Abstract
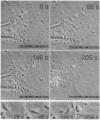
Upon entering the host cell's cytoplasm, the pathogen Listeria monocytogenes can subvert the normal contractile system of the host cell; subsequent assembly of polar actin-filament structures is likely to provide the force for rapid intracellular bacterial movement and its cell-to-cell spread. We have now investigated the functional consequences of microinjecting Listeria-infected PtK2 cells with a synthetic peptide, CFEFPPPPTDE. This peptide represents one of four related oligoproline stretches in ActA, a bacterial surface protein necessary for Listeria-induced actin assembly. Over an estimated intracellular concentration range of 80 nM to 0.8 microM, this analogue rapidly blocks the formation of the actin-filament tails and arrests intracellular bacterial motility. Over the same time scale and concentration range, introduction of the ActA analogue also causes host cell membrane retraction. Bodipyphallacidin staining reveals that microinjection of the ActA analogue results in massive retraction of the actin cytoskeleton. Microinjection of 1-20 microM poly(L-proline) (intracellular concentration) fails to block Listeria intracellular movement or polar actin-filament assembly. As observed with ActA, however, poly(L-proline) does cause membrane retraction. Our findings demonstrate the efficacy of low molecular weight peptides in efforts to distinguish mechanistic features in Listeria motility and PtK2 host cell membrane reorganization. These observations also suggest that a cytoskeletal component sensitive to specific oligoproline peptides may participate in protein-protein interactions essential for both of these actin-associated processes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dabiri G. A., Sanger J. M., Portnoy D. A., Southwick F. S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddette D. W., Frieden C. Actin polymerization. The mechanism of action of cytochalasin D. J Biol Chem. 1986 Dec 5;261(34):15974–15980. [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Doberstein S. K., Pollard T. D. Mechanism of the interaction of human platelet profilin with actin. J Cell Biol. 1991 Jun;113(5):1081–1089. doi: 10.1083/jcb.113.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Synthesis and secretion of interferon by murine fibroblasts in response to intracellular Listeria monocytogenes. Infect Immun. 1986 Dec;54(3):787–792. doi: 10.1128/iai.54.3.787-792.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly J. C., Flynn G., Purich D. L. The microtubule-binding fragment of microtubule-associated protein-2: location of the protease-accessible site and identification of an assembly-promoting peptide. J Cell Biol. 1989 Nov;109(5):2289–2294. doi: 10.1083/jcb.109.5.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Metz P., Hof H., Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987 Mar;169(3):1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Gouin E., Tabouret M., Berche P., Ohayon H., Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992 Feb 7;68(3):521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- Kocks C., Hellio R., Gounon P., Ohayon H., Cossart P. Polarized distribution of Listeria monocytogenes surface protein ActA at the site of directional actin assembly. J Cell Sci. 1993 Jul;105(Pt 3):699–710. doi: 10.1242/jcs.105.3.699. [DOI] [PubMed] [Google Scholar]
- Kuhn M., Prévost M. C., Mounier J., Sansonetti P. J. A nonvirulent mutant of Listeria monocytogenes does not move intracellularly but still induces polymerization of actin. Infect Immun. 1990 Nov;58(11):3477–3486. doi: 10.1128/iai.58.11.3477-3486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Schutt C. E., Hellsten E., Tjäder A. C., Hult T. The use of poly(L-proline)-Sepharose in the isolation of profilin and profilactin complexes. Biochim Biophys Acta. 1988 Dec 15;967(3):391–400. doi: 10.1016/0304-4165(88)90102-x. [DOI] [PubMed] [Google Scholar]
- Mockrin S. C., Korn E. D. Acanthamoeba profilin interacts with G-actin to increase the rate of exchange of actin-bound adenosine 5'-triphosphate. Biochemistry. 1980 Nov 11;19(23):5359–5362. doi: 10.1021/bi00564a033. [DOI] [PubMed] [Google Scholar]
- Peskin C. S., Odell G. M., Oster G. F. Cellular motions and thermal fluctuations: the Brownian ratchet. Biophys J. 1993 Jul;65(1):316–324. doi: 10.1016/S0006-3495(93)81035-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. M., Sanger J. W., Southwick F. S. Host cell actin assembly is necessary and likely to provide the propulsive force for intracellular movement of Listeria monocytogenes. Infect Immun. 1992 Sep;60(9):3609–3619. doi: 10.1128/iai.60.9.3609-3619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre P. H., Chang H. C., Hussey R. E., Brown N. R., Richardson N. E., Spagnoli G., Clayton L. K., Reinherz E. L. Molecular cloning and expression of T11 cDNAs reveal a receptor-like structure on human T lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(9):2941–2945. doi: 10.1073/pnas.84.9.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. The structure of the human synapsin I gene and protein. J Biol Chem. 1990 May 15;265(14):7849–7852. [PubMed] [Google Scholar]
- Tanaka M., Shibata H. Poly(L-proline)-binding proteins from chick embryos are a profilin and a profilactin. Eur J Biochem. 1985 Sep 2;151(2):291–297. doi: 10.1111/j.1432-1033.1985.tb09099.x. [DOI] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J., Tilney L. G., Portnoy D. A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992 May 21;357(6375):257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Connelly P. S., Portnoy D. A. Actin filament nucleation by the bacterial pathogen, Listeria monocytogenes. J Cell Biol. 1990 Dec;111(6 Pt 2):2979–2988. doi: 10.1083/jcb.111.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Feramisco J. R., Ash J. F. Fluorescent localization of contractile proteins in tissue culture cells. Methods Enzymol. 1982;85(Pt B):514–562. doi: 10.1016/0076-6879(82)85050-7. [DOI] [PubMed] [Google Scholar]
- Weller P. A., Ogryzko E. P., Corben E. B., Zhidkova N. I., Patel B., Price G. J., Spurr N. K., Koteliansky V. E., Critchley D. R. Complete sequence of human vinculin and assignment of the gene to chromosome 10. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5667–5671. doi: 10.1073/pnas.87.15.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. L., Southwick F. S., Weber A. Kinetics of the interaction of a 41-kilodalton macrophage capping protein with actin: promotion of nucleation during prolongation of the lag period. Biochemistry. 1990 Mar 6;29(9):2232–2240. doi: 10.1021/bi00461a005. [DOI] [PubMed] [Google Scholar]