Summary
Cells make accurate decisions in the face of molecular noise and environmental fluctuations by relying not only on present pathway activity, but also on their memory of past signaling dynamics. Once a decision is made, cellular transitions are often rapid and switch-like due to positive feedback loops in the regulatory network. While positive feedback loops are good at promoting switch-like transitions, they are not expected to retain information to inform subsequent decisions. However, this expectation is based on our current understanding of network motifs that accounts for temporal, but not spatial dynamics. Here, we show how spatial organization of the feedback-driven yeast G1/S switch enables the transmission of memory of past pheromone exposure across this transition. We expect this to be one of many examples where the exquisite spatial organization of the eukaryotic cell enables previously well-characterized network motifs to perform new and unexpected signal processing functions.
Introduction
Cellular signaling pathways are used to transmit information about the extra- and intra-cellular environment. Specific outputs from such signaling pathways are then used by decision-making networks to determine a proper cellular response. Currently, signaling pathways are most often described as static schematics based on a combination of genetic dependencies and biochemical interactions. While a good first step, such a characterization can neither describe nor predict the pathway dynamics that determine cellular response to time-dependent input signals (Behar et al., 2008; Yosef and Regev, 2011). Indeed, outputs of the regulatory networks controlling proliferation and apoptosis depend on the history of dynamic input signals, not only current levels (Doncic and Skotheim, 2013; Lee et al., 2012; Purvis et al., 2012). This strongly suggests that the ability to retain information from prior states is a key determinant informing cellular decision making.
Signaling dynamics play important roles in many networks regulating switch-like transitions between distinct states. The switch-like nature of transitions often arises from positive feedback loops that quickly increase the activity of key regulatory proteins when triggered by input signals above a specific threshold. Networks containing positive feedback loops frequently give rise to bistability, i.e., for a range of input signals, the output will be one of two possible values depending on the history of the input signal. However, this is a very simple form of history-dependence as all possible time-dependent input signals get mapped onto only two possible outputs, i.e., history-dependence is collapsed onto only a single bit of information. This implies that while positive feedback loops may be good at promoting switch-like transitions, they appear unable to retain more than rudimentary information about signaling pathway history. It is therefore improbable that a positive feedback driven switch can be used to transmit information to inform future cellular decisions. However, this conclusion is based on the current framework for analyzing network motifs such as feedback-loops or feed-forward interactions (Alon, 2007), which accounts for temporal but not spatial dynamics. Thus, while it is well-known that spatial organization plays an important role in signal transduction, we do not currently know how or if the eukaryotic cell’s spatial organization can affect existing motif functions or give rise to entirely new motif functions (Howell et al., 2012; Kholodenko et al., 2010; Santos et al., 2012).
To better understand how spatial organization might affect cellular signal processing, we decided to examine the cell cycle control network responsible for the decision to divide in budding yeast. In yeast, the decision to commit to cell division takes place in late G1, prior to DNA replication at a point called Start (Hartwell et al., 1974). Multiple internal and external signals are integrated to determine when a cell passes Start, beyond which cells no longer respond to mating pheromone (α-factor). Start is a switch-like, irreversible transition that corresponds to the activation of a positive feedback loop of cyclin-dependent kinase (Cdk1) activity (Doncic et al., 2011). Specifically, Cln3-Cdk partially inactivates Whi5, a transcriptional inhibitor of the expression of the G1 cyclins CLN1 and CLN2. The expression of Cln1 and Cln2 complete inactivation of Whi5 by forming a positive feedback loop (Costanzo et al., 2004; de Bruin et al., 2004; Skotheim et al., 2008).
Prior to Start, cells can be arrested by pheromone-dependent activation of the mitogen activated protein kinase (MAPK) mating pathway (Chen and Thorner, 2007). Upon pheromone exposure, the MAPK Fus3 phosphorylates and activates the Cdk inhibitor Far1, which inhibits the G1 cyclins essential for progression through Start (Chang and Herskowitz, 1990; Gartner et al., 1998; Jeoung et al., 1998; Peter et al., 1993; Pope et al., 2014; Tyers and Futcher, 1993). Conversely, post-Start, the G1 cyclins inhibit the mating pathway by targeting the upstream scaffold protein Ste5 as well as Far1 (Garrenton et al., 2009; Henchoz et al., 1997; Peter and Herskowitz, 1994; Strickfaden et al., 2007; Tyers and Futcher, 1993) (Figure S1A). Thus, progression through Start drives an increase in cyclin expression that results in Far1 degradation, whereas pre-Start exposure to pheromone leads to Far1 activation, G1 cyclin inhibition, and G1 arrest (Doncic et al., 2011; McKinney et al., 1993; Pope et al., 2014). In other words, the regulatory network underlying Start is bistable, where a well-defined commitment point separates stable low- and high-Cdk activity states, and only the low-Cdk activity state can be inhibited by MAPK signaling (Doncic et al., 2011).
Although this characterization of Start is accurate for a step input of high pheromone concentration, cells exposed to low or intermediate pheromone concentrations do not arrest permanently, but rather delay progression through G1 (Hao et al., 2008; Malleshaiah et al., 2010; Moore, 1984). This suggests a more complex decision making machinery that balances the benefits of successful mating with the costs of staying arrested and both failing to mate and proliferate. Thus, while the Start network remains bistable, its output changes from a digital response to arrest or not, to an analog computation determining how long to arrest before reentering the cell division cycle. We previously showed that in this analog computation, yeast cells decide to reenter the cell cycle based on their history of exposure to pheromone during an arrest, not just the current pathway activity. Time-dependent pheromone signals are processed by the MAPK pathway using a coherent feedforward motif in which the MAPK Fus3 activates Far1 both by direct phosphorylation and by increasing its expression via the Ste12 transcription factor (Chang and Herskowitz, 1990; Errede and Ammerer, 1989; Gartner et al., 1998) (Figure S1A; red arrows). This architecture allows a robust yet rapidly reversible cellular state. Far1 accumulates to provide a memory so that cells exposed to pheromone for longer durations have more Far1 rendering them more reluctant to reenter the cell cycle. In addition, fast dephosphorylation allow Far1 to be rapidly inactivated so that cells can rapidly reenter the cell cycle if the MAPK signal plummets (Doncic and Skotheim, 2013).
However, while the accumulation of Far1 provides a mechanism to remember the history of pheromone exposure during a single arrest, it does not suggest a mechanism to transmit this information to subsequent generations after cell cycle reentry. This is because the mutual inhibition of Cdk and Far1 activity underlying the bistable Start switch is expected to target all Far1 for degradation once the cell cycle has been reentered. Similarly, the sharp switch at mitotic exit also employs ultra-sensitive protein degradation (Yang and Ferrell, 2013). Protein degradation may be useful to sharpen switches and reset regulatory circuits, but comes at the cost of losing cellular memory. Thus, while bistable regulatory networks are excellent at generating all-or-none transitions, they limit the amount of information that can be propagated across these transitions.
Here, we show how spatial compartmentalization of the bistable G1 control network allows cellular memory to traverse the Start switch. Far1 is split into nuclear and cytoplasmic pools that combat distinct sets of cyclin-Cdk complexes allowing these two compartments of the Far1-Cdk switch to have distinct dynamics. Upon reentering the cell cycle from pheromone arrest, nuclear Far1 is rapidly degraded, while cytoplasmic Far1 is degraded much more slowly so that a substantial pool remains at the beginning of the next division cycle. We show that this inherited pool contributes to cell cycle arrest in the daughter cells so that the mother cells are able to transmit their memory of pheromone exposure to the next generation. This intergenerational memory depends on the anchoring of Far1 to cytoplasmic Cdc24, a regulator of cell polarization. Thus, we demonstrate how compartmentalization of a bistable regulatory circuit enables an entirely new function to be performed by this well-characterized signaling motif. More broadly, our results argue that spatial organization can greatly enhance the function of regulatory motifs and is therefore just as integral to pathway function as network topology and chemical kinetics.
Results
Nuclear Far1 and nuclear Cln2 function in cell cycle commitment
To determine if and how signal information could be propagated across a bistable switch, we examined the network regulating Start, the point of commitment to cell division in budding yeast (Figure 1A). Since cyclin-Cdk complexes phosphorylate Far1 to target it for degradation, we expected that Far1 would be rapidly degraded upon progression through Start.
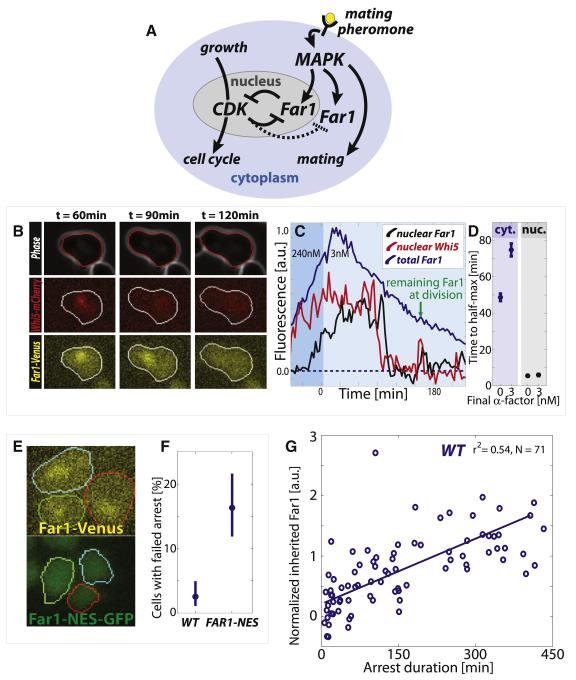
Figure 1. Cytoplasmic Far1 is inherited to provide intergenerational memory across the Start switch.
(A) Schematic of the double-negative feedback (equivalent to positive feedback) network that regulates the switch between cell cycle progression and pheromone arrest. (B) Example images of segmented phase, Whi5-mCherry (red) and Far1-Venus (yellow) channels for cells reentering the cell cycle. Whi5-mCherry is nuclear in arrested cells. (C) Example time series of nuclear Whi5-mCherry, and nuclear and cytoplasmic Far1-Venus that corresponds to the cell shown in (B). Nuclear Far1 is much more rapidly degraded than cytoplasmic Far1. (D) Time from peak to half-maximum for cytoplasmic and nuclear Far1 in cells arrested in 240nM and released into either 3 or 0 nM pheromone. (E) Example FAR1-Venus and FAR1-NES-GFP cells arrested in 240nM for 2h show that the nuclear localization is diminished in the FAR1-NES-GFP cells. (F) A larger fraction of pre-Start FAR1-NES cells fails to arrest when abruptly exposed to 240nM pheromone, where Start is defined as removal of 50% of nuclear Whi5-mCherry. (G) Inherited Far1 in daughter cells is correlated with arrest duration. Error bars in (D) denote standard error of the mean, while error bars in (F) denote 95% confidence intervals from 10,000 bootstrap iterations.
To examine the localization and dynamics of Far1, we used a Far1-Venus fusion protein expressed from the endogenous locus (Figure 1B). This FAR1-Venus strain exhibited the same arrest kinetics as an unlabeled WT strain, and we will subsequently refer to FAR1-Venus strains as WT (Doncic and Skotheim, 2013). Unless specified otherwise, all strains are in a background lacking the Bar1 protease that cleaves mating pheromone (for strain list see Table S1). Cells were arrested in high pheromone (240nM α-factor) and released into pheromone-free medium using a previously described microfluidics-based assay (Doncic et al., 2011). Consistent with previous results (McKinney et al., 1993), Far1 was synthesized during mating arrest and mostly degraded post-Start after release into pheromone-free medium. However, the examination of Far1-Venus using time-lapse microscopy revealed a striking spatial dichotomy in Far1 degradation kinetics. The nuclear pool of Far1 is rapidly degraded in less than 10 minutes approximately 7 minutes after Start, which is defined as when 50% of Whi5 has been exported from the nucleus (Doncic et al., 2011). Nuclear Far1 is degraded at approximately the same time as the Cdk-B-type cyclin inhibitor Sic1, which we previously measured as occurring ~8 minutes after Start (see Figures S1B and S1C for Far1 degradation timing and (Doncic et al., 2011) for Sic1 degradation timing). This implies that Far1 degradation is likely coincident with the appearance of B-type cyclin activity in the nucleus. However, the cytoplasmic pool lingered and reached half-maximum ~50 minutes after Start (Figures 1C and 1D). This observed difference in Far1 degradation kinetics is consistent with previous reports and most likely due to the nuclear F-box protein Cdc4 that mediates Far1 degradation (Blondel et al., 2000). This demonstrates that there are two separate pools of Far1 protein being degraded on very different time scales.
The rapid degradation of nuclear Far1 upon progression through Start suggests that it is primarily this nuclear pool that contributes to the commitment decision in response to exposure to pheromone (Blondel et al., 1999; Blondel et al., 2000). To test this, we added a nuclear export sequence to the endogenous FAR1 allele (FAR1-NES), which greatly reduced the nuclear pool without affecting expression levels (Figure 1E and S1D). We then examined the cellular response to an abrupt increase in pheromone in the framework we previously developed to examine Start (Doncic et al., 2011). When exposed to a step-increase of pheromone, pre-Start FAR1-NES cells were over six times more likely than WT cells to fail to arrest despite not having traversed the Whi5-threshold (16% FAR1-NES vs 2.5% WT, Figure 1F). In addition, we found that nuclear, but not cytoplasmic Cln2 participated in Start (Figures S1E-I). Taken together, these results support a role for nuclear Far1 in Start.
Cytoplasmic Far1 provides intergenerational memory of pheromone exposure
Even though nuclear Far1 was important for Start, most Far1 in arrested cells (~90%), is cytoplasmic and is not degraded rapidly upon cell cycle reentry (Figure S2A-E). In fact, cytoplasmic Far1 is so slowly degraded after cell cycle reentry that appreciable quantities are passed on to subsequent generations (Figures 1C and S2F). This is surprising because once cells reenter the cell cycle, these mother cells are desensitized to that level of pheromone and divide repeatedly without delay (Figure S2G) (Caudron and Barral, 2013; Doncic and Skotheim, 2013; Moore, 1984). To examine the role of inherited Far1 in daughter cells, we briefly arrested cells at high pheromone concentration (240nM) before releasing the cells into an intermediate pheromone concentration (3nM). The time to reach half-maximum Far1 post-Start in 3nM pheromone was ~5 minutes in the nuclear pool and ~75 minutes in the cytoplasmic pool (Figure 1D). Thus, the time to reach cytoplasmic half-maximum post-Start is increased relative to cells reentering in pheromone-free medium. Consequently, daughter cells entering the cell cycle in 3nM pheromone inherited an increased amount of Far1 compared to daughter cells entering the cell cycle in pheromone-free medium (Figure S2H). This finding, that cells cycling in higher pheromone concentrations pass increasing amounts of Far1 on to their daughter cells, led us to hypothesize that inheritance of cytoplasmic Far1 is the molecular basis of an intergenerational memory of pheromone exposure.
To test the intergenerational memory hypothesis, we measured both the amount of inherited Far1 and subsequent G1 duration for daughter cells cycling in 3nM, an intermediate mating pheromone concentration (see methods and Figure S2I). The more Far1 a daughter cell inherited, the longer it delayed progression through G1, supporting the hypothesis that mother cells transmit information about pheromone exposure to their daughters through cytoplasmic Far1 (Figure 1G). We also examined if differential inheritance of MAPK pathway scaffold Ste5, which affects pheromone signaling in a dosage-dependent manner (Thomson et al., 2011), could affect arrest duration, but found no effect (Figure S2J and S2K).
Compartmentalization is supported by a fixed fraction of cytoplasmic Far1
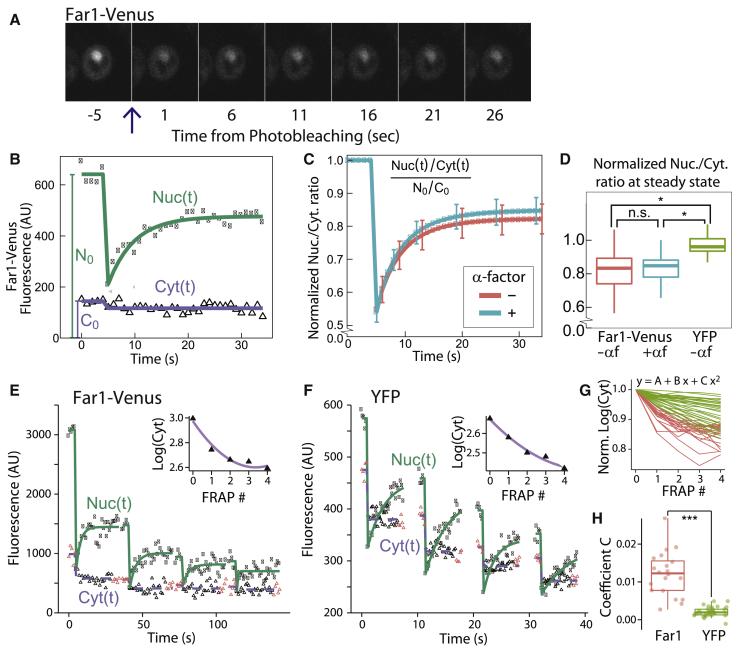
The rapid degradation of the nuclear, but not cytoplasmic pool of Far1 requires a slow exchange between these two pools. Indeed, it would be impossible to maintain Far1 post-Start if Far1 were exchanged rapidly between the two pools as the half-life of nuclear Far1 is ~5 minutes. To investigate this requirement, we photobleached the nucleus of FAR1-Venus cells and measured the recovery of nuclear fluorescence (Figure 2A). Pre-Start cells were identified by examining the localization of Whi5-mKO fusion proteins expressed from the endogenous locus. After photobleaching, a significant recovery of the nuclear fraction on the 10 second time scale was seen. However, the nuclear to cytoplasmic fluorescence ratio did not recover to its initial level and reached a plateau prior to 30 seconds (Figures 2B-D). Note that there is little new protein synthesis or degradation over the time frame of the experiment so that nearly all the recovery is due to protein translocation. The incomplete recovery of the nuclear-to-cytoplasmic ratio of Far1-Venus indicates that there is a pool of Far1 molecules that does not shuttle between the nucleus and the cytoplasm. As a control, we examined recovery of the yellow fluorescent protein YFP expressed from an ACT1 promoter. The YFP nuclear-to-cytoplasmic ratio completely recovered after bleaching, which is consistent with rapid and unencumbered shuttling between nucleus and cytoplasm (Figure 2D). Taken together, we here identify both a rapidly shuttling and a fixed pool of Far1, which supports our model for how compartmentalization is used to generate intergenerational memory.
Figure 2. A pool of Far1 is fixed in the cytoplasm.
(A) Fluorescence images from a typical time course, where the nuclear Far1-Venus was photobleached at t=0. (B) Data and model fit for the nuclear, Nuc(t), and cytoplasmic, Cyt(t), fractions of Far1. N0 and C0 denote the initial nuclear and cytoplasmic fractions. (C) Mean nuclear-to-cytoplasmic ratio of Far1-Venus normalized to its initial value, N0/C0. Bars denote the 95% confidence interval of the mean. We examined pre-Start G1 cells either not exposed to α-factor (red) or exposed to 760nM α-factor (blue). (D) Distribution of the estimated steady-state value of the normalized Nuc/Cyt ratio after photobleaching. Cells expressing Far1-Venus (blue/red) do not recover the initial nuclear-to-cytoplasmic ratio, while cells expressing the fluorescent protein YFP from an integrated ACT1 promoter (green) recover the initial ratio. (E-F) Nuclear and cytoplasmic fluorescence from Far1-Venus or YFP following 4 sequential photobleaching events. Inset shows logarithm base 10 of the mean steady state cytoplasmic fluorescence following the indicated photobleaching event and the associated quadratic fit. Red triangles denote data points used for steady state estimates. (G) Single cell data for cytoplasmic steady state fluorescence after normalization to its value prior to the first bleaching event for Far1-Venus (red) and YFP (green). (H) Distribution of coefficients C for the quadratic term of the quadratic fit. C = 0 indicates a linear relationship between the logarithm of the cytoplasmic fluorescence and the number of photobleaching events, which corresponds to the case with no fixed cytoplasmic pool. C > 0 indicates the presence of a fixed cytoplasmic pool (see supporting material). *denotes p<0.05, ***denotes p<0.001, n.s denotes p>0.05.
To transmit intergenerational memory, there should be a fixed cytoplasmic pool of Far1. While a single FRAP experiment indicates the presence of a fixed Far1 pool, it does not identify its location. To determine if there is fixed Far1 in the cytoplasm, we photobleached the nucleus four times sequentially and measured depletion of cytoplasmic Far1-Venus or YFP (Figures 2E and 2F). In the case of YFP, there is no fixed pool, so that the nuclear-to-cytoplasmic ratio recovers after each photobleaching event. Thus, each bleaching of the nucleus bleaches a constant fraction of the total still fluorescing protein. This leads to a linear relationship between the logarithm of the fluorescence and the number of photobleaching events (see supporting material). A fixed cytoplasmic pool would result in a deviation from this linear fit. To test for a fixed cytoplasmic pool, we fit the normalized cytoplasmic fluorescence to a quadratic equation for each cell (Figure 2G). We find positive quadratic coefficients for Far1-Venus fits indicating the presence of a pool of Far1 that is fixed in the cytoplasm (Figure 2H).
Pheromone exposure is remembered across the entire cell cycle
To better understand intergenerational memory, we sought to investigate the mechanisms responsible for the increased time to half-maximum in 3nM relative to 0nM pheromone (Figure 1D). Such an increase could arise due to either increased Far1 synthesis or decreased Far1 degradation, or both. To test for regulated protein degradation, we expressed a FAR1-Venus fluorescent fusion protein from a galactose-inducible GAL1 promoter. We inactivated Far1 synthesis by switching the carbon source from galactose to glucose and measured Far1 half-life post-Start (Figure 3A). Far1 stability post-Start is only weakly sensitive to pheromone concentration, which suggests that continued synthesis is more likely than increased protein stability to underlie increased inheritance of cytoplasmic Far1 at intermediate pheromone concentrations (Figure 3B, S4A). To test this possibility, we used single molecule fluorescence in situ hybridization (smFISH) (Raj et al., 2008), to measure the amount of FAR1 mRNA transcripts (Figure 3C). Indeed, FAR1 transcription was higher in 3nM compared to 0nM for cells with small and medium sized buds, corresponding to S and G2 cells respectively (Figures 3D). For large budded cells, likely about to divide, the number of FAR1 transcripts was similarly high for both conditions, consistent with previous work showing that FAR1 and other Ste12 transcription factor targets are transcribed at the M/G1 transition, even at 0nM pheromone (Doncic and Skotheim, 2013; McKinney et al., 1993; Oehlen et al., 1996).
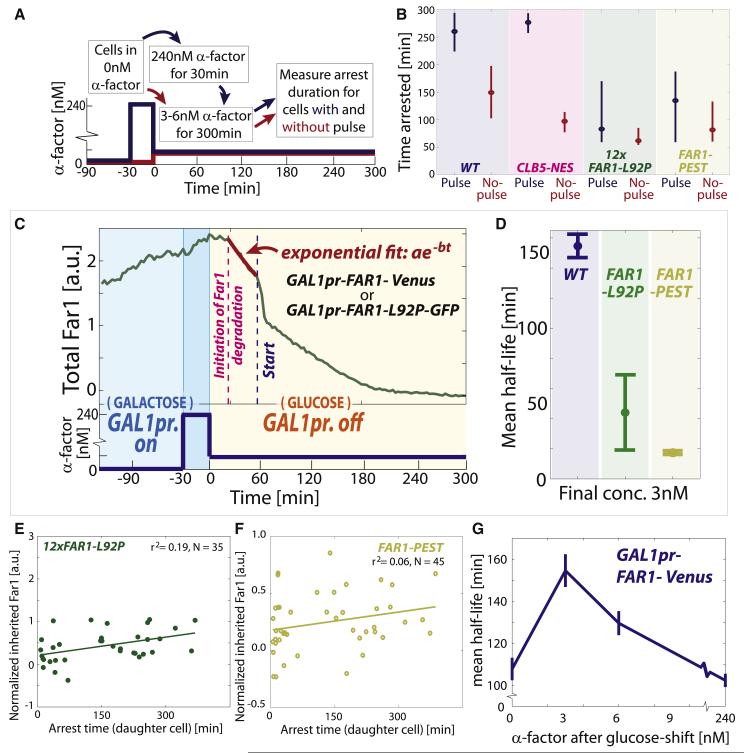
Figure 3. Pheromone exposure post-Start is remembered.
(A) Experiment schematic for measuring stability of Far1 protein post-Start. (B) Post-Start half-life measured after release from pheromone arrest in 240nM to 0, 3, 6 and 240nM. (C) Example of segmented phase image of mother cell body (red) and bud (green) and their corresponding smFISH maximal intensity projections. Each dot represents a single FAR1 mRNA. (D) Mean number of FAR1 mRNA in cells having small, medium and large buds. Error bars denote standard error of the mean. (E) Example segmented phase and Ste5-YFP fluorescence images for a cell arrested in 3nM α-factor. Ste5-YFP localizes to the site of polarized growth. (F) Kymograph of example cell in (E). The amount of Ste5 at the site of polarized growth, whose location was determined using the Viterbi algorithm (Forney, 1973). (G) Ste5-YFP trace of example cell shown in (F) indicating levels before, after, and during arrest. (H) Mean Ste5-YFP intensity at the site of polarized growth for pre- and post-Start cells in 3nM. Membrane fluorescence prior to pheromone addition was background subtracted. p<0.05 for all comparisons. (I-L) Cells were arrested in G1 using pheromone and released synchronously through the cell cycle. 50 minutes after release, after commitment to division, cells were re-exposed to pheromone (see methods). (I) Bud index. (J) (top) Western blot time course with a phospho-specific antibody indicates presence of phosphorylated Fus3 in S/G2/M cells exposed to pheromone; (bottom) Ponceau stained blots are provided as loading controls. (K-L) Fus3-TAP was immunoprecipitated at the 75 minute time point, when nearly all cells were in S/G2/M, for cells in 0, 3 or 240 nM pheromone. (K) Western blot for this IP indicating increasing Fus3 phosphoshifts in 3 and 240 nM pheromone. (L) Fus3 activity on MBP was measured in an in vitro kinase assay using radiolabeling.
To test if the increased Far1 transcription in intermediate pheromone concentrations results from MAPK pathway activity, we examined STE5-YFP cells expressing the mating pathway scaffold protein Ste5 fused to a yellow fluorescent protein (Yu et al., 2008). Ste5 localizes to the site of polarized growth when the mating pathway is active (Pryciak and Huntress, 1998; Strickfaden et al., 2007). STE5-YFP cells were arrested in 3nM pheromone and tracked through a cell cycle. The cell perimeter was segmented, linearized, and plotted on a kymograph to visualize the location and intensity of Ste5-YFP on the cell membrane (Figures 3E and 3F). As expected, we observed a transition from a low to a high level of Ste5-YFP at the site of polarized growth upon pheromone arrest (Figure 3G). Upon reentering the cell cycle, Ste5-YFP only partially dissociates from the membrane suggesting that the MAPK pathway remains active through the cell cycle at intermediate pheromone concentrations (p<0.05 for all comparisons, Figure 3H). We also tested if the MAPK Fus3 is active in S/G2/M cells exposed to pheromone as implied by the above results. Fus3 activity correlates with increased nuclear localization and phosphorylation (Blackwell et al., 2003). We therefore measured Fus3 activity using time lapse microscopy and Western blot with a phosphospecific antibody (Nagiec and Dohlman, 2012). Consistent with MAPK (Fus3) activity being responsive to pheromone concentration in cycling cells, Fus3-GFP nuclear localization quickly decreased in cells in S/G2/M phases of the cell cycle that experienced a drop in extracellular pheromone concentration (Figures S4B-D; p<10−4). Similarly, exposure of cells in S/G2/M phases of the cell cycle to pheromone increased the amount of phosphorylated Fus3 (Figures 3I-K). Moreover, we immunoprecipitated Fus3 from S/G2/M cells exposed to 0, 3 and 240 nM pheromone. IP-Fus3 phosphorylated a substrate (MBP) at a rate increasing with pheromone concentration (Figure 3L).
Taken together, our data support a model in which cells cycling in intermediate pheromone concentrations have increased cytoplasmic Far1 levels due to a partially active MAPK pathway post-Start. Thus, while it is clear that cell cycle progression inhibits pheromone signaling (Garrenton et al., 2009; Strickfaden et al., 2007; Torres et al., 2011), this inhibition is not complete at intermediate pheromone concentrations. Our data thus shows that intergenerational memory is composed of Far1 accumulated from the entire previous cell division cycle. In other words, cells remember pheromone exposure post-Start as well as pre-Start from the previous cell division cycle.
Decreasing cytoplasmic Far1 reduces intergenerational memory
Our results so far support the model in which an intergenerational memory of pheromone exposure is transmitted to newborn daughter cells via stable cytoplasmic Far1. If true, we predict that reducing inherited Far1 by genetic manipulation would result in shorter arrest durations in daughter cells. It was previously shown that deletion of the S-phase cyclins CLB5 and CLB6, but not the G1 cyclins CLN1 and CLN2, resulted in longer arrest durations in 3nM pheromone (Doncic and Skotheim, 2013) and that ectopic expression of Clb5 downregulates Far1 (Oehlen et al., 1998). In addition, the S-phase cyclins are nuclear, where Far1 is rapidly degraded (Blondel et al., 2000; Shirayama et al., 1999). We therefore constructed a CLB5-NES strain by adding a nuclear export sequence to CLB5 (Figure 4A). In this strain, the time to half-maximum post-Start of cytoplasmic Far1 in 3nM pheromone was ~45 minutes, a significant reduction from the ~75 minute half-maximum of wild type cells (Figures 4B, 4C and S5A,B; p<0.01).
Figure 4. Reduction of cytoplasmic Far1 decreases intergenerational memory.
(A) Clb5 targets Far1 for degradation and is predominantly nuclear in WT cells. Adding a nuclear exclusion sequence (NES) to CLB5 translocates a fraction to the cytoplasm to target cytoplasmic Far1. (B) Example time series of Far1 concentration in a CLB5-NES cell used to calculate the time to half-maximum post-Start for cytoplasmic Far1. (C) Mean time to cytoplasmic Far1 half-maximum post-Start for WT and CLB5-NES cells first arrested in 240nM and then released into 3nM α-factor. (D) CLB5-NES cells inherit less Far1 than WT and (E) arrest significantly shorter duration (p<10−5). (FG) The relationship between the amount of inherited Far1 and the duration of the subsequent arrest is statistically indistinguishable for CLB5-NES and WT cells (p>0.05). (H) WT and CLB5-NES cells exhibit significantly different mating frequencies (p<0.05). Error bars denote standard error of the mean of cells in panels (C,D) or of replica experiments in panel (G)
In G1, Far1 will be stable because Clb5 is targeted for degradation by the APC/C following mitosis (Shirayama et al., 1999). Thus, while CLB5-NES cells have less cytoplasmic Far1, we expect the smaller amount of inherited Far1 to be just as functional in restraining passage through Start as in WT cells. That is, given the same amount of inherited Far1, CLB5-NES cells would arrest for similar durations as WT cells. Consistent with these predictions, CLB5-NES cells inherited less Far1 and remained arrested for shorter durations relative to WT (Figure 4D, 4E, and S5C). Also as predicted, the relationship between inherited Far1 and arrest duration was statistically similar to WT (Figures 4F and 4G; P>0.1). These data support the interpretation that the CLB5-NES allele affects intergenerational memory through a reduction in inherited cytoplasmic Far1 prior to cytokinesis.
Reducing inherited Far1 lowers mating efficiency
While our results indicate an intergenerational memory of pheromone exposure from mother to daughter cells, it remains unclear if this intergenerational memory plays a role under other physiological conditions. To test this possibility, we performed a quantitative mating assay using WT and CLB5-NES strains. WT cells are able to mate more frequently than CLB5-NES cells (Figure 4H, p<0.05). To test that this decrease in mating frequency was not due to a polarization defect we verified that CLB5-NES cells polarize similarly to WT cells in presence of a pheromone gradient (Figure S5D). These experiments are consistent with a role for intergenerational memory in physiological conditions.
Far1 binding to Cdc24 is required for intergenerational memory
Our results so far identify an intergenerational memory that is correlated with the stability of cytoplasmic Far1. This suggests a non-shuttling cytoplasmic pool of Far1 is inherited to transmit intergenerational memory. Consistent with this model, Far1 has binding partners in the cytoplasm, which we hypothesize serve to anchor Far1. A prime candidate for anchoring is Cdc24, a GTP exchange factor (GEF) regulating cell polarization. Far1 binding to Cdc24 is necessary for pheromone gradient sensing, but not for cell cycle arrest (Nern and Arkowitz, 1999; Valtz et al., 1995) (Figure 5A). Moreover, Cdc24 is nuclear in G1, but is partially exported to the cytoplasm and plasma membrane during mating arrest in a Far1-dependent manner (Nern and Arkowitz, 2000; Shimada et al., 2000).
Figure 5. Far1 binding to cytoplasmic Cdc24 is required for intergenerational memory.
(A) Schematic of the role of Cdc24 with respect to Far1. (B) Fraction of nuclear Far1 in arrested cells. (C) Time to half-maximum Far1 after cell cycle reentry in 6nM pheromone. (D) The post-Start stability of Far1-D1A measured as described in Figure 3A. (E) Inherited Far1 in FAR1-D1A cells compared to WT (p<10−4). (F-G) No intergenerational memory was observed for FAR1-D1A and bni1Δ cells.
To test whether the interaction between Cdc24 and Far1 is required for intergenerational memory, we created strains with the endogenous FAR1 allele replaced by either FAR1-D1A or FAR1-H7 mutant alleles that express Far1 proteins whose interaction with Cdc24 is greatly reduced (Nern and Arkowitz, 2000; Valtz et al., 1995). As previously reported, both strains arrest in pheromone. However, we identified a slight arrest deficiency and 6nM pheromone was required to arrest cells for similar durations as WT cells in 3nM (p>0.05). We therefore used 6nM for the analysis of FAR1-D1A and FAR1-H7 strains. Consistent with Cdc24 anchoring Far1 in the cytoplasm during arrest, the nuclear fraction of Far1 was increased in FAR1-D1A and FAR1-H7 cells compared to WT cells (p < 10−3; Figures 5B, S6A,B). Since nuclear Far1 is rapidly degraded in the cell cycle (Figure 1D), we expected that reduction of cytoplasmic anchoring results in a more rapidly degraded Far1 protein. Indeed, Far1 proteins with reduced Cdc24 interactions reach half-maximum concentration more rapidly following cell cycle entry (Figures 5C, S6C and S6D). Finally, we examined the relationship between intergenerational memory and inherited Far1 in FAR1-D1A and FAR1-H7 cells. Consistent with the requirement of a cytoplasmic anchor, and the model that Cdc24 fills this role, post-Start Far1 was less stable, less Far1 was inherited, and the intergenerational memory was abolished or greatly reduced in cells expressing Far1 proteins with reduced ability to bind Cdc24 (Figures 5D-F and S6E-G).
To further test the Cdc24 anchoring model, we sought to examine bni1Δ cells that are unable to export Cdc24 from the nucleus to the shmoo tip during pheromone arrest (Qi and Elion, 2005). Bni1 is a formin that regulates the polarization of actin cables during mating arrest and is required for cell polarization (Evangelista et al., 1997). We found that bni1Δ cells arrested as round cells for significant periods of time in G1 when exposed to 6nM pheromone (Figure S6H). Under these conditions, bni1Δ cells contained a higher fraction of nuclear Far1, and degraded Far1 more rapidly upon cell cycle entry compared to WT cells (Figures 5B, 5C, S6B and S6C). Finally, bni1Δ cells exhibited no intergenerational memory (Figure 5G). That the localization of Cdc24 outside the nucleus was required for intergenerational memory further supports the role of cytoplasmic Cdc24 as a Far1 anchor.
Far1 stability pre- and post-Start is required for intra- and inter-generational memory respectively
The intergenerational memory that we describe here is in addition to the intragenerational memory of pheromone exposure encoded in Far1 that we previously described (Doncic and Skotheim, 2013). Intragenerational memory allows cells to remember their history of exposure to pheromone during an arrest via the accumulation of Far1. Since Clb5 is targeted for degradation in mitosis, and is therefore not active during pheromone arrest, we do not expect cytoplasmic Clb5 to affect intragenerational memory. To test this prediction, we examined cell cycle progression in cells exposed to different histories of mating pheromone during G1. Cells were either exposed to a brief pulse of high pheromone followed by an intermediate pheromone concentration or just to the intermediate pheromone concentration (Figure 6A). As predicted, both WT and CLB5-NES cells experiencing the high pheromone pulse greatly extended arrest duration indicating that while CLB5-NES cells have reduced intergenerational memory, their intragenerational memory remains firmly intact (Figures 6B and S7A-D). Furthermore, these experiments demonstrate how intergenerational memory is distinct from intragenerational memory and affected by different mutations.
Figure 6. Protein stability is required for intra- and inter-generational memory.
(A) Experimental schematic for intragenerational memory experiment. (B) WT and CLB5-NES cells have intragenerational memory, where the decision to reenter the cell cycle is based on the history of pheromone exposure during the arrest, while 12xFAR1-L92P and FAR1-PEST cells do not. Medians plotted with 95% confidence intervals computed using 10,000 bootstrap iterations. Note that about half of both the CLB5-NES and WT cells exposed to a pulse of high mating pheromone are arrested for the duration of the experiment (Fig. S7B). We therefore do not compare arrest durations for WT and CLB5-NES cells exposed to a pheromone pulse. (C) Conditional expression of FAR1 from a GAL1 promoter is used to measure half-life pre-Start in a series of pheromone concentrations. (D) Far1 half-life pre-Start in WT, FAR1-L92P, and FAR1-PEST cells in 3nM α-factor. (E-F) 12xFAR1-L92P and FAR1-PEST cells lack intergenerational memory as their arrest duration is independent of the amount of inherited Far1. (G) Conditional expression from a GAL1 promoter is used to measure Far1 half-life ± standard error pre-Start as in (C), but for a range of pheromone concentrations.
Just as intergenerational memory depends on the stability of Far1 throughout the cell cycle, intragenerational memory should depend on the stability of Far1 during arrest. To destabilize Far1 during pheromone arrest, we generated a FAR1 allele with the 92nd residue mutated from Leucine to Proline (FAR1-L92P). This mutation is predicted to generate an additional Cdk consensus phosphorylation site to enhance the degradation of Far1 (Valk and Loog in preparation; Figure S7E). To control for the potentially pleiotropic effects of the L92P mutation, we also generated a FAR1-PEST allele, where an otherwise WT FAR1 allele was fused to the C-terminus of CLN2, which destabilizes this cyclin (Lanker et al., 1996). To determine the stability of Far1-L92P and Far1-PEST proteins during arrest, we fused them to GFP and expressed them from a GAL1 promoter. Consistent with these mutations reducing protein stability, the pre-Start half-lives of Far1-L92P and Far1-PEST were reduced to ~50 and ~20 minutes respectively compared to over 130 minutes for WT Far1 (Figures 6C and 6D).
To test the dependence of intragenerational memory on Far1 stability, we next constructed a strain containing a single copy of FAR1-L92P expressed from its endogenous locus. However, FAR1-L92P cells failed to arrest even at high pheromone concentrations. We therefore constructed a strain containing 10-12 copies of FAR1-L92P that arrested as WT cells (72±4 minutes for WT and 61±7 minutes for FAR1-L92P in 2.7nM pheromone, p = 0.17). To verify that the activity of Far1 remains unaltered in the FAR1-L92P strain we also showed that the ability of 12xFAR1-L92P cells to polarize towards pheromone gradients was similar to WT cells (Fig. S7F). Consistent with memory depending on Far1 stability, 12xFAR1-L92P cells exhibited little if any intragenerational memory despite retaining the ability to arrest at this pheromone concentration (Figures 6B and S7A-C). In addition, 12xFAR1-L92P cells also exhibit no intergenerational memory, most likely because this phenomenon also depends on Far1 stability (Figure 6E). Similarly, the destabilized FAR1-PEST strain greatly reduced both intra- and inter-generational memory (Figures 6B, 6F, S7A and S7G). Taken together, these experiments demonstrate the requirement of Far1 stability pre- and post-Start for intra- and inter-generational memory respectively.
Far1 stability during arrest peaks at intermediate pheromone concentrations
The clear connection between Far1-based memory and protein stability suggested the possibility that WT cells might modulate Far1 stability to regulate memory. To test this possibility, we measured FAR1 half-life by expressing it from the GAL1 promoter and shutting off transcription (Figure 6C). However, we now measure the half-life during pre-Start for cells growing in 0, 3, 6, or 240nM pheromone. We found that Far1 stability peaked at 3nM with a ~150 minute half-life (Figures 6G and S7H-I). Far1 stability was reduced to ~100 minutes in 0 and 240nM pheromone. We speculate that this decreased stability might arise from increased G1 cyclin and Fus3 MAPK kinase activities. It is interesting to note that the maximum half-life, i.e., maximal memory, occurs right where the decision to reenter the cell cycle is most sensitive to pheromone concentration.
Discussion
To inform our decisions, our experiences are sensed, encoded and stored as memories. Like us, individual cells also rely on past experience to inform their most important decisions. In budding yeast, one of the most important decisions is whether or not to proliferate or to arrest division and attempt to mate with another haploid cell. Budding yeast invest heavily in this decision, as mutations eliminating the ability to mate provide a ~2% growth rate advantage in pheromone-free conditions (Lang et al., 2009). Perhaps not surprisingly, several distinct types of memory regulate this proliferation-differentiation decision.
The simplest type of memory informing yeast mating is binary and stored as a single bit of information. For example, due to its asymmetric division pattern, a budding yeast cell is either a mother or a daughter. With regards to mating, this bit matters because mother cells are less sensitive to pheromone than daughter cells (Moore, 1984). In part, this is likely due to the differential expression of CLN3, the upstream cyclin driving cell cycle progression in G1 (Laabs et al., 2003). Mother cells produce a burst of CLN3 expression at the transition from mitosis to G1, while daughter cells do not due to the daughter-specific transcription factors, Ace2 and Ash1 (Di Talia et al., 2009). In addition, differential post-transcriptional regulation of CLN3 in mother and daughter cells is may be effected by Whi3 (Caudron and Barral, 2013), which both decreases CLN3 message stability and translation rate (Cai and Futcher, 2013; Gari et al., 2001; Holmes et al., 2013). Yet, all this information pertaining being a mother or daughter cell is binary.
Previously, we identified a continuous, analog form of memory of past pheromone exposure that informs the decision to reenter the cell cycle from pheromone arrest (Doncic and Skotheim, 2013). Cells experiencing higher pheromone concentrations over longer periods of time are more reluctant to reenter the cell cycle. While MAPK pathway activity rapidly responds to reflect the current extracellular pheromone concentration, proteins are more stable so that their level will reflect an integral of past pathway activity (Colman-Lerner et al., 2005; Takahashi and Pryciak, 2008; Yu et al., 2008). More specifically, Far1 accumulates at a monotonically increasing rate with pheromone concentration so that its total amount reflects a combination of arrest duration and pheromone concentration (Chang and Herskowitz, 1990; Doncic and Skotheim, 2013). Thus, the amount of Far1 accumulated during pheromone arrest reflects an integral of pathway activity over time that encodes the history of pheromone exposure into a continuous analog rather than binary variable. However, the mutual inhibition of Far1 and Cdk activities suggested that this analog memory, i.e., the accumulated Far1, would be lost upon flipping the cell cycle switch (Figure 7A-C).
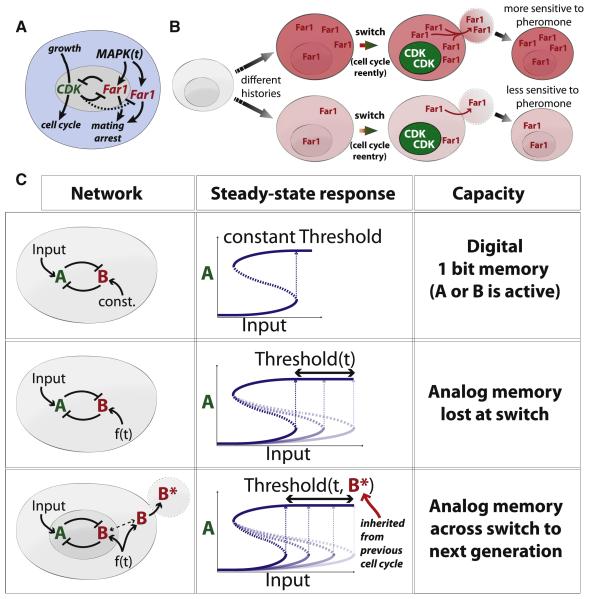
Figure 7. Compartmentalization enables analog memory to pass through a bistable switch.
(A) The Start regulatory network regulating the proliferation-differentiation decision in budding yeast is a bistable switch. (B) Inheritance of cytoplasmic Far1 forms the mechanistic basis of intergenerational memory of pheromone exposure. (C) Top: a double-negative switch with constant signal activating B results in a bistable switch with a single bit of memory, i.e., did the input approach its current level from above or below. Middle: a time-dependent f(t) signal activates B to allow the time-dependent threshold to encode an analog memory of f(t). However, this memory is lost by inactivation upon triggering the double-negative switch. Bottom: compartmentalization allows transmission of analog memory of the time-dependent f(t) signal across the double-negative (positive) feedback switch.
Here, we show how the distribution of Far1 into nuclear and cytoplasmic compartments is used to transmit the analog memory of pheromone exposure across the cell cycle switch to the next generation. While nuclear Far1 is rapidly degraded, as expected by the double-negative switch, cytoplasmic Far1 is longer lived and continually synthesized so that a significant amount remains at the end of the cell cycle to be inherited by daughter cells. We show here that this inherited Far1 contributes to increased pheromone sensitivity and thereby allows mother cells to transmit intergenerational memory of pheromone exposure to their daughter cells. We also identified a fraction of fixed cytoplasmic Far1 as key to storing intergenerational memory. If some Far1 were not fixed in the cytoplasm, it would likely be rapidly degraded post-Start due to the high nuclear B-type cyclin activity. While this Far1 fraction is fixed on the minute time scale, we suspect that it is not permanently fixed because nuclear Far1 is important for maintaining the cell cycle arrest to which the inherited cytoplasmic Far1 eventually contributes. Thus, the slow dissociation of the fixed Far1 is likely central to reading the intergenerational memory of pheromone exposure.
Protein stability is central to both intra- and intergenerational Far1-based analog memory as destabilizing mutations eliminate both. In general, protein stability determines the time scale on which the cell can remember past events. For rapidly degraded proteins, levels will simply reflect the current state of the cell, while for stable proteins, levels will reflect their synthesis over longer periods of time so that their amount can be used to store long-term memories on the time scale of dilution due to cell growth. The demonstrated ability of the cell to regulate protein stability over a wide range of temporal and spatial scales suggests that the analog memory mechanisms discussed here can be easily tuned through mutation and selection.
More broadly, our work illustrates how spatial organization can greatly expand the functionality of signaling motifs. Recently, it has been shown how positive feedback can be enhanced by protein transport within the mammalian mitotic switch (Santos et al., 2012). Activation of Cdk1-Cyclin B complexes within the nucleus recruit additional such complexes to dramatically ramp up nuclear Cdk activity without protein synthesis. However, this represents an enhancement of the well-known ability of positive feedback circuits to generate sharp switches. Here, we have shown how spatial organization allows memory to be transmitted across a positive feedback-driven switch to enable an entirely new and unexpected property of this well-characterized signaling motif. Given the extensive spatial organization within cells, we expect this example to be the first of many in which new signal-processing properties of network motifs are enabled by compartmentalization.
Materials and Methods
See additional supporting information for methods regarding confocal microscopy, FRAP, western blot and kinase assays shown in Figures 2 and 3.
Wide-field timelapse microscopy and analysis
A Zeiss Observer Z1 microscope with an automated stage using a plan-apo 63X/1.4NA oil immersion objective and Definite Focus hardware was used to take images every 3 minutes (6 minutes for the FAR1-L92P strains). We used a Cellasic microfluidics device (http://www.cellasic.com/) with Y04C plates. WHI5-mCherry, FAR1-Venus and FAR1-GFP strains were exposed for 750ms, 300ms or 150-300ms using the Colibri 540-80, 505 or 470 LED modules respectively at 25% power. There was no significant photobleaching at our sampling rate (Figure S7J). FAR1 activity is not affected by fusion to a fluorescent protein (Doncic and Skotheim, 2013). Image segmentation and quantification was performed as described in (Doncic et al., 2013). We often plot mean values and their associated standard error because this gives a graphical representation of statistical significance. Corresponding full distributions can be found in the supporting material.
Measurement of inherited Far1
For each cell we determine inherited Far1 to be (Far1-Venus signal – baseline)/(baseline). ‘Normalized inherited Far1′ is the amount at the beginning of G1 above what that cell would be expected to have when cycling in pheromone-free media (see also schematic S2I and supporting information for details).
Strains and media
All strains are congenic with W303 (see Table S1) and were constructed using standard methods. Yeast were grown in synthetic complete media with 2% glucose unless otherwise stated (2% galactose were used for the Far1 stability experiments in Figures 3 and 6). Before an experiment, cells were grown to an OD <0.1 after which they were sonicated for ~5s at 3W intensity. All media were mixed with 20 mg/ml casein (Sigma) to inhibit α-factor surface adhesion (Colman-Lerner et al., 2005).
Supplementary Material
Acknowledgements
Research in the Skotheim laboratory was supported by the Burroughs Wellcome Fund (CASI) and the NIH (GM092925). The Colman-Lerner laboratory was supported by grant PICT2010-2248 from the Argentine Agency of Research and Technology (ANPCyT) and the NIH (GM097479), while the Loog laboratory was supported by Estonian Science Agency Grant IUT2-21.
Footnotes
Author contributions AD & JS designed the study. AD and OA performed all experiments with the following exceptions. EV & ML identified of the FAR1-L92P mutant and performed the Fus3 activity measurements (Fig. 3I-L). AG, AB, GV & ACL performed the FRAP experiments (Fig. 2). AD, OA & JS wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alon U. Network motifs: theory and experimental approaches. Nat Rev Genet. 2007;8:450–461. doi: 10.1038/nrg2102. [DOI] [PubMed] [Google Scholar]
- Behar M, Hao N, Dohlman HG, Elston TC. Dose-to-duration encoding and signaling beyond saturation in intracellular signaling networks. PLoS Comput Biol. 2008;4:e1000197. doi: 10.1371/journal.pcbi.1000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell E, Halatek IM, Kim HJ, Ellicott AT, Obukhov AA, Stone DE. Effect of the pheromone-responsive G(alpha) and phosphatase proteins of Saccharomyces cerevisiae on the subcellular localization of the Fus3 mitogen-activated protein kinase. Mol Cell Biol. 2003;23:1135–1150. doi: 10.1128/MCB.23.4.1135-1150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel M, Alepuz PM, Huang LS, Shaham S, Ammerer G, Peter M. Nuclear export of Far1p in response to pheromones requires the export receptor Msn5p/Ste21p. Genes Dev. 1999;13:2284–2300. doi: 10.1101/gad.13.17.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel M, Galan JM, Chi Y, Lafourcade C, Longaretti C, Deshaies RJ, Peter M. Nuclear-specific degradation of Far1 is controlled by the localization of the F-box protein Cdc4. Embo J. 2000;19:6085–6097. doi: 10.1093/emboj/19.22.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Futcher B. Effects of the yeast RNA-binding protein Whi3 on the half-life and abundance of CLN3 mRNA and other targets. PLoS One. 2013;8:e84630. doi: 10.1371/journal.pone.0084630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudron F, Barral Y. A super-assembly of Whi3 encodes memory of deceptive encounters by single cells during yeast courtship. Cell. 2013;155:1244–1257. doi: 10.1016/j.cell.2013.10.046. [DOI] [PubMed] [Google Scholar]
- Chang F, Herskowitz I. Identification of a gene necessary for cell cycle arrest by a negative growth factor of yeast: FAR1 is an inhibitor of a G1 cyclin, CLN2. Cell. 1990;63:999–1011. doi: 10.1016/0092-8674(90)90503-7. [DOI] [PubMed] [Google Scholar]
- Chen RE, Thorner J. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2007;1773:1311–1340. doi: 10.1016/j.bbamcr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman-Lerner A, Gordon A, Serra E, Chin T, Resnekov O, Endy D, Pesce CG, Brent R. Regulated cell-to-cell variation in a cell-fate decision system. Nature. 2005;437:699–706. doi: 10.1038/nature03998. [DOI] [PubMed] [Google Scholar]
- Costanzo M, Nishikawa JL, Tang X, Millman JS, Schub O, Breitkreuz K, Dewar D, Rupes I, Andrews B, Tyers M. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell. 2004;117:899–913. doi: 10.1016/j.cell.2004.05.024. [DOI] [PubMed] [Google Scholar]
- de Bruin RA, McDonald WH, Kalashnikova TI, Yates J, 3rd, Wittenberg C. Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell. 2004;117:887–898. doi: 10.1016/j.cell.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Di Talia S, Wang H, Skotheim JM, Rosebrock AP, Futcher B, Cross FR. Daughter-specific transcription factors regulate cell size control in budding yeast. PLoS Biol. 2009;7:e1000221. doi: 10.1371/journal.pbio.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncic A, Eser U, Atay O, Skotheim JM. An Algorithm to Automate Yeast Segmentation and Tracking. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncic A, Falleur-Fettig M, Skotheim JM. Distinct interactions select and maintain a specific cell fate. Mol Cell. 2011;43:528–539. doi: 10.1016/j.molcel.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncic A, Skotheim JM. Feedforward Regulation Ensures Stability and Rapid Reversibility of a Cellular State. Molecular Cell. 2013;50:856–868. doi: 10.1016/j.molcel.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errede B, Ammerer G. STE12, a protein involved in cell-type-specific transcription and signal transduction in yeast, is part of protein-DNA complexes. Genes Dev. 1989;3:1349–1361. doi: 10.1101/gad.3.9.1349. [DOI] [PubMed] [Google Scholar]
- Evangelista M, Blundell K, Longtine MS, Chow CJ, Adames N, Pringle JR, Peter M, Boone C. Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- Forney GD. Viterbi Algorithm. P Ieee. 1973;61:268–278. [Google Scholar]
- Gari E, Volpe T, Wang H, Gallego C, Futcher B, Aldea M. Whi3 binds the mRNA of the G1 cyclin CLN3 to modulate cell fate in budding yeast. Genes Dev. 2001;15:2803–2808. doi: 10.1101/gad.203501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrenton LS, Braunwarth A, Irniger S, Hurt E, Kunzler M, Thorner J. Nucleus-specific and cell cycle-regulated degradation of mitogen-activated protein kinase scaffold protein Ste5 contributes to the control of signaling competence. Mol Cell Biol. 2009;29:582–601. doi: 10.1128/MCB.01019-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A, Jovanovic A, Jeoung DI, Bourlat S, Cross FR, Ammerer G. Pheromone-dependent G1 cell cycle arrest requires Far1 phosphorylation, but may not involve inhibition of Cdc28-Cln2 kinase, in vivo. Mol Cell Biol. 1998;18:3681–3691. doi: 10.1128/mcb.18.7.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao N, Nayak S, Behar M, Shanks RH, Nagiec MJ, Errede B, Hasty J, Elston TC, Dohlman HG. Regulation of cell signaling dynamics by the protein kinase-scaffold Ste5. Mol Cell. 2008;30:649–656. doi: 10.1016/j.molcel.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Culotti J, Pringle JR, Reid BJ. Genetic control of the cell division cycle in yeast. Science. 1974;183:46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Henchoz S, Chi Y, Catarin B, Herskowitz I, Deshaies RJ, Peter M. Phosphorylation- and ubiquitin-dependent degradation of the cyclin-dependent kinase inhibitor Far1p in budding yeast. Genes Dev. 1997;11:3046–3060. doi: 10.1101/gad.11.22.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KJ, Klass DM, Guiney EL, Cyert MS. Whi3, an S. cerevisiae RNA-binding protein, is a component of stress granules that regulates levels of its target mRNAs. PLoS One. 2013;8:e84060. doi: 10.1371/journal.pone.0084060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AS, Jin M, Wu CF, Zyla TR, Elston TC, Lew DJ. Negative feedback enhances robustness in the yeast polarity establishment circuit. Cell. 2012;149:322–333. doi: 10.1016/j.cell.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeoung DI, Oehlen LJ, Cross FR. Cln3-associated kinase activity in Saccharomyces cerevisiae is regulated by the mating factor pathway. Mol Cell Biol. 1998;18:433–441. doi: 10.1128/mcb.18.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko BN, Hancock JF, Kolch W. Signalling ballet in space and time. Nat Rev Mol Cell Biol. 2010;11:414–426. doi: 10.1038/nrm2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laabs TL, Markwardt DD, Slattery MG, Newcomb LL, Stillman DJ, Heideman W. ACE2 is required for daughter cell-specific G1 delay in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2003;100:10275–10280. doi: 10.1073/pnas.1833999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang GI, Murray AW, Botstein D. The cost of gene expression underlies a fitness tradeoff in yeast. Proc Natl Acad Sci U S A. 2009;106:5755–5760. doi: 10.1073/pnas.0901620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanker S, Valdivieso MH, Wittenberg C. Rapid degradation of the G1 cyclin Cln2 induced by CDK-dependent phosphorylation. Science. 1996;271:1597–1601. doi: 10.1126/science.271.5255.1597. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Ye AS, Gardino AK, Heijink AM, Sorger PK, MacBeath G, Yaffe MB. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149:780–794. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleshaiah MK, Shahrezaei V, Swain PS, Michnick SW. The scaffold protein Ste5 directly controls a switch-like mating decision in yeast. Nature. 2010;465:101–105. doi: 10.1038/nature08946. [DOI] [PubMed] [Google Scholar]
- McKinney JD, Chang F, Heintz N, Cross FR. Negative regulation of FAR1 at the Start of the yeast cell cycle. Genes Dev. 1993;7:833–843. doi: 10.1101/gad.7.5.833. [DOI] [PubMed] [Google Scholar]
- Moore SA. Yeast cells recover from mating pheromone alpha factor-induced division arrest by desensitization in the absence of alpha factor destruction. J Biol Chem. 1984;259:1004–1010. [PubMed] [Google Scholar]
- Nagiec MJ, Dohlman HG. Checkpoints in a yeast differentiation pathway coordinate signaling during hyperosmotic stress. PLoS Genet. 2012;8:e1002437. doi: 10.1371/journal.pgen.1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nern A, Arkowitz RA. A Cdc24p-Far1p-Gbetagamma protein complex required for yeast orientation during mating. J Cell Biol. 1999;144:1187–1202. doi: 10.1083/jcb.144.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nern A, Arkowitz RA. Nucleocytoplasmic shuttling of the Cdc42p exchange factor Cdc24p. J Cell Biol. 2000;148:1115–1122. doi: 10.1083/jcb.148.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlen LJ, Jeoung DI, Cross FR. Cyclin-specific START events and the G1-phase specificity of arrest by mating factor in budding yeast. Mol Gen Genet. 1998;258:183–198. doi: 10.1007/s004380050722. [DOI] [PubMed] [Google Scholar]
- Oehlen LJ, McKinney JD, Cross FR. Ste12 and Mcm1 regulate cell cycle-dependent transcription of FAR1. Mol Cell Biol. 1996;16:2830–2837. doi: 10.1128/mcb.16.6.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M, Gartner A, Horecka J, Ammerer G, Herskowitz I. FAR1 links the signal transduction pathway to the cell cycle machinery in yeast. Cell. 1993;73:747–760. doi: 10.1016/0092-8674(93)90254-n. [DOI] [PubMed] [Google Scholar]
- Peter M, Herskowitz I. Direct inhibition of the yeast cyclin-dependent kinase Cdc28-Cln by Far1. Science. 1994;265:1228–1231. doi: 10.1126/science.8066461. [DOI] [PubMed] [Google Scholar]
- Pope PA, Bhaduri S, Pryciak PM. Regulation of Cyclin-Substrate Docking by a G1 Arrest Signaling Pathway and the Cdk Inhibitor Far1. Curr Biol. 2014 doi: 10.1016/j.cub.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryciak PM, Huntress FA. Membrane recruitment of the kinase cascade scaffold protein Ste5 by the Gbetagamma complex underlies activation of the yeast pheromone response pathway. Genes Dev. 1998;12:2684–2697. doi: 10.1101/gad.12.17.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis JE, Karhohs KW, Mock C, Batchelor E, Loewer A, Lahav G. p53 dynamics control cell fate. Science. 2012;336:1440–1444. doi: 10.1126/science.1218351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M, Elion EA. Formin-induced actin cables are required for polarized recruitment of the Ste5 scaffold and high level activation of MAPK Fus3. J Cell Sci. 2005;118:2837–2848. doi: 10.1242/jcs.02418. [DOI] [PubMed] [Google Scholar]
- Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods. 2008;5:877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SD, Wollman R, Meyer T, Ferrell JE., Jr. Spatial positive feedback at the onset of mitosis. Cell. 2012;149:1500–1513. doi: 10.1016/j.cell.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Gulli MP, Peter M. Nuclear sequestration of the exchange factor Cdc24 by Far1 regulates cell polarity during yeast mating. Nat Cell Biol. 2000;2:117–124. doi: 10.1038/35000073. [DOI] [PubMed] [Google Scholar]
- Shirayama M, Toth A, Galova M, Nasmyth K. APC(Cdc20) promotes exit from mitosis by destroying the anaphase inhibitor Pds1 and cyclin Clb5. Nature. 1999;402:203–207. doi: 10.1038/46080. [DOI] [PubMed] [Google Scholar]
- Skotheim JM, Di Talia S, Siggia ED, Cross FR. Positive feedback of G1 cyclins ensures coherent cell cycle entry. Nature. 2008;454:291–296. doi: 10.1038/nature07118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickfaden SC, Winters MJ, Ben-Ari G, Lamson RE, Tyers M, Pryciak PM. A mechanism for cell-cycle regulation of MAP kinase signaling in a yeast differentiation pathway. Cell. 2007;128:519–531. doi: 10.1016/j.cell.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Pryciak PM. Membrane localization of scaffold proteins promotes graded signaling in the yeast MAP kinase cascade. Curr Biol. 2008;18:1184–1191. doi: 10.1016/j.cub.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson TM, Benjamin KR, Bush A, Love T, Pincus D, Resnekov O, Yu RC, Gordon A, Colman-Lerner A, Endy D, et al. Scaffold number in yeast signaling system sets tradeoff between system output and dynamic range. Proc Natl Acad Sci U S A. 2011;108:20265–20270. doi: 10.1073/pnas.1004042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MP, Clement ST, Cappell SD, Dohlman HG. Cell cycle-dependent phosphorylation and ubiquitination of a G protein alpha subunit. J Biol Chem. 2011;286:20208–20216. doi: 10.1074/jbc.M111.239343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyers M, Futcher B. Far1 and Fus3 link the mating pheromone signal transduction pathway to three G1-phase Cdc28 kinase complexes. Mol Cell Biol. 1993;13:5659–5669. doi: 10.1128/mcb.13.9.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtz N, Peter M, Herskowitz I. FAR1 is required for oriented polarization of yeast cells in response to mating pheromones. J Cell Biol. 1995;131:863–873. doi: 10.1083/jcb.131.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Ferrell JE., Jr. The Cdk1-APC/C cell cycle oscillator circuit functions as a time-delayed, ultrasensitive switch. Nat Cell Biol. 2013;15:519–525. doi: 10.1038/ncb2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosef N, Regev A. Impulse control: temporal dynamics in gene transcription. Cell. 2011;144:886–896. doi: 10.1016/j.cell.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RC, Pesce CG, Colman-Lerner A, Lok L, Pincus D, Serra E, Holl M, Benjamin K, Gordon A, Brent R. Negative feedback that improves information transmission in yeast signalling. Nature. 2008;456:755–761. doi: 10.1038/nature07513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.