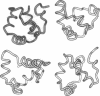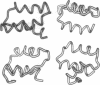Abstract
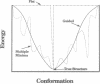
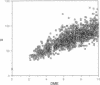
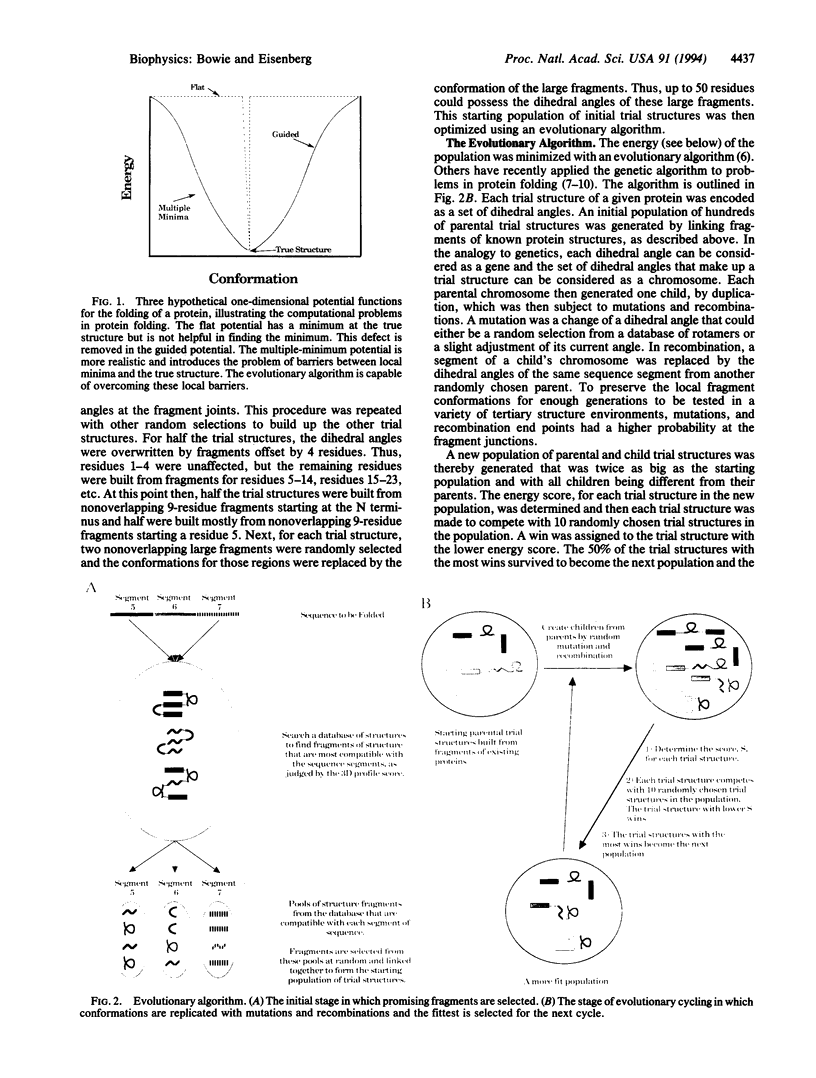
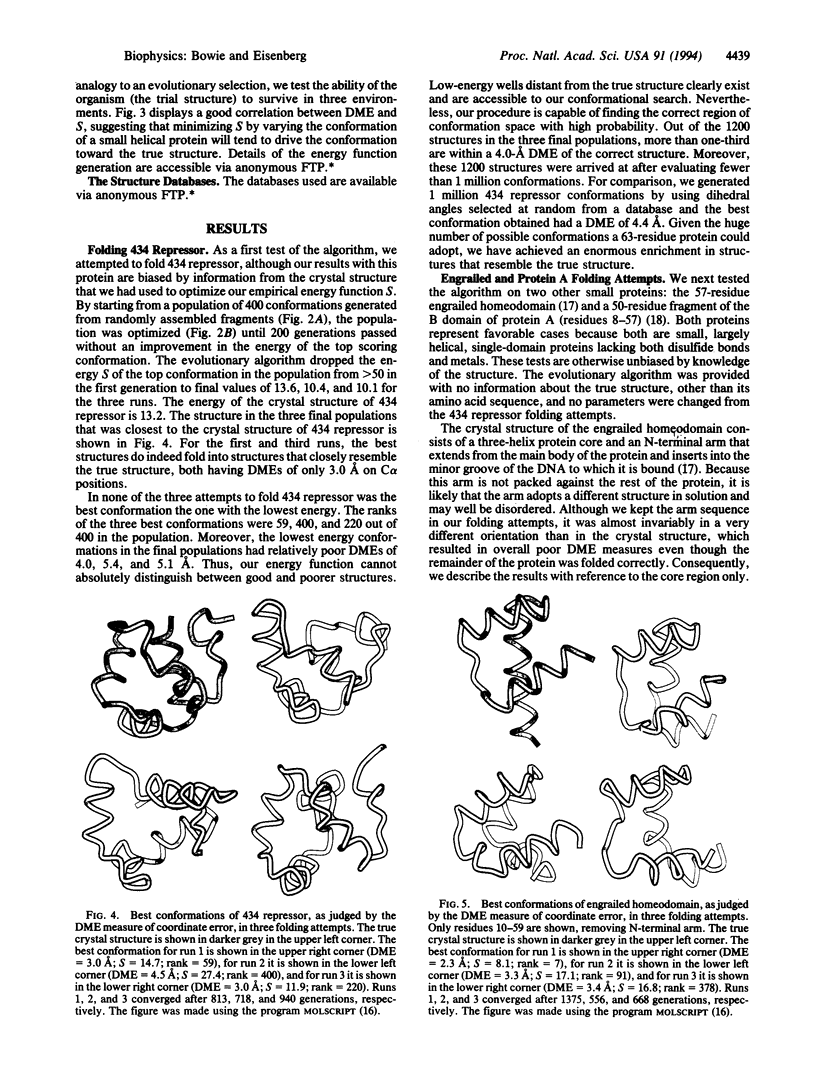
Three short protein sequences have been guided by computer to folds resembling their crystal structures. Initially, peptide fragment conformations ranging in size from 9 to 25 residues were selected from a database of known protein structures. A fragment was selected if it was compatible with a segment of the sequence to be folded, as judged by three-dimensional profile scores. By linking the selected fragment conformations together, hundreds of trial structures were generated of the same length and sequence as the protein to be folded. These starting trial structures were then improved by an evolutionary algorithm. Selection pressure for improving the structures was provided by an energy function that was designed to guide the conformational search procedure toward the correct structure. We find that by evolution of only 400 structures for fewer than 1400 generations, the overall fold of some small helical proteins can be computed from the sequence, with deviations from observed structures of 2.5-4.0 A for C alpha atoms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Bowie J. U., Lüthy R., Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure. Science. 1991 Jul 12;253(5016):164–170. doi: 10.1126/science.1853201. [DOI] [PubMed] [Google Scholar]
- Dandekar T., Argos P. Potential of genetic algorithms in protein folding and protein engineering simulations. Protein Eng. 1992 Oct;5(7):637–645. doi: 10.1093/protein/5.7.637. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981 Apr 28;20(9):2361–2370. [PubMed] [Google Scholar]
- Gouda H., Torigoe H., Saito A., Sato M., Arata Y., Shimada I. Three-dimensional solution structure of the B domain of staphylococcal protein A: comparisons of the solution and crystal structures. Biochemistry. 1992 Oct 13;31(40):9665–9672. doi: 10.1021/bi00155a020. [DOI] [PubMed] [Google Scholar]
- Kissinger C. R., Liu B. S., Martin-Blanco E., Kornberg T. B., Pabo C. O. Crystal structure of an engrailed homeodomain-DNA complex at 2.8 A resolution: a framework for understanding homeodomain-DNA interactions. Cell. 1990 Nov 2;63(3):579–590. doi: 10.1016/0092-8674(90)90453-l. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Mondragón A., Subbiah S., Almo S. C., Drottar M., Harrison S. C. Structure of the amino-terminal domain of phage 434 repressor at 2.0 A resolution. J Mol Biol. 1989 Jan 5;205(1):189–200. doi: 10.1016/0022-2836(89)90375-6. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Suchanek E. G. Computer-aided model-building strategies for protein design. Biochemistry. 1986 Oct 7;25(20):5987–5991. doi: 10.1021/bi00368a023. [DOI] [PubMed] [Google Scholar]
- Sander C., Schneider R. Database of homology-derived protein structures and the structural meaning of sequence alignment. Proteins. 1991;9(1):56–68. doi: 10.1002/prot.340090107. [DOI] [PubMed] [Google Scholar]
- Sun S. Reduced representation model of protein structure prediction: statistical potential and genetic algorithms. Protein Sci. 1993 May;2(5):762–785. doi: 10.1002/pro.5560020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R., Moult J. Genetic algorithms for protein folding simulations. J Mol Biol. 1993 May 5;231(1):75–81. doi: 10.1006/jmbi.1993.1258. [DOI] [PubMed] [Google Scholar]
- Yamashita M. M., Wesson L., Eisenman G., Eisenberg D. Where metal ions bind in proteins. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5648–5652. doi: 10.1073/pnas.87.15.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehfus M. H., Seltzer J. P., Rose G. D. Fast approximations for accessible surface area and molecular volume of protein segments. Biopolymers. 1985 Dec;24(12):2511–2519. doi: 10.1002/bip.360241222. [DOI] [PubMed] [Google Scholar]