Abstract
Background
Silent brain infarcts (SBIs) and white matter hyperintensities are subclinical cerebrovascular lesions associated with incident stroke and cognitive decline. Left ventricular ejection fraction (LVEF) is a predictor of stroke in patients with heart failure, but its association with subclinical brain disease in the general population is unknown. Left ventricular global longitudinal strain (GLS) can detect subclinical cardiac dysfunction even when LVEF is normal. We investigated the relationship of LVEF and GLS with subclinical brain disease in a community-based cohort.
Methods and Results
LVEF and GLS were assessed by 2-dimensional and speckle-tracking echocardiography in 439 participants free of stroke and cardiac disease from the Cardiovascular Abnormalities and Brain Lesions (CABL) study. SBIs and white matter hyperintensities were assessed by brain MRI. Mean age of the study population was 69±10 years, 61% were women, LVEF was 63.8±6.4%, GLS was −17.1±3.0%. SBIs were detected in 53 participants (12%), white matter hyperintensity volume was 0.63±0.86%. GLS was significantly lower in participants with SBI versus those without (−15.7±3.5% versus −17.3±2.9%, P<0.01), whereas no difference in LVEF was observed (63.3±8.6% versus 63.8±6.0%, P=0.60). In multivariate analysis, lower GLS was associated with SBI (odds ratio/unit decrease=1.18; 95% confidence interval, 1.05–1.33; P<0.01), whereas LVEF was not (odds ratio/unit increase=1.00; 95% confidence interval, 0.96–1.05; P=0.98). Lower GLS was associated with greater white matter hyperintensity volume (adjusted β=0.11, P<0.05), unlike LVEF (adjusted β=−0.04, P=0.42).
Conclusions
Lower GLS was independently associated with subclinical brain disease in a community-based cohort without overt cardiac disease. GLS can provide additional information on cerebrovascular risk burden beyond LVEF assessment.
Keywords: brain infarction, echocardiography, global longitudinal strain, magnetic resonance imaging, speckle-tracking, ventricular ejection fraction, white matter diseases
Stroke prevalence in the adult population of the United States is estimated at 3.0%, with an average of 1 person every 4 minutes dying of a stroke.1 The morbidity, mortality, and economic burden associated with stroke is huge, making its prevention a public health priority.1 In population-based studies, the prevalence of asymptomatic vascular brain lesions is substantially higher than that of clinically overt disease. Silent brain infarcts (SBIs) have been documented in 7% to 28% of subjects without previous stroke, with a steep increase in prevalence observed with aging.2–8 Cerebral white matter hyperintensities (WMHs) have also been described in asymptomatic people in the general population.9–12 SBIs and WMHs are associated with an increased risk of future clinical stroke, cognitive impairment, and dementia.10,11,13–15
Left ventricular (LV) systolic function measured as ejection fraction (LVEF) is strongly associated with incident stroke and with cognitive impairment in the setting of congestive heart failure and coronary artery disease.16,17 The prevalence of SBI in patients with heart failure is higher than that observed in unselected populations.18,19 LV dysfunction has also been found more often in patients with stroke than in stroke-free controls.20 However, in the general population, and in the absence of heart disease, it is not known whether LV systolic function is associated with subclinical brain disease. In a previous study, LVEF was not associated with WMH volume (WMHV) in the general population.21 LV global longitudinal strain (GLS) is an echocardiographic measure of LV function that can unmask subclinical systolic abnormalities, even when LVEF is in the normal range, in a variety of conditions that are also potent risk factors for stroke, such as arterial hypertension and diabetes mellitus. In fact, GLS has prognostic value for cardiovascular events and mortality that is independent and additive to that of LVEF.22,23 It is not known whether GLS is associated with the presence of subclinical brain disease in subjects without heart disease. If demonstrated, this association might be of value in the identification of cerebrovascular disease at an asymptomatic stage. Accordingly, the aim of the present study was to investigate the association of LV systolic function, measured by LVEF and GLS, with subclinical cerebrovascular disease in a community-based cohort without clinical stroke or overt cardiac disease.
Methods
Study Population
The Cardiovascular Abnormalities and Brain Lesion (CABL) study is a community-based epidemiological study designed to investigate the cardiovascular predictors of silent cerebrovascular disease in the community. CABL based its recruitment on the Northern Manhattan Study (NOMAS), a population-based prospective study that enrolled 3298 participants from the community living in northern Manhattan between 1993 and 2001. The study design and recruitment details of NOMAS have been described previously.24 Beginning in 2003, participants were invited to participate in an MRI substudy if they (1) were at least 55 years of age, (2) had no contraindications to MRI, and (3) did not have a previous diagnosis of stroke. From September 2005 to July 2010, NOMAS MRI participants that voluntarily agreed to undergo a more extensive cardiovascular evaluation including transthoracic echocardiography were included in CABL. Participants for whom echocardiography and brain MRI information was available constitute the sample of the present study. Participants with nonsinus rhythm at the time of the echocardiogram, history of atrial fibrillation or atrial flutter, significant mitral or aortic valve disease, and coronary artery disease were excluded from the present analysis. Written informed consent was obtained from all study participants. The study was approved by the Institutional Review Boards of Columbia University Medical Center and of the University of Miami.
Risk Factor Assessment
Cardiovascular risk factors were ascertained through direct examination and interview by trained research assistants. Systolic and diastolic blood pressures were measured at the nondominant arm in a sitting position after 5 minutes of rest, using a mercury sphygmomanometer and a proportioned arm cuff. Study participants were not asked to discontinue antihypertensive medications on the day of the visit. Two blood pressure measurements were performed and averaged. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, or the participant’s self-reported history of hypertension or use of antihypertensive medication. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or the participant’s self-reported history of diabetes mellitus or use of diabetes mellitus medications. Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL, a patient’s self-report of hypercholesterolemia or use of lipid-lowering treatment. Cigarette smoking, either at the time of the interview or in the past, was recorded. Coronary artery disease was defined as a history of myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, typical angina, or use of anti-ischemic medications. Atrial fibrillation was defined from ECG at the time of echocardiography or from self-reported history. The race-ethnicity classification was based on self-identification, and categorized as black, Hispanic, and white.
Echocardiographic Assessment
Two-Dimensional Echocardiography
Transthoracic echocardiography was performed by using a commercially available system (iE 33, Philips, Andover, MA) by a trained, registered cardiac sonographer according to a standardized protocol. Interventricular septum and posterior wall thickness, LV end-diastolic diameter, and left atrial anteroposterior diameter were measured from a parasternal long-axis view according to the recommendations of the American Society of Echocardiography.25 LV end-diastolic diameter and left atrial diameter were indexed by body surface area. LVEF was calculated by using the biplane modified Simpson’s rule, replaced by a semiquantitative method or visual estimation in the case of technically suboptimal images. LV mass was calculated with a validated method26 and indexed by body surface area. LV relative wall thickness was calculated as: 2 × posterior wall thickness/LV end-diastolic diameter. LV diastolic function assessment has been previously described in detail.27 In brief, color Doppler imaging was used to visualize the transmitral flow from an apical 4-chamber view; the pulsed-wave Doppler sample volume was placed perpendicular to the inflow jet at the level of mitral valve leaflet tips. The peak early velocity (E), late velocity (A), and deceleration time of the mitral inflow were measured, and the E/A ratio was calculated. Mitral annular velocities were evaluated by pulsed-wave tissue Doppler imaging and sampled on the longitudinal axis from the apical 4-chamber view. The peak early diastolic velocity (e′) of the lateral and the septal mitral annulus were measured and the average value was calculated. Diastolic dysfunction definition was published previously.27
Speckle-Tracking Strain Imaging
Speckle-tracking analysis was performed off-line by using commercially available software (QLAB Advanced Quantification Software version 8.1, Philips) as previously described.28 In brief, speckle-tracking analysis of LV myocardial deformation over the longitudinal axis was performed from 2-dimensional gray-scale loops by automatic tracking of myocardial speckles after manual selection of landmark points. Global longitudinal systolic strain (GLS) was calculated averaging the negative peak of longitudinal strain from 12 ventricular segments from the apical 4-chamber and 2-chamber views. At least 2 cardiac cycles were recorded at a frame rate ≥ 45 fps, and were averaged for strain analysis. Aortic valve opening and closing times were measured from the LV outflow Doppler profile and were incorporated in the speckle-tracking strain profile to exclude postsystolic components. Because GLS is represented by negative values, with more negative numbers expressing greater systolic shortening and therefore better function, we adopted the terminology lower GLS referring to higher, less negative values, therefore expressing smaller systolic shortening. Abnormal GLS was defined as a GLS lower than the 95th percentile of the GLS distribution in the healthy subgroup of subjects free of hypertension, diabetes mellitus, coronary artery disease, arrhythmias, and with body mass index ≤25 kg/m2, corresponding to a GLS > −14%. Reproducibility of speckle-tracking measurements has been reported previously.29
Brain MRI
A detailed description of the assessment of subclinical cerebrovascular lesions has been published previously.9,30 In brief, brain imaging was performed on a 1.5-T MRI system (Philips Medical Systems). Median time between MRI and echocardiographic examination was 2 days (75th percentile: 5 days). SBIs were rated by 2 of the authors (C.D. and M.Y.) and defined as either a cavitation on the fluid-attenuated inversion recovery sequence of at least 3 mm in size, distinct from a vessel (owing to the lack of signal void on T2 sequence), and of equal intensity to cerebrospinal fluid in the case of lacunar infarction, or as a wedge shaped cortical or cerebellar area of encephalomalacia with surrounding gliosis consistent with infarction attributable to distal arterial branch occlusion. Interobserver agreement for SBI detection was 93.3%.30 WMHV analysis was based on a fluid attenuated inversion recovery image and performed by using the Quantum 6.2 package on a Sun Microsystems Ultra 5 workstation. WMHV was expressed as proportion of total cranial volume to correct for differences in head size, and log-transformed (log-WMHV) to achieve a normal distribution for analysis as a continuous variable. All measurements were performed blinded to participant identifying and clinical information.
Statistical Analysis
Data are presented as means±standard deviation for continuous variables and as percentages for categorical variables. The Student t test was used to assess differences in GLS, LVEF, and WMHV between groups (presence/absence of SBI; normal/abnormal GLS and LVEF). Logistic and linear regression models were used to test the association of demographic and clinical variables with parameters of LV function, and the association of LV function parameters with SBI and log-WMVH. Unstandardized (B) and standardized (β) parameter estimates, standard errors, Pearson partial correlation coefficients, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated and reported. Covariates for multivariate models were selected based on their univariate association with LV function parameters, with a threshold for entry in the multivariate models set at a probability value of <0.2. For all statistical analyses, a 2-tailed P<0.05 was considered significant. Statistical analyses were performed by using SAS software version 9.2 (SAS Institute Inc, Cary, NC).
Results
Study Population
Clinical and demographic characteristics of the study population are shown in Table 1. We detected SBI in 53 study participants (12%); in 9 cases (17%), the SBIs were located in cortical areas and 44 (83%) were in subcortical areas. Mean WMHV was 0.63±0.86% (median=0.32%, interquartile range=0.47%). Echocardiographic data, including LVEF and GLS of the study population are shown in Table 2.
Table 1.
General Characteristics of the Study Population
| n=439 | |
|---|---|
| Age, y | 69.3±9.7 |
| Women, n (%) | 266 (60.6) |
| Race-ethnicity | |
| White, n (%) | 59 (13.4) |
| Black, n (%) | 70 (15.9) |
| Hispanic, n (%) | 306 (69.7) |
| Other, n (%) | 4 (0.9) |
| Body mass index, kg/m2 | 27.8±4.5 |
| SBP, mm Hg | 133.8±16.8 |
| DBP, mm Hg | 78.4±9.3 |
| Hypertension, n (%) | 319 (72.7) |
| Diabetes mellitus, n (%) | 115 (26.2) |
| Hypercholesterolemia, n (%) | 270 (61.5) |
| Smoking history, n (%) | 238 (54.2) |
| Current smoking, n (%) | 32 (7.3) |
DBP indicates diastolic blood pressure; and SBP, systolic blood pressure.
Table 2.
Echocardiographic Data
| LV septal thickness, mm | 11.3±1.7 |
| LVEDi, mm/m2 | 25.6±2.9 |
| LV posterior wall thickness, mm | 11.0±1.5 |
| LV mass index, g/m2 | 102.5±23.8 |
| Relative wall thickness | 0.49±0.08 |
| LVEF, % | 63.8±6.4 |
| LVEF <55%, n (%) | 29 (6.6) |
| GLS, % | −17.1±3.0 |
| GLS > −14%, n (%) | 52 (11.8) |
| Left atrial diameter, mm/m2 | 21.9±2.7 |
| LV diastolic dysfunction, n (%) | 240 (54.7) |
| Heart rate, beats/min | 70.3±11.2 |
GLS indicates global longitudinal strain; LV, left ventricular; LVEDi, LV end-diastolic dimension index; and LVEF, LV ejection fraction.
Factors Associated With LV Function
Table 3 shows the univariate associations of LVEF and GLS with demographics, risk factors, and echocardiographic variables. Lower LVEF was significantly associated with male sex, greater LV mass index, smaller relative wall thickness (all P<0.01), and higher body mass index (P<0.05). Lower GLS was significantly associated with older age, higher systolic and diastolic blood pressures, hypertension, diabetes mellitus, greater LV mass index, LV diastolic dysfunction (all P<0.01), and greater relative wall thickness (P<0.05).
Table 3.
Factors Univariately Associated With LV Function
| LVEF (per % point) | GLS (per % point) | |||
|---|---|---|---|---|
| B (SE) | P Value | B (SE) | P Value | |
| Age (per year) | −0.03 (0.03) | 0.34 | 0.04 (0.02) | <0.01 |
| Male sex | −3.11 (0.61) | <0.01 | 0.47 (0.29) | 0.11 |
| Body mass index (per kg/m2) | −0.17 (0.07) | <0.05 | 0.04 (0.03) | 0.25 |
| SBP (per mm Hg) | 0.02 (0.02) | 0.29 | 0.03 (0.01) | <0.01 |
| DBP (per mm Hg) | 0.02 (0.03) | 0.61 | 0.06 (0.02) | <0.01 |
| Hypertension | 0.37 (0.68) | 0.59 | 1.11 (0.32) | <0.01 |
| Diabetes mellitus | 0.27 (0.69) | 0.70 | 0.86 (0.32) | <0.01 |
| Hypercholesterolemia | 0.73 (0.62) | 0.25 | 0.41 (0.29) | 0.17 |
| Smoking history | −1.01 (0.61) | 0.10 | 0.28 (0.29) | 0.33 |
| Current smoking | −0.56 (1.17) | 0.64 | −0.30 (0.55) | 0.59 |
| LV mass index (per g/m2) | −0.04 (0.01) | <0.01 | 0.03 (0.01) | <0.01 |
| Relative wall thickness | 12.61 (3.56) | <0.01 | 3.48 (1.69) | <0.05 |
| Left atrial diameter (per mm/m2) | 0.06 (0.11) | 0.61 | 0.01 (0.05) | 0.83 |
| LV diastolic dysfunction | −1.14 (0.61) | 0.06 | 1.45 (0.28) | <0.01 |
Values in table are parameter estimates (B) and relative standard error (SE). DBP indicates diastolic blood pressure; GLS, global longitudinal strain; LV, left ventricular; LVEF, LV ejection fraction; and SBP, systolic blood pressure.
LV Systolic Function and Subclinical Cerebrovascular Disease
GLS was significantly lower in participants with SBI than in those without (−15.7±3.5% versus −17.3±2.9%, P<0.01), whereas LVEF was not significantly different in the 2 groups (63.3±8.6% versus 63.8±6.0%, P=0.60). Data on the association between measures of LV systolic function and the presence of SBI are shown in Table 4. LVEF showed no association with SBI in either univariate analysis (OR per unit increase of LVEF=0.99; 95% CI, 0.95–1.03; P=0.53) or after adjustment for relevant covariates (adjusted OR=1.00; 95% CI, 0.96–1.05; P=0.98). A LVEF <55% also did not show any significant relationship with SBI (adjusted OR=0.64; 95% CI, 0.19–2.16; P=0.47). Lower GLS was significantly associated with SBI in univariate analysis (OR per unit decrease of GLS=1.21; 95% CI, 1.10–1.34; P<0.01), and this association was confirmed after adjusting for covariates (adjusted OR=1.16; 95% CI, 1.04–1.30; P<0.01). The addition of LVEF to the model did not affect the significant relationship between GLS and SBI (adjusted OR=1.18; 95% CI, 1.05–1.33; P<0.01). An abnormal GLS was associated with a greater prevalence of SBI both in univariate (OR=4.44; 95% CI, 2.24–8.81) and in multivariate analysis (adjusted OR=3.56; 95% CI, 1.62–7.83; P<0.01).
Table 4.
Association of LV Systolic Function With Silent Brain Infarcts
| Predictor | Model 1 OR (95% CI) |
P Value | Model 2 OR (95% CI) |
P Value | Model 3 OR (95% CI) |
P Value |
|---|---|---|---|---|---|---|
| LVEF* | 0.99 (0.95–1.03) | 0.53 | 1.00 (0.96–1.05) | 0.98 | — | — |
| Abnormal LVEF (<55%)† | 1.20 (0.40–3.60) | 0.75 | 0.64 (0.19–2.16) | 0.47 | — | — |
| GLS‡ | 1.21 (1.10–1.34) | <0.01 | 1.16 (1.04–1.30) | <0.01 | 1.18 (1.05–1.33) | <0.01 |
| Abnormal GLS (>−14%)§ | 4.44 (2.24–8.81) | <0.01 | 3.28 (1.53–7.03) | <0.01 | 3.56 (1.62–7.83) | <0.01 |
Values in table are odds ratios (OR) and 95% confidence intervals (CI). Each predictor is evaluated in a separate model. Model 1, unadjusted; model 2, adjusted for age, sex, body mass index, SBP, DBP, hypertension, diabetes mellitus, hypercholesterolemia, smoking history, LV mass index, relative wall thickness, and LV diastolic dysfunction; model 3, adjusted for covariates in model 2 plus LVEF. DBP indicates diastolic blood pressure; GLS indicate global longitudinal strain; LV, left ventricular; LVEF, left ventricular ejection fraction; and SBP, systolic blood pressure.
OR per 1 LVEF unit increase.
Reference group: normal LVEF (≥55%).
OR per 1 GLS unit decrease (less negative values).
Reference group: normal GLS (≤ −14%).
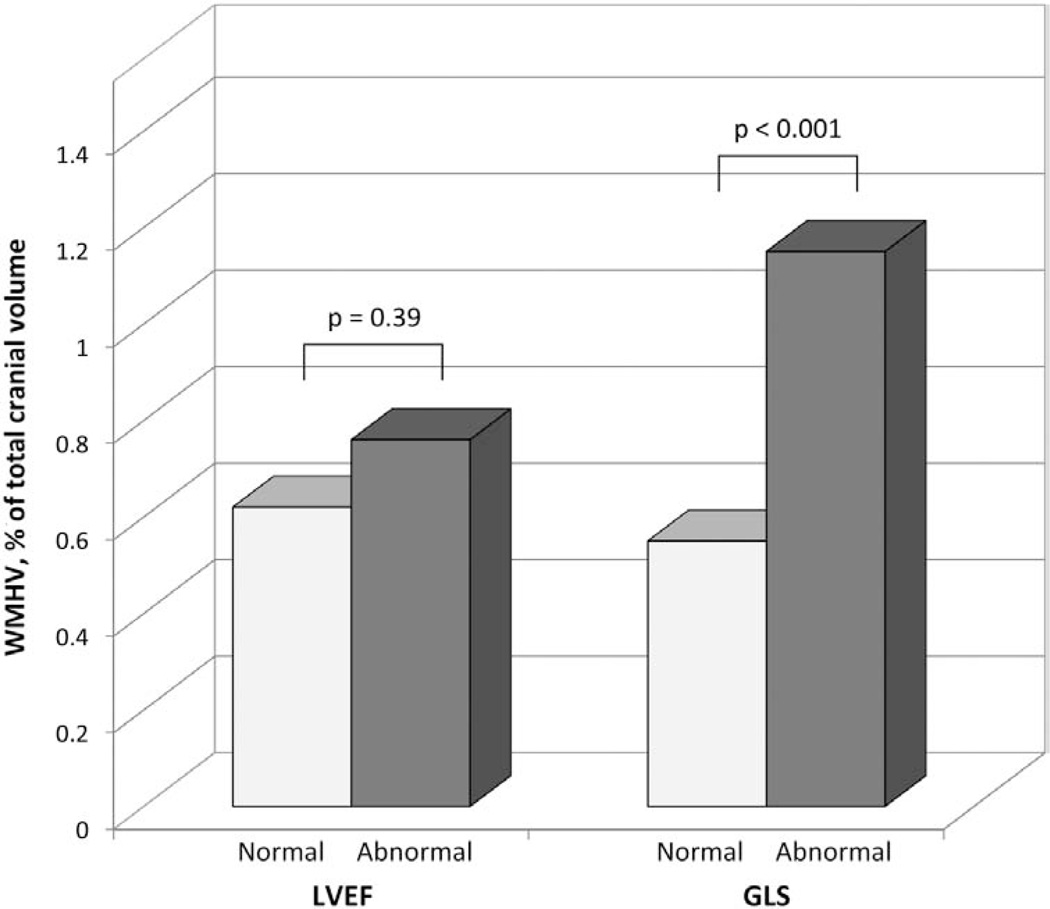
WMHV was not significantly different in subjects with normal versus low LVEF (0.62±0.85% versus 0.76±0.81%, P=0.39), whereas it was significantly greater in subjects with abnormal versus normal GLS (1.15±1.46% versus 0.56±0.71%, P<0.001) (Figure). The association between LV systolic function and log-WMHV is shown in Table 5. LVEF was not associated with log-WMHV in either univariate analysis (β=−0.06, P=0.25) or after adjustment for covariates (β=−0.04, P=0.42). Lower GLS was significantly associated with greater log-WMHV both in univariate (β=0.18, P<0.01) and multivariate analysis (β=0.11, P<0.05). In a model further adjusted for LVEF, a lower GLS remained significantly associated with greater log-WMHV (β=0.10, P<0.05). No significant interaction was found between GLS and LVEF in the association with either SBI or log-WMHV.
Figure 1.
Association between WMHV, expressed as a percentage of the total cranial volume, and LV systolic function. GLS indicates global longitudinal strain; LV, left ventricular; LVEF, left ventricular ejection fraction; and WMHV, white matter hyperintensity volume.
Table 5.
Association of LV Systolic Function With log-WMHV
| Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | β (SE) | P Value | β (SE) | Part. R | P Value | β (SE) | Part. R | P Value |
| LVEF | −0.06 (0.05) | 0.25 | −0.04 (0.04) | −0.06 | 0.42 | — | — | — |
| GLS | 0.18 (0.05) | <0.01 | 0.11 (0.04) | 0.12 | <0.05 | 0.10 (0.05) | 0.11 | <0.05 |
Values in table are parameter estimates per standard deviation increase (β), standard errors (SE), and partial correlation coefficients (Part. R). Each predictor is evaluated in a separate model. Model 1, unadjusted; model 2, adjusted for age, sex, body mass index, SBP, DBP, hypertension, diabetes mellitus, hypercholesterolemia, smoking history, LV mass index, relative wall thickness, and LV diastolic dysfunction; model 3, adjusted for covariates from model 2 plus LVEF. DBP indicates diastolic blood pressure; GLS indicate global longitudinal strain; LV, left ventricular; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; and WMHV, white matter hyperintensity volume.
Discussion
In this study, we demonstrated for the first time that GLS is associated with subclinical brain disease in a community-based cohort free of stroke and of overt cardiac disease. This association was significant for both SBI and WMHV, and was independent of confounders, cardiovascular risk factors, and LVEF.
The mechanism underlying the association between a lower GLS and subclinical brain disease is not known. It is important to note that the vast majority (93.4%) of the study participants had a normal LVEF, making cerebral hypoperfusion, one of the proposed etiologic mechanisms for white matter lesions,31,32 an unlikely contributor to our findings. In this respect, we confirm the findings of the Framingham Heart Study, which showed no association between LVEF and WMHV in a large sample of subjects free from clinical stroke and with largely normal LVEF.21 GLS, however, is an indicator of myocardial deformation that has a different meaning and behaves differently from LVEF, especially in the early subclinical stages of LV dysfunction.29 GLS is mainly a measure of the contraction of the longitudinally oriented myocardial fibers, that are mostly located in the LV subendocardium. Because the LV subendocardium is especially vulnerable to hypoperfusion and hemodynamic overload,33 GLS can be a sensitive maker of LV ischemia.34 In previous studies, a lower GLS has been shown to be associated with several traditional cardiovascular risk factors such as hypertension, LV hypertrophy, and diabetes mellitus.29,35,36 Furthermore, in a previous study we demonstrated that GLS, but not LVEF, was reduced in subjects with increased arterial stiffness.37 The association of GLS with the aforementioned risk factors and with increased arterial stiffness, in otherwise asymptomatic subjects, may suggest GLS as an indicator of subclinical atherosclerosis. Consistent with this hypothesis, a study in asymptomatic diabetic patients with normal LVEF showed that a reduced GLS was associated with higher coronary artery calcium score, a direct marker of coronary atherosclerosis.38 This association might therefore provide a pathophysiological explanation for the association between GLS and subclinical brain disease shown in the present study. Indeed, several studies showed that SBI and WMHV are associated with atherosclerotic and cardiovascular risk factors, such as hypertension, LV hypertrophy, diabetes mellitus, cigarette smoking, hyperhomocysteinemia, carotid plaque, and intima-media thickness.2,6,9,39,40 The fact that the association between GLS and subclinical brain disease persisted after adjusting for many known risk factors for atherosclerotic disease may be compatible with the concept that, once atherosclerosis has developed, adjusting for these factors may no longer affect the risk estimates, or not as much. Furthermore, it is also possible that other unmeasured factors may be involved in mediating the observed associations.
Although in patients with overt heart failure the presence of SBI has been hypothesized to be, in part, of thromboembolic origin18 (because of the low cardiac output favoring blood stasis in the cardiac chambers), in the present study population, this does not appear to be a likely cause, given the largely normal LVEF, the exclusion of participants with history of coronary artery disease, and the absence of LV thrombus in all study participants. Cardioembolism from paroxysmal atrial arrhythmias can theoretically be another mechanism for SBI occurrence. Although we excluded participants with a history and ECG evidence of atrial arrhythmias, the presence of undocumented episodes of paroxysmal atrial fibrillation that may have contributed to our findings in participants with lower GLS cannot be excluded. It is known that patients with isolated paroxysmal atrial fibrillation but normal LVEF may have lower GLS in comparison with healthy controls, and that catheter ablation of the atrial fibrillation restores GLS to normal levels.41 Additionally, we have previously demonstrated that a lower GLS is associated with reduced left atrial reservoir function,28 which in turn is associated with the development of both atrial arrhythmias and subclinical brain disease.42,43 Although cardioembolism may be a theoretically possible link between lower GLS and SBI, it is unlikely that it played a significant role in explaining our findings. In fact, the number of cortical brain infarcts, generally considered of more likely embolic origin, in our study population was very low.
Our study has potential clinical implications. For the first time, we show that an echocardiographic parameter of LV systolic function can provide useful information about the risk of subclinical brain disease. We found that an abnormal GLS was associated with an >3-fold increase in risk of having a SBI in multivariate analysis, and, importantly, this association was independent of LVEF. The negative prognostic value of a reduced LVEF has been well documented in patients with heart failure, in patients with dilated cardiomyopathy, and in patients surviving myocardial infarctions.16,44,45 It is also known that lower LVEF is associated with subclinical brain disease in the context of heart failure18,19; however, probably because of our study sample’s mostly normal LVEF values, LVEF did not provide useful information on stroke risk stratification, nor did it show a significant interaction with GLS on the risk of subclinical brain disease. GLS, on the other hand, may detect the rearrangement in LV mechanics described in the early stages of LV remodeling, when a reduction in longitudinal shortening can be compensated by either an increase in circumferential strain or the development of myocardial hypertrophy, both resulting in a preserved LVEF46,47; hence, the use of speckle-tracking echocardiography might be of help in the early identification of subjects with subclinical LV dysfunction and who might also be at higher risk of developing cerebrovascular disease. Whether more aggressive treatment strategies and risk factor control may reduce the future incidence of clinical stroke and cognitive decline in those individuals is a hypothesis that deserves further investigation.
Strengths and Limitations
The main strengths of our study are the large number of subjects studied with advanced imaging techniques (speckle-tracking echocardiography and brain MRI), the wide range of cardiovascular risk profiles present in our study population, and the confirmation of our findings in multivariable models. However, our study also has limitations. The study sample included subjects >55 years of age with a large representation of Hispanic ethnicity, which might preclude the generalization of our findings to populations with a different demographic composition. However, because silent brain disease is more commonly found in the older adults, our study cohort was the ideal setting for this study. Furthermore, the cross-sectional design of our study allows us to document associations that do not necessarily imply direct cause–effect relationships. Finally, although we performed multivariate analyses adjusted for many known risk factors for atherosclerosis and brain disease, the possibility that unmeasured confounders might be involved in the observed associations cannot be excluded.
Conclusions
A lower GLS was independently associated with subclinical brain lesions, both SBI and WMHV, in a stroke-free community-based cohort without overt cardiac disease. The traditional assessment of LV systolic function by LVEF was not associated with either SBI or WMHV. GLS can provide additional information on cerebrovascular risk even when LVEF is in the normal range. The early identification of subjects at higher risk for subclinical brain disease may be crucial for intervention aiming at reducing the global burden of clinical stroke and cognitive decline.
Clinical Perspective.
In population studies, the prevalence of silent brain infarcts has been estimated from 7% to 28%, depending on the age of the studied population. Cerebral white matter hyperintensity volume has also been described in asymptomatic participants in population studies. Both silent brain infarct and white matter hyperintensity volume have been associated with future incidence of stroke, cognitive impairment, and dementia. We conducted a study in a stroke-free community-based cohort without overt cardiac disease from the Cardiac Abnormalities and Brain Lesions (CABL) study to assess the relationship between left ventricular (LV) systolic function measured by speckle-tracking global longitudinal strain and subclinical brain disease. For the first time, we showed that an abnormal global longitudinal strain was associated with an >3-fold increase in risk of having a silent brain infarct and a higher white matter hyperintensity volumes, and these associations were independent of LV ejection fraction and other confounders. The traditional assessment of LV systolic function by LV ejection fraction was not associated with subclinical cerebrovascular disease. Our findings suggest that measuring global longitudinal strain, even in a population without overt cardiovascular disease, may allow early identification of subjects with subclinical LV dysfunction who might also be at higher risk of developing cerebrovascular disease. Whether targeted treatment strategies and risk factor control may reduce the future incidence of clinical stroke and cognitive decline in those individuals needs further study.
Acknowledgments
We thank Janet De Rosa, MPH (project manager), Rafi Cabral, MD, Michele Alegre, RDCS, and Palma Gervasi-Franklin (collection and management of the data).
Sources of Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (grant R01 NS36286 to Dr Di Tullio and R37 NS29993 to Drs Sacco and Elkind).
Footnotes
Disclosures
None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, Sacco RL. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70:425–430. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, Niessen WJ, Breteler MM, van der Lugt A. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 4.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2002;33:21–25. doi: 10.1161/hs0102.101629. [DOI] [PubMed] [Google Scholar]
- 5.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Howard G, Wagenknecht LE, Cai J, Cooper L, Kraut MA, Toole JF. Cigarette smoking and other risk factors for silent cerebral infarction in the general population. Stroke. 1998;29:913–917. doi: 10.1161/01.str.29.5.913. [DOI] [PubMed] [Google Scholar]
- 7.Ylikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R. White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke. 1995;26:1171–1177. doi: 10.1161/01.str.26.7.1171. [DOI] [PubMed] [Google Scholar]
- 8.Price TR, Manolio TA, Kronmal RA, Kittner SJ, Yue NC, Robbins J, Anton-Culver H, O’Leary DH. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1997;28:1158–1164. doi: 10.1161/01.str.28.6.1158. [DOI] [PubMed] [Google Scholar]
- 9.Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, DeCarli C. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke. 2005;36:1207–1211. doi: 10.1161/01.STR.0000165923.02318.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM Rotterdam Scan Study. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 11.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ, Jr Cardiovascular Health Study Collaborative Research Group. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 12.Mosley TH, Jr, Knopman DS, Catellier DJ, Bryan N, Hutchinson RG, Grothues CA, Folsom AR, Cooper LS, Burke GL, Liao D, Szklo M. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology. 2005;64:2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 13.Bernick C, Kuller L, Dulberg C, Longstreth WT, Jr, Manolio T, Beauchamp N, Price T Cardiovascular Health Study Collaborative Research Group. Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi S, Okada K, Koide H, Bokura H, Yamaguchi S. Subcortical silent brain infarction as a risk factor for clinical stroke. Stroke. 1997;28:1932–1939. doi: 10.1161/01.str.28.10.1932. [DOI] [PubMed] [Google Scholar]
- 15.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 16.Dries DL, Rosenberg YD, Waclawiw MA, Domanski MJ. Ejection fraction and risk of thromboembolic events in patients with systolic dysfunction and sinus rhythm: evidence for gender differences in the studies of left ventricular dysfunction trials. J Am Coll Cardiol. 1997;29:1074–1080. doi: 10.1016/s0735-1097(97)00019-3. [DOI] [PubMed] [Google Scholar]
- 17.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 18.Siachos T, Vanbakel A, Feldman DS, Uber W, Simpson KN, Pereira NL. Silent strokes in patients with heart failure. J Card Fail. 2005;11:485–489. doi: 10.1016/j.cardfail.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Kozdag G, Ciftci E, Vural A, Selekler M, Sahin T, Ural D, Kahraman G, Agacdiken A, Demirci A, Komsuoglu S, Komsuoglu B, Fici F. Silent cerebral infarction in patients with dilated cardiomyopathy: echocardiographic correlates. Int J Cardiol. 2006;107:376–381. doi: 10.1016/j.ijcard.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 20.Hays AG, Sacco RL, Rundek T, Sciacca RR, Jin Z, Liu R, Homma S, Di Tullio MR. Left ventricular systolic dysfunction and the risk of ischemic stroke in a multiethnic population. Stroke. 2006;37:1715–1719. doi: 10.1161/01.STR.0000227121.34717.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jefferson AL, Himali JJ, Au R, Seshadri S, Decarli C, O’Donnell CJ, Wolf PA, Manning WJ, Beiser AS, Benjamin EJ. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study) Am J Cardiol. 2011;108:1346–1351. doi: 10.1016/j.amjcard.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 23.Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WH, Thomas JD, Klein AL. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol. 2012;60:2074–2081. doi: 10.1016/j.jacc.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 24.Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden-Albala B, Di Tullio MR, Homma S, Elkind MS, Paik MC. Improving global vascular risk prediction with behavioral and anthropometric factors. The multiethnic NOMAS (Northern Manhattan Cohort Study) J Am Coll Cardiol. 2009;54:2303–2311. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 27.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98:813–820. doi: 10.1136/heartjnl-2011-301388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Relationship of multidirectional myocardial strain with radial thickening and ejection fraction and impact of left ventricular hypertrophy: a study in a community-based cohort. Echocardiography. 2013;30:794–802. doi: 10.1111/echo.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willey JZ, Moon YP, Paik MC, Yoshita M, Decarli C, Sacco RL, Elkind MS, Wright CB. Lower prevalence of silent brain infarcts in the physically active: the Northern Manhattan Study. Neurology. 2011;76:2112–2118. doi: 10.1212/WNL.0b013e31821f4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 32.Yoshizaki K, Adachi K, Kataoka S, Watanabe A, Tabira T, Takahashi K, Wakita H. Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Exp Neurol. 2008;210:585–591. doi: 10.1016/j.expneurol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 34.Choi JO, Cho SW, Song YB, Cho SJ, Song BG, Lee SC, Park SW. Longitudinal 2D strain at rest predicts the presence of left main and three vessel coronary artery disease in patients without regional wall motion abnormality. Eur J Echocardiogr. 2009;10:695–701. doi: 10.1093/ejechocard/jep041. [DOI] [PubMed] [Google Scholar]
- 35.Kang SJ, Lim HS, Choi BJ, Choi SY, Hwang GS, Yoon MH, Tahk SJ, Shin JH. Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase- 1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr. 2008;21:907–911. doi: 10.1016/j.echo.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, Nucifora G, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ, Bax JJ. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2009;104:1398–1401. doi: 10.1016/j.amjcard.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 37.Russo C, Jin Z, Takei Y, Hasegawa T, Koshaka S, Palmieri V, Elkind MS, Homma S, Sacco RL, Di Tullio MR. Arterial wave reflection and subclinical left ventricular systolic dysfunction. J Hypertens. 2011;29:574–582. doi: 10.1097/HJH.0b013e328342ca56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scholte AJ, Nucifora G, Delgado V, Djaberi R, Boogers MJ, Schuijf JD, Kharagjitsingh AV, Jukema JW, van der Wall EE, Kroft LJ, de Roos A, Bax JJ. Subclinical left ventricular dysfunction and coronary atherosclerosis in asymptomatic patients with type 2 diabetes. Eur J Echocardiogr. 2011;12:148–155. doi: 10.1093/ejechocard/jeq165. [DOI] [PubMed] [Google Scholar]
- 39.Manolio TA, Burke GL, O’Leary DH, Evans G, Beauchamp N, Knepper L, Ward B. Relationships of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults: the Cardiovascular Health Study. CHS Collaborative Research Group. Arterioscler Thromb Vasc Biol. 1999;19:356–365. doi: 10.1161/01.atv.19.2.356. [DOI] [PubMed] [Google Scholar]
- 40.Vermeer SE, Den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM Rotterdam Scan Study. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2003;34:392–396. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- 41.Reant P, Lafitte S, Bougteb H, Sacher F, Mignot A, Douard H, Blanc P, Hocini M, Clementy J, Haissaguerre M, Roudaut R, Jais P. Effect of catheter ablation for isolated paroxysmal atrial fibrillation on longitudinal and circumferential left ventricular systolic function. Am J Cardiol. 2009;103:232–237. doi: 10.1016/j.amjcard.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 42.Abhayaratna WP, Fatema K, Barnes ME, Seward JB, Gersh BJ, Bailey KR, Casaclang-Verzosa G, Tsang TS. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. Am J Cardiol. 2008;101:1626–1629. doi: 10.1016/j.amjcard.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 43.Russo C, Jin Z, Liu R, Iwata S, Tugcu A, Yoshita M, Homma S, Elkind MS, Rundek T, Decarli C, Wright CB, Sacco RL, Di Tullio MR. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) study. JACC Cardiovasc Imaging. 2013;6:313–323. doi: 10.1016/j.jcmg.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–742. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 45.Møller JE, Hillis GS, Oh JK, Reeder GS, Gersh BJ, Pellikka PA. Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. Am Heart J. 2006;151:419–425. doi: 10.1016/j.ahj.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 46.Palmon LC, Reichek N, Yeon SB, Clark NR, Brownson D, Hoffman E, Axel L. Intramural myocardial shortening in hypertensive left ventricular hypertrophy with normal pump function. Circulation. 1994;89:122–131. doi: 10.1161/01.cir.89.1.122. [DOI] [PubMed] [Google Scholar]
- 47.Aurigemma GP, Silver KH, Priest MA, Gaasch WH. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol. 1995;26:195–202. doi: 10.1016/0735-1097(95)00153-q. [DOI] [PubMed] [Google Scholar]