Abstract
The asymmetric BODIPY 1a (BODIPY=4,4-difluoro-4-bora-3a,4a-diaza-s-indacene), containing two chloro substituents at the 3,8-positions and a reactive 5-methyl group, was synthesized from the asymmetric dipyrroketone 3, which was readily obtained from available pyrrole 2a. The reactivity of 3,8-dichloro-6-ethyl-1,2,5,7-tetramethyl-BODIPY 1a was investigated by using four types of reactions. This versatile BODIPY undergoes regioselective Pd0-catalyzed Stille coupling reactions and/or regioselective nucleophilic addition/elimination reactions, first at the 8-chloro and then at the 3-chloro group, using a variety of organostannanes and N-, O-, and S-centered nucleophiles. On the other hand, the more reactive 5-methyl group undergoes regioselective Knoevenagel condensation with an aryl aldehyde to produce a monostyryl-BODIPY, and oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) gives the corresponding 5-formyl-BODIPY. Investigation of the reactivity of asymmetric BODIPY 1a led to the preparation of a variety of functionalized BODIPYs with λmax of absorption and emission in the ranges 487–587 and 521–617 nm, respectively. The longest absorbing/emitting compound was the monostyryl-BODIPY 16, and the largest Stokes shift (49 nm) and fluorescence quantum yield (0.94) were measured for 5-thienyl-8-phenoxy-BODIPY 15. The structural properties (including 16 X-ray structures) of the new series of BODIPYs were investigated.
Keywords: addition/elimination, BODIPY, fluorescence, Pd0-catalyzed coupling, oxidation, substitution
Introduction
4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene dyes, known generically as borondipyrromethenes or BODIPYs, are very versatile compounds that have attracted much attention since they were first reported in 1968.[1] Due to their remarkable properties, including high extinction coefficients, and generally high fluorescence quantum yields, low molecular weights, and high thermal and photochemical stabilities,[2] BODIPYs have been actively investigated in protein[3] and DNA-labeling,[3a, 4] drug delivery,[3a, 5] light-harvesting arrays,[6] fluorescent switches,[7] and in medical imaging and theranostics.[8]
A traditional approach to the synthesis of BODIPY scaffolds involves the condensation reaction between excess α-free pyrroles and aldehydes[9] in the presence of a Lewis acid, followed by DDQ oxidation to dipyrromethene, and then boron complexation, using boron trifluoride diethyl etherate under basic conditions. Alternatively, α-free pyrroles react with acid chlorides[10] or anhydrides[11] to directly yield dipyrromethenes. Another efficient methodology for the preparation of both symmetric and asymmetric meso-free BODIPYs is by condensation of a 2-formylpyrrole with an α-free pyrrole by using POCl3 as a catalyst, followed by boron complexation.[12] Similarly, meso-aryl BODIPYs can be prepared from the reaction of 2-ketopyrrole[13] with an α-free pyrrole fragment.[14] Alternatively, the synthesis of symmetric 8-functionalized BODIPYs has been reported via a dipyrrothioketone[15] or dipyrroketone[16] intermediate; this route offers expeditious access to 8-thio or 8-halo-BODIPYs, respectively.
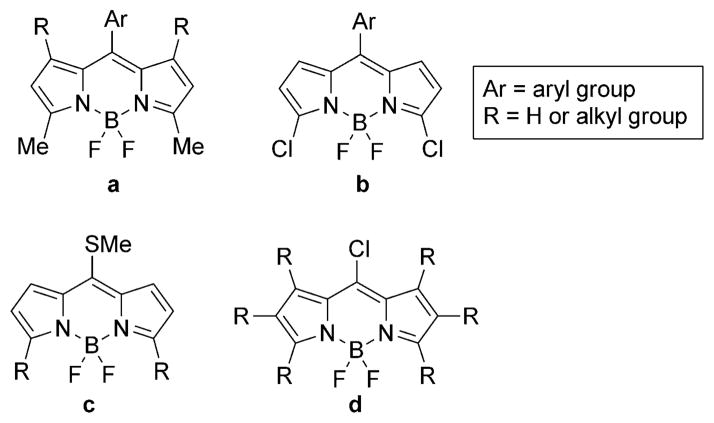
Using the above methods, a number of BODIPY platforms, such as those in Figure 1, have been synthesized and their reactivity investigated.[2] For example, the α-methyl groups on BODIPY platform a undergo Knoevenagel condensations with various aldehydes, to produce the corresponding styryl-BODIPYs that display significant redshifted absorption and emission spectra.[17] On the other hand, BODIPY platform b bearing 3,5-chloro groups undergoes milder Pd0-catalyzed cross-coupling reactions and nucleophilic substitution reactions at these positions, producing a variety of functionalized BODIPYs for various applications.[18] Functionalizations at the 8-position of BODIPYs are reported for platform c,[15, 19] which undergoes nucleophilic addition/elimination and Liebeskind–Srogl cross-coupling reactions, and also for platform d bearing an 8-chloro group.[16] Compared with the 8-methylthio-BODIPY c, platform d is more versatile, reacting with a variety of N-, O-, and S-centered nucleophiles, as well as under a variety of Pd0-catalyzed Stille, Suzuki, and Sonogashira coupling conditions, producing the corresponding functionalized BODIPYs in good to quantitative yields.
Figure 1.
Examples of reported BODIPY platforms.
We have recently synthesized a symmetric 3,5,8-trichloro-BODIPY dye and explored its regioselectivity in Stille cross-coupling conditions.[20] Herein we report the synthesis of a versatile, asymmetric BODIPY 1a bearing two chloro substituents at positions 3 and 8, as well as a reactive 5-methyl group, starting from an asymmetric dipyrroketone. We show that BODIPY 1a can be regioselectively functionalized using four types of reactions, at positions 3, 5 and 8, to produce new conjugated BODIPYs. The nucleophilic addition/elimination and cross-coupling reactions both occur regioselectively at the 8-chloro followed by the 3-chloro position, allowing stepwise functionalization using various organometallic reagents and N-, O-, and S-centered nucleophiles. This is the first report on the meso-versus α-position regioselectivity of BODIPY nucleophilic reactions. Furthermore, the 5-methyl group undergoes regioselective Knoevenagel and oxidation reactions, providing access to additional functionalized BODIPY platforms. The structural and spectroscopic properties of the new BODIPYs were investigated.
Results and Discussion
Synthesis and structural characterization
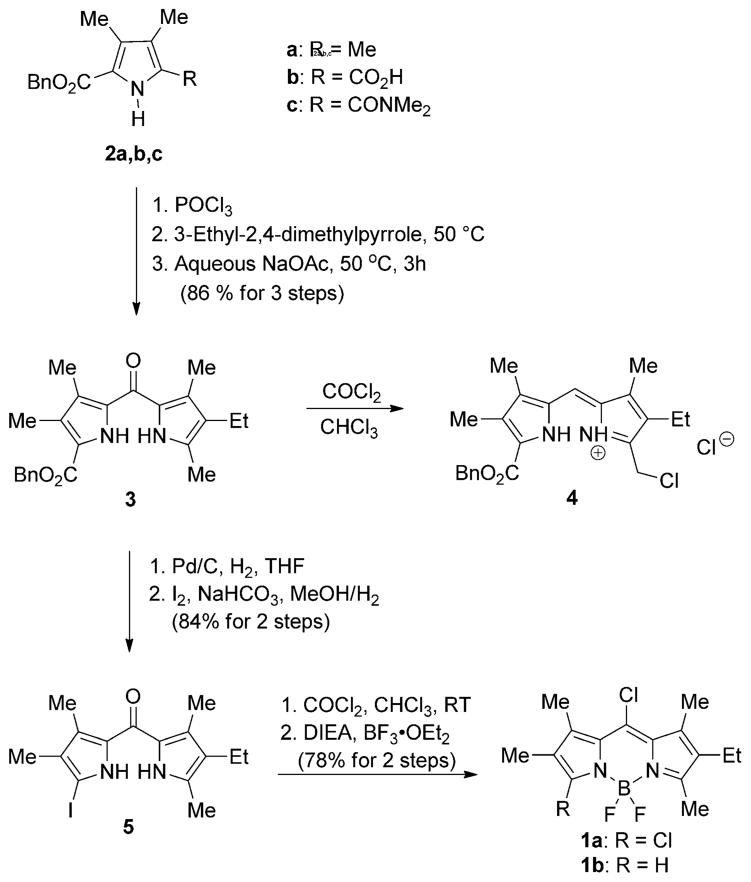
The synthetic route to asymmetric 3,8-dichloro-BODIPY 1a is outlined in Scheme 1. The conversion of the α-methyl group of readily available pyrrole 2a[21] to a carboxylic acid produced 2b,[22] which was acylated with thionyl chloride followed by reaction with dimethylamine gas[22b] affording 5-(N,N-dimethylamido) pyrrole 2c in 85% yield. The asymmetric dipyrroketone 3 was prepared by reaction of pyrrole 2c with phosphoryl chloride, followed by addition of commercially available 3-ethyl-2,4-dimethylpyrrole and subsequent hydrolysis using aqueous sodium acetate[23] in 86% overall yield. When excess phosgene was used to convert dipyrroketone 3 to the corresponding 5-chlorodipyrrin salt, the dipyrryl salt 4 was obtained instead by chlorination of the the α-methyl group of 3, probably due to the presence of the electron-withdrawing benzyl ester.[20] Therefore, the benzyl ester was converted to an iodide by debenzylation followed by decarboxylative iodination[24] producing dipyrroketone 5 in 84% overall yield. The reaction of 5 with excess phosgene (15% in toluene) was accompanied by a color change from yellow to dark red, along with a down-field shift of the NH protons in the 1H NMR spectrum. Subsequent boron complexation using excess BF3·OEt2 and N,N-diisopropylethylamine (DIEA) produced BODIPY 1a in 78% overall yield. The trans-chlorination of the 1-iododipyrroketone has been previously observed under these reaction conditions;[20] in addition, the 8-monochloro-BODIPY 1b was obtained as a minor product from the reaction, in 6% yield.
Scheme 1.
Synthesis of asymmetric 3,8-dichloro-BODIPY 1a.
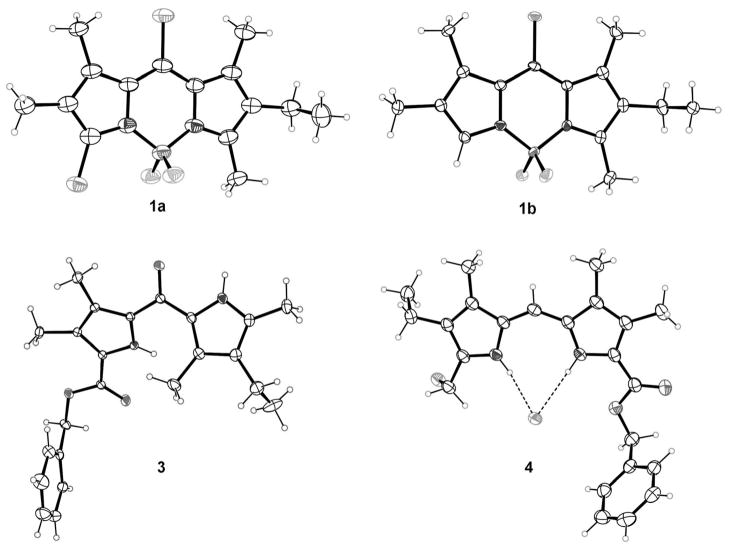
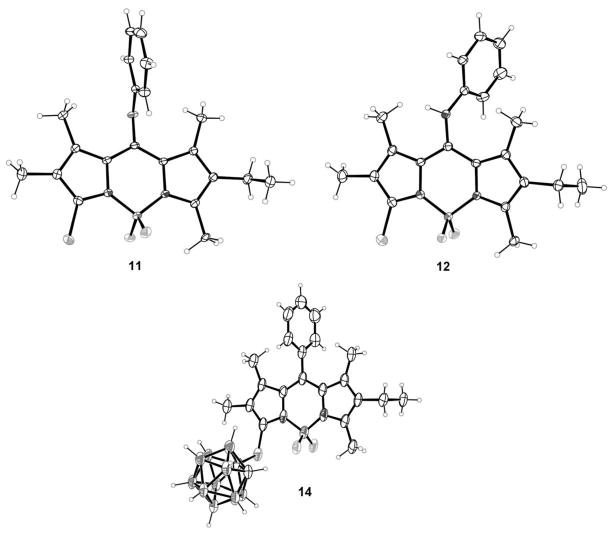
The structures of BODIPYs 1a,b, dipyrroketones 3 and 5 and dipyrrin 4 were confirmed by 1H, 13C NMR spectroscopy, HRMS (ESI-TOF) and, in the case of 1a,b, 3, and 4 also by X-ray crystallography (Figure 2). The structure of 1a has two independent molecules and a disordered chloroform solvent molecule. The two molecules are almost identical with mean deviations from planarity of their 12-atom BODIPY cores of 0.027 and 0.036 Å, and a 9.8° torsional difference in the conformation of the ethyl group. BODIPY 1b has four independent molecules, and mean deviations of their BODIPY cores are in the range of 0.023–0.054 Å. As in BODIPY 1a, the conformations of the ethyl groups are essentially perpendicular to the BODIPY planes, with C-C-C-C torsion angles differing from 90° by 0.3–1.7°.
Figure 2.
X-ray crystal structures of BODIPYs 1a,b dipyrroketone 3 and dipyrryl salt 4 with 50% probability ellipsoids. Only one of the independent molecules is shown for both 1a and 1b.
The conformation of dipyrroketone 3 is such that one pyrrole NH group is syn to the central carbonyl (N-C-C-O torsion angle 15.5°), while the other is syn to the benzyl ester carbonyl (torsion angle 6.0°). Both form intermolecular hydrogen bond dimers. On the other hand, dipyrrin 4 is the hydrochloride salt, with both N atoms protonated, forming hydrogen bonds to the chloride. The C9N2 dipyrryl group is planar to within a mean deviation of 0.017 Å.
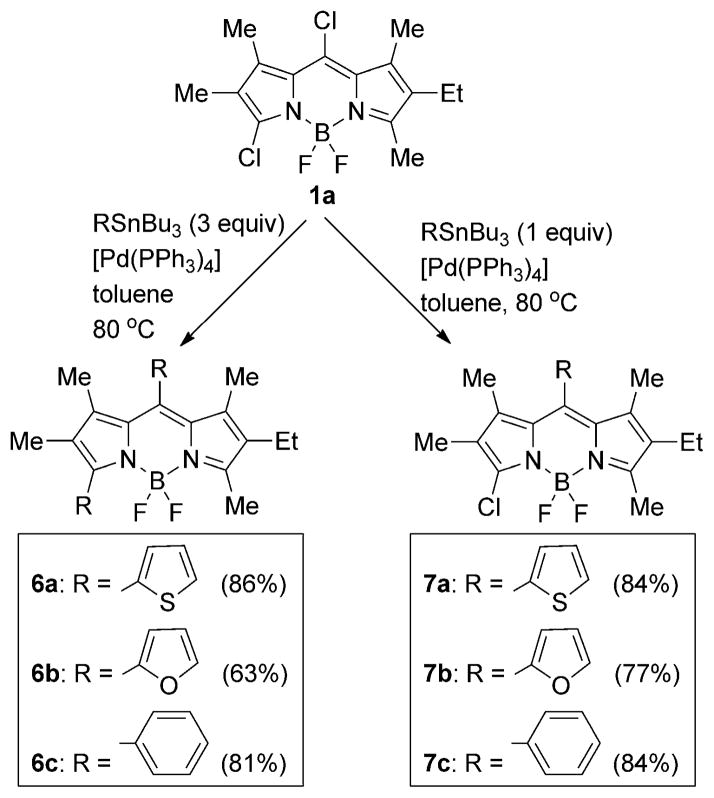
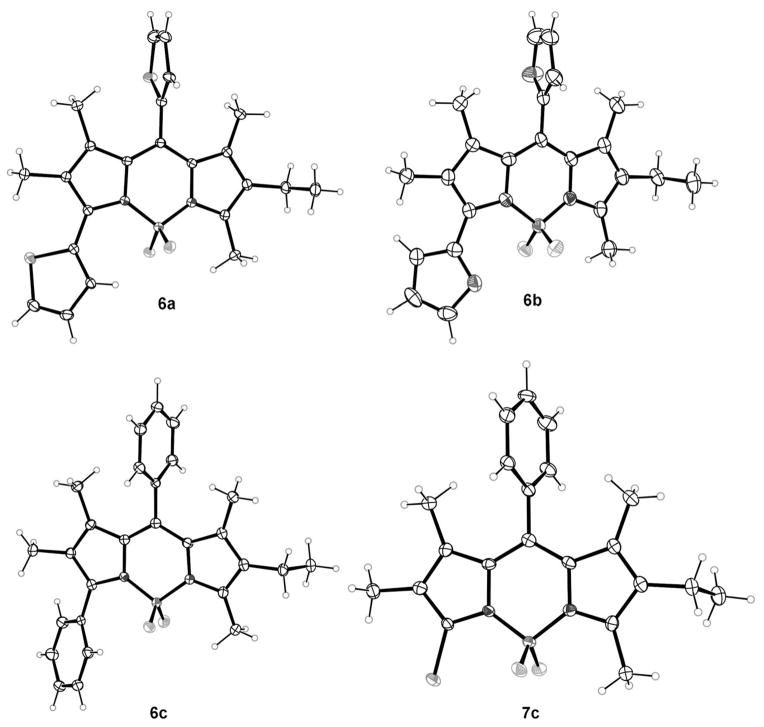
Previous work has shown that symmetric 8-chloro-,[16] 3,5-dichloro-[18c,d], and 3,5,8-trichloro-BODIPYs[20] undergo Pd0-catalyzed cross-coupling reactions, including Suzuki, Sonogashira, Heck, and Stille reactions to produce the corresponding aryl-, alkyl-, alkenyl- and alkynyl-BODIPYs. Among these, the mild Stille cross-couplings generally give high yields of the targeted functionalized BODIPYs. Under similar conditions, BODIPY 1a reacted in the presence of one or three equivalents of aryltin reagent and 10 mol% of [Pd(PPh3)4] in toluene, to produce the corresponding 8-aryl-3-chloro- and 3,8-diaryl-BODIPYs, as shown in Scheme 2. The 3,8-diaryl-BODIPYs 6a–c were produced in 63–86% yields when an excess of organotin reagent was used, whereas the reaction occurred regioselectively at the most reactive 8-chloride when only one equivalent of organotin was used in a twice diluted solution (ca. 10−3M) to produce BODIPYs 7a–c in 77–84% yields.
Scheme 2.
Global and regioselective Stille coupling reactions of 3,8-dichloro-BODIPY 1a.
The regioselectivity of these Stille reactions was confirmed by X-ray crystallography, as shown in Figure 3. Crystals of BODIPYs 6a–c and 7c were grown from slow diffusion of hexane into a solution of BODIPY in dichloromethane. Except for BODIPY 7c, all structures exhibit disorder, with the thiophene at the 8-position in 6a having two conformations, as do both furans in 6b and the ethyl group in 6c. Mean deviations from planarity of the C9BN2 BODIPY cores are 0.072 Å for 6a, 0.087 for 6b, 0.054 for 6c, and 0.013 Å for 7c. The substituents at the 8-positions deviate by varying amounts from being perpendicular to the BODIPY cores, forming dihedral angles of 87.7° for 6a, 83.0° for 6b, 79.7° for 6c, and 75.6° for 7c, to minimize steric interactions with the 1,7-methyl groups. On the other hand, the aryl substituents at the 3-position deviate much more from orthogonality with the BODIPY core, forming dihedral angles of 41.7° for the thiophene in 6a, 36.9° for the furan in 6b, and 42.7° for the phenyl group in 6c.
Figure 3.
X-ray crystal structures of asymmetric BODIPY dyes 6a–c and 7c (50% probability ellipsoids). Only the major conformer is shown for disordered groups.
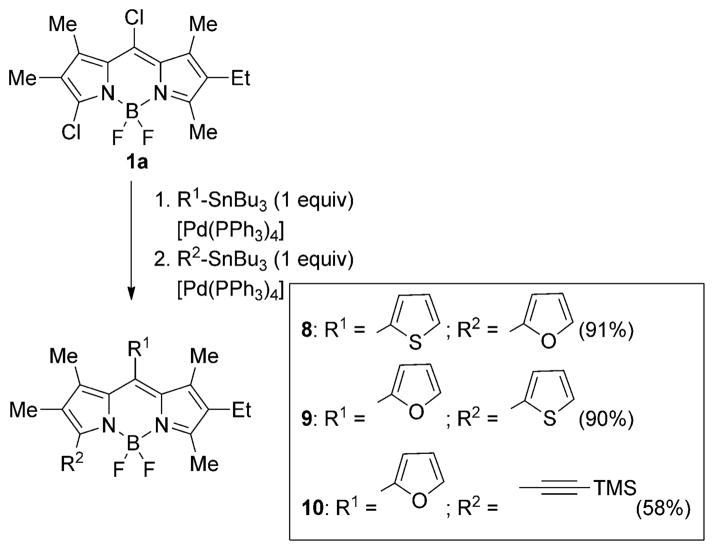
We took advantage of the regioselectivity observed in the Stille cross-coupling reactions to prepare BODIPYs 8–10 containing two different aryl groups at the 3- and 8-positions, by performing two consecutive Stille couplings using two different organotin reagents (Scheme 3). Therefore, BODIPY 1a reacted with one equivalent of either 2-(tributylstannyl)thiophene or 2-(tributylstannyl)furan under Stille conditions to pro- duce the 3-monochloro-BODIPYs 7a and 7b respectively; these BODIPYs were subjected to a second Stille coupling with a different organotin producing BODIPYs 8–10 in 58–91% yields. While BODIPY 7a bearing a 8-thienyl group readily reacted with 2-(tributylstannyl)furan producing 8 in high yield, BODIPY 7b reacted slower and required three equivalents of organotin and a more concentrated reaction mixture (ca. 10−2M) to produce BODIPYs 9 and 10 in moderate to high yields.
Scheme 3.
Sucessive Stille coupling reactions of 3,8-dichloro-BODIPY 1a.
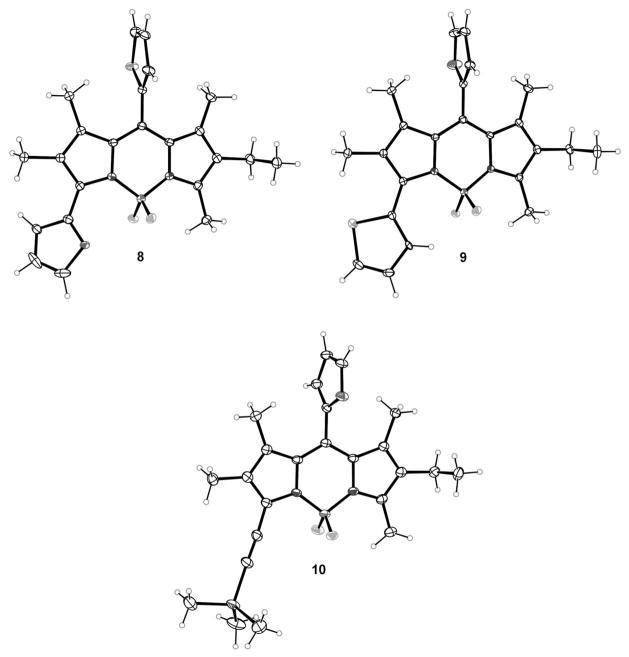
Crystals suitable for X-ray analysis were obtained for all three BODIPYs 8–10 and their structures are shown in Figure 4. In BODIPY 8, both the furan and thiophene rings exhibit rotational disorder. In these three structures, the planarity of the BODIPY core and the dihedral angles formed by the planar substituents at the 3- and 8-positions closely resemble the features in compounds 6a–c and 7c. Mean deviations for the C9BN2 cores are 0.082 Å for 8, 0.071 Å for 9, and 0.018 Å for 10. In BODIPY 8, the thiophene at the 8-position forms an 87.2° angle with the core, and the 8-furyl groups form slightly lower corresponding dihedral angles of 84.8° in 9 and 78.0° in 10. On the other hand the 3-furyl forms a dihedral angle of 38.6° with the BODIPY core plane in 8, and the 3-thienyl forms an angle of 39.6° in 9.
Figure 4.
X-ray crystal structures of asymmetric BODIPY dyes 8–10 (50% probability ellipsoids). Only the main conformers are shown for the disordered thiophene and furan in 8.
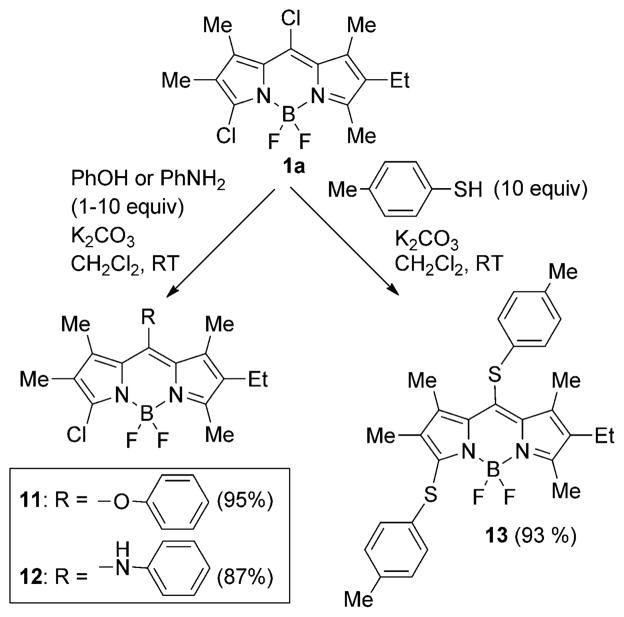
Nucleophilic addition/elimination reactions of BODIPY 1a were also investigated using N-, O-, and S-centered nucleophiles, as shown in Scheme 4. Such reactions have been previously reported on 8-chloro-BODIPYs,[16a,b] and on 3,5-dichloro-BODIPYs,[18a,b] although the regioselectivity (i.e. meso vs. α-pyrrolic position) of these reactions has never been investigated. BODIPY 1a reacted at room temperature, in the presence of potassium carbonate, with up to 10 equivalents of either phenol or aniline as the nucleophile, regioselectively affording the 3-chloro-BODIPYs 11 and 12, respectively, in high yields (87–95%). Similar conditions but using p-methylthiophenol as the nucleophile produced both the mono- and disubstituted products, showing the higher reactivity of the sulphur-centered nucleophile in these reactions. When 10 equivalents of p-methylthiophenol were used, the disubstituted product 13 was the major product, isolated in 93% yield. With 1.5 equivalents of thiol, the nucleophilic substitution reaction proceeded with regioselectivity at only the meso-position. These results illustrate the higher reactivity of the 8-chloro versus the 3-chloro group towards nucleophilic reactions.
Scheme 4.
Nucleophilic additions/eliminations of BODIPY 1a.
X-ray crystallography confirmed the regioselectivity of these reactions; Figure 5 shows the structures of BODIPYs 11 and 12. In both, there is a disorder in which the methyl and chloro substituents at the 3- and 5-positions are swapped, the minor component being present with about 8% population in both cases. Mean deviations from coplanarity of the C9BN2 core atoms are 0.033 Å for 11 and 0.061 Å for 12. Interestingly, the plane of the 8-phenoxy group forms a dihedral angle of 86.7° with the BODIPY core in 11, whereas the aniline plane forms an angle of 68.6° with the core in 12.
Figure 5.
X-ray crystal structures of asymmetric BODIPYS 11, 12, and 14 (50% probability ellipsoids). The disorder in 11 and 12 is not shown.
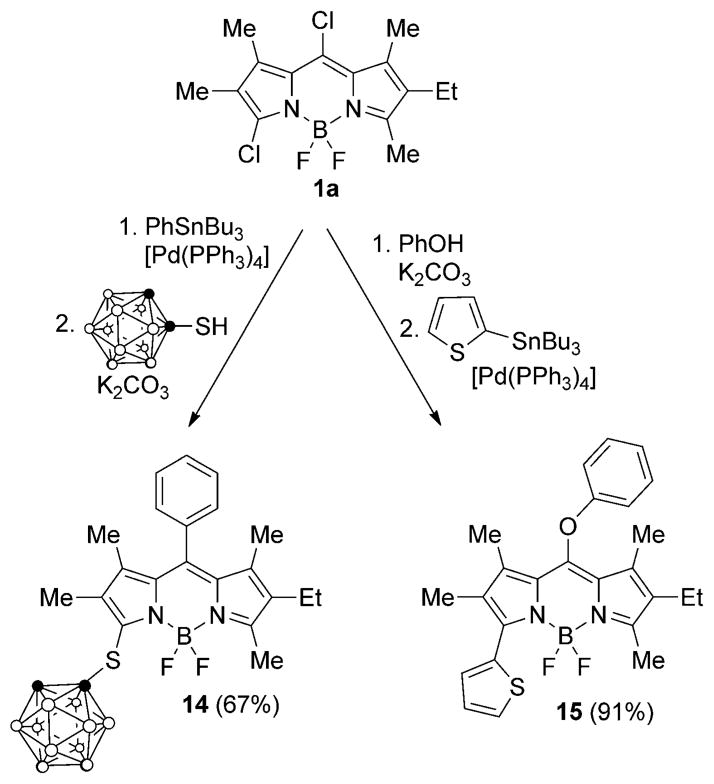
Additionally, we investigated sucessive Stille cross-coupling followed by nucleophilic substitution reactions, and vice-versa, of BODIPY 1a, as shown in Scheme 5. Using this approach and using similar conditions as described above for the two types of reactions, BODIPYs 14 and 15 were synthesized in 67 and 91% overall yields, respectively. The first reaction always occurs regioselectively at the most reactive 8-chloro, followed by the second reaction at the 3-chloro group.
Scheme 5.
Sucessive Stille cross-coupling and nucleophilic reactions of BODIPY 1a.
The X-ray structure for BODIPY 14 was also obtained to confirm the regioselectivity of these reactions, and it is shown in Figure 5. The BODIPY core atoms are coplanar to within a mean deviation of 0.019 Å, and it forms a dihedral angle of 77.9° with the 8-phenyl group plane.
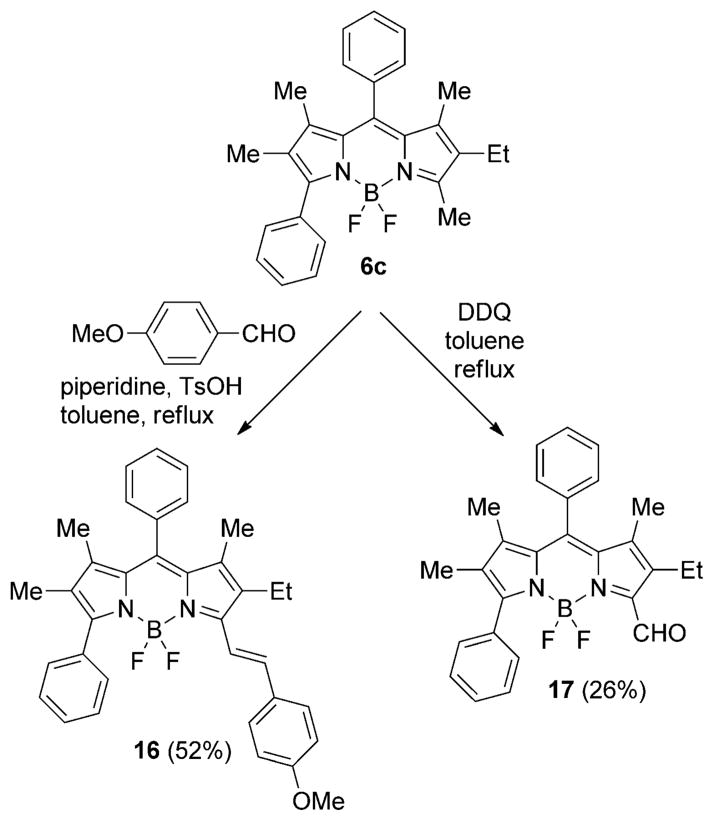
The 5-methyl group of BODIPY 1a was unreactive under the Stille cross-coupling and nucleophilic addition/elimination reactions described above. However, it is known that the α-methyl groups of BODIPYs can react with aldehydes under Knoevenagel condensation conditions,[17] providing an additional reactive site for functionalization of BODIPY 1a. Furthermore, we anticipated that this methyl group could be regioselectively oxidized under mild conditions, providing functionality for further derivatization and/or conjugation of the BODIPY.[25] To illustrate these reactions, BODIPY 6c reacted with 4-formylanisole in the presence of p-toluenesulfonic acid and piperidine, in refluxing toluene for 72 h, regioselectively affording monostyryl-BODIPY 16 in 52% yield (Scheme 6). The monostyryl-BODIPY displays further redshifted absorbance and emission spectra (see below, Table 1 and Figure 7) and potential applications as a fluorescent sensor and photosensitizer.[17]
Scheme 6.
Knoevenagel and oxidation reactions of BODIPY 6c.
Table 1.
Spectroscopic properties of BODIPYs 1a,b, 6a–c, 7a–c, and 8–17 in dichloromethane at room temperature.[a]
| BODIPY | Absorption λmax [nm] | log ε [M−1cm−1] | Emission λmax [nm] | Φf | Stokes shift [nm] |
|---|---|---|---|---|---|
| 1a | 517 | 4.80 | 540 | 0.52 | 23 |
| 1b | 513 | 4.95 | 536 | 0.33 | 23 |
| 6a | 547 | 4.64 | 591 | 0.097 | 44 |
| 6b | 586 | 4.78 | 611 | 0.022 | 25 |
| 6c | 523 | 4.71 | 551 | 0.46 | 28 |
| 7a | 529 | 4.73 | 548 | 0.09 | 19 |
| 7b | 535 | 4.78 | 555 | 0.0097 | 20 |
| 7c | 515 | 4.71 | 536 | 0.48 | 21 |
| 8 | 577 | 4.53 | 592 | 0.097 | 15 |
| 9 | 556 | 4.66 | 577 | 0.011 | 21 |
| 10 | 563 | 4.84 | 582 | 0.022 | 19 |
| 11 | 505 | 4.52 | 521 | 0.89 | 16 |
| 12 | 487 | 4.52 | 531 | 0.0017 | 44 |
| 13 | 582 | 4.35 | 610 | 0.13 | 28 |
| 14 | 512 | 4.41 | 532 | 0.41 | 20 |
| 15 | 521 | 4.62 | 570 | 0.94 | 49 |
| 16 | 587 | 4.22 | 617 | 0.73 | 30 |
| 17 | 538 | 4.46 | 562 | 0.24 | 24 |
For BODIPYs 1a,b, 6c, 7a, 7c, 11, 12, 14, and 15 the calculation of fluorescence quantum yield used rhodamine 6G in ethanol (0.95) as standard; for BODIPYs 6a, 7b, and 17, rhodamine B in ethanol (0.49) was used as a standard; for BODIPYs 6b, 8–10, 13, and 16, crystal violet perchlorate in methanol (0.54) was used as standard.
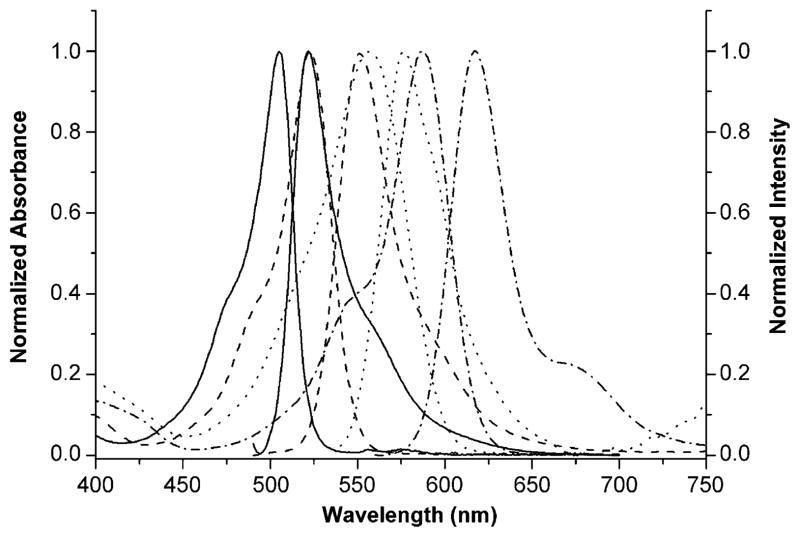
Figure 7.
Normalized UV/Vis and fluorescence spectra of BODIPYs 6c (dash), 9 (dot), 11 (solid) and 16 (dash dot) in dichloromethane at room temperature.
The 3-methyl group of BODIPY 6c was also regioselectively oxidized in the presence of DDQ[26] giving formyl-BODIPY 17 in 26% yield. Formyl-BODIPYs are useful materials with potential applications as fluorescent sensors,[27] and have been shown to undergo Wittig reactions with alkyl or aryl ylides,[28a] as well as condensations with malononitrile, methyl acetoacetate,[28b] and pyrrole[28c] to produce further functionalized compounds.
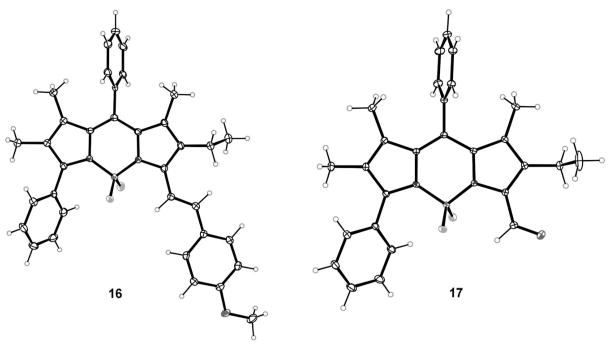
BODIPYs 16 and 17 were characterized by X-ray crystallography, as shown in Figure 6. BODIPY 16 crystallizes with two independent molecules in the asymmetric unit, and 17 as the chloroform solvate. In 16, the BODIPY core atoms of the two molecules have mean deviations of 0.064 and 0.078 Å from co-planarity. The 8-phenyl group forms dihedral angles of 84.0 and 75.0° with the BODIPY core, the 3-phenyl group forms dihedral angles of 59.8 and 65.9° with the BODIPY core, and the styryl group forms angles of 26.2 and 22.7° with the BODIPY core. In BODIPY 17, the core atoms have mean deviation of 0.075 Å from coplanarity, the 8-phenyl group forms a dihedral angle of 72.7° with the BODIPY core and the formyl C–C=O group forms a small dihedral angle of 6.2° with the BODIPY core.
Figure 6.
X-ray crystal structures of asymmetric BODIPYS 16 and 17 (50% probability ellipsoids). Only one of the two independent molecules of 16 is shown.
Spectroscopic properties
The spectroscopic properties of BODIPYs 1, 6a–c, 7a–c, and 8–17 in dichloromethane, namely their maximum absorption and fluorescence wavelengths, Stokes shifts, molar extinction coefficients and fluorescence quantum yields, are summarized in Table 1. Figure 7 shows the normalized absorption and fluorescence spectra of four representative BODIPYs (see the Supporting Information for additional BODIPY spectra). The BODIPYs have characteristic absorption and emission spectra, showing high molar absorption coefficients (logε=4.22–4.95) and Stokes-shifted fluorescence emission bands. Relative to BODIPY 1a bearing 3,8-chloro groups, slight blueshifts (1–5 nm) were observed in the absorption and emission bands of BODIPYs 7c and 14 bearing 8-phenyl groups, due to the large dihedral angles between this group and the BODIPY core (see Figures 3 and 5) as a result of 1,7-dimethyl substitution.[20] Larger blue-shifts were observed as a result from 8-phenoxy and 8-phenylamino substitution, as previously observed for 8-aryloxy and 8-arylamino-BODIPYs.[16, 29] This is in part due to the destabilization of the LUMO as a result of the electron-donating effect of these groups, which increases the HOMO–LUMO gap. However in contrast, the 8-phenoxy-BODIPY 11 showed a high fluorescence quantum yield (Φf=0.89), whereas the 8-phenylamino analogue 12 was practically nonfluorescent (Φf<0.002), probably due to intramolecular charge transfer in the excited state. The largest quantum yield was observed for the 8-phenoxy-BODIPY 15, bearing a 5-thienyl group (Φf=0.94). On the other hand, substitution at the 8-position with an arylthio group, as in BODIPY 13, induced a low quantum yield (Φf=0.13), as previously observed.[16, 29, 30]
The introduction of 8-thienyl and 8-furyl groups into the BODIPY core caused redshifts in the absorption and emission bands relative to BODIPY 1a, particularly for the furan group, due to a decrease in the HOMO–LUMO gap. The largest redshifts in the 3,8-diaryl-BODIPYs were induced by furan groups at the 3-position, as in BODIPYs 6b and 8. However, these compounds show low fluorescence quantum yields (Φf<0.1), as a result of the greater freedom of rotation of the thienyl and furyl groups in comparison with phenyl, which increases the amount of energy lost to nonradiative decay to the ground state.[20, 31] The monostyryl-BODIPY 16 showed the most redshifted fluorescence emission (λmax=617 nm) of all the BODIPYs investigated, and a high quantum yield (Φf=0.73). On the other hand, the significant redshift in the absorption and emission of formyl-BODIPY 17 relative to 6c is due to the conjugation of the carbonyl group with the BODIPY π-system, as indicated by the nearly co-planarity seen in the X-ray structure (Figure 6). The lower quantum yield determined for 17 relative to 6c is in agreement with previous reports.[26]
The Stokes shifts of the 3-, 5-, and/or 8-substituted BODIPYs were generally larger than that of the starting BODIPY 1a, and they were the largest for the 5-thienyl-BODIPYs 6a and 15 (44 and 49 nm, respectively), in agreement with previous studies, probably due to increased geometry relaxation upon photoexcitation of these compounds.[20, 32] The 8-phenylamino-BODIPY 12 also showed larger Stokes shift (44 nm) than other functionalized BODIPYs, in agreement with previous investigations.[29]
Conclusion
The total synthesis of 3,8-dichloro-6-ethyl-1,2,5,7-tetramethyl-BODIPY 1a from an unsymmetric dipyrroketone was accomplished in good yield. BODIPY 1a undergoes four types of reactions: nucleophilic addition/elimination using N-, O-, and S-centered nucleophiles and Stille cross-coupling reactions at the 3- and 8-chloro groups, as well as Knoevenagel condensation and oxidation at the most reactive 5-methyl group. Excellent selectivity for the meso-8- over the 3-chloro group of 1a was observed using both nucleophilic and Stille coupling reactions. This regioselectivity is likely the result of the higher electrophilicity of the meso-position versus the α-pyrrolic position based on consideration of mesomeric resonance structures, and of the different mechanistic pathways: SNAr for α-pyrrolic and addition/elimination for the meso-position. X-ray crystallography (16 structures were obtained) was used to confirm the regioselectivity of the reactions.
The BODIPYs with 8-phenyl, 8-phenoxy, and 8-phenylamino groups showed blueshifted absorption and emission bands relative to BODIPY 1a. All other BODIPYs displayed redshifted bands, with the monostyryl-BODIPY 16 showing the most redshifted absorption and emission of all BODIPYs. The Stokes shifts varied between 15–49 nm, with the 5-thienyl-8-phenoxy-BODIPY 15 showing the largest Stokes shift, and also the highest fluorescence quantum yield. In contrast to the phenyl- and phenoxy-substitued BODIPYs, the compounds bearing phenylamino, methylphenylthio, thienyl, and furyl groups at the 8-position of the BODIPY core showed very low fluorescence.
Experimental Section
Syntheses
General
All reagents and solvents were purchased from Sigma–Aldrich, Fisher Scientific or Alfa Aesar as reagent grade and used without further purification. Argon was used to protect the air-sensitive reactions. Analytical TLC (polyester backed, 60 Å, 0.2 mm, precoated, Sorbent Technologies) was used to monitor the reactions. Column chromatography was performed on silica gel (60 Å, 230–400 mesh, Sorbent Technologies). All 1H and 13C NMR spectra were obtained using Bruker AV-400 nanobay or AV-500 spectrometers (400 or 500 MHz for 1H and 100 MHz for 13C NMR, and 128 MHz for 11B NMR spectra) in CDCl3 with tetramethylsilane as an internal standard, at room temperature. Chemical shifts (δ) are given in parts per million (ppm) versus CDCl3 (7.27 ppm for 1H NMR, 77.0 ppm for 13C NMR). All high-resolution mass spectra (ESI-TOF) were obtained using a 6210 ESI-TOF mass spectrometer (Agilent Technologies). All UV/Vis spectra were recorded on a Varian Cary 50 spectrophotometer at room temperature. Fluorescence spectra were studied on a PTI QuantaMaster4/2006SE spectrofluorimeter with the slit width set at 3 nm at room temperature. A 10 mm path length quartz cuvette and spectroscopic grade solvents were used for the measurements. The determination of optical density (ε) was used for the solutions with an absorbance of λmax between 0.5–1. The dilute solutions with absorbance of particular excitation wavelength between 0.02–0.05 were used for fluorescence quantum yield measurements. Rhodamine 6G (0.95 in ethanol), rhodamine B (0.49 in ethanol) and crystal violet perchlorate (0.54 in methanol) were used as external standards for calculation of the relative fluorescence quantum yields of the BODIPYs. All fluorescence quantum yields (Φf) were determined using the following equation:[33]
in which Φx and Φs are the fluorescence quantum yields of the test samples and standards; Fx and Fs are the areas under the test samples’ and standards’ emission peaks; Ax is the absorbance at which test samples were excited; As the absorbance at which standards were excited; nx and ns are refractive indexes of test samples and standards.
5-Benzyloxycarbonyl-3,4-dimethylpyrrole-2-carboxylic acid (2b)
Benzyl-3,4,5-trimethylpyrrole-2-carboxylate (2a)[21] (4.34 g, 17.84 mmol) was dissolved in carbon tetrachloride (270 mL). Sulfuryl chloride (7.69 g, 57 mmol) was added dropwise and the resulting solution was stirred overnight at room temperature. The reaction was stopped when the signals for RCH2Cl (δ=4.6 ppm) and CHCl2 (δ=6.7 ppm) disappeared from the 1H NMR spectra measured in CCl4. Organic solvents were removed under reduced pressure to give a red oil residue. The oil residue was dissolved in dioxane (70 mL) and a solution of sodium acetate (11 g) in water (60 mL) was added. The solution was stirred at 60–65°C for 3 h. The solution was then cooled to room temperature and extracted using diethyl ether (2×70 mL). The organic layers were combined and washed with 5% Na2CO3 aqueous solution. All the aqueous layers were then combined and acidified by slowly adding acetic acid (10 %). A white precipitate was filtered and washed thoroughly with water. The solid was redissovled in THF and dried over anhydrous Na2SO4. The organic solvents were removed under reduced pressure to give the title product (3.64 g) in 74.7% yield. 1H NMR (CDCl3, 400 MHz): δ=9.48 (1 H, s), 7.40–7.44 (5 H, m), 5.36 (2 H, s), 2.30 ppm (6 H, s); 13C NMR (CDCl3, 100 MHz): δ=165.7, 160.7, 135.7, 129.2, 128.7, 128.4, 128.3, 127.7, 122.5, 120.8, 66.5, 10.1 ppm; MS (ESI-TOF) m/z: calcd for C15H16NO4: 274.1074; found: 274.1076 [M+H]+.
Benzyl 5-N,N-dimethylamido-3,4-dimethylpyrrole-2-carboxylate (2c)
5-Benzyloxycarbonyl-3,4-dimethylpyrrole-2-carboxylic acid (2b) (4.6 g, 16.8 mol) was added portionwise into thionyl chloride (65 mL) over 20 min. The mixture was stirred for 30 min at 40–45°C. The solvent was removed under reduced pressure to give an oily product. The oily residue was redissolved in anhydrous benzene (130 mL). Then, dimethylamine gas was passed into the mixture for 10 min, and the mixture was stirred for another 2 h. The reaction was monitored by TLC analysis. After the reaction was completed, the organic solution was washed with water (100 mL), dilute acetic acid (10 %), and water again (100 mL), and then dried over anhydrous Na2SO4. The organic solvents were removed under reduced pressure to give a yellow oil. Purification by silica gel column chromatography with CH2Cl2/MeOH as the eluent gave the yellow titled product (4.3 g, 85.1 %). 1H NMR: (CDCl3, 400 MHz): δ= 9.05 (1 H, s), 7.34–7.42 (5 H, m), 5.31 (2 H, s), 3.06 (6 H, s), 2.28 (3 H, s), 2.04 ppm (3 H, s); 13C NMR (CDCl3, 100 MHz): δ=164.7, 160.9, 136.1, 128.6, 128.2, 127.2, 126.3, 120.6, 119.8, 66.0, 10.3, 10.3 ppm; MS (ESI-TOF): m/z: calcd for C17H21N2O3: 301.1547; found: 301.1548 [M+H]+.
Benzyl 8-ethyl-2,3,7,9-tetramethyl-5-dipyrroketone-1-carboxylate (3)
Benzyl 5-N,N-dimethylamido-3,4-dimethylpyrrole-2-carboxylate (2c) (1.808 g, 6.02 mmol) was dissolved in warm POCl3 (9.23 g, 60.2 mmol) The mixture was stirred at 50°C for 5 h and then cooled to room temperature. Excess POCl3 was removed by evaporation using a ethylene dibromide under reduced pressure to give a red oily product that was then redissolved in CH2Cl2 (4 mL). A solution of 3-ethyl-2,4-dimethylpyrrole (0.82 mL, 6.02 mmol) in CH2Cl2 (4 mL) was added into the mixture under argon. The mixture was then stirred at 30°C overnight. The reaction was monitored by UV/Vis (reaction was stopped when extinctions at 351/399 nm reached a maximum). A solution of sodium acetate (10 g) in water (25 mL) was added into the mixture, which was then refluxed for 2–3 h. After the mixture was cooled to room temperature, chloroform (50 mL×3) was used to extract the organic components, which were washed with water, aqueous Na2CO3 solution (10 %), water again, and finally dried over anhydrous Na2SO4. The organic solvents were removed under reduced pressure and then the residue was crystallized from Et2O. Further purification by silica gel chromatography (CH2Cl2/MeOH as the eluent) gave a pale yellow product (1.95 g), in 85.7% yield. 1H NMR (CDCl3, 400 MHz): δ=9.20 (1 H, s), 8.78 (1 H, s), 7.35–7.44 (5 H, m), 5.33 (2 H, s), 2.38–2.42 (2 H, q, 3J(H,H)=7.6 Hz), 2.36 (3 H, s), 2.31 (3 H, s), 2.25 (3 H, s), 2.15 (3 H, s), 1.04–1.08 ppm (3 H, t, 3J(H,H)=7.5 Hz); 13C NMR (CDCl3, 100 MHz): δ=175.6, 161.2, 136.0, 133.0, 131.4, 128.6, 128.3, 128.2, 127.8, 127.7, 127.4, 125.4, 124.3, 120.4, 66.1, 17.3, 15.0, 11.5, 10.8, 10.3, 9.9 ppm; MS (ESI-TOF): m/z: calcd for C23H27N2O3: 379.2016: found: 379.2017 [M+H]+.
8-Ethyl-1-iodo-2,3,7,9-tetramethyl-5-dipyrroketone (5)
Pd/C (165 mg, 10%) was added to a 250 mL flask equipped with a magnetic stirrer. The flask was evacuated and refilled with THF (10 mL) and then with H2. The mixture was stirred strongly for 15 min under a H2 atmosphere. A THF (150 mL) solution of dipyrroketone 3 (1.65 g, 4.36 mmol) was added to the flask under H2. The mixture was stirred at room temperature for 12 h. The reaction was stopped when the starting material disappeared according to TLC analysis. The palladium catalyst was filtered through a celite cake and washed with THF (100 mL×3). The organic solutions were combined and the solvents were removed under reduced pressure to yield a white solid. The solid products were dissolved in a mixture of NaHCO3 (1.87 g, 22.3 mmol)/H2O (110 mL) and MeOH (44 mL). A solution of I2 (1.63 g, 6.42 mmol) in MeOH (26 mL) was added dropwise into the mixture at room temperature and brown solids precipitated out immediately. The mixture was stirred for another 2 h at room temperature after the addition was completed. The solids were filtered and washed with water, saturated NaHCO3 (aq), water again, and hexanes to remove excess iodine. The solids were redissolved in CH2Cl2 and dried over anhydrous Na2SO4. The organic solvents were removed under reduced pressure to give a pure pale yellow product (1.36 g, 84.2 %). 1H NMR: (CDCl3, 400 MHz): δ=8.79 (1 H, s), 8.64 (1 H, s), 2.38–2.43 (2 H, q, 3J(H,H)=6.9 Hz), 2.45 (3 H, s), 2.17 (3 H, s), 2.13 (3 H, s), 2.00 (3 H, s), 1.06–1.10 ppm (3 H, t, 3J(H,H)=6.8 Hz); 13C NMR (CDCl3, 100 MHz): δ=174.5, 133.6, 131.1, 126.9, 126.5, 126.4, 124.9, 124.8, 73.2, 17.3, 15.1, 11.9, 11.5, 11.2, 10.8 ppm; MS (ESI-TOF): m/z: calcd for C15H20IN2O: 371.0615; found: 371.0613 [M+H]+.
3,8-Dichloro-6-ethyl-1,2,5,7-tetramethyl-BODIPY (1a)
Iododipyrroketone 5 (1.36 g, 3.67 mmol) was dissolved in chloroform (300 mL). The flask was evacuated and refilled with argon. Phosgene solution (15% in toluene, 26 mL) was added into the mixture and then it was stirred overnight at room temperature. The reaction was stopped when the starting materials were totally consumed. N2 was purged into the flask to remove extra phosgene into a beaker containing saturated NaHCO3 solution. The organic solvents were removed under reduced pressure to obtain a red solid. The red solid was added into another 500 mL round-bottom flask equipped with a stirrer. The flask was evacuated and refilled with argon. Chloroform (300 mL) and N,N-diisopropylethylamine (3.32 g, 25.7 mmol) were added under argon. The mixture was then stirred for 30 min. BF3·OEt2 (5.2 g, 36.7 mmol) was added into the mixture which was stirred for another 10 h. The organic solution was washed with water, saturated NaHCO3 solution, and brine. After drying over anhydrous Na2SO4, the organic solvents were removed under reduced pressure. Further purification by silica gel column chromatography with CH2Cl2/hexanes as the eluent gave the titled BODIPY 1a (0.99 g, 78.2%) and its 8-monochloro-BODIPY byproduct (68 mg, 6 %).
For BODIPY 1a
1H NMR (CDCl3, 400 MHz): δ=2.54 (3 H, s), 2.40–2.45 (8 H, m, overlapped), 2.01(3 H, s), 1.05–1.09 ppm (3 H, t, 3J(H,H)= 7.5 Hz); 13C NMR (CDCl3, 100 MHz): δ=158.4, 140.4, 138.2, 137.5, 135.4, 135.2, 130.8, 127.6, 124.5, 17.1, 14.5, 14.2, 14.1, 12.9, 8.9 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.18 ppm (t, 1J(B,F)= 30.2 Hz); MS (ESI-TOF): m/z: calcd for C15H17BCl2F2N2: 344.0939; 344.0937 [M+H]+.
For the 8-monochloro-BODIPY byproduct 1b
1H NMR (CDCl3, 400 MHz): δ=7.40 (1 H, s), 2.53 (3 H, s), 2.40–2.45 (8 H, m, overlapped), 2.04 (3 H, s), 1.05–1.09 ppm (3 H, t, 3J(H,H)=7.5 Hz); 13C NMR (CDCl3, 400 MHz): δ=157.8, 140.6, 138.7, 137.5, 137.0, 134.6, 131.0, 129.2, 126.7, 17.1, 14.6, 14.1, 13.4, 12.9, 10.0 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.05 ppm (t, 1J(B,F)=30.3 Hz); MS (ESI-TOF): m/z: calcd for C15H19BClF2N2: 310.1329; found: 310.1307 [M+H]+.
General procedure for the preparation of BODIPYs 6a–c
Into a 50 mL round-bottom flask was added 3,8-dichloro-1,2,5,7- tetramethyl-BODIPY 1a (34.4 mg, 0.1 mmol) and [Pd(PPh3)4] (10 mol%). The flask was then evacuated and refilled with argon 3 times. Dry toluene (30 mL) and organostannane regent (0.3 mmol) were introduced into the flask. The mixture was refluxed for 6 h under an argon atmosphere. The reaction was stopped when TLC analysis showed the disappearance of starting material. Toluene was removed under reduced pressure. A flash column chromatography (CH2Cl2 as the eluent) was used to separate the crude products. Further purification by using a silica gel column with CH2Cl2/hexanes or ethyl acetate/hexanes as the eluent gave the desired disubstituted BODIPY products.
BODIPY 6a
Yield: 37.7 mg, 85.6%; 1H NMR (CDCl3, 400 MHz): δ= 7.50–7.55 (3 H, m), 7.02–7.19 (3 H, m), 2.51 (3 H, s), 2.31–2.36 (2 H, q, 3J(H,H)=7.6 Hz), 1.97 (3 H, s), 1.53 (6 H, s), 0.99–1.03 ppm (3 H, t, 3J(H,H)=7.6 Hz); 13C NMR (CDCl3, 100 MHz): δ=158.6, 145.4, 140.4, 138.2, 135.6, 134.9, 133.5, 133.0, 132.8, 132.1, 130.6, 130.5, 128.1, 127.7, 127.7, 127.5, 127.0, 17.1, 14.4, 13.0, 11.3, 11.1, 10.4 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.66 ppm (t, 1J(B,F)=32.3 Hz); MS (ESI-TOF): m/z: calcd for C23H23BF2N2S2: 440.1473; found: 440.1445 [M+H]+.
BODIPY 6b
Yield: 25.8 mg, 63.2%; 1H NMR (CDCl3, 400 MHz): δ= 7.49–7.64 (3 H, m), 6.44–6.59 (3 H, m), 2.57 (3 H, s), 2.32–2.38 (2 H, q, 3J(H,H)=7.4 Hz), 2.17 (3 H, s), 1.52 (3 H, s), 1.50 (3 H, s), 1.01–1.05 ppm (3 H, t, 3J(H,H)=7.4 Hz); 13C NMR (CDCl3, 100 MHz): δ=157.9, 147.3, 146.0, 143.6, 142.5, 142.4, 139.2, 138.7, 134.4, 133.8, 133.5, 127.7, 126.3, 115.2(t), 112.3, 111.7, 111.5, 17.1, 14.4, 13.0, 10.9, 10.4 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.87 ppm (t, 1J(B,F)=32.7 Hz); MS (ESI-TOF): m/z: calcd for C23H23BF2N2O2: 408.193; found: 408.1905 [M+H]+.
BODIPY 6c
Yield: 34.6 mg, 80.8 %; 1H NMR (CDCl3, 400 MHz): δ= 7.35–7.49 (10 H, m), 2.46 (3 H, s), 2.27–2.33 (2 H, q, 3J(H,H)=7.4 Hz), 1.79 (3 H, s), 1.32 (3 H, s), 1.35 (3 H, s), 0.96–0.99 ppm (3 H, t, 3J(H,H)= 7.4 Hz); 13C NMR (CDCl3, 100 MHz): δ=156.8, 153.2, 141.3, 139.8, 138.2, 135.8, 134.0, 132.9, 131.8, 130.9, 130.0, 129.1, 128.9, 128.4, 128.3, 127.6, 126.4, 17.1, 14.4, 12.8, 12.1, 11.8, 9.7 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.67 ppm (t, 1J(B,F)=32.4 Hz); MS (ESI-TOF): m/z: calcd for C27H26BFN2: 408.2282; found: 408.2284 [M−F]+.
General procedure for the preparation of BODIPYs 7a–c
Into a 100 mL round-bottom flask was added BODIPY 1a (34.4 mg, 0.1 mmol) and [Pd(PPh3)4] (10 mol%). The flask was then evacuated and refilled with argon 3 times. Dry toluene (60 mL) and organostannane regent (0.1 mmol) were introduced into the flask. The mixture was refluxed at 80°C under Ar. The reaction was stopped when TLC analysis showed the disappearance of starting material. Toluene was removed under reduced pressure. Silica gel flash column chromatography was used for purification of the products, eluting with dichloromethane/hexanes or ethyl acetate/hexanes.
BODIPY 7a
Yield: 33 mg, 84%; 1H NMR (CDCl3, 400 MHz): δ= 7.52–7.54 (1 H, q, 3J(H,H)=4.0, 4J(H,H)=1.1 Hz), 7.14–7.16 (1 H, q, 3J(H,H)=3.5, 3J(H,H)=1.5 Hz); 6.99–7.00 (1 H, q, 3J(H,H)=2.3, 4J(H,H)= 1.1 Hz), 2.58 (3 H, s), 2.31–2.37 (2 H, q, 3J(H,H)=7.5 Hz), 1.92 (3 H, s), 1.52 (3 H, s), 1.48 (3 H, s), 1.00–1.03 ppm (3 H, t, 3J(H,H)=7.6 Hz); 13C NMR (CDCl3, 100 MHz): δ=159.4, 141.1, 139.0, 138.1, 135.1, 134.8, 133.4, 132.7, 130.2, 128.1, 127.7, 127.6, 124.3, 17.1, 14.3, 13.0, 11.3, 11.1, 8.8 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.35 ppm (t, 1J(B,F)=31.0 Hz); MS (ESI-TOF): m/z: calcd for C19H20BClF2N2S: 392.1206; found: 392.1208 [M+H]+.
BODIPY 7b
Yield: 29 mg, 77%; 1H NMR (CDCl3, 400 MHz): 7.63–7.64 (1 H, m), 6.56–6.57 (1 H, q, 3J(H,H)=1.8, 3J(H,H)=1.5 Hz), 6.45–6.46 (1 H, m), 2.57 (3 H, s), 2.32–2.38 (2 H, q, 3J(H,H)=7.6 Hz), 1.93 (3 H, s), 1.52 (3 H, s), 1.49 (3 H, s), 1.01–1.05 ppm (3 H, t, 3J(H,H)=7.5 Hz); 13C NMR (CDCl3, 100 MHz): δ=160.3, 145.1, 142.8, 140.8, 139.3, 137.7, 135.1, 134.1, 130.6, 126.9, 124.3, 111.8, 111.6, 17.1, 14.3, 13.1, 10.8, 10.6, 8.8 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.32 ppm (t, 1J(B,F)=30.9 Hz); MS (ESI-TOF): m/z: calcd for C19H20BClF2N2O: 376.1434; found: 376.1410 [M+H]+.
BODIPY 7c
Yield: 32.4 mg, 83.8 %; 1H NMR (CDCl3, 400 MHz): δ= 7.28–7.50 (5 H, m), 2.58(3 H, s), 2.29–2.35 (2 H, q, 3J(H,H)=7.5 Hz), 1.90 (3 H, s), 1.28 (3 H, s), 1.30 (3 H, s), 0.98–1.02 ppm (3 H, t, 3J(H,H)= 7.5 Hz); 13C NMR (CDCl3, 100 MHz): δ=158.5, 140.8, 140.5, 138.4, 137.9, 135.1, 134.7, 132.2, 129.4, 129.2, 129.1, 128.1, 124.0, 17.1, 14.4, 12.9, 12.1, 11.9, 8.8 ppm; 11B NMR (CDCl3, 128 MHz): δ= 0.41 ppm (t, 1J(B,F)=31.1 Hz); MS (ESI-TOF): m/z: calcd for C21H22BClFN2: 366.1579; 366.1567 [M−F]+.
BODIPY 8
Into a 50 mL round-bottom flask was added BODIPY 7a (19.6 mg, 0.05 mmol) and [Pd(PPh3)4] (10 mol%). The flask was then evacuated and refilled with argon 3 times. Dry toluene (20 mL) and tributyl-(2-furyl)stannane (0.1 mmol) were introduced into the flask. The mixture was refluxed for 6 h under Ar. The reaction was stopped when TLC analysis showed the disappearance of starting materials. Toluene was removed under reduced pressure. A flash column chromatography (CH2Cl2 as the eluent) was used to separate the crude products. Further purification by silica gel column chromatography with ethyl acetate/hexanes as the eluent gave the desired disubstituted BODIPY product. Yield: 19.3 mg, 91 %; 1H NMR (CDCl3, 400 MHz): δ=7.61 (1 H, m), 7.52–7.53 (1 H, m), 7.46–7.47 (1 H, m), 7.15–7.17 (1 H, m), 7.00–7.01 (1 H, m), 6.59–6.60 (1 H, m), 2.57 (3 H, s), 2.30–2.37 (2 H, q, 3J(H,H)=7.4 Hz), 2.15 (3 H, s), 1.52 (6 H, s), 1.00–1.04 ppm (3 H, t, 3J(H,H)=7.5 Hz); 13C NMR (CDCl3, 100 MHz): δ=157.2, 147.2, 143.5, 141.8, 139.7, 139.0, 135.8, 134.5, 133.1, 132.8, 128.2, 127.8, 127.6, 127.4, 114.9 (t), 112.2, 17.1, 14.4, 12.9, 11.1, 11.0, 10.9 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.84 ppm (t, 1J(B,F)=32.8 Hz); MS (ESI-TOF): m/z calcd for C23H23BF2N2NaOS: 446.1521; 446.1517 [M+Na]+.
BODIPY 9
Into a 50 mL round bottom flask was added BODIPY 7b (18.8 mg, 0.05 mmol) and [Pd(PPh3)4] (10 mol%). The flask was then evacuated and refilled with argon for 3 times. Dry toluene (20 mL) and 2-(tributylstannyl)thiophene (0.15 mmol) were introduced into the flask. The mixture was refluxed for 6 h under Ar. The reaction was stopped when TLC analysis showed the disappearance of starting material. Toluene was removed under reduced pressure. A flash column chromatography (CH2Cl2 as eluent) was used to give the crude products. Further purification by silica gel column chromatography with ethyl acetate/hexanes as the eluent gave the desired disubstituted BODIPY product. Yield: 19 mg, 89.6%; 1H NMR (CDCl3, 400 MHz): δ=7.67 (1 H, m), 7.51–7.56 (2 H, m), 7.18–7.20 (1 H, m), 6.59 (1 H, m), 6.48–6.49 (1 H, m), 2.51 (3 H, s), 2.32–2.37 (2 H, q, 3J(H,H)=7.6 Hz), 1.99 (3 H, s), 1.53 (6 H, s), 1.00–1.04 ppm (3 H, t, 3J(H,H)=7.5 Hz); 13C NMR (CDCl3, 100 MHz): δ=159.4, 146.0, 145.8, 142.6, 140.0, 137.9, 134.8, 134.1, 132.8, 132.6, 130.6 (t), 127.8, 127.6, 127.1, 127.0, 111.7, 112.5, 17.1, 14.4, 13.1, 10.7, 10.5, 10.4 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.68 ppm (t, 1J(B,F)=32.2 Hz); MS (ESI-TOF): m/z: calcd for C23H24BF2N2OS: 424.1701; found: 424.1674 [M+H]+.
BODIPY 10
Into a 50 mL round-bottom flask was added meso-substituted BODIPY 7b (18.8 mg, 0.05 mmol) and [Pd(PPh3)4] (10 mol%). The flask was then evacuated and refilled with argon 3 times. Dry toluene (4 mL) and trimethyl[(tributylstannyl)ethynyl]silane (0.06 mmol) were introduced into the flask. The mixture was refluxed for 6 h under an argon atmosphere. The reaction was stopped when TLC analysis showed the disappearance of starting material. Toluene was removed under reduced pressure. A flash column chromatography (CH2Cl2 as eluent) was used to separate the crude products. Further purification by silica gel column chromatography with ethyl acetate/hexanes as the eluent gave the desired disubstituted BODIPY product. Yield: 12.7 mg, 58%; 1H NMR (CDCl3, 400 MHz): δ=7.62 (1 H, m), 6.54–6.56 (1 H, q, 3J(H,H)=1.7 Hz, 3J(H,H)=1.5 Hz), 6.43–6.44 (1 H, m), 2.59 (3 H, s), 2.32–2.37 (2 H, q, 3J(H,H)=7.5 Hz), 1.99 (3 H, s), 1.52 (3 H, s), 1.45 (3 H, s), 1.00–1.04 (3 H, t, 3J(H,H)=7.5 Hz), 0.31 ppm (9 H, s); 13C NMR (CDCl3, 100 MHz): δ= 162.1, 145.8, 143.0, 141.1, 136.3, 135.8, 132.7, 132.6, 132.2, 126.9, 112.0, 111.8, 109.3, 97.3, 17.5, 14.6, 13.7, 10.9, 10.7, 10.0, 0.2 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.28 ppm (t, 1J(B,F)=30.0 Hz); MS (ESI-TOF): m/z: calcd for C24H30BF2N2OSi: 438.2219; found: 438.2223 [M+H]+.
General procedure for the preparation of BODIPYs 11–13
Into a 5 mL round-bottom flask was added BODIPY 1a (17.2 mg, 0.05 mmol), nucleophile (1–10 equiv), and K2CO3 (13.8 mg, 0.1 mmol). CH2Cl2 (0.5 mL) was added into the flask. The mixture was stirred at room temperature. The reaction was stopped when TLC analysis showed the disappearance of starting material. The crude product was subjected to a short flash column chromatography (CH2Cl2 as eluents) to remove polar byproducts. Further purification by silica gel column chromatography with CH2Cl2/hexanes or ethyl acetate/hexanes as the eluent gave the desired BODIPY products.
BODIPY 11
Yield: 19.1 mg, 94.9 %; 1H NMR (CDCl3, 400 MHz): δ= 6.99–7.34 (5 H, m), 2.57 (3 H, s), 2.34–2.38 (2 H, q, 3J(H,H)=7.1 Hz), 2.01 (3 H, s), 1.98 (3 H, s), 1.93 (3 H, s), 1.01–1.04 ppm (3 H, t, 3J(H,H)= 7.3 Hz); 13C NMR (CDCl3, 100 MHz): δ=158.6, 157.5, 150.5, 138.2, 137.8, 135.2, 133.9, 130.5, 128.3, 125.6, 123.5, 123.0, 114.7, 16.9, 14.4, 13.0, 12.0, 11.7, 8.6 ppm; 11B NMR (CDCl3, 128 MHz): δ= 0.36 ppm (t, 1J(B,F)=30.5 Hz); MS (ESI-TOF): m/z: calcd for C21H23BClF2N2O: 402.1591; found: 402.1564 [M+H]+.
BODIPY 12
Yield: 17.4 mg, 86.6%; 1H NMR (400 MHz, CDCl3): δ= 6.93–7.25 (5 H, m), 6.55 (1 H, s), 2.55 (3 H, s), 2.31–2.37 (2 H, q, 3J(H,H)=7.4 Hz), 1.98 (3 H, s), 1.91 (3 H, s), 1.89 (3 H, s), 1.00–1.03 ppm (3 H, t, 3J(H,H)=7.6 Hz); 13C (100 MHz, CDCl3): δ=153.5, 143.5, 140.6, 135.3, 134.9, 133.0, 132.6, 129.8, 127.2, 125.5, 122.8, 118.0, 17.0, 14.7, 12.9, 12.8, 12.64, 8.8 ppm; 11B NMR (CDCl3, 128 MHz): δ= 0.47 ppm (t, 1J(B,F)=31.1 Hz); MS (ESI-TOF): m/z: calcd for C21H24BClF2N3: 401.1751; found: 401.1725 [M+H]+.
BODIPY 13
Yield: 24.2 mg, 93%; 1H NMR (400 MHz, CDCl3): δ= 7.05–7.22 (8 H, m), 2.60 (3 H, s), 2.36–2.41 (5 H, m, overlapped), 2.31 (6 H, s), 2.29 (3 H, s), 1.72 (3 H, s), 1.01–1.04 ppm (3 H, t, 3J(H,H)= 6.0 Hz); 13C NMR (100 MHz, CDCl3): δ=160.4, 143.6, 141.9, 138.2, 137.2, 136.3, 135.9, 134.3, 133.0, 132.8, 132.1, 131.7, 130.3, 129.8, 129.4, 126.3, 21.1, 21.0, 17.2, 14.5, 14.4, 13.2, 10.1 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.51 ppm (t, 1J(B,F)=31.5 Hz); MS (ESI-TOF): m/z: calcd for C29H32BF2N2S2: 520.2099; found: 520.2085 [M+H]+.
BODIPY 14
Into a 5 mL round-bottom flask were added BODIPY 7c (19.3 mg, 0.05 mmol), thiolcarborane (88 mg, 0.5 mmol), and K2CO3 (13.8 mg, 0.1 mmol). CH2Cl2 (1 mL) was added into the flask. The mixture was then stirred at room temperature. The reaction was quenched with water when TLC analysis showed the disappearance of starting material. CH2Cl2 (10 mL×3) was used to extract the organic components. Organic solvents were combined, dried over anhydrous Na2SO4, and evaporated under reduced pressure to give the crude product. Further purification by silica gel column chromatography with ethyl acetate/hexanes as the eluent gave the titled product (17.7 mg, 67.3 %). 1H NMR (400 MHz, CDCl3): δ=7.24–7.5 (5 H, m), 4.59 (1 H, s), 1.76–3.34 (10 H, m), 2.63 (3 H, s), 2.32–2.38 (2 H, q, 3J(H,H)=7.6 Hz), 2.04 (3 H, s), 1.36 (3 H, s), 1.29 (3 H, s), 0.99–1.03 ppm (3 H, t, 3J(H,H)=7.5 Hz); 13C NMR (100 MHz, CDCl3): δ=165.0, 143.4, 141.6, 137.3, 135.5, 134.9, 134.7, 134.2, 133.3, 132.5, 129.4(t), 128.0, 127.9, 66.3, 66.2, 17.1, 14.1, 13.6, 12.2, 12.1, 11.1 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.29 (1 B, t, 1J(B,F)=31.1 Hz), −0.85 (1 B, d, 1J(B,H)=142 Hz), −4.74 (1 B, d, 1J(B,H)= 128 Hz), −10.1 to −12.92 ppm (8 B, m); MS (ESI-TOF): m/z: calcd for C23H24B11F2N2S: 527.3520; found: 527.3512 [M+H]+.
BODIPY 15
Into a 50 mL round-bottom flask was added meso-substiutued BODIPY 11 (10 mg, 0.025 mmol) and [Pd(PPh3)4] (10 mol%). The flask was then evacuated and refilled with argon 3 times. Dry toluene (20 mL) and 2-(tributylstannyl)thiophene (0.05 mmol) were introduced into the flask. The mixture was refluxed for 6 h under an argon atmosphere. The reaction was stopped when TLC analysis showed the disappearance of starting material. Toluene was removed under reduced pressure. A flash column chromatography (CH2Cl2 as the eluent) was used to obtain the crude product. Further purification by silica gel column chromatography with ethyl acetate/hexanes as the eluent gave the desired product. Yield: 10.2 mg, 90.6%; 1H NMR (400 MHz, CDCl3): δ= 7.50–7.53 (2 H, m), 7.32–7.36 (2 H, m), 7.17–7.19 (1 H, q, 3J(H,H)=3.6, 3J(H,H)=1.5 Hz), 7.05–7.10 (3 H, m), 2.50 (3 H, s), 2.31–2.37 (2 H, q, 3J(H,H)=7.6 Hz), 2.04 (3 H, s), 2.02 (3 H, s), 1.99 (3 H, s), 0.99–1.03 ppm (3 H, t, 3J(H,H)=7.6 Hz); 13C (100 MHz, CDCl3): δ=157.8, 157.7, 150.9, 145.4, 137.4, 135.3, 133.6, 132.7, 130.4 (t), 130.1, 128.3, 127.6, 127.0, 126.7, 123.0, 122.8, 114.9, 17.0, 14.5, 13.0, 11.9, 11.7, 10.2 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.64 ppm (t, 1J(B,F)=32.0 Hz); MS (ESI-TOF): m/z: calcd for C25H26BF2N2OS: 450.1858; found: 450.1831 [M+H]+.
BODIPY 16
Into a 50 mL round-bottom flask was added BODIPY 6c (21.4 mg, 0.05 mmol), molecular sieves, and methyl 4-formylanisole (68 mg, 0.5 mmol) in toluene (10 mL). p-TsOH (10 mg) and piperidine (0.1 mL) were added into the mixture. The mixture was stirred and refluxed for 72 h under an argon atmosphere. The reaction was stopped when TLC analysis showed the disappearance of starting material. The mixture was cooled to room temperature and filtered to remove the moleculer sieves. The filtrate was poured into water (20 mL) and CH2Cl2 (20 mL×3) was used to extract the organic components. The organic solvents were combined, dried over anhydrous Na2SO4, and evaporated under reduced pressure to give the crude product. Further purification by silica gel column chromatography with ethyl acetate/hexanes as the eluent gave the styryl-BODIPY 16 (14.2 mg, 52 %). 1H NMR (400 Hz, CDCl3): δ=7.13–7.60 (14 H, m), 6.85–6.87 (2 H, d, 3J(H,H)= 8 Hz), 3.83 (3 H, s), 2.56–2.62 (2 H, q, 3J(H,H)=7.5 Hz), 1.80 (3 H, s), 1.36 (6 H, s), 1.13–1.16 ppm (3 H, t, 3J(H,H)=7.3 Hz); 13C NMR (400 Hz, CDCl3): δ=160.3, 154.1, 152.1, 140.3, 140.1, 138.4, 136.2, 136.1, 133.9, 132.9, 132.8, 131.9, 130.0, 129.1, 128.9, 128.5, 127.7, 127.2, 117.9, 114.1, 55.4, 18.4, 14.0, 12.2, 11.6, 9.8 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.90 ppm (t, 1J(B,F)=32.7 Hz); MS (ESI-TOF): m/z: calcd for C35H33BF2N2O: 545.2685; found: 545.2687 [M+H]+.
BODIPY 17
Into a 50 mL round-bottom flask was added BODIPY 6c (21.4 mg, 0.05 mmol). The flask was then evacuated and refilled with argon 3 times. Dry toluene (10 mL) was added. The temperature was raised to 110°C. A solution of DDQ (56.8 mg, 0.25 mmol) in toluene (10 mL) was added slowly into the mixture, which was stirred and refluxed under an argon atmosphere. The reaction was stopped when TLC analysis showed the disappearance of starting material. Solvents were removed under reduced pressure to give the crude product. Further purification by silica gel column chromatography or preparative TLC plates with ethyl acetate/hexanes as the eluent gave BODIPY 17 (5.7 mg, 25.8% yield). 1H NMR (400 Hz, CDCl3): δ=10.30 (1 H, s), 7.37–7.59 (5 H, m), 2.67–2.71 (2 H, q, 3J(H,H)=7.4 Hz), 1.84 (3 H, s), 1.44 (3 H, s), 1.31 (3 H, s), 1.00–1.03 ppm (3 H, t, 3J(H,H)=7.1 Hz); 13C NMR (400 Hz, CDCl3): δ=186.3, 164.5, 144.7, 144.5, 141.2, 137.2, 136.3, 135.8, 134.9, 132.6, 131.5, 130.9, 130.0, 129.6, 129.5, 129.3, 128.1, 127.8, 17.6, 14.3, 12.9, 10.7, 10.0 ppm; 11B NMR (CDCl3, 128 MHz): δ=0.69 ppm (t, 1J(B,F)= 32.1 Hz); MS (ESI-TOF): m/z: calcd for C27H26BF2N2O: 442.2137; found: 442.2145 [M+H]+.
Crystal data
Diffraction data were collected at low temperature (90–105 K) on either a Nonius KappaCCD diffractometer equipped with MoKα radiation (λ=0.71073 Å) or a Bruker Kappa Apex-II DUO diffractometer equipped with Mo or CuKα radiation (λ=1.54184 Å). Refinement was by full-matrix least squares using SHELXL, with H atoms in idealized positions, except for those on N in 3, 4, and 12, which were refined. BODIPYS 1a and 16 have two independent molecules, and 1b has four. 1a, 6b, and 16 were nonmerohedral twins, and disorder was present in 1a, 6a–c, 8, 11, and 12. Compounds 1a and 17 were chloroform solvates. The absolute structures of both noncentrosymmetric crystals 1b and 7c were determined. For 1a: C15H17BCl2F2N2·0.5CHCl3, triclinic P-1, a=8.4888(3), b=13.9111(6), c=14.8676(6) Å, α=86.609(3), β=88.646(2), γ=89.580(2)°, Z=4, T=90 K, R=0.067; 1b: C15H18BClF2N2, monoclinic P21, a= 15.4384(5), b=11.5896(4), c=16.8479(5) Å, β=99.215(2)°, Z=8, T= 90 K, R=0.047; 3: C23H26N2O3, monoclinic P21/n, a=7.7071(6), b= 12.3770(9), c=21.4131(17) Å, β=92.673(4)°, Z=4, T=100 K, R= 0.045; 4: [C23H26ClN2O2]Cl, triclinic P-1, a=8.1637(4), b=9.5889(5), c=13.6082(8) Å, α=94.850(3), β=97.737(2), γ=92.855(2)°, Z=2, T=100 K, R=0.033; 6a: C23H23BF2N2S2, triclinic P-1, a=9.5771(14), b=10.3269(15), c=11.567(2) Å, α=77.851(8), β=66.885(6), γ= 79.384(7)°, Z=2, T=100 K, R=0.041; 6b: C23H23BF2N2O2, triclinic P-1, a=9.362(2), b=10.155(2), c=11.682(3) Å, α=76.840(6), β= 66.796(6), γ=77.094(6)°, Z=2, T=90 K, R=0.089; 6c: C27H27BF2N2, monoclinic P21/n, a=11.4039(6), b=7.9247(4), c=24.2010(12) Å, β=91.650(3)°, Z=4, T=90 K, R=0.047; 7c: C21H22BClF2N2, monoclinic Pc, a=7.6918(2), b=8.2818(2), c=14.8858(4) Å, β= 94.762(2)°, Z=2, T=105 K, R=0.035; 8: C23H23BF2N2OS, triclinic P-1, a=9.5378(3), b=10.1969(3), c=11.5554(4) Å, α=76.414(2), β= 66.800(2), γ=78.800(2)°, Z=2, T=90 K, R=0.040; 9: C23H23BF2N2OS, triclinic P-1, a=9.4222(2), b=10.2488(2), c= 11.6233(3) Å, α=78.492(2), β=66.845(2), γ=77.813(2)°, Z=2, T= 90 K, R=0.043; 10: C24H29BF2N2OSi, monoclinic P21/c, a=6.9871(2), b=22.5959(8), c=14.6041(6) Å, β=93.098(2)°, Z=4, T=90 K, R= 0.041; 11: C21H22BClF2N2O, triclinic P-1, a=9.4115(3), b=10.4033(4), c=11.3995(4) Å, α=66.223(2), β=77.459(2), γ=68.527(2)°, Z=2, T=90 K, R=0.051; 12: C21H23BClF2N3, monoclinic C2/c, a= 21.8367(10), b=8.9694(4), c=21.9939(8) Å, β=114.299(2)°, Z=8, T=90 K, R=0.050; 14: C23H33B11F2N2S, triclinic P-1, a=6.9030(7), b=12.0111(12), c=17.1906(14) Å, α=77.599(7), β=79.990(7), γ= 83.195(7)°, Z=2, T=90 K, R=0.070; 16: C35H33BF2N2O, triclinic P-1, a=11.5598(5), b=13.2476(6), c=19.1537(8) Å, α=85.869(2), β= 86.135(2), γ=72.967(2)°, Z=4, T=90 K, R=0.048; 17: C27H25BF2N2O·CHCl3, triclinic P-1, a=9.6902(5), b=10.7446(6), c= 13.9386(8) Å, α=68.727(3), β=75.951(3), γ=73.728(3)°, Z=2, T= 90 K, R=0.037;
CCDC 1038325, 1038326, 1038327, 1038328, 1038329, 1038330, 1038331, 1038332, 1038333, 1038334, 1038335, 1038336, 1038337, 1038338, 1038339, and 1038340 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation, grant CHE-1362641, and the National Institutes of Heath, grant CA132861.
Footnotes
BODIPY=4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/chem.201406550.
References
- 1.Treibs A, Kreuzer FH. Justus Liebigs Ann Chem. 1968;718:208–223. doi: 10.1002/jlac.19687180118. [DOI] [PubMed] [Google Scholar]
- 2.a) Loudet A, Burgess K. Chem Rev. 2007;107:4891–4932. doi: 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]; b) Ulrich G, Ziessel R, Harriman A. Angew Chem Int Ed. 2008;47:1184–1201. doi: 10.1002/anie.200702070. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2008;120:1202–1219. [Google Scholar]; c) Lu H, Mack J, Yang Y, Shen Z. Chem Soc Rev. 2014;43:4778–4823. doi: 10.1039/c4cs00030g. [DOI] [PubMed] [Google Scholar]
- 3.a) Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. Chem Rev. 2010;110:2620–2640. doi: 10.1021/cr900263j. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Crivellato E, Candussio L, Rosati AM, Bartoli-Klugmann F, Mallardi F, Decorti G. J Histochem Cytochem. 2002;50:731–734. doi: 10.1177/002215540205000514. [DOI] [PubMed] [Google Scholar]; c) Lee JJ, Lee SC, Zhai D, Ahn YH, Yeo HY, Tan YL, Chang YT. Chem Commun. 2011;47:4508–4510. doi: 10.1039/c1cc10362h. [DOI] [PubMed] [Google Scholar]
- 4.a) Kurata S, Kanagawa T, Yamada K, Torimura M, Yokomaku T, Kamagata Y, Kurane R. Nucleic Acids Res. 2001;29:34e. doi: 10.1093/nar/29.6.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jang HG, Park M, Wishnok JS, Tannenbaum SR, Wogan GN. Anal Biochem. 2006;359:151–160. doi: 10.1016/j.ab.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 5.a) Carten JD, Bradford MK, Farber SA. Developmental Biology. 2011;360:276–285. doi: 10.1016/j.ydbio.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Peters C, Billich A, Ghobrial M, Högenauer K, Ullrich T, Nussbaumer P. J Org Chem. 2007;72:1842–1845. doi: 10.1021/jo062347b. [DOI] [PubMed] [Google Scholar]
- 6.a) Ziessel R, Ulrich G, Haefele A, Harriman A. J Am Chem Soc. 2013;135:11330–11344. doi: 10.1021/ja4049306. [DOI] [PubMed] [Google Scholar]; b) Yilmaz MD, Bozdemir OA, Akkaya EU. Org Lett. 2006;8:2871–2873. doi: 10.1021/ol061110z. [DOI] [PubMed] [Google Scholar]
- 7.a) Coskun A, Deniz E, Akkaya EU. Org Lett. 2005;7:5187–5189. doi: 10.1021/ol052020h. [DOI] [PubMed] [Google Scholar]; b) Golovkova TA, Kozlov DV, Neckers DC. J Org Chem. 2005;70:5545–5549. doi: 10.1021/jo050540k. [DOI] [PubMed] [Google Scholar]
- 8.a) Liu S, Lin T, Li D, Leamer L, Shan H, Li Z, Gabbaï FP, Conti PS. Theranostics. 2013;3:181–189. doi: 10.7150/thno.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bernhard C, Goze C, Rousselin Y, Denat F. Chem Commun. 2010;46:8267–8269. doi: 10.1039/c0cc02749a. [DOI] [PubMed] [Google Scholar]; c) Tasan S, Zava O, Bertrand B, Bernhard C, Goze C, Picquet M, Le Gendre P, Harvey P, Denat F, Casini A, Bodio E. Dalton Trans. 2013;42:6102–6109. doi: 10.1039/c2dt32055j. [DOI] [PubMed] [Google Scholar]
- 9.a) Wagner RW, Lindsey JS. Pure Appl Chem. 1996;68:1373–1380. [Google Scholar]; b) Lee CH, Lindsey JS. Tetrahedron. 1994;50:11427–11440. doi: 10.1016/j.tet.2008.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burghart A, Kim H, Welch MB, Thoresen LH, Reibenspies J, Burgess K. J Org Chem. 1999;64:7813–7819. [Google Scholar]
- 11.a) Li Z, Mintzer E, Bittman R. J Org Chem. 2006;71:1718–1721. doi: 10.1021/jo052029x. [DOI] [PubMed] [Google Scholar]; b) Wang D, Fan J, Gao X, Wang B, Sun S, Peng X. J Org Chem. 2009;74:7675–7683. doi: 10.1021/jo901149y. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Burgess K. Chem Commun. 2008:4933–4935. doi: 10.1039/b810503k. [DOI] [PubMed] [Google Scholar]
- 13.a) White J, McGillivray G. J Org Chem. 1977;42:4248–4251. [Google Scholar]; b) Nicolaou KC, Claremon DA, Papahatjis DP. Tetrahedron Lett. 1981;22:4647–4650. [Google Scholar]; c) Wallace DM, Leung SH, Senge MO, Smith KM. J Org Chem. 1993;58:7245–7257. [Google Scholar]
- 14.Tahtaoui C, Thomas C, Rohmer F, Klotz P, Duportail G, Mély Y, Bonnet D, Hibert M. J Org Chem. 2007;72:269–272. doi: 10.1021/jo061567m. [DOI] [PubMed] [Google Scholar]
- 15.a) Goud TV, Tutar A, Biellmann JF. Tetrahedron. 2006;62:5084–5091. [Google Scholar]; b) Gómez-Durán CFA, Garcia-Moreno I, Costela A, Martin V, Sastre R, Banuelos J, Arbeloa FL, Arbeloa IL, Pena-Cabrera E. Chem Commun. 2010;46:5103–5105. doi: 10.1039/c0cc00397b. [DOI] [PubMed] [Google Scholar]; c) Bañuelos J, Martín V, Gómez-Durán CFA, Córdoba IJA, Peña-Cabrera E, García-Moreno I, Costela Á, Pérez-Ojeda ME, Arbeloa T, Arbeloa L. Chem Eur J. 2011;17:7261–7270. doi: 10.1002/chem.201003689. [DOI] [PubMed] [Google Scholar]
- 16.a) Wang H, Vicente MGH, Fronczek FR, Smith KM. Chem Eur J. 2014;20:5064–5074. doi: 10.1002/chem.201304310. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Leen V, Yuan P, Wang L, Boens N, Dehaen W. Org Lett. 2012;14:6150–6153. doi: 10.1021/ol3028225. [DOI] [PubMed] [Google Scholar]; c) Misra R, Dhokale B, Jadhav T, Mobin SM. Dalton Trans. 2013;42:13658–13666. doi: 10.1039/c3dt51374b. [DOI] [PubMed] [Google Scholar]
- 17.a) Rurack K, Kollmannsberger M, Daub J. Angew Chem Int Ed. 2001;40:385–387. [PubMed] [Google Scholar]; Angew Chem. 2001;113:396–399. [Google Scholar]; b) Dost Z, Atilgan S, Akkaya EU. Tetrahedron. 2006;62:8484–8488. [Google Scholar]; c) He H, Lo PC, Yeung SL, Fong WP, Ng DKP. J Med Chem. 2011;54:3097–3102. doi: 10.1021/jm101637g. [DOI] [PubMed] [Google Scholar]; d) Uppal T, Bhupathiraju NVSDK, Vicente MGH. Tetrahedron. 2013;69:4687–4693. [Google Scholar]; e) Gibbs JH, Zhou Z, Kessel D, Fronczek FR, Pakhomova S, Vicente MGH. J Photochem Photobiol B: Biol. doi: 10.1016/j.jphotobiol.2015.02.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Baruah M, Qin W, Vallée RAL, Beljonne D, Rohand T, Dehaen W, Boens N. Org Lett. 2005;7:4377–4380. doi: 10.1021/ol051603o. [DOI] [PubMed] [Google Scholar]; b) Rohand T, Baruah M, Qin W, Boens N, Dehaen W. Chem Commun. 2006:266–268. doi: 10.1039/b512756d. [DOI] [PubMed] [Google Scholar]; c) Rohand T, Qin W, Boens N, Dehaen W. Eur J Org Chem. 2006:4658–4663. [Google Scholar]; d) Qin W, Rohand T, Dehaen W, Clifford JN, Driesen K, Beljonne D, Van Averbeke B, Van der Auweraer M, Boens N. J Phys Chem A. 2007;111:8588–8597. doi: 10.1021/jp073547+. [DOI] [PubMed] [Google Scholar]
- 19.a) Peña-Cabrera E, Aguilar-Aguilar A, González-Domínguez M, Lager E, Zamudio-Vázquez R, Godoy-Vargas J, Villanueva-García F. Org Lett. 2007;9:3985–3988. doi: 10.1021/ol7016615. [DOI] [PubMed] [Google Scholar]; b) Arroyo IJ, Hu R, Merino G, Tang BZ, Peña-Cabrera E. J Org Chem. 2009;74:5719–5722. doi: 10.1021/jo901014w. [DOI] [PubMed] [Google Scholar]; c) Arroyo IJ, Hu R, Tang BZ, López FI, Peña-Cabrera E. Tetrahedron. 2011;67:7244–7250. [Google Scholar]
- 20.Wang H, Fronczek FR, Vicente MGH, Smith KM. J Org Chem. 2014;79:10342–10352. doi: 10.1021/jo501969z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson AW, Markham E, Price R, Shaw KB. J Chem Soc. 1958:4254–4257. [Google Scholar]
- 22.a) Smith KM, Craig GW, Eivazi F, Martynenko Z. Synthesis. 1980:493–495. [Google Scholar]; b) Jackson AH, Supphayen D. J Chem Soc Perkin Trans 1. 1987:277–286. [Google Scholar]
- 23.Ballantine JA, Jackson AH, Kenner GW, McGillivray G. Tetrahedron. 1966;22:241–259. [Google Scholar]
- 24.Jiao L, Yu C, Uppal T, Liu M, Li Y, Zhou Y, Hao E, Hu X, Vicente MGH. Org Biomol Chem. 2010;8:2517–2519. doi: 10.1039/c001068e. [DOI] [PubMed] [Google Scholar]
- 25.de Rezende LCD, Emery FS. Orbital Elec J Chem. 2013;5:62–83. [Google Scholar]
- 26.a) Haefele A, Zedde C, Retailleau P, Ulrich G, Ziessel R. Org Lett. 2010;12:1672–1675. doi: 10.1021/ol100109j. [DOI] [PubMed] [Google Scholar]; b) Uppal T. Ph D Dissertation. Louisiana State University; 2012. [Google Scholar]
- 27.Madhu S, Rao MR, Shaikh MS, Ravikanth M. Inorg Chem. 2011;50:4392–4400. doi: 10.1021/ic102499h. [DOI] [PubMed] [Google Scholar]
- 28.a) Lakshmi V, Ravikanth M. J Org Chem. 2013;78:4993–5000. doi: 10.1021/jo4006969. [DOI] [PubMed] [Google Scholar]; b) Jiao L, Yu C, Li J, Wang Z, Wu M, Hao E. J Org Chem. 2009;74:7525–7528. doi: 10.1021/jo901407h. [DOI] [PubMed] [Google Scholar]; c) Madhu S, Ravikanth M. Inorg Chem. 2012;51:4285–4292. doi: 10.1021/ic202758r. [DOI] [PubMed] [Google Scholar]
- 29.Boens N, Wang L, Leen V, Yuan P, Verbelen B, Dehaen W, Van der Auweraer M, De Borggraeve WD, Van Meervelt L, Jacobs J, Beljonne D, Tonnele C, Lazzaroni R, Rueds-Rama MJ, Orte A, Crovetto L, Talavera EM, Alvarez-Pez JM. J Phys Chem A. 2014;118:1576–1594. doi: 10.1021/jp412132y. [DOI] [PubMed] [Google Scholar]
- 30.Jiao L, Pang W, Zhou J, Wei Y, Mu X, Bai G, Hao E. J Org Chem. 2011;76:9988–9996. doi: 10.1021/jo201754m. [DOI] [PubMed] [Google Scholar]
- 31.a) Kee HL, Kirmaier C, Yu L, Thamyongkit P, Youngblood WJ, Calder ME, Ramos L, Noll BC, Bocian DF, Scheidt WR, Birge RR, Lindsey JS, Holten D. J Phys Chem B. 2005;109:20433–20443. doi: 10.1021/jp0525078. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Brizet B, Desbois N, Bonnot A, Langlois A, Dubois A, Barbe JM, Gros CP, Goze C, Denat F, Harvey PD. Inorg Chem. 2014;53:3392–3403. doi: 10.1021/ic402798f. [DOI] [PubMed] [Google Scholar]; c) Xu HJ, Bonnot A, Karsenti PL, Langlois A, Abdelhameed M, Barbe JM, Gros CP, Harvey PD. Dalton Trans. 2014;43:8219–8229. doi: 10.1039/c3dt53630k. [DOI] [PubMed] [Google Scholar]
- 32.a) Chen Y, Zhao J, Guo H, Xie L. J Org Chem. 2012;77:2192–2206. doi: 10.1021/jo202215x. [DOI] [PubMed] [Google Scholar]; b) Chen Y, Zhao J, Xie L, Guo H, Li Q. RSC Adv. 2012;2:3942–3953. [Google Scholar]
- 33.Didier P, Ulrich G, Mely Y, Ziessel R. Org Biomol Chem. 2009;7:3639–3642. doi: 10.1039/b911587k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.