Abstract
Oocytes isolated from female rhesus monkeys following standard ovarian stimulation protocols during the summer months displayed a reduced capacity to mature compared with stimulation during the normal breeding season. Because the gene expression profiles of oocyte-associated cumulus cells and mural granulosa cells (CCs and GCs) are indicative of altered oocyte quality and can provide insight into intrafollicular processes that may be disrupted during oogenesis, we performed array-based transcriptome comparisons of CCs and GCs from summer and normal breeding season stimulation cycles. Summer CCs and GCs both display deficiencies in expression of mRNAs related to cell proliferation, angiogenesis, and endocrine signaling, as well as reduced expression of glycogen phosphorylase. Additionally, CCs display deficiencies in expression of mRNAs related to stress response. These results provide the first insight into the specific molecular pathways and processes that are disrupted in the follicles of rhesus macaque females during the summer season. Some of the changes seen in summer GCs and CCs have been reported in humans and in other model mammalian species. This suggests that the seasonal effects seen in the rhesus monkey may help us to understand better the mechanisms that contribute to reduced oocyte quality and fertility in humans.
Keywords: cumulus cells, granulosa cells, reproduction, seasonality
the time of highest fertility of the female rhesus monkey has long been recognized to be in the fall and winter when animals are housed outdoors in feral or seminatural environments (11, 19). This is referred to as the breeding season and occurs in the months of mid-October to mid-April in the northern hemisphere and is reversed by 6 mo in the southern hemisphere (2). Because most outdoor-housed monkeys have free access to males and conceive in the first few ovulatory cycles in each annual breeding season, it was not known initially how long the breeding season might extend. When females were housed outdoors with vasectomized males, ovulation occurred from mid-November to mid-April, and menses and serum estradiol levels were decreased from May to September (50). Levels of bioactive luteinization hormone (LH) are also reduced in the summer months (51).
With the increased availability of indoor-housed, time-mated animals it was noted that under controlled environmental conditions such as temperature and light, many indoor-housed, time-mated females still display reduced menses and fertility during the summer months. Ovulation is dramatically reduced; in one study only two of seven monkeys ovulated (39). Although serum estradiol levels are similar throughout the year, progesterone levels are reduced during the summer (24). Furthermore, during the summer the development of the dominant follicle appears to be delayed and the ratio of follicle stimulating hormone (FSH) to LH is altered, which may lead to deficient luteal progesterone production (24). Thus, the maturation of the entire follicle appears to be compromised.
The advent of controlled ovarian stimulation (COS - “superovulation”), with injected recombinant human gonadotropins has allowed the natural serum levels of FSH and LH to be superseded. Although it is now possible to obtain oocytes during the summer months and embryo development to the blastocyst stage, especially when fertilization is accomplished via sperm injection, there is only one report of successful in vitro fertilization in summer (34). Because so few summer oocytes have been collected with these assisted reproduction techniques, the overall quality of summer oocytes has not been well studied.
In this report, we present data documenting reduced quality of rhesus monkey oocytes obtained during the summer months. To examine the basis for this seasonal effect on oocyte quality, we compared global transcriptomes between cumulus and mural granulosa cells (CCs and GCs) associated with oocytes produced by COS during the winter and summer months. Previous studies in the rhesus monkey note that the gene expression patterns of CCs were more predictive of oocyte quality than transcriptome profiles of oocytes (28, 29) and that CCs and GCs share many features after ovulation (9). This relationship between follicular somatic cells and oocyte quality reflects the intimate bidirectional interactions between oocyte and CCs and the role of the GCs in defining the follicular environment, both of which support the development of high-quality oocytes. Similar correlations have been reported in other species, including humans (21). These transcriptome comparisons of CCs revealed significant effects on the expression of mRNAs related to cell morphology, angiogenesis, proliferation, hypoxic stress response, lipid metabolism, and endocrine signaling.
METHODS
Collection of cells.
Adult female rhesus macaques (Macaca mulatta) were housed as previously described (35) at the California National Primate Research Center. All procedures for maintenance and handling of the animals were reviewed and approved in advance by the Institutional Animal Use and Care Administrative Advisory Committee at the University of California at Davis. The criteria for selection included age range from 6 to 12 yr, history of successful pregnancy, and normal menstrual cycles.
Controlled ovarian stimulation.
COS was performed with twice daily injections of human recombinant FSH (37.5 IU) for 7 days (begun within 4 days after menses) and 1,000 IU of human chorionic gonadotropin (hCG) on day 8. On day 9, oocyte-CC complexes were obtained by ultrasound-guided needle aspiration of follicles as described (49). Follicle sizes collected ranged from 3 to 10 mm. Oocyte and CCs were processed according to established procedures (28, 29). GCs were also separated from the follicular aspirate and processed as described (9). Oocytes were observed for maturation status and only CCs from mature oocytes (MII) were used for this study. Thus, GCs were pooled from all follicles, but CCs were pooled from only MII oocytes. Cells representing the normal breeding season were collected from three females from October to April. Cells representing the summer season were collected from three females from mid-June to mid-August.
Array data acquisition.
Affymetrix rhesus genome arrays were used to create whole transcriptome mRNA expression profiles for CCs and GCs obtained during the normal breeding season and summer. To minimize the impact of interfollicle or interfemale variability on array results, we adopted a pooling strategy. Samples from three females were combined to yield each array sample. The GCs and CCs from an individual female were pooled according to cell type, and then aliquots of cells from two to three females were pooled to create the final samples for array analysis (summer CC, 3 females/sample; control CC, three females for one sample, two females for other samples; summer GC, three females/sample; control GC, two females for one sample, three females/sample for the other samples). In this way, we obtained three or four replicate arrays for each cell type and condition.
Total RNA was isolated from cells with the PicoPure RNA isolation kit (Life Technologies). Up to 50 ng of total RNA from each array sample were subjected to two rounds of cDNA synthesis with the Arcturus RiboAmp HS Plus kit (Life Technologies). Labeled cRNA was produced using the Affymetrix GeneChip Expression 3′ Amplification for IVT Labeling Kit. The biotin-labeled cRNA samples were fragmented, and 10 μg were hybridized onto arrays. Posthybridization washing, staining, and scanning were performed as described in the Affymetrix GeneChip Expression Analysis Technical Manual.
Array data analysis.
Array data were analyzed with scripts written in R (http://www.R-project.org), utilizing routines from Bioconductor (18) and Significance Analysis of Microarrays (SAM) (48) packages. We assessed the quality of array data by examining standard indicators: minimum, maximum, and average background; percentage of present calls by MAS5 algorithm; scaling factor; and ratios of expression between 3′ and 5′ probes for spike-in probe-sets. Table 1 contains the summary of these parameters for samples that passed quality control. We summarized and normalized probe-set expression values with robust multiarray analysis (25), using custom probe-set definitions (explained below). Samples from summer and breeding season were compared (separately for each cell type) to identify differentially expressed genes with the SAM algorithm (48), with false discovery rate (FDR) threshold 0.01 and 1,000 permutations per comparison. Probe-sets with all expression values <100 were excluded, as well as the probe-sets with at least one absent call in both treatment groups. To eliminate the effects of random sampling, we repeated SAM analysis five times, and a probe-set was deemed significantly differentially expressed only if it passed FDR control all five times. Full datasets were deposited in the National Center for Biotechnology Information's (NCBI's) Gene Expression Omnibus. Data are also available in a searchable format at the Primate Embryo Gene Expression Resource (http://www.preger.org).
Table 1.
Quality control parameters for expression arrays
| Assays, n | Present Calls, % | Probe-sets with Present Calls, n | Scale Factor | Average Background | |
|---|---|---|---|---|---|
| Control granulosa | 3 | 34.19 (32.66–35.86) | 18,075 (17,268–18,959) | 1.08 (0.93–1.27) | 68.99 (60.77–76.14) |
| Summer granulosa | 3 | 35.29 (34.21–36.11) | 18,655 (18,087–19,089) | 0.92 (0.79–1.17) | 72.13 (69.23–73.88) |
| Control cumulus | 4 | 35.03 (34.15–36.12) | 18,521 (18,055–19,097) | 1.13 (0.97–1.30) | 49.72 (46.06–53.23) |
| Summer cumulus | 4 | 34.27 (33.41–35.65) | 18,116 (17,662–18,848) | 1.47 (1.35–1.58) | 47.78 (44.29–51.97) |
A custom set of probe-set definitions was used to reduce the potential impact of cross-hybridization to off-target mRNAs and to maximize the confidence and number of gene annotations applied to probe-sets. We validated probe-set definitions by aligning probe sequences to rhesus macaque mRNA Reference Sequences (RefSeq) and removing probes not aligned to any mRNA sequences or aligned to two or more unrelated mRNA sequences. Updated probe-set annotations were compiled from gene and mRNA records downloaded through NCBI Entrez eUtils system and FTP server (http://www.ncbi.nlm.nih.gov/) on July 17, 2013. The gene symbol assignments were combined with assignments based on alignment of rhesus assay probes to human mRNA sequences, provided by the laboratory of Prof. Robert Norgren (http://www.unmc.edu/rhesusgenechip/RhesusGeneChipAnnot3.xlsx). This was especially important for those probe-sets that were not assigned to any rhesus gene (possibly due to incomplete or wrong RefSeq mRNA sequences) or for which the initially assigned rhesus genes had generic symbols of the form LOCnumber. Human-to-rhesus alignment data (ftp://hgdownload.cse.ucsc.edu/goldenPath/rheMac2/vsHg19/) were used to resolve ambiguities or inconsistencies between symbols for homologous human and rhesus genes.
To evaluate possible cooperative effects among genes showing significant differences in expression between summer and breeding season samples, we used the Ingenuity Pathway Analysis (IPA) program (Ingenuity Systems, Rockwood, CA) to analyze lists of affected genes separately for each cell type. IPA provides the opportunity to identify statistically significant effects on biological functions, canonical pathways, and potential upstream regulators. Statistical significance is evaluated by Fisher's exact test (P value threshold 0.05). The z-score, which measures the congruence between the observed change (increased or decreased) in gene expression and previously reported gene activities, is also used to evaluate and characterize significance of effects on specific biofunctions and the activation/inhibition states of predicted upstream regulators. IPA also constructs networks that incorporate genes from the target lists with previously reported relationships that can provide insight into possible pathways leading to observed changes in gene expression.
Quantitative RT-PCR analysis.
For CC RNA, which was very limited in amount, an aliquot of each RNA pool was subjected to reverse transcription and then whole transcriptome amplification using the QuantiTect whole transcriptome kit (Qiagen, Valencia, CA), and ∼160 ng of cDNA were used for each quantitative (q)PCR measurement. For GCs, where a more abundant supply of RNA was available, cDNA from reverse transcription of each RNA pool was used directly for qPCR measurement (initial total RNA input 50–250 ng). We assayed each gene by completing seven technical replicates for each sample. The mRNA abundance of each target gene was initially normalized to the endogenous control mRNA encoding GOT2 (mitochondrial glutamate oxaloacetate transaminase). Two additional endogenous controls were employed for confirmation, as described in results (mitochondrial ribosomal protein S18C gene–MRPS18C, and the serine/arginine rich splicing factor 7 -SRSF7). TaqMan qRT-PCR gene expression assays were performed with primers from Life Technologies/ABI and a QuantStudio 7 platform according to the manufacturer's recommendations. Primer efficiencies were estimated by performing qPCR on a seven-point 10× dilution series with four technical replicates. qPCR amplification data were preprocessed in Quant Studio 7 software: baseline adjustment, threshold identification, Ct calculation, outlier identification were performed. Wells marked as outliers (where discrepancy in Ct value was too large to be attributed to technical variation, but rather to a pipetting or amplification problem) were omitted from further analysis. Ct values were exported to a proprietary Microsoft Excel workbook for relative quantity (RQ) and fold-change (FC) calculation and statistical analysis (RQ values were calculated relative to the first control sample). A random sampling procedure implemented in Excel/VBA was used to account for variation in technical replicates: 1) one technical replicate was randomly selected for each sample/gene combination, 2) ΔCt, ΔΔCt, RQ, and FC were calculated and two-sided unpaired t-test performed for Ct values of randomly selected technical replicates, 3) steps 1 and 2 were repeated 10,000 times, 4) mean of P values for 10,000 t-tests was calculated, as well as the median, the 2.5th percentile, and the 97.5th percentile for FC and RQs. Mean of P values and median for FC and RQs were reported as the outcome of the procedure, while the 2.5th and 97.5th percentiles served as an estimate of 95% confidence interval for FC and RQs. The same pools of RNA employed for arrays were employed for qRT-PCR. Data for one of summer CC samples were discarded because five genes were not detected at all, while the remaining genes, including the endogenous control GOT2, were detected in only a small number of wells, making all calculations unreliable.
Taqman primers and assays.
Some assays were performed with predesigned primer and probe combinations already available from Life Technologies and were identified by Assay ID: CTSL2 (Rh02838259_m1), CYP19A1 (Rh02787020_m1), GOT2 (Rh02808768_gH), MRPS18C (Mf02798136_g1), PYGL (Rh00958092_m1), SHISA2 (Rh02911434_m1), SRSF7 (Rh02835409_m1), STC2 (Rh01063214_m1), and TNFAIP6 (Rh02862767_m1). Other genes were assayed with custom primers: ATP8 (forward, CCACCAACCACCCCTTACAAA; reverse, ATTTTGGTTGTCAGCGGACATTG; reporter, CCCACCCTTCAGTCTCA), and ND2 (forward, AGCCCCCTTCCACTTCTGA; reverse, GGGTGAATTTGGTACATGATTGAGATG; reporter, CCAAGGAACACCCCTAACC), ND4L (forward, CGCTCTTTATTATGGCCACCCTTAT; reverse, AGGCGGCAAATACTAACAAAATGATG; reporter, CAAACACACACTTCCC).
RESULTS
Quality of oocytes from summer cycles.
Oocyte collections performed during the summer exhibited a large percentage that did not mature to MII; oocytes underwent germinal vessel breakdown but failed to mature and appeared to have darker cytoplasm. The average percentage of oocytes maturing to MII was 36% (range 26–48%) in the summer, which was much lower than the >80% MII oocytes obtained in our studies during the previous breeding season (8).
Transcriptome comparisons between summer and breeding season cells.
The above data indicate that oocytes from summer cycles are reduced in overall quality. To explore the basis for this, we conducted a global transcriptome analysis of the somatic cells (CCs and GCs) associated with ovarian follicles; previous studies indicate that somatic cell transcriptomes, particularly those of CCs, are predictive of oocyte quality and that their analysis can reveal alterations in follicle biology that underlie reduced oocyte quality.
We obtained four high-quality arrays for summer and breeding CCs and three high-quality arrays for summer and breeding season GCs. One of the four processed summer GC arrays was omitted from the analysis as an outlier, with a highly dissimilar global expression profile compared with both the remaining three summer GC arrays as well as with three breeding season GC arrays (average correlation coefficient is 0.983 ± 0.002 within three summer GC arrays, 0.978 ± 0.0001 within three breeding season GC arrays, but only 0.967 ± 0.004 between outlier GC array and six other GC). The percentage of P calls (present calls) and average backgrounds were all within acceptable ranges and similar between arrays. The scaling factors were close to each other, consistent with acceptable quality (Table 1). SAM analysis revealed significant effects of season on 106 probe-sets with the GC samples and on 292 probe-sets with the CC samples (Supplemental Tables S1 and S2).1
Among the probe-sets showing reduced signals in summer GCs, 18 (including 14 probe-sets with assigned gene annotations) were also reduced in summer CCs; three of these corresponded to the liver form of glycogen phosphorylase (PYGL); three affected probe-sets correspond to mRNAs encoding mitochondrial proteins NADH dehydrogenase subunits 2 and 4L and ATP synthase F0 subunit 8, and a fourth was a nuclear gene encoding a mitochondrial ATP synthase complex component (Table 2). Another affected probe-set with reduced expression corresponded to a modulator of potassium channel function (KCNAB1). Another probe-set showing reduced expression in GCs and CCs represented SHISA2, an inhibitor of WNT signaling.
Table 2.
Genes showing significantly reduced expression in both summer granulosa and summer cumulus cells
| Granulosa Cells |
Cumulus Cells |
|||||
|---|---|---|---|---|---|---|
| Gene Symbol | Ctrl | Summ | Ratio Ctrl:Summ | Ctrl | Summ | Ratio Ctrl:Summ |
| ATP8 | 2,768 | 469 | 5.906 | 2,293 | 53 | 43.185 |
| ND4L | 4,125 | 740 | 5.574 | 2,117 | 77 | 27.330 |
| ND2 | 7,886 | 1,207 | 6.536 | 6,547 | 402 | 16.300 |
| STC2 | 82 | 18 | 4.438 | 1,153 | 100 | 11.547 |
| SHISA2 | 2,932 | 444 | 6.600 | 1,643 | 226 | 7.283 |
| C8orf47 | 670 | 181 | 3.709 | 462 | 137 | 3.374 |
| PYGL | 401 | 86 | 4.654 | 638 | 237 | 2.686 |
| PYGL | 352 | 83 | 4.249 | 602 | 233 | 2.587 |
| HMCN1 | 343 | 58 | 5.942 | 290 | 161 | 1.797 |
| PYGL | 203 | 56 | 3.604 | 259 | 91 | 2.853 |
| KCNAB1 | 249 | 51 | 4.867 | 299 | 143 | 2.093 |
| VAT1L | 194 | 56 | 3.503 | 888 | 320 | 2.774 |
| LOC100423785 | 15,638 | 4,510 | 3.467 | 14,663 | 5,407 | 2.712 |
| SLC26A2 | 2,031 | 585 | 3.473 | 681 | 331 | 2.055 |
Probe-sets are sorted in decreasing order according to the product of granulosa cell (GC) and cumulus cell (CC) fold-change ratios. Additional information about probe-sets and genes is available in Supplemental Tables S1 and S2. Four additional probe-sets with significantly reduced expression in both summer GCs and CCs are not listed in this table because they lack gene assignments.
Of the 63 probe-sets showing increased hybridization signals in summer GC, 22 were also increased in summer CCs (Table 3). Among these, the probe-sets with largest FCs include those for the genes COL14A1, SYNE1, and ANKRD28. ANGPTL1.
Table 3.
Genes showing significantly increased expression in both summer granulosa and summer cumulus cells
| Granulosa Cells |
Cumulus Cells |
|||||
|---|---|---|---|---|---|---|
| Gene Symbol | Ctrl | Summ | Ratio Summ:Ctrl | Ctrl | Summ | Ratio Summ:Ctrl |
| COL14A1 | 39 | 627 | 16.268 | 31 | 384 | 12.200 |
| SYNE1 | 12 | 127 | 10.560 | 34 | 255 | 7.438 |
| ANKRD28 | 35 | 502 | 14.490 | 35 | 179 | 5.079 |
| DNAJC1 | 20 | 147 | 7.454 | 28 | 83 | 2.950 |
| FAM13A | 40 | 290 | 7.283 | 52 | 154 | 2.978 |
| VWF | 226 | 971 | 4.290 | 154 | 761 | 4.935 |
| NEAT1 | 190 | 887 | 4.674 | 231 | 922 | 3.987 |
| RORA | 281 | 1,284 | 4.575 | 181 | 702 | 3.875 |
| SLC20A1 | 135 | 696 | 5.140 | 216 | 615 | 2.848 |
| ANXA4 | 152 | 479 | 3.146 | 178 | 720 | 4.057 |
| ALCAM | 48 | 151 | 3.139 | 38 | 139 | 3.622 |
| TFPI | 42 | 215 | 5.112 | 51 | 109 | 2.141 |
| KCTD12 | 1,226 | 2,618 | 2.136 | 545 | 2,013 | 3.693 |
| ANGPTL1 | 75 | 164 | 2.187 | 24 | 85 | 3.597 |
| EID3 | 109 | 329 | 3.027 | 108 | 256 | 2.371 |
| ALCAM | 1,179 | 2,839 | 2.409 | 993 | 2,687 | 2.707 |
| ALDH9A1 | 722 | 1,850 | 2.561 | 846 | 1,997 | 2.359 |
| INPP4A | 174 | 409 | 2.350 | 134 | 340 | 2.538 |
| PRICKLE1 | 209 | 492 | 2.353 | 146 | 339 | 2.326 |
| AKR1C2 | 974 | 2,012 | 2.066 | 1,775 | 4,299 | 2.422 |
| A2BP1 | 115 | 310 | 2.686 | 158 | 290 | 1.833 |
| AUTS2 | 337 | 823 | 2.444 | 245 | 469 | 1.914 |
Probe-sets are sorted in decreasing order according to the product of GC and CC fold-change ratios. Additional information about probesets and genes is available in Supplemental Tables S1 and S2.
Individual gene expression analysis by qRT-PCR.
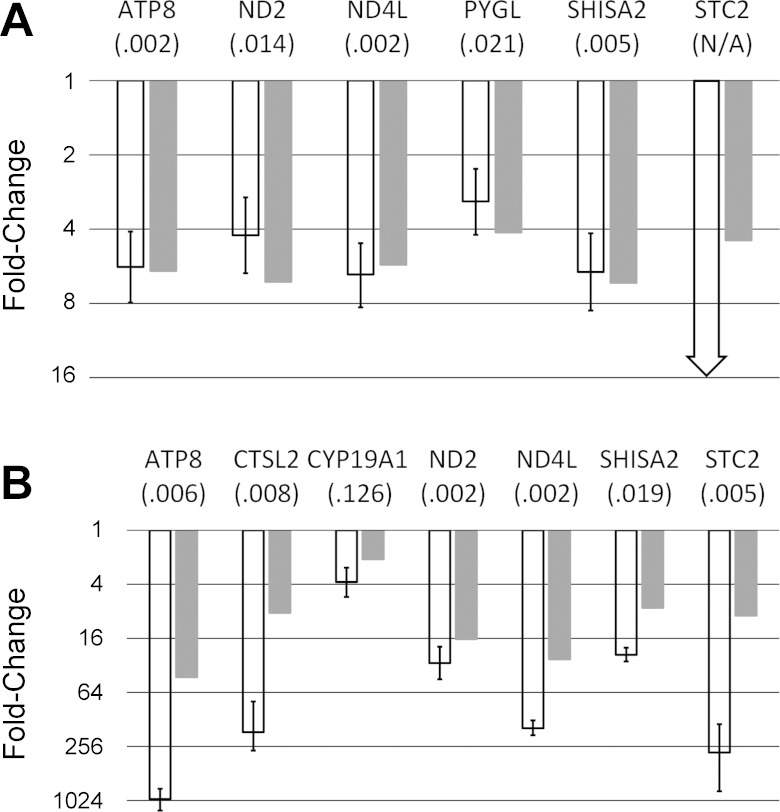
From the genes with statistically significant differential expression detected in GCs and CCs by microarrays, six were selected for follow-up with qPCR in GCs, and nine were selected for follow-up in CCs. All six genes assayed with qPCR in GCs were confirmed to be differentially expressed (Fig. 1A). Stanniocalcin 2 mRNA (STC2) was detected in control GC samples but not detected in summer GC samples, confirming differential expression.
Fig. 1.
Evaluation by quantitative (q)RT-PCR of expression of 9 genes in summer granulosa cells (A) and 6 genes in summer cumulus cells (B). White bars show fold-changes estimated by qRT-PCR, with error bars representing 95% confidence intervals. Gray bars show fold-changes estimated by microarrays. The P values for statistical comparison of expression (qRT-PCR) between breeding season samples and summer samples are given in parentheses below gene symbol. STC2 expression in summer granulosa cells (A) was below the detection threshold for qRT-PCR, confirming downregulation (fold-change and P value could not be calculated, as indicated by arrow). Results for CYP19A1 indicate downregulation in cumulus cells (B), but with a P value >0.05 significance threshold. Results for glycogen phosphorylase (PYGL) and tumor necrosis factor alpha-induced protein 6 (TNFAIP6) in cumulus cells (B) did not indicate a significant difference (P < 0.05) and are not shown.
Six of nine genes assayed with qPCR in CCs were confirmed to be differentially expressed (Fig. 1B). Three genes for which qPCR confirmation failed to be confirmed at a confidence level of P < 0.05 were cytochrome P450 19A1 (CYP19A1), PYGL, and tumor necrosis factor alpha-induced protein 6 (TNFAIP6). These are also the three selected genes with the lowest FC as estimated by the microarray analysis. Results for CYP19A1 revealed the expected trend toward downregulation in summer samples, but due to higher variance among samples and the small magnitude of difference, the obtained P value (0.126) was above the significance threshold. PYGL mRNA was flagged as significantly reduced in CCs by multiple probe-sets on the microarray with FC ranging from 2.59 to 2.93, less than the over fourfold reduction predicted on arrays for GCs. PYGL qPCR measurements were highly variable for CCs and failed to confirm downregulation for these cells. However, differential expression of PYGL was confirmed for GCs.
To confirm the results obtained using GOT2 as the endogenous control, we repeated GC measurement for six selected genes and GOT2 twice, using SRSF7 and MRPS18C as internal standards. Obtained P values and FC ratios for six genes were all similar as when GOT2 was used, confirming the validity of results. Furthermore, there was no significant difference in expression of GOT2 between control and summer samples, validating its use as the endogenous control. Cross-checking GOT2 expression to the other two housekeeping genes revealed variance in GOT2 expression within normal samples and within summer samples; however, GOT2 offered the least variance between control and summer samples of the three candidate housekeeping genes tested. This small variance in endogenous control gene expression may have obscured the detection of small FCs in expression of some of the target genes (e.g., CYP19A1, PYGL, and TNFAIP6 in CCs).
IPA of gene expression differences.
To gain further insight into the biological functions, pathways, and processes likely to be most highly affected in CCs and GCs by season, we subjected the lists of affected probe-sets to IPA analysis. The top affected biofunctions emerging from the IPA analysis for GC cells were associated with cardiovascular system development, cell proliferation, fatty acid metabolism, and cytoskeleton dynamics (Table 4 and Supplemental Table S3). Genes appearing repeatedly among the affected biofunctions include angiopoietin like 1 (ANGPTL1), coagulation factor II receptor like 1 (F2RL1), activated leukocyte cell adhesion molecule (ALCAM), prostaglandin E receptor 3 EPs subtype (PTGER3), protein tyrosine phosphatase receptor type Mu (PTPRM), and transforming growth factor beta type III (TGFBR3) for cardiovascular and cell proliferation functions. Three of the five affected IPA networks retrieved for GC analyses were also related to cell proliferation (Supplemental Table S4).
Table 4.
Predicted increased/decreased biofunctions identified by IPA in summer granulosa cells
| Function Annotation | Predicted Activation State | Activation z-Score | Molecules |
|---|---|---|---|
| Development of cardiovascular tissue | decreased | −1.969 | ⇈ANGPTL1, ⇊F2RL1, ⇈PTPRM, ⇈TFPI, ⇈TGFBR3 |
| Proliferation of endothelial cells | decreased | −1.969 | ⇈ANGPTL1, ⇊F2RL1, ⇈PTPRM, ⇈TFPI |
Symbols for genes with increased (or decreased) expression are preceded with ⇈ (or ⇊); changes in expression for all genes listed in this table are 2-fold or higher.
The IPA upstream regulator analysis is a useful tool for discovering potential mechanistic roots of observed effects on biofunctions and pathways. The analysis can predict upstream regulators that control the expression of affected genes (Supplemental Table S5). Predicted upstream regulators that regulate the highest number of genes in IPA networks are shown in Table 5. Predicted upstream regulators can themselves be affected (Supplemental Table S5, top) or unaffected (Supplemental Table S5, bottom) by the season. Unaffected upstream regulators can include cellular regulators that are not expressed in the cell type analyzed or even exogenous chemical or other inhibitory factors. Thus, the upstream regulator analysis can reveal potentially relevant factors outside of just the range of expressed or differentially expressed genes.
Table 5.
Overview of the most prominent upstream regulators in IPA networks for summer granulosa cells
| # | Upstream Regulator | Networks, n | Networks (molecules regulated, n) | # | Upstream Regulator | Networks, n | Networks (molecules regulated, n) |
|---|---|---|---|---|---|---|---|
| 1 | ⇊MAFB | 1 | N1(M/2) | 14 | SYVN1 | 1 | N2(2) |
| 2 | TGFB1 | 3 | N1(7), N2(3), N3(2) | 15 | ERG | 1 | N2(2) |
| 3 | TP53 | 2 | N1(4), N2(2) | 16 | CCND1 | 1 | N2(M/3) |
| 4 | Vegf | 1 | N1(M/5) | 17 | PPARG | 1 | N2(2) |
| 5 | hydrogen peroxide | 1 | N1(5) | 18 | TCF7L1 | 1 | N3(M/1) |
| 6 | EGF | 1 | N1(4) | 19 | RAF1 | 1 | N3(2) |
| 7 | Cg | 1 | N1(M/5) | 20 | HOXA10 | 1 | N5(2) |
| 8 | OSM | 1 | N1(4) | 21 | miR-101-3p | 1 | N5(M/1) |
| 9 | PI3K (family) | 1 | N1(M/3) | 22 | calmodulin | 1 | N5(M/2) |
| 10 | FGF2 | 1 | N1(4) | 23 | ELAVL4 | 1 | N5(M/1) |
| 11 | IL15 | 1 | N1(4) | 24 | GLI1 | 1 | N5(2) |
| 12 | prostaglandin E2 | 1 | N1(4) | 25 | progesterone | 1 | N5(2) |
| 13 | dihydrotestosterone | 1 | N1(4) |
MAFB has >2-fold reduced expression in summer GCs. Regulators are ordered by the number of IPA networks that they regulate. One regulator (MAFB) that is itself affected in summer GCs is listed separately on top. The numbers in parenthesis next to network IDs in the 4th/8th column signify how many molecules in those networks the regulators are affecting (e.g., TP53 regulates 4 molecules in network 1 and 2 molecules in network 2). Labels (M/. . .) in the 4th/8th column signify that the regulators are also member of those networks, e.g., Vegf regulates 5 molecules in network 1 and is also a member of network 1.
One upstream regulator that was itself affected (reduced expression in summer GCs) was retrieved (musculoaponeurotic fibrosarcoma oncogene family protein B, MAFB) but was not associated with a significant z-score. The only predicted upstream regulator with a significant (>1.96 or <−1.96) z-score (in this case negative, indicating inhibition of the pathway) and a predicted activation state for GCs was the cell proliferation regulator oncostatin M (OSM). The second highest ranked upstream regulator was vascular endothelial growth factor (VEGF). The upstream regulator analysis thus confirms likely negative effects of summer season on the process of cell proliferation via OSM (which exerts pleiotropic effects, either inhibitory or mitogenic) and suggests additional effects on GC cell proliferation signaling may occur via other regulators.
Of the 292 affected probe-sets in CC samples, 200 displayed reduced expression in the summer CCs, and 92 displayed increased expression (Supplemental Table S2). Among the probe-sets indicating reduced mRNA expression, the three most highly affected again correspond to mitochondrial mRNAs encoding NADH dehydrogenase subunits 2 and 4L and ATP synthase F0 subunit 8. Other prominent affected probe-sets indicating reduced mRNA expression include nuclear encoded genes, with 83 probe-sets displaying twofold or greater changes. The genes affected at the level of twofold or more include ones related to a variety of processes, such as calcium regulation, cellular signaling, cell-cell interactions, transcription, angiopoiesis, and endocrine signaling, apoptosis, transport and metabolism (e.g., stanniocalcin 2, cathepsin L2, alpha-2 actinin, alpha-3 soluble guanylate cyclase, LH/hCG receptor, angiopoietin 2, PYGL, SIN3A associated protein, 30 kDa, and CYP19A1).
Of the probe-sets displaying increased expression in summer CCs, 72 display twofold or greater changes (Supplemental Table S2). These correspond to genes related to cell-matrix interaction, cytoskeleton, angiopoiesis, cellular signaling, metabolism, translation transcription, and transport.
To gain further insight into the biological functions, pathways, and processes likely to be most highly affected in CCs by season, we subjected the lists of affected probe-sets to IPA analysis. We first evaluated effects of season on biological functions (Table 6 and Supplemental Table S6). The IPA biofunction analysis revealed inhibition of 11 biological functions, with both significant z-scores and significant P values. Of these, six relate to cell morphology and movement, three relate to cell proliferation, along with angiogenesis, and lipid synthesis. Effects on several genes appear repeatedly among these affected biofunctions, including: reduced expression in summer CCs of angiopoietin 2 (ANGPT2), CYP19A1, LHCGR, high mobility group box 1 (HMGB1), caveolin 1 (CAV1), periostin (POSTN), cathepsin L2 (CTSL2, a.k.a. CTSV), and vascular endothelial growth factor C (VEGFC) mRNAs and the microRNA mir-210, and increased expression of ANGPTL1 and forkhead box O1A (FOXO1) mRNAs. An additional 17 biofunctions were assigned z-scores at the level of 1.5 or higher (or −1.5 or lower for negative z-scores) but below the threshold 1.96 (or above −1.96) required for biofunctions to be characterized as activated or inhibited. These scores may possibly be lower due to insufficient annotations in the knowledge database or inconsistency relative to anticipated gene expression changes among affected member genes (Supplemental Table S6), and the lack of activated/inhibited characterization may possibly be false for some of these biofunctions. These additional categories echoed the above 11 but included in addition effects related to cell death. The IPA network analysis (Supplemental Table S7) reinforces many of the processes highlighted in the biofunction analysis, including cell movement and morphology, cardiovascular system development, cell proliferation, and cell death.
Table 6.
Predicted increased/decreased biofunctions identified by IPA in summer cumulus cells
| Function Annotation | Predicted Activation State | Activation z-Score | Molecules |
|---|---|---|---|
| Sprouting | decreased | −2.930 | ⇈ADAMTS1, ⇊ANGPT2, ↓CAV1, ⇊CRIP1, ⇊CYP19A1, ⇈DCN, ↓HMGB1, ⇈RGS4, ↓SEPT11, ↓VEGFC |
| Invasion of tumor | decreased | −2.630 | ⇈ALCAM, ↓CAV1, ⇊CTSV, ↓HMGB1, ⇊LHCGR, ⇊MMP1, ↓POSTN, ↓ZEB1 |
| Angiogenesis | decreased | −2.509 | ⇈ADAMTS1, ⇈ALCAM, ⇊ANGPT2, ⇈ANGPTL1, ⇈BTC, ↓CAV1, ↑COL4A2, ⇈COL4A3, ⇈DCN, ↓EGLN1, ↓ELK3, ⇈FOXO1, ↓HMGB1, ⇈KAT6A, ↓mir-210, ⇊MMP1, ⇈RGS4, ↓SMAD7, ⇈TFPI, ↓VEGFC, ↓ZEB1 |
| Synthesis of lipid | decreased | −2.493 | ⇊ABCA8, ⇈AKR1C1/AKR1C2, ⇊ANGPT2, ↓ARV1, ↓BHMT, ⇊BNIP3, ↓CAV1, ↓CAV2, ↓CES1, ⇊CYP19A1, ⇈FOXO1, ↓GGPS1, ⇈HPGD, ↓INSIG2, ⇊LHCGR, ↓PON2, ⇊PTGIS, ⇈RHOQ, ⇊SGMS2, ⇊ST3GAL5, ⇈VWF |
| Migration of Vascular smooth Muscle cells | decreased | −2.414 | ⇊ANGPT2, ↓CKLF, ↓HMGB1, ⇈NOV, ↓POSTN, ⇈TFPI |
| Migration of Smooth muscle cells | decreased | −2.361 | ⇊ANGPT2, ↓CKLF, ⇊CTSV, ↓HMGB1, ⇈NOV, ↓POSTN, ⇈TFPI |
| Proliferation of vascular smooth muscle cells | decreased | −2.236 | ⇊ANGPT2, ↓CKLF, ⇈NOV, ↓POSTN, ↓SKP2, ⇈TFPI |
| Migration of endothelial cells | decreased | −2.223 | ⇈ADAMTS1, ⇊ANGPT2, ⇈ANGPTL1, ⇈BTC, ↓CAV1, ↑COL4A2, ⇈DCN, ⇈FOXO1, ↓HMGB1, ↓MAP2K6, ↓mir-210, ⇈TFPI, ↓VEGFC |
| Branching of endothelial cells | decreased | −2.173 | ⇊ANGPT2, ⇈DCN, ↓HMGB1, ⇈RGS4, ↓VEGFC |
| Proliferation of endothelial cells | decreased | −2.159 | ⇈ADAMTS1, ⇊ANGPT2, ⇈ANGPTL1, ↓CAV1, ↓CAV2, ↑COL4A2, ⇈COL4A3, ⇈FOXO1, ↓HMGB1, ⇊MMP1, ↓SKP2, ⇈TFPI, ⇈TNFRSF11A, ↓VEGFC |
| Proliferation of muscle cells | decreased | −2.137 | ⇊ANGPT2, ↓CAV1, ↓CKLF, ⇈FOXO1, ↓HMGB1, ⇊MMP1, ⇈NOV, ↓POSTN, ⇈RGS4, ↓SKP2, ⇈TFPI |
| Motor function | decreased | −1.982 | ⇊ANGPT2, ↓CANX, ↓HMGB1, ↓RIMS2, ↓SLC12A2 |
| Glycolysis of cells | decreased | −1.963 | ↓CAV1, ⇊ENO2, ↓mir-210, ↓SKP2, ⇊SLC2A1 |
Symbols for genes with increased (or decreased) expression and fold-change ≥2 are preceded with ⇈ (or ⇊). Symbols for genes with increased (or decreased) expression and fold-change <2 are preceded with ↑ (or ↓).
Pathway analysis for CCs (Table 7) yielded additional effects related to NAD salvage/phosphorylation/dephosphorylation pathways, nitric oxide signaling, HMG1B signaling, hypoxia inducible factor (HIF1A) signaling, pyrimidine metabolism, interleukin-6 (IL-6) signaling, and estrogen signaling. Affected molecules in these pathways included some of those highlighted in the biofunction analysis, such as CYP19A1, VEGFC, FOXO1, ANGPT2, and CAV1.
Table 7.
Significant IPA canonical pathways for genes affected in summer cumulus cells
| Ingenuity Canonical Pathways | P Value | Ratio | Molecules |
|---|---|---|---|
| NAD salvage pathway II | 0.00214 | 8.82 × 10−2 | ⇈NMRK1, ↑ACPL2, ↑ACPP |
| Nitric oxide signaling in the cardiovascular system | 0.00407 | 4.00 × 10−2 | ⇊GUCY1A3, ⇊RYR2, ↓CAV1, ↓VEGFC, ↓GUCY1B3 |
| NAD phosphorylation and dephosphorylation | 0.00661 | 1.05 × 10−1 | ↑ACPL2, ↑ACPP |
| Heparan sulfate biosynthesis (late stages) | 0.01514 | 4.84 × 10−2 | ⇊UST, ↓CHST7, ↓GLCE |
| HMGB1 signaling | 0.01622 | 3.67 × 10−2 | ↓MAP2K6, ⇈RHOQ, ↓HMGB1, ⇈KAT6A |
| Nicotine degradation III | 0.01698 | 4.11 × 10−2 | ⇊CYP19A1, ↑CYP2B6, ⇊UGT1A9 (includes others) |
| Salvage pathways of pyrimidine Ribonucleotides | 0.01738 | 3.54 × 10−2 | ↓MAP2K6, ⇊DAPK1, ↓CDK8, ↓NME5 |
| Phototransduction pathway | 0.01778 | 4.48 × 10−2 | ⇊GUCY1A3, ⇊OPN3, ↓GUCY1B3 |
| Melatonin degradation I | 0.01950 | 4.55 × 10−2 | ⇊CYP19A1, ↑CYP2B6, ⇊UGT1A9 (includes others) |
| Heparan sulfate biosynthesis | 0.02239 | 4.00 × 10−2 | ⇊UST, ↓CHST7, ↓GLCE |
| HIF1α signaling | 0.02239 | 3.57 × 10−2 | ↓EGLN1, ⇊SLC2A1, ↓VEGFC, ⇊MMP1 |
| Xenobiotic metabolism signaling | 0.02344 | 2.43 × 10−2 | ↓MAP2K6, ⇊UST, ↓CES1, ↓CHST7, ↑CYP2B6, ⇈ALDH9A1, ⇊UGT1A9 (includes others) |
| Superpathway of melatonin degradation | 0.02455 | 3.70 × 10−2 | ⇊CYP19A1, ↑CYP2B6, ⇊UGT1A9 (includes others) |
| Pyrimidine deoxyribonucleotides de novo Biosynthesis I | 0.02570 | 4.55 × 10−2 | ↓TYMS, ↓NME5 |
| Nicotine degradation II | 0.02570 | 3.53 × 10−2 | ⇊CYP19A1, ↑CYP2B6, ⇊UGT1A9 (includes others) |
| Antiproliferative role of TOB in T cell signaling | 0.02951 | 7.69 × 10−2 | ⇈PABPC1, ↓SKP2 |
| Bupropion degradation | 0.02951 | 6.06 × 10−2 | ⇊CYP19A1, ↑CYP2B6 |
| Angiopoietin signaling | 0.03020 | 4.00 × 10−2 | ⇈ANGPTL1, ⇊ANGPT2, ⇈FOXO1 |
| Pyridoxal 5¢-phosphate salvage pathway | 0.03162 | 3.95 × 10−2 | ↓MAP2K6, ⇊DAPK1, ↓CDK8 |
| Acetone degradation I (to methylglyoxal) | 0.03162 | 5.56 × 10−2 | ⇊CYP19A1, ↑CYP2B6 |
| PXR/RXR activation | 0.03236 | 3.26 × 10−2 | ⇈FOXO1, ↑CYP2B6, ⇊UGT1A9 (includes others) |
| IL-6 signaling | 0.03311 | 3.23 × 10−2 | ↓MAP2K6, ⇊TNFAIP6, ⇊CYP19A1, ⇈MAP4K4 |
| Estrogen receptor signaling | 0.04365 | 2.94 × 10−2 | ⇊PELP1, ↓H3F3A/H3F3B, ↓CDK8, ⇈HNRNPD |
| eNOS signaling | 0.04786 | 2.58 × 10−2 | ⇊GUCY1A3, ↓CAV1, ↓VEGFC, ↓GUCY1B3 |
| Inhibition of angiogenesis by TSP1 | 0.04898 | 4.76 × 10−2 | ⇊GUCY1A3, ↓GUCY1B3 |
Symbols for genes with increased (or decreased) expression and fold-change ≥2 are preceded with ⇈ (or ⇊). Symbols for genes with increased (or decreased) expression and fold-change <2 are preceded with ↑ (or ↓).
Predicted upstream regulators in CCs are shown in Supplemental Table S8, their associations with specific IPA networks in CCs are shown in Supplemental Table S7, and a summary of regulators that regulate the highest number of IPA network members is given in Table 8. The upstream regulator analysis identified 13 regulators that were altered in summer CCs (Supplemental Table S8); one of these (FOXO1) is associated with a significant z-score, indicating a predicted inhibition state, but all are associated with significant P values. Effects on upstream regulator expression include decreased expression of the LHCGR mRNA and decreased expression of the mRNA encoding transcription regulator SIN3A associated factor, 30 kDa (SAP30; Supplemental Table S8), which regulates LHCGR. The microRNA mir-210 also appears as an affected upstream regulator. Effects were also observed on the mRNA encoding the interacting transcriptional regulators HMGB1 and Mothers against decapentaplegic, Drosophila homolog 7 (SMAD7). The caveolin mRNAs (CAV1 and CAV2) are also listed as affected regulators. Additional affected upstream regulators modulate expression of CYP19A1.
Table 8.
Overview of the most prominent upstream regulators in IPA networks for summer cumulus cells
| # | Upstream Regulator | Networks, n | Networks (molecules regulated, n) | # | Upstream Regulator | Networks, n | Networks (molecules regulated, n) |
|---|---|---|---|---|---|---|---|
| 1 | ↓miR-210 | 1 | N2(M/3) | 33 | Notch | 1 | N3(M/3) |
| 2 | ↓SMAD7 | 1 | N2(M/3) | 34 | HISTONE | 1 | N3(M/1) |
| 3 | ↓CAV1 | 1 | N4(M/3) | 35 | estrogen receptor | 1 | N4(M/2) |
| 4 | ↓CAV2 | 1 | N4(M/1) | 36 | d-glucose | 1 | N4(4) |
| 5 | ⇈BMPR1B | 1 | N4(M/1) | 37 | AGT | 1 | N4(4) |
| 6 | ↓SKP2 | 1 | N7(M/1) | 38 | ERK | 1 | N4(M/2) |
| 7 | ⇈FOXO1 | 1 | N7(M/2) | 39 | Ras | 1 | N4(M/1) |
| 8 | ↑TOX | 1 | N7(M/1) | 40 | PDGF BB | 1 | N4(M/3) |
| 9 | ⇈RORA | 1 | N7(M/3) | 41 | WNT3A | 1 | N5(2) |
| 10 | ↓HMGB1 | 1 | N7(M/1) | 42 | ZNF100 | 1 | N5(M/1) |
| 11 | ⇊LHCGR | 1 | N13(M/1) | 43 | ZNF85 | 1 | N5(M/1) |
| 12 | TGFB1 | 7 | N1(7), N2(9), N4(5), N6(M/3), N7(4), N9(2), N10(2) | 44 | ZNF254 | 1 | N5(M/1) |
| 45 | ZNF431 | 1 | N5(M/1) | ||||
| 46 | ZNF708 | 1 | N5(M/1) | ||||
| 13 | β-Estradiol | 6 | N1(5), N3(7), N4(7). | 47 | ZNF665 | 1 | N5(M/1) |
| N7(5), N10(2), N13(2) | 48 | ZNF528 | 1 | N5(M/1) | |||
| 14 | HNF4A | 5 | N1(6), N5(2), N6(M/3), N7(8), N9(2) | 49 | ZNF43 | 1 | N5(M/1) |
| 50 | ZNF429 | 1 | N5(M/1) | ||||
| 15 | TNF | 4 | N1(9), N2(6), N4(5), N13(2) | 51 | ZNF91 | 1 | N5(M/1) |
| 52 | LONP1 | 1 | N5(M/1) | ||||
| 16 | TP53 | 4 | N2(5), N4(4), N7(4), N9(2) | 53 | FGF1 | 1 | N5(2) |
| 54 | E2F1 | 1 | N6(M/1) | ||||
| 17 | Tretinoin | 3 | N1(5), N2(6), N4(5) | 55 | CEBPA | 1 | N7(4) |
| 18 | IL1B | 2 | N1(5), N7(5) | 56 | progesterone | 1 | N7(4) |
| 19 | OSM | 2 | N1(5), N7(4) | 57 | IL1 | 1 | N7(M/3) |
| 20 | MYC | 2 | N3(4), N13(2) | 58 | butyric acid | 1 | N7(5) |
| 21 | PD98059 | 2 | N4(4), N5(2) | 59 | P38 MAPK | 1 | N7(M/2) |
| 22 | Cg | 1 | N1(M/12) | 60 | proinsulin | 1 | N7(M/1) |
| 23 | FSH | 1 | N1(M/10) | 61 | PI3K (complex) | 1 | N7(M/2) |
| 24 | Lh | 1 | N1(M/9) | 62 | KISS1R | 1 | N10(M/1) |
| 25 | ERK1/2 | 1 | N1(M/3) | 63 | testosterone | 1 | N10(M/1) |
| 26 | α-Catenin | 1 | N1(M/4) | 64 | IL13 | 1 | N11(2) |
| 27 | SB203580 | 1 | N1(5) | 65 | PKD1 | 1 | N11(M/2) |
| 28 | AHR | 1 | N2(5) | 66 | SUMO2 | 1 | N12(M/1) |
| 29 | Tgf-β | 1 | N2(M/3) | 67 | NUPR1 | 1 | N12(M/3) |
| 30 | Integrin α V β | 1 | N2(M/1) | 68 | Vegf | 1 | N13(M/2) |
| 31 | IL6 | 1 | N2(5) | 69 | PRL | 1 | N13(2) |
| 32 | Akt | 1 | N2(M/2) | 70 | GATA4 | 1 | N13(2) |
Symbols for regulators with increased (or decreased) expression and fold-change ≥2 are preceded with ⇈ (or ⇊). Symbols for genes with increased (or decreased) expression and fold-change <2 are preceded with ↑ (or ↓). Regulators are ordered by the number of IPA networks that they are regulate. Regulators that are themselves affected in summer cumulus cells are listed separately on top. The numbers in parenthesis next to network IDs in the 4th/8th column signify how many molecules in those networks the regulators are affecting (e.g., IL1B regulates 5 molecules in network 1 and 5 molecules in network 7). Labels (M/. . .) in the 4th/8th column signify that the regulators are also member of those networks, e.g., miR210 regulates 3 molecules in network 2 and is also a member of network 2.
The upstream regulator analysis revealed predicted inhibition of pathways downstream of 14 upstream regulators and predicted activation of pathways downstream of five regulators; none of these upstream regulators show significantly different expression in summer CCs (Supplemental Table S8). One of these was an inhibition in signaling via HIF1A, which works with miR-210 in hypoxia response. The other upstream regulators displaying significant negative z-scores indicative of downstream inhibition include angiotensinogen 2 (AGT), avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2), OSM, testosterone, aryl hydrocarbon receptor nuclear translocator 2 (ARNT2), signal transducer and activator of transcription 5B (STAT5B), p38 mitogen activated protein kinase MAPK (a.k.a. MAPK14), CD38 antigen (CD38), E2F transcription factor 1 (E2F1), fibroblast growth factor 1(FGF1), extracellular signal-related kinase 1/2 (ERK1/2), neural precursor cell expressed developmentally downregulated 9 (NEDD9), V-Ha-Ras Harvey rat sarcoma viral oncogene homolog (RAS), and prostaglandin endoperoxide synthase 2 (PTGS2). The apparent effects thus echo the effects projected by biofunction analysis for control of vasculogenesis and cell proliferation for CCs. Additional upstream regulators with negative z-scores close to the threshold for inhibition (−1.96) and known associations with CC function include inhibin beta A (INHBA), peroxisome proliferator-activated receptor gamma (PPARG), epidermal growth factor receptor (EGFR), and interleukin 1B (IL1B). The upstream regulators displaying significant positive z-scores indicative of downstream activation include wingless type MMTV integration site family member 3A (WNT3A), the PI3 kinase inhibitor LY294002, the copper metabolism gene COMM domain containing 1 (COMMD1), tumor suppressor VHL, and kinase inhibitor H89.
DISCUSSION
We report here that the developmental potential of oocytes obtained during summer months from rhesus macaques is reduced. The transcriptomes of oocyte-associated CCs and GCs are disrupted. Summer CCs and GCs both display evidence of deficiencies in expression of mRNAs related to cell proliferation, angiogenesis and endocrine signaling, as well as reduced expression of PYGL. Additionally, CCs display deficiencies in expression of mRNAs related to stress response. These results provide the first insight into the specific molecular pathways and processes that are disrupted in the follicles of rhesus macaque females during the summer season. Although humans do not display seasonality in breeding, the seasonality effect in rhesus monkeys provides one important model of reduced oocyte quality in a primate species. Accordingly, the molecular changes contributing to reduced oocyte quality during the summer season in rhesus macaques may also contribute to our understanding of reduced reproductive performance in humans.
Reduced expression of PYGL mRNA could inhibit the ability of the GCs and CCs to break down glycogen and provide glucose or other carbon substrates to oocytes. Studies in mice have demonstrated clearly the essential role of CCs in metabolizing glucose via glycolysis, passing metabolites to the oocyte and promoting oocyte meiosis (14). Studies in the macaque demonstrated that PYGL expression increases after an ovulatory stimulus and may promote a rise in intrafollicular glucose availability, which is spared for oocyte utilization (4). Reducing the follicular supply of glucose through reduced PYGL expression would be expected to compromise oocyte developmental competence.
The endocrine profile of rhesus macaque females is significantly altered during the summer months, with reduced serum levels of progesterone, LH, and estradiol reported (24, 50, 51). Our data indicate that disruptions in intraovarian endocrine signaling likely also exist. We observed reduced expression of LH receptor mRNA and other genes residing in an LH regulated network in summer CCs. We also observed reduced expression of the mRNA for SAP30, a cofactor that works with SIN3A in transcriptional repression. Superficially, reduced expression of SAP30 could be viewed as promoting LHR expression (15, 31, 53); it also participates as a component of other transcriptional repressor complexes, such as those participating in retinoid receptor, nuclear hormone receptor, and JNK-mediated gene activation. Reduced LH receptor mRNA expression in CCs is associated with reduced oocyte quality in cattle (6). Progesterone produced by FSH and LH-stimulated CCs promotes maturation in pig oocytes (42). Reduced LHR expression in the monkey could thus compromise oocyte quality by effects on steroidogenesis in summer CCs. Moreover, mouse GV-stage oocytes suppress LH receptor expression in GCs, whereas MII-stage oocytes are less potent in this capability (16). These effects are consistent with an oocyte role in promoting the CC phenotype (and thus suppressing the GC phenotype), but a loss of this effect as maturation progresses. We note that monkey CCs come to resemble GCs quite closely after maturation (9). This may allow CCs to replace GC functions after ovulation and move toward a steroidogenic phenotype that supports production of quality oocytes. Reduced LHR signaling in the summer CC could indicate an overall disruption or discoordination of the key oocyte-CC interactions that orchestrate these transitions, thereby contributing to lower quality oocytes.
We observed lower expression of mRNA for CYP19A1 (aromatase, required for conversion of androgens to estrogen) in summer CCs. CYP19A1 mRNA was not altered in summer GCs. CYP19A1 expression is typically observed in the context of GC production of intrafollicular estrogen. Its expression in human GC cells is positively correlated with pregnancy outcomes (20), but its mRNA expression is higher in CCs from in vitro matured rhesus monkey CC-oocyte complexes with lower oocyte quality, due to an apparent failure to be downregulated during maturation (29). Bovine CC-oocyte complexes produce estradiol in vitro in the presence of aromatizable androgen (1), and androstenedione in rhesus monkey in vitro maturation medium enhances oocyte quality (40). CYP19A1 expression in the CC may provide a protective function by reducing exposure of the oocyte to excess androgen. The lower expression of CYP19A1 mRNA in CCs of summer follicles may thus contribute to reduced oocyte quality. It is also worth noting that, given the differential response of CYP19A1 mRNA expression under different modes of reduced oocyte quality, more than one underlying cause of reduced oocyte quality likely exists, with different associated CC transcriptome profiles.
In addition to disruptions in endocrine signaling, disruptions in signaling via other key ligands may also arise in the summer follicle. We observed negative z-scores indicative of potential inhibition of signaling networks associated with upstream regulators PTGS2, INHBA, FGF1, FGF2, FSH, EGFR, and IL1B. Upstream regulator analysis indicates modest roles for other growth actors, such as TGFB3. FSH and PTGS2 are key regulators of CC and GC differentiation (21). Signaling via EGFR may promote favorable metabolic changes (38), steroidogenesis (26), and production of high-quality rhesus monkey oocytes (36). Expression of EGF family members in CCs and GCs may regulate ovulation (22, 52) and is sensitive to FSH in mice (41). Inhibition of EGFR signaling following FSH stimulation can inhibit oocyte maturation in the pig (37), and EGFR induction following gonadotropin stimulation promotes EGFR expression in sheep GCs and CCs (12). Cross talk between the FSH and EGF signaling pathways is important in pig CC-oocyte complexes (37). FGF receptor mRNAs are expressed in response to FSH stimulation in bovine CCs (5). Inhibition of cell proliferation functions was a hallmark of gene expression changes seen for both GCs and CCs from summer follicles, an effect that may arise from reduced signaling through TGF, EGF, and FGF pathways. The IPA analysis also indicated activated signaling downstream of WNT3A in CCs and reduced expression of the negative regulator of WNT signaling SHISA (which acts cell-autonomously) in GCs. WNT signaling can negatively affect folliculogenesis via FOXO3 (7, 30), and excessive signaling through beta-catenin can be detrimental (17). FOXO3 suppresses primordial follicle development in mice but in nonrodent species FOXO1 (which is elevated in summer CCs) may play a redundant role (47). It is noted that oocyte-derived R-spondin promotes folliculogenesis via WNT signaling but that R-spondin can act through either TGF or WNT pathways (10, 13). These observations indicate that changes in signaling through the WNT pathway in GCs and CCs could affect oocyte development. Angiotensin (AGT) contributes to LH-induced progesterone production in bovine GCs (44). Our data implicate AGT signaling as being reduced in summer CCs. Overall, the inhibition of signaling via AGT, PTGS2, INHBA, FGF1, FGF2, FSH, EGFR, and IL1B-related pathways, and activated WNT-FOXO1 signaling in summer CCs could all contribute to seasonal reduction in oocyte quality.
Disruptions in endocrine and other signaling pathways may underlie another key deficiency implicated in the IPA analysis of transcriptomes for summer GCs and CCs, namely reduced angiogenesis and vascular development. Reduced expression of ANGPT2 and VEGF would reflect a reduced capacity for neovascularization. Changes in gene expression related to angiogenesis were previously reported for GCs in the human (3). Indeed, other studies in the monkey and other species implicate GCs in promoting angiogenesis (45) and the beneficial effects of this on folliculogenesis and production of quality oocytes. Interestingly, CCs promote angiogenesis in rat ovaries at early stages of folliculogenesis, but this function is taken over by the GCs during later stages of folliculogenesis (43). We observed inhibition in expression of angiogenesis functions in both cell types, indicating that reduced vascularization of ovarian follicles likely contributes to reduced oocyte quality.
The deficiency in angiogenesis may relate to a broader deficiency in CC responsiveness to hypoxic stress. Reduced signaling through HIF1A was implicated for CCs in the IPA analysis. Reduced expression of mir-210, which cooperates with HIF1A (23), was seen for summer CCs. Hypoxia promotes neovascularization (32). HIF1A promotes hormonally regulated follicular differentiation and luteinization (46) and is important for vascularization in the ovary (33). Inhibition of HIF1A function impedes oocyte maturation (27). Reduced HIF1A signaling and reduced expression of genes related to vascularization in summer ovaries may thus be mechanistically linked in contributing to compromised folliculogenesis and reduced oocyte quality.
In summary, our data demonstrate reduced quality of oocytes obtained from rhesus macaques during the summer months, combined with altered patterns of gene expression in GCs and CCs. Perhaps the most significant effect observed was reduced expression of PYGL in both CCs and GCs, which may effectively starve the summer oocyte for essential carbon substrates. Additionally, disruptions in angiogenesis, reduced LHR signaling, reduced CYP19A1 expression, and changes in signaling through numerous other pathways were implicated in the IPA analysis of seasonal gene expression differences in GCs and CCs. Some of the changes seen in summer GCs and CCs have been reported in humans and in other model mammalian species. This suggests that the seasonal effects seen in the rhesus monkey may help us to understand better the mechanisms that contribute to reduced oocyte quality and fertility in humans.
GRANTS
This work was supported by grants from the Office of Research Infrastructure Programs Division of Comparative Medicine Grants R24 OD-012221 (to K. E. Latham) and OD011107/RR00169 (California National Primate Research Center) and OD010967/RR025880 (to C. A. VandeVoort), by MSU AgBioResearch, and Michigan State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.A.V. and K.E.L. conception and design of research; C.A.V. and N.R.M. performed experiments; C.A.V., U.M., and K.E.L. drafted manuscript; C.A.V., N.R.M., U.M., and K.E.L. edited and revised manuscript; C.A.V., N.R.M., U.M., and K.E.L. approved final version of manuscript; U.M. and K.E.L. analyzed data; K.E.L. interpreted results of experiments.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dana Hill, Bela Patel, and Jeff Cabello for their technical assistance.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Bernuci MP, Bulgareli DL, Vireque AA, Pitangui CP, de Sa MF, Rosa-e-Silva AC. Exposing bovine cumulus-oocyte complexes to aromatizable androgen restore serum-induced low estradiol production in vitro. Zygote 22: 496–499, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Bielert C, Vandenbergh JG. Seasonal influences of births and male sex skin coloration in rhesus monkeys (Macaca mulatta) in the southern hemisphere. J Reprod Fert 61: 229–233, 1981. [DOI] [PubMed] [Google Scholar]
- 3.Borgbo T, Povlsen BB, Andersen CY, Borup R, Humaidan P, Grondahl ML. Comparison of gene expression profiles in granulosa and cumulus cells after ovulation induction with either human chorionic gonadotropin or a gonadotropin-releasing hormone agonist trigger. Fertil Steril 100: 994–1001, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Brogan RS, MacGibeny M, Mix S, Thompson C, Puttabyatappa M, VandeVoort CA, Chaffin CL. Dynamics of intra-follicular glucose during luteinization of macaque ovarian follicles. Mol Cell Endocrinol 332: 189–195, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caixeta ES, Machado MF, Ripamonte P, Price C, Buratini J. Effects of FSH on the expression of receptors for oocyte-secreted factors and members of the EGF-like family during in vitro maturation in cattle. Reprod Fertil Dev 25: 890–899, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Calder MD, Caveney AN, Smith LC, Watson AJ. Responsiveness of bovine cumulus-oocyte-complexes (COC) to porcine and recombinant human FSH, and the effect of COC quality on gonadotropin receptor and Cx43 marker gene mRNAs during maturation in vitro. Reprod Biol Endocrinol 1: 14, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301: 215–218, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Chaffin CL, Latham KE, Mtango NR, Midic U, VandeVoort CA. Dietary sugar in healthy female primates perturbs oocyte maturation and in vitro preimplantation embryo development. Endocrinology 155: 2688–2695, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaffin CL, Lee YS, Vandevoort CA, Patel BG, Latham KE. Rhesus monkey cumulus cells revert to a mural granulosa cell state after an ovulatory stimulus. Endocrinology 153: 5535–5545, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y, Kawamura K, Takae S, Deguchi M, Yang Q, Kuo C, Hsueh AJ. Oocyte-derived R-spondin2 promotes ovarian follicle development. FASEB J 27: 2175–2184, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conaway DH, Koford CB. Estrous cycles and mating behavior in a free-ranging band of rhesus monkeys. J Mammal 45: 577–588, 1964. [Google Scholar]
- 12.Cotterill M, Catt SL, Picton HM. Characterisation of the cellular and molecular responses of ovine oocytes and their supporting somatic cells to pre-ovulatory levels of LH and FSH during in vitro maturation. Reproduction 144: 195–207, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harbor Perspect Biol 5: a015081, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downs SM, Utecht AM. Metabolism of radiolabeled glucose by mouse oocytes and oocyte-cumulus cell complexes. Biol Reprod 60: 1446–1452, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Dufau ML, Liao M, Zhang Y. Participation of signaling pathways in the derepression of luteinizing hormone receptor transcription. Mol Cell Endocrinol 314: 221–227, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod 56: 976–984, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Fan HY, O'Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS. Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endocrinol 24: 1529–1542, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentleman RC, Carey V, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon TP. Reproductive behavior in the rhesus monkey: social and endocrine variables. Am Zool 21: 185–195, 1981. [Google Scholar]
- 20.Hamel M, Dufort I, Robert C, Gravel C, Leveille MC, Leader A, Sirard MA. Identification of differentially expressed markers in human follicular cells associated with competent oocytes. Hum Reprod 23: 1118–1127, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Hao L, Midic U, Garriga J, Latham KE. Contribution of CBX4 to cumulus oophorus cell phenotype in mice and attendant effects in cumulus cell cloned embryos. Physiol Genomics 46: 66–80, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27: 1914–1924, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Zuo J. Emerging roles of miR-210 and other non-coding RNAs in the hypoxic response. Acta Biochim Biophys Sin 46: 220–232, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Hutz RJ, Dierschke DJ, Wolf RC. Seasonal effects on ovarian folliculogenesis in rhesus monkeys. Biol Reprod 33: 653–659, 1985. [DOI] [PubMed] [Google Scholar]
- 25.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamnongjit M, Gill A, Hammes SR. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci USA 102: 16257–16262, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Bagchi IC, Bagchi MK. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology 150: 3392–3400, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YS, Latham KE, Vandevoort CA. Effects of in vitro maturation on gene expression in rhesus monkey oocytes. Physiol Genomics 35: 145–158, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YS, VandeVoort CA, Gaughan JP, Midic U, Obradovic Z, Latham KE. Extensive effects of in vitro oocyte maturation on rhesus monkey cumulus cell transcriptome. Am J Physiol Endocrinol Metab 301: E196–E209, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Ji SY, Yang JL, Li XX, Zhang J, Zhang Y, Hu ZY, Liu YX. Wnt/beta-catenin signaling regulates follicular development by modulating the expression of Foxo3a signaling components. Mol Cell Endocrinol 382: 915–925, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Liao M, Zhang Y, Dufau ML. Protein kinase Calpha-induced derepression of the human luteinizing hormone receptor gene transcription through ERK-mediated release of HDAC1/Sin3A repressor complex from Sp1 sites. Mol Endocrinol 22: 1449–1463, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meidan R, Klipper E, Zalman Y, Yalu R. The role of hypoxia-induced genes in ovarian angiogenesis. Reprod Fertil Dev 25: 343–350, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura R, Okuda K. Hypoxia is important for establishing vascularization during corpus luteum formation in cattle. J Reprod Dev 56: 110–116, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Nusser KD, Mitalipov S, Widmann A, Gerami-Naini B, Yeoman RR, Wolf DP. Developmental competence of oocytes after ICSI in the rhesus monkey. Hum Reprod 16: 130–137, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Nyholt de Prada JK, Kellam LD, Patel BG, Latham KE, Vandevoort CA. Growth hormone and gene expression of in vitro-matured rhesus macaque oocytes. Mol Reprod Dev 77: 353–362, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyholt de Prada JK, Lee YS, Latham KE, Chaffin CL, VandeVoort CA. Role for cumulus cell-produced EGF-like ligands during primate oocyte maturation in vitro. Am J Physiol Endocrinol Metab 296: E1049–E1058, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prochazka R, Blaha M, Nemcova L. Signaling pathways regulating FSH- and amphiregulin-induced meiotic resumption and cumulus cell expansion in the pig. Reproduction 144: 535–546, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Richani D, Sutton-McDowall ML, Frank LA, Gilchrist RB, Thompson JG. Effect of epidermal growth factor-like peptides on the metabolism of in vitro- matured mouse oocytes and cumulus cells. Biol Reprod 90: 49, 2014. [DOI] [PubMed] [Google Scholar]
- 39.Riesen JW, Meyer RK, Wolf RC. The effect of season on occurrence of ovulation in the rhesus monkey. Biol Reprod 5: 111–114, 1971. [DOI] [PubMed] [Google Scholar]
- 40.Schramm RD, Paprocki AM, VandeVoort CA. Causes of developmental failure of in-vitro matured rhesus monkey oocytes: impairments in embryonic genome activation. Hum Reprod 18: 826–833, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20: 1352–1365, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Shimada M, Terada T. FSH and LH induce progesterone production and progesterone receptor synthesis in cumulus cells: a requirement for meiotic resumption in porcine oocytes. Mol Hum Reprod 8: 612–618, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Shweiki D, Itin A, Neufeld G, Gitay-Goren H, Keshet E. Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J Clin Invest 91: 2235–2243, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siqueira LC, dos Santos JT, Ferreira R, Souza dos Santos R, dos Reis AM, Oliveira JF, Fortune JE, Goncalves PB. Preovulatory changes in the angiotensin II system in bovine follicles. Reprod Fertil Dev 25: 539–546, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Stouffer RL, Martinez-Chequer JC, Molskness TA, Xu F, Hazzard TM. Regulation and action of angiogenic factors in the primate ovary. Arch Med Res 32: 567–575, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Tam KK, Russell DL, Peet DJ, Bracken CP, Rodgers RJ, Thompson JG, Kind KL. Hormonally regulated follicle differentiation and luteinization in the mouse is associated with hypoxia inducible factor activity. Mol Cell Endocrinol 327: 47–55, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Tarnawa ED, Baker MD, Aloisio GM, Carr BR, Castrillon DH. Gonadal expression of Foxo1, but not Foxo3, is conserved in diverse mammalian species. Biol Reprod 88: 103, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VandeVoort CA, Leibo SP, Tarantal AF. Improved collection and developmental competence of immature macaque oocytes. Theriogenology 59: 699–707, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Walker ML, Gordon TP, Wilson ME. Menstrual cycle characteristics of seasonally breeding rhesus monkeys. Biol Reprod 29: 841–848, 1983. [DOI] [PubMed] [Google Scholar]
- 51.Wilson ME, Pope NS, Gordon TP. Seasonal modulation of luteinizing-hormone secretion in female rhesus monkeys. Biol Reprod 36: 975–984, 1987. [DOI] [PubMed] [Google Scholar]
- 52.Yamashita Y, Shimada M. The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process. J Reprod Dev 58: 510–514, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Dufau ML. Silencing of transcription of the human luteinizing hormone receptor gene by histone deacetylase-mSin3A complex. J Biol Chem 277: 33431–33438, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.