Abstract
Objective:
To investigate the effects of vascular risk factors and APOE status on white matter microstructure, and subsequent cognitive decline among older people.
Methods:
This study included 241 participants (age 60 years and older) from the population-based Swedish National Study on Aging and Care in Kungsholmen in central Stockholm, Sweden, who were free of dementia and stroke at baseline (2001–2004). We collected data through interviews, clinical examinations, and laboratory tests. We measured fractional anisotropy (FA) and mean diffusivity (MD) on diffusion tensor imaging, and estimated volume of white matter hyperintensities using automatic segmentation. We assessed global cognitive function with the Mini-Mental State Examination at baseline and at 3- and/or 6-year follow-up. We analyzed the data using multivariate linear regression and linear mixed models.
Results:
Heavy alcohol consumption, hypertension, and diabetes were significantly associated with lower FA or higher MD (p < 0.05). When aggregating heavy alcohol consumption, hypertension, and diabetes together with current smoking, having an increasing number of these 4 factors concurrently was associated with decreasing FA and increasing MD (ptrend < 0.01), independent of white matter hyperintensities. Vascular risk factors and APOE ε4 allele interacted to negatively affect white matter microstructure; having multiple (≥2) vascular factors was particularly detrimental to white matter integrity among APOE ε4 carriers. Lower tertile of FA and upper tertile of MD were significantly associated with faster Mini-Mental State Examination decline.
Conclusions:
Vascular risk factors are associated with reduced white matter integrity among older adults, which subsequently predicted faster cognitive decline. The detrimental effects of vascular risk factors on white matter microstructure were exacerbated among APOE ε4 carriers.
Population-based studies have linked some individual vascular risk factors (e.g., hypertension and diabetes) to reduced microstructural white matter integrity.1–3 However, few studies have explored the relationship of clustering vascular risk factors to white matter microstructure. This is important because vascular factors often occur concurrently in older people. Moreover, as white matter hyperintensities (WMH) and reduced white matter microstructure may share a common ischemic origin, it is of interest to explore whether associations between vascular risk factors and lower microstructural white matter integrity are independent of WMH.
Vascular risk factors contribute to cognitive decline in aging, but the mechanisms are poorly understood.4 One possibility is that vascular risk factors may cause cognitive decline by conferring detrimental impact on white matter microstructure. Indeed, population-based studies have reported a cross-sectional association between microstructural white matter deterioration and cognitive deficits,5,6 but evidence from longitudinal studies is scarce. In addition, APOE ε4 allele exacerbates cognitive decline associated with vascular risk factors, possibly by affecting the extent to which vascular factors influence white matter integrity.7–9 However, no previous research has examined the interactive effect of APOE ε4 and vascular factors on white matter microstructure.
We therefore hypothesize that vascular risk factors influence cognitive function by reducing microstructural white matter integrity, in which APOE ε4 may reinforce the effect. We tested this hypothesis using data from a population-based longitudinal study. Specifically, we investigated whether (1) individual and aggregated vascular risk factors are associated with reduced microstructural white matter integrity, considering demographics, APOE genotype, and WMH, and (2) reduced white matter integrity is associated with exacerbated cognitive decline.
METHODS
Study population.
The study sample was from the population-based Swedish National Study on Aging and Care in Kungsholmen (SNAC-K), as described in detail elsewhere.10,11 Briefly, the SNAC-K population consisted of an age-stratified random sample of people who were aged 60 years and older, living at home or in institutions in the Kungsholmen district of Stockholm. The sample included 4 younger age groups, with a 6-year interval (60, 66, 72, and 78 years) and 7 older age groups, with a 3-year interval (81, 84, 87, 90, 93, 96, and 99+ years). Of the 4,590 persons who were eligible to participate in SNAC-K, 1,227 declined, and 3,363 (73.3%) undertook examination for SNAC-K at baseline (March 2001 to June 2004). During September 2001 to October 2003, we invited participants who were nondisabled, noninstitutionalized, and without dementia to undertake a structural MRI scan (n = 2,204); 555 participants underwent the scan.11 This study included 263 subjects who had the same diffusion tensor imaging (DTI) sequence,12 and a baseline Mini-Mental State Examination (MMSE) score >24. We performed follow-up examinations at 3 years (2004–2007), 6 years (2007–2010), or both. Figure e-1 on the Neurology® Web site at Neurology.org shows a flowchart of the study.
Standard protocol approvals, registrations, and patient consents.
The Regional Ethical Review Board and the Central Ethical Review Board in Stockholm, Sweden, approved the protocols for data collection at baseline and follow-ups.11 All participants provided written informed consent.
MRI measurements.
Participants underwent MRI scans on a 1.5T system (Philips Intera; Philips Medical Systems, Best, the Netherlands) at baseline.11 We acquired DTI data using a single-shot diffusion-weighted echoplanar imaging sequence with the following parameters: repetition time = 6,838 milliseconds; field of view = 230 × 138 mm2; echo time = 104 milliseconds; 128 × 77 matrix; slice thickness = 5 mm with 1-mm gap; b value = 600 s/mm2.12 We applied a scheme with 6 noncollinear diffusion-weighted gradient directions to determine the diffusion tensor set.12
We analyzed DTI data using an iterative optimization algorithm, as previously reported.12,13 Briefly, we processed the fractional anisotropy (FA) data using tract-based spatial statistics, and mean diffusivity (MD), axial diffusivity, and radial diffusivity based on the processing results of FA images.12–14 We used masks of 7 tracts of interest in each hemisphere to extract mean FA, MD, axial diffusivity, and radial diffusivity from the skeleton image of each individual, including the cingulate gyrus part of cingulum, the portion of the cingulum that extends to the hippocampus, the corticospinal tract, the forceps major, the forceps minor, the inferior fronto-occipital fasciculus, and the superior longitudinal fasciculus.12–14 These are major tracts that can be assessed with high reliability.12,13
We first estimated global WMH volume using the Lesion Segmentation Toolbox in the Statistical Parametric Mapping 8 software. Then, we visually scrutinized and manually corrected the volume for greater volumetric precision in MRIcroN.11 The correlation coefficient of repeated assessments of WMH volume for 10 randomly selected images after 1 month was 0.99. We corrected WMH volume using total intracranial volume,4 and then, we made a log-transformation because of skewed distribution.
Baseline data collection.
At baseline, we collected data on age, sex, education, lifestyles (e.g., smoking, alcohol consumption), medical history (e.g., hypertension, diabetes), and current use of medications (e.g., antihypertensive drugs) through interviews by nurses and physicians.10,11 We categorized educational level into elementary school, high school, or university.10
We recorded smoking status as never, former, or current smoking.11 We assessed and classified alcohol consumption into no or occasional, light to moderate, or heavy drinking.11 We categorized physical activity into inactivity or health-/fitness-enhancing activities.10 We defined obesity as a body mass index ≥30 kg/m2,11 hypertension as blood pressure ≥140/90 mm Hg or current use of antihypertensive agents,15 diabetes as having a self-reported history of diabetes, records in the inpatient register, use of hypoglycemic agents, or hemoglobin A1c ≥6.5%,16,17 and high cholesterol as nonfasting total cholesterol ≥6.22 mmol/L or use of cholesterol-lowering agents, and borderline high cholesterol as total cholesterol 5.18 to 6.21 mmol/L and no use of cholesterol-lowering agents.18 We categorized the APOE gene as carriers or noncarriers of the ε4 allele.
Assessment of global cognitive function at baseline and follow-ups.
We assessed global cognitive functioning with the MMSE at baseline (2001–2004) for all participants, at 3-year (2004–2007) and 6-year (2007–2010) follow-ups for participants aged 78 years and older, and at 6-year follow-up (2007–2010) for those aged 60 to 72 years at baseline (figure e-1).
Statistical analysis.
We compared characteristics of participants by APOE ε4 status using t tests for continuous variables with normal distribution and χ2 tests for categorical variables. We used confirmatory factor analysis to extract scores for global mean FA and MD, respectively, from the 7 regions of interest in both hemispheres.12,19 The FA and MD factor scores were multiplied by 100 in the regression analyses. We performed multivariate linear regressions to estimate β coefficients and 95% confidence interval of FA and MD scores associated with APOE ε4 allele, and with individual and aggregated vascular factors. We assessed the clustering of vascular risk factors by counting those factors that were associated with lower FA, higher MD, or both in multivariate linear regression models. Here, we adopted a liberal statistical threshold (p < 0.10) for inclusion of variables in order to not miss any potential risk factors for reduced white matter integrity. In the initial analysis, we controlled for age, sex, education, APOE ε4, and all examined vascular factors. In further analysis, we also controlled for WMH volume to explore whether the relationship of vascular risk factors to FA and MD was dependent on WMH load. We tested multiplicative interactions by simultaneously including the 2 independent variables and their cross-product term in the same model. We used linear mixed-effect models to estimate the associations of global FA and MD scores to changes in MMSE over the follow-up periods, taking into account the effect of random intercept and random slope.20 We included FA/MD, time (i.e., follow-up time in years from the date of baseline survey to the date of follow-up), covariates, and an interaction term of FA/MD × time. The coefficient for time represents the average annual rate of change in MMSE score, and the coefficient for the interaction term estimates the difference in average annual rate of MMSE change associated with the difference in FA/MD score. Finally, in additional analyses, we assessed the associations of axial diffusivity and radial diffusivity with vascular risk factors and MMSE decline. SAS 9.3 (SAS Institute Inc., Cary, NC) and Stata 12.0 (StataCorp 2011; StataCorp LP, College Station, TX) for Windows were used for all statistical analyses.
RESULTS
Compared with the remainder of the SNAC-K sample (n = 3,122), the analytical sample was younger (mean age 72.0 vs 74.5 years, p < 0.01) and had achieved a higher educational level (for university, 38.6% vs 32.3%, p = 0.02), but did not differ in sex distribution (p = 0.54).
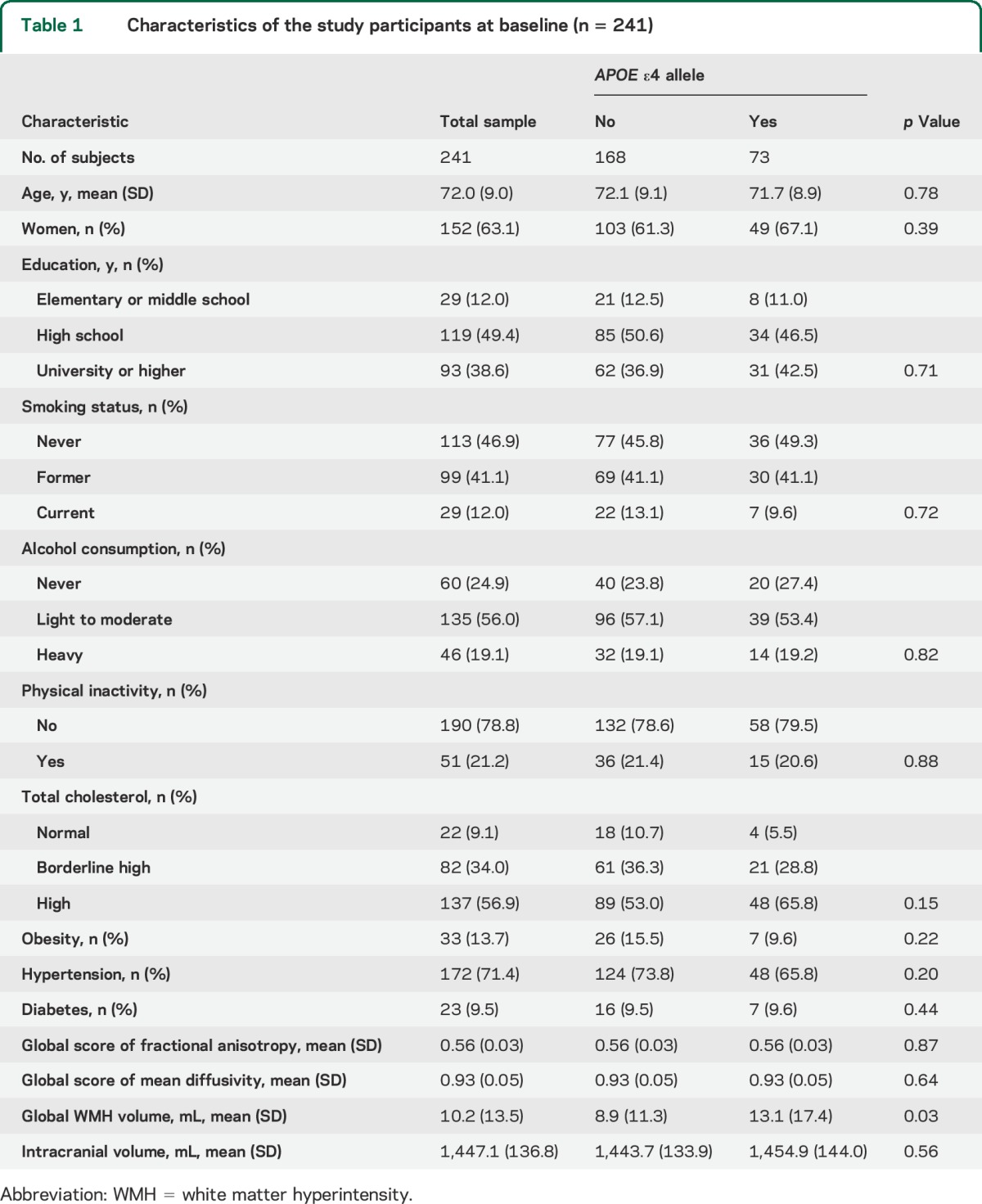
APOE ε4 carriers had larger WMH volume than noncarriers (p = 0.03), but carriers and noncarriers did not differ in mean age, FA and MD, total brain tissue volume, or in the distribution of sex, education, smoking, alcohol consumption, physical inactivity, obesity, hypertension, high cholesterol, or diabetes (all p > 0.05) (table 1).
Table 1.
Characteristics of the study participants at baseline (n = 241)
The proportions of common variance of regional DTI measurements accounted for by the global latent factor were 63.7% for FA and 68.6% for MD. In the multivariate model that included age, sex, education, APOE ε4, and all targeted vascular factors, heavy alcohol consumption and hypertension were significantly associated with lower FA, whereas current smoking was associated with lower FA, albeit nonsignificantly (table 2, model 1). When further controlling for WMH volume, the associations of FA with current smoking and heavy alcohol drinking were slightly attenuated, whereas the association with hypertension was substantially reduced and became nonsignificant (table 2, model 2). Diabetes was not significantly associated with FA.
Table 2.
Associations of global scores of fractional anisotropy and mean diffusivity with vascular risk factors and APOE ε4 status
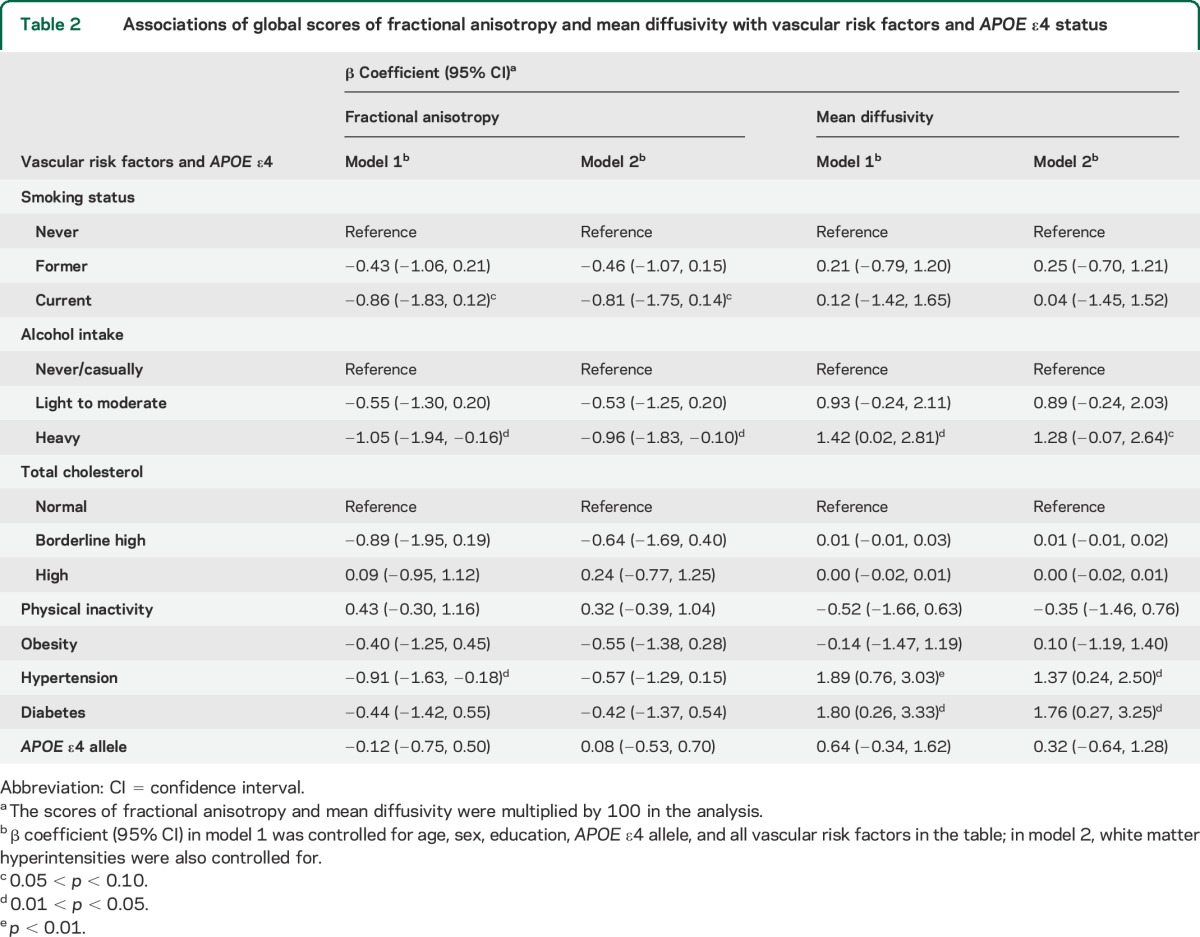
Heavy alcohol drinking, hypertension, and diabetes were significantly associated with higher MD (table 2, model 1); after further controlling for WMH volume, these associations remained significant for hypertension and diabetes, and there was a tendency toward significance for the association with heavy alcohol consumption (table 2, model 2). Smoking was not associated with MD. Physical inactivity, high cholesterol, obesity, and APOE status were not associated with FA or MD.
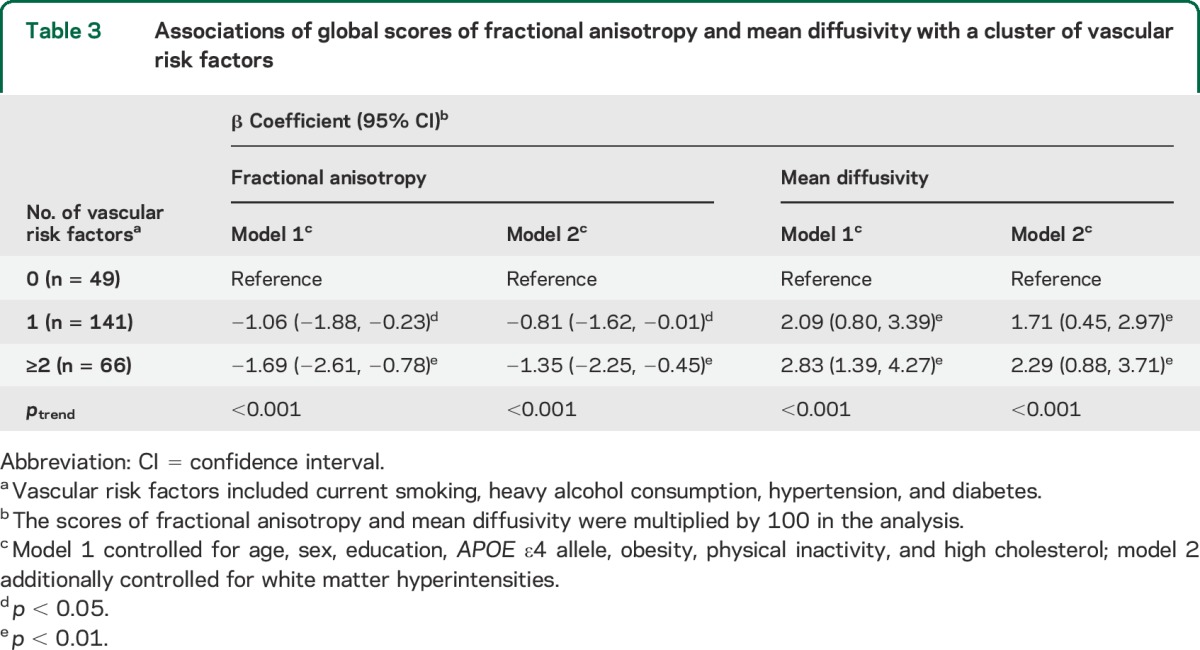
When aggregating current smoking, heavy alcohol consumption, hypertension, and diabetes, having 1 and having 2 or more of these factors, in comparison with having none, were significantly associated with lower FA and higher MD, even after controlling for multiple factors including WMH volume (ptrend < 0.01) (table 3, models 1 and 2).
Table 3.
Associations of global scores of fractional anisotropy and mean diffusivity with a cluster of vascular risk factors
There was a significant interaction between APOE ε4 and the presence of multiple (≥2) vascular factors for MD (pinteraction = 0.02), and the p value of this interaction was 0.07 for FA. Further analysis stratified by APOE ε4 indicated that, among ε4 carriers, having 1 of these 4 vascular factors, especially having multiple vascular factors, was significantly associated with lower FA and higher MD, even in the fully adjusted model (figure 1, model 2). By contrast, among ε4 noncarriers, when further controlling for WMH volume, the associations of having multiple vascular factors with FA or MD were reduced and became nonsignificant.
Figure 1. β Coefficient (95% CI) of FA (A) and MD (B) related to a cluster of vascular risk factors by APOE ε4 status.
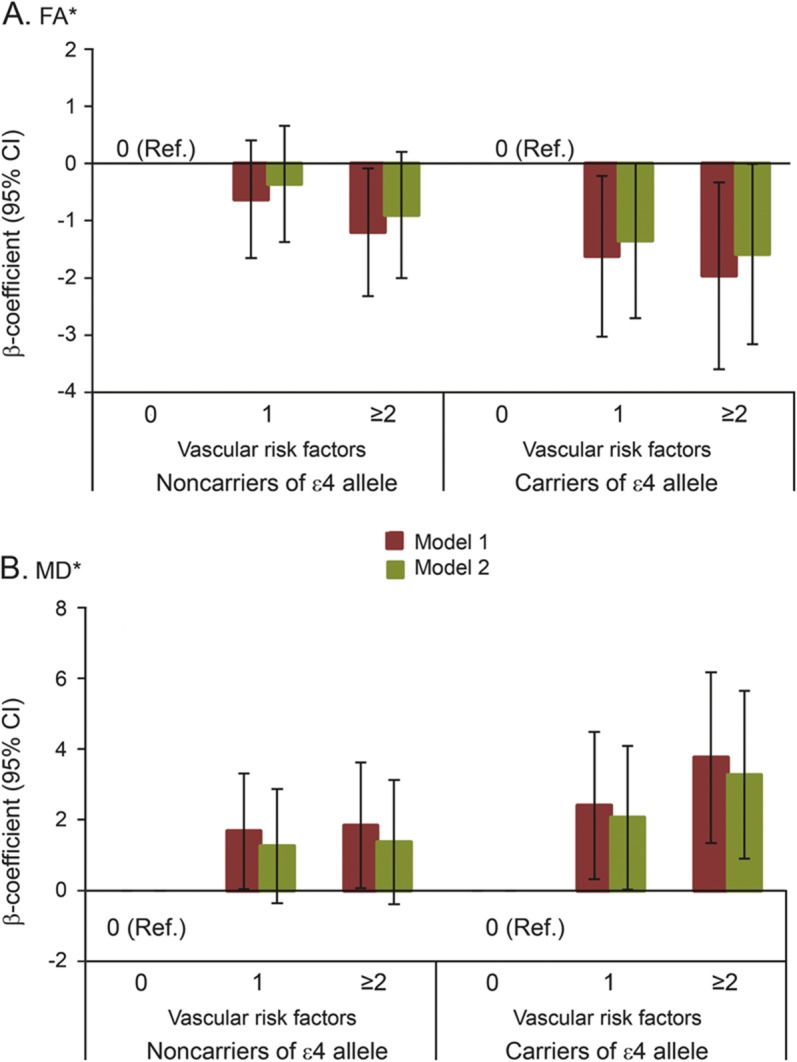
*The scores of FA and MD were multiplied by 100 in the analysis. Vascular risk factors included current smoking, heavy alcohol consumption, hypertension, and diabetes. Model 1 was adjusted for age, sex, education, obesity, high cholesterol, and physical inactivity; model 2 was additionally adjusted for white matter hyperintensities. CI = confidence interval; FA = fractional anisotropy; MD = mean diffusivity; Ref. = reference.
The mean follow-up period was 4.98 years (range, 2.25–6.88 years). Figure 2 shows the predictive trajectory of MMSE score by baseline tertiles of FA and MD, adjusting for demographics, APOE ε4, and WMH volume. For FA, compared with the bottom tertile, the multiadjusted β coefficients for the annual rate of change in MMSE in relation to the median and top tertiles were 0.11 (95% confidence interval: −0.07, 0.29) and 0.29 (0.10, 0.47), respectively (ptrend < 0.01). Likewise, the multiadjusted β coefficients of the annual rate of change in MMSE associated with the median and top tertiles of MD score relative to the bottom tertile were −0.17 (−0.35, 0.00) and −0.40 (−0.57, −0.22), respectively (ptrend < 0.01). When we analyzed FA and MD as continuous variables, the multiadjusted β coefficients of annual change in MMSE score over time were 5.21 (2.58, 7.85) for FA and −3.94 (−5.46, −2.43) for MD.
Figure 2. Adjusted means for MMSE score over a 7-year period by tertiles of FA (A) and MD (B) at baseline.

The means were adjusted for age, sex, education, white matter hyperintensities, and APOE ε4 status. FA = fractional anisotropy; MD = mean diffusivity; MMSE = Mini-Mental State Examination.
Additional analyses.
We further investigated the association of vascular risk factors with other nonfractional measures of diffusivity (i.e., axial and radial diffusivity), and subsequent cognitive decline. Diabetes was significantly associated with higher axial diffusivity, while there was a tendency toward a significant association of hypertension with increased axial diffusivity, even in the model that included WMH volume (table e-1). Similarly, heavy alcohol drinking and hypertension were significantly associated with higher radial diffusivity, while there was a tendency toward a significant association between diabetes and increased radial diffusivity. When aggregating hypertension, diabetes, current smoking, and heavy alcohol consumption, there was a significant linear trend of association, such that having more of these factors was significantly associated with higher axial diffusivity and higher radial diffusivity (ptrend < 0.05), although the strength of associations was reduced and the linear trend became nonsignificant for axial diffusivity (ptrend = 0.08) when additionally adjusting for WMH (table e-1). Compared with the bottom tertile, the top tertiles of axial diffusivity and radial diffusivity were significantly associated with faster MMSE decline: the multiadjusted β coefficients were −0.37 (−0.55, −0.19) and −0.41 (−0.58, −0.23), respectively (figure e-2).
DISCUSSION
The main findings of this community-based longitudinal study of older Swedish adults were as follows: (1) current smoking, heavy alcohol consumption, hypertension, and diabetes, especially when occurring concurrently, were associated with reduced microstructural white matter integrity, independent of WMH; (2) the association between aggregated vascular risk factors and lower white matter integrity was most pronounced in APOE ε4 carriers; and (3) reduced white matter integrity was linked to exacerbated global cognitive decline.
Consistent with our findings, other population-based studies of middle-aged and older adults have reported associations of hypertension, diabetes, and current smoking with lower white matter integrity.2,3,21 Our data extend past research, showing that current smoking, hypertension, and diabetes were associated with reduced white matter integrity, even after adjusting for WMH. In addition, we found that heavy alcohol consumption was associated with reduced white matter integrity. Moreover, the detrimental effects of multiple vascular risk factors on white matter microstructure appeared to be cumulative. In additional analyses, we observed that these vascular risk factors were also associated with increased axial and radial diffusivity, which reinforced the link between vascular risk factors and white matter integrity. Altogether, our data revealed that vascular risk factors, especially when occurring concurrently, were associated with a decrease in FA and an increase in MD, radial diffusivity, and axial diffusivity. Based on a previous study,22 the observed pattern of diffusivity measures in our study might indicate an age-related chronic white matter degeneration, such as loss of myelin and axons. We additionally found that this pattern could be a function of vascular risk factors. This supports the notion that interventions targeting multiple modifiable vascular risk factors may be particularly effective in slowing the process of white matter deterioration in aging, which in turn may postpone the onset of cognitive dysfunction and dementia.
APOE ε4 allele has been associated with reduced integrity of microstructural white matter in specific brain regions, but few studies investigated the association between APOE status and global white matter integrity.23 A recent study of people aged 55 years and older found no effect of APOE ε4 allele on global microstructural white matter integrity,24 consistent with our finding. However, our study revealed an interactive effect between APOE ε4 and vascular risk factors on the DTI indices, suggesting that APOE ε4 may exacerbate the deleterious effect of vascular factors on chronic white matter degeneration.
A hospital-based study of patients with mild cognitive impairment suggested that deficits in microstructural white matter integrity predicted cognitive decline.25 Our population-based study revealed that reduced microstructural white matter integrity was also predictive of subsequent cognitive decline, independent of WMH load. Although previous research has suggested that reduced microstructural white matter integrity in aging is largely explained by WMH,26 our data indicated that the association between reduced white matter integrity and cognitive decline could not be accounted for by global WMH load. This may reflect potential effects of brain lesions that are not visible on conventional MRI, such as microinfarcts.5,27 That said, the link between reduced white matter integrity and cognitive decline suggests that DTI-derived white matter indices may be more sensitive in detecting subtle cognitive changes than WMH. This might be particularly true in population-based studies targeting older adults from the general community such as SNAC-K, where most participants exhibit WMH.12 Furthermore, this finding is consistent with emerging evidence that microstructural white matter changes may serve as a potential marker for early Alzheimer-related brain changes.28,29
The mechanisms underlying the association between vascular risk factors and white matter damage are not fully understood. It is possible that vascular dysautoregulation related to vascular factors such as smoking, hypertension, and diabetes can cause transient reductions in blood flow to white matter watershed areas of vascular supply, which in turn results in transient hyporemia, hypoxemia, and subtle myelin injury.22,30,31 We found an interaction between APOE ε4 and vascular risk factors that affected white matter microstructure. ApoE has a major role in transporting lipid components that contribute to building up the myelin sheath. Vascular risk factors, such as long-lasting hypertension, may lead to ischemia in white matter tissue,32 and the APOE ε4 allele is associated with impaired response to cerebral damage.33 Thus, reduced white matter integrity caused by vascular risk factors is likely to be more severe among APOE ε4 carriers than in noncarriers.
Strengths of this study include a relatively large sample of community-dwelling older adults with data on extensive vascular factors, DTI indices, and WMH, as well as a longitudinal assessment of cognitive performance. However, there are limitations to note. First, the study sample was healthier than the general older population, which may lead to weaker associations among vascular factors, DTI indices, and cognitive function. Second, the suboptimal quality of the images may have resulted in partial-volume effects (gray/white mixture) for large and anisotropic voxels, which could not be completely accounted for by tract-based spatial statistics.12 In addition, lack of free-water elimination may cause measurement bias in our study.34 Finally, although the global factor scores for FA and MD accounted for more than 60% of the common variance of regional DTI measurements, some specific associations with regional tracts that are related to vascular risk factors, APOE status, or cognitive decline may still be missed.
This population-based DTI study provides evidence suggesting that current smoking, heavy alcohol consumption, hypertension, and diabetes are associated with reduced white matter integrity in old age, independent of WMH load. The effects were exacerbated by the aggregation of vascular risk factors and further reinforced by the APOE ε4 allele. Deficits in white matter integrity were linked to global cognitive decline. These results suggest that both vascular mechanisms and genetic susceptibility are involved in microstructural white matter degradation and cognitive decline in aging.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful to the SNAC-K participants and to the colleagues in the SNAC-K group for their collaboration in data collection and management. In addition, the authors are grateful to Emerald Heiland for further checking the English in the final version of the manuscript.
GLOSSARY
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- MD
mean diffusivity
- MMSE
Mini-Mental State Examination
- SNAC-K
Swedish National Study on Aging and Care in Kungsholmen
- WMH
white matter hyperintensity
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Study concept and design: R. Wang, L. Fratiglioni, and C. Qiu. Funding: L. Fratiglioni, L. Bäckman, and C. Qiu. Data acquisition: L. Fratiglioni, M. Lövdén, E.J. Laukka, G. Kalpouzos, A. Salami, L. Keller, and C. Graff. Data analysis: R. Wang. Interpretation of data: R. Wang, L. Fratiglioni, E.J. Laukka, M. Lövdén, G. Kalpouzos, L. Keller, C. Graff, A. Salami, L. Bäckman, and C. Qiu. Drafting of the manuscript: R. Wang and C. Qiu. Critical revision of the manuscript: all the authors. R. Wang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
STUDY FUNDING
SNAC-K is financially supported by the Swedish Ministry of Health and Social Affairs, the participating County Councils and Municipalities, and the Swedish Research Council. This work was further supported by grants from the Swedish Research Council, Swedish Council for Working Life and Social Research, and Karolinska Institutet (KID-funding). Lars Bäckman was also supported by an Alexander von Humboldt Research Award and by a donation from the af Jochnick Foundation.
DISCLOSURE
R. Wang is supported in part by KID-funding from Karolinska Institutet. L. Fratiglioni, E. Laukka, M. Lövdén, and G. Kalpouzos report no disclosures relevant to the manuscript. L. Keller received financial support from Emil and Wera Cornells Foundation. C. Graff and A. Salami report no disclosures relevant to the manuscript. L. Bäckman was supported by an Alexander von Humboldt Research Award and by a donation from the af Jochnick Foundation. C. Qiu received grants from the Swedish Research Council, Swedish Research Council for Health, Working Life and Welfare, and Karolinska Institutet (KID-funding). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res 2009;1297:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgmans S, van Boxtel MP, Gronenschild EH, et al. Multiple indicators of age-related differences in cerebral white matter and the modifying effects of hypertension. Neuroimage 2010;49:2083–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falvey CM, Rosano C, Simonsick EM, et al. Macro- and microstructural magnetic resonance imaging indices associated with diabetes among community-dwelling older adults. Diabetes Care 2013;36:677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu C, Sigurdsson S, Zhang Q, et al. Diabetes, markers of brain pathology and cognitive function: the Age, Gene/Environment Susceptibility–Reykjavik Study. Ann Neurol 2014;75:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernooij MW, Ikram MA, Vrooman HA, et al. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry 2009;66:545–553. [DOI] [PubMed] [Google Scholar]
- 6.Charlton RA, Barrick TR, McIntyre DJ, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology 2006;66:217–222. [DOI] [PubMed] [Google Scholar]
- 7.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes 2002;51:1256–1262. [DOI] [PubMed] [Google Scholar]
- 8.Bangen KJ, Beiser A, Delano-Wood L, et al. APOE genotype modifies the relationship between midlife vascular risk factors and later cognitive decline. J Stroke Cerebrovasc Dis 2013;22:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Sr, Diaz-Arrastia R, Park DC. Risk factors for beta-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurol 2013;70:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rydwik E, Welmer AK, Kareholt I, Angleman S, Fratiglioni L, Wang HX. Adherence to physical exercise recommendations in people over 65: the SNAC-Kungsholmen Study. Eur J Public Health 2013;23:799–804. [DOI] [PubMed] [Google Scholar]
- 11.Wang R, Fratiglioni L, Laveskog A, et al. Do cardiovascular risk factors explain the link between white matter hyperintensities and brain volumes in old age? A population-based study. Eur J Neurol 2014;21:1076–1082. [DOI] [PubMed] [Google Scholar]
- 12.Lövdén M, Laukka EJ, Rieckmann A, et al. The dimensionality of between-person differences in white matter microstructure in old age. Hum Brain Mapp 2013;34:1386–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 14.Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer's disease. Brain 2010;133:529–539. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 16.Goodall I. HbA1c standardisation destination: global IFCC standardisation. How, why, where and when: a tortuous pathway from kit manufacturers, via inter-laboratory lyophilized and whole blood comparisons to designated national comparison schemes. Clin Biochem Rev 2005;26:5–19. [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 18.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–3421. [PubMed] [Google Scholar]
- 19.Penke L, Munoz Maniega S, Murray C, et al. A general factor of brain white matter integrity predicts information processing speed in healthy older people. J Neurosci 2010;30:7569–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology 2014;83:1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gons RA, van Norden AG, de Laat KF, et al. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain 2011;134:2116–2124. [DOI] [PubMed] [Google Scholar]
- 22.Burzynska AZ, Preuschhof C, Bäckman L, et al. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage 2010;49:2104–2112. [DOI] [PubMed] [Google Scholar]
- 23.Kanchibhotla SC, Mather KA, Wen W, Schofield PR, Kwok JB, Sachdev PS. Genetics of ageing-related changes in brain white matter integrity: a review. Ageing Res Rev 2013;12:391–401. [DOI] [PubMed] [Google Scholar]
- 24.Nyberg L, Salami A. The APOE ε4 allele in relation to brain white-matter microstructure in adulthood and aging. Scand J Psychol 2014;55:263–267. [DOI] [PubMed] [Google Scholar]
- 25.Selnes P, Aarsland D, Bjornerud A, et al. Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment. J Alzheimers Dis 2013;33:723–736. [DOI] [PubMed] [Google Scholar]
- 26.Vernooij MW, de Groot M, van der Lugt A, et al. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage 2008;43:470–477. [DOI] [PubMed] [Google Scholar]
- 27.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol 2012;11:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuang L, Sachdev PS, Trollor JN, et al. Microstructural white matter changes in cognitively normal individuals at risk of amnestic MCI. Neurology 2012;79:748–754. [DOI] [PubMed] [Google Scholar]
- 29.Sachdev PS, Zhuang L, Braidy N, Wen W. Is Alzheimer's disease of the white matter? Curr Opin Psychiatry 2013;26:244–251. [DOI] [PubMed] [Google Scholar]
- 30.Maillard P, Seshadri S, Beiser A, et al. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol 2012;11:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yau PL, Hempel R, Tirsi A, Convit A. Cerebral white matter and retinal arterial health in hypertension and type 2 diabetes mellitus. Int J Hypertens 2013;2013:329602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Leeuw FE, Richard F, de Groot JC, et al. Interaction between hypertension, apoE, and cerebral white matter lesions. Stroke 2004;35:1057–1060. [DOI] [PubMed] [Google Scholar]
- 33.Ryan L, Walther K, Bendlin BB, Lue LF, Walker DG, Glisky EL. Age-related differences in white matter integrity and cognitive function are related to APOE status. Neuroimage 2011;54:1565–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med 2009;62:717–730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


