Abstract
Multiple cellular systems exist to prevent uncontrolled inflammation in brain tissues; the Suppressor of cytokine signaling (SOCS) proteins have key roles in these processes. SOCS proteins are involved in restricting cellular signaling pathways by enhancing the degradation of activated receptors and removing the stimuli for continued activation. There are eight separate SOCS genes that code for proteins with similar structures and properties. All SOCS proteins can reduce signaling of activated transcription factors JAK and STAT, but they also regulate many other signaling pathways. SOCS-1 and SOCS-3 have particular roles in regulating inflammatory processes. Chronic inflammation is a key feature of the pathology present in Alzheimer’s disease (AD)-affected brains resulting from responses to amyloid plaques or neurofibrillary tangles, the pathological hallmarks of AD. The goal of this study was to examine SOCS gene expression in human non-demented (ND) and AD brains and in human brain-derived microglia to determine if AD-related pathology resulted in a deficit of these critical molecules. We demonstrated that SOCS-1, SOCS-2, SOCS-3 and CIS mRNA expression was increased in Aβ– and inflammatory-stimulated microglia, while SOCS-6 mRNA expression was decreased by both types of treatments. Using human brain samples from temporal cortex from ND and AD cases, SOCS-1 through SOCS-7 and CIS mRNA and SOCS-1 through SOCS-7 protein could be detected constitutively in ND and AD human brain samples. Although, the expression of key SOCS genes did not change to a large extent as a result of AD pathology, there were significantly increased levels of SOCS-2, SOCS-3 and CIS mRNA and increased protein levels of SOCS-4 and SOCS-7 in AD brains. In summary, there was no evidence of a deficit of these key inflammatory regulating proteins in aged or AD brains.
Keywords: inflammation, amyloid, microglia, gene expression, anti-inflammatory, cellular signaling
1
Neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS) and rarer tauopathies, can be characterized by loss of selective populations of neurons in brain regions involved with cognition or motor functions. Inflammatory responses to neurodegeneration include increased expression of cytokines, complement proteins, degradative enzymes, adhesion molecules, increased production of reactive oxygen species along with prominent cellular activation of microglia and astrocytes (recent reviews (Rampa et al, 2013;Lynch, 2014;Lane et al, 2012;Sastre et al, 2011)). Inflammatory responses can also be an early result of neurodegenerative insults even before significant neuronal loss is evident, and could be contributing to the establishment and acceleration of neurodegenerative processes (Ferretti and Cuello, 2011).
The primary cellular mediators of inflammation in brains affected by neurodegenerative processes are microglia, a population of central nervous system (CNS) resident macrophages that become activated in response to cell death and aggregated proteins (Lue et al, 2010;Lynch, 2014). Vascular endothelial cells, astrocytes and perivascular macrophages can also be involved in brain inflammatory responses (Mosher and Wyss-Coray, 2014). The innate immune system inflammatory response has evolved to be balanced and self-limiting to prevent damage and loss of viable cells and tissues. However, chronic inflammation, which becomes enhanced with aging and in age-related diseases, is a perpetuated form of inflammation with continued elevated expression of proinflammatory cytokines and related damaging enzymes (Mosher and Wyss-Coray, 2014). Chronic inflammation is a feature of most neurodegenerative and peripheral diseases (e.g. AD, heart disease, atherosclerosis, cancer, diabetes) (Frostegard, 2013). A feature of chronic age-related inflammation is a failure of endogenous anti-inflammatory systems to function effectively. As examples, the fractalkine and fractalkine receptor and the CD200 and CD200 receptor systems, which interact to downregulate microglial inflammation, become deficient with aging and in AD brains thus contributing to the perpetuation of chronic inflammation (Cribbs et al, 2012;Walker et al, 2009).
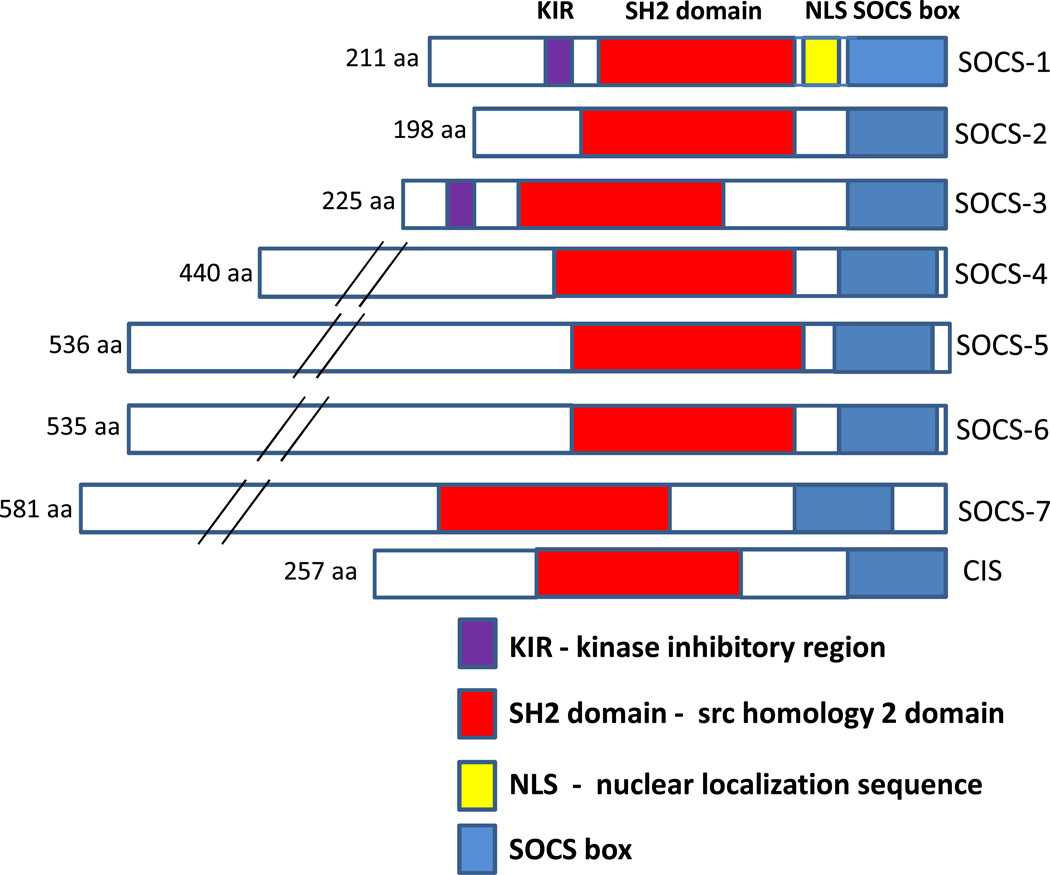
Many cytokines, including interleukins (IL) and interferons (IFN), and growth factors exert their biological effects via the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathways following interactions with cell surface receptors (Carow and Rottenberg, 2014;Dimitriou et al, 2008;Kershaw et al, 2013). Binding to certain classes of receptors (e.g. IL-6 to IL6R/gp130, IFN-γ to IFNGR1/IFNGR2) leads to the activation of receptor-associated JAK kinases, of which there are 4 (JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2)), and phosphorylation of receptor cytoplasmic domains. Phosphorylated receptors recruit STAT proteins, of which there are 7 (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6). STAT proteins become activated by phosphorylation, translocate to the nucleus and induce gene transcription of a range of inflammatory-associated genes (O'Shea and Murray, 2008). Suppressor of cytokine signaling (SOCS) proteins are the key anti-inflammatory regulators of JAK-STAT pathways with their expression being upregulated as a consequence of JAK-STAT activation (Kimura et al, 2005;Kershaw et al, 2013). The SOCS family of proteins has eight members – SOCS-1, SOCS-2, SOCS-3, SOCS-4, SOCS-5, SOCS-6, SOCS-7 and CIS (cytokine-inducible SH2 containing protein). The relative structures of these proteins are shown in Figure 1. These proteins interact with activated receptors and provide negative feedback to cytokine/growth factor signaling by inhibiting activated JAK or STAT interactions with receptors, and also by promoting the proteosomal degradation of activated receptors. The two most intensively studied SOCS genes from functional aspects are SOCS-1 and SOCS-3, which have key roles in regulating inflammatory responses. Since their initial identification, the range of factors that induce and are regulated by SOCS genes has greatly expanded from those involved in inflammatory signaling to include many growth factors (Trengove and Ward, 2013).
Fig. 1. Diagram showing relative sizes and structures of SOCS family of proteins.
A series of studies showed how SOCS-1 and SOCS-3 modulated inflammatory signaling in microglia/monocytes and astrocytes. SOCS-1 expression induced by IFN-β in astrocytes negatively regulated immune cell migration by reducing expression of key chemokines (Qin et al, 2008). SOCS-1 induced in microglia by IFN-β led to downregulation of CD40 expression (Qin et al, 2006). By contrast, a similar reduced CD40 inflammatory response was seen following lipopolysaccharide (LPS) treatment of microglia, which induced IL-10 causing increased expression of SOCS-3 (Qin et al, 2007). However, when expressed in neurons, SOCS-1 and SOCS-3 had different properties. SOCS-1 inhibited IFN-γ induction of MHC-II protein, while SOCS-3 inhibited insulin growth factor-1 (IGF-1)-induced neurite outgrowth (Yadav et al, 2005;Miao et al, 2006). Overexpression of SOCS-3 in neurons inhibited IGF-1 mediated neuroprotection from tumor necrosis factor (TNF)-α induced cell death (Yadav et al, 2005). Overexpression of SOCS-3 in oligodendrocytes and neurons following traumatic brain injury or spinal cord injury could be detrimental as this inhibited protective STAT3 mechanisms (Emery et al, 2006). The significance of SOCS-1 and SOCS-3 to CNS disease has been shown in models of MS. Administration of the SOCS-1 mimetic peptide tyrosine kinase inhibitor (TKIP), which inhibits JAK2 phosphorylation of STAT1a, protected mice from developing experimental autoimmune encephalomyelitis (EAE) and induced remission of those with active disease (Mujtaba et al, 2005). Conditional deletion of SOCS-3 from myeloid cells resulted in animals developing more severe EAE due to enhanced STAT-3 activation (Qin et al, 2012b). In relation to human disease, increased SOCS-3 expression in monocytes or T cells appears to be protective as MS patients with relapsing remitting form of the disease had lower levels of SOCS-3 expression than those in remission (Frisullo et al, 2007). A similar study showed a decrease of SOCS-1 mRNA, but an increase in SOCS-3 mRNA, expression in MS patients’ peripheral blood leukocytes (Sedeno-Monge et al, 2014). Data from cell culture and animal experiments indicated that SOCS expression should be transient with induction resulting from an acute increase of inflammation in the localized environment, followed by downregulation of inflammator signaling, and then degradation of the SOCS-receptor complexes in the proteasome. When moving our studies to human brain tissues, we had hypothesized that if SOCS expression could be detected it would be selectively in an environment of ongoing inflammatory activation. As there is evidence that manipulating SOCS expression, particularly SOCS-1 and SOCS-3, could be a target for drug development aimed at inflammation, we wanted to first address the question whether there was any alteration in SOCS expression in AD brains or in Aβ-stimulated human microglia that would permit chronic JAK/STAT signaling and therefore chronic inflammation.
2. EXPERIMENTAL PROCEDURES
2.1 Human brain tissue resources
All brain samples were from subjects who had been participants in the Arizona Study of Aging and Neurodegenerative Disorders and were autopsied by the Brain and Body Donation Program (BBDP) of the Banner Sun Health Research Institute, Sun City, Arizona. This longitudinal clinicopathological study has been running for 27 years with continuous Institutional Review Board approval. All cases were diagnosed according to National Institutes on Aging/Reagan criteria for AD (Newell et al, 1999) and accepted clinicopathological criteria for PD (McKeith et al, 2005;Gelb et al, 1999). The demographic data on cases used in this study are shown in Table 1. Brain tissue used was from inferior temporal gyrus (ITG), middle temporal gyrus (MTG) and cerebellum. For isolation of human microglia, samples of frontal cortex provided within 3 hours of death by the BBDP from consented donors were used. As part of the neuropathology diagnostic process, each brain was assessed for plaque and tangle load using histological procedures to measure severity of AD pathology (Dugger et al, 2012;Beach et al, 2012). In brief, 80 µm sections containing the requisite brain regions were stained using Campbell-Switzer or Gallyas methods to identify plaques or neurofibrillary tangles respectively. Plaque density scores were obtained by assigning values of none (0), sparse (1), moderate (2) and frequent (3), according to the published CERAD templates; a similar ranking score for neurofibrillary tangles using Braak and Braak criteria were used. This ranking score (0–3) for each of 5 brain regions (entorhinal cortex, hippocampus, temporal cortex, parietal cortex and frontal cortex) gives a potential summary score of 0–15 for frequency of plaques and tangles in each brain (Beach et al, 2012).
Table 1.
Demographic details of cases used in study
| Inferior Temporal Gyrus | ||||||
|---|---|---|---|---|---|---|
| Disease Groups (n) | M/F | Age (yrs) | PMI | Plaque Score | Tangle Score | MMSE |
| Non-Demented (11) | 8/3 | 84.4±8.1 | 2.5±0.5 (1.5–3.5) |
3.0±4.0 | 3.6±1.5 | 29 (27–30) |
| Mild Cognitively (13) Impaired |
10/3 | 88.6±7.3 | 2.8±0.8 (1.75–4.3) |
9.9±3.3 | 6.4±2.5 | 27.9 (25–30) |
| Alzheimer’s Disease (12) | 6/6 | 78.9±8.3 | 3.3±1.1 (1.83–5) |
14.0±1.5 | 14.7±0.5 | 9 (0–26) |
| Middle Temporal Gyrus | ||||||
|---|---|---|---|---|---|---|
| Disease Groups (n) | M/F | Age (yrs) | PMI | Plaque Score | Tangle Score | MMSE |
| Non-Demented (12) Low Plaque |
9/3 | 84.0±8.0 | 2.5±1.3 (1.5–3.25) |
3.1±2.1 | 2.6±0.6 | 28.6 (27–30) |
| Non-demented (12) High Plaque |
8/4 | 84.6±5.4 | 3.2±0.7 (2–4.8) |
9.2±1.6 | 3.5±1.3 | 27.8 (26–29) |
| Alzheimer’s Disease (12) | 6/6 | 78.9±9.4 | 2.9±0.9 (2.4–5) |
14.5±0.7 | 13.4±3.1 | 12 (0–21) |
Data represent: (mean± standard deviation). No significant differences between age or PMI of groups. Numbers in parentheses represent range.
2.2 Human autopsy brain cultures and experimental treatments
Human autopsy brain microglia were isolated from frontal cortex according to our standard protocols (Walker et al, 2006;Walker et al, 2009). After isolation, microglia were cultured for 10–14 days prior to use in experiments. The one difference from previous experiments was the culture of microglia in low glucose Dulbecco’s modified Eagles Medium (1 g/L) and not high glucose DMEM (4.5 g/L) as higher concentrations of glucose have been shown to affect SOCS-1 and SOCS-3 expression in vitro (Gonzalez et al, 2012;Venieratos et al, 2010). For this study, microglia from ND cases only were used. Data were combined from each experiment for analyses. Microglia cultures (n=3 per treatment) were stimulated for 24 hours with fibril/oligomer-containing Aβ(1–42) peptide (Walker et al, 2006) (CPG Scientific, Sunnyvale, CA) at 0.5-5 µM, cytokines interferon-gamma (IFN-γ), interleukin (IL)-1β, IL-4, IL-6, IL-10 and transforming growth factor (TGF)β-1 (20 ng/ml) (all cytokines from R&D Systems, Minneapolis, MN) or lipopolysaccharide (LPS)(Sigma)(100 ng/ml). Treated cells were used for preparation of RNA as described below.
2.3 RNA isolation and Quantitative Real Time Polymerase Chain Reaction
RNA was prepared from the human brain tissue samples and cultured human microglia using RNAeasy Plus Mini kits (Qiagen, Valencia, CA) according to the manufacturer’s directions. Concentrations of RNA samples were determined using a Nanodrop 1000 spectrophotometer, and integrity using an Agilent Bioanalyzer and Nano 6000 kits (Agilent, Wilmington, DE). All samples used for qPCR had RIN values greater than 7.0. RNA from each brain sample (0.5 µg) or from cultured cell sample (0.2 µg) was reverse transcribed using a Quantitect reverse transcription kit (Qiagen). Prior to use in quantitative PCR (qPCR), each cDNA was diluted 1:1 with water. Appropriate numbers of no reverse transcriptase controls were prepared in parallel for each batch of samples. For qPCR, cDNA samples were amplified using Perfecta SYBR Green Fast Mix 2x reaction mixture (Quanta Biosciences, Gaithersburg, MD) and mRNA-specific primers for SOCS/CIS genes (listed in Table 2). All primer pair sequences were verified for absence of hairpin formations or primer-dimers that interfere with amplification efficiency (NetPrimer, Premier Biosoft), and had amplification efficiencies within the range of 95%-105%. QPCR was carried out using a Stratagene Mx3000p machine and expression levels quantified relative to a standard curve. All PCR values were normalized against values for β-actin mRNA expression as described previously (Walker et al, 2009). QPCR analyses followed most of the recommended criteria for minimal information for publication of quantitative real time PCR experiments (MIQE) (Bustin et al, 2009).
Table 2.
SOCS PCR primer sequences
| Gene | Primer Sequences | Amplicon/bp | Ref Seq | |
|---|---|---|---|---|
| SOCS-1 | AS: | GGATGCCGTGTTATTTTGTT | 242 bp | NM_00345.1 |
| S | TAGGAGGTGCGAGTTCAGGT | |||
| SOCS-2 | AS: | GCAAGGATAAGCGGACAG | 107 bp | NM_003877.4 |
| S: | TAATGGTGAGCCTACAGAGAT | |||
| SOCS-3 | AS: | CCTCGCCACCTACTGAACC | 174 bp | NM_003955.4 |
| S: | TCCGACAGAGATGCTGAAGA | |||
| SOCS-4 | AS: | CGGTTATGTGTGGAGTGGAA | 273 bp | NM_199421.1 |
| S: | TTAGACGGAAGCCCTGAAGA | |||
| SOCS-5 | AS: | GAGGGAGGAAGCCGTAGTG | 104 bp | NM_014011.3 |
| S: | CTTTGTTGCTGAGGAGTTGAG | |||
| SOCS-6 | AS: | TCTCACCATTGCTACCTCCA | 299 bp | NM_004232.3 |
| S: | CCCGAACAAGAAAAGAACCA | |||
| SOCS-7 | AS: | CTCTCCACACCCTCCAACTC | 219 bp | NM_014598.3 |
| S: | CCAACCACACTTCTCCAACTC | |||
| CIS | AS: | CCAGCCCAGACAGAGAGTG | 106 bp | NM_013324.5 |
| S: | AACCCCAATACCAGCCAG |
2.4 Western Blot Analysis
Protein extracts from temporal cortex (MTG) were analyzed using western blot methodology for measuring levels of proteins (Walker et al, 2013). Samples were dissolved at a concentration of 1 µg/µl protein in western blot sample buffer (NUPAGE LDS—Life Technologies, Carlsbad, CA) containing 0.1 M DTT and heated at 70 °C for 10 minutes. Samples were separated on 4–12% NuPAGE Bis–Tris Mini gels using MOPS-SDS running buffer (Life Technologies). Proteins were transferred to nitrocellulose membranes according to the manufacturer's suggestion (30 V for 90 minutes). Following drying, membranes were blocked in 5% skim milk solution dissolved in Tris-buffered saline (TBST—50 mM Tris–HCl (pH 8.0), 250 mM NaCl, 0.05% (w/v) Tween 20), and then reacted for 18 hours in appropriate dilutions of antibodies in TBST containing 2% milk. The following antibodies were employed in this study: SOCS-1 (Zymed: ZMD385, rabbit polyclonal; 1:2000 dilution); SOCS-2 (R&D Systems, AF4979, goat polyclonal; 0.5 µg/ml); SOCS-3 (R&D Systems, MAB5696, mouse monoclonal; 0.25 µg/ml); SOCS-4 (R&D Systems, MAB5628, mouse monoclonal; 0.1 µg/ml); SOCS-5 (R&D Systems, AF4796, goat polyclonal; 0.25 µg/ml); SOCS-6 (R&D Systems, MAB5806, mouse monoclonal; 0.25 µg/ml); SOCS-7 (R&D Systems, MAB5809, mouse monoclonal; 0.5 µg/ml); phosphorylated tau (Ser 202/Thr 205)(Pierce Antibodies, MN1020, mouse monoclonal; 0.05 µg/ml). We were not able to identify a satisfactory antibody for detecting CIS in human brain samples. SOCS-1 and SOCS-3 antibody specificity were confirmed using HEK 293 cells transfected with SOCS-1 and SOCS-3 expressing plasmids (Genecopoeia, Gaithersburg, MD). Following washes of membranes with TBST, bound antibody was detected by reaction for 2 hours with the appropriate horseradish peroxidase (HRP) labeled anti-immunoglobulin (Thermo-Fisher - 1:10,000 dilution). Bound antibodies were detected by reaction of membranes with HRP chemiluminescent substrate (Advansta Western Bright chemiluminescent substrate, Advansta, Menlo Park, CA) with direct imaging using a Fluorochem Q imaging system (Cell Biosciences, Santa Clara, CA). Protein loading was normalized by reaction of all blots with an antibody to β-actin (mouse monoclonal: Sigma (St. Louis, MO) at 1:5000 dilution). Intensities of chemiluminescence bands were quantified using Fluorochem Q SA software (Cell Biosciences). To ensure reproducible measurements across multiple blots, each set of gels included a repeated series of samples that were used to verify consistency between blots.
2.5 Statistical Analysis
Statistical analyses, except multiple linear regression analyses, were performed using Graphpad Prism version 6 (Graphpad software, La Jolla, CA: www.graphpad.com). Non-parametric measures were analyzed using the Kruskall-Wallis test with Dunn’s test for comparison between groups, while parametric measures were analyzed by One Way Analysis of Variance (ANOVA) with the Fisher LSD test for post-hoc comparison between groups. Correlation analysis between SOCS measures and non-parametric ranking measures (plaques scores or tangle scores) employed the Spearman non-parametric correlation test. To determine the potential interaction of different factors (disease, plaque numbers, tangle numbers, post mortem interval), multiple linear regression analyses were carried out (SigmaPlot, Systat Software, San Jose, CA.). For statistical tests, significance levels were set at P< 0.05.
3. RESULTS
3.1 SOCS mRNA expression by human microglia
In a previous study that had employed gene expression profiling of amyloid beta (Aβ) peptide stimulated human microglia, we had observed a consistent increase in mRNA expression of SOCS-3 (Walker et al, 2006). Since there have been no studies of SOCS/CIS in human brains or brain-derived microglia, a survey of these genes in relation to expression in stimulated human brain-derived microglia was initiated.
We extended our earlier studies by measuring SOCS-1 to SOCS-7 and CIS mRNA expression in a series of human microglia samples stimulated with different doses of aggregated Aβ(1–42). The primers are listed in Table 2. Each set of primers was specific for the indicated SOCS gene, and would detect all transcript variants of the particular SOCS gene.
The method used to aggregate Aβ produces a mixture of Aβ oligomers and fibrils, which will represent species of Aβ found in AD-affected brains (Masters and Selkoe, 2012). We designed the in vitro Aβ stimulation experiments to model chronic inflammation by using a 24 hour-treatment paradigm. As it had also been shown that induction of SOCS-1, SOCS-2, SOCS-3 and CIS mRNA in LPS-treated human monocyte-derived dendritic cells appeared to be protracted with significant elevations of expression still detectable after 24 hours, this one time point was used for all in vitro treatments (Posselt et al, 2011). As the number of microglia available from each human case is limited, use of multiple time points or experimental doses is not possible.
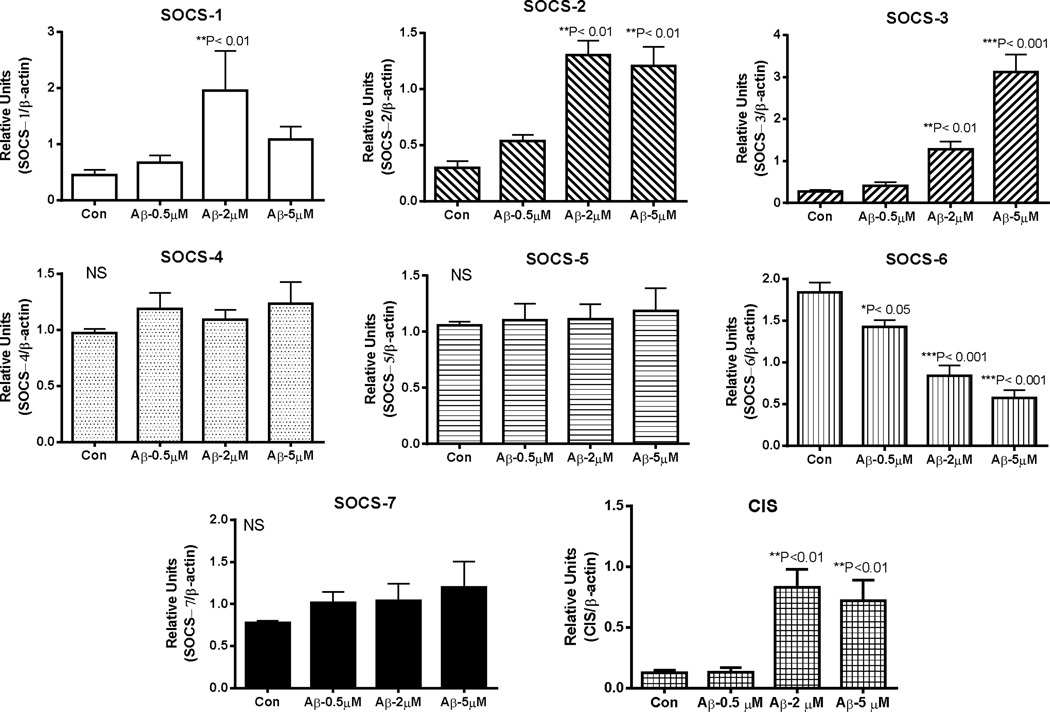
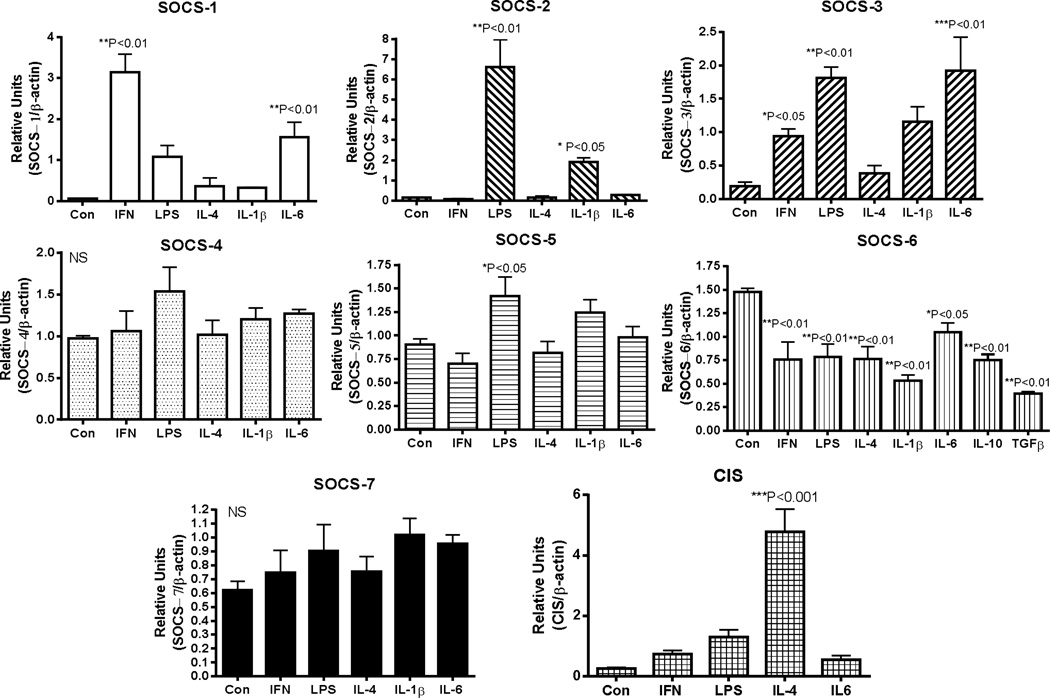
Induction of SOCS-1, SOCS-2, SOCS-3 and CIS mRNA expression by Aβ stimulation of human microglia was evident, but not for SOCS-4 – SOCS-7 (Fig. 2). SOCS-1 – 3 and CIS have significant roles in modulating inflammation, while SOCS-4–7 have been less characterized. SOCS-1 mRNA expression was induced by 4.3-fold, SOCS-2 mRNA by 4.3-fold, SOCS-3 mRNA by 11.1-fold and CIS mRNA by 6.6-fold. Downregulation of SOCS-6 mRNA expression was a unique feature of these studies. Comparing the effects of Aβ with other inflammatory cytokines and regulators (Fig. 3), the maximal responses for SOCS-1, SOCS-2, SOCS-3 and CIS mRNA were to different cytokines. SOCS-1 mRNA expression was strongly induced by IFN-γ, while SOCS-2 mRNA was most strongly stimulated by LPS and IL-1β, but not by IFN-γ or IL-6. By comparison, stimulation of expression of SOCS-3 mRNA occurred in response to IL-6, LPS, and IFN-γ, while CIS mRNA was stimulated most strongly by IL-4. Consistent with the downregulation of SOCS-6 mRNA expression by Aβ stimulation, this feature was also observed with a range of cytokines. It is noticeable that the anti-inflammatory cytokines IL-4, IL-10 and TGF-β1 caused downregulation of SOCS-6 mRNA expression similar to a range of proinflammatory agents. All data were obtained from the same group of microglia cases with the exception of treatments with IL-10 and TGFβl where the analyses were carried out with additional cases. It can be seen that expression of SOCS-4, SOCS-5 and SOCS-7 mRNA expression was not significantly affected by Aβ or the tested cytokines, with only LPS having a small but significant effect on SOCS-5 mRNA expression (P<0.05).
Fig. 2. Relative effects of amyloid beta (Aβ) peptide stimulation of human microglia on expression of mRNA for SOCS genes.
Bar charts show mean ± standard error of mean (SEM) of mRNA levels for SOCS-1, SOCS-2, SOCS-3, SOCS-4, SOCS-5, SOCS-6, SOCS-7 and CIS following 24 hour-treatment of human microglia (n=4 cases) with indicated doses of aggregated Aβ (1–42). Significant differences between treatment and control cultures indicated. Statistical analysis one-way Analysis of Variance (ANOVA) followed by Dunnett’s post hoc test for between group significance.
Fig. 3. Relative effects of cytokine stimulation of human microglia on expression of mRNA for SOCS genes.
Bar charts show mean ± standard error of mean (SEM) for SOCS-1, SOCS-2, SOCS-3, SOCS-4, SOCS-5, SOCS-6, SOCS-7 and CIS mRNA following treatment of human microglia (n=4 cases) with indicated cytokines and inflammatory agents. All cytokines were used at 20 ng/ml doses, while lipopolysaccharide (LPS) was used at 100 ng/ml. Significant differences between treatment and control cultures indicated. Statistical analysis one-way Analysis of Variance (ANOVA) followed by Dunnett’s post hoc test for between group significance.
Abbreviations: IFN: Interferon-γ. LPS: lipopolysaccharide. IL: interleukin. TGF: transforming growth factor β-1.
The qPCR analysis method used produces relative values for each gene with the samples tested. Although there were significant changes in expression with many of the treatments, a noticeable feature was abundant expression of SOCS/CIS mRNA in unstimulated microglia.
3.2 SOCS mRNA expression in human brains
As we had shown that human brain-derived microglia responded strongly to treatment with aggregated Aβ(1–42), which would be present in vulnerable areas of AD brains, with altered expression of SOCS-1, SOCS-2, SOCS-3, CIS and SOCS-6 mRNA, we continued our survey by measuring expression of all these genes in cDNA samples derived from inferior temporal gyrus (ITG) of ND, mild cognitively impaired (MCI) and AD cases (Table 1). The goal was to determine if there was evidence of similar altered patterns of gene expression in human brains with significant amounts of AD pathology. These samples were derived from elderly donors that were separated into these three groups based on degree of cognitive decline. The relative levels of neuropathology and cognitive levels (MMSE) in each group are shown in Table 1. MCI can be considered an intermediate stage between ND and AD in terms of cognitive decline and degree of pathology. The results of analyses for ITG were compared to cerebellum, a brain region that does not develop significant AD pathology.
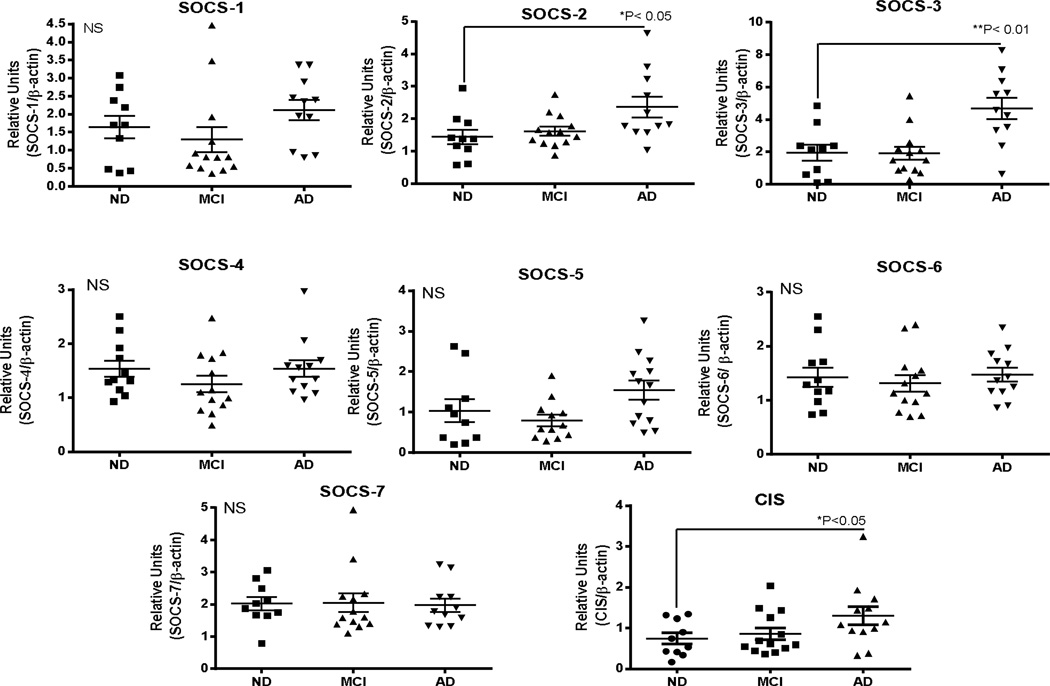
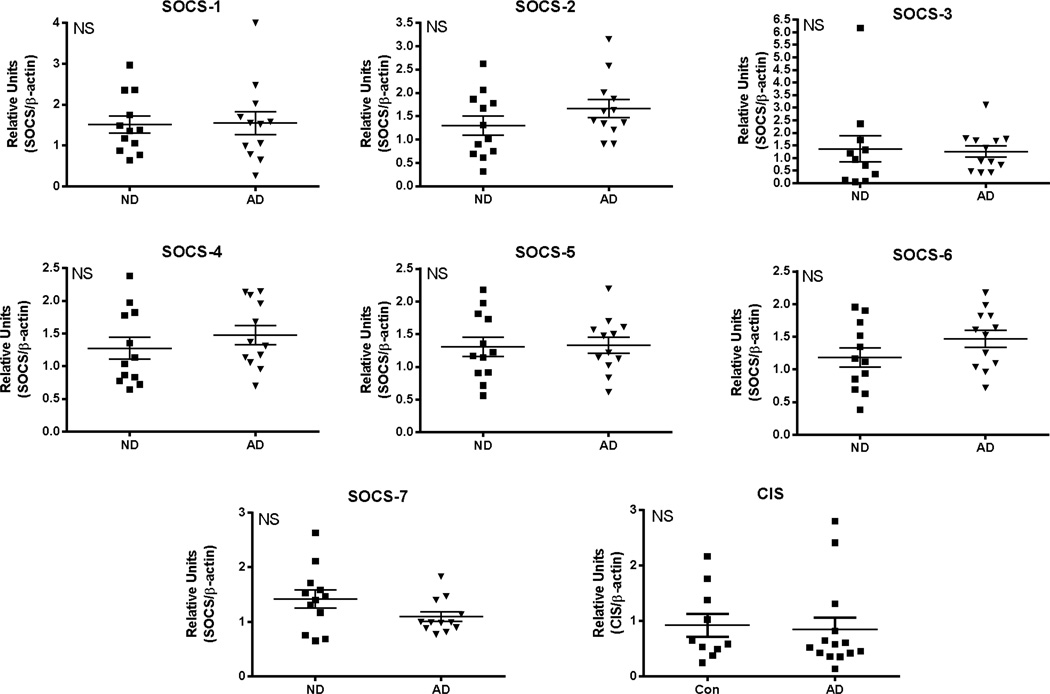
Significantly increased expression of SOCS-2 (*P< 0.05), SOCS-3 (**P<0.01) and CIS (*P<0.05) was detected in the AD cases compared to the MCI or ND cases, but for the other SOCS genes, there were no significant differences between the groups (Fig. 4). Multiple linear regression analysis showed that only SOCS-3 mRNA expression was significantly affected by PMI (*P=0.03). If expression levels of SOCS-3 mRNA were controlled for PMI, a disease difference was still achieved (*P<0.05). There were no differences in expression levels in cerebellum between the ND and AD groups for any SOCS genes (Fig. 5).
Fig. 4. Expression of SOCS genes in inferior temporal gyrus samples from non-demented (ND), mild cognitive impaired (MCI) and Alzheimer’s disease (AD) cases.
Scatter plots show mRNA levels for SOCS-1, SOCS-2, SOCS-3, SOCS-4, SOCS-5, SOCS-6, SOCS-7 and CIS in indicated human brain samples. ND (n=11); MCI (n=13); AD (n=12). Lines represent mean values ± SEM. Significant differences between groups indicated for SOCS-2 and SOCS3. Statistical analysis one-way Analysis of Variance (ANOVA) followed by Fisher LSD post hoc test for between group significance.
Fig. 5. Expression of SOCS genes in cerebellum samples from non-demented (ND), Parkinson’s disease (PD) and Alzheimer’s disease (AD) cases.
Scatter plots show mRNA levels for SOCS-1, SOCS-2, SOCS-3, SOCS-4, SOCS-5, SOCS-6, SOCS-7 and CIS in indicated human brain samples. ND (n=11); MCI (n=12); AD (n=12). Lines represent mean values + SEM. Significant differences between groups indicated for SOCS-2 and SOCS3. Statistical analysis one-way Analysis of Variance (ANOVA) followed by Fisher LSD post hoc test for between group significance.
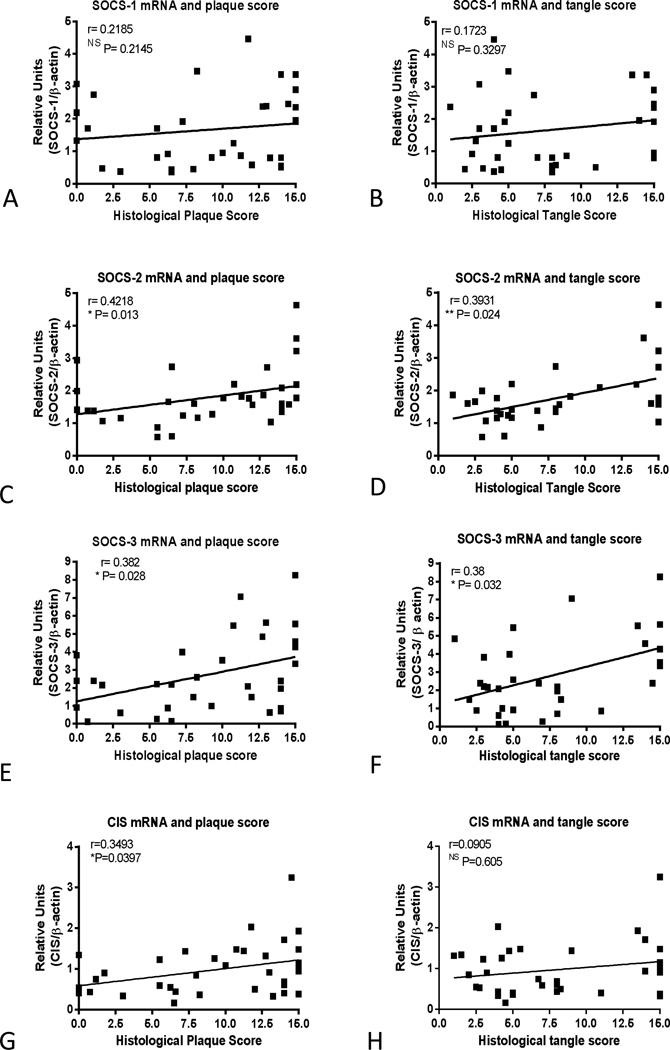
Correlation analyses were carried out between levels of SOCS mRNAs and plaque and tangle scores to determine if there were interactions between these factors (Fig. 6). There were significant correlations between SOCS-2 mRNA levels and plaque scores (Fig. 6C) (Spearman r=0.4218, P=0.013) and tangle scores (Fig. 6D) (Spearman r=0.3931, P=0.024); between SOCS-3 mRNA levels and plaque scores (Fig. 6E) (Spearman r=0.382, P=0.028) and tangle scores (Fig. 6F) (Spearman r=0.38, P=0.032); and between CIS mRNA levels and plaque scores (Fig. 6G) (Spearman r=0.349, P=0.039), but not tangle scores (Spearman r=0.09, P=0.605)(Fig. 6H). There were no correlations between these measures for SOCS-1 (Fig. 6A or 6B) or for SOCS-4, SOCS-5, SOCS-6 or SOCS-7 (not shown).
Fig. 6. Correlation analysis of SOCS-1, SOCS-2, SOCS-3 and CIS mRNA levels with histological plaque and tangle scores.
Scatter plots showing SOCS mRNA levels plotted against histological plaque scores (A, C, F, G) or histological tangle scores (B, D, F, H) with linear regression lines displayed for each chart. Statistical analysis Spearman r non-parametric correlation coefficient with indicated P values showing significance. Significance correlations between SOCS-2 mRNA and plaque (Spearman r =0.4218, P=0.013) and tangle scores (Spearman r=0.3931, P=0.024) , between SOCS-3 mRNA and plaque (Spearman r =0.382, P=0.028) and tangle scores (Spearman r=0.38, P=0.032) and between CIS mRNA and plaque (Spearman r =0.349, P=0.04) but not tangle scores (Spearman r=0.09, P=0.605). Lack of correlation between SOCS-1 mRNA and plaque and tangle scores (A and B) and also for SOCS-4, SOCS-5, SOCS-6, SOCS-7 (not shown).
3.3 SOCS protein expression in human brains
To further the characterization of SOCS expression in human brains, we used a series of brain samples derived from middle temporal gyrus (MTG), another region that shows vulnerability to AD pathology, to measure changes in protein levels with disease (Table 1). These cases had been selected to provide samples with a progressive change in amounts of plaque pathology. One ND group had low plaque scores (<5) (ND-LP), the other ND group had high plaque scores (>5) (ND-HP), but no diagnosis of AD (Table 1). All of the ND-HP cases were classified as cognitively normal, except one with a diagnosis of MCI. The ND-LP and ND-HP groups had similar tangle scores (Table 1).
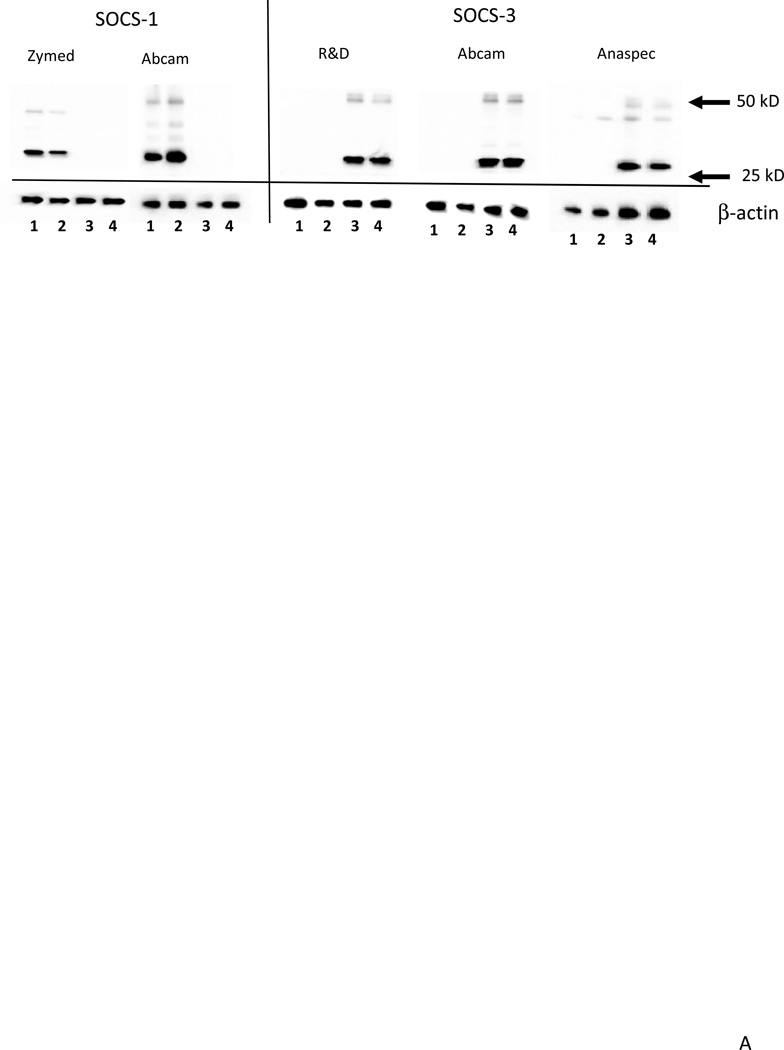
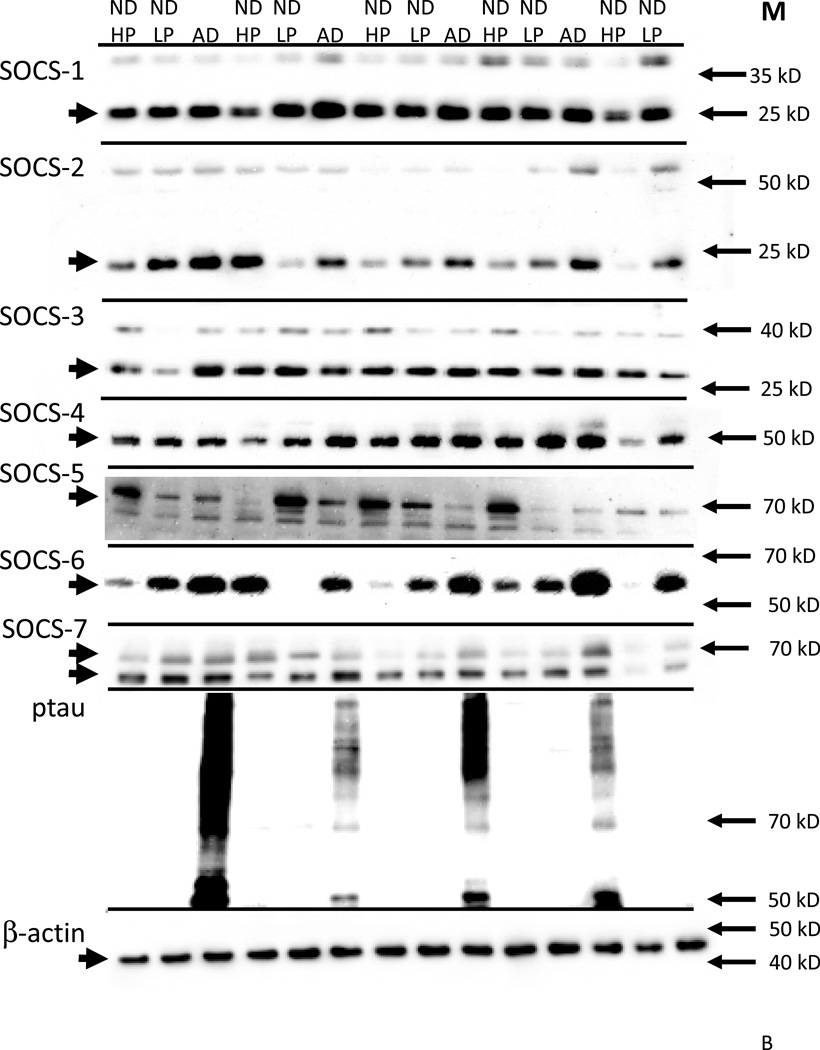
Western blots were carried out with this series of samples to measure levels of each SOCS proteins. The specificity of different antibodies to SOCS-1 and SOCS-3 were further tested and verified using cell lysates from SOCS-1 or SOCS-3 overexpressing cells (Fig. 7A). In brain samples, each of the SOCS antibodies identified a band of the expected molecular weight for the particular SOCS protein (Fig. 7B). SOCS-5 and SOCS-7 antibodies identified two closely migrating bands; both bands were quantified. As SOCS proteins tend to be short-lived proteins, and can interact with ubiquitin complexes and various receptors, we considered these bands to represent the functional brain-forms of these proteins. The presence of higher molecular weight forms of SOCS-1, SOCS-2 and SOCS-3 were also consistently identified in brain samples and transfected cells and could also represent complexes of SOCS with other proteins. These upper bands were not included in the measurements as they represented minor species. To confirm the differences between the ND-LP, ND-HP groups and AD group in relation to pathology, western blots were reacted with the antibody AT8 that recognizes tau phosphorylated at serine 202 and threonine 205 and specifically identifies tangles. Phosphorylated tau was only detectable in the AD samples (Fig. 7B).
Fig. 7.
A: Validation of antibodies to SOCS-1 and SOCS-3 using HEK293 cells transfected with SOCS-1 or SOCS-3 plasmids.
Panel shows specificity of a range of antibodies to SOCS-1 or SOCS-3. . Antibodies shown (left to right) are SOCS-1 (Zymed; rabbit polyclonal dilution 0.5 µg/ml); SOCS-1 (Abcam cat no AB83493; rabbit polyclonal dilution 1:2000); SOCS-3 (R&D; mouse monoclonal dilution 0.2 µg/ml); SOCS-3 (Abcam AB53984, rabbit polyclonal dilution 1:1000) and SOCS-3 (Anaspec rabbit polyclonal dilution 0.5 µg/ml). All gels. Lane 1: HEK-293 cells transfected with SOCS-1 plasmid (1 µg): Lane 2: HEK-293 cells transfected with SOCS-1 plasmid (0.5 µg): Lane 3: HEK-293 cells transfected with SOCS-3 plasmid (1 µg): Lane 4: HEK-293 cells transfected with SOCS-3 plasmid (1 µg):
B: Representative western blot results for detection of SOCS family of proteins in human middle temporal gyrus samples.
Figure shows relative molecular weights of indicated SOCS proteins, phosphorylated tau and β-actin for a subset of brain samples analyzed. The positions of adjacent marker proteins are indicated on each panel. The presence of additional bands is shown for SOCS-1, SOCS-2 and SOCS-3 and SOCS-7. These bands were a consistent feature and could represent ubiquitin modified SOCS proteins conjugated with other target proteins. The major bands for each protein are of the expected molecular weight. Antibodies used were as follows: SOCS-1 (Zymed; rabbit polyclonal) ; SOCS-1 (Zymed; rabbit polyclonal) SOCS-1 (Zymed; rabbit polyclonal) SOCS-1 (Zymed; rabbit polyclonal) SOCS-1 (Zymed; rabbit polyclonal) SOCS-1 (Zymed; rabbit polyclonal) SOCS-1 (Zymed; rabbit polyclonal) SOCS-1 (Zymed; rabbit polyclonal) SOCS-1 (Zymed; rabbit polyclonal)
Abbreviations: ND: non-demented. AD: Alzheimer’s disease. HP: high plaque case (scores >5). LP: low plaque case (scores<5).
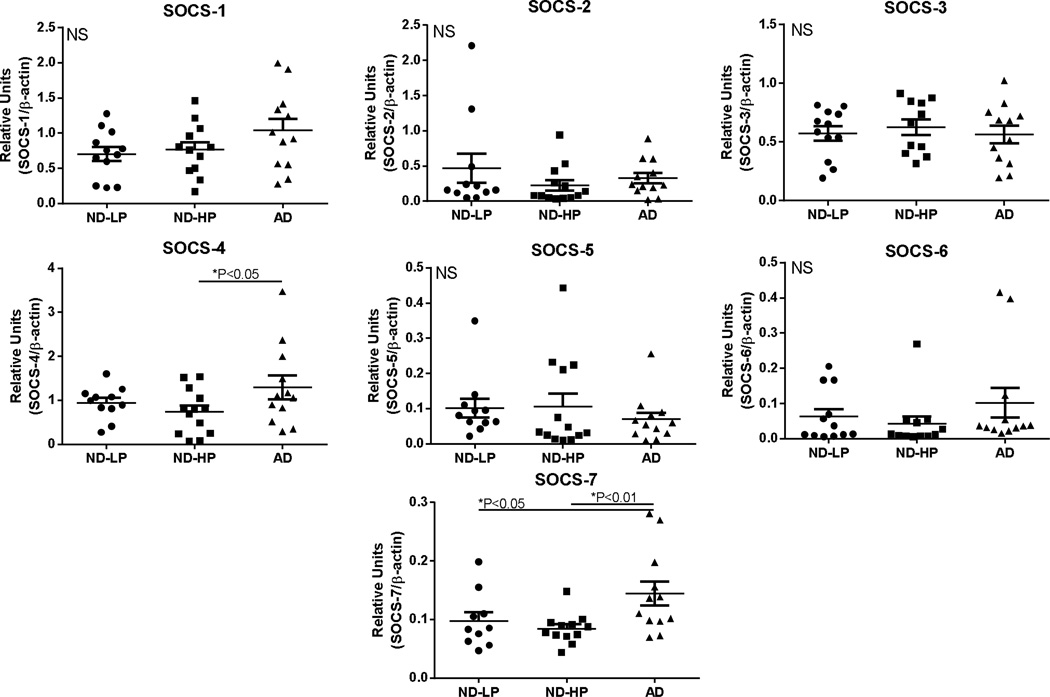
Western blot quantifications of SOCS protein levels in these MTG samples, divided into ND-LP, ND-HP and AD groups, are shown (Fig. 8). Although there were differences in mRNA levels for SOCS-2 and SOCS-3 in AD cases compared to ND cases, this was not reflected in differences in their protein levels. By comparison, significantly increased protein levels were detected for SOCS-4 (one way ANOVA; *P<0.05) between ND-HP and AD group, and for SOCS-7 (one way ANOVA; **P<0.01) between both the ND-LP and ND-HP and AD group. Analyses showed that the differences in any SOCS protein levels between the ND-LP group compared to the ND-HP group did not reach statistical significance.
Fig. 8. Relative levels of SOCS proteins in middle temporal gyrus samples from non-demented (ND) and Alzheimer’s disease (AD) cases.
Scatter plots show protein levels for SOCS-1, SOCS-2, SOCS-3, SOCS-4, SOCS-5, SOCS-6 and SOCS-7 in indicated human brain samples. ND-LP (n=12); ND-HP (n=12); AD (n=12). Lines represent mean values ± SEM. ND cases contained 12 LP cases and 12 HP cases. Significant differences between groups indicated for SOCS-4 and SOCS-7. Statistical analysis was one-way Analysis of Variance (ANOVA) followed by Fisher LSD post-hoc test for between group significance.
Abbreviations: ND-LP: non-demented low plaque (scores <5): ND-HP: non-demented high plaque (scores >5). AD: Alzheimer’s disease.
4. DISCUSSION
This is the first report to profile SOCS genes in human aged and AD brains and their regulation in human microglia. SOCS proteins as key regulators of receptor-mediated-inflammation and insulin signaling could have pivotal roles in controlling these processes in human brains affected by neurodegenerative diseases (Jorgensen et al, 2013;Wada et al, 2011). There is significant evidence that increased inflammation is involved in AD pathogenic processes (Ferreira et al, 2014;Kamal et al, 2014). Identifying the features of SOCS protein expression in human brains would be the first stage in determining whether they represent potential therapeutic targets for preventing neuroinflammation in AD. This had been speculated based on the many regulatory properties of SOCS-1 and SOCS-3 (Baker et al, 2009;Wang and Campbell, 2002), but overall there have been limited numbers of studies on the neurobiology of these molecules, particularly in human brains.
The goal of this study was not only to investigate expression of SOCS family of genes in human brains in relation to AD, but also in microglia as the primary inflammatory-associated cells in the brain. The major findings of this study are that all SOCS mRNA and proteins are readily detectable in human brain samples, even in the ND samples, and there is increased expression of key SOCS genes in brains affected by AD. The constitutive expression of SOCS mRNA and protein suggest that factors leading to their induction and persistence are ubiquitous as part of normal cellular functions not just during inflammation. SOCS-1, SOCS-2, SOCS-3 and CIS mRNA expression was upregulated in microglia treated with Aβ; changes in expression of SOCS-2, SOCS-3 and CIS mRNA expression was also detected in AD brains where increased levels of Aβ and inflammation are features.
Considering the in vitro data from human microglia; while increased expression of SOCS-1, SOCS-2, SOCS-3 and CIS mRNA in microglia showed dose responses to Aβ.the patterns of responses to cytokines for these genes indicated different features of regulation. SOCS-1 mRNA induction was greatest in response to IFN-γ, SOCS-2 mRNA to LPS, CIS mRNA induction to IL-4, and SOCS-3 mRNA was greatest to IL-6 but also affected by IFN-γ and LPS. The differential patterns of responses to cytokines could be related to their effects on different isoforms of JAK or STAT. SOCS-1 has been found to respond preferentially to and regulate STAT1, while SOCS-3 tends to preferentially respond to and regulate STAT3. The functions of SOCS-2 and CIS share many features and been shown to respond preferentially to STAT5 activation, however the induction of CIS by IL-4 would suggest that this was primarily due to STAT6 activation in microglia (Trengove and Ward, 2013). A prominent induction of CIS by IL-4 was also observed as part of the generation of T helper cells (Yang et al, 2013). CIS was also shown to be induced in macrophages from humans and mice treated with IL-4 (Martinez et al, 2013). The presence of classical activated (M1) and alternatively activated (M2a, M2b, M2c) microglia are a feature in the AD brain and controlling the polarization of microglia towards the reparative anti-inflammatory phenotype is now believed central to reducing neuroinflammation (Colton and Wilcock, 2010;Colton et al, 2006;Wilcock, 2014). CIS may prove to be a useful biomarker for following this process as SOCS proteins have key roles in this process (Qin et al, 2012a;Whyte et al, 2011).
In addition to being induced by activated JAK/STAT pathways, SOCS-3 can be induced by TNFα through the MKK6/MAPK cascade (Ehlting et al, 2007), and by LPS via the MAPK-ERK1/2 and JNK pathways downstream of toll-like receptor (TLR)-4 activation (Gorina et al, 2011). This latter pathway could be the mechanism for Aβ induction of SOCS-1, SOCS-2, SOCS-3 and CIS mRNA in human microglia. Certain aggregated forms of Aβ can directly interact with microglial TLR-4 resulting in its activation (Reed-Geaghan et al, 2009;Walter et al, 2007). It was observed that a number of different TLR ligands could activate expression of SOCS-1 and SOCS-3 in murine macrophage, resulting in the downregulation of TLR signaling due to inhibition of type 1 interferon signaling not NFκB activation (Baetz et al, 2004). As this study indicated that TLR ligands can also directly activate STAT1, it needs to be determined if Aβ stimulation of microglia results directly in STAT activation, or whether SOCS gene expression induction is by an alternative pathway.
The pronounced downregulation of SOCS-6 mRNA with Aβ and cytokine treatments was unique compared to other SOCS genes studied, and suggests a constitutive regulatory function that is being disrupted by inflammatory processes (Trengove and Ward, 2013;Li et al, 2004). There appear to be no studies on the role of SOCS-6 in innate immune responses so further experimentation will be required to define how reduction in SOCS-6 affects the nature of microglia inflammatory responses. SOCS-6 appears to have a number of unique regulator functions including regulation of T-cell signaling by binding to p56lck (Choi et al, 2010), promoting neurite outgrowth (Gupta et al, 2011) and regulating phosphoinositide 3-kinase (PI3K) (Li et al, 2004). These responses of microglia might be cell-type specific as we have observed that SOCS-6 mRNA expression in human astrocytes, neurons and brain vascular endothelial cells was not downregulated by inflammatory stimulation (unpublished observations). Rodent astrocytes have also been shown to express all SOCS genes constitutively; SOCS-6 mRNA expression did not change with inflammatory stimulation in this cell type (Hwang et al, 2007).
In human AD ITG samples, there were significantly increased levels of SOCS-2, SOCS-3 and CIS mRNA compared to ND and/or MCI samples, but not for the other SOCS genes, while there were no significant changes in SOCS mRNA levels in cerebellum samples. AD pathology, including microglial activation and inflammation, is not a significant feature in cerebellum, while most features of AD pathology are found in temporal cortex. The induction of these particular genes in AD brains, where there is a large increase in amounts of Aβ as the major constituent of amyloid plaques, is consistent these genes being induced by Aβ in human microglia, however other brain cell types that respond to Aβ can also express SOCS/CIS genes. The expression changes of SOCS-2, SOCS-3 and CIS mRNA in AD brains correlated with increasing amounts of pathology. We showed that expression levels for SOCS-2 and SOCS-3 mRNA significantly correlated with histological plaque and tangle scores suggesting that both pathologies were inducing SOCS expression, while CIS mRNA levels only correlated with plaque scores. This last piece of data would support the induction of CIS mRNA in AD brain being a response to Aβ or Aβ–induced inflammatory responses. Correlations between all of these features were not strong, which would suggest that other factors are involved in maintaining or inducing SOCS/CIS expression. The lack of change of SOCS-1 mRNA likely represents differences in the cytokine environment in the human brain, particular the lack of IFN-γ. SOCS-1, SOCS-2, SOCS-3 and CIS have been shown to be more significantly involved in inflammation regulation compared to other SOCS genes whose primary functions involve growth factor regulation, however this discrimination is not as clear now as it used to be as the range of SOCS regulating receptors and pathways keeps expanding (Trengove and Ward, 2013;Dalpke et al, 2008;Labuzek et al, 2012). The downregulation of SOCS-6 mRNA expression observed in activated microglia was not replicated in the human brain studies suggesting other factors were also regulating SOCS-6 expression (Li et al, 2004).
A significant finding was that changes in SOCS mRNA levels did not correspond to changes in SOCS protein levels in AD brains. There were increased levels of SOCS-4 and SOCS-7 protein, not SOCS-2 and SOCS-3. The differences in SOCS mRNA and protein levels might be due to different regions of temporal cortex being used for the mRNA and protein studies. This seems unlikely as it is known that the inferior and middle temporal gyri generally show the same degree of vulnerability to AD (Galton et al, 2001), and the neuropathology of the cases in both groups was very similar. However, based on the transient nature of SOCS proteins, it seems likely that newly synthesized SOCS proteins, particularly SOCS-3, are rapidly utilized and metabolized. The largest increase in SOCS mRNA in AD brains compared to ND was for SOCS-3, while the steady state protein levels were not different. There appear to be multiple factors promoting rapid turnover of SOCS proteins. Firstly, they will be metabolized after interacting with activated receptors and being targeted for proteosomal degradation, and secondly these proteins appear to have short half-lifes. As the half-life of SOCS-3 protein in macrophages induced by LPS and IFN-γ was only 0.7 hours (Fletcher et al, 2010), this short half-life might be common for all SOCS proteins. SOCS-1, SOCS-2, SOCS-3 and CIS proteins contain proline, glutamine, serine and threonine (PEST) motifs rendering them susceptible to proteolysis by calpain proteases (Babon et al, 2006). In addition, several recent studies have identified certain micro RNAs (miRs) that have complex roles in regulating the stability of SOCS mRNA and levels of SOCS proteins under inflammatory conditions (Amodio et al, 2013;Cardoso et al, 2012;Collins et al, 2013;Noguchi et al, 2013) . For example, inflammatory induction resulted in increased levels of miR-155 leading to reduced levels of SOCS-1 mRNA and protein. Restoring SOCS-1 protein by inhibiting miR-155 transcription reduced certain inflammatory markers (Cardoso et al, 2012). Increased miR-155 in the triple transgenic model of AD correlated with enhanced neuroinflammation and neuropathology and reduced levels of SOCS-1 (Guedes et al, 2014). Additional studies have shown SOCS-6 expression can be regulated by miRNA-424-5p and miRNA-431 in different cell types (Tanaka et al, 2014;Wu et al, 2013). Recent findings on the myeloid protein CD33 affecting the risk of developing AD have also pointed to a possible role for SOCS-3 in this process, and also indicate how new synthesized SOCS-3 induced under inflammatory conditions could be rapidly metabolized. It had been shown recently that a single nucleotide polymorphism for CD33 (rs3865444) was associated with reduced risk of AD, and expression levels of CD33 by microglia and monocytes affect their efficiency of amyloid uptake (Malik et al, 2013;Griciuc et al, 2013). It had been demonstrated in peripheral monocytes that SOCS-3 has a central role in removing activated CD33. SOCS-3 binds with the activated immunoreceptor tyrosine-inhibitor motif (ITIM) of CD33 resulting in its endocytosis and targeting this complex for proteosomal degradation (Orr et al, 2007;Gonzalez et al, 2012). Monocytes from diabetic patients or monocytes cultured under high glucose conditions had increased SOCS-3 expression and reduced levels of CD33. Reduced CD33 under some circumstances can result in enhanced levels of proinflammatory cytokines (Gonzalez et al, 2012), however in the context of AD, reduced microglia CD33 was associated with enhanced phagocytosis of Aβ (Griciuc et al, 2013). Although CD33 is just one of many receptors regulated by SOCS-3, this mechanism would enhance degradation of SOCS-3 and suggest an additional reason why increased expression of SOCS-3 mRNA in AD brains does not correspond with increased SOCS-3 protein. Further studies are ongoing to determine if SOCS-3 and CD33 interactions can be demonstrated in human brain AD samples.
At present, SOCS-4 has not been extensively studied and its function in brain is unclear, so the significance of increased protein levels in AD requires further studies. SOCS-4 mRNA expression was increased in hippocampus of rats after focal cerebral ischemia and reperfusion (Sun et al, 2007). SOCS-4 can be induced by epidermal growth factor (EGF) and regulates EGF receptor (EGFR) by competitively binding to phosphotyrosine sites with STAT3 (Bullock et al, 2007). Induction of EGFR expression in the vasculature and in neurons of AD brains has been observed (Styren et al, 1990;Ferrer et al, 1996). In addition, biomarker studies have shown increased levels of EGF in plasma of AD cases (Bjorkqvist et al, 2012;Marksteiner et al, 2011). As for SOCS-7, the function of this protein in brain also requires further studies. SOCS-7 can regulate growth hormone, leptin, insulin and EGFR by interaction with activated STAT3 and STAT5, but its regulation of inflammatory receptors is not known (Martens et al, 2005).
Considering the neurobiology of SOCS, a study employing gene expression profiling of young, elderly ND and AD brains showed that SOCS-3 mRNA expression in hippocampus increased significantly between the young and elderly ND cases, but not between the elderly ND and AD cases (Cribbs et al, 2012). An in situ hybridization study of SOCS-1, SOCS-2 and SOCS-3 mRNA in developing and adult mouse brains demonstrated predominant localization in neurons, particularly for SOCS-2 (Polizzotto et al, 2000). Increased neuronal and glial expression of SOCS-2 and SOCS-3 was detected in rats induced to have seizures; similarly increased astrocyte and neuronal expression of SOCS-2 occurred following transient forebrain ischemia (Rosell et al, 2003;Choi et al, 2009). A study using transgenic mice that overexpressed IL-12 on the astrocyte-specific glial fibrillary acidic protein promoter hadsignificant induction and overexpression of SOCS-1 and SOCS-3. By comparison, SOCS-2 and SOCS-5 mRNA were constitutively expressed even in non-transgenic mice brains and their expression did not alter in the presence of excess IL-12 (Maier et al, 2002). The same types of analyses were carried out using non-transgenic mice treated to develop EAE. Maximal induction of SOCS-1 and SOCS-3 mRNA was detected at 14 days in cortex, cerebellum and spinal cord with the largest induction occurring in the cerebellum. There was no change in expression of SOCS-2 or SOCS-5 mRNA in these samples. In these models, it appeared that SOCS-1 and SOCS-3 expression was primarily induced in microglia or macrophages under acute inflammatory conditions (Maier et al, 2002).
The therapeutic potential of manipulating SOCS expression has been implied from studies of diseases with inflammatory components. In one recent study using the MPTP model of PD in mice, a neuroprotective effect of resveratrol was associated with anti-inflammatory consequences of SOCS-1 induction (Lofrumento et al, 2014). Statins also have SOCS modulating properties. Treatment of murine macrophages with lovastatin and fluvastatin specifically induced protein levels of SOCS-3, but not SOCS-1, SOCS-2, SOCS-5 or SOCS-6 and attenuated STAT1, STAT3 and STAT5 activation (Huang et al, 2003). Treatment of MS patients with relapsing remitting multiple sclerosis with simvastatin resulted in reduction in cytokines IL-1β, IL-23, TGF-β, IL-21, IL-12p70 and induction of IL-27 secretion from dendritic cells; these measures correlated with increased levels of SOCS-1, SOCS-3 and SOCS-7 (Zhang et al, 2013). Initial findings of anti-inflammatory effects of SOCS-1 and SOCS-3 had suggested that these could represent therapeutic anti-inflammatory targets, however with the accumulation of studies showing SOCS expression by most cell types and control of many cellular signaling processes, it seems unlikely that targeted manipulation of SOCS could be feasible (Dimitriou et al, 2008).
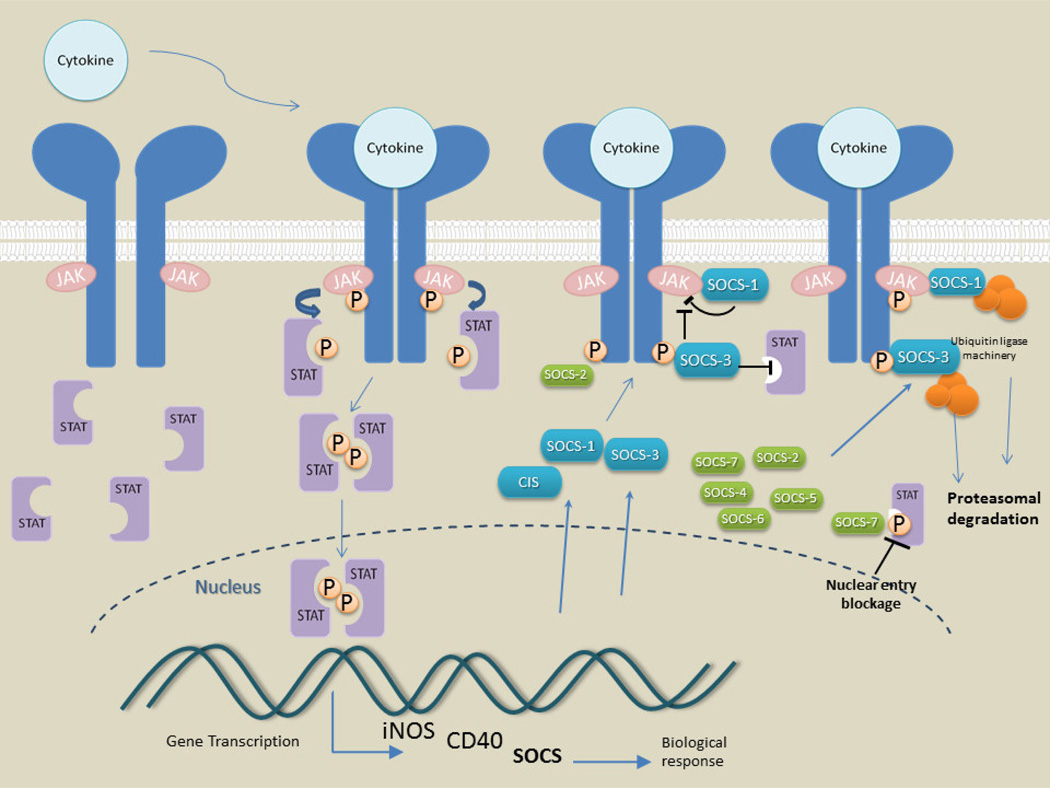
In summary, this study showed an association of increased SOCS expression with features of AD pathology and inflammation, and now further mechanistic focused studies are required. The central features of SOCS regulation of activated receptors are illustrated in Figure 9. The scheme focuses on SOCS-1 and SOCS-3 responses to inflammatory receptor activation, but the feature of targeting activated receptors for proteosomal degradation is shared with all SOCS genes. With the expanding list of SOCS-inducing and -regulating factors and pathway affected, there are many additional features of SOCS expression in human brain that have to be considered, including expressing and responding cell types. Maintaining normal cellular functions in human brains and also preventing damaging signaling from pathology induced inflammation are ongoing but separate processes with SOCS proteins being continuously involved in many of these processes. From this study, many additional questions on the role of SOCS in normal and diseased human brains have been raised. The findings on microglia are compelling, but further studies of effects of manipulating levels of all SOCS genes in relevant complex human neural cell models and also multiple brain regions are first needed. Studies on the involvement of SOCS in cells vulnerable to inflammatory or neurodegenerative pathology, namely neurons, endothelial cells and oligodendrocytes, not just cells that mediate inflammation, will be informative to develop models for determining the interaction of all features of AD pathology with these molecules.
Fig. 9. Diagram illustrating the key features of SOCS induction, function and processing.
The diagram is based on properties of cytokine stimulation and function of SOCS-1 and SOCS-3 but also illustrates the common features of SOCS protein properties, namely the targeting and degradation of receptor and associated adaptor molecules in the proteasome.
5. CONCLUSIONS
This study has provided a detailed survey of expression of SOCS genes in human microglia and human brains. Although there are many similarities in the regulatory properties of these proteins, each one has distinct patterns of expression and regulation. We have demonstrated that Aβ peptide, inflammatory cytokines and AD pathology affects expression of key members of this group of protein. As these proteins affect many aspects of cellular signaling beyond inflammation, including insulin signaling and growth factors, which have been linked to AD pathology, further studies must involve more complex cell models that include other brain cell types.
Highlights.
We identified SOCS-1 through SOCS-7 and CIS mRNA expression in human brains
We identified SOCS-1 through SOCS-7 protein expression in human brains
We showed increased expression of SOCS-2, SOCS-3 and CIS mRNA in AD brains
SOCS-2, SOCS-3 and CIS mRNA levels in brains correlated with plaque pathology
Amyloid beta increased SOCS-1, SOCS-2, SOCS-3 and CIS mRNA in human microglia
Acknowledgements
This study was supported by grants R21AG034409-A1 and R21AG044068-1 from the National Institutes of Health to DGW.
We thank Dr. Thomas G. Beach, Ms Lucia Sue and Dr. Geidy Serrano for providing brain tissue samples. The Brain and Body Donation Program at BSHRI is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Abbreviations
- AD
Alzheimer’s disease
- Aβ
amyloid beta peptide
- CIS
cytokine-inducible SH2 containing protein
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- ERK
extracellular signal regulated kinase
- IFN
interferon
- ITG
inferior temporal gyrus
- IGF-1
insulin growth factor-1
- IL
interleukin
- JAK
Janus kinase
- LPS
lipopolysaccharide
- JNK
Jun N-terminal kinase
- MAPK
mitogen-activated protein kinases
- MS
multiple sclerosis
- MTG
middle temporal gyrus
- PD
Parkinson’s disease
- PMI
postmortem interval
- qPCR
quantitative polymerase chain reaction
- SOCS
suppressor of cytokine signaling
- STAT
signal transducer and activator of transcription
- TGF
transforming growth factor
- TLR
toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests
None of the authors have financial conflicts of interest.
Contributor Information
D.G. Walker, Email: Douglas.Walker@bannerhealth.com.
A. M. Whetzel, Email: Alexis.whetzel@bannerhealth.com.
L-F Lue, Email: Lihfen.lue@bannerhealth.com.
Literature Cited
- Amodio N, Bellizzi D, Leotta M, Raimondi L, Biamonte L, D'Aquila P, Di Martino MT, Calimeri T, Rossi M, Lionetti M, Leone E, Passarino G, Neri A, Giordano A, Tagliaferri P, Tassone P. miR-29b induces SOCS-1 expression by promoter demethylation and negatively regulates migration of multiple myeloma and endothelial cells. Cell Cycle. 2013;12:3650–3662. doi: 10.4161/cc.26585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babon JJ, McManus EJ, Yao S, DeSouza DP, Mielke LA, Sprigg NS, Willson TA, Hilton DJ, Nicola NA, Baca M, Nicholson SE, Norton RS. The structure of SOCS3 reveals the basis of the extended SH2 domain function and identifies an unstructured insertion that regulates stability. Mol Cell. 2006;22:205–216. doi: 10.1016/j.molcel.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J Biol Chem. 2004;279:54708–54715. doi: 10.1074/jbc.M410992200. [DOI] [PubMed] [Google Scholar]
- Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30:392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Sue LI, Walker DG, Sabbagh MN, Serrano G, Dugger BN, Mariner M, Yantos K, Henry-Watson J, Chiarolanza G, Hidalgo JA, Souders L. Striatal amyloid plaque density predicts Braak neurofibrillary stage and clinicopathological Alzheimer's disease: implications for amyloid imaging. J Alzheimers Dis. 2012;28:869–876. doi: 10.3233/JAD-2011-111340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkqvist M, Ohlsson M, Minthon L, Hansson O. Evaluation of a previously suggested plasma biomarker panel to identify Alzheimer's disease. PLoS One. 2012;7:e29868. doi: 10.1371/journal.pone.0029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock AN, Rodriguez MC, Debreczeni JE, Songyang Z, Knapp S. Structure of the SOCS4-ElonginB/C complex reveals a distinct SOCS box interface and the molecular basis for SOCS-dependent EGFR degradation. Structure. 2007;15:1493–1504. doi: 10.1016/j.str.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Cardoso AL, Guedes JR, Pereira de AL, Pedroso de Lima MC. miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology. 2012;135:73–88. doi: 10.1111/j.1365-2567.2011.03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carow B, Rottenberg ME. SOCS3, a Major Regulator of Infection and Inflammation. Front Immunol. 2014;5:58. doi: 10.3389/fimmu.2014.00058. eCollection@2014.:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Shin YJ, Lee JY, Choi JY, Cha JH, Chun MH, Lee MY. Enhanced expression of SOCS-2 in the rat hippocampus after transient forebrain ischemia. J Neurotrauma. 2009;26:2097–2106. doi: 10.1089/neu.2008.0793. [DOI] [PubMed] [Google Scholar]
- Choi YB, Son M, Park M, Shin J, Yun Y. SOCS-6 negatively regulates T cell activation through targeting p56lck to proteasomal degradation. J Biol Chem. 2010;285:7271–7280. doi: 10.1074/jbc.M109.073726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AS, McCoy CE, Lloyd AT, O'Farrelly C, Stevenson NJ. miR-19a: an effective regulator of SOCS3 and enhancer of JAK-STAT signalling. PLoS One. 2013;8:e69090. doi: 10.1371/journal.pone.0069090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton C, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9:179. doi: 10.1186/1742-2094-9-179. 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpke A, Heeg K, Bartz H, Baetz A. Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology. 2008;213:225–235. doi: 10.1016/j.imbio.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Dimitriou ID, Clemenza L, Scotter AJ, Chen G, Guerra FM, Rottapel R. Putting out the fire: coordinated suppression of the innate and adaptive immune systems by SOCS1 and SOCS3 proteins. Immunol Rev. 2008;224:265–283. doi: 10.1111/j.1600-065X.2008.00659.x. 265–283. [DOI] [PubMed] [Google Scholar]
- Dugger BN, Serrano GE, Sue LI, Walker DG, Adler CH, Shill HA, Sabbagh MN, Caviness JN, Hidalgo J, Saxon-Labelle M, Chiarolanza G, Mariner M, Henry-Watson J, Beach TG. Presence of Striatal Amyloid Plaques in Parkinson's Disease Dementia Predicts Concomitant Alzheimer's Disease: Usefulness for Amyloid Imaging. J Parkinsons Dis. 2012;2:57–65. doi: 10.3233/JPD-2012-11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlting C, Lai WS, Schaper F, Brenndorfer ED, Matthes RJ, Heinrich PC, Ludwig S, Blackshear PJ, Gaestel M, Haussinger D, Bode JG. Regulation of suppressor of cytokine signaling 3 (SOCS3) mRNA stability by TNF-alpha involves activation of the MKK6/p38MAPK/MK2 cascade. J Immunol. 2007;178:2813–2826. doi: 10.4049/jimmunol.178.5.2813. [DOI] [PubMed] [Google Scholar]
- Emery B, Cate HS, Marriott M, Merson T, Binder MD, Snell C, Soo PY, Murray S, Croker B, Zhang JG, Alexander WS, Cooper H, Butzkueven H, Kilpatrick TJ. Suppressor of cytokine signaling 3 limits protection of leukemia inhibitory factor receptor signaling against central demyelination. Proc Natl Acad Sci U S A. 2006;103:7859–7864. doi: 10.1073/pnas.0602574103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira ST, Clarke JR, Bomfim TR, De Felice FG. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer's disease. Alzheimers Dement. 2014;10:S76–S83. doi: 10.1016/j.jalz.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Alcantara S, Ballabriga J, Olive M, Blanco R, Rivera R, Carmona M, Berruezo M, Pitarch S, Planas AM. Transforming growth factor-alpha (TGF-alpha) and epidermal growth factor-receptor (EGF-R) immunoreactivity in normal and pathologic brain. Prog Neurobiol. 1996;49:99–123. doi: 10.1016/0301-0082(96)00009-3. [DOI] [PubMed] [Google Scholar]
- Ferretti MT, Cuello AC. Does a pro-inflammatory process precede Alzheimer's disease and mild cognitive impairment? Curr Alzheimer Res. 2011;8:164–174. doi: 10.2174/156720511795255982. [DOI] [PubMed] [Google Scholar]
- Fletcher TC, DiGiandomenico A, Hawiger J. Extended anti-inflammatory action of a degradation-resistant mutant of cell-penetrating suppressor of cytokine signaling 3. J Biol Chem. 2010;285:18727–18736. doi: 10.1074/jbc.M109.095216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisullo G, Mirabella M, Angelucci F, Caggiula M, Morosetti R, Sancricca C, Patanella AK, Nociti V, Iorio R, Bianco A, Tomassini V, Pozzilli C, Tonali PA, Matarese G, Batocchi AP. The effect of disease activity on leptin, leptin receptor and suppressor of cytokine signalling-3 expression in relapsing-remitting multiple sclerosis. J Neuroimmunol. 2007;192:174–183. doi: 10.1016/j.jneuroim.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Frostegard J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. doi: 10.1186/1741-7015-11-117. 117-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, Sahakian BJ, Hodges JR. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology. 2001;57:216–225. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Gonzalez Y, Herrera MT, Soldevila G, Garcia-Garcia L, Fabian G, Perez-Armendariz EM, Bobadilla K, Guzman-Beltran S, Sada E, Torres M. High glucose concentrations induce TNF-alpha production through the down-regulation of CD33 in primary human monocytes. BMC Immunol. 2012;13:19. doi: 10.1186/1471-2172-13-19. 19-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorina R, Font-Nieves M, Marquez-Kisinousky L, Santalucia T, Planas AM. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkappaB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59:242–255. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes JR, Custodia CM, Silva RJ, de Almeida LP, de Lima MC, Cardoso AL. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer's disease triple transgenic mouse model. Hum Mol Genetddu. 2014:348. doi: 10.1093/hmg/ddu348. [DOI] [PubMed] [Google Scholar]
- Gupta S, Mishra K, Surolia A, Banerjee K. Suppressor of cytokine signalling-6 promotes neurite outgrowth via JAK2/STAT5-mediated signalling pathway, involving negative feedback inhibition. PLoS One. 2011;6:e26674. doi: 10.1371/journal.pone.0026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KC, Chen CW, Chen JC, Lin WW. Statins induce suppressor of cytokine signaling-3 in macrophages. FEBS Lett. 2003;555:385–389. doi: 10.1016/s0014-5793(03)01297-3. [DOI] [PubMed] [Google Scholar]
- Hwang MN, Kim KS, Choi YW, Jou I, Yoon S. PMA activates Stat3 in the Jak/Stat pathway and induces SOCS5 in rat brain astrocytes. Mol Cells. 2007;23:94–99. [PubMed] [Google Scholar]
- Jorgensen SB, O'Neill HM, Sylow L, Honeyman J, Hewitt KA, Palanivel R, Fullerton MD, Oberg L, Balendran A, Galic S, van der Poel C, Trounce IA, Lynch GS, Schertzer JD, Steinberg GR. Deletion of skeletal muscle SOCS3 prevents insulin resistance in obesity. Diabetes. 2013;62:56–64. doi: 10.2337/db12-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal MA, Priyamvada S, Anbazhagan AN, Jabir NR, Tabrez S, Greig NH. Linking Alzheimer's disease and type 2 diabetes mellitus via aberrant insulin signaling and inflammation. CNS Neurol Disord Drug Targets. 2014;13:338–346. doi: 10.2174/18715273113126660137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw NJ, Murphy JM, Lucet IS, Nicola NA, Babon JJ. Regulation of Janus kinases by SOCS proteins. Biochem Soc Trans. 2013;41:1042–1047. doi: 10.1042/BST20130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Naka T, Muta T, Takeuchi O, Akira S, Kawase I, Kishimoto T. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT. Proc Natl Acad Sci U S A. 2005;102:17089–17094. doi: 10.1073/pnas.0508517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuzek K, Suchy D, Gabryel B, Pierzchala O, Okopien B. Role of the SOCS in monocytes/macrophages-related pathologies. Are we getting closer to a new pharmacological target? Pharmacol Rep. 2012;64:1038–1054. doi: 10.1016/s1734-1140(12)70902-7. [DOI] [PubMed] [Google Scholar]
- Lane RF, Shineman DW, Steele JW, Lee LB, Fillit HM. Beyond amyloid: the future of therapeutics for Alzheimer's disease. Adv Pharmacol. 2012;64:213–271. doi: 10.1016/B978-0-12-394816-8.00007-6. 213–271. [DOI] [PubMed] [Google Scholar]
- Li L, Gronning LM, Anderson PO, Li S, Edvardsen K, Johnston J, Kioussis D, Shepherd PR, Wang P. Insulin induces SOCS-6 expression and its binding to the p85 monomer of phosphoinositide 3-kinase, resulting in improvement in glucose metabolism. J Biol Chem. 2004;279:34107–34114. doi: 10.1074/jbc.M312672200. [DOI] [PubMed] [Google Scholar]
- Lofrumento DD, Nicolardi G, Cianciulli A, De NF, La P, V, Carofiglio V, Dragone T, Calvello R, Panaro MA. Neuroprotective effects of resveratrol in an MPTP mouse model of Parkinson's-like disease: possible role of SOCS-1 in reducing pro-inflammatory responses. Innate Immun. 2014;20:249–260. doi: 10.1177/1753425913488429. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Beach T, Walker DG. Microglia activation and anti-inflammatory regulation in Alzheimer's disease. Mol Neurobiol. 2010;41:115–128. doi: 10.1007/s12035-010-8106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. The impact of neuroimmune changes on development of amyloid pathology; relevance to Alzheimer's disease. Immunology. 2014;141:292–301. doi: 10.1111/imm.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier J, Kincaid C, Pagenstecher A, Campbell IL. Regulation of signal transducer and activator of transcription and suppressor of cytokine-signaling gene expression in the brain of mice with astrocyte-targeted production of interleukin-12 or experimental autoimmune encephalomyelitis. Am J Pathol. 2002;160:271–288. doi: 10.1016/S0002-9440(10)64371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, Simpson JF, Parikh I, Wilfred BR, Fardo DW, Nelson PT, Estus S. CD33 Alzheimer's risk-altering polymorphism, CD33 expression, and exon 2 splicing. J Neurosci. 2013;33:13320–13325. doi: 10.1523/JNEUROSCI.1224-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marksteiner J, Kemmler G, Weiss EM, Knaus G, Ullrich C, Mechtcheriakov S, Oberbauer H, Auffinger S, Hinterholzl J, Hinterhuber H, Humpel C. Five out of 16 plasma signaling proteins are enhanced in plasma of patients with mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2011;32:539–540. doi: 10.1016/j.neurobiolaging.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens N, Uzan G, Wery M, Hooghe R, Hooghe-Peters EL, Gertler A. Suppressor of cytokine signaling 7 inhibits prolactin, growth hormone, and leptin signaling by interacting with STAT5 or STAT3 and attenuating their nuclear translocation. J Biol Chem. 2005;280:13817–13823. doi: 10.1074/jbc.M411596200. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Milde R, Varin A, Melgert BN, Draijer C, Thomas B, Fabbri M, Crawshaw A, Ho LP, Ten Hacken NH, Cobos J, V, Kootstra NA, Hamann J, Greaves DR, Locati M, Mantovani A, Gordon S. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood. 2013;121:e57–e69. doi: 10.1182/blood-2012-06-436212. [DOI] [PubMed] [Google Scholar]
- Masters CL, Selkoe DJ. Biochemistry of amyloid beta-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del ST, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Miao T, Wu D, Zhang Y, Bo X, Subang MC, Wang P, Richardson PM. Suppressor of cytokine signaling-3 suppresses the ability of activated signal transducer and activator of transcription-3 to stimulate neurite growth in rat primary sensory neurons. J Neurosci. 2006;26:9512–9519. doi: 10.1523/JNEUROSCI.2160-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher KI, Wyss-Coray T. Microglial dysfunction in brain aging and Alzheimer’s disease. Biochem Pharmacol. 2014;88:594–604. doi: 10.1016/j.bcp.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujtaba MG, Flowers LO, Patel CB, Patel RA, Haider MI, Johnson HM. Treatment of mice with the suppressor of cytokine signaling-1 mimetic peptide, tyrosine kinase inhibitor peptide, prevents development of the acute form of experimental allergic encephalomyelitis and induces stable remission in the chronic relapsing/remitting form. J Immunol. 2005;175:5077–5086. doi: 10.4049/jimmunol.175.8.5077. [DOI] [PubMed] [Google Scholar]
- Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:1147–1155. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Noguchi S, Yamada N, Kumazaki M, Yasui Y, Iwasaki J, Naito S, Akao Y. socs7, a target gene of microRNA-145, regulates interferon-beta induction through STAT3 nuclear translocation in bladder cancer cells. Cell Death Dis. 2013;4:e482. doi: 10.1038/cddis.2013.11. e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SJ, Morgan NM, Elliott J, Burrows JF, Scott CJ, McVicar DW, Johnston JA. CD33 responses are blocked by SOCS3 through accelerated proteasomal-mediated turnover. Blood. 2007;109:1061–1068. doi: 10.1182/blood-2006-05-023556. [DOI] [PubMed] [Google Scholar]
- Polizzotto MN, Bartlett PF, Turnley AM. Expression of "suppressor of cytokine signalling" (SOCS) genes in the developing and adult mouse nervous system. J Comp Neurol. 2000;423:348–358. [PubMed] [Google Scholar]
- Posselt G, Schwarz H, Duschl A, Horejs-Hoeck J. Suppressor of cytokine signaling 2 is a feedback inhibitor of TLR-induced activation in human monocyte-derived dendritic cells. J Immunol. 2011;187:2875–2884. doi: 10.4049/jimmunol.1003348. [DOI] [PubMed] [Google Scholar]
- Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012a;189:3439–3448. doi: 10.4049/jimmunol.1201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Niyongere SA, Lee SJ, Baker BJ, Benveniste EN. Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J Immunol. 2008;181:3167–3176. doi: 10.4049/jimmunol.181.5.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Roberts KL, Niyongere SA, Cong Y, Elson CO, Benveniste EN. Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia. J Immunol. 2007;179:5966–5976. doi: 10.4049/jimmunol.179.9.5966. [DOI] [PubMed] [Google Scholar]
- Qin H, Wilson CA, Lee SJ, Benveniste EN. IFN-beta-induced SOCS-1 negatively regulates CD40 gene expression in macrophages and microglia. FASEB J. 2006;20:985–987. doi: 10.1096/fj.05-5493fje. [DOI] [PubMed] [Google Scholar]
- Qin H, Yeh WI, De SP, Holdbrooks AT, Liu Y, Muldowney MT, Reynolds SL, Yanagisawa LL, Fox TH, III, Park K, Harrington LE, Raman C, Benveniste EN. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci U S A. 2012b;109:5004–5009. doi: 10.1073/pnas.1117218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampa A, Gobbi S, Belluti F, Bisi A. Emerging targets in neurodegeneration: new opportunities for Alzheimer's disease treatment? Curr Top Med Chem. 2013;13:1879–1904. doi: 10.2174/15680266113139990143. [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell DR, Akama KT, Nacher J, McEwen BS. Differential expression of suppressors of cytokine signaling-1, −2, and −3 in the rat hippocampus after seizure: implications for neuromodulation by gp130 cytokines. Neuroscience. 2003;122:349–358. doi: 10.1016/s0306-4522(03)00594-3. [DOI] [PubMed] [Google Scholar]
- Sastre M, Richardson JC, Gentleman SM, Brooks DJ. Inflammatory risk factors and pathologies associated with Alzheimer's disease. Curr Alzheimer Res. 2011;8:132–141. doi: 10.2174/156720511795256062. [DOI] [PubMed] [Google Scholar]
- Sedeno-Monge V, Arcega-Revilla R, Rojas-Morales E, Santos-Lopez G, Perez-Garcia JC, Sosa-Jurado F, Vallejo-Ruiz V, Solis-Morales CL, Aguilar-Rosas S, Reyes-Leyva J. Quantitative analysis of the suppressors of cytokine signaling 1 and 3 in peripheral blood leukocytes of patients with multiple sclerosis. J Neuroimmunol. 2014;273:117–119. doi: 10.1016/j.jneuroim.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Styren SD, Mufson EJ, Styren GC, Civin WH, Rogers J. Epidermal growth factor receptor expression in demented and aged human brain. Brain Res. 1990;512:347–352. doi: 10.1016/0006-8993(90)90647-t. [DOI] [PubMed] [Google Scholar]
- Sun SL, Li TJ, Yang PY, Qiu Y, Rui YC. Modulation of signal transducers and activators of transcription (STAT) factor pathways during focal cerebral ischaemia: a gene expression array study in rat hippocampus after middle cerebral artery occlusion. Clin Exp Pharmacol Physiol. 2007;34:1097–1101. doi: 10.1111/j.1440-1681.2007.04679.x. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Arai M, Jiang X, Sugaya S, Kanda T, Fujii K, Kita K, Sugita K, Imazeki F, Miyashita T, Kaneda A, Yokosuka O. Downregulation of microRNA-431 by human interferon-beta inhibits viability of medulloblastoma and glioblastoma cells via upregulation of SOCS6. Int J Oncol. 2014;44:1685–1690. doi: 10.3892/ijo.2014.2317. [DOI] [PubMed] [Google Scholar]
- Trengove MC, Ward AC. SOCS proteins in development and disease. Am J Clin Exp Immunol. 2013;2:1–29. [PMC free article] [PubMed] [Google Scholar]
- Venieratos PD, Drossopoulou GI, Kapodistria KD, Tsilibary EC, Kitsiou PV. High glucose induces suppression of insulin signalling and apoptosis via upregulation of endogenous IL-1beta and suppressor of cytokine signalling-1 in mouse pancreatic beta cells. Cell Signal. 2010;22:791–800. doi: 10.1016/j.cellsig.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Wada T, Hoshino M, Kimura Y, Ojima M, Nakano T, Koya D, Tsuneki H, Sasaoka T. Both type I and II IFN induce insulin resistance by inducing different isoforms of SOCS expression in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2011;300:E1112–E1123. doi: 10.1152/ajpendo.00370.2010. [DOI] [PubMed] [Google Scholar]
- Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF. Decreased expression of CD200 and CD200 receptor in Alzheimer's disease: a potential mechanism leading to chronic inflammation. Exp Neurol. 2009;215:5–19. doi: 10.1016/j.expneurol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Link J, Lue LF, Dalsing-Hernandez JE, Boyes BE. Gene expression changes by amyloid beta peptide-stimulated human postmortem brain microglia identify activation of multiple inflammatory processes. J Leukoc Biol. 2006;79:596–610. doi: 10.1189/jlb.0705377. [DOI] [PubMed] [Google Scholar]
- Walker DG, Lue LF, Adler CH, Shill HA, Caviness JN, Sabbagh MN, Akiyama H, Serrano GE, Sue LI, Beach TG. Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol. 2013 Nov 28;240:190–204. doi: 10.1016/j.expneurol.2012.11.020. Epub@2012 :190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Wang J, Campbell IL. Cytokine signaling in the brain: putting a SOCS in it? J Neurosci Res. 2002;67:423–427. doi: 10.1002/jnr.10145. [DOI] [PubMed] [Google Scholar]
- Whyte CS, Bishop ET, Ruckerl D, Gaspar-Pereira S, Barker RN, Allen JE, Rees AJ, Wilson HM. Suppressor of cytokine signaling (SOCS)1 is a key determinant of differential macrophage activation and function. J Leukoc Biol. 2011;90:845–854. doi: 10.1189/jlb.1110644. [DOI] [PubMed] [Google Scholar]
- Wilcock DM. Neuroinflammatory phenotypes and their roles in Alzheimer's disease. Neurodegener Dis. 2014;13:183–185. doi: 10.1159/000354228. [DOI] [PubMed] [Google Scholar]
- Wu K, Hu G, He X, Zhou P, Li J, He B, Sun W. MicroRNA-424-5p suppresses the expression of SOCS6 in pancreatic cancer. Pathol Oncol Res. 2013;19:739–748. doi: 10.1007/s12253-013-9637-x. [DOI] [PubMed] [Google Scholar]
- Yadav A, Kalita A, Dhillon S, Banerjee K. JAK/STAT3 pathway is involved in survival of neurons in response to insulin-like growth factor and negatively regulated by suppressor of cytokine signaling-3. J Biol Chem. 2005;280:31830–31840. doi: 10.1074/jbc.M501316200. [DOI] [PubMed] [Google Scholar]
- Yang XO, Zhang H, Kim BS, Niu X, Peng J, Chen Y, Kerketta R, Lee YH, Chang SH, Corry DB, Wang D, Watowich SS, Dong C. The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat Immunol. 2013;14:732–740. doi: 10.1038/ni.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tao Y, Wang J, Garcia-Mata R, Markovic-Plese S. Simvastatin inhibits secretion of Th17-polarizing cytokines and antigen presentation by DCs in patients with relapsing remitting multiple sclerosis. Eur J Immunol. 2013;43:281–289. doi: 10.1002/eji.201242566. [DOI] [PubMed] [Google Scholar]