Murine γδ T cells promote a pro-inflammatory response to high fat diet by regulating adipose tissue macrophages and systemic insulin resistance during obesity.
Keywords: macrophage, adipose tissue, liver, skeletal muscle, CCL2, IL-6
Abstract
γδ T cells are resident in AT and increase during diet-induced obesity. Their possible contribution to the inflammatory response that accompanies diet-induced obesity was investigated in mice after a 5 to 10 week milk HFD. The HFD resulted in significant increases in CD44hi, CD62Llo, and TNF-α+ γδ T cells in eAT of WT mice. Mice deficient in all γδ T cells (TCRδ−/−) or only Vγ4 and Vγ6 subsets (Vγ4/6−/−) were compared with WT mice with regard to proinflammatory cytokine production and macrophage accumulation in eAT. Obesity among these mouse strains did not differ, but obese TCRδ−/− and Vγ4/6−/− mice had significantly reduced eAT expression of F4/80, a macrophage marker, and inflammatory mediators CCL2 and IL-6 compared with WT mice. Obese TCRδ−/− mice had significantly reduced CD11c+ and TNF-α+ macrophage accumulation in eAT after 5 and 10 weeks on the HFD, and obese Vγ4/6−/− mice had significantly increased CD206+ macrophages in eAT after 5 weeks on the diet and significantly reduced macrophages after 10 weeks. Obese TCRδ−/− mice had significant reductions in systemic insulin resistance and inflammation in liver and skeletal muscle after longer-term HFD feeding (10 and 24 weeks). In vitro studies revealed that isolated γδ T cells directly stimulated RAW264.7 macrophage TNF-α expression but did not stimulate inflammatory mediator expression in 3T3-L1 adipocytes. These findings are consistent with a role for γδ T cells in the proinflammatory response that accompanies diet-induced obesity.
Introduction
Obesity leads to a state of chronic, low-grade systemic inflammation, which links obesity and various metabolic abnormalities, such as insulin resistance and cardiovascular diseases [1–3]. Diet-induced AT inflammation appears to play a prime role in this process [4]. Increased chemokines and proinflammatory cytokines in AT, chiefly produced by adipocytes and macrophages, contribute to the development of inflammation in AT and signal the recruitment of several immune cell populations [5].
Macrophages are recruited during obesity into AT of mice and humans [6, 7]. AT macrophages in lean mice express anti-inflammatory and tissue-reparative characteristics of the alternatively activated M2 macrophages, including surface markers, such as the mannose receptor CD206 [8, 9]. During obesity, CD11c+ classically activated M1 macrophages infiltrate AT and produce various mediators of inflammation, such as TNF-α and IL-6 that promote insulin resistance [8, 10, 11]. Macrophages are recruited into AT by various mechanisms, including increased chemotactic factors (CCL2) and activation of innate and adaptive immune components, such as pattern recognition receptors and CD8+ T and B cells, among others [11–14]. Along with macrophages, various lymphocytes infiltrate the AT after HFD feeding, including NKT, B, αβ T, and γδ T cells [15]. Whereas NKT, B, and αβ T cells have been the subject of intense study [16], the role of γδ T cells in AT inflammation remains unknown.
γδ T cells bridge the innate and adaptive immune inflammatory responses [17]. In the periphery, γδ T cells are naturally activated and rapidly secrete cytokines, such as IFN-γ, TNF-α, and IL-17a [18]. Specific subsets of γδ T cells are localized in organs to play specific roles, such as protecting host against injury and pathogens, and they are either pro- or anti-inflammatory, based on the environmental stimuli [19]. More recently, it has been observed that γδ T cells are also present in AT [15, 20, 21]. After a long-term lard HFD, γδ T cells in the AT are a chief source of IL-17a, a cytokine that regulates adipogenesis and glucose metabolism in AT [20]. The question we address in this study is whether γδ T cells contribute to AT inflammation during obesity.
To determine the contribution of γδ T cells to milk HFD-induced inflammatory changes in the AT in mice, we studied mice deficient in all γδ T cells (TCRδ−/−) and WT mice treated with antibodies to block the γδ TCR. To study further the role of some subsets of γδ T cells found in AT, Vγ4/6−/− mice were used. In each experimental condition, inflammatory changes in AT were reduced significantly. Additionally, in TCRδ−/− mice, the systemic increase in insulin resistance that normally results from the obesogenic diet was reduced significantly, and inflammation in liver and skeletal muscle was reduced significantly. These results are consistent with an important role for γδ T cells in the developing metabolic syndrome of diet-induced obesity.
MATERIALS AND METHODS
Mice
TCRδ−/− [22] and C57BL/6J (WT) control mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Littermate TCRδ+/+ mice were used as controls for experiments with TCRδ−/− mice. Vγ4/6−/− mice [23], on a C57BL/6J background, were a kind gift from Dr. Rebecca O’Brien (University of Colorado, Denver, CO, USA). Mice were maintained in the Children’s Nutrition Research Center vivarium in a specific pathogen-free, temperature-controlled, and 12 h light-dark cycle environment. Starting at 5 weeks of age, male mice were fed ad libitum either a ND (16% kcal fat; Teklad #2020; Harlan Laboratories, Madison, WI, USA) or a Western HFD (42% kcal fat; Dyet 112734; Dyets, Bethlehem, PA, USA) for 5, 10, or 24 weeks. All animal studies and experimental procedures were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
Body composition analysis and food intake measurement
Body composition of live, conscious mice was determined by use of a quantitative magnetic resonance machine, Echo MRI-100 (EchoMRI, Houston, TX, USA). Daily food intake was measured by use of feeding cups (Research Products International, Mount Prospect, IL, USA). Mice were housed individually starting at 4 weeks of age and were placed on a diet at 5 weeks of age. After 3 weeks of diet feeding, feeding cups were placed in the cages, and the food in the cups was measured daily at 10 AM. The food intake for each day was determined by subtracting the weight of food in the cup on the day of measurement from that measured on the previous day. Average food intake was calculated for daily measurements taken over 1 week of diet feeding.
Tissue harvesting and preparation
At designated time-points, mice were euthanized, and the eAT, liver, and soleus and gastrocnemius skeletal muscles were dissected. Tissue was frozen in liquid nitrogen and stored at –80°C. For flow cytometry experiments, eAT was collected in 1% BSA in PBS, spleen was collected in PBS, and blood was drawn from the inferior vena cava into a syringe containing 3.2% sodium citrate. For histologic examination, eAT was preserved in Z-fix (BD Biosciences, San Jose, CA, USA), embedded in paraffin, sectioned (5 µm), and stained with H&E. For each animal, the number of adipocytes was counted in 5 randomly selected fields.
Real-time PCR
Whole eAT was crushed in TRIzol reagent (Qiagen, Valencia, CA, USA) by use of mortar and pestle and homogenized further via sonication. Total RNA was isolated by use of RNeasy columns (Qiagen), according to the manufacturer’s instructions. RNA (500 ng) was reverse transcribed into cDNA by use of Moloney murine leukemia virus RT (Applied Biosystems, Foster City, CA, USA) for qPCR by use of TaqMan probes (Applied Biosystems). Gene expression levels were calculated after normalization to housekeeping gene GAPDH.
PCR for TCR Vγ and Vδ chains
cDNA was amplified by use of Taq DNA polymerase (Qiagen), and the primers used for detecting Vγ1, -2, -4, -5, -6, and -7 and Vδ1, -2, -5, and -6 were as described previously [24] by use of the nomenclature of Heilig and Tonegawa [25]. Other primers used were Vδ3-forward, AAGCCAAACGCTTGTGTCAG, Vδ3-reverse, CTGAGCATTGTTGTTGGAATG, and Vδ4-forward, GAACCAGTTGCCAAAACTT, Vδ4-reverse, TTGTTGCCTTCTGAATGTCG. The PCR products were run on a 2% agarose gel and visualized by use of ethidium bromide.
Isolation of eAT SVF and flow cytometry
eAT was digested by use of collagenase type I (Worthington Biochemical, Lakewood, NJ, USA) as previously described [26]. In brief, eAT was weighed, minced into small pieces, and subsequently digested in 1% BSA in PBS containing 280 U/ml collagenase I for 75 min at 37°C, while shaking at 180 rpm. Tissue was then filtered through nylon fiber, and SVF cells were pelleted and washed with PBS. The following florescent-labeled anti-mouse antibodies were used to stain the SVF cells: CD45 (30-F11; BD PharMingen, San Diego, CA, USA), CD3e (145-2c11; BD PharMingen), TCRδ (GL3; BD PharMingen), CD69 (H1.2F3; eBioscience, San Diego, CA, USA), CD44 (IM7; eBioscience), CD62L (MEL-14; BD PharMingen), CD27 (LG.3A10; BioLegend, San Diego, CA, USA), CCR6 (140706; R&D Systems, Minneapolis, MN, USA), F4/80 (BM8; eBioscience), CD11b (M1/70; BD PharMingen), CD11c (HL3; BD PharMingen), and CD206 (MR5D3; AbD Serotec, Kidlington, United Kingdom). For intracellular staining, cells were incubated overnight at 37°C in complete DMEM (Sigma-Aldrich, St. Louis, MO, USA), containing 10% FBS, 1% nonessential amino acids, 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin, and supplemented with 2µg/ml Brefeldin A (BD PharMingen). Cells were then fixed and permeabilized by use of the BD Cytofix/Cytoperm kit (BD PharMingen) and stained with the following antibodies: TNFα (MP6-XT22; eBioscience), IL-6 (MP5-20F3; eBioscience), IFN-γ (XMG1.2; eBioscience), and IL-17a (TC11-18H10.1; BioLegend). Cells were fixed in BD FACS lysing solution (BD PharMingen), and CountBright absolute counting beads (Life Technologies, Carlsbad, CA, USA) were added to evaluate the number of cells analyzed. Cells were analyzed by LSRII flow cytometry by use of BD FACSDiva software (Becton Dickinson, Franklin Lakes, NJ, USA). Five thousand bead events were collected, and the absolute number of cells within the sample was calculated as a ratio of number of cell events:5000 beads by use of the manufacturer’s instructions, to calculate number of cells/fat pads. This absolute number was divided by the grams of fat to calculate number of cells/fat pads/grams of fat.
Isolation of intrahepatic leukocytes from liver for flow cytometry analysis
Intrahepatic cells were isolated by use of collagenase type IV (Sigma-Aldrich), as described previously [27]. In brief, hepatic perfusion of mice was performed by use of PBS and collagenase type IV solution. The liver was excised, minced into small pieces, filtered through a 70 um filter, and digested in RPMI containing 0.02% collagenase IV for 40 min at 37°C, while shaking at 60 rpm. After digestion, hepatocytes were pelleted and discarded, and the supernatant was centrifuged further to pellet the leukocytes. The cells were stained with desired antibodies and analyzed by LSRII flow cytometry by use of counting beads.
In vivo anti-TCRγδ antibody treatment
C57BL/6J male mice were started on HFD at 5 weeks of age and maintained on it for 4.5 weeks. After being on the diet for 2.5 weeks, mice were given 2 doses of the anti-TCRγδ antibody UC7-13D5 (BD PharMingen), 1 week apart. The antibody was delivered i.v. by retro-orbital injection at doses 250 μg, each. Studies suggest that this antibody clone blocks the TCRγδ and does not deplete γδ T cells [28]. As a control, another group of mice was given i.v. injection of anti-hamster IgG isotype control antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at a similar dose and frequency.
Insulin tolerance test
Mice were fasted for 6 h. To perform the ITT, mice were i.p. injected with human insulin (Humulin R; Eli Lilly, Indianapolis, IN, USA) at a dose of 0.75 U/kg body weight. Blood samples were obtained from the tail at the following time intervals: 0, 5, 10, 20, 30, 45, 60, 90, and 120 min. Blood glucose levels were determined by use of a OneTouch Ultra glucometer (LifeScan, Milpitas, CA, USA). Plasma insulin levels were measured by use of a mouse insulin ELISA kit (EMD Millipore, Billerica, MA, USA).
Primary γδ T cell isolation
Spleen, harvested from male C57BL/6J mice, was dispersed and RBCs lysed by use of ammonium-chloride-potassium lysis buffer. Cells were resuspended in MACS buffer, and magnetic beads were used to isolate γδ T cells, according to the manufacturer’s protocol (Miltenyi Biotec, Auburn, CA, USA). γδ T cells were cultured in complete DMEM.
In vitro 3T3-L1 preadipocyte culture and differentiation to adipocytes
Murine 3T3-L1 preadipocyte cells (ATCC, Manassas, VA, USA) were cultured to confluence in DMEM, supplemented with 10% calf serum in 5% CO2 at 37°C. 3T3-L1 preadipocytes were stimulated to undergo differentiation by use of DMEM, supplemented with 10% FBS, 1 μM dexamethasone, 5 mM 3-isobutyl-1-methylxanthine, and 1 μg/ml bovine insulin. Days 8–12 differentiated adipocytes were used for all experiments. Cells were harvested in TRIzol reagent for RNA isolation and further qPCR analysis.
Effect of γδ T cells on 3T3-L1 adipocytes
γδ T cells isolated from spleens of WT mice fed ND or HFD for 5 weeks were cultured overnight to collect γδ T cell CM. Differentiated 3T3-L1 cells were cocultured with γδ T cells or treated with γδ T cell CM. After 24 h, γδ T cells and CM were washed away by use of PBS, and TRIzol was used for isolating RNA from adipocytes for qPCR analysis. As a control, just before adipocyte RNA isolation, 3T3-L1 adipocytes were mixed with γδ T cells or treated with γδ T cell CM, which were then washed away.
Oil Red O staining for 3T3-L1 cells
3T3-L1 adipocytes were fixed in 10% formaldehyde for 1 h and incubated further with 0.2% (w/v) Oil Red O solution prepared in 60% (v/v) isopropanol for 10 min. The cells were washed with water, and lipid stain was extracted by use of isopropanol. Lipid content was quantified by measuring absorbance at 500 nm.
AT explant culture
eAT was dissected from mice and minced and incubated in DMEM, supplemented with low endotoxin, fatty acid-free 1% BSA (Sigma-Aldrich) at a ratio of 3 ml DMEM/g AT at 37°C. After 24 h incubation, the eAT-ex was collected and stored at –20°C. 3T3-L1-differentiated adipocytes were treated with eAT-ex media from WT or TCRδ−/− mice fed ND or HFD for 5 weeks. After 24 h of treatment, cells were harvested and RNA isolated.
Experiments with RAW 264.7 cells
Murine macrophage cell line RAW264.7 cells (ATCC) were cultured in DMEM in vitro. γδ T cells, isolated from spleen of WT mice, fed ND or HFD for 5 weeks, were cocultured with RAW264.7 cells at a ratio of 20:1 (RAW264.7:γδ T cells) in a 48-well plate for 24 h. To test the noncontact-mediated effect of γδ T cells on macrophages, RAW264.7 cells were treated with γδ T cell CM for 24 h and RNA isolated for qPCR analysis. As a control, just before macrophage RNA isolation, RAW264.7 cells were mixed with γδ T cells or treated with γδ T cell CM, which were then washed away.
Statistical analysis
Values are reported as mean ± sem. Results were analyzed by use of a two-tailed unpaired Student’s t-test or one-way ANOVA with Tukey’s post hoc test, as indicated, by use of GraphPad Prism software (San Diego, CA, USA). Significance was accepted at P < 0.05.
RESULTS
Characterization of γδ T cells in eAT after 5 weeks of HFD
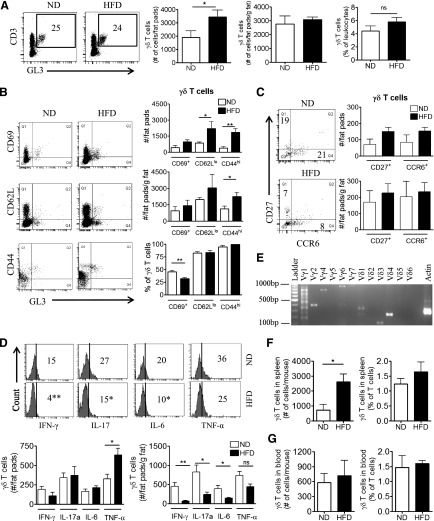
The response of AT γδ T cells to diet-induced inflammation was assessed in WT mice placed on a milk HFD for 5 weeks. γδ T cells made up ∼5% of total leukocytes and ∼25% of the total T cell population in the SVF of both lean and obese eAT (Fig 1A). The absolute number of γδ T cells in the eAT was increased following the HFD, as determined by flow cytometry analysis (Fig 1A). Furthermore, this increase in γδ T cells was proportional to an increase in fat depot mass during obesity, as suggested by expressing the number of γδ T cells relative to grams of fat (Fig. 1A). The activation status of γδ T cells in eAT was assessed by flow cytometry analysis by use of the gating strategy outlined in Supplemental Fig. 1A. Approximately 75% and 90% of γδ T cells in eAT of lean and obese mice were CD44hi and CD62Llo, respectively, and increased in number/mouse after HFD (Fig. 1B). The number of CD44hi γδ T cells was increased proportional to grams of eAT after 5 weeks of HFD, although there was no difference in the CD62Llo γδ T cells population. CD69+ γδ T cells did not increase in number.
Figure 1. Characterization of γδ T cells in eAT of mice after 5 weeks of HFD.
(A) Analysis of CD3+GL3+ γδ T cells in SVF isolated by collagenase digestion of eAT from male C57BL/6J mice on 5 weeks of ND (white bars) or HFD (black bars). Cells were gated on the CD3+ population and analyzed for coexpression of GL3 by flow cytometry. Data are represented as total number of γδ T cells in eAT/mouse (# of cells/fat pads) and number of cells relative to grams of AT mass (# of cells/fat pads/g fat) and as percentage of total leukocytes in eAT, and numbers inside boxes refer to percentage of γδ T cells of total T cells; n = 8–10 mice in each group. *P < 0.05, ns, Not significant. (B) Flow cytometric analysis of CD69, CD62L and CD44 expression on γδ T cells in eAT of mice after 5 weeks of ND or HFD. (C) Flow cytometric analysis of CD27 and CCR6 expression on γδ T cells in eAT of mice after 5 weeks of ND or HFD. (D) Flow cytometric analysis of intracellular cytokine IFN-γ, IL-17, IL-6, and TNF-α expression by γδ T cells in eAT after 5 weeks of ND or HFD. SVF cells were isolated from eAT and cultured overnight in DMEM containing Brefeldin A. (B–D) Data are represented as number of cells/fats pads and number of cells/fat pads/gram of fat and as a percentage of γδ T cells in eAT (numbers inside box). *P < 0.05, and **P < 0.01 comparing ND- and HFD-fed groups of mice; n = 4–5 mice in each group. (E) PCR analysis to determine the γ and δ TCR chains expressed in eAT of C57BL/6J mice after 5 weeks of HFD. Representative gel picture of at least 3 repeat experiments. (F and G) Flow cytometric analysis of γδ T cells in (F) spleen and (G) whole blood after 5 weeks of ND or HFD, represented as number of cells/tissue and as a percent of total T cells; n = 3–5 mice in each group. Values are mean ± sem. *P < 0.05 by Student’s t-test.
γδ T cells were characterized functionally by expression of CD27 and CCR6, surface markers used for distinguishing cells that express IFN-γ and IL-17, respectively [29, 30]. Both markers were detected on a relatively small fraction of γδ T cells in eAT with no significant increase in numbers after HFD (Fig. 1C). In agreement, the number of intracellular IFN-γ+ and IL-17a+ γδ T cells did not increase in eAT after HFD (Fig. 1D). Similar results were obtained with IL-6+ γδ T cells (Fig. 1D). The drop in percent of IFN-γ+, IL-17a+, and IL-6+ γδ T cells after HFD suggests that the negative population increased more than the positive and that there may be an increase in another cytokine-producing γδ T cell population. Notably, ∼35–40% of γδ T cells in eAT express TNF-α, with a significant increase in numbers proportional to the increase in fat depot mass after 5 weeks of HFD (Fig. 1D). TNF-α has been shown to play an important role in the AT dysfunction and insulin resistance associated with obesity [31–33]. The γδ T cells in eAT were characterized further by evaluating subsets of γδ T cells based on the rearrangements of the TCRγ and -δ chains. By PCR, we identified the following subsets of γδ T cells in the eAT of obese mice: Vγ1, -2, -4, and -6 and Vδ1, -3, and -4 (Fig. 1E). Similar subsets were observed in eAT of lean mice (data not shown). Of note, a smear was observed in the Vγ1 lane, along with a band at the predicted size, suggesting the presence of multiple subpopulations within the Vγ1 population. This is in contrast to the single Vγ1 subset present in murine spleen (see refs. [24, 34]).
The systemic effects of HFD on γδ T cells in whole blood and spleen were evaluated. There was an increase in total number of γδ T cells in spleen after 5 weeks of HFD (Fig. 1F), although there was no change in the blood (Fig. 1G). γδ T cells made up a relatively small percentage of T cells in spleen and blood, which was not changed after HFD (Fig. 1F and G).
Diet-induced inflammatory changes in TCRδ−/− mice, Vγ4/6−/− mice, and mice treated with anti-TCRγδ antibody
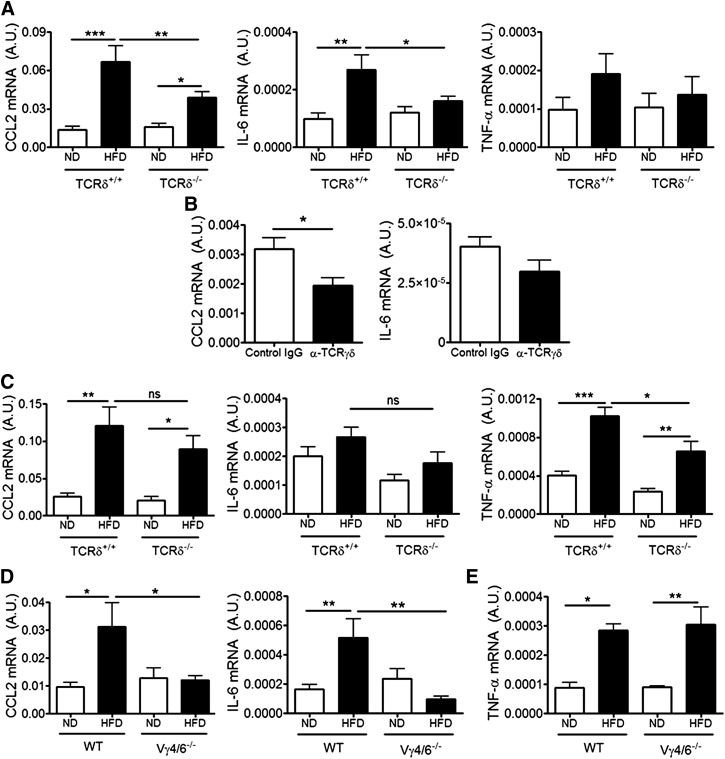
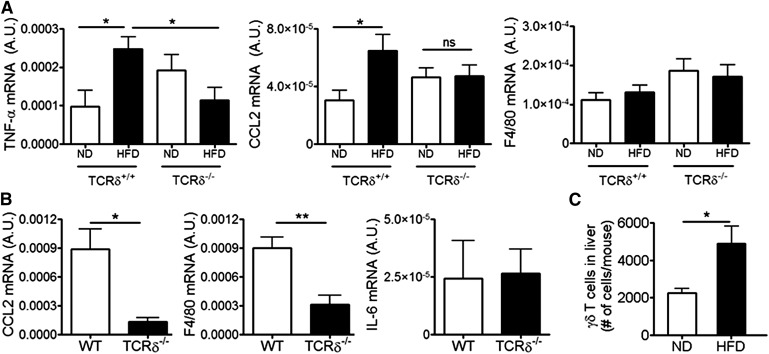
The requirement for γδ T cells in HFD-induced eAT inflammation was assessed in TCRδ−/− and littermate control mice placed on 5 weeks of HFD. Total body weight, eAT weight, and lean and fat mass distribution were similar between TCRδ+/+ and TCRδ−/− mice (Supplemental Fig. 2A and B). TCRδ−/− mice ate similar amounts of food as WT mice (Supplemental Fig. 2C), and there were no significant differences in the adipocyte size, as determined by adipocyte count/unit area (Supplemental Fig. 2D). Despite the similarity in weight gain, TCRδ−/− mice had significantly lower mRNA expression of CCL2 and IL-6 in eAT after 5 weeks of HFD compared with HFD-fed TCRδ+/+ mice (Fig. 2A). TNF-α mRNA levels were not elevated in whole eAT tissue from WT or TCRδ−/− mice at this time-point (Fig. 2A).
Figure 2. CCL2, IL-6, and TNF-α expression in eAT of HFD-fed TCRδ−/− mice, Vγ4/6−/− mice, and mice treated with anti-TCRγδ antibody.
(A) mRNA expression of CCL2, IL-6, and TNF-α in eAT of TCRδ−/− and TCRδ+/+ mice after 5 weeks of ND or HFD, as determined by qPCR analysis, expressed relative to housekeeping gene GAPDH; n = 6–9 mice in each group. (B) mRNA expression of CCL2 and IL-6 in eAT of HFD-fed C57BL/6J mice treated with IgG isotype control antibody (white bar) or anti-TCRγδ antibody (black bar), as determined by qPCR analysis; n = 5 mice in each group. *P < 0.05 by Student’s t-test. (C) mRNA expression of CCL2, IL-6, and TNF-α in eAT of TCRδ−/− and TCRδ+/+ mice after 10 weeks of ND or HFD, as determined by qPCR analysis; n = 5–6 mice in each group. (D) mRNA expression of CCL2 and IL-6 in eAT of Vγ4/6−/− and WT mice after 5 weeks of ND or HFD, as determined by qPCR; n = 4–6 mice in each group. (E) mRNA expression of TNF-α in eAT of Vγ4/6−/− and WT mice after 10 weeks of ND or HFD, as determined by qPCR; n = 4–6 mice in each group. Values are mean ± sem., (A, C–E) *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Tukey’s post hoc test. One representative cohort of 2 independent cohorts of mice.
WT mice were treated i.v. with anti-TCRγδ antibody, twice during the HFD feeding period. CCL2 expression was reduced compared with isotype control antibody-treated mice, although IL-6 was not (Fig. 2B). Similar results were obtained in another cohort of mice treated i.p. with the antibody and by use of saline as control (Supplemental Fig. 3B). GL3+ cells could not be detected by flow cytometry in the blood of anti-TCRγδ antibody-treated mice, suggesting that at the time of death, the antibody had access to the γδ TCR (Supplemental Fig. 3A).
To evaluate time-dependent changes in eAT inflammation, TCRδ−/− mice were placed on the HFD for 10 weeks, a time when TNF-α expression was increased in WT mice. Body weight, eAT weight, and adipocyte size were not different between TCRδ+/+ and TCRδ−/− mice (Supplemental Fig. 2F and G). The eAT of TCRδ−/− mice had significantly lower mRNA expression of TNF-α compared with TCRδ+/+ after 10 weeks of HFD, but in contrast to the 5 week feeding studies, CCL2 and IL-6 expression was not reduced (Fig. 2C).
The potential contributions of the two γδ T cell subsets found in the eAT (Fig. 1E) to HFD-induced AT inflammation were assessed in Vγ4/6−/− mice, which had similar body weight and eAT weight as C57BL/6J WT mice after 5 weeks (Supplemental Fig. 2H) and 10 weeks of HFD (Supplemental Fig. 2I). Obese Vγ4/6−/− eAT had reduced mRNA expression of CCL2 and IL-6 after 5 weeks of HFD compared with WT eAT (Fig. 2D). After 10 weeks of HFD, there were no significant differences in TNF-α expression between WT and Vγ4/6−/− mice (Fig. 2E). These data suggest that Vγ4/6−/− mice respond to HFD in a manner similar to TCRδ−/− mice by failing to produce cytokines in eAT at 5 weeks of feeding but not at 10 weeks.
γδ T cells and cytokine expression by 3T3-L1 adipocytes in vitro
A chief source of CCL2 and IL-6 in the AT is the adipocyte [5]. Treatment of the differentiated 3T3-L1 adipocyte with eAT explant media from TCRδ+/+ mice, fed for 5 weeks on the HFD, significantly increased adipocyte CCL2 and IL-6 gene expression compared with treatment with media from ND-fed TCRδ+/+ mice (Fig. 3A). There was also a significant difference in gene expression observed for 3T3-L1 cells treated with media taken from eAT explants of HFD-fed TCRδ+/+ versus TCRδ−/− mice (Fig. 3A), suggesting that γδ T cells contributed to the HFD-induced cytokine increase from adipocytes. TNF-α expression could not be detected in any of the 3T3-L1 samples.
Figure 3. Effect of γδ T cells on cytokine expression by 3T3-L1 adipocytes and RAW264.7 macrophages in vitro.
(A) qPCR gene expression of CCL2 and IL-6 by differentiated 3T3-L1 adipocytes treated for 24 h with eAT explant media from TCRδ+/+ or TCRδ−/− mice after 5 weeks of ND or HFD; n = 3–5 replicates/group. (B) qPCR gene expression of CCL2 and IL-6 by differentiated 3T3-L1 adipocytes treated with CM from γδ T cells or cocultured with γδ T cells for 24 h. As control, γδ T cells were plated onto 3T3-L1 cells and washed away immediately before harvesting the 3T3-L1 cells for RNA isolation (0 h). γδ T cells were isolated from spleen of C57BL/6J mice fed HFD for 5 weeks; n = 3 replicates/group. (C) Preadipocyte (Pre-AD)-to-adipocyte differentiation of 3T3-L1 cells treated with eAT explant media from WT or TCRδ−/− mice fed ND or HFD for 5 weeks. Undifferentiated preadipocytes and adipocytes treated with DMEM or eAT explant control media or mouse recombinant TNF-α used as controls for 3T3-L1 adipocyte differentiation. Oil Red O staining performed on Day 8 of adipocyte differentiation; n = 6 replicates/group. (D) TNF-α gene expression by RAW264.7 murine macrophage cell line cocultured with γδ T cells from spleens of male WT mice on ND or HFD, as analyzed by qPCR. RAW264.7 cells were cocultured with γδ T cells at a ratio of 20:1 (macrophages:γδ T cells) for 24 h. As control, γδ T cells were plated onto RAW264.7 cells and washed away immediately before harvesting the RAW264.7 cells for RNA isolation; n = 3 replicates/group. Data from a representative experiment of 3 independent experiments. Values are mean ± sem. *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Tukey’s post hoc test.
A possible direct effect of γδ T cells was evaluated when 3T3-L1 adipocytes were treated in vitro with γδ T cell CM or cocultured with γδ T cells, isolated from spleen of HFD-fed WT mice. There was no increase in CCL2 and IL-6 gene expression by adipocytes (Fig. 3B), suggesting that γδ T cells may not have a direct effect on adipocytes. It appears that γδ T cells require the presence of other cells to increase adipocyte CCL2 and IL-6 expression. A caveat of this experiment was that γδ T cells were isolated from murine spleen. γδ T cells were insufficient in number to isolate from eAT (Fig. 1A). The number of γδ T cells in spleen increased after HFD (Fig. 1F), and others have shown Vγ1, Vγ2, and Vγ4 to be the major subsets of γδ T cells in spleen [24, 34]. We confirmed the presence of these subsets in the γδ T cell isolate obtained from spleen after magnetic separation (Supplemental Fig. 4). Vγ1, Vγ2, and Vγ4 subsets were found in eAT as well (Fig 1E), thus potentially supporting the use of splenic γδ T cells.
Treatment of 3T3-L1 preadipocytes with eAT explant media from WT or TCRδ−/− mice during the differentiation process did not significantly influence preadipocyte-to-adipocyte differentiation, as determined by the amount of lipid content within adipocytes (Fig. 3C), suggesting that γδ T cells do not modulate adipogenesis. Treatment with recombinant TNF-α blocked preadipocyte differentiation (Fig. 3C) [35] and was used as an experimental control.
γδ T cells directly increase TNF-α expression by RAW 264.7 macrophages in vitro
Another chief source of proinflammatory cytokines during obesity is AT macrophages [6]. To determine if γδ T cells can directly influence cytokine production by macrophages, we used RAW264.7 murine macrophage cell line. RAW264.7 cells were treated for 24 h with γδ T cell CM or cocultured with γδ T cells isolated from spleens of WT mice. Such treatment increased the mRNA expression of TNF-α from macrophages compared with the control treatment (Fig. 3D). There was no difference between γδ T cells isolated from spleen of ND-fed and HFD-fed mice (Fig. 3D). This might be a result of the isolation process of γδ T cells, which involves a positive selection step that could potentially activate γδ T cells. Alternatively, γδ T cells in the murine spleen may be naturally activated, as has been suggested by others [18], and the HFD chiefly increases their infiltration into spleen as shown in Fig 1F. Coculturing γδ T cells with RAW264.7 cells increased the expression of TNF-α significantly more than just treatment with γδ T cell CM, suggesting that γδ T cells increase macrophage TNF-α expression by cell contact-dependent and -independent means in vitro (Fig. 3D).
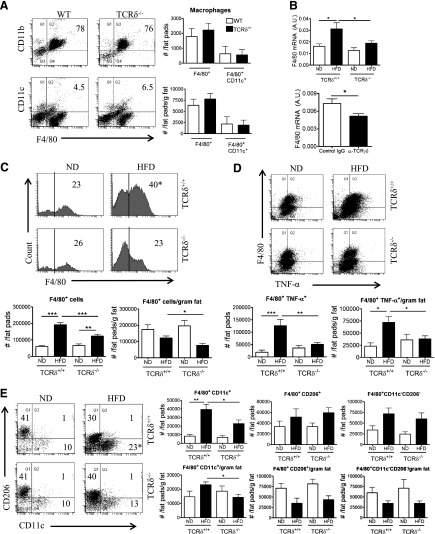
TCRδ−/− mice fed the HFD have fewer proinflammatory macrophages in eAT
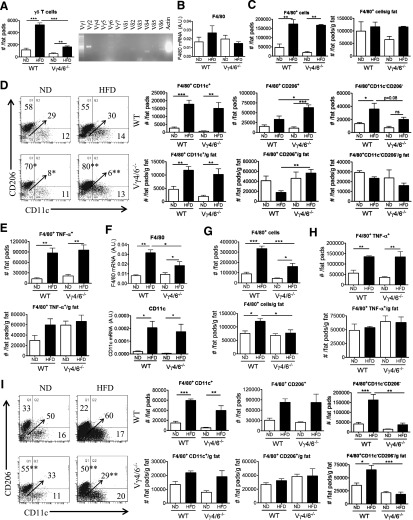
The effect of γδ T cells in vivo on macrophages was evaluated by use of HFD-fed control or TCRδ−/− mice by flow cytometry analysis by use of the gating strategy outlined in Supplemental Fig. 1B. There were no significant baseline differences in numbers of total and CD11c+ macrophages in the eAT of young WT and TCRδ−/− mice before starting the HFD (Fig. 4A). eAT from TCRδ−/− mice failed to show an increase in F4/80 gene expression after 5 weeks of HFD compared with TCRδ+/+ mice, which had significantly elevated F4/80 expression (Fig. 4B). In agreement, obese mice treated with anti-TCRγδ antibody had significantly reduced F4/80 mRNA expression relative to control-treated mice (Fig. 4B).
Figure 4. HFD-fed TCRδ−/− mice have fewer proinflammatory macrophages in eAT after 5 weeks of HFD.
(A) Flow cytometric analysis of F4/80+CD11b+ macrophages and F4/80+CD11c+ M1 macrophages in eAT of 5-week-old WT and TCRδ−/− mice at baseline before starting on HFD. Data are represented as number of cells/fat pads and number of cells/fat pads/gram fat; n = pool of eAT from 5 mice, 2–3 pools/group. (B) F4/80 mRNA expression in eAT of TCRδ−/− and TCRδ+/+ mice after 5 weeks of ND or HFD (n = 6–9 mice in each group) and in eAT of HFD-fed C57BL/6J mice treated with IgG isotype control antibody or anti-TCRγδ antibody (n = 5 mice in each group). *P < 0.05 by Student’s t-test, as determined by qPCR analysis. (C–E) Flow cytometric analysis of SVF cells in eAT of TCRδ−/− and TCRδ+/+ mice after 5 weeks of ND or HFD. (C) Total F4/80+ macrophages, represented as percentage of cells within macrophage gate based on scatter pattern, (D) TNF-α+ macrophages, and (E) F4/80+CD11c+CD206– M1, F4/80+CD11c–CD206+ M2, and F4/80+CD11c–CD206– DN macrophages; n = 6–9 mice in each group. Data from a representative experiment of 2 independent experiments. Data are represented as number of cells/fat pads and number of cells/fat pads/gram of fat and as a percentage of macrophages. Values are mean ± sem. *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Tukey’s post hoc test. Numbers inside boxes refer to percent of cells, and *P < 0.05 comparing HFD-fed groups of mice.
eAT of HFD-fed TCRδ−/− mice had significantly reduced numbers of macrophages/fat pads, numbers/fat pads/gram of fat, and percentage of total macrophages compared with HFD-fed TCRδ+/+ mice (Fig. 4C). Obese TCRδ−/− mice had significantly reduced CD11c+CD206– M1 macrophage in eAT than TCRδ+/+ mice (Fig. 4E). There was no significant difference in CD206+CD11c– M2 macrophage and CD11c–CD206– DN macrophage populations (Fig. 4E). Moreover, TCRδ−/− mice had fewer TNF-α+ macrophages in eAT compared with TCRδ+/+ mice after 5 weeks on HFD (Fig. 4D). Taken together, these results suggest that γδ T cells modulate the macrophage content in AT during HFD feeding.
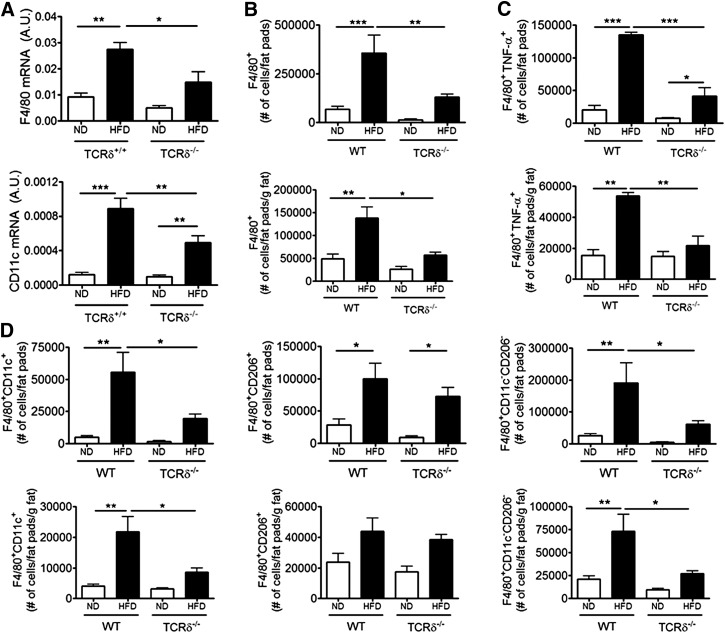
TCRδ−/− mice on 10 weeks HFD were also studied. The eAT from TCRδ−/− mice had significantly reduced gene expression of F4/80 and CD11c compared with TCRδ+/+ mice after 10 weeks of HFD (Fig. 5A). Flow cytometry analysis revealed that obese TCRδ−/− mice had significantly reduced total macrophages in eAT compared with TCRδ+/+ mice (Fig. 5B). Additionally, there was a defect in CD11c+CD206– M1, CD11c–CD206– DN, and TNF-α+ macrophage numbers in obese TCRδ−/− mice (Fig. 5C and D). There was no difference in the CD206+CD11c– M2 macrophage population between obese TCRδ−/− and TCRδ+/+ mice (Fig. 5D). These results suggest that γδ T cells are required for proinflammatory macrophage accumulation in eAT, even in the context of longer-term HFD feeding.
Figure 5. Quantification of macrophages in eAT of TCRδ−/− mice after 10 weeks of HFD.
(A) mRNA expression of F4/80 and CD11c in eAT of TCRδ−/− and TCRδ+/+ mice after 10 weeks of ND or HFD, as determined by qPCR analysis; n = 5–6 mice in each group. (B–D) Flow cytometric analysis of SVF cells in eAT of TCRδ−/− and WT mice after 10 weeks of ND or HFD. (B) Total F4/80+ macrophages, represented as percentage of cells within macrophage gate based on scatter pattern, (C) TNF-α+ macrophages, and (D) F4/80+CD11c+CD206– M1, F4/80+CD11c–CD206+ M2, and F4/80+CD11c–CD206– DN macrophages; n = 4–5 mice in each group. Data from a representative experiment of 2 independent experiments. Data are represented as number of cells/fat pads and number of cells/fat pads/gram of fat. Values are mean ± sem. *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Tukey’s post hoc test.
Vγ4/6−/− mice fed a HFD exhibit increased CD206+ M2 macrophages in eAT
The requirement for two specific subsets of γδ T cells in regulating macrophage accumulation in eAT after HFD was studied by use of Vγ4/6−/− mice, which had significantly reduced numbers of total γδ T cells after 5 weeks of HFD compared with WT mice (Fig. 6A). The expected subsets of γδ T cells from eAT of WT mice (Fig. 1E), except Vγ4 and Vγ6, were present in eAT of obese Vγ4/6−/− mice (Fig. 6A). In contrast to TCRδ−/−, 5 weeks of HFD did not alter F4/80 mRNA expression (Fig. 6B). In fact, by flow cytometry analysis, it was noted that after 5 weeks of HFD, Vγ4/6−/− mice eAT did not have a defect in accumulation of total, CD11c+CD206– M1 and TNF-α+ macrophages, unlike TCRδ−/− mice (Fig. 6C–E). However, HFD-fed Vγ4/6−/− mice eAT had significantly more CD206+CD11c– M2 macrophages, expressed in terms of numbers/fat pads and numbers/fat pads/gram fat and as a percentage of total macrophages compared with WT controls (Fig. 6D). Additionally, the eAT from obese Vγ4/6−/− mice had reduced CD11c–CD206– DN macrophages, expressed as a percentage of total macrophages (Fig. 6D).
Figure 6. HFD-fed Vγ4/6−/− mice have increased CD206+ M2 macrophages in eAT.
(A) Quantification of γδ T cells in eAT of Vγ4/6−/− and WT mice after 5 weeks of ND or HFD by flow cytometry analysis (n = 4–5 mice in each group; data from a representative experiment of 3 independent experiments) and PCR analysis to determine the γ and δ TCR chains expressed in eAT of Vγ4/6−/− mice after 5 weeks of HFD (representative gel picture of at least 3 repeat experiments). (B) mRNA expression of F4/80 in eAT of Vγ4/6−/− and WT mice after 5 weeks of ND or HFD, as determined by qPCR; n = 4–6 mice in each group. (C–E) Flow cytometric analysis of SVF cells in eAT of Vγ4/6−/− and WT mice after 5 weeks of ND or HFD. (C) Total F4/80+ macrophages, (D) F4/80+CD11c+CD206– M1, F4/80+CD11c–CD206+ M2, and F4/80+CD11c–CD206– DN macrophages, and (E) TNF-α+ macrophages; n = 4–5 mice in each group. Data from a representative experiment of 3 independent experiments. (F) mRNA expression of F4/80 and CD11c in eAT of Vγ4/6−/− and WT mice after 10 weeks of ND or HFD, as determined by qPCR; n = 4–6 mice in each group. (G–I) Flow cytometric analysis of SVF cells in eAT of Vγ4/6−/− and WT mice after 10 weeks of ND or HFD. (G) Total F4/80+ macrophages, (H) TNF-α+ macrophages, and (I) F4/80+CD11c+CD206– M1, F4/80+CD11c–CD206+ M2, and F4/80+CD11c–CD206– DN macrophages; n = 4–5 mice in each group. Data from a representative experiment of 2 independent experiments. Data are represented as number of cells/fat pads and number of cells/fat pads/gram of fat and as a percentage of macrophages. Values are mean ± sem. *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Tukey’s post hoc test. Numbers inside boxes refer to percent of cells, arrow points to value of double negative quadrant, and *P < 0.05, and **P < 0.01 comparing WT and Vγ4/6−/− mice on the same diet.
After 10 weeks of HFD, F4/80 gene expression was reduced in obese Vγ4/6−/− mice compared with obese WT mice, although there was no difference in CD11c expression between these two genotypes (Fig. 6F). Furthermore, there was now a defect in accumulation of total macrophages but not CD11c+CD206– M1 and TNF-α+ macrophages in eAT of obese Vγ4/6−/− mice after 10 weeks of HFD (Fig. 6G–I). The percentage of CD206+CD11c– M2 macrophages was increased in eAT of Vγ4/6−/− mice compared with WT mice on ND and HFD, although there was no difference in number of cells (Fig. 6I). The CD11c–CD206– DN macrophage population was reduced in terms of number of cells and percentage of total macrophages in eAT of obese Vγ4/6−/− mice (Fig. 6I). Hence, Vγ4 and Vγ6 subsets of γδ T cells apparently influence the presence of CD206+ macrophages in eAT, even after a longer term HFD.
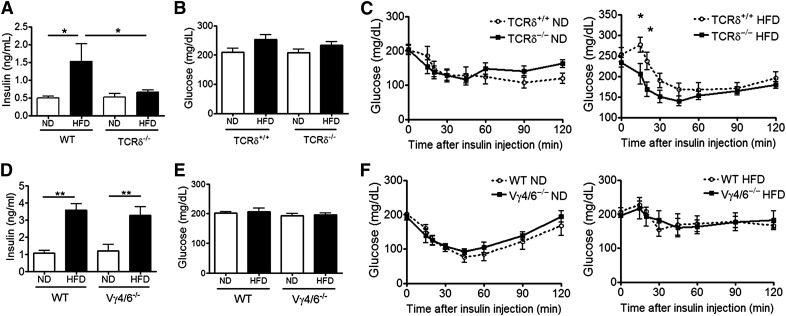
Obese TCRδ−/− mice but not Vγ4/6−/− mice are more insulin sensitive than WT mice
HFD-induced AT inflammation has been suggested to be one of the factors that contributes to systemic insulin resistance and Type 2 diabetes during obesity [1–3]. As obese TCRδ−/− mice have lowered inflammatory status in AT, we hypothesized that these mice will be more insulin sensitive than WT controls. C57BL/6J WT mice, after 5 weeks of HFD feeding, had elevated plasma insulin levels compared with ND-fed WT mice after 6 h of fasting (Fig 7A). The insulin levels in HFD-fed TCRδ−/− mice were reduced significantly compared with HFD-fed WT control mice (Fig 7A). To evaluate systemic insulin sensitivity, an ITT was conducted on mice fed the HFD for 10 weeks. The fasting blood glucose levels were not significantly different between groups (Fig. 7B). Both TCRδ+/+ and TCRδ−/− ND-fed mice were better able to clear blood glucose over time after insulin injection than HFD-fed mice (Fig. 7C). We observed significantly better clearance of blood glucose after insulin injection in HFD-fed TCRδ−/− mice than HFD-fed TCRδ+/+ mice, indicating that obese TCRδ−/− mice are more insulin sensitive than TCRδ+/+ mice (Fig. 7C).
Figure 7. Obese TCRδ−/− mice but not Vγ4/6−/− mice are more insulin sensitive than WT mice.
(A) Fasting insulin levels in plasma of nonlittermates TCRδ−/− and WT mice on 5 weeks of ND or HFD after a 6 h fast; n = 5–6 mice in each group. (B) Fasting blood glucose levels and (C) blood glucose levels during ITT (0.75 U insulin/kg body weight) of TCRδ−/− and TCRδ+/+ mice on 10 weeks of ND or HFD after a 6 h fast; n = 8–11 mice in each group. (D) Fasting plasma insulin levels of Vγ4/6−/− and WT mice on 5 weeks of ND or HFD after a 6 h fast; n = 4 mice in each group. (E) Fasting blood glucose levels and (F) blood glucose levels during ITT (0.75 U insulin/kg body weight) of Vγ4/6−/− and WT mice on 10 weeks of ND or HFD after a 6 h fast; n = 4 mice in each group. Values are mean ± sem, (A and D) *P < 0.05, and **P < 0.01 by one-way ANOVA with Tukey's post hoc test. (C and F) *P < 0.05 by two-way ANOVA with Bonferroni’s post hoc test.
The insulin sensitivity of obese Vγ4/6−/− mice was also tested. After 5 weeks of HFD, C57BL/6J WT, as well as Vγ4/6−/−, mice had similarly elevated insulin levels in the plasma after 6 h of fasting compared with ND-fed mice (Fig. 7D). After 10 weeks of feeding, the fasting blood glucose levels were not significantly different between groups (Fig. 7E). After insulin injection during the ITT, WT and Vγ4/6−/− ND-fed mice were better able to clear blood glucose over time than HFD-fed mice (Fig. 7F). Furthermore, there was no significant difference between glucose levels after insulin injection in HFD-fed WT and Vγ4/6−/− mice (Fig. 7F), suggesting that obese Vγ4/6−/− mice are insulin resistant, similar to obese WT mice, which is different from TCRδ−/− mice.
Obese TCRδ−/− mice exhibit reduced inflammation in skeletal muscle and liver after long-term HFD
The systemic effects of HFD in TCRδ−/− mice were studied further by evaluating inflammation in liver and skeletal muscle, two other tissues apart from AT that have been shown to influence whole-body insulin sensitivity [2]. An inflammatory response could not be detected in WT mice in either tissue after 5 weeks HFD (data not shown). After 10 weeks of HFD, soleus muscle of TCRδ+/+ mice showed significantly increased mRNA expression of TNF-α and CCL2, whereas TCRδ−/− mice failed to show this increase (Fig. 8A). F4/80 expression did not differ between groups (Fig. 8A). Gastrocnemius muscle did not show an inflammatory response, even after 10 weeks HFD (data not shown). Furthermore, livers of obese TCRδ−/− mice, after 6 months of HFD, had significantly lowered gene expression of CCL2 and F4/80 in liver than obese WT mice (Fig. 8B). IL-6 gene expression in liver did not differ between groups (Fig. 8B). Additionally, the total number of γδ T cells in the liver of C57BL/6J mice after 5 weeks of HFD was increased compared with the liver of ND-fed mice, suggesting that there is infiltration of γδ T cells into liver during obesity (Fig. 8C). Thus, along with eAT, liver and soleus muscle of obese TCRδ−/− mice exhibited reduced expression of certain inflammatory markers compared with WT controls.
Figure 8. Obese TCRδ−/− mice have reduced inflammation in skeletal muscle and liver after long-term HFD.
(A) mRNA expression of TNF-α, CCL2, and F4/80 in soleus muscle of TCRδ−/− and TCRδ+/+ mice after 10 weeks of ND or HFD, as determined by qPCR analysis; n = 5–6 mice in each group. *P < 0.05 by one-way ANOVA with Tukey’s post hoc test. (B) mRNA expression of CCL2, F4/80, and IL-6 in liver of TCRδ−/− and WT mice after 24 weeks on HFD; n = 5–6 mice in each group. *P < 0.05, and **P < 0.01 by Student’s t-test. (C) Flow cytometry analysis of CD3+GL3+ γδ T cells in liver of male C57BL/6J mice after 5 weeks of ND (white bars) or HFD (black bars). Data are represented as total number of γδ T cells in liver/mouse; n = 7–8 mice in each group. *P < 0.05 by Student’s t-test. Values are mean ± sem.
DISCUSSION
AT has resident populations of immune cells, including regulatory T, Th2, and innate lymphoid cells and eosinophils that help maintain alternatively activated macrophages [36]. During obesity, the balance shifts, with proinflammatory cells accumulating within AT, including myeloid cells, such as neutrophils, mast cells, classically activated macrophages, and dendritic cells and lymphocytes, such as αβ T (CD8+ T, CD4+ Th1, and Th17 cells), B, NKT, NK, and γδ T cells [15, 16, 36]. The role of NKT and NK cells in the inflammatory response in obese AT remains unclear, but CD8+ T cells apparently contribute to the recruitment and activation of macrophages [13], CD4+ Th1 cells contribute to the inflammatory cytokines milieu [37, 38], and B cells may promote inflammation by modulating T cells and macrophages and secreting pathogenic autoantibodies and cytokines [14, 39].
Previous reports have shown the presence of γδ T cells in eAT of lean mice and mice fed 36% fat diet and 60% lard fat diet for 12–16 weeks [15, 20, 21]. γδ T cells are innate-like cells that quite often play a role early on in inflammatory processes [17]. We confirmed the presence of γδ T cells in eAT of lean mice, and their numbers increased proportional to the increased eAT fat mass in mice fed a 42% milk HFD for 5 weeks. The percentage of γδ T cells, as expressed, relative to total leukocytes and total T cells in the eAT did not change, probably as these other cell populations also increase in number during obesity. These γδ T cells are in a CD44hi- and CD62Llo-activated state. Most peripheral γδ T cells are naturally activated CD44hi and CD62Llo [18] and spontaneously express cytokines that can be rapidly up-regulated upon TCR activation [40, 41]. We found that TNF-α+ γδ T cells are increased in eAT relative to fat mass increase after HFD, whereas IL-17+ γδ T cells represent a very small subpopulation of γδ T cells in eAT that did not increase after 5 weeks of HFD, although others have reported increases in this subpopulation after a long-term lard fat diet [20]. It remains to be understood how a HFD increases TNF-α+ γδ T cells in eAT.
Our studies, by use of TCRδ−/− mice, which lack all γδ T cells, and Vγ4/6−/− mice, which lack specific subsets of γδ T cells, suggest that γδ T cells contribute to AT inflammation by regulating chemokine and cytokine expression after 5 weeks (CCL2 and IL-6) and 10 weeks (TNF-α) of HFD feeding. Experiments, by use of an anti-TCRγδ antibody to block γδ TCR, suggest that γδ T cells, through their TCR, contribute to AT inflammation. It is unclear what in the AT may activate γδ TCR. We also show that eAT from TCRδ−/− mice has a reduced accumulation of macrophages, specifically CD11c+CD206– M1 and TNF-α+ macrophages, after HFD feeding compared with WT controls. This is consistent with a previously recognized ability of γδ T cells to promote proinflammatory macrophages [42]. During obesity, monocytes infiltrate and differentiate into proinflammatory M1-like macrophages in AT, which play an important role in development of inflammation and insulin resistance [10–12]. It is clear from other studies that B cells and CD4+, and CD8+ T cells are important for regulating proinflammatory macrophage accumulation and activation in AT [13, 14, 43], and the current study provides evidence for a significant influence of γδ T cells to this inflammatory cascade. The finding that different markers of inflammation show elevated gene expression in eAT after different time periods of HFD feeding, CCL2, and IL-6 after 5 weeks and TNF-α and CD11c after 10 weeks suggests that during obesity, there may be progressive development of inflammation over time, as has been suggested by others [7, 44]. Importantly, TCRδ−/− mice show reduced markers of inflammation, as well as reduced macrophage accumulation even after 10 weeks of HFD, indicating an influence of γδ T cells at both the progressing and later stages of development of AT inflammation during obesity.
Murine γδ T cells can be classified into subsets based on their γ and δ TCR chains [25]. Subsets prominently home to particular tissues or organs, where they perform specialized functions [19]. We identified Vγ1, Vγ2, Vγ4, and Vγ6 subsets and Vδ1, Vδ3, and Vδ4 subsets of the γδ T cell in the AT. The smear in the Vγ1 lane during PCR gel electrophoresis suggests clonality within the Vγ1 γδ T cell subset, probably as a result of junctional diversity, a process well-documented in the γδ TCR by others [45]. To assess functions of two of the subsets, we studied Vγ4/6−/− mice. These mice have significantly reduced numbers of γδ T cells in eAT compared with WT mice after HFD, suggesting that most of γδ T cells accumulating in WT mice are Vγ4 and Vγ6 subsets themselves, or they require these subsets for their accumulation. Furthermore, Vγ4/6−/− mice do not have a defect in accumulation of CD11c+ M1 macrophages but instead, have increased numbers of CD206+ macrophages (consistent with M2-polarized macrophages), suggesting that Vγ4 and Vγ6 subsets contribute to HFD-induced eAT inflammation by preventing accumulation of anti-inflammatory M2 macrophages, thus allowing the development of a proinflammatory environment in AT of HFD-fed WT mice. Additionally, Vγ4/6−/− mice have reduced CD11c–CD206– DN macrophages in eAT, which have been identified by others to express various chemokine receptors and thus, could potentially play a role in AT inflammation [46]. Our results are consistent with other studies, indicating that Vγ4 γδ T cells regulate the polarization of macrophages [42, 47, 48]. We speculate that the other subsets of γδ T cells present in eAT may play a role in the diet-induced accumulation of M1 macrophages. These data show that Vγ4 and Vγ6 subsets play a previously unidentified, proinflammatory role in AT inflammation during obesity.
We found that obese TCRδ−/− mice have reduced hyperinsulinemia and are more insulin sensitive than TCRδ+/+ mice, in contrast to a previous study that showed no difference in insulin resistance between TCRδ−/− and WT mice [20]. This may be attributed to differences in HFD, length of feeding study, or difference in WT control mice. However, obese Vγ4/6−/− mice are not protected from hyperinsulinemia and insulin resistance, suggesting that Vγ4 and Vγ6 subsets may not contribute to insulin resistance and that other subsets may play a greater role in promoting it during obesity. With progressing obesity, other metabolically active organs, such as liver and skeletal muscle, have been shown to develop signs of inflammation as well, which may further contribute to insulin resistance [2]. γδ T cells, especially the Vγ1 subset, are found abundantly in the liver and change in number and function with ongoing inflammation [19]. γδ T cells have been shown to infiltrate into inflamed muscle [49]. We show that obese TCRδ−/− mice have reduced inflammation in liver and soleus skeletal muscle, suggesting that γδ T cells promote HFD-induced inflammation in these tissues. These phenotypic differences were noted after longer-term HFD feeding studies, indicating that observable inflammation in liver and muscle and systemic insulin resistance in WT mice occur after more prolonged exposure to obesogenic conditions. It is not yet clear if the proinflammatory effect of γδ T cells on inflammation in these organs is a direct influence of γδ T cells infiltrating into these organs during obesity and/or the effect of γδ T cells located elsewhere. γδ T cell accumulation was detectable in the livers of obese mice (Fig 8C), although was not detectable in skeletal muscle (data not shown).
TNF-α, CCL2, and IL-6 are elevated in AT during obesity and play key roles in mediating inflammation, as well as insulin resistance [5, 50–52]. Adipocytes and macrophages are the major sources of these inflammatory mediators in AT [5, 6]. Our results with in vitro experiments suggest that γδ T cells directly activate macrophage TNF-α expression in a cell contact-dependent and -independent manner and indirectly activate adipocyte CCL2 and IL-6 expression. We speculate that this indirect effect of γδ T cells on adipocytes may be mediated through macrophages, which are decreased significantly in HFD-fed TCRδ−/− mice. Macrophages, through their ability to produce various inflammatory mediators, such as TNF-α, have been shown to enhance adipocyte inflammation and diminish insulin sensitivity [12, 53]. Thus, we propose that γδ T cells may be an important additional player in the inflammatory crosstalk between adipocytes and macrophages.
To summarize, in this study, we report that total and TNF-α-expressing γδ T cells are increased in eAT of mice fed HFD. We describe the novel finding that mice that lack γδ T cells have reduced HFD-induced inflammation, as measured by CCL2, IL-6, and TNF-α gene expression in AT. We made the novel observation that Vγ4 and Vγ6 subsets of γδ T cells contribute to AT inflammation induced by HFD. Additionally, we show that γδ T cells positively contribute to AT inflammation by regulating the AT macrophage populations. Importantly, we report that obese TCRδ−/− mice show improved insulin sensitivity and lowered liver and skeletal muscle inflammation, indicating that γδ T cells contribute to the development of the metabolic syndrome. These data reveal that γδ T cells play an important role in the well-established inflammatory response in AT and the increased insulin resistance in the context of obesity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK078847-01A2 and U.S. Department of Agriculture/Agricultural Research Service Children's Nutrition Research Center grant 6250-51000-046.
Glossary
- −/−
deficient
- AT
adipose tissue
- ATCC
American Type Culture Collection
- CD
cluster of differentiation
- CD62L
cluster of differentiation 62 ligand
- CM
conditioned medium
- DN
double-negative
- eAT
epididymal adipose tissue
- eAT-ex
epididymal adipose tissue explant culture medium
- HFD
high fat diet
- ITT
insulin tolerance test
- ND
normal diet
- qPCR
quantitative PCR
- SVF
stromal vascular fraction
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
AUTHORSHIP
P.M. designed the experiments. P.M. and A.N.A. performed the experiments. P.M. and C.W.S. analyzed the data, interpreted the results, designed the project, and wrote the paper.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Hotamisligil G. S. (2006) Inflammation and metabolic disorders. Nature 444, 860–867. [DOI] [PubMed] [Google Scholar]
- 2.Lumeng C. N., Saltiel A. R. (2011) Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoelson S. E., Lee J., Goldfine A. B. (2006) Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg A. S., Obin M. S. (2006) Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 83, 461S–465S. [DOI] [PubMed] [Google Scholar]
- 5.Ouchi N., Parker J. L., Lugus J. J., Walsh K. (2011) Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W. Jr. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., Chen H. (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris D. L., Singer K., Lumeng C. N. (2011) Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr. Opin. Clin. Nutr. Metab. Care 14, 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patsouris D., Li P. P., Thapar D., Chapman J., Olefsky J. M., Neels J. G. (2008) Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisberg S. P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K., Charo I., Leibel R. L., Ferrante A. W. Jr. (2006) CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chawla A., Nguyen K. D., Goh Y. P. (2011) Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 11, 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S., Yoshimura K., Kadowaki T., Nagai R. (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920. [DOI] [PubMed] [Google Scholar]
- 14.Winer D. A., Winer S., Shen L., Wadia P. P., Yantha J., Paltser G., Tsui H., Wu P., Davidson M. G., Alonso M. N., Leong H. X., Glassford A., Caimol M., Kenkel J. A., Tedder T. F., McLaughlin T., Miklos D. B., Dosch H. M., Engleman E. E. (2011) B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17, 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caspar-Bauguil S., Cousin B., Galinier A., Segafredo C., Nibbelink M., André M., Casteilla L., Pénicaud L. (2005) Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 579, 3487–3492. [DOI] [PubMed] [Google Scholar]
- 16.Chatzigeorgiou A., Karalis K. P., Bornstein S. R., Chavakis T. (2012) Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia 55, 2583–2592. [DOI] [PubMed] [Google Scholar]
- 17.Bonneville M., O’Brien R. L., Born W. K. (2010) Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 10, 467–478. [DOI] [PubMed] [Google Scholar]
- 18.Tough D. F., Sprent J. (1998) Lifespan of gamma/delta T cells. J. Exp. Med. 187, 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carding S. R., Egan P. J. (2002) Gammadelta T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2, 336–345. [DOI] [PubMed] [Google Scholar]
- 20.Zúñiga L. A., Shen W. J., Joyce-Shaikh B., Pyatnova E. A., Richards A. G., Thom C., Andrade S. M., Cua D. J., Kraemer F. B., Butcher E. C. (2010) IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 185, 6947–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang E., Perrard X. D., Yang D., Khan I. M., Perrard J. L., Smith C. W., Ballantyne C. M., Wu H. (2014) Essential role of CD11a in CD8+ T-cell accumulation and activation in adipose tissue. Arterioscler. Thromb. Vasc. Biol. 34, 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itohara S., Mombaerts P., Lafaille J., Iacomini J., Nelson A., Clarke A. R., Hooper M. L., Farr A., Tonegawa S. (1993) T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell 72, 337–348. [DOI] [PubMed] [Google Scholar]
- 23.Sunaga S., Maki K., Komagata Y., Miyazaki J., Ikuta K. (1997) Developmentally ordered V-J recombination in mouse T cell receptor gamma locus is not perturbed by targeted deletion of the Vgamma4 gene. J. Immunol. 158, 4223–4228. [PubMed] [Google Scholar]
- 24.Andrew E. M., Newton D. J., Dalton J. E., Egan C. E., Goodwin S. J., Tramonti D., Scott P., Carding S. R. (2005) Delineation of the function of a major gamma delta T cell subset during infection. J. Immunol. 175, 1741–1750. [DOI] [PubMed] [Google Scholar]
- 25.Heilig J. S., Tonegawa S. (1986) Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature 322, 836–840. [DOI] [PubMed] [Google Scholar]
- 26.Brake D. K., Smith C. W. (2008) Flow cytometry on the stromal-vascular fraction of white adipose tissue. Methods Mol. Biol. 456, 221–229. [DOI] [PubMed] [Google Scholar]
- 27.Mehal W. Z., Crispe I. N. (1998) TCR ligation on CD8+ T cells creates double-negative cells in vivo. J. Immunol. 161, 1686–1693. [PubMed] [Google Scholar]
- 28.Koenecke C., Chennupati V., Schmitz S., Malissen B., Förster R., Prinz I. (2009) In vivo application of mAb directed against the gammadelta TCR does not deplete but generates “invisible” gammadelta T cells. Eur. J. Immunol. 39, 372–379. [DOI] [PubMed] [Google Scholar]
- 29.Haas J. D., González F. H., Schmitz S., Chennupati V., Föhse L., Kremmer E., Förster R., Prinz I. (2009) CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur. J. Immunol. 39, 3488–3497. [DOI] [PubMed] [Google Scholar]
- 30.Ribot J. C., deBarros A., Pang D. J., Neves J. F., Peperzak V., Roberts S. J., Girardi M., Borst J., Hayday A. C., Pennington D. J., Silva-Santos B. (2009) CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 10, 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotamisligil G. S., Shargill N. S., Spiegelman B. M. (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91. [DOI] [PubMed] [Google Scholar]
- 32.Uysal K. T., Wiesbrock S. M., Marino M. W., Hotamisligil G. S. (1997) Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389, 610–614. [DOI] [PubMed] [Google Scholar]
- 33.Ventre J., Doebber T., Wu M., MacNaul K., Stevens K., Pasparakis M., Kollias G., Moller D. E. (1997) Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes 46, 1526–1531. [DOI] [PubMed] [Google Scholar]
- 34.Pereira P., Gerber D., Huang S. Y., Tonegawa S. (1995) Ontogenic development and tissue distribution of V gamma 1-expressing gamma/delta T lymphocytes in normal mice. J. Exp. Med. 182, 1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pape M. E., Kim K. H. (1988) Effect of tumor necrosis factor on acetyl-coenzyme A carboxylase gene expression and preadipocyte differentiation. Mol. Endocrinol. 2, 395–403. [DOI] [PubMed] [Google Scholar]
- 36.Ferrante A. W., Jr. (2013) The immune cells in adipose tissue. Diabetes Obes. Metab. 15(Suppl 3), 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H., Ghosh S., Perrard X. D., Feng L., Garcia G. E., Perrard J. L., Sweeney J. F., Peterson L. E., Chan L., Smith C. W., Ballantyne C. M. (2007) T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 115, 1029–1038. [DOI] [PubMed] [Google Scholar]
- 38.Strissel K. J., DeFuria J., Shaul M. E., Bennett G., Greenberg A. S., Obin M. S. (2010) T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 18, 1918–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeFuria J., Belkina A. C., Jagannathan-Bogdan M., Snyder-Cappione J., Carr J. D., Nersesova Y. R., Markham D., Strissel K. J., Watkins A. A., Zhu M., Allen J., Bouchard J., Toraldo G., Jasuja R., Obin M. S., McDonnell M. E., Apovian C., Denis G. V., Nikolajczyk B. S. (2013) B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. USA 110, 5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin Z., Chen C., Szabo S. J., Glimcher L. H., Ray A., Craft J. (2002) T-Bet expression and failure of GATA-3 cross-regulation lead to default production of IFN-gamma by gammadelta T cells. J. Immunol. 168, 1566–1571. [DOI] [PubMed] [Google Scholar]
- 41.He W., Hao J., Dong S., Gao Y., Tao J., Chi H., Flavell R., O’Brien R. L., Born W. K., Craft J., Han J., Wang P., Zhao L., Wu J., Yin Z. (2010) Naturally activated V gamma 4 gamma delta T cells play a protective role in tumor immunity through expression of eomesodermin. J. Immunol. 185, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tramonti D., Andrew E. M., Rhodes K., Newton D. J., Carding S. R. (2006) Evidence for the opposing roles of different gamma delta T cell subsets in macrophage homeostasis. Eur. J. Immunol. 36, 1729–1738. [DOI] [PubMed] [Google Scholar]
- 43.Winer S., Chan Y., Paltser G., Truong D., Tsui H., Bahrami J., Dorfman R., Wang Y., Zielenski J., Mastronardi F., Maezawa Y., Drucker D. J., Engleman E., Winer D., Dosch H. M. (2009) Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 15, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregor M. F., Hotamisligil G. S. (2011) Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445. [DOI] [PubMed] [Google Scholar]
- 45.Raulet D. H. (1989) The structure, function, and molecular genetics of the gamma/delta T cell receptor. Annu. Rev. Immunol. 7, 175–207. [DOI] [PubMed] [Google Scholar]
- 46.Zeyda M., Gollinger K., Kriehuber E., Kiefer F. W., Neuhofer A., Stulnig T. M. (2010) Newly identified adipose tissue macrophage populations in obesity with distinct chemokine and chemokine receptor expression. Int. J. Obes. (Lond). 34, 1684–1694. [DOI] [PubMed] [Google Scholar]
- 47.Huber S. A., Born W., O’Brien R. (2005) Dual functions of murine gammadelta cells in inflammation and autoimmunity in coxsackievirus B3-induced myocarditis: role of Vgamma1+ and Vgamma4+ cells. Microbes Infect. 7, 537–543. [DOI] [PubMed] [Google Scholar]
- 48.Mosser D. M. (2003) The many faces of macrophage activation. J. Leukoc. Biol. 73, 209–212. [DOI] [PubMed] [Google Scholar]
- 49.O’Hanlon T. P., Messersmith W. A., Dalakas M. C., Plotz P. H., Miller F. W. (1995) Gamma delta T cell receptor gene expression by muscle-infiltrating lymphocytes in the idiopathic inflammatory myopathies. Clin. Exp. Immunol. 100, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cawthorn W. P., Sethi J. K. (2008) TNF-alpha and adipocyte biology. FEBS Lett. 582, 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eder K., Baffy N., Falus A., Fulop A. K. (2009) The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res. 58, 727–736. [DOI] [PubMed] [Google Scholar]
- 52.Panee J. (2012) Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine 60, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suganami T., Nishida J., Ogawa Y. (2005) A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler. Thromb. Vasc. Biol. 25, 2062–2068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.