Abstract
Large animal models of genetic diseases are rapidly becoming integral to biomedical research as technologies to manipulate the mammalian genome improve. The creation of cystic fibrosis (CF) ferrets and pigs is an example of such progress in animal modeling, with the disease phenotypes in the ferret and pig models more reflective of human CF disease than mouse models. The ferret and pig CF models also provide unique opportunities to develop and assess the effectiveness of gene and cell therapies to treat affected organs. In this review, we examine the organ disease phenotypes in these new CF models and the opportunities to test gene therapies at various stages of disease progression in affected organs. We then discuss the progress in developing recombinant replication-defective adenoviral, adeno-associated viral, and lentiviral vectors to target genes to the lung and pancreas in ferrets and pigs, the two most affected organs in CF. Through this review, we hope to convey the potential of these new animal models for developing CF gene and cell therapies.
Introduction
Cystic fibrosis (CF) is a common lethal autosomal-recessive disorder caused by mutations in a single gene encoding a protein, the cystic fibrosis transmembrane conductance regulator (CFTR).1–3 CFTR is an anion channel, located in the apical membrane of epithelial cells, that conducts chloride and bicarbonate across the cell membrane.4,5 CF affects at least 70,000 people worldwide and almost 2000 sequence variations have been identified in the CFTR gene.6,7 The most common CFTR mutant is the deletion of a nucleotide triplet that results in the loss of a phenylalanine residue at position 508 of the CFTR protein (ΔF508CFTR). Approximately 70% of patients with CF carry two copies of the ΔF508 mutation, whereas 90% carry one.8–10 CFTR gene mutations result in a wide range of organ-level dysfunction, including severe lung infections, pancreatic failure, intestinal obstruction, male infertility, and nutritional deficits.11,12 A recurrent theme in CF organ disease is thick secretions and reduced pH caused by impaired bicarbonate transport.
Although CF affects multiple organs, lung failure due to chronic bacterial infections and inflammation is responsible for most morbidity and mortality.13 Because CF is a monogenic fatal disorder, and the airway epithelium is an easily accessible target for gene therapy vectors, CF lung disease is an ideal genetic disorder for treatment by gene therapy.14 Twenty-five clinical trials for CF lung disease have been implemented in approximately 450 patients with CF since the mid-1990s,15 including those using recombinant adenovirus vector (rAD) targeting the nasal and bronchial epithelium16–22; recombinant adeno-associated virus (rAAV) with aerosolized administration to nose, sinuses, and lungs23–27; as well as cationic liposome or formulated DNA nanoparticles for nonviral CFTR gene transfer.28–31 Despite the success of preclinical studies demonstrating efficacy of these recombinant vectors to correct CFTR channel defects, using ex vivo and in vitro airway model systems, all CF gene therapy trials to date have failed either to meet molecular end points or to improve lung function in patients with CF.32–34 These failures are likely due to several issues, including (1) the lack of efficient gene transfer to cellular targets required to correct in vivo CFTR function,35 (2) the animal models in which various preclinical vectors were tested,36–39 and (3) previously unknown intracellular and extracellular barriers that limit viral transduction.40–43
Basic research on airway biology has found that gene delivery to airway epithelial cells in vivo must overcome a number of intracellular and extracellular barriers that physically or biologically hinder the delivery of DNA or viral vectors to the nucleus,40,41,44,45 or target clearance of the vectors or infected cells through host immune surveillance.46–51 Importantly, lung infection and inflammation in CF lung disease enhance these barriers. Challenges surrounding the physical barriers in the airway of a patient with CF, such as the thick layer of airway mucus secretion and the mechanisms of mucociliary clearance, were not completely recognized when the early CF lung gene therapy trials were conducted. Of note, the gene transfer agents used in these early trials were also not fully validated at that time42,43 because of the lack of an animal model system that fully recapitulates the pathological condition of human CF lung disease.
Research on vector biology and virology has also revealed some inherent weaknesses that required solutions before applications in CFTR gene therapy. For example, in the initial rAAV2 clinical trials, the relative small package capacity (<5.0 kb)52 of the AAV genome necessitated the use of a weak cryptic promoter in the AAV2 inverted terminal repeat (ITR) to enable packaging of the 4.44-kb CFTR genome.24,53 It was also not known in early trials that rAAV2 has relatively high airway tropism in the preclinical rhesus monkey model54 in contrast to human airway.36,38 Subsequent studies demonstrated that rAAV2 has low tropism for transduction from the apical surface of the human airway epithelium40 and has impaired proteasome-dependent intracellular processing and trafficking to the nucleus for productive transduction.41,55,56 A major limitation of first-generation rAD vectors is the leaky expression of vector-encoded viral proteins that elicit strong humoral and cellular immune responses.57,58 In addition, it is now known that the type 5 rAD receptor (coxsackievirus–adenovirus receptor) is not presented on the apical surface of human airway epithelium,59,60 despite the fact that type 5 rAD highly transduced the murine airways used in preclinical studies.50,61 In contrast, nonvirus-mediated approaches for CFTR delivery lack many of the limitations of viral vectors, including immunological barriers and tropism-specific features defined by species-specific receptors. However, liposome-plasmid based formulations are generally much less efficient at transfecting airway epithelium than viral vectors.62,63
Two larger animal models of cystic fibrosis in the ferret and pig have been generated by either disruption of the CFTR gene or introduction of the ΔF508 CFTR mutation.64,65 Both CF ferrets and pigs spontaneously develop the lung disease phenotype, as well as pancreatic, gallbladder, and intestinal disease.66,67 These models will be useful to test CF gene therapy in the context of disease that reproduces the human condition. In this review, we first briefly review the history of the development of these CF animal models and then describe their disease phenotypes at the organ level, with a focus on similarities and differences in organ phenotypes that will differentiate the gene therapy approaches that can be tested in the various affected organs. We then review the progress of using viral vectors to deliver foreign genes to the lung and pancreas in ferrets and pigs, which are two key target organs for future CF gene therapy efforts. We also discuss the prospects and practical issues of using the CF ferret and pig models for the development of CF gene therapies.
The Development of CF Animal Models
Mouse models of CF have been invaluable tools in the study of CFTR physiology in multiple organs for more than two decades.68 More recently, the development of conditional CFTR knockout mice69 has aided in dissecting novel CFTR functions in myeloid-derived cells and T cells.70,71 However, major limitations of CF mouse models are the lack of spontaneous lung infections and pancreatic disease observed in patients with CF.66,67 Several biologic reasons may account for the lack of pathology in these organs of CF mice. From an anatomical perspective, airway submucosal glands, which express abundant CFTR in human cartilaginous airways72 and play important roles in lung innate immunity through the secretion of antimicrobials,73,74 are present only in the proximal trachea of mice.75 From an ion channel perspective, alternative non-CFTR, cAMP-activated, chloride channel activity appears to compensate for the lack of CFTR in the trachea39,76 and pancreas in CF mice.76–78
In contrast to mice, ferrets and pigs share a high level of similarity in airway cytoarchitecture with humans79–82 and have a similar composition of chloride channels in the airway.83 The use of the ferret as an animal model for hypersecretory diseases such as CF and chronic bronchitis was first suggested in 1982, based on the properties of mucus, goblet cells, and submucosal glands throughout the tracheobronchial tree.84 However, until somatic cell nuclear transfer (SCNT) cloning of mammals was first demonstrated in 1996 with the cloning of the sheep named Dolly,85 this hypothesis could not be tested. The approach of combining SCNT with rAAV-mediated gene targeting in primary fibroblasts led to the successful generation of CF ferret64 and pig models65 in 2008.
Multiorgan Disease in CF Ferrets and Pigs Is Similar to the Human CF Phenotype
Although the lung is the primary organ that leads to mortality in CF, CF is a multiorgan disease for which disease in secondary organs such as the gut and pancreas can influence the health of the lung. For instance, CF-related diabetes and malnutrition are two examples by which pancreatic and intestinal health can negatively impact lung health in patients with CF. Thus, each of these organs is a potential target for gene therapy. Disease phenotypes in the CF pig and ferret have subtle differences in disease severity and time of onset. Hence, these two models present unique opportunities for testing gene therapies at various stages of CF organ disease.
Intestinal disease
Whereas only 15% of infants with CF suffer from meconium ileus (MI) at birth,86,87 MI occurs in 100% of CFTR knockout piglets88,89 and 75% of CFTR knockout ferret kits.90 The phenotype of MI in CF ferret kits and piglets is extremely similar to that which occurs in CF infants, including intestinal atresia, diverticulosis, and microcolon. Although intestinal surgery in newborn CF ferrets has not been possible due to size, it has been successfully used in CF pigs to rescue newborn animals. Malnutrition and distal intestinal obstruction syndrome in older CF ferrets and pigs is similar to that observed in patients with CF.91,92 The creation of gut-corrected CFTR knockout ferrets and pigs harboring a wild-type CFTR transgene under the direction of the fatty acid-binding promoter will be useful models with reduced intestinal pathologies.90,92 Furthermore, gut-corrected CFTR knockout models provide the opportunity to test gene therapies without the potential complication of developing cellular immunity against the CFTR transgene.
Pancreatic disease
At birth, only 3% of patients with CF have severe lesions associated with exocrine pancreatic destruction (EPD), whereas 72–92% infants demonstrate mild lesions associated with exocrine acinar duct dilatation (ADD).93,94 However, damage to the pancreas continues after birth and ultimately leads to complete destruction of pancreatic exocrine function in most patients with CF, with 82% of adult patients with CF suffering pancreatic insufficiency. Inflammatory damage to the pancreas of CFTR knockout pigs begins in utero on embryonic day 83 (pigs have a 114-day gestation) and 100% of CF pigs have EPD at birth.95,96 By contrast, newborn CFTR knockout ferrets exhibit relatively mild histopathology of the exocrine pancreas characterized by ADD similar to that seen in CF infants.90,97 Interestingly, a small subset of CFTR knockout ferrets (<1%) demonstrates pancreatic sufficiency throughout life with normal weight gain and only minor exocrine damage.91 These findings suggest that pancreatic modifier genes exist in CF ferrets, as has also been suggested in patients with CF. However, the exocrine pancreas of most CFTR knockout ferrets undergoes rapid destruction over the first month of life, leading to extensive fibrosis, loss of exocrine pancreas, islet remodeling, and diabetes.91,97 Both CF ferrets and pigs also show abnormalities in insulin secretion at birth,97,98 suggesting that abnormal islet function initiates early in CF. Because CF ferret and pig models have differing degrees of exocrine disease severity at birth, they present opportunities to test CF gene therapies that target early and late disease processes, respectively. Furthermore, both models may have unique utilities for testing gene therapies for CF-related diabetes.
Gallbladder and hepatic disease
Biliary cirrhosis and gallbladder disease are observed in 15–30% of patients with CF.11,99,100 Both CF pigs and ferrets develop moderate hepatic lesions, including biliary cirrhosis, ductal hyperplasia, steatosis, and fibrosis.88,89,91 Newborn CF ferrets also have abnormally elevated plasma alanine aminotransferase and bilirubin levels,90 similar to those observed in CF infants,101 and suggestive of early liver disease. Like the pancreas, gallbladder disease in CF ferrets and pigs progresses at different rates, with disease in the CF pig beginning in utero and in the CF ferret beginning postnatally. Microgallbladder with thick mucus secretions is found in 100% of CF piglets at birth,88,89 whereas the newborn CF ferret gallbladder is histologically normal despite the electrophysiologic absence of cAMP-mediated chloride currents.90,102 However, with age, the majority of CF ferrets develop gallbladder disease characterized by cystic mucosal hyperplasia as seen in humans.91 The liver represents a tractable target for gene therapy in both CF pigs and ferrets, whereas gallbladder-directed gene therapy would likely be limited to CF ferrets because of the extent of disease observed in CF pigs at birth.
Lung and airway diseases
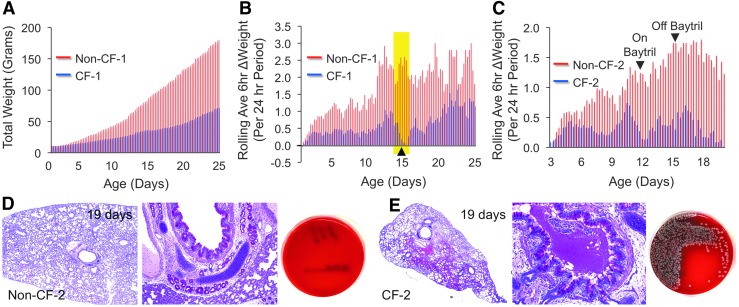
The CF lung is the primary target for gene therapy, as it is the most severely affected organ in CF. Both CF pig and ferret models develop spontaneous lung infections similar to that in human patients with CF. Although CF pigs lack lung inflammation at birth, they fail to eradicate bacteria and eventually develop lung disease within the first few months of life characterized by airway inflammation, remodeling, mucus accumulation, and infection.88,103 CF ferrets also have a lung bacterial eradication defect, but demonstrate an abnormally elevated inflammatory response at birth.104 Proteomics analysis of bronchoalveolar fluid from sterile Caesarean-sectioned and natural-born CF and non-CF ferrets suggests that alterations to lung immunity may begin before birth in CF kits and prime the lung for hyperinflammation after the first bacterial exposure during birth.104 The onset of lung infections in CF ferrets is rapid and if animals are not reared on antibiotics, they succumb to polymicrobial lung infections within the first week of life.90 However, improved methods of rearing CF ferrets on multiple antibiotics from birth to 6 months of age have allowed for the study of a more slowly progressive lung disease that recapitulates human CF lung disease.105 Both CF pig and ferret models represent unique opportunities to evaluate gene therapies to the CF lung. Gene therapy end points to the neonatal CF ferret lung may also benefit from methods of closely monitored weight gain, for which small decreases have been demonstrated to be indicative of the onset of a lung infection (Fig. 1).105
FIG. 1.
Weight monitoring as a surrogate for lung infections in young cystic fibrosis (CF) ferrets. The weights of kits were measured every 6 hr and were compared between paired CF and non-CF kits born to the same jill. (A) Typical patterns of total daily weight gain for CF kits (blue bars) and non-CF kits (red bars). (B) The rolling average 6-hr delta weight gain over a 24-hr period (calculated as the average of five measurements over a 24-hr period) is plotted for a CF (blue bars)/non-CF (red bars) pair. A decline in this rolling average indicated early lung infection (yellow shaded region), and thus a second antibiotic (Baytril) was applied (at arrowhead). (C) A second set of CF and non-CF animals reared on Zosyn from birth, given Baytril on day 12 because of weight loss in the CF animal, and then removed from Baytril on day 15. The CF animal succumbed to lung infection at 19 days. (D and E) Lung histology and bacterial colony-forming units (CFU) in lung lysates from the 19-day-old (D) non-CF control and (E) CF animal shown in (C). (A) and (B) are reproduced with permission from Sun et al.105
Progress in Viral Vector Development for CFTR Gene Transfer
Over the last two decades, a significant effort has been made to solve the challenges encountered in early CF gene therapy clinical trials. Areas of focus have included the identification of viral vectors with the appropriate tropisms to infect the apical membrane of polarized human airway epithelia, understanding the intracellular barriers that limit viral transduction, and improving vector design to both limit immune recognition and allow for packaging of the large CFTR cDNA. Here we review some of the most significant advances in viral vectors for CF gene therapy.
Recombinant adenoviral vectors
The major limitation of recombinant adenoviruses for gene therapy is the immune recognition and T cell-mediated responses that lead to clearance of virally infected cells. Next-generation rAD vectors, also called helper-dependent adenoviral (HD-AD) vectors, have been developed.106 HD-AD vectors have all viral protein-coding sequences deleted, which significantly reduces the host immune response.107,108 Thus, HD-AD confers longer term transgene expression in mouse lung and can be readministered through transient immunosuppression in mice.109 Viral vector-mediated airway gene transfer has also been greatly improved by pharmacological interventions. Mucolytic agents have been used to break down the mucus layer and improve rAD transduction in mice.110 A variety of pharmacological agents, such as sodium caprate,111 ethylene glycol tetraacetic acid,59 and lysophosphatidylcholines,112 have also been used to transiently open the tight junction of airway epithelium, allowing access of rAD to the coxsackievirus–adenovirus receptor on the basolateral membrane of the airway epithelium.
Recombinant adeno-associated viral vectors
New rAAV vector serotypes, such as rAAV1113,114 and rAAV6,115 have demonstrated improved transduction efficiency after apical infection of polarized human airway epithelium compared with the rAAV2 vector used in initial clinical trials. Other rAAV capsid variants with enhanced tropism from the apical membrane of human airway epithelial cells were obtained by directed evolution in human airway cell cultures116,117 or through genetic modification of the AAV6115 and AAV2 capsid.118 Other advances have focused on vector issues pertaining to the small packaging capacity of rAAV genomes. In this regard, shortened CFTR minigenes have allowed for the incorporation of stronger promoter/enhancer elements with rAAV vectors.119 These have included deletion of 52 amino acid residues (156 bp) from the R-domain to create a CFTRΔR protein that retains ∼80% Cl− channel activity in comparison with the full-length CFTR.120 In addition, a novel cross-genus hybrid parvoviral vector was developed that packages the rAAV2 genome into human bocavirus type 1 (HBoV1) capsids, a human respiratory virus that naturally infects human airway epithelium in infancy.121 rAAV2/HBoV1 vectors have greater apical transduction of polarized human airway epithelium than rAAV1 vectors. Furthermore, rAAV2/HBoV1 vectors retain 20% greater packaging capacity than rAAV vectors because of the larger HBoV1 virion. The rAAV2/HBoV1 vector harboring a full-length CFTR expression cassette driven by the strong CBA promoter (a combination of the cytomegalovirus immediate-early [CMV IE] enhancer and chicken β-actin promoter) has been shown to efficiently correct CFTR-mediated chloride currents in CF human airway epithelium after apical infection.121 Advances in our understanding of the intracellular barriers to rAAV transduction have also led to pharmacologic methods of enhancing transduction from the apical membrane of polarized airway epithelia. The use of proteasome inhibitors during or after infection can dramatically increase apical rAAV transduction of human airway epithelial by enhancing nuclear viral translocation.41,122
Recombinant lentiviral vectors
Lentiviral vectors are able to transduce dividing and nondividing cells, conferring long-term expression through the integration of a transgene expression cassette into host chromosomal DNA.123,124 The most commonly used lentiviral vector is derived from the human immunodeficiency virus (HIV),125 but in the context of lung gene transfer feline (FIV)126 and simian (SIV)127 immunodeficiency viruses, and equine infectious anemia virus (EIAV),128 have also been studied. These viruses do not have a natural tropism for the airway, and lentiviral vectors commonly pseudotyped with an envelope glycoprotein from the vesicular stomatitis virus (VSV-G) are relatively inefficient at transducing polarized human airway epithelial cultures from the apical membrane.129–131 However, promising new advances in developing retargeted lentiviral vectors for airway transduction have been made. Lentiviral vectors pseudotyped with GP64 glycoprotein from baculovirus of Autographa californica,131 or M2 envelop protein and hemagglutinin (HA) from influenza virus,128 demonstrated fairly high vector production yields and apical tropism to transduce polarized airway epithelial cultures in vitro and mouse airway epithelium in vivo. Similarly, SIV vector pseudotyped with Sendai virus hemagglutinin-neuraminidase (HN) and fusion (F) protein can efficiently transduce polarized human airway epithelia from the apical membrane and also efficiently transduces mouse nasal epithelial cells in vivo, resulting in transgene expression sustained for periods far beyond the proposed life span of differentiated airway epithelial cells.132 Additional studies with lentiviruses demonstrate the feasibility of repeated administration to the respiratory tract without blocking antibody immune responses.133,134
Lung and Pancreatic Gene Therapy in Ferrets and Pigs
Before testing CFTR-mediated lung and pancreatic gene therapies in the CF ferret and pig models, it is necessary first to understand the optimal vector design for each species. Although efficacy studies in the CF models have yet to be completed, there has been a significant amount of research to aid in vector choice. Here we present both published and unpublished data that are being used to build a framework for future studies of CFTR gene delivery to important affected organs in these two animal CF models.
Gene transfer to ferret and pig airways, using replication-defective adenovirus
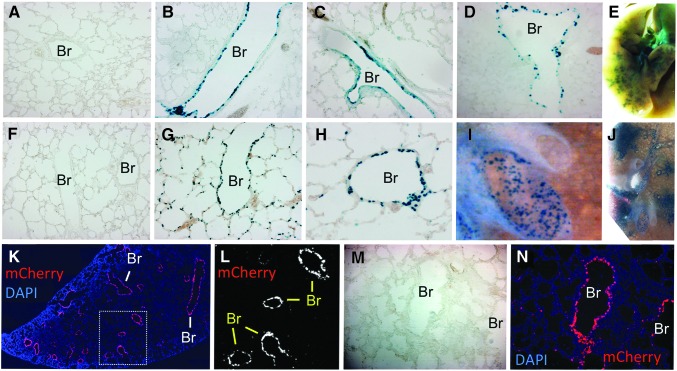
HD-AD vectors have been tested for their ability to deliver reporter genes to the airways of both normal pigs and ferrets. The HD-AD has been tested in 3- to 4-day-old newborn ferrets (∼10–13 g body weight) by intratracheal injection of 3×1011 particles of HD-AD virus formulated with lysophosphatidylcholine and DEAE-dextran in a volume of 40 μl. The vector harbored a nuclear-targeted β-galactosidase reporter gene (nt-LacZ) driven by the human cytokeratin 18 (K18) promoter, which confers conducting airway epithelial cell-specific transgene expression. At 8 days postinfection, reporter LacZ expression was seen in the surface airway epithelial cells of intralobar conducting airways with little expression in alveolar regions of the lung (Fig. 2A–E). Similar HD-AD vectors carrying an K18 promoter driving nt-LacZ or human CFTR cDNA were delivered to 25- to 30-kg pigs as an aerosol under bronchoscopic guidance.135 Aerosol delivery of 5×1012 particles of HD-AD vector formulated with 0.01% lysophosphatidylcholine in 5 ml was directed to the left lung through the left mainstem bronchus. One week after infection, X-Gal staining revealed strong LacZ reporter expression in the surface epithelium of the segmental bronchus, bronchioles, and respiratory bronchioles from the infected left lung, whereas the right lung lacked reporter expression. Histological examination of tissue sections demonstrated that ∼20% of the epithelial cells expressed LacZ transgene in the infected lobes. Importantly, LacZ expression was also found in the airway submucosal glands, which are considered an important target for CF lung gene therapy. HD-AD virus encoding the human CFTR gene also demonstrated expression throughout the infected lobes by real-time RT-PCR for the exogenous hCFTR mRNA and immunostaining using anti-hCFTR antibody for expression of the exogenous hCFTR protein. Although acute inflammatory cytokine and chemokine production was observed after HD-AD administration, as well as the infiltration of neutrophils into the pig airway epithelium 24 hr after vector delivery, there was no systemic toxicity observed after aerosol delivery of the HD-AD vectors, and no significant difference in inflammatory cell infiltration in the bronchi and alveolar regions before and after 1 week of vector delivery. The mRNA levels for cytokines and chemokines from the bronchoalveolar lavage cells and the lung tissue were also not significantly different on day 7 between the infected and noninfected animals.
FIG. 2.
Gene transfer in the airways of 3- to 6-day-old ferrets. (A–E) Airways after intratracheal delivery of (A) vehicle or (B–E) helper-dependent Ad5 (i.e., gutted adenovirus) with X-Gal staining 8 days postinfection. This vector expresses nt-LacZ under the control of the K18 promoter. (A–D) are sections and (E) is a whole-mount preparation. The transgene is expressed predominantly in the small airways. (F–J) Airways after intratracheal delivery of (F) vehicle or (G–J) equine infectious anemia virus (EIAV) pseudotyped with influenza A virus subtype H7 hemagglutinin (HA) with X-Gal staining 8 days postinfection. This EIAV vector expresses nt-LacZ under the control of the CBA promoter. (F–H) are sections and (I and J) are whole-mount preparations. The transgene is expressed in the large and small airways as well as in alveolar regions. (K–N) Newborn ferrets were infected with an RSVmCherry-encoding FIV-GP64 virus. mCherry expression (red) was observed almost exclusively in bronchioles (Br) at 7 days postinfection. (L) shows only the mCherry channel of the boxed region in (K). (M) is the bright-field image of the fluorescence panel in (N). (K) and (N) are counterstained with DAPI to mark nuclei.
Gene transfer to ferret and pig airways, using rAAV
Studies evaluating the use of rAAV for gene transfer to the ferret and pig lung have also demonstrated the feasibility of this vector for use in gene therapy in the CF animal models. Studies comparing rAAV1, rAAV2, and rAAV5 serotypes for their ability to transduce polarized human, pig, and ferret airway epithelial cultures suggest that these three species share a similar apical tropism for these serotypes, with rAAV2/1 being the most efficient.37 Our studies have found that the newborn ferret airway is resistant to rAAV transduction by different serotypes including types 1, 2, 6, and 9 (our unpublished data). However, in vivo transduction with rAAV2/1 was significantly enhanced by the addition of 200 μM doxorubicin in the vector inoculum.136 When the infections were conducted in 5- and 12-day-old ferrets, transgene expression was observed in the tracheobronchial epithelium, bronchioles, and scattered alveolar cells, whereas transgene expression was significantly lower in 18-day-old animals and undetectable in adult animals.136 Interestingly, the resistance to rAAV2/1 transduction in older ferrets appears to be due, at least in part, to the increased abundance of a secreted inhibitory factor(s) in the ferret airway. The identity of the secreted inhibitory factor(s) remains unknown; however, resistance of the airway to rAAV1 infection appears to develop at 18 days after birth, a time point when submucosal glands are nearing a mature state in ferrets. Nevertheless, our studies demonstrated that rAAV2/1 may be a suitable viral vector to test gene therapy to the lung of neonatal CF ferrets. Notably, rAAV2/1 is also one of the most efficient natural serotypes of rAAV for transduction of polarized human airway epithelial cultures from the apical membrane.
Capsid-directed evolution of rAAV in polarized human airway epithelial cultures has yielded some AAV variants with better apical tropism to the human airway.116 This strategy was also used to direct the evolution of an AAV capsid library in pig airway in vivo and isolated a new AAV variant, AAV2H22.137 The capsid sequence of this variant was identical to that of AAV2 except for five mutations of amino acid residues as E67A, S207G, Q598L, I648V, and V708I. A new rAAV vector generated by pseudotyping rAAV2 genome into the AAV2H22 capsid demonstrated its ability to selectively and efficiently transduce pig airway epithelium in vitro and in vivo. This vector will be useful in delivery of the porcine CFTR gene to test gene therapy for lung disease in the CF pigs.
Gene transfer to ferret and pig airways, using lentiviral vectors
Studies comparing HIV with FIV lentiviral vectors in well-differentiated human and pig airway epithelia screened a number of envelope glycoproteins and identified baculovirus protein GP64 as one of the most efficient pseudotypes for transduction from the apical membrane by both HIV and FIV vectors.129 Furthermore, this study also demonstrated that FIV-GP64 recombinant virus was effective at transducing the airways of pigs in vivo. We tested the efficiency of two lentiviral vectors for gene transfer to the newborn ferret lung, including EIAV pseudotyped with hemagglutinin (HA) from avian influenza A virus138 and FIV pseudotyped with GP64.133 In vivo airway infection was conducted by intratracheal injection of 40 μl containing 7.5×106 infectious units (IU) of EIAV/HA-H7.CBAntLacZ into 3-day-old ferrets. Eight days after infection, tracheas and lungs were harvested and stained with X-Gal (Fig. 2F–J). Grossly, significant transgene expression was seen in all lobes and the large and small conducting airways of the lungs, but not in the trachea. Histologic analysis demonstrated that EIAV/HA-H7 virus efficiently transduced bronchi, bronchioles, and alveoli, ranging from ∼10 to 70% of cells in these regions (Fig. 2F–J). Similarly, infection of 6-day-old ferrets with 1×108 IU (100 μl) of an RSVmCherry-encoding GP64-pseudotyped FIV vector efficiently transduced intralobar small airways at 7 days postinfection (Fig. 2K–N). These results suggested the HA-H7- and/or GP64-pseudotyped lentiviral vectors may be useful in testing lung gene therapies in the CF ferret and pig models.
Gene transfer to the pancreas of ferrets and pigs
The pancreas represents another important target organ for CF gene therapy. Several routes of viral gene delivery to the pancreas have been tested in animal models including direct pancreas injection, systemic delivery with temporary clamping of portal vein and hepatic artery, retrograde pancreaticobiliary duct delivery, intraperitoneal delivery, and intravenous delivery.139–142
A minimally invasive procedure to deliver rAAV to the pancreas via the celiac artery, the vessel that supplies major branches to the pancreas, was developed and tested in newborn pigs.143 In this study, the celiac artery was used for vector delivery within 24 hr of birth and accessed via umbilical artery catheterization. One month after delivering 2×1012 particles of rAAV9-EGFP through the celiac artery, reporter expression was found in pancreatic ducts, including the intercalated and intralobular ducts; these ducts express the highest levels of CFTR in pig and human pancreatic tissue. rAAV2/9 also transduced pancreatic polypeptide (PP) cells of the islets, but not α, β, or δ cells. Celiac artery delivery of rAAV2/9 also transduced a number of other organs, as indexed by enhanced green fluorescent protein (EGFP) mRNA, including the liver, gallbladder, heart, spleen, salivary glands, trachea, and lung, but not stomach or duodenum. Notably, systemic venous delivery of rAAV2/9 did not transduce the pancreas in newborn pigs.143
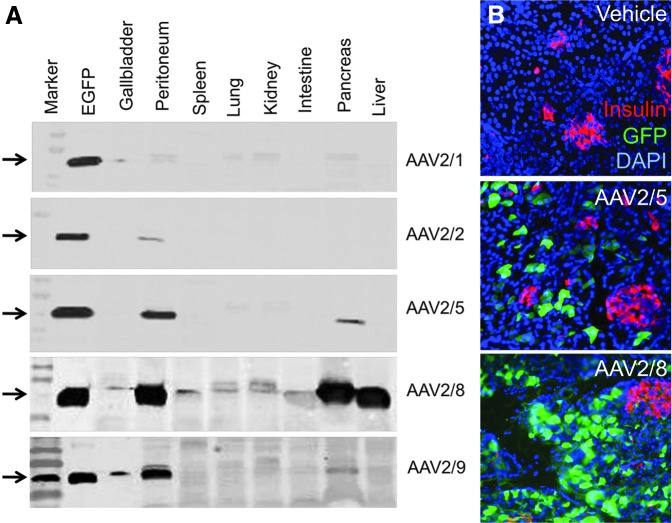
We have also tested rAAV transduction of the pancreas in newborn ferrets. Because newborn ferrets are much smaller than piglets, celiac artery or umbilical artery cannulation is not feasible. However, it was previously reported that intraperitoneal injection of rAAV2/6 and rAAV2/8 can effectively transduce the pancreatic acinar cells and islets in mice.142 We adopted this method and delivered 2×1011 particles of various serotypes of rAAV vectors (rAAV1, 2, 5, 8, and 9) to 3-day-old newborn ferrets by intraperitoneal injection. Western blot analysis of EGFP expression in various organs demonstrated that rAAV2/8 effectively delivered EGFP to the pancreas and liver (Fig. 3A). rAAV2/5 was the next most effective serotype at pancreatic gene delivery and demonstrated no hepatic gene transfer. Immunofluorescence staining for EGFP and insulin demonstrated that neither rAAV2/5 nor rAAV2/8 effectively transduced β cells of the islets (Fig. 3B). These findings suggest that the AAV8 serotype vectors may be most suitable for gene therapy to the ferret exocrine pancreas.
FIG. 3.
rAAV transduction of the pancreas in 3-day-old ferrets. Three-day-old ferrets were injected intraperitoneally with 200 μl of PBS (vehicle) or the indicated serotype rAAV vector encoding EGFP (2×1011 particles/20 g body weight in 200 μl of PBS). Ferrets were sacrificed 2 weeks after infection. (A) Tissue homogenates of the various organs were used for Western blot detection of EGFP. The lane marked “EGFP” contained the same amount of EGFP protein (marked by arrows) on each blot. (B) Pancreatic tissue sections from animals injected with vehicle, rAAV2/5-EGFP, or rAAV2/8-EGFP were immunofluorescently stained for insulin (red) and EGFP (green) followed by mounting in the presence of DAPI (blue) to mark nuclei.
Conclusions and Perspective
The creation of CF pig and ferret models presents a unique opportunity to evaluate the ability of gene therapies to slow the progression of disease in multiple target organs. Differences in the rate of disease progression in certain organs of these CF models (e.g., pancreatic and gallbladder disease) also provide opportunities to understand at what stages of disease gene therapy can be effective. Both models appear to develop disease at an accelerated rate compared with humans, facilitating a variety of studies. These models may also be useful in testing stem cell-based gene therapies, either using induced pluripotent stem cells or adult somatic stem cells. Gene-editing technologies using engineered zinc finger nucleases,144 transcription activator-like effector nucleases,145 and clustered regularly interspaced short palindromic repeats/Cas9 nucleases146 have begun to demonstrate the potential to correct endogenous mutations at the CFTR locus by homologous recombination.147,148 Notably, the ΔF508-CFTR pig model exists149 and new CFTR mutant ferret models are in the pipeline. Such models will further expand the usefulness of CF pigs and ferrets to test innovative gene and cell therapy strategies.
Acknowledgments
This work was supported by NIH grants HL108902 and DK096518 (to J.F.E.), DK092284 (to Z.A.S.), P01 HL051670 (to P.B.M.), P01 HL091842 (to P.B.M.), the Cystic Fibrosis Foundation RDP (to M.J.W.), the University of Iowa Center for Gene Therapy (DK054759), and the National Ferret Research and Resource Center on Lung Disease (HL123482), the Roy J. Carver Chair in Pulmonary Research (to P.B.M.), the Canadian Institute of Human Research (to J.H.), and the Roy J. Carver Chair in Molecular Medicine (to J.F.E.).
Author Disclosure Statement
The authors declare no financial conflict of interest.
References
- 1.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066–1073 [DOI] [PubMed] [Google Scholar]
- 2.Rommens JM, Iannuzzi MC, Kerem B, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 1989;245:1059–1065 [DOI] [PubMed] [Google Scholar]
- 3.Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science 1989;245:1073–1080 [DOI] [PubMed] [Google Scholar]
- 4.Rich DP, Anderson MP, Gregory RJ, et al. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature 1990;347:358–363 [DOI] [PubMed] [Google Scholar]
- 5.Tang L, Fatehi M, and Linsdell P. Mechanism of direct bicarbonate transport by the CFTR anion channel. J Cyst Fibros 2009;8:115–121 [DOI] [PubMed] [Google Scholar]
- 6.Boucher RC, Knowles MR, and Yankaskas JR. Cystic fibrosis. In: Murry and Nadel's Textbook of Respiratory Medicine. Mason RJ, Martin T, King TEJ, Schraufnagel D, Murray JF, and Nadel JA, eds. (Elsevier, Philadelphia, PA: ). 2010; pp. 985–1022 [Google Scholar]
- 7.Fanen P, Wohlhuter-Haddad A, and Hinzpeter A. Genetics of cystic fibrosis: CFTR mutation classifications toward genotype-based CF therapies. Int J Biochem Cell Biol 2014;52:94–102 [DOI] [PubMed] [Google Scholar]
- 8.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem 2008;77:701–726 [DOI] [PubMed] [Google Scholar]
- 9.Cutting GR. Modifier genes in mendelian disorders: the example of cystic fibrosis. Ann N Y Acad Sci 2010;1214:57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sosnay PR, Siklosi KR, Van Goor F, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet 2013;45:1160–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welsh MJ, Ramsey BW, Accurso F, et al. Cystic fibrosis. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, and Valle D, eds. (McGraw–Hill, New York, NY: ). 2001; pp. 5121–5188 [Google Scholar]
- 12.Rowe SM, Miller S, and Sorscher EJ. Cystic fibrosis. N Engl J Med 2005;352:1992–2001 [DOI] [PubMed] [Google Scholar]
- 13.Welsh MJ, and Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 1993;73:1251–1254 [DOI] [PubMed] [Google Scholar]
- 14.Driskell RA, and Engelhardt JF. Current status of gene therapy for inherited lung diseases. Annu Rev Physiol 2003;65:585–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griesenbach U, and Alton EW. Gene transfer to the lung: lessons learned from more than 2 decades of CF gene therapy. Adv Drug Deliv Rev 2009;61:128–139 [DOI] [PubMed] [Google Scholar]
- 16.Zabner J, Couture LA, Gregory RJ, et al. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 1993;75:207–216 [DOI] [PubMed] [Google Scholar]
- 17.Knowles MR, Hohneker KW, Zhou Z, et al. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N Engl J Med 1995;333:823–831 [DOI] [PubMed] [Google Scholar]
- 18.Crystal RG, Mcelvaney NG, Rosenfeld MA, et al. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet 1994;8:42–51 [DOI] [PubMed] [Google Scholar]
- 19.Zabner J, Ramsey BW, Meeker DP, et al. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J Clin Invest 1996;97:1504–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boucher RC, Knowles MR, Johnson LG, et al. Gene therapy for cystic fibrosis using E1-deleted adenovirus: a phase I trial in the nasal cavity. The University of North Carolina at Chapel Hill. Hum Gene Ther 1994;5:615–639 [DOI] [PubMed] [Google Scholar]
- 21.Wilson JM, Engelhardt JF, Grossman M, et al. Gene therapy of cystic fibrosis lung disease using E1 deleted adenoviruses: a phase I trial. Hum Gene Ther 1994;5:501–519 [DOI] [PubMed] [Google Scholar]
- 22.Zuckerman JB, Robinson CB, Mccoy KS, et al. A phase I study of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator gene to a lung segment of individuals with cystic fibrosis. Hum Gene Ther 1999;10:2973–2985 [DOI] [PubMed] [Google Scholar]
- 23.Bellon G, Michel-Calemard L, Thouvenot D, et al. Aerosol administration of a recombinant adenovirus expressing CFTR to cystic fibrosis patients: a phase I clinical trial. Hum Gene Ther 1997;8:15–25 [DOI] [PubMed] [Google Scholar]
- 24.Aitken ML, Moss RB, Waltz DA, et al. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum Gene Ther 2001;12:1907–1916 [DOI] [PubMed] [Google Scholar]
- 25.Wagner JA, Nepomuceno IB, Messner AH, et al. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther 2002;13:1349–1359 [DOI] [PubMed] [Google Scholar]
- 26.Flotte TR, Zeitlin PL, Reynolds TC, et al. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: a two-part clinical study. Hum Gene Ther 2003;14:1079–1088 [DOI] [PubMed] [Google Scholar]
- 27.Moss RB, Milla C, Colombo J, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther 2007;18:726–732 [DOI] [PubMed] [Google Scholar]
- 28.Caplen NJ, Alton EW, Middleton PG, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med 1995;1:39–46 [DOI] [PubMed] [Google Scholar]
- 29.Porteous DJ, Dorin JR, Mclachlan G, et al. Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther 1997;4:210–218 [DOI] [PubMed] [Google Scholar]
- 30.Konstan MW, Davis PB, Wagener JS, et al. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther 2004;15:1255–1269 [DOI] [PubMed] [Google Scholar]
- 31.Alton EW, Boyd AC, Cheng SH, et al. A randomised, double-blind, placebo-controlled phase IIB clinical trial of repeated application of gene therapy in patients with cystic fibrosis. Thorax 2013;68:1075–1077 [DOI] [PubMed] [Google Scholar]
- 32.Conese M, Ascenzioni F, Boyd AC, et al. Gene and cell therapy for cystic fibrosis: from bench to bedside. J Cyst Fibros 2011;10Suppl 2:S114–S128 [DOI] [PubMed] [Google Scholar]
- 33.Sumner-Jones SG, Gill DR, and Hyde SC. Gene therapy for cystic fibrosis lung disease. In: Gene Therapy for Autoimmune and Inflammatory Diseases. Chernajovsky Y. and Robbins PD, eds. (Springer, Basel, Switzerland: ). 2010; pp. 47–64 [Google Scholar]
- 34.Griesenbach U, and Alton EW. Progress in gene and cell therapy for cystic fibrosis lung disease. Curr Pharm Des 2012;18:642–662 [DOI] [PubMed] [Google Scholar]
- 35.Jiang Q, and Engelhardt JF. Cellular heterogeneity of CFTR expression and function in the lung: implications for gene therapy of cystic fibrosis. Eur J Hum Genet 1998;6:12–31 [DOI] [PubMed] [Google Scholar]
- 36.Flotte TR, Fischer AC, Goetzmann J, et al. Dual reporter comparative indexing of rAAV pseudotyped vectors in chimpanzee airway. Mol Ther 2010;18:594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Luo M, Guo C, et al. Comparative biology of rAAV transduction in ferret, pig and human airway epithelia. Gene Ther 2007;14:1543–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Luo M, Trygg C, et al. Biological differences in rAAV transduction of airway epithelia in humans and in old world non-human primates. Mol Ther 2007;15:2114–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Yan Z, Luo M, et al. Species-specific differences in mouse and human airway epithelial biology of recombinant adeno-associated virus transduction. Am J Respir Cell Mol Biol 2006;34:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan D, Yue Y, Yan Z, et al. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum Gene Ther 1998;9:2761–2776 [DOI] [PubMed] [Google Scholar]
- 41.Duan D, Yue Y, Yan Z, et al. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest 2000;105:1573–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griesenbach U, Inoue M, Hasegawa M, et al. Viral vectors for cystic fibrosis gene therapy: what does the future hold? Virus Adapt Treat 2010;2:159–171 [Google Scholar]
- 43.Mueller C, and Flotte TR. Gene therapy for cystic fibrosis. Clin Rev Allergy Immunol 2008;35:164–178 [DOI] [PubMed] [Google Scholar]
- 44.Sanders N, Rudolph C, Braeckmans K, et al. Extracellular barriers in respiratory gene therapy. Adv Drug Deliv Rev 2009;61:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knowles MR, and Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 2002;109:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrari S, Griesenbach U, Geddes DM, et al. Immunological hurdles to lung gene therapy. Clin Exp Immunol 2003;132:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Figueredo J, Limberis MP, and Wilson JM. Prediction of cellular immune responses against CFTR in patients with cystic fibrosis after gene therapy. Am J Respir Cell Mol Biol 2007;36:529–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Limberis MP, Figueredo J, Calcedo R, et al. Activation of CFTR-specific T cells in cystic fibrosis mice following gene transfer. Mol Ther 2007;15:1694–1700 [DOI] [PubMed] [Google Scholar]
- 49.Chirmule N, Propert K, Magosin S, et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther 1999;6:1574–1583 [DOI] [PubMed] [Google Scholar]
- 50.Engelhardt JF, Litzky L, and Wilson JM. Prolonged transgene expression in cotton rat lung with recombinant adenoviruses defective in E2a. Hum Gene Ther 1994;5:1217–1229 [DOI] [PubMed] [Google Scholar]
- 51.Goldman MJ, Litzky LA, Engelhardt JF, et al. Transfer of the CFTR gene to the lung of nonhuman primates with E1-deleted, E2a-defective recombinant adenoviruses: a preclinical toxicology study. Hum Gene Ther 1995;6:839–851 [DOI] [PubMed] [Google Scholar]
- 52.Dong JY, Fan PD, and Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther 1996;7:2101–2112 [DOI] [PubMed] [Google Scholar]
- 53.Flotte TR, Afione SA, Solow R, et al. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem 1993;268:3781–3790 [PubMed] [Google Scholar]
- 54.Conrad CK, Allen SS, Afione SA, et al. Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung. Gene Ther 1996;3:658–668 [PubMed] [Google Scholar]
- 55.Yan Z, Zak R, Zhang Y, et al. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J Virol 2004;78:2863–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding W, Zhang L, Yan Z, et al. Intracellular trafficking of adeno-associated viral vectors. Gene Ther 2005;12:873–880 [DOI] [PubMed] [Google Scholar]
- 57.Shimizu K, Sakurai F, Machitani M, et al. Quantitative analysis of the leaky expression of adenovirus genes in cells transduced with a replication-incompetent adenovirus vector. Mol Pharm 2011;8:1430–1435 [DOI] [PubMed] [Google Scholar]
- 58.Wivel NA, Gao GP, and Wilson JM. In: Adenoviral Vectors. (Cold Spring Harbor Laboratory Press, San Diego, CA: ). 1999 [Google Scholar]
- 59.Walters RW, Grunst T, Bergelson JM, et al. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem 1999;274:10219–10226 [DOI] [PubMed] [Google Scholar]
- 60.Pickles RJ, Mccarty D, Matsui H, et al. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol 1998;72:6014–6023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y, Nunes FA, Berencsi K, et al. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet 1994;7:362–369 [DOI] [PubMed] [Google Scholar]
- 62.Lemoine JL, Farley R, and Huang L. Mechanism of efficient transfection of the nasal airway epithelium by hypotonic shock. Gene Ther 2005;12:1275–1282 [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Jiang Q, Dudus L, et al. Vector-specific complementation profiles of two independent primary defects in cystic fibrosis airways. Hum Gene Ther 1998;9:635–648 [DOI] [PubMed] [Google Scholar]
- 64.Sun X, Yan Z, Yi Y, et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest 2008;118:1578–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rogers CS, Hao Y, Rokhlina T, et al. Production of CFTR-null and CFTR-deltaf508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest 2008;118:1571–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keiser NW, and Engelhardt JF. New animal models of cystic fibrosis: what are they teaching us? Curr Opin Pulm Med 2011;17:478–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fisher JT, Zhang Y, and Engelhardt JF. Comparative biology of cystic fibrosis animal models. Methods Mol Biol 2011;742:311–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guilbault C, Saeed Z, Downey GP, et al. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol 2007;36:1–7 [DOI] [PubMed] [Google Scholar]
- 69.Hodges CA, Cotton CU, Palmert MR, et al. Generation of a conditional null allele for CFTR in mice. Genesis 2008;46:546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonfield TL, Hodges CA, Cotton CU, et al. Absence of the cystic fibrosis transmembrane regulator (CFTR) from myeloid-derived cells slows resolution of inflammation and infection. J Leukoc Biol 2012;92:1111–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mueller C, Braag SA, Keeler A, et al. Lack of cystic fibrosis transmembrane conductance regulator in CD3+lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am J Respir Cell Mol Biol 2011;44:922–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engelhardt JF, Yankaskas JR, Ernst SA, et al. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 1992;2:240–248 [DOI] [PubMed] [Google Scholar]
- 73.Dajani R, Zhang Y, Taft PJ, et al. Lysozyme secretion by submucosal glands protects the airway from bacterial infection. Am J Respir Cell Mol Biol 2005;32:548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wine JJ, and Joo NS. Submucosal glands and airway defense. Proc Am Thorac Soc 2004;1:47–53 [DOI] [PubMed] [Google Scholar]
- 75.Pack RJ, Al-Ugaily LH, Morris G, et al. The distribution and structure of cells in the tracheal epithelium of the mouse. Cell Tissue Res 1980;208:65–84 [DOI] [PubMed] [Google Scholar]
- 76.Clarke LL, Grubb BR, Yankaskas JR, et al. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in CFTR(−/−) mice. Proc Natl Acad Sci U S A 1994;91:479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gray MA, Winpenny JP, Verdon B, et al. Chloride channels and cystic fibrosis of the pancreas. Biosci Rep 1995;15:531–541 [DOI] [PubMed] [Google Scholar]
- 78.Pascua P, Garcia M, Fernandez-Salazar MP, et al. Ducts isolated from the pancreas of CFTR-null mice secrete fluid. Pflugers Arch 2009;459:203–214 [DOI] [PubMed] [Google Scholar]
- 79.Wang X, Zhang Y, Amberson A, et al. New models of the tracheal airway define the glandular contribution to airway surface fluid and electrolyte composition. Am J Respir Cell Mol Biol 2001;24:195–202 [DOI] [PubMed] [Google Scholar]
- 80.Rogers CS, Abraham WM, Brogden KA, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;295:L240–L263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Widdicombe JH. Transgenic animals may help resolve a sticky situation in cystic fibrosis. J Clin Invest 2010;120:3093–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X, Driskell RR, and Engelhardt JF. Airway glandular development and stem cells. Curr Top Dev Biol 2004;64:33–56 [DOI] [PubMed] [Google Scholar]
- 83.Liu X, Luo M, Zhang L, et al. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am J Respir Cell Mol Biol 2007;36:313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jacob S, and Poddar S. Mucous cells of the tracheobronchial tree in the ferret. Histochemistry 1982;73:599–605 [DOI] [PubMed] [Google Scholar]
- 85.Wilmut I, Schnieke AE, Mcwhir J, et al. Viable offspring derived from fetal and adult mammalian cells. Nature 1997;385:810–813 [DOI] [PubMed] [Google Scholar]
- 86.Oppenheimer EH, and Esterly JR. Observations in cystic fibrosis of the pancreas. II. Neonatal intestinal obstruction. Bull Johns Hopkins Hosp 1962;111:1–13 [PubMed] [Google Scholar]
- 87.Gaillard D, Bouvier R, Scheiner C, et al. Meconium ileus and intestinal atresia in fetuses and neonates. Pediatr Pathol Lab Med 1996;16:25–40 [PubMed] [Google Scholar]
- 88.Rogers CS, Stoltz DA, Meyerholz DK, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 2008;321:1837–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meyerholz DK, Stoltz DA, Pezzulo AA, et al. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol 2010;176:1377–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun X, Sui H, Fisher JT, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest 2010;120:3149–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun X, Olivier AK, Yi Y, et al. Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets. Am J Pathol 2014;184:1309–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stoltz DA, Rokhlina T, Ernst SE, et al. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest 2013;123:2685–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oppenheimer EH, and Esterly JR. Cystic fibrosis of the pancreas. Morphologic findings in infants with and without diagnostic pancreatic lesions. Arch Pathol 1973;96:149–154 [PubMed] [Google Scholar]
- 94.Sturgess JM. Structural and developmental abnormalities of the exocrine pancreas in cystic fibrosis. J Pediatr Gastroenterol Nutr 1984;3Suppl 1:S55–S66 [DOI] [PubMed] [Google Scholar]
- 95.Abu-El-Haija M, Ramachandran S, Meyerholz DK, et al. Pancreatic damage in fetal and newborn cystic fibrosis pigs involves the activation of inflammatory and remodeling pathways. Am J Pathol 2012;181:499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abu-El-Haija M, Sinkora M, Meyerholz DK, et al. An activated immune and inflammatory response targets the pancreas of newborn pigs with cystic fibrosis. Pancreatology 2011;11:506–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olivier AK, Yi Y, Sun X, et al. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest 2012;122:3755–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uc A, Olivier AK, Griffin MA, et al. Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci (Lond) 2015;128:131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oppenheimer EH, and Esterly JR. Pathology of cystic fibrosis review of the literature and comparison with 146 autopsied cases. Perspect Pediatr Pathol 1975;2:241–278 [PubMed] [Google Scholar]
- 100.Chaudry G, Navarro OM, Levine DS, et al. Abdominal manifestations of cystic fibrosis in children. Pediatr Radiol 2006;36:233–240 [DOI] [PubMed] [Google Scholar]
- 101.Lindblad A, Glaumann H, and Strandvik B. Natural history of liver disease in cystic fibrosis. Hepatology 1999;30:1151–1158 [DOI] [PubMed] [Google Scholar]
- 102.Fisher JT, Tyler SR, Zhang Y, et al. Bioelectric characterization of epithelia from neonatal CFTR knockout ferrets. Am J Respir Cell Mol Biol 2013;49:837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stoltz DA, Meyerholz DK, Pezzulo AA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2010;2:29ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keiser NW, Birket SE, Evans IA, et al. Defective innate immunity and hyper-inflammation in newborn CFTR-knockout ferret lungs. Am J Respir Cell Mol Biol 2014. DOI: 10.1165/rcmb.2014-0250OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun X, Olivier AK, Liang B, et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am J Respir Cell Mol Biol 2014;50:502–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brunetti-Pierri N, and Ng P. Progress and prospects: gene therapy for genetic diseases with helper-dependent adenoviral vectors. Gene Ther 2008;15:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O'neal WK, Rose E, Zhou H, et al. Multiple advantages of alpha-fetoprotein as a marker for in vivo gene transfer. Mol Ther 2000;2:640–648 [DOI] [PubMed] [Google Scholar]
- 108.Toietta G, Koehler DR, Finegold MJ, et al. Reduced inflammation and improved airway expression using helper-dependent adenoviral vectors with a K18 promoter. Mol Ther 2003;7:649–658 [DOI] [PubMed] [Google Scholar]
- 109.Cao H, Yang T, Li XF, et al. Readministration of helper-dependent adenoviral vectors to mouse airway mediated via transient immunosuppression. Gene Ther 2011;18:173–181 [DOI] [PubMed] [Google Scholar]
- 110.Kushwah R, Oliver JR, Cao H, et al. Nacystelyn enhances adenoviral vector-mediated gene delivery to mouse airways. Gene Ther 2007;14:1243–1248 [DOI] [PubMed] [Google Scholar]
- 111.Gregory LG, Harbottle RP, Lawrence L, et al. Enhancement of adenovirus-mediated gene transfer to the airways by DEAE dextran and sodium caprate in vivo. Mol Ther 2003;7:19–26 [DOI] [PubMed] [Google Scholar]
- 112.Koehler DR, Frndova H, Leung K, et al. Aerosol delivery of an enhanced helper-dependent adenovirus formulation to rabbit lung using an intratracheal catheter. J Gene Med 2005;7:1409–1420 [DOI] [PubMed] [Google Scholar]
- 113.Yan Z, Lei-Butters DC, Liu X, et al. Unique biologic properties of recombinant AAV1 transduction in polarized human airway epithelia. J Biol Chem 2006;281:29684–29692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yan Z, Lei-Butters DC, Keiser NW, et al. Distinct transduction difference between adeno-associated virus type 1 and type 6 vectors in human polarized airway epithelia. Gene Ther 2013;20:328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Limberis MP, Vandenberghe LH, Zhang L, et al. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol Ther 2009;17:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Excoffon KJ, Koerber JT, Dickey DD, et al. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc Natl Acad Sci U S A 2009;106:3865–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li W, Asokan A, Wu Z, et al. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Mol Ther 2008;16:1252–1260 [DOI] [PubMed] [Google Scholar]
- 118.White AF, Mazur M, Sorscher EJ, et al. Genetic modification of adeno-associated viral vector type 2 capsid enhances gene transfer efficiency in polarized human airway epithelial cells. Hum Gene Ther 2008;19:1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang L, Wang D, Fischer H, et al. Efficient expression of CFTR function with adeno-associated virus vectors that carry shortened CFTR genes. Proc Natl Acad Sci U S A 1998;95:10158–10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ostedgaard LS, Rokhlina T, Karp PH, et al. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc Natl Acad Sci U S A 2005;102:2952–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yan Z, Keiser NW, Song Y, et al. A novel chimeric adenoassociated virus 2/human bocavirus 1 parvovirus vector efficiently transduces human airway epithelia. Mol Ther 2013;21:2181–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ding W, Yan Z, Zak R, et al. Second-strand genome conversion of adeno-associated virus type 2 (AAV-2) and AAV-5 is not rate limiting following apical infection of polarized human airway epithelia. J Virol 2003;77:7361–7366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Naldini L, Blomer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996;272:263–267 [DOI] [PubMed] [Google Scholar]
- 124.Delenda C. Lentiviral vectors: optimization of packaging, transduction and gene expression. J Gene Med 2004;6Suppl 1:S125–S138 [DOI] [PubMed] [Google Scholar]
- 125.Naldini L, Blomer U, Gage FH, et al. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A 1996;93:11382–11388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang G, Slepushkin V, Zabner J, et al. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J Clin Invest 1999;104:R55–R62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kobayashi M, Iida A, Ueda Y, et al. Pseudotyped lentivirus vectors derived from simian immunodeficiency virus SIVagm with envelope glycoproteins from paramyxovirus. J Virol 2003;77:2607–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mckay T, Patel M, Pickles RJ, et al. Influenza M2 envelope protein augments avian influenza hemagglutinin pseudotyping of lentiviral vectors. Gene Ther 2006;13:715–724 [DOI] [PubMed] [Google Scholar]
- 129.Sinn PL, Cooney AL, Oakland M, et al. Lentiviral vector gene transfer to porcine airways. Mol Ther Nucleic Acids 2012;1:e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Johnson LG, Olsen JC, Naldini L, et al. Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Ther 2000;7:568–574 [DOI] [PubMed] [Google Scholar]
- 131.Sinn PL, Burnight ER, Hickey MA, et al. Persistent gene expression in mouse nasal epithelia following feline immunodeficiency virus-based vector gene transfer. J Virol 2005;79:12818–12827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mitomo K, Griesenbach U, Inoue M, et al. Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with Sendai virus envelopes. Mol Ther 2010;18:1173–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sinn PL, Arias AC, Brogden KA, et al. Lentivirus vector can be readministered to nasal epithelia without blocking immune responses. J Virol 2008;82:10684–10692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Griesenbach U, Inoue M, Meng C, et al. Assessment of F/HN-pseudotyped lentivirus as a clinically relevant vector for lung gene therapy. Am J Respir Crit Care Med 2012;186:846–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cao H, Machuca TN, Yeung JC, et al. Efficient gene delivery to pig airway epithelia and submucosal glands using helper-dependent adenoviral vectors. Mol Ther Nucleic Acids 2013;2:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yan Z, Sun X, Evans IA, et al. Postentry processing of recombinant adeno-associated virus type 1 and transduction of the ferret lung are altered by a factor in airway secretions. Hum Gene Ther 2013;24:786–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Steines BR, Dickey DD, Gansemer N, et al. Efficient gene transfer to pig airway epithelia by a vector evolved using directed evolution of an adeno-associated virus library in vivo. American Society for Cell and Gene Therapy Conference Simultaneous Oral Abstract Sessions: Cardiovascular and Pulmonary Therapies Abstract 2014; p. 503 [Google Scholar]
- 138.Patel M, Giddings AM, Sechelski J, et al. High efficiency gene transfer to airways of mice using influenza hemagglutinin pseudotyped lentiviral vectors. J Gene Med 2013;15:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ayuso E, Chillon M, Garcia F, et al. In vivo gene transfer to healthy and diabetic canine pancreas. Mol Ther 2006;13:747–755 [DOI] [PubMed] [Google Scholar]
- 140.Mcclane SJ, Hamilton TE, Burke CV, et al. Functional consequences of adenovirus-mediated murine pancreatic gene transfer. Hum Gene Ther 1997;8:739–746 [DOI] [PubMed] [Google Scholar]
- 141.Wang AY, Peng PD, Ehrhardt A, et al. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum Gene Ther 2004;15:405–413 [DOI] [PubMed] [Google Scholar]
- 142.Wang Z, Zhu T, Rehman KK, et al. Widespread and stable pancreatic gene transfer by adeno-associated virus vectors via different routes. Diabetes 2006;55:875–884 [DOI] [PubMed] [Google Scholar]
- 143.Griffin MA, Restrepo MS, Abu-El-Haija M, et al. A novel gene delivery method transduces porcine pancreatic duct epithelial cells. Gene Ther 2014;21:123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Urnov FD, Rebar EJ, Holmes MC, et al. Genome editing with engineered zinc finger nucleases. Nat Rev Genet 2010;11:636–646 [DOI] [PubMed] [Google Scholar]
- 145.Joung JK, and Sander JD. Talens: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol 2013;14:49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hsu PD, Scott DA, Weinstein JA, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 2013;31:827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schwank G, Koo BK, Sasselli V, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 2013;13:653–658 [DOI] [PubMed] [Google Scholar]
- 148.Lee CM, Flynn R, Hollywood JA, et al. Correction of the deltaf508 mutation in the cystic fibrosis transmembrane conductance regulator gene by zinc-finger nuclease homology-directed repair. Biores Open Access 2012;1:99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ostedgaard LS, Meyerholz DK, Chen JH, et al. The deltaf508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med 2011;3:74ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]