870. [DOI] [PubMed] [Google Scholar]
- 34.Jang MH, Seth NP, Wucherpfennig KW. Ex vivo analysis of thymic CD4 T cells in nonobese diabetic mice with tetramers generated from I-A(g7)/class II-associated invariant chain peptide precursors. J Immunol. 2003;171(8):4175–4186. doi: 10.4049/jimmunol.171.8.4175. [DOI] [PubMed] [Google Scholar]
- 35.Reijonen H, Kwok WW, Nepom GT. Detection of CD4+ autoreactive T cells in T1D using HLA class II tetramers. Ann N Y Acad Sci. 2003;1005:82–87. doi: 10.1196/annals.1288.009. [DOI] [PubMed] [Google Scholar]
- 36.Kotzin BL, et al. Use of soluble peptide-DR4 tetramers to detect synovial T cells specific for cartilage antigens in patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97(1):291–296. doi: 10.1073/pnas.97.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ráki M, et al. Tetramer visualization of gut-homing gluten-specific T cells in the peripheral blood of celiac disease patients. Proc Natl Acad Sci USA. 2007;104(8):2831–2836. doi: 10.1073/pnas.0608610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Contin-Bordes C, et al. Expansion of myelin autoreactive CD8+ T lymphocytes in patients with neuropsychiatric systemic lupus erythematosus. Ann Rheum Dis. 2011;70(5):868–871. doi: 10.1136/ard.2010.140012. [DOI] [PubMed] [Google Scholar]
- 39.Kattah MG, Wong MT, Yocum MD, Utz PJ. Cytokines secreted in response to Toll-like receptor ligand stimulation modulate differentiation of human Th17 cells. Arthritis Rheum. 2008;58(6):1619–1629. doi: 10.1002/art.23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinman L. A rush to judgment on Th17. J Exp Med. 2008;205(7):1517–1522. doi: 10.1084/jem.20072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60(5):1472–1483. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 42.Nalbandian A, Crispín JC, Tsokos GC. Interleukin-17 and systemic lupus erythematosus: Current concepts. Clin Exp Immunol. 2009;157(2):209–215. doi: 10.1111/j.1365-2249.2009.03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183(5):3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu HC, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9(2):166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 45.Jacobs JP, Wu HJ, Benoist C, Mathis D. IL-17–producing T cells can augment autoantibody-induced arthritis. Proc Natl Acad Sci USA. 2009;106(51):21789–21794. doi: 10.1073/pnas.0912152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Odegard JM, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205(12):2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozaki K, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298(5598):1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 48.Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282(48):34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mallone R, Nepom GT. MHC class II tetramers and the pursuit of antigen-specific T cells: Define, deviate, delete. Clin Immunol. 2004;110(3):232–242. doi: 10.1016/j.clim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Altman JD, Reay PA, Davis MM. Formation of functional peptide complexes of class II major histocompatibility complex proteins from subunits produced in Escherichia coli. Proc Natl Acad Sci USA. 1993;90(21):10330–10334. doi: 10.1073/pnas.90.21.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98(9):5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.