Abstract
Heightened protection from infectious disease as conferred by vaccination or pathogen exposure relies on the effective generation and preservation of specific immunological memory. T cells are irreducibly required for the control of most viral infections, and maintenance of CD8+T cell memory is regulated by at least two cytokines, IL-7 and IL-15, which support survival (IL-7, IL-15) and basal homeostatic proliferation (IL-15) of specific CD8+ memory T cells (TM). In contrast, the factors governing the homeostasis of pathogen-specific CD4+TM remain at present unknown. Here, we used a physiologic in vivo model system for viral infection to delineate homeostatic features and mechanisms of antiviral CD4+TM preservation in direct juxtaposition to CD8+T cell memory. Basal homeostatic proliferation is comparable between specific CD4+ and CD8+TM and independent of immunodominant determinants and functional avidities but regulated in a tissue-specific fashion. IL-7, identified as the dominant cytokine, and IL-15, an accessory cytokine, regulate basal homeostatic proliferation and survival of antiviral CD4+TM. Interestingly, a role for these cytokines in regulation of CD4+T cell memory is not readily discernible in the generic “memory-phenotype” population, apparently a consequence of its heterogeneous composition. We also describe a prominent, nonredundant role for IL-7 in supporting basal homeostatic proliferation of CD8+TM. We propose that homeostatic control of antiviral CD4+ and CD8+ T cell memory is fundamentally similar and characterized by quantitative, rather than qualitative, differences.
Among the most striking attributes of adaptive immunity is the phenomenon of immunological memory (1), the basis for enhanced protection against disease upon reexposure to previously encountered pathogens and the efficacy of vaccination as a tool for the global control of infectious diseases (2). Although specific antibody titers often correlate well with protective immunity, control of most viral and many bacterial infections requires the participation of T cells (3, 4). The two major subsets recruited into specific T cell response and memory, CD8+ and CD4+T lymphocytes, perform overlapping and distinct functions in the control of pathogens, and emerging evidence indicates that regulation of their specific activity and maintenance also follows both common and divergent rules (5–7). CD8+T cells constitute the major effector population by virtue of their capacity for cytolysis and cytokine production (4), yet CD4+T cells provide an array of additional functions for successful resolution of infection that comprise direct effector activities as well as modulation of CD8+T cell, B cell, antigen-presenting cell and natural killer cell function. Studies on human diseases, such as HIV, Epstein–Barr virus, cytomegalovirus, hepatitis B virus, and hepatitis C virus infection, have illustrated the importance of virus-specific CD4+T cells, and direct evidence for their indispensable role has been obtained in multiple murine models of viral infection (8). Furthermore, even successful pathogen control in the absence of CD4+T cells can lead to defective CD8+T cell memory and compromised protection upon secondary infection (9).
T cell memory is an active process that regulates the preservation of specific memory T cell (TM) populations in a dynamic environment (10–12). Its cardinal feature is arguably the stem cell-like, antigen-independent self-renewal of TM, termed “basal homeostatic proliferation” because it assures specific T cell survival at the population level by continued propagation of its own constituents. Basal homeostatic proliferation is defined as the in vivo turnover of TM observed under steady-state conditions in T cell-replete compartments (13). Thus, basal homeostatic proliferation is “nonproductive” (i.e., cell numbers are not increased) and to be distinguished from proliferation under conditions of lymphopenia (“acute homeostatic proliferation”) (13) or in response to antigenic stimuli (antigen-driven proliferation).
Work over the past few years has established a critical role for cytokines in maintenance of CD8+T cell memory (10–12, 14). IL-15 promotes basal homeostatic proliferation and survival of CD8+TM in different experimental systems (13, 15–21), whereas IL-7 performs an overlapping and complementary role during acute homeostatic proliferation in lymphopenic environments (13, 18, 22). In intact lymphatic compartments, IL-7 is thought to function primarily as a survival factor without providing substantial support for basal homeostatic proliferation (13, 20, 23). In addition, a dual function for IL-2 is documented by the findings that both administration of IL-2 (24) and blockade of the IL-2/IL-2R system can enhance proliferation, the latter phenomenon apparently due to depletion of CD4+CD25+ regulatory T cells (20, 25). Less information is available about homeostatic regulation of CD4+T cell memory which has been proposed to rely on different mechanisms. Although human CD4+TM proliferate in vitro in response to IL-7 and IL-15 (26), studies in mice showed that acute homeostatic proliferation of “memory-phenotype” CD4+T cells is independent of IL-7 and IL-15 (18). Both cytokines were also ruled out to participate in CD4+TM survival, because CD4+TM deficient for CD132 (γc-chain, jointly used by IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 receptors) are effectively maintained in vivo (27), but more recent work has challenged this assumption by demonstrating that IL-7 is in fact required for survival of both memory-phenotype and T cell receptor transgenic CD4+TM (28–30). So far, however, the factors governing basal homeostatic proliferation of CD4+TM have remained elusive, and a detailed analysis of homeostatic features and mechanisms operative in the preservation of pathogen-specific CD4+T cell memory under physiological conditions has not been performed.
Materials and Methods
Mice and Virus. C57BL/6J (B6) mice were obtained from the Rodent Breeding Colony at The Scripps Research Institute. IL-15-/- mice on a B6 background (B6.IL-15-/-) were obtained from Taconic Farms. All mice were bred and maintained under specific pathogen-free conditions at The Scripps Research Institute. Eight- to 10-week-old mice were infected with a single i.p. dose of 1.5 × 105 plaque-forming units of lymphocytic chorio-meningitis virus (LCMV) Armstrong 53b (7).
Tissue Preparation. Lymphocytes were isolated from spleen, lymph nodes, blood, and bone marrow according to standard procedures. Peritoneal cavity cells were obtained by peritoneal lavage. Liver, lung, and kidney were removed after total body perfusion, and lymphocytes were enriched by using a Percoll gradient.
Antibodies, Cytokines, and Peptides. The antibodies, fluorochromes, and peptides used have been described (7). The IL-7Rα-specific A7R34 hybridoma was generated by S. Nishikawa (Kumamoto University School of Medicine, Kumamoto, Japan; see ref. 31); we obtained the hybridoma from C. Surh (The Scripps Research Institute) and produced the purified antibody by using established protocols (32). Recombinant murine cytokines were purchased from eBioscience, San Diego (mIL-2, mIL-7, and mIL-15), R & D Systems [mIL-21, murine thymic stromal lymphopoietin (TSLP), and mIL-17], or Peprotech, Rocky Hill, NJ (mIL-7 and mIL-15). Cytokines dissolved in PBS were injected in a volume of 200 μl into the tail vein of LCMV-immune mice.
Flow Cytometry. Reagents and procedures for 5 h in vitro restimulation and surface and intracellular staining have been described (7).
In Vivo Proliferation Assay. LCMV-immune mice were injected with 2 mg of BrdUrd (Sigma) i.p. and supplied with daily prepared drinking water containing 0.8 mg/ml BrdUrd. To combine detection of cytokine production and BrdUrd incorporation by specific TM, we used the BrdUrd-specific B44 antibody (BD Biosciences) and modified protocols for intracellular staining (7).
Further procedural details are provided as Supporting Materials and Methods and Figs. 6 and 7, which are published as supporting information on the PNAS web site.
Results and Discussion
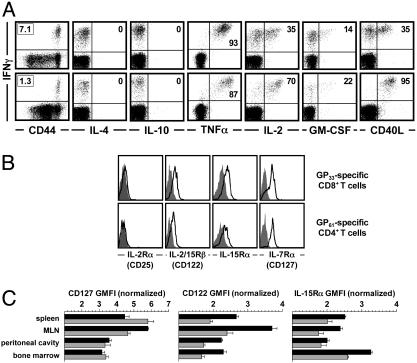
Functional and Phenotypic Profiling of Virus-Specific TM. To study homeostasis of pathogen-specific CD4+T cell memory, we selected a natural, T cell-dependent host–pathogen system that allowed us to compare and contrast cardinal aspects of CD4+ and CD8+TM in the absence of persisting antigen (33, 34). Upon infection with LCMV, B6 mice generate and maintain specific T cell immunity distributed over six MHC-I-restricted and two MHC-II-restricted T cell populations that recognize epitopes derived from the viral glycoprotein (GP) or nucleoprotein (NP) (7, 35). As a population phenomenon, T cell memory exhibits multiple specificities and a broad range of T cell functionalities and phenotypes that may provide clues to its homeostatic regulation. By virtue of their uniform CD44hi expression, LCMV-specific CD8+ and CD4+TM are part of the generic memory-phenotype population, and rapid induction of IFN-γ in the absence of appreciable IL-4 and IL-10 production confirms the clear skewing toward Tc1/TH1 responses in the LCMV system (Fig. 1A). The inducible expression of tumor necrosis factor α, IL-2, granulocyte/macrophage colony-stimulating factor, and CD40L in distinct subpopulations underscores the functional similarities and quantitative differences between specific CD8+ and CD4+TM (Fig. 1 A).
Fig. 1.
Phenotypic and functional profiling of CD4+ and CD8+TM.(A) Spleen cells from LCMV-immune mice (7–9 weeks after challenge) were restimulated for 5 h with the dominant MHC-I-restricted (GP33) or MHC-II-restricted (GP61) LCMV epitopes and stained for intracellular IFN-γ in combination with indicated surface or intracellular markers. (Upper) CD8+T cell gates. (Lower) CD4+T cell gates. Boxed numbers indicate frequencies of GP33-specifc CD8+ and GP61-specific CD4+TM; numbers in upper right quadrants are percentages of all epitope-specific (IFN-γ+) T cells coexpressing indicated additional markers. Values are means of 6–10 mice analyzed. TNFα, tumor necrosis factor α. GM-CSF, granulocyte/macrophage colony-stimulating factor. (B) Cytokine receptor expression by splenic TM. Black traces indicate staining for cytokine receptors; gray histograms are isotype control stains or polyclonal goat Ig stains (IL-15Rα histograms). (C) CD127/IL-7Rα, CD122/IL-2Rβ, and IL-15Rα expression by virus-specific CD8+ (black) and CD4+ (gray) TM in different organs. Cytokine receptor expression levels were normalized by dividing the geometric mean of fluorescence intensity (GMFI) of experimental stains by that of the isotype control stains. CD127 expression by specific CD4+TM was significantly higher as compared with specific CD8+TM in spleen (P = 0.0367) and lower in the MLN (P = 0.0011). Comparative CD122 expression by specific CD4+TM was reduced in all organs (P < 0.006), as was IL-15Rα expression (P < 0.02), with the exception of the peritoneal cavity.
Because an analysis of cytokine receptor expression may provide information about the capacity of defined T cell subsets to respond to corresponding cytokines in vivo, we evaluated receptor expression levels for cytokines that have been shown to regulate CD8+TM homeostasis (IL-2, IL-7, and IL-15). The high-affinity IL-2Rα (CD25) was not expressed by specific CD8+ or CD4+TM, but IL-7Rα (CD127), CD122 (jointly used by IL-15 and IL-2), and the high-affinity IL-15Rα were detected on both TM populations (Fig. 1B). However, relative expression levels differed between specific CD4+ and CD8+TM and according to anatomical location (Fig. 1C). Although these differences may indicate differential sensitivity to IL-7 and IL-15, the altered expression levels in distinct tissues were regulated in a comparable fashion among specific CD4+ and CD8+TM. Thus, cytokine-dependent basal homeostatic proliferation may be regulated in a similar yet tissue-specific manner for CD4+ and CD8+TM.
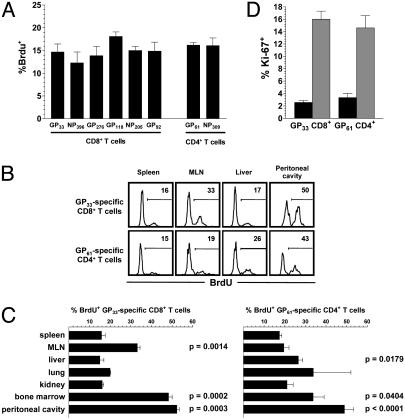
Basal Homeostatic Proliferation As a Function of T Cell Lineage, Immunodominant Determinants, and Functional Avidity. To evaluate basal homeostatic proliferation rates of complex TM populations, LCMV-immune mice were given the nucleotide analog BrdUrd, which is incorporated into the DNA of proliferating cells and can be visualized in specific TM by combined intracellular IFN-γ and BrdUrd staining. As shown in Fig. 2A, basal homeostatic proliferation was comparable between specific CD8+ and CD4+TM and independent of immunodominant determinants. Additional determination of the peptide concentrations required to induce IFN-γ production in half of the corresponding epitope-specific TM populations revealed a broad range of “functional avidities” among the eight LCMV-specific TM populations (≈0.2–20 nM for specific CD8+ and ≈65–650 nM for specific CD4+TM). This finding provides indirect evidence against a role for specific T cell receptor-dependent interactions in basal homeostatic proliferation and supports the notion that maintenance of TM is independent of MHC-mediated interactions (33, 36). Based on the results in Fig. 2 A, we calculated that ≈2% of all TM are proliferating on any given day. Indeed, a 24-h BrdUrd pulse confirmed this estimate and demonstrated that ≈50,000 LCMV-specific CD8+ and ≈5,000 specific CD4+TM in the spleen entered the cell cycle every day (Fig. 6A).
Fig. 2.
Delineating basal homeostatic proliferation of CD4+ and CD8+TM.(A) Basal homeostatic proliferation of TM specific for MHC-I-restricted (GP33, NP396, GP276, GP118, NP205, and GP92) and MHC-II-restricted (GP61 and NP309) LCMV epitopes was evaluated ≈7 weeks after infection and 7-d BrdUrd administration before combined IFN-γ/BrdUrd staining. Bars (SEM) indicate the fraction of BrdUrd+TM among respective epitope-specific populations that are displayed in order of immunodominance. Experiment (n = three mice per group) was performed twice with similar results. (B) Histograms gated on GP33-specific CD8+TM (Upper) and GP61-specific CD4+TM (Lower) show percentage of BrdUrd incorporation (7-d pulse) by TM recovered from different tissues. (C) Summary of basal homeostatic proliferation (7-d BrdUrd pulse) as a function of anatomic location. Data (SEM; n = three mice per group) from one of three similar experiments. Statistical analyses for proliferative turnover in reference to spleen were performed by Student's t test; P values are indicated. (D) Ki-67 expression by virus-specific TM ≈10 weeks after LCMV challenge in spleen (black) and peritoneal cavity (gray). Shown are the combined data from two of four separate experiments (n = three mice per group; differences between Ki-67+ splenic and peritoneal TM, P < 0.001).
Basal Homeostatic Proliferation As a Function of Anatomic Localization. Surprisingly, turnover rates differed significantly according to anatomic location (Fig. 2 B–D). Proliferation of specific CD8+TM was comparable to the spleen in nonlymphatic organs, such as liver, lung, and kidney, but was accelerated in lymph nodes and dramatically enhanced in the peritoneal cavity and bone marrow (Fig. 2 B–D). Overall, specific CD4+TM exhibited similar variations in tissue-dependent proliferation rates. However, increased turnover was also found in liver, whereas lymph node CD4+TM proliferated at slightly but not significantly higher rates and enhanced proliferation in the bone marrow was less pronounced. These observations were also confirmed for other epitope-specific CD4+ and CD8+TM populations (data not shown). The possibility that accelerated proliferation was an artifact of differential BrdUrd incorporation in distinct tissues was ruled out by determination of Ki-67 expression in LCMV-specific TM. Ki-67, a nuclear antigen expressed in all active stages of the cell cycle, was detectable in 2–3% of splenic but in ≈15% of peritoneal cavity CD8+ and CD4+TM (Fig. 2D).
Because preliminary analyses revealed partial associations between cytokine mRNA expression in tissue-specific microenvironments, proliferation rates, and/or cytokine-receptor expression by specific TM (data not shown), we evaluated proliferation of spleen-derived TM exposed to the microenvironment of the peritoneal cavity by adoptive transfer. Proliferation of transferred spleen TM indeed increased in the host peritoneal cavity but not to levels observed for endogenous resident TM, possibly because of the limited exposure time chosen to prevent extensive redistribution of transferred TM (Fig. 6B). In separate experiments, we also observed faster proliferation among endogenous splenic CD62L+ as compared with CD62L- TM subsets extending a recent observation for CD8+TM (37) to the specific CD4+TM compartment, but this distinction did not apply uniformly to all organs (data not shown). The precise interaction between tissue-specific microenvironments and preferential migration and/or residence of defined TM subsets clearly remains to be investigated in greater detail. However, because differences in tissue-specific proliferation rates applied in a similar fashion to both specific CD8+ and CD4+TM, the notion of joint regulatory mechanisms operative in the control of CD8+ and CD4+TM homeostasis is further emphasized.
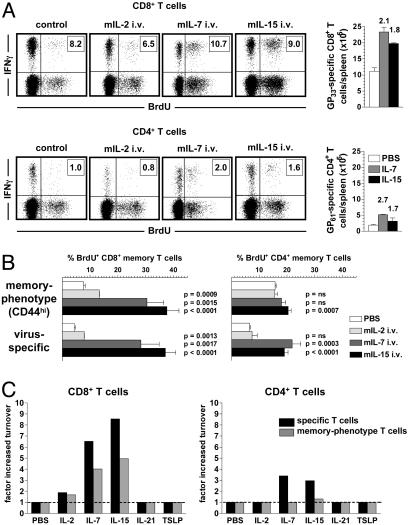
Cytokine-Driven Proliferation of Specific TM. We next assessed the impact of γc-cytokines on TM homeostasis in vivo by injecting recombinant murine cytokines into LCMV-immune mice. As anticipated, administration of IL-15 led to accelerated proliferation of specific CD8+TM (Fig. 3 A–C). Although some studies have observed enhanced T cell cycling after administration of IL-7 (38, 39), the extent of IL-7-driven proliferation among specific CD8+TM in our experiments was unexpected (Fig. 3 A–C). In contrast, IL-2 administration promoted only a small although significant acceleration of CD8+TM turnover. Simultaneous analysis of CD44hiCD8+T cells confirmed previous findings for IL-15 and IL-2 treatment (15) and illustrates that memory-phenotype CD8+T cell analysis for cytokine-driven T cell proliferation, including IL-7, appropriately reflects the responsiveness of specific CD8+TM (Fig. 3A–C). Proliferation of CD4+TM was also increased by IL-7 and IL-15 treatment, however with three important distinctions: (i) Enhanced proliferation of specific CD4+TM was less pronounced than in the specific CD8+ compartment and (ii) not readily discernible in the memory-phenotype CD4+ population, and (iii), in the absence of exogenous cytokine administration, CD44hiCD4+T cells exhibited higher proliferation rates as compared with CD44hiCD8+T cells and specific CD4+ or CD8+TM.
Fig. 3.
Cytokine-driven proliferation of TM. (A) Memory mice (6–9 weeks after infection) were injected with PBS (control) or 2 μg of recombinant murine IL-2, IL-7, or IL-15 and pulsed with BrdUrd for 3 d. Plots are gated on CD8+ (Upper)orCD4+ (Lower) T cells. Virus-specific TM are identified by IFN-γ stains. Additional controls included mice injected with mIL-17, which displayed BrdUrd uptake identical to PBS control mice. Boxed numbers indicate frequencies of GP33-specifc CD8+ and GP61-specific CD4+TM. Bar diagrams display absolute numbers of specific TM in spleen. Values indicate the factor by which cytokine administration increased specific TM numbers. No significant changes were noted after IL-2 administration. (B) Cytokine-driven proliferation of memory-phenotype (CD44hi) and specific TM as induced by i.v. administration of 2 μg of IL-2, IL-7, or IL-15. P values were calculated in relation to PBS-injected control mice. (C) The “factor increased turnover” after cytokine injection was calculated only for specific and memory-phenotype (CD44hi)TM populations that demonstrated significantly altered proliferation and is compared to proliferation in control mice (PBS injected, given a value of 1). Data in B and C are combined from four separate experiments evaluating three to nine mice total.
IL-7 and IL-15 treatment furthermore increased specific TM frequencies and absolute numbers, demonstrating that these cytokines can also induce productive proliferation (Fig. 3A). In contrast, IL-2 given at the same dosage was not associated with enhanced proliferation or significant changes in specific CD4+TM frequencies or numbers. We also evaluated the in vivo impact of TSLP [not previously associated with T cell proliferation but included because of its usage of the IL-7Rα subunit (40)] and IL-21, a novel γc-cytokine similar in domain organization and primary sequence to IL-2 and IL-15. Although IL-21 can enhance the proliferative effects of IL-2, IL-7, or IL-15 on peripheral T cells (41), it also antagonizes the effects of IL-15 on memory-phenotype CD8+T cells in vitro (42). In our experiments, neither TSLP nor IL-21 promoted discernible changes of TM turnover (Fig. 3C), indicating that their significance for TM homeostasis in vivo, if any, is more limited.
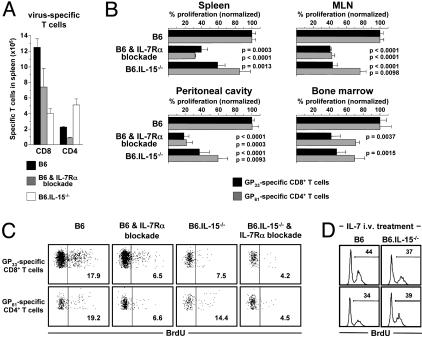
Regulation of Basal Homeostatic Proliferation of Specific TM by IL-7 and IL-15. To assess whether IL-7 regulates TM homeostasis under physiological conditions, we used IL-7Rα-blockade in LCMV-immune mice because reduced cellularity and altered homeostatic conditions in IL-7Rα-/- and IL-7-/- mice (43, 44) prevented their usage in our study. Treatment with the IL-7Rα-blocking antibody A7R34 decreased numbers of both virus-specific CD8+ and CD4+TM populations (Fig. 4A). Concomitant analyses of LCMV-immune IL-15-/- mice confirmed previous reports about reduced specific CD8+TM counts in the spleen (16, 17) and revealed that numbers of specific CD4+TM generated in the absence of IL-15 were in fact increased (Fig. 4A), resulting in part from an enhanced primary CD4+T cell response (data not shown). Similar results were obtained in other organs tested (data not shown).
Fig. 4.
Regulation of TM proliferation by IL-7 and IL-15. (A) Enumeration of GP33-specific CD8+ and GP61-specific CD4+TM (≈9 weeks after LCMV) in spleens of control mice, mice receiving four separate i.v. injections of 450 μg of IL-7Rα-blocking antibody over a period of 7 d, and IL-15-/- mice. Shown are the representative data from one of four similar experiments, with error bars indicating SEM (n = three mice per group per experiment). (B) Basal homeostatic proliferation of GP33-specific CD8+ and GP61-specific CD4+TM in spleen, MLN, peritoneal cavity, and bone marrow in control mice and under conditions of IL-7Rα-blockade (4 × 450 μg of IL-7Rα antibody i.v.) or IL-15-deficiency (7-d BrdUrd pulse). To directly compare the relative impact of receptor blockade or cytokine deficiency, proliferation of respective TM populations in control mice (B6) was set to 100%; P values were calculated in comparison to respective TM populations in B6 control mice. Data are from one of two experiments (n = three mice per group); similar results were obtained by employing 3 × 300 μg of IL-7Rα antibody in two additional experiments. (C) Proliferation of GP33-specific CD8+ and GP61-specific CD4+TM under conditions of IL-7Rα-blockade (4 × 450 μg of IL-7Rα antibody i.v.) and/or IL-15-deficiency (7-d BrdUrd pulse). Values indicate the fraction of BrdUrd+ GP33- or GP61-specific TM. (D) Proliferation of GP33-specific CD8+ (Upper) and GP61-specific CD4+ (Lower)TM after treatment of B6 and B6.IL-15-/- mice (≈12 weeks after LCMV) with 2 μg of mIL-7 (3-d BrdUrd pulse). Consistent with the data displayed in B, proliferation of specific CD8+ but not CD4+TM was reduced in PBS-injected B6.IL-15-/- as compared with B6 control mice (data not shown).
Although the A7R34 antibody has been used in multiple studies of in vivo blockade, an obvious concern is the potential opsonization of T cells coated with the IL-7Rα antibody, an effect that cannot be fully controlled by injections of isotype antibodies. Thus, it becomes imperative to analyze the effect of IL-7Rα-blockade at the level of surviving TM. Here, IL-7Rα-blockade reduced basal homeostatic proliferation rates of specific CD4+ and CD8+TM by ≈60% in spleen and mesenteric lymph node (MLN) and ≈80% in the peritoneal cavity, indicating that IL-7 regulates homeostatic proliferation of TM in lymphatic and nonlymphatic tissues alike (Fig. 4B). These observations were confirmed by the finding that IL-7Rα-blockade resulted in a 2- to 3-fold reduction of Ki-67+ specific TM populations (data not shown). Interestingly, inhibition of proliferation was less pronounced in the bone marrow, suggesting that accelerated turnover of TM in this compartment depends on other factors that may include IFN-γ (data not shown).
Analysis of TM homeostasis in IL-15-/- mice confirmed reduced proliferation rates in the specific CD8+ compartment (16, 17) and demonstrated a modest, tissue-dependent proliferative inhibition of specific CD4+TM (Fig. 4B). Although CD4+TM are responsive to IL-15 (Fig. 3), they may rely on IL-15 activity only under homeostatic conditions associated with higher IL-15 concentrations. This hypothesis is supported by reports that transgenic IL-15 expression can enhance pathogen-specific CD4+T cell responses (45, 46) and our observation that IL-15-deficiency preferentially affects proliferation of specific CD4+TM in the peritoneal cavity, which contains ≈5-fold higher IL-15 mRNA levels as compared with the spleen (data not shown).
Further experiments indicated a synergistic effect of IL-7 and IL-15 function, because the most pronounced inhibition of proliferation among specific TM was observed in IL-15-/- mice treated with IL-7Rα-blockade (Fig. 4C). Because the observed effect was only modest, we tested the notion of IL-15-independent IL-7 activity by administration of IL-7 to IL-15-/- and control mice. The results displayed in Fig. 4D demonstrate that IL-7-induced proliferation of specific TM is largely independent of endogenous IL-15. Thus, IL-7 dominates the regulation of basal homeostatic proliferation in the specific CD4+TM compartment, whereas both IL-7 and IL-15 function independently to control homeostatic turnover in the specific CD8+TM compartment.
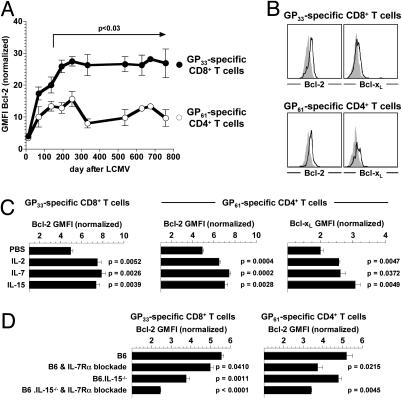
Regulation of Specific TM Survival by IL-7 and IL-15. In addition to their effect on T cell proliferation, IL-7 and IL-15 can provide survival signals, in particular by induction of antiapoptotic members in the Bcl-2 family (10, 11). Bcl-2 expression was previously found to be increased in memory as compared with effector CD8+T cells (23, 47), and aging memory-phenotype CD8+T cells gradually up-regulated Bcl-2 levels (48). Although CD4+ memory-phenotype T cells did not conform to these dynamics (47, 48), we recently reported that specific CD4+TM do, in fact, up-regulate Bcl-2 expression, albeit to a lesser degree than specific CD8+TM (7). We now demonstrate in an extended kinetic analysis that Bcl-2 expression by specific CD8+ and CD4+TM is progressively increased over an ≈8-month period after the peak of the primary response. The difference between Bcl-2 expression by specific CD4+ and CD8+TM was already apparent in early memory but attained statistical significance only ≈5 months after virus challenge (Fig. 5A). To evaluate the general capacity of γc-cytokines to induce Bcl-2 and Bcl-xL in specific TM, expression levels of these proteins were quantified after a 24-h in vitro culture in the presence of IL-2, IL-7, or IL-15. All cytokines promoted a modest yet significant increase of Bcl-2 and Bcl-xL in both virus-specific CD8+ and CD4+TM (Fig. 5 B and C), suggesting that the gradual increase of Bcl-2 levels in vivo (Fig. 5A) represents the cumulative result of long-term exposure to physiological cytokine concentrations during the extended maturation of established T cell memory. When tested in vivo, IL-7Rα-blockade reduced Bcl-2 levels predominantly in specific CD4+TM, whereas combined IL-7Rα-blockade/IL-15-deficiency was most effective in lowering Bcl-2 in specific CD8+TM (Fig. 5D). Thus, the impact of IL-7Rα-blockade and/or IL-15-deficiency on ex vivo Bcl-2 expression by specific TM mirrors their biological effect on basal homeostatic proliferation (Fig. 4 B and C).
Fig. 5.
Regulation of TM survival by IL-7 and IL-15. (A) Bcl-2 expression by specific CD8+ and CD4+TM in peripheral blood as a function of time after virus challenge. Bcl-2 expression was normalized as indicated in the legend to Fig. 1C. (B) Up-regulation of Bcl-2 and Bcl-xL expression by specific CD8+ and CD4+TM (≈9 weeks after LCMV challenge) after 24 h in vitro treatment with 100 ng/ml mIL-7. Black traces represent data for mIL-7, and gray histograms represent data for PBS. (C) Summary of in vitro cytokine-induced Bcl-2 and Bcl-xL up-regulation by specific TM (100 ng/ml, 24-h stimulation); P values were calculated in comparison to respective TM populations in PBS-treated control cultures. (D) Ex vivo Bcl-2 expression by virus-specific TM 7 weeks after LCMV infection and under conditions of IL-7Rα-blockade (4 × 450 μg of antibody i.v. over 7 d) and/or IL-15-deficiency. Significant differences in Bcl-2 expression were also observed after administration of 4 × 300 μg of antibody (data not shown). Experiments (n = three mice per group) were performed four times; P values were calculated in comparison to respective TM populations from PBS-injected or uninjected control mice (B6).
Regulation of Memory-Phenotype T Cell Homeostasis. To further evaluate the extent to which regulation is comparable between specific and memory-phenotype TM, we enumerated memoryphenotype CD8+T cells under conditions of IL-7Rα-blockade and/or IL-15-deficiency. In agreement with previous studies (13, 22, 49, 50) we found that these conditions reduced numbers of peripheral CD44hiCD8+T cells and observed in addition a pattern of decreased Bcl-2 expression commensurate to specific CD8+TM (Fig. 7 A and C). Interestingly, diverging results were obtained in regards to basal homeostatic proliferation as IL-7Rα-blockade but not IL-15-deficiency reduced turnover of CD44hiCD8+T cells to some extent (Fig. 7B). Together with the comparable cytokine-responsiveness of memory-phenotype and specific CD8+TM (Fig. 3 B and C), and with the notable exception of basal homeostatic turnover of CD44hiCD8+T cells generated in an IL-15-/- environment (50), analysis of memory-phenotype CD8+T cells thus provides a good approximation of the cytokine-dependent homeostatic characteristics of specific CD8+TM.
However, several differences emerged in comparisons of specific and memory-phenotype CD4+T cells. We observed an expected decrease of CD44hiCD4+T cell numbers after IL-7Rα-blockade (29, 30), but this effect was not associated with reduced Bcl-2 levels among surviving memory-phenotype CD4+T cells, and basal homeostatic proliferation rates remained unaffected by IL-7Rα-blockade or administration of IL-7. Similarly, IL-15-deficiency also had no impact on Bcl-2 expression or proliferation of CD44hiCD4+T cells (Fig. 7 A–C). Because it appears implausible that physical loss of memory-phenotype CD4+T cells after treatment with the nondepleting A7R34 antibody occurs without preceding functional impairments, an explanation for these findings may come from the heterogeneous composition of the CD44hiCD4+T cell compartment. In fact, our data show that memory-phenotype CD4+T cells proliferate faster and express lower Bcl-2 levels as compared with specific CD4+TM (Fig. 7 B and C). Therefore, functional attributes of IL-7-dependent subpopulations (e.g., antiviral CD4+TM), such as enhanced proliferation after IL-7 administration or reduced proliferation and Bcl-2 expression after IL-7Rα-blockade, may be masked by faster proliferation and low Bcl-2 expression among IL-7-independent populations in the CD44hi compartment. The precise nature of the putative IL-7-independent populations remains unclear but may include effector-type cells reactive to ubiquitous environmental antigens in gut flora or nutrients. This hypothesis is supported by the recent observation that ablation of T cell receptor-mediated signals substantially reduces homeostatic proliferation of memory-phenotype CD4+T cells (30). Although we have ruled out that activated effector or regulatory T cells expressing CD69 or CD25 contribute to the accelerated proliferation in the CD44hi compartment (data not shown), it should be noted that these markers are quickly down-regulated in later stages of primary CD4+ effector responses (7). It appears, therefore, that memory-phenotype CD4+T cells exhibit differential reliance on cytokines depending on the context of their generation.
Conclusion. Our study demonstrates that the regulation of antiviral CD4+ and CD8+TM homeostasis is remarkably similar and relies on the same antigen-independent factors but exhibits definable quantitative rather than qualitative differences: IL-7 controls basal homeostatic proliferation and survival of specific CD4+TM and performs nonredundant functions that regulate, in addition to survival (23), basal homeostatic proliferation of CD8+TM. These conclusions appear in partial contrast to recent reports that have documented a role for IL-7 in CD4+TM survival but not proliferation (28, 29). However, in the experimental systems used, T cell receptor-transgenic CD4+TM showed minimal or no proliferation, even in the presence of IL-7, indicating a crucial difference to the endogenously generated CD4+TM in our model.
Our results further suggest that IL-15 performs accessory functions in regulation of specific CD4+TM homeostasis, whereas the related cytokines IL-21 and TSLP do not contribute to basal homeostatic TM proliferation. Interestingly, memoryphenotype CD4+T cells, apparently because of their extensive heterogeneity, exhibit homeostatic characteristics that differ from specific CD4+TM. We therefore caution against the use of memory-phenotype CD4+T cells as convenient substitute for analyses of CD4+T cell memory. The challenging task for the immune system to preserve the diversity and specificity of T cell memory in an ever-changing environment (51) is likely regulated and supported by additional factors that may include other extracellular components and cellular interactions that jointly preserve the integrity and functionality of TM. Our results also suggest that IL-7, given its central role for both specific CD4+ and CD8+TM, may be particularly useful for immunotherapies aimed at embellishing complex T cell memory.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants AI09484, AI45927, and DK58541 (to M.B.A.O.) and CA38355, AI21487, AI46710, and AG01743 (to J.S.) and National Institutes of Health Training Grant 00080 (to D.H.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: LCMV, lymphocytic choriomeningitis virus; TM, memory T cell; MLN, mesenteric lymph node; GP, glycoprotein; NP, nucleoprotein; TSLP, thymic stromal lymphopoietin.
References
- 1.Sprent, J. & Surh, C. D. (2002) Annu. Rev. Immunol. 20, 551-579. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, R. & Gray, D. (1996) Science 272, 54-60. [DOI] [PubMed] [Google Scholar]
- 3.Zinkernagel, R. M. (2002) Curr. Opin. Immunol. 14, 523-536. [DOI] [PubMed] [Google Scholar]
- 4.Whitton, J. L. & Oldstone, M. B. (2001) in Field's Virology, eds. Fields, B., Howley, P. M., Griffin, D. E., Lamb, R. A., Martin, M. A., Roizman, B., Straus, S. E. & Knipe, D. (Lippincott, Philadelphia), pp. 285-320.
- 5.Beverley, P. C. & Maini, M. K. (2000) Philos. Trans. R. Soc. London B 355, 401-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitmire, J. K., Murali-Krishna, K., Altman, J. & Ahmed, R. (2000) Philos. Trans. R. Soc. London B 355, 373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homann, D., Teyton, L. & Oldstone, M. B. (2001) Nat. Med. 7, 913-919. [DOI] [PubMed] [Google Scholar]
- 8.Norris, P. J. & Rosenberg, E. S. (2002) J. Mol. Med. 80, 397-405. [DOI] [PubMed] [Google Scholar]
- 9.Kaech, S. M. & Ahmed, R. (2003) Science 300, 263-265. [DOI] [PubMed] [Google Scholar]
- 10.Jameson, S. C. (2002) Nat. Rev. Immunol. 2, 547-556. [DOI] [PubMed] [Google Scholar]
- 11.Schluns, K. S. & Lefrancois, L. (2003) Nat. Rev. Immunol. 3, 269-279. [DOI] [PubMed] [Google Scholar]
- 12.Marrack, P., Bender, J., Hildeman, D., Jordan, M., Mitchell, T., Murakami, M., Sakamoto, A., Schaefer, B. C., Swanson, B. & Kappler, J. (2000) Nat. Immunol. 1, 107-111. [DOI] [PubMed] [Google Scholar]
- 13.Goldrath, A.W., Sivakumar, P.V., Glaccum, M., Kennedy, M. K., Bevan, M. J., Benoist, C., Mathis, D. & Butz, E. A. (2002) J. Exp. Med. 195, 1515-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprent, J., Zhang, X., Sun, S. & Tough, D. (2000) Philos. Trans. R. Soc. London B 355, 317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang, X., Sun, S., Hwang, I., Tough, D. F. & Sprent, J. (1998) Immunity 8, 591-599. [DOI] [PubMed] [Google Scholar]
- 16.Schluns, K. S., Williams, K., Ma, A., Zheng, X. X. & Lefrancois, L. (2002) J. Immunol. 168, 4827-4831. [DOI] [PubMed] [Google Scholar]
- 17.Becker, T. C., Wherry, E. J., Boone, D., Murali-Krishna, K., Antia, R., Ma, A. & Ahmed, R. (2002) J. Exp. Med. 195, 1541-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan, J. T., Ernst, B., Kieper, W. C., LeRoy, E., Sprent, J. & Surh, C. D. (2002) J. Exp. Med. 195, 1523-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yajima, T., Nishimura, H., Ishimitsu, R., Watase, T., Busch, D. H., Pamer, E. G., Kuwano, H. & Yoshikai, Y. (2002) J. Immunol. 168, 1198-1203. [DOI] [PubMed] [Google Scholar]
- 20.Ku, C. C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. (2000) Science 288, 675-678. [DOI] [PubMed] [Google Scholar]
- 21.Berard, M., Brandt, K., Paus, S. B. & Tough, D. F. (2003) J. Immunol. 170, 5018-5026. [DOI] [PubMed] [Google Scholar]
- 22.Kieper, W. C., Tan, J. T., Bondi-Boyd, B., Gapin, L., Sprent, J., Ceredig, R. & Surh, C. D. (2002) J. Exp. Med. 195, 1533-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schluns, K. S., Kieper, W. C., Jameson, S. C. & Lefrancois, L. (2000) Nat. Immunol. 1, 426-432. [DOI] [PubMed] [Google Scholar]
- 24.Blattman, J. N., Grayson, J. M., Wherry, E. J., Kaech, S. M., Smith, K. A. & Ahmed, R. (2003) Nat. Med. 9, 540-547. [DOI] [PubMed] [Google Scholar]
- 25.Murakami, M., Sakamoto, A., Bender, J., Kappler, J. & Marrack, P. (2002) Proc. Natl. Acad. Sci. USA 99, 8832-8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geginat, J., Sallusto, F. & Lanzavecchia, A. (2001) J. Exp. Med. 194, 1711-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lantz, O., Grandjean, I., Matzinger, P. & Di Santo, J. P. (2000) Nat. Immunol. 1, 54-58. [DOI] [PubMed] [Google Scholar]
- 28.Li, J., Huston, G. & Swain, S. L. (2003) J. Exp. Med. 198, 1807-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondrack, R. M., Harbertson, J., Tan, J. T., McBreen, M. E., Surh, C. D. & Bradley, L. M. (2003) J. Exp. Med. 198, 1797-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seddon, B., Tomlinson, P. & Zamoyska, R. (2003) Nat. Immunol. 4, 680-686. [DOI] [PubMed] [Google Scholar]
- 31.Sudo, T., Nishikawa, S., Ohno, N., Akiyama, N., Tamakoshi, M. & Yoshida, H. (1993) Proc. Natl. Acad. Sci. USA 90, 9125-9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoenberger, S. P., Toes, R. E., van der Voort, E. I., Offringa, R. & Melief, C. J. (1998) Nature 393, 480-483. [DOI] [PubMed] [Google Scholar]
- 33.Murali-Krishna, K., Lau, L. L., Sambhara, S., Lemonnier, F., Altman, J. & Ahmed, R. (1999) Science 286, 1377-1381. [DOI] [PubMed] [Google Scholar]
- 34.Lau, L. L., Jamieson, B. D., Somasundaram, T. & Ahmed, R. (1994) Nature 369, 648-652.7516038 [Google Scholar]
- 35.Oxenius, A., Bachmann, M. F., Ashton-Rickardt, P. G., Tonegawa, S., Zinkernagel, R. M. & Hengartner, H. (1995) Eur. J. Immunol. 25, 3402-3411. [DOI] [PubMed] [Google Scholar]
- 36.Swain, S. L., Hu, H. & Huston, G. (1999) Science 286, 1381-1383. [DOI] [PubMed] [Google Scholar]
- 37.Wherry, E. J., Teichgraber, V., Becker, T. C., Masopust, D., Kaech, S. M., Antia, R., von Andrian, U. H. & Ahmed, R. (2003) Nat. Immunol. 4, 225-234. [DOI] [PubMed] [Google Scholar]
- 38.Geiselhart, L. A., Humphries, C. A., Gregorio, T. A., Mou, S., Subleski, J. & Komschlies, K. L. (2001) J. Immunol. 166, 3019-3027. [DOI] [PubMed] [Google Scholar]
- 39.Fry, T. J., Moniuszko, M., Creekmore, S., Donohue, S. J., Douek, D. C., Giardina, S., Hecht, T. T., Hill, B. J., Komschlies, K., Tomaszewski, J., et al. (2003) Blood 101, 2294-2299. [DOI] [PubMed] [Google Scholar]
- 40.Pandey, A., Ozaki, K., Baumann, H., Levin, S. D., Puel, A., Farr, A. G., Ziegler, S. F., Leonard, W. J. & Lodish, H. F. (2000) Nat. Immunol. 1, 59-64. [DOI] [PubMed] [Google Scholar]
- 41.Parrish-Novak, J., Dillon, S. R., Nelso, A., Hammond, A., Sprecher, C., Gross, J. A., Johnston, J., Madden, K., Xu, W., West, J., et al. (2000) Nature 408, 57-63. [DOI] [PubMed] [Google Scholar]
- 42.Kasaian, M. T., Whitters, M. J., Carter, L. L., Lowe, L. D., Jussif, J. M., Deng, B., Johnson, K. A., Witek, J. S., Senices, M., Konz, R. F., et al. (2002) Immunity 16, 559-569. [DOI] [PubMed] [Google Scholar]
- 43.Peschon, J. J., Morrissey, P. J., Grabstein, K. H., Ramsdell, F. J., Maraskovsky, E., Gliniak, B. C., Park, L. S., Ziegler, S. F., Williams, D. E., Ware, C. B., et al. (1994) J. Exp. Med. 180, 1955-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Freeden-Jeffry, U., Vieira, P., Lucian, L. A., McNeil, T., Burdach, S. E. & Murray, R. (1995) J. Exp. Med. 181, 1519-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimura, H., Yajima, T., Naiki, Y., Tsunobuchi, H., Umemura, M., Itano, K., Matsuguchi, T., Suzuki, M., Ohashi, P. S. & Yoshikai, Y. (2000) J. Exp. Med. 191, 157-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yajima, T., Nishimura, H., Ishimitsu, R., Yamamura, K., Watase, T., Busch, D. H., Pamer, E. G., Kuwano, H. & Yoshikai, Y. (2001) Eur. J. Immunol. 31, 757-766. [DOI] [PubMed] [Google Scholar]
- 47.Grayson, J. M., Zajac, A. J., Altman, J. D. & Ahmed, R. (2000) J. Immunol. 164, 3950-3954. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, X., Fujii, H., Kishimoto, H., LeRoy, E., Surh, C. D. & Sprent, J. (2002) J. Exp. Med. 195, 283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kennedy, M. K., Glaccum, M., Brown, S. N., Butz, E. A., Viney, J. L., Embers, M., Matsuki, N., Charrier, K., Sedger, L., Willis, C. R., et al. (2000) J. Exp. Med. 191, 771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Judge, A.D., Zhang, X., Fujii, H., Surh, C. D. & Sprent, J. (2002) J. Exp. Med. 196, 935-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welsh, R. M. & Selin, L. K. (2002) Nat. Rev. Immunol. 2, 417-426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.