Abstract
Calcium-binding synaptotagmins (Syts) are membrane proteins that are conserved from nematode to human. Fifteen Syts (Syts I–XV) have been identified in mammalian species. Syt I has been well studied and is a candidate for the Ca2+-sensor that triggers evoked exocytosis underlying fast synaptic transmission. Whereas the functions of the other Syts are unclear, Syt IV is of particular interest because it is rapidly up-regulated after chronic depolarization or seizures, and because null mutations exhibit deficits in fine motor coordination and hippocampus-dependent memory. Screening Syts I–XIII, which are enriched in brain, we find that Syt IV is located in processes of astroglia in situ. Reduction of Syt IV in astrocytes by RNA interference decreases Ca2+-dependent glutamate release, a gliotransmission pathway that regulates synaptic transmission. Mutants of the C2B domain, the only putative Ca2+-binding domain in Syt IV, act in a dominant-negative fashion over Ca2+-regulated glial glutamate release, but not gliotransmission induced by changes in osmolarity. Because we find that Syt IV is expressed predominantly by astrocytes and is not in the presynaptic terminals of the hippocampus, and because Syt IV knockout mice exhibit hippocampal-based memory deficits, our data raise the intriguing possibility that Syt IV-mediated gliotransmission contributes to hippocampal-based memory.
Considerable evidence supports the notion that astrocytes are active signaling elements at tripartite synapses (1). In addition to removing transmitters from the extracellular space by the action of neurotransmitter transporters (2), astrocytes, in response to elevated internal Ca2+, release gliotransmitters, which modulate neuronal actions (3–7). At least three distinct gliotransmitters can be released from astrocytes: glutamate, ATP, and d-serine. In the hippocampus, astrocyte-derived glutamate modulates γ-aminobutyric acid-mediated inhibitory synaptic transmission (8). At the neuromuscular junction, perisynaptic Schwann cells regulate nerve terminal acetylcholine release (9) through a mechanism consistent with glutamate as the messenger (10). ATP released from astrocytes, acting through adenosine, which accumulates after ATP degradation, can lead to a presynaptic inhibition of glutamate release from synaptic terminals (11, 12). Similarly, glial cell-derived ATP, acting through adenosine, suppresses neuronal excitability (13). Finally, astrocytes selectively express the enzyme serine racemase (14), which converts l-serine to d-serine. Induced release of d-serine facilitates synaptic transmission by binding to the glycine-binding site of the N-methyl-d-aspartate receptor (15, 16).
Although gliotransmitters regulate neuronal function, there is little information regarding how these transmitters are released. Various hypotheses have been proposed including the release of transmitters through Ca2+-dependent exocytosis, transporter reversal, anion channels, or gap junction hemichannels (17). Although more than one mechanism recruited under different physiological or pathological conditions likely mediates gliotransmitter release, recent evidence supports an exocytosis-mediated pathway for glutamate release from astrocytes (18). This evidence includes the findings that vesicular glutamate transporters are expressed within astrocytes in situ, that perturbation of N-ethylmaleimide-sensitive factor attachment protein receptor complex formation prevents astrocyte glutamate release, and that whole-cell capacitance measurements demonstrate that receptor-induced Ca2+ elevations lead to a clostridial toxin-sensitive fusion of vesicles with the plasma membrane, which is coincident with the release of glutamate (18, 19). Although this recent evidence supports a vesicle-mediated mechanism of Ca2+-evoked glutamate release from astrocytes, the protein target of action for elevated internal Ca2+ is undefined.
Calcium-binding synaptotagmins (Syts) are conserved from nematode to human (20). Fifteen Syts (Syts I-XV) have been identified in mammalian species (21–23). Syt I has been well studied and is a candidate for the Ca2+ sensor that triggers evoked exocytosis (24, 25), underlying fast synaptic transmission. We therefore asked which Syts are expressed by astrocytes and whether they play a critical role in the regulation of glutamate release from astrocytes. We demonstrate that Syt IV is expressed in astrocytes and that the expression of mutant forms of Syt IV act as dominant-negative inhibitors of Ca2+-evoked glial glutamate release. We propose that Syt IV is essential for the regulated release of glutamate from astrocytes and is a candidate Ca2+ sensor for gliotransmission by these nonneuronal cells.
Methods
Cell Culture. Animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. Cultures of purified murine astrocytes and mixed cultures of astrocytes and neurons were generated as described (26). Cortices were dissected from 0- to 2-day-old postnatal mice, treated with papain (20 units/ml for 20 min at room temperature), triturated in minimum essential medium, and transferred into 25-cm2 flasks. They were grown to confluence at 37°C in a humidified 5% CO2 atmosphere. After 7–10 days, flasks were washed with cold Earle's balanced salt solution and fed with cold modified minimum essential medium before shaking at 260 rpm (ROSI 1000, Thermolyne reciprocating orbital shaking incubator). Remaining adherent cells were dissociated by using trypsin (0.1%), and were pelleted (100 × g for 10 min), resuspended, and plated onto coverslips. Cells were used after 2–4 days in culture.
Acute Isolation of Cells. As described (18), 10- to 14-day old pGFAP-GFP mice (The Jackson Laboratory) were used to obtain hippocampal slices. After 30-min treatment with protease IV, tissues were dissociated and plated onto poly-l-lysine-coated coverslips for immunostaining or single-cell multiplex RT-PCR.
Multiplex RT-PCR. Total RNA was extracted from whole brain or from astrocyte cultures, or RNA was collected from single cells and was subjected to two-step RT-PCR as described (18), with primers listed in Table 1, which is published as supporting information on the PNAS web site. All PCR products were examined by using DNA electrophoresis and were sequenced to confirm identity.
Western Blotting and Immunocytochemistry. Rabbit polyclonal antibodies against Syts I–XIII were used in Western blots (27, 28). Specificity of reactivity was confirmed by testing each antibody against recombinant Syts I–XIII (Fig. 6, which is published as supporting information on the PNAS web site). Specificity was corroborated by the observation that treatment with Syt IV- and V-specific small interfering (si)RNA abolished immunolabeling by the respective antibody in immunofluorescence studies of cultured astrocytes. Western blots and immunocytochemistry were performed by using standard approaches (18, 22). Confocal images were obtained by using a three-channel confocal microscope (Prairie Technologies, Middleton, WI).
Immunoelectron Microscopy. Male Sprague–Dawley rats (Harlan, Indianapolis; 200–250 g) were used for immunoelectron microscopy. Standard reactions were performed as described (18). Immunoperoxidase was used to detect either anti-GFAP or anti-S100β, and silver-enhanced gold was used to detect anti-Syt IV and anti-Syt I reactivity.
RNA Interference. Syt IV-siRNA and scrambled siRNA were chemically synthesized and purified by HPLC (Qiagen, Valencia, CA). Syt IV-siRNA: sense 5′-r(GAAGCACAGAGUGAAGACCA)d(TT)-3′ and antisense 5′-r(UGGUCUUCACUCUGUGCUUC)d(TT)-3′. Syt V-siRNA: sense 5′-r(CGAGCAGGAGA ACAGCGAG)d(T T)-3′ and antisense 5′-r(CUCGCUGUUCUCCUGCUCG)d(TT)-3′. Transfections of siRNAs were carried out with a TransMessenger transfection kit (Qiagen) and RNA concentration was 3.2 μg/ml during a 4-h transfection period.
Mutagenesis. Mutations were generated by using a QuikChange site-directed mutagenesis kit (Stratagene). The following sets of primers were used: (i) SS-Syt IV, 5′-ACAATCTTACCAGAGAAAAAACATCGGGTCAAAACAAGAGTGCTCAGGAAGACGCTGGACCC-3′ and 5′-TCTTCCTGAGCACTCTTGTTTTGACCCGATGTTTTTTCTCTGGTAAGATTGTCATTTTGATGTACG-3′; (ii) B-D1,2N, 5′-CTACCGA A ATCTA ACGTGTCTGGACT T TCA A ACCCCTACGTCAAAGTGAACC-3′ and 5′-TTTGACGTAGGGGTTTGAAAGTCCAGACACGTTAGATTTCGGTAGGTGCCGC-3′; and (iii) B-D3N,E4Q, 5′-GAATTCTTGGTTTTGAACTCTCAGAGGGGATCCCGAAATGAGG-3′ and 5′-CAT T TCGGGATCCCCTCTGAGAGT TCA A A ACCA AGAATTCAAC-3′.
Virus Preparation. To ensure that effects of transgenes are mediated by effects on astrocytes and are not due to other potential contaminating cell types, we used purified cultures of astrocytes and the avian leukosis virus to selectively deliver transgenes to astrocytes (18, 29). DF-1 cells (American Type Culture Collection, catalog no. CRL-12203), were propagated in modified minimum essential medium and were transfected with constructs to produce avian leukosis virus containing the desired genes. Virus-containing medium was collected, filtered, and directly applied onto cultures of astrocytes obtained from Gtv-a mice, in which the GFAP promoter drives the expression of the avian leukosis virus receptor.
Extracellular Glutamate Measurement. An enzymatic assay was used to visualize glutamate released from purified cultured astrocytes (18). Purified cultures of astrocytes were found to be free of neuronal contamination by RT-PCR, Western blot, and immunocytochemistry, using probes for the neuronal markers synaptophysin and Syt I. With minor modifications discussed earlier (18), we obtained real-time images of extracellular glutamate change with a 2-s time resolution. Statistical significance was determined by ANOVA followed by Student's t test.
Results
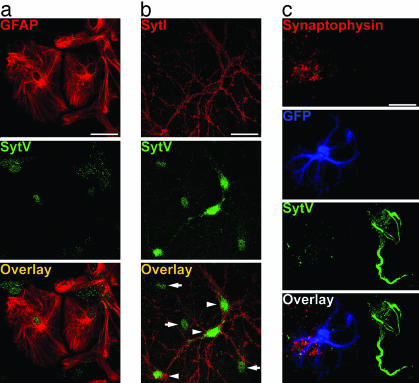
To determine which Syts are expressed by astrocytes, we first performed Western blots that detected the presence of Syts IV, V, and XI in cultured astrocytes (Fig. 1a, black arrowhead). Subsequent studies focused on Syts IV and V, because anti-Syt XI recognized multiple bands on Western blots (Fig. 1a, gray arrowheads). RT-PCR performed on RNA from whole brain and purified astrocyte cultures confirmed the results of Western blots by demonstrating the presence of Syts IV and V transcripts in astrocytes, but not Syt I or II (Fig. 1b). Immunofluorescence demonstrated Syt IV expression in a punctate pattern throughout cultured glial fibrillary acidic protein (GFAP)-positive astrocytes (Fig. 1c). In mixed cultures of astrocytes and neurons, Syt IV is expressed predominantly in astrocytes, although it is also detected in a perinuclear region of neurons, as reported (ref. 30 and Fig. 1d). Syt I is expressed only in neuronal processes, presumably presynaptic terminals (Fig. 1d). Syt V is expressed in a perinuclear region of astrocytes and in neuronal cell bodies and proximal processes (Fig. 2).
Fig. 1.
Expression of Syt IV in cultured astrocytes. (a) Western blots demonstrate the presence of Syts IV, V, and XI (black arrowheads) in cultured astrocytes (recombinant protein is shown in the left lane). Anti-Syt XI reacts with three bands, one of the appropriate molecular mass and two that are smaller (gray arrowheads). (b) RT-PCR on brain (Upper) and cultured astrocytes (Lower) shows that Syts IV and V, but not Syts I or II, transcripts were amplified from astrocyte cultures. (c) Double label of GFAP (red) and Syt IV (green) on pure astrocyte culture. (Bar, 10 μm.) (d) Double label of Syts I (red) and IV (green) on astrocyte-neuron coculture shows that Syt IV is expressed predominantly in astrocytes (white arrows), although some reactivity in neuronal somata is seen in cultures (arrowheads). (Bar, 20 μm.)
Fig. 2.
Syt V is predominantly expressed in neuronal cell bodies. (a) Double labeling of cultured astrocytes with anti-GFAP (red) and anti-Syt V (green) shows Syt V localization to the cell body region of cultured astrocytes. (b) Double labeling of mixed cultures of astrocytes and neurons with anti-Syt I (red) and Syt V (green) shows that Syt V is expressed predominantly in neuronal cell bodies (white arrowheads). (c) Labeling of an FIA from pGFAP-GFP mice shows the virtual absence of Syt V (green) in GFP-containing astrocytes (blue). Attached presynaptic nerve terminals (red; synaptophysin) are also negative for Syt V. In contrast, an adjacent GFP-negative FIN cell body and primary dendrites exhibit Syt V reactivity. (Bar, 20 μm.)
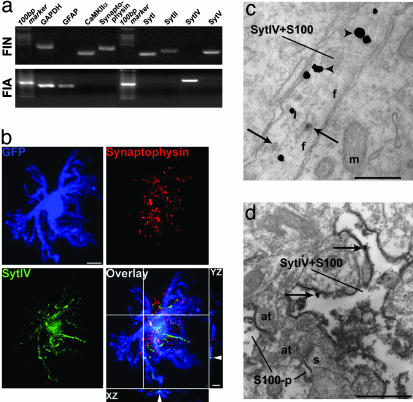
Because the expression of syt4 is regulated by seizures (31) and chronic depolarization (32), we examined the expression of this gene in situ to determine localization in unperturbed tissue. Syt IV immunoreactivity was sporadically detected within neuron cell bodies in sections of hippocampus, although clearly identified cells were observed within the dentate gyrus (Fig. 7, which is published as supporting information on the PNAS web site). Immunoreactivity for syt4 was diffuse in other parts of the hippocampal formation and could not be localized to specific cellular structures by using light microscopy. To ask whether this outcome represented neuronal or glial localization, we used individual identified cells freshly isolated from living hippocampi. Within 45 min after killing animals, cells were isolated, at which point they maintained their in vivo properties (33). By using pGFAP-GFP-transgenic mice in which the human GFAP promoter drives the expression of GFP selectively in astrocytes (ref. 34, and Fig. 8, which is published as supporting information on the PNAS web site) to assist in cell identification, individual freshly isolated astrocytes (FIAs) and freshly isolated neurons (FINs) were aspirated into a micropipette and subjected to single-cell multiplex RT-PCR (Table 1). Sample purity was confirmed by the amplification of GFAP transcript from astrocytes only (n = 12), and amplification of Ca2+/calmodulin-dependent protein kinase II α subunit (CaMKIIα) and synaptophysin transcripts from isolated neurons only (n = 9; Fig. 3a), as determined (18). Simultaneously, we asked whether we could detect transcript for Syts. In accordance with previous evidence, we amplified Syt I and II transcripts from FINs only (n = 9). These transcripts were never detected in astrocytes (n = 12; Fig. 3a). In contrast, Syt IV transcript was reliably detected in astrocytes, but was never detected in FINs (Table 2, which is published as supporting information on the PNAS web site, and Fig. 3a). These results were confirmed by using multiplex PCR after whole-cell recordings made from area CA1 pyramidal neurons and astrocytes in brain slice preparations (Table 3, which is published as supporting information on the PNAS web site). Immunofluorescence studies on freshly isolated cells confirmed these results. Syt IV was present within GFP-containing processes of FIAs, but not in the associated nerve terminals that remain attached to the isolated astrocyte and are disclosed by synaptophysin immunostaining (Fig. 3b). Syt IV was present in GFP-containing astrocytic processes of 82.4% FIAs (n = 17) but was absent in FINs (n = 11), whereas Syt V was present in 93.3% FINs (n = 15) and only weak and mostly nuclear-located signals were found in 66.7% FIAs (n = 12; Fig. 2c). This initial evaluation supports the notion that Syt IV is expressed in astrocytes with sparse neuronal expression in situ.
Fig. 3.
Selective expression of Syt IV in astrocytes in situ. (a) Single-cell multiplex RT-PCR of FIAs and FINs demonstrates selective detection of Syt IV transcripts in astrocytes (500-bp ladder). Cell identities were confirmed by GAPDH, GFAP, CaMKIIα, and synaptophysin. (b) Triple-label immunostaining of an FIA shows the localization of Syt IV (green) is mainly in GFP (blue)-containing astrocytic process. XZ and YZ sections at the crosshair area confirmed the presence of Syt IV within the GFP-labeled processes. (Bar, 5 μm.) (c) Electron micrograph showing immunoperoxidase labeling for Syt IV (arrows) in a glial profile containing ImmunoGold-silver labeling for S100β (arrowheads) in the rat hippocampal formation. (Bar, 0.25 μm.) (d) Reverse labeling of the antigens where peroxidase labeling was used to identify S100β and gold-silver labeling for was used to identify Syt IV (arrows). Irrespective of the labeling protocol used, Syt IV was identified in glial processes (Bar, 0.7 μm.) In d, glial processes exhibiting Syt IV in the neuropil are adjacent to axon terminals that form synapses with spinous processes.
Based on the localization of Syt IV within astrocytic processes. we subsequently focused on this protein. By using silver-enhanced gold and anti-Syt IV and anti-S-100β and peroxidase labeling to disclose astrocytic processes (as well as reverse labeling of antigens), we found that Syt IV was located in 22% of the S-100β-positive processes of astrocytes (n = 265). Reactivity was never seen in presynaptic terminals (Fig. 3 c and d). By using a similar double-labeling method, we confirmed that Syt I was seen only in presynaptic terminals, but never in astrocytic processes (Fig. 9, which is published as supporting information on the PNAS web site). Together, the results of these single-cell RT-PCR, immunofluorescence, and immunoelectron microscopy studies demonstrate that syt4 is expressed predominantly by hippocampal astrocytes and is not localized in presynaptic terminals in situ.
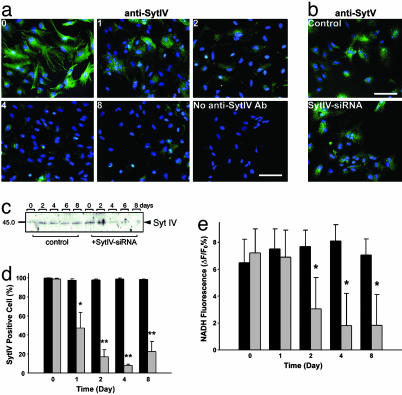
Because Syt IV can functionally replace Syt I in Ca2+-dependent exocytosis (35–37), we asked whether Syt IV is necessary for Ca2+-evoked glutamate release from cultured astrocytes. By using siRNA, which can silence gene expression in a sequence-selective manner (38), we reduced the expression of Syt IV in astrocytes (Fig. 4 a–d) with a maximal reduction after 4 days without obvious effects on the expression of other proteins involved in the regulation of transmitter release (Fig. 4b, and Fig. 10, which is published as supporting information on the PNAS web site). Scrambled siRNA had no effect on Syt IV expression.
Fig. 4.
siRNA knockdown of Syt IV leads to a reduction of astrocytic glutamate release. (a) Immunolabeling of Syt IV (green) by anti-Syt IV antibody at 0–8 days after transfection of siRNA against Syt IV. Expression of Syt IV in most astrocytes is reduced to background level comparable with control staining without anti-Syt IV antibody (blue is nuclear labeling by Hoechst 33342; Bar, 50 μm). Note that the bright spots are due to nonspecific labeling of debris. (b) No significant change can be detected by immunostaining with anti-Syt V 4 days after Syt IV-siRNA treatment (blue is nuclear labeling by Hoechst 33342; Bar, 50 μm). (c) Western blots of protein isolated from astrocyte cultures after 0–8 days of treatment with Syt IV-siRNA or with scrambled RNA (control) confirm that Syt IV-siRNA greatly reduces the expression of Syt IV. (d) Percentage of cells with detectable Syt IV immunoreactivity after treatment with Syt IV-siRNA (gray histograms) and scrambled siRNA (black histograms) (*, P < 0.05; **, P < 0.01). (e) Glutamate release induced by 20 μM ATP is significantly decreased from 2 days after Syt IV-siRNA application (gray bar) compared with scrambled siRNA (black bar; P < 0.01). Data shown in d and e were collected from 18 trials obtained from two independent sets of cultures.
By using a real-time optical assay (18) in which the extracellular accumulation of fluorescent NADH indicates that glutamate has been released from astrocytes, we asked whether Syt IV is essential for Ca2+-dependent gliotransmission. Application of 20 μM ATP, which, by acting through P2Y receptors, leads to the release of Ca2+ from inositol trisphosphate-sensitive internal stores, stimulated an increase of NADH fluorescence (Fig. 4e), requiring the presence of both the enzyme glutamate dehydrogenase and the cofactor β-NAD+ (data not shown), demonstrating that this Ca2+-mobilizing stimulus evokes glutamate release (average concentration of 8 μM glutamate; Fig. 11, which is published as supporting information on the PNAS web site). Treatment of cultures with Syt IV siRNA exhibited decreased glutamate release after 2 or more days of treatment, with no effects resulting from transfection with scrambled siRNA (Fig. 4e). Although Ca2+-evoked glutamate release was significantly depressed by Syt IV-siRNA treatment, this effect was selective; constitutive membrane recycling, assayed by FM4–64 uptake, was unaffected (data not shown).
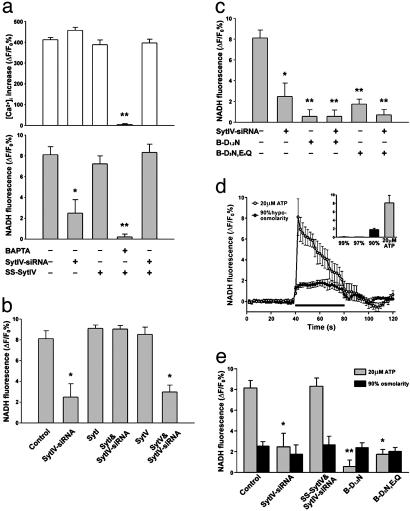
Because siRNA-induced RNA interference in mammalian systems may induce off-target effects (39) and IFN response (40), we asked whether Ca2+-dependent glutamate release can be recovered by reintroducing Syt IV into astrocytes. We generated a FLAG-tagged Syt IV with an 8-bp same-sense mutation at the siRNA-targeting region, which maintained the wild-type Syt IV protein sequence (SS-Syt IV). To achieve persistent and specific expression in astrocytes, we used an astrocyte-specific gene delivery system (29). As predicted, the expression of SS-Syt IV (>95% of astrocytes) was unaffected by Syt IV-siRNA treatment (data not shown). SS-Syt IV-expressing astrocytes exhibit the same properties as uninfected controls; ATP induces Ca2+ elevations and glutamate release that can be blocked by loading astrocytes with the Ca2+ chelator, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (Fig. 5a). By using SS-Syt IV, we determined that reexpression of Syt IV in the presence of Syt IV-siRNA led to a full recovery of glutamate release (Fig. 5a). In agreement with previous studies (35–37) that demonstrate that Syts I and IV are functionally interchangeable, release was also recovered by expression of Syt I, but not by Syt V (Fig. 5b).
Fig. 5.
Syt IV is necessary for Ca2+-dependent glutamate release from astrocytes. (a) Expression of SS-Syt IV does not affect glutamate release or Ca2+ signaling induced by ATP (20 μM), but leads to a recovery of release when expressed in the presence of Syt IV-siRNA. Because the changes in glutamate release are independent of the amplitude of the Ca2+ signal, they support the notion that Syt IV is required downstream of the Ca2+ signal to control transmitter release (*, P < 0.05; **, P < 0.01). Data were collected from 18 (calcium imaging) and 12 (glutamate assays) trials obtained from two independent sets of cultures. (b) Syt I but not Syt V can rescue glutamate release after Syt IV-siRNA treatment (P < 0.01). (c) Mutations of amino acids homologous to Syt I C2B domain amino acids that coordinate Ca2+ binding (B-D1,2N and B-D3N,E4Q) lead to dominant-negative effects on astrocytic glutamate release (*, P < 0.05; *, P < 0.01). All modifications to release (except N,N,N′,N′-tetraacetate) are independent of changes in ATP-evoked Ca2+ mobilization. (d) Hypoosmotic saline induces the release of glutamate from astrocytes (black circles), which is comparable with ATP-evoked glutamate release (gray circles). The black horizontal bar represents the application of stimuli (ATP or hypoosmotic saline). (e) Syt IV-siRNA and Syt IV dominant-negative mutations significantly depress ATP-induced, Ca2+-dependent glutamate release (gray bar, *, P < 0.05; **, P < 0.01) but have no effect on hypoosmotic saline-induced glutamate release (black bar). Data shown in b–e were collected from 12 trials obtained from two independent cultures.
To further explore the role of Syt IV in mediating Ca2+-dependent glutamate release from astrocytes, we manipulated the C2B domain, a putative Ca2+-binding domain of Syt IV (41). Because of a conserved substitution of an aspartate residue with serine in the C2A domain, the Syt IV C2A domain does not bind Ca2+ (42). However, because Syt IV can substitute for Syt I in recovering Ca2+-evoked transmitter release from nerve terminals and because the Syt IV C2B domain is responsible for Ca2+-dependent homooligomerization (41), we made mutations to conserved amino acids in the Syt IV C2B domain that are essential for coordinating Ca2+ binding by Syt I and for Ca2+-evoked transmitter release (35, 36). As with Syt I, the first and the second aspartate residues were mutated to asparagines (D318,324N, referred to henceforth as B-D1,2N), or the third aspartate and the fourth glutamate residues were mutated to asparagine and glutamine, respectively (D378N,E380Q, referred to henceforth as B-D3N,E4Q). Expression of either the B-D1,2N or the B-D3N,E4Q mutant did not change ATP-stimulated [Ca2+]i elevation but did block glutamate release in a dominantnegative fashion (Fig. 5c).
Several pathways of transmitter release have been proposed for astrocytes (17). Changes in osmolarity evoke the release of glutamate from astrocytes (Fig. 5d) by a mechanism that is thought to involve anion transporters (43, 44). Because it is possible that ATP-stimulated Ca2+-dependent glutamate release may involve the exocytotic insertion into the plasma membrane of such transporters, we determined whether hypo-osmolarity-induced loss of this transmitter from astrocytes was affected by manipulations of Syt IV expression. Neither reductions in Syt IV expression through the action of siRNA nor the expression of dominant-negative Syt IV (Fig. 5e) affected the amount of glutamate released from astrocytes when the extracellular saline was reduced to 90% of its normal osmolarity. This result demonstrates the specificity of Syt IV action on distinct gliotransmitter release pathways and shows that osmolaritysensitive anion transmitter release pathways are distinct from the Syt IV-dependent pathway of Ca2+-induced glutamate release.
Discussion
Taken together, our data support the notion that Syt IV is essential for the regulated release of glutamate from astrocytes. We demonstrate that astrocytes in culture, freshly isolated from the nervous system, as well as in situ express Syt IV. That reductions in Syt IV expression lead to reduced glutamate release and that the expression of point mutants causes a dominant-negative inhibition of glutamate release indicate that this protein is required for the release of glutamate from astrocytes.
Because there is considerable evidence to support the notion that Syt I is the Ca2+ sensor for synaptic transmission (23, 45), and because Syt IV is structural homologous to Syt I, it is tempting to speculate that Syt IV is the equivalent Ca2+ sensor for the release of glutamate from astrocytes. However, it is important to understand that there are distinctions between the properties of these two Syts. Members of the Syt family contain two C2 domains, which, in some cases, including that of Syt I, have been shown to bind Ca2+. However, Syt IV is known to have different Ca2+-binding properties than those of Syt I. Syt IV has a single amino acid substitution in the C2A domain in one of the five amino acids known to be critical for coordinating Ca2+ binding. This substitution results in the Syt IV C2A domain being insensitive to Ca2+ (42). However, in contrast, the C2B domain of Syt IV mediates Ca2+-dependent homooligomerization (41), suggesting that although the C2A domain is Ca2+ insensitive, the whole protein does sense Ca2+ through the C2B domain. Supporting this notion is the fact that Syt IV can replace Syt I in mediating Ca2+-dependent fast synaptic transmission (35–37). Additionally mutations of the homologous amino acids in the Syt IV C2B domain that coordinate Ca2+ binding by Syt I lead to a block of glutamate release from astrocytes. Despite this evidence, it will still be important to directly demonstrate this Ca2+-binding activity and to show that these mutations prevent a Ca2+-sensing function for this protein. Nonetheless, these data collectively suggest that Syt IV is the Ca2+ sensor for glutamate release from astrocytes.
Previous studies (46, 47) employing Syt I knockout mice and Drosophila have shown that Syt I is a low-affinity Ca2+ sensor that is essential for fast synchronized transmitter release but not for the delayed asynchronous release of neurotransmitter. When synaptic release of transmitter is integrated over a 1-s time period, although fast release is significantly depressed, the total release is unchanged in Syt I knockout mice. Because Syt I can functional replace Syt IV in mediating glutamate release from astrocytes, and the temporal resolution of optical assay for glutamate is 2 s, it is surprising, therefore, that release is significantly suppressed by reducing Syt IV expression because we are measuring on a time scale that, at the synapse, detects the Syt I-insensitive asynchronous component of release. However, in contrast to synaptic release of neurotransmitter where the action potential synchronizes the opening of voltage-sensitive calcium channels for a period of a few milliseconds, the Ca2+ flux in astrocytes is due to the repeated and asynchronous opening of inositol trisphosphate receptors over several seconds. If these receptors are localized adjacent to release sites they could provide locally elevated calcium to activate a low-affinity Ca2+ sensor. Although the Kd for glutamate release is not known for astrocytes, the Kd for exocytosis from these cells is ≈20 μM (19). Thus, it is entirely feasible that the slow integrated release of transmitter detected with our assay is due to the summation of temporally asynchronous release events that individually are due to Ca2+ released through an inositol trisphosphate receptor locally associated with the release machinery to provide the Ca2+ elevation necessary to activate a low-affinity Ca2+ sensor.
In nerve terminals, the endocytotic retrieval of synaptic vesicles is regulated by Syt I. Thus, a loss of Syt I impairs endocytosis (48). We have been unable to monitor the regulated component of endocytosis to ask whether Syt IV is necessary for this retrieval process. Unlike the case for nerve terminals, there is a high level of constitutive membrane cycling in cultured astrocytes, resulting in significant uptake of FM4–64 in the absence of stimulation. This cycling results in a high background signal, making the study of endocytosis after glutamate release extremely difficult. Thus, although Syt IV is not necessary for constitutive membrane retrieval, we are unable, at this time, to determine whether it plays a role in endocytosis after regulated release of glutamate.
The predominant expression of Syt IV in astrocytes and its absence from nerve terminals is of great interest because hippocampal-based memory formation has been reported to be impaired in syt4 knockout mice (49). Because glutamate released from astrocytes has been shown to activate extrasynaptic N-methyl-d-aspartate receptors of hippocampal pyramidal neurons (56) it will be extremely interesting to determine whether this gliotransmission pathway is critical for the induction of processes such as long-term potentiation.
Although Syts I and IV appear to be interchangeable in terms of supporting fast synaptic transmission (35–37) as well as Ca2+-dependent glutamate release from astrocytes (Fig. 5b), it is worth noting that these two proteins have been reported to support different forms of transmitter release. By using PC-12 cells in which either Syt I or Syt IV was expressed, amperometric measurements of catecholamine release allowed the detection of two forms of transmitter release: kiss and run and full fusion release. Syt IV expression increased the probability of kiss-and-run release, whereas Syt I biased the system toward full fusion release. These two forms of release, which have been shown to occur in cultured hippocampal neurons (50, 51), provide the opportunity for different kinetics of transmission. In the kiss-and-run mode, where a vesicle forms an ephemeral fusion pore that rapidly releases transmitter, it would be expected that such transmitter-depleted vesicles could more rapidly become available to release transmitter again than could those that fully fuse with the plasma membrane; an expectation that is supported by recent data (50, 51). Although there is considerable evidence to support the concept of vesicle-mediated exocytosis of glutamate from these glial cells (18, 52–54), it is of concern that, in situ, there are fewer vesicles within their processes than in nerve terminals. Perhaps Syt IV-dependent transmission by glia provides these cells with a rapid recycling pool of vesicles so that fewer vesicles are required for gliotransmission than in Syt I-dependent transmission.
In addition to its role in transmitter release, Syt IV was identified as an immediate early gene that is up-regulated after seizures and through depolarization and elevations of cAMP (31, 32). In addition, because the human syt4 gene has been mapped to a locus associated with bipolar disorder and schizophrenia (55), it will be of great interest to determine whether deficits in gliotransmission contribute to psychiatric and seizure disorders as well as to impairments in memory formation.
Supplementary Material
Acknowledgments
We thank Dr. Marc Dichter and Margie Maronski for providing neuronal cultures. This work was supported by National Institutes of Health Grants RO1 NS43142 and R37 NS37585 (to P.G.H.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Syt, synaptotagmin; siRNA, small interfering RNA; GFAP, glial fibrillary acidic protein; FIA, freshly isolated astrocyte; FIN, freshly isolated neuron.
References
- 1.Araque, A., Parpura, V., Sanzgiri, R. P. & Haydon, P. G. (1999) Trends Neurosci. 22, 208-215. [DOI] [PubMed] [Google Scholar]
- 2.Bergles, D. E. & Jahr, C. E. (1997) Neuron 19, 1297-1308. [DOI] [PubMed] [Google Scholar]
- 3.Newman, E. A. (2003) Trends Neurosci. 26, 536-542. [DOI] [PubMed] [Google Scholar]
- 4.Nedergaard, M., Ransom, B. & Goldman, S. A. (2003) Trends Neurosci. 26, 523-530. [DOI] [PubMed] [Google Scholar]
- 5.Haydon, P. G. (2001) Nat. Rev. Neurosci. 2, 185-193. [DOI] [PubMed] [Google Scholar]
- 6.Carmignoto, G. (2000) Prog. Neurobiol. 62, 561-581. [DOI] [PubMed] [Google Scholar]
- 7.Auld, D. S. & Robitaille, R. (2003) Neuron 40, 389-400. [DOI] [PubMed] [Google Scholar]
- 8.Kang, J., Jiang, L., Goldman, S. A. & Nedergaard, M. (1998) Nat. Neurosci. 1, 683-692. [DOI] [PubMed] [Google Scholar]
- 9.Robitaille, R. (1998) Neuron 21, 847-855. [DOI] [PubMed] [Google Scholar]
- 10.Pinard, A., Levesque, S., Vallee, J. & Robitaille, R. (2003) Eur. J. Neurosci. 18, 3241-3250. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, J. M., Wang, H. K., Ye, C. Q., Ge, W., Chen, Y., Jiang, Z. L., Wu, C. P., Poo, M. M. & Duan, S. (2003) Neuron 40, 971-982. [DOI] [PubMed] [Google Scholar]
- 12.Pascual, O. & Haydon, P. G. (2003) Neuron 40, 873-875. [DOI] [PubMed] [Google Scholar]
- 13.Newman, E. A. (2003) J. Neurosci. 23, 1659-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schell, M. J., Molliver, M. E. & Snyder, S. H. (1995) Proc. Natl. Acad. Sci. USA 92, 3948-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mothet, J. P., Parent, A. T., Wolosker, H., Brady, R. O., Jr., Linden, D. J., Ferris, C. D., Rogawski, M. A. & Snyder, S. H. (2000) Proc. Natl. Acad. Sci. USA 97, 4926-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang, Y., Ge, W., Chen, Y., Zhang, Z., Shen, W., Wu, C., Poo, M. & Duan, S. (2003) Proc. Natl. Acad. Sci. USA 100, 15194-15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evanko, D., Zhang, Q., Zorec, R. & Haydon, P. G. (2004) Glia., in press. [DOI] [PubMed]
- 18.Zhang, Q., Pangric, T., Kreft, M., Kran, M., Li, N., Sul, J. Y., Halassa, M., Van Bockstaele, E., Zorec, R. & Haydon, P. G. (2004) J. Biol. Chem. 279, 12724-12733. [DOI] [PubMed] [Google Scholar]
- 19.Kreft, M., Stenovec, M., Rupnik, M., Grilc, S., Krzan, M., Potokar, M., Pangrsic, T., Haydon, P. G. & Zorec, R. (2004) Glia 46, 437-445. [DOI] [PubMed] [Google Scholar]
- 20.Craxton, M. (2001) Genomics 77, 43-49. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda, M. (2003) J Biochem. (Tokyo) 133, 641-649. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda, M. (2003) Biochem. Biophys. Res. Commun. 306, 64-71. [DOI] [PubMed] [Google Scholar]
- 23.Sudhof, T. C. (2002) J. Biol. Chem. 277, 7629-7632. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Chacon, R., Konigstorfer, A., Gerber, S. H., Garcia, J., Matos, M. F., Stevens, C. F., Brose, N., Rizo, J., Rosenmund, C. & Sudhof, T. C. (2001) Nature 410, 41-49. [DOI] [PubMed] [Google Scholar]
- 25.Geppert, M., Goda, Y., Hammer, R. E., Li, C., Rosahl, T. W., Stevens, C. F. & Sudhof, T. C. (1994) Cell 79, 717-727. [DOI] [PubMed] [Google Scholar]
- 26.Araque, A., Parpura, V., Sanzgiri, R. P. & Haydon, P. G. (1998) Eur. J. Neurosci. 10, 2129-2142. [DOI] [PubMed] [Google Scholar]
- 27.Ibata, K., Fukuda, M., Hamada, T., Kabayama, H. & Mikoshiba, K. (2000) J. Neurochem. 74, 518-526. [DOI] [PubMed] [Google Scholar]
- 28.Saegusa, C., Fukuda, M. & Mikoshiba, K. (2002) J. Biol. Chem. 277, 24499-24505. [DOI] [PubMed] [Google Scholar]
- 29.Holland, E. C. & Varmus, H. E. (1998) Proc. Natl. Acad. Sci. USA 95, 1218-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berton, F., Cornet, V., Iborra, C., Garrido, J., Dargent, B., Fukuda, M., Seagar, M. & Marqueze, B. (2000) Eur. J. Neurosci. 12, 1294-1302. [DOI] [PubMed] [Google Scholar]
- 31.Tocco, G., Bi, X. N., Vician, L., Lim, I. K., Herschman, H. & Baudry, M. (1996) Mol. Brain Res. 40, 229-239. [DOI] [PubMed] [Google Scholar]
- 32.Vician, L., Lim, I. K., Ferguson, G., Tocco, G., Baudry, M. & Herschman, H. R. (1995) Proc. Natl. Acad. Sci. USA 92, 2164-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimelberg, H. K., Schools, G. P., Cai, Z. & Zhou, M. (2000) J. Neurosci. Res. 61, 577-587. [DOI] [PubMed] [Google Scholar]
- 34.Zhuo, L., Sun, B., Zhang, C. L., Fine, A., Chiu, S. Y. & Messing, A. (1997) Dev. Biol. 187, 36-42. [DOI] [PubMed] [Google Scholar]
- 35.Mackler, J. M., Drummond, J. A., Loewen, C. A., Robinson, I. M. & Reist, N. E. (2002) Nature 418, 340-344. [DOI] [PubMed] [Google Scholar]
- 36.Robinson, I. M., Ranjan, R. & Schwarz, T. L. (2002) Nature 418, 336-340. [DOI] [PubMed] [Google Scholar]
- 37.Wang, C. T., Lu, J. C., Bai, J., Chang, P. Y., Martin, T. F., Chapman, E. R. & Jackson, M. B. (2003) Nature 424, 943-947. [DOI] [PubMed] [Google Scholar]
- 38.Hannon, G. J. (2002) Nature 418, 244-251. [DOI] [PubMed] [Google Scholar]
- 39.Jackson, A. L., Bartz, S. R., Schelter, J., Kobayashi, S. V., Burchard, J., Mao, M., Li, B., Cavet, G. & Linsley, P. S. (2003) Nat. Biotechnol. 21, 635-637. [DOI] [PubMed] [Google Scholar]
- 40.Sledz, C. A., Holko, M., de Veer, M. J., Silverman, R. H. & Williams, B. R. (2003) Nat. Cell Biol. 5, 834-839. [DOI] [PubMed] [Google Scholar]
- 41.Thomas, D. M., Ferguson, G. D., Herschman, H. R. & Elferink, L. A. (1999) Mol. Biol. Cell 10, 2285-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Poser, C., Ichtchenko, K., Shao, X., Rizo, J. & Sudhof, T. C. (1997) J. Biol. Chem. 272, 14314-14319. [DOI] [PubMed] [Google Scholar]
- 43.Mongin, A. A. & Kimelberg, H. K. (2002) Am. J. Physiol. 283, C569-C578. [DOI] [PubMed] [Google Scholar]
- 44.Nedergaard, M., Takano, T. & Hansen, A. J. (2002) Nat. Rev. Neurosci. 3, 748-755. [DOI] [PubMed] [Google Scholar]
- 45.Koh, T. W. & Bellen, H. J. (2003) Trends Neurosci. 26, 413-422. [DOI] [PubMed] [Google Scholar]
- 46.Shin, O. H., Rhee, J. S., Tang, J., Sugita, S., Rosenmund, C. & Sudhof, T. C. (2003) Neuron 37, 99-108. [DOI] [PubMed] [Google Scholar]
- 47.Yoshihara, M. & Littleton, J. T. (2002) Neuron 36, 897-908. [DOI] [PubMed] [Google Scholar]
- 48.Poskanzer, K. E., Marek, K. W., Sweeney, S. T. & Davis, G. W. (2003) Nature 426, 559-563. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson, G. D., Anagnostaras, S. G., Silva, A. J. & Herschman, H. R. (2000) Proc. Natl. Acad. Sci. USA 97, 5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aravanis, A. M., Pyle, J. L. & Tsien, R. W. (2003) Nature 423, 643-647. [DOI] [PubMed] [Google Scholar]
- 51.Gandhi, S. P. & Stevens, C. F. (2003) Nature 423, 607-613. [DOI] [PubMed] [Google Scholar]
- 52.Bezzi, P., Carmignoto, G., Pasti, L., Vesce, S., Rossi, D., Rizzini, B. L., Pozzan, T. & Volterra, A. (1998) Nature 391, 281-285. [DOI] [PubMed] [Google Scholar]
- 53.Pasti, L., Zonta, M., Pozzan, T., Vicini, S. & Carmignoto, G. (2001) J. Neurosci. 21, 477-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Araque, A., Li, N., Doyle, R. T. & Haydon, P. G. (2000) J. Neurosci. 20, 666-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferguson, G. D., Vician, L. & Herschman, H. R. (2001) Mol. Neurobiol. 23, 173-185. [DOI] [PubMed] [Google Scholar]
- 56.Araque, A., Sanzgiri, R. P., Parpura, V. & Haydon, P. G. (1998) J. Neurosci. 18, 6822-6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.