Abstract
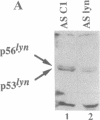
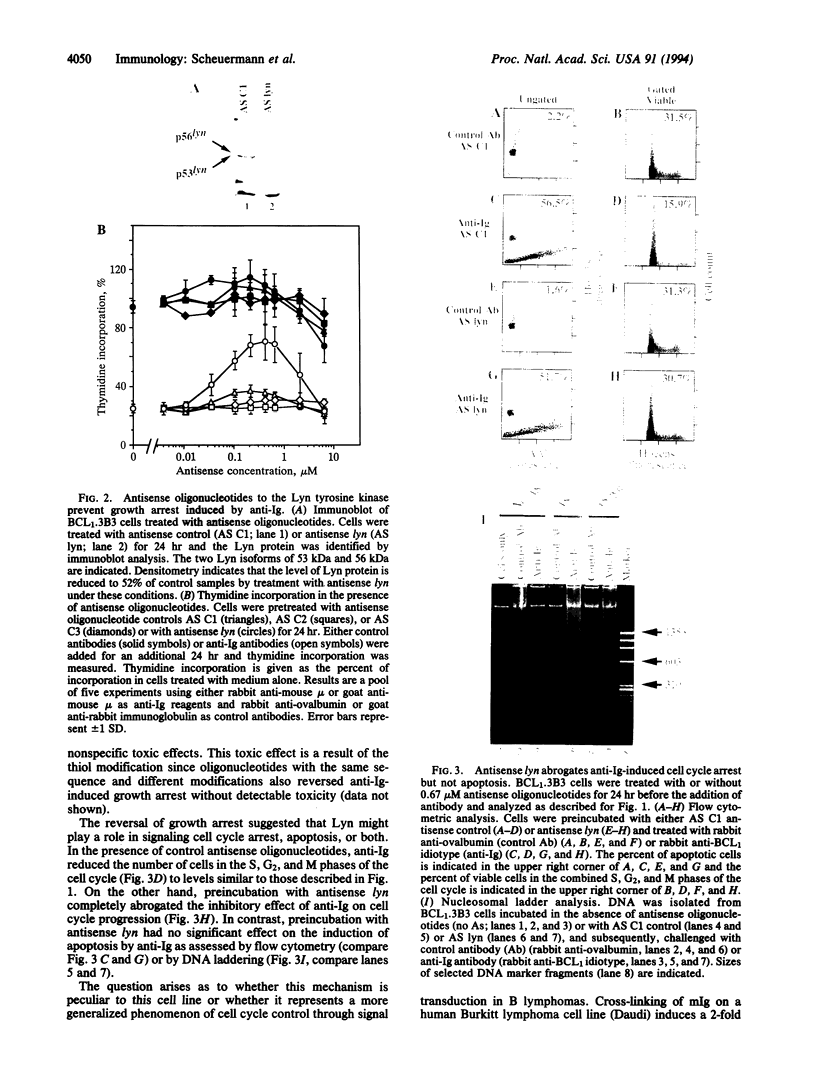
Signal transduction initiated by binding of antibodies to cell surface molecules can have an important impact on the growth of tumor cells. The malignant behavior of the murine lymphoma BCL1 can be suppressed and the neoplastic cells can be induced to enter a dormant state by in vivo ligation of membrane immunoglobulin. Anti-CD19 antibodies can prolong the survival of SCID mice challenged with the human Burkitt lymphoma cell line, Daudi. Here, we show that cross-linking of membrane immunoglobulin on both murine BCL1 and human Daudi cells initiates a cascade of signals leading to the induction of both apoptosis and cell cycle arrest in vitro. Using antisense oligonucleotides, we demonstrate that the immunoglobulin-associated Lyn tyrosine kinase is required for anti-immunoglobulin-mediated cell cycle arrest but is not required for the signal leading to apoptosis. These results define a branch point in the cytosolic signaling pathways mediating cell cycle arrest and apoptosis. In Daudi cells, Lyn is also critical for cell cycle arrest induced by anti-CD19 signaling. Thus, the Lyn tyrosine kinase may be an important mediator of cell cycle arrest in neoplastic B lymphocytes and, perhaps, other cell types.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks K., Yuan D., Uhr J. W., Krammer P. H., Vitetta E. S. Lymphokine-induced IgM secretion by clones of neoplastic B cells. Nature. 1983 Apr 28;302(5911):825–826. doi: 10.1038/302825a0. [DOI] [PubMed] [Google Scholar]
- Cambier J. C., Campbell K. S. Membrane immunoglobulin and its accomplices: new lessons from an old receptor. FASEB J. 1992 Oct;6(13):3207–3217. doi: 10.1096/fasebj.6.13.1397843. [DOI] [PubMed] [Google Scholar]
- Cooke M. P., Perlmutter R. M. Expression of a novel form of the fyn proto-oncogene in hematopoietic cells. New Biol. 1989 Oct;1(1):66–74. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Bruno S., Del Bino G., Gorczyca W., Hotz M. A., Lassota P., Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13(8):795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Dymecki S. M., Niederhuber J. E., Desiderio S. V. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science. 1990 Jan 19;247(4940):332–336. doi: 10.1126/science.2404338. [DOI] [PubMed] [Google Scholar]
- George A. J., Tutt A. L., Stevenson F. K. Anti-idiotypic mechanisms involved in suppression of a mouse B cell lymphoma, BCL1. J Immunol. 1987 Jan 15;138(2):628–634. [PubMed] [Google Scholar]
- Hekman A., Honselaar A., Vuist W. M., Sein J. J., Rodenhuis S., ten Bokkel Huinink W. W., Somers R., Rümke P., Melief C. J. Initial experience with treatment of human B cell lymphoma with anti-CD19 monoclonal antibody. Cancer Immunol Immunother. 1991;32(6):364–372. doi: 10.1007/BF01741331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. M., Alber G., Weiser P., Reth M. Signalling function of the B-cell antigen receptors. Immunol Rev. 1993 Apr;132:125–146. doi: 10.1111/j.1600-065x.1993.tb00840.x. [DOI] [PubMed] [Google Scholar]
- Krolick K. A., Uhr J. W., Slavin S., Vitetta E. S. In vivo therapy of a murine B cell tumor (BCL1) using antibody-ricin A chain immunotoxins. J Exp Med. 1982 Jun 1;155(6):1797–1809. doi: 10.1084/jem.155.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker T., Lowder J., Cleary M. L., Stewart S., Warnke R., Sklar J., Levy R. Emergence of idiotype variants during treatment of B-cell lymphoma with anti-idiotype antibodies. N Engl J Med. 1985 Jun 27;312(26):1658–1665. doi: 10.1056/NEJM198506273122602. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Cellular and molecular mechanisms of B lymphocyte tolerance. Adv Immunol. 1992;52:283–331. doi: 10.1016/s0065-2776(08)60878-0. [DOI] [PubMed] [Google Scholar]
- Pesando J. M., Bouchard L. S., McMaster B. E. CD19 is functionally and physically associated with surface immunoglobulin. J Exp Med. 1989 Dec 1;170(6):2159–2164. doi: 10.1084/jem.170.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. A., Church J. G. EGF-dependent growth inhibition in MDA-468 human breast cancer cells is characterized by late G1 arrest and altered gene expression. Exp Cell Res. 1991 Jul;195(1):20–26. doi: 10.1016/0014-4827(91)90495-g. [DOI] [PubMed] [Google Scholar]
- Prchal J. T., Prchal J. F. Evolving understanding of the cellular defect in polycythemia vera: implications for its clinical diagnosis and molecular pathophysiology. Blood. 1994 Jan 1;83(1):1–4. [PubMed] [Google Scholar]
- Press O. W., Appelbaum F., Ledbetter J. A., Martin P. J., Zarling J., Kidd P., Thomas E. D. Monoclonal antibody 1F5 (anti-CD20) serotherapy of human B cell lymphomas. Blood. 1987 Feb;69(2):584–591. [PubMed] [Google Scholar]
- Shaw J. P., Kent K., Bird J., Fishback J., Froehler B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucleic Acids Res. 1991 Feb 25;19(4):747–750. doi: 10.1093/nar/19.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Yuan D., Krolick K., Isakson P., Knapp M., Slavin S., Strober S. Characterization of the spontaneous murine B cell leukemia (BCL1). III. Evidence for monoclonality by using an anti-idiotype antibody. J Immunol. 1979 May;122(5):1649–1654. [PubMed] [Google Scholar]
- Yamanashi Y., Fukui Y., Wongsasant B., Kinoshita Y., Ichimori Y., Toyoshima K., Yamamoto T. Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1118–1122. doi: 10.1073/pnas.89.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Fukushige S., Semba K., Sukegawa J., Miyajima N., Matsubara K., Yamamoto T., Toyoshima K. The yes-related cellular gene lyn encodes a possible tyrosine kinase similar to p56lck. Mol Cell Biol. 1987 Jan;7(1):237–243. doi: 10.1128/mcb.7.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Mori S., Yoshida M., Kishimoto T., Inoue K., Yamamoto T., Toyoshima K. Selective expression of a protein-tyrosine kinase, p56lyn, in hematopoietic cells and association with production of human T-cell lymphotropic virus type I. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6538–6542. doi: 10.1073/pnas.86.17.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X. R., Scott D. W. Expression of protein tyrosine kinases in the Ig complex of anti-mu-sensitive and anti-mu-resistant B-cell lymphomas: role of the p55blk kinase in signaling growth arrest and apoptosis. Immunol Rev. 1993 Apr;132:163–186. doi: 10.1111/j.1600-065x.1993.tb00842.x. [DOI] [PubMed] [Google Scholar]
- Yefenof E., Picker L. J., Scheuermann R. H., Tucker T. F., Vitetta E. S., Uhr J. W. Cancer dormancy: isolation and characterization of dormant lymphoma cells. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1829–1833. doi: 10.1073/pnas.90.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T. L., Bolen J. B., Ihle J. N. Hematopoietic cells express two forms of lyn kinase differing by 21 amino acids in the amino terminus. Mol Cell Biol. 1991 May;11(5):2391–2398. doi: 10.1128/mcb.11.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noesel C. J., Lankester A. C., van Schijndel G. M., van Lier R. A. The CR2/CD19 complex on human B cells contains the src-family kinase Lyn. Int Immunol. 1993 Jul;5(7):699–705. doi: 10.1093/intimm/5.7.699. [DOI] [PubMed] [Google Scholar]