Abstract
Citrobacter rodentium is a murine intestinal pathogen used as a model for the foodborne human pathogens enterohemorrhagic Escherichia coli and enteropathogenic E. coli. During infection, these pathogens use two-component signal transduction systems to detect and adapt to changing environmental conditions. In E. coli, the CpxRA two-component signal transduction system responds to envelope stress by modulating the expression of a myriad of genes. Quantitative real-time PCR showed that cpxRA was expressed in the colon of C57BL/6J mice infected with C. rodentium. To determine whether CpxRA plays a role during C. rodentium infection, a cpxRA deletion strain was generated and found to have a colonization defect during infection. This defect was independent of an altered growth rate or a defective type III secretion system, and single-copy chromosomal complementation of cpxRA restored virulence. The C. rodentium strains were then tested in C3H/HeJ mice, a lethal intestinal infection model. Mice infected with the ΔcpxRA strain survived infection, whereas mice infected with the wild-type or complemented strains succumbed to infection. Furthermore, we found that the cpxRA expression level was higher during early infection than at a later time point. Taken together, these data demonstrate that the CpxRA two-component signal transduction system is essential for the in vivo virulence of C. rodentium. In addition, these data suggest that fine-tuned cpxRA expression is important for infection. This is the first study that identifies a C. rodentium two-component transduction system required for pathogenesis. This study further indicates that CpxRA is an interesting target for therapeutics against enteric pathogens.
INTRODUCTION
Bacteria must be able to detect changes in their environment and adapt accordingly to survive. One way in which bacteria detect changes is through the use of two-component signal transduction systems (TCSs) (1, 2). The typical TCS consists of a histidine kinase (HK) sensor in the bacterial plasma membrane and a cytoplasmic response regulator (RR) protein that usually acts as a transcriptional regulator (2). Changes in the environment are detected by the HK, which results in autophosphorylation of the HK cytoplasmic domain. The subsequent transfer of the phosphoryl group to the RR affects its DNA-binding properties, resulting in changes in the expression of specific genes. The CpxRA TCS is mainly involved in the detection of and adaptation to cell envelope stress (3–6). In the absence of envelope stress, the CpxA HK exhibits phosphatase activity and maintains the CpxR RR in an inactive state (7). In some bacteria, such as Escherichia coli, the small periplasmic protein CpxP maintains the CpxA HK in an inactive state through a negative feedback loop (8, 9). The exact in vivo signal leading to CpxRA activation is unknown; however, some in vitro conditions that activate this TCS have been identified, including the overexpression of pilus components, alkaline pH, and alterations in membrane composition (3, 10, 11). Other studies have shown that CpxRA is activated in E. coli cells that overexpress the outer membrane lipoprotein NlpE when they adhere to hydrophobic surfaces (12). Altogether, these studies provide evidence that CpxRA may detect adherence to specific surfaces in addition to its role in monitoring protein folding in the periplasm. More recent studies have shown that the CpxRA signal transduction network affects the expression of hundreds of genes with a plethora of functions, indicating that CpxRA activation affects multiple functions within the cell (13–15).
Citrobacter rodentium is a natural murine pathogen used to model infections caused by the human pathogens enterohemorrhagic E. coli (EHEC) and enteropathogenic E. coli (EPEC) (16). These pathogens share many virulence factors, including those required for the formation of attaching and effacing (A/E) lesions characterized by intimate attachment to intestinal epithelial cells, localized effacement of microvilli, and the formation of actin-rich pedestals beneath sites of bacterial adherence (16). To date, the TCS involved in fucose sensing has been implicated in the in vivo virulence of EHEC (17). Less is known about the in vivo role of the other TCSs, including CpxRA.
The CpxRA TCS appears to have contrasting effects on the pathogenesis of different bacterial species. For example, activation of CpxRA in Shigella sonnei results in increased expression of virulence genes (18). Further supporting a role for the CpxRA TCS during infection, the insect pathogen Xenorhabdus nematophila and the human pathogens Yersinia pestis and uropathogenic E. coli require the CpxRA TCS for full virulence (19–21). These results are in direct contrast to what was observed for Haemophilus ducreyi, where activation of the CpxRA TCS results in the downregulation of known virulence genes (14). More importantly, in H. ducreyi, the constitutive activation of CpxRA leads to in vivo virulence defects, and the deletion of this TCS has no effect on infection (22, 23). These studies are consistent with what was observed during experimental infection with Salmonella enterica serovar Typhimurium and Vibrio cholerae (24, 25). Therefore, the in vivo contribution of the CpxRA TCS to virulence appears to vary among species.
In the A/E pathogen EPEC, CpxRA has been implicated in the in vitro regulation of virulence genes (26). The bundle-forming pilus, a structure unique to EPEC, is downregulated following the activation of CpxRA (27). This pilus is required for infection and is involved in early attachment to intestinal cells (28). Other evidence supports a role for CpxRA in the indirect downregulation of type III secretion system (TTSS) genes common to all A/E pathogens (29). Therefore, the constitutive activation of CpxRA negatively impacts virulence gene expression in vitro. In addition to these findings, the deletion of cpxR and subsequent inactivation of CpxRA leads to decreases in cell adherence and in vitro infectivity of EPEC (30). These studies provide compelling evidence that CpxRA is involved in the regulation of essential colonization and virulence factors of EPEC and that the activation of this TCS must be carefully fine-tuned to protect cells against cell envelope stress while allowing for virulence gene expression. The CpxRA proteins in C. rodentium are highly homologous to those found in E. coli K-12 and EPEC, suggesting that they may have some shared functions (see Table S1 in the supplemental material). Furthermore, the cpxRA gene organization is similar, with cpxR and cpxA forming an operon and the cpxP gene located next to cpxR in the opposite orientation. To date, there have been no studies investigating the role of CpxRA in the A/E pathogen C. rodentium. In this study, we examine the in vivo contribution of CpxRA to C. rodentium virulence. We show that the C. rodentium CpxRA TCS is essential for in vivo infection and provide evidence that this virulence defect is independent of a major defect in type III secretion (TTS).
MATERIALS AND METHODS
Media and reagents.
Bacteria were routinely cultured at 37°C with aeration (200 rpm) in Luria-Bertani (LB) broth (1% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract, 1% [wt/vol] NaCl). When appropriate, LB broth was supplemented with chloramphenicol (Cm) (30 μg/ml), ampicillin (Amp) (300 μg/ml), gentamicin (Gent) (30 μg/ml), kanamycin (Kan) (50 μg/ml), or dl-diaminopimelic acid (DAP) (50 μg/ml).
Construction of the C. rodentium cpxRA deletion strain.
The bacterial strains and plasmids used in this study are listed in Table 1. DH5α and DH5αλpir were routinely used for genetic manipulations. DNA purification, cloning, and transformation were performed according to standard procedures (31). The C. rodentium ΔcpxRA strain was generated by sacB gene-based allelic exchange (32). Genomic DNA from C. rodentium was used as a template to PCR amplify the upstream (primer pair cpxRA1/cpxRA2) (Table 2) sequence of the cpxRA operon. The resultant PCR product was treated with XbaI and XhoI and ligated into pBluescript SK+ that had been treated with the same restriction enzymes, generating pBScpxRA. In a similar manner, the downstream sequence of the cpxRA operon was PCR amplified by using primer pair cpxRA3/cpxRA4 (Table 2). The resultant PCR product was treated with XhoI and KpnI and ligated into pBScpxRA that had been treated with the same restriction enzymes, generating pBSΔcpxRA. Plasmid pΔcpxRA was generated by subcloning the DNA fragment from pBSΔcpxRA into the XbaI and KpnI sites of pRE112. The sequence of pΔcpxRA was verified by sequencing (Genome Quebec). Plasmid pΔcpxRA was conjugated into wild-type C. rodentium by using E. coli χ7213 as the donor strain. Integration of the plasmid into the chromosome was selected for by plating bacteria onto LB agar supplemented with Cm. Cm-resistant transformants of C. rodentium were then plated onto peptone agar containing 5% sucrose to isolate colonies that were sucrose resistant. The resultant colonies were also tested for Cm sensitivity. Gene deletions were verified by PCR using primers cpxRA1 and cpxRA4.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| C. rodentium | ||
| DBS100 | Wild-type C. rodentium DBS100 | 48 |
| ΔcpxRA | DBS100 ΔcpxRA | This study |
| ΔcpxRA::cpxRA | DBS100 ΔcpxRA with a single copy of cpxRA integrated at the att Tn7 site; Gentr | This study |
| ΔcpxRA(pSTNSK) | DBS100 ΔcpxRA + pSTNSK; Kanr | This study |
| spy::lacZ | DBS100 with a chromosomal insertion of spy::lacZ | This study |
| ΔcpxRA::spy::lacZ | ΔcpxRA strain with a chromosomal insertion of spy::lacZ | This study |
| ΔcpxRA::cpxRA::spy::lacZ | ΔcpxRA::cpxRA strain with a chromosomal insertion of spy::lacZ | This study |
| E. coli | ||
| DH5α | HuA2 Δ(lac)U169 phoA glnV44 ϕ80lacZΔM15 endA recA hsdR17 (rM− mK+) thi-1 gyrA96 relA1 | Invitrogen |
| DH5α(pBSΔcpxRA) | DH5α containing pBSΔcpxRA | This study |
| DH5αλpir | K-12 F− ϕ80lacZΔM15 endA recA hsdR17 (rM− mK+) supE44 thi-1 gyrA96 relA1 Δ(lacZYA-argF)U169 λpir | |
| DH5αλpir(pΔcpxRA) | DH5αλpir containing pΔcpxRA | This study |
| DH5αλpir(pcpxRA) | DH5αλpir containing pcpxRA | This study |
| DH5αλpir(pspylacZ) | DH5αλpir containing pspylacZ | This study |
| χ7213 | thr-1 leuB6 fhuA21 lacY1 glnV44 recA1 asdA4 thi-1 RP4-2-Tc::Mu [-pir] Kanr | 49 |
| χ7213(pΔcpxRA) | χ7213 containing pΔcpxRA | This study |
| χ7213(pcpxRA) | χ7213 containing pcpxRA | This study |
| χ7213(pspy::lacZ) | χ7213 containing pspy::lacZ | This study |
| Plasmids | ||
| pSTNSK | pST76-K::tnsABCD; Kanr | 33 |
| pBluescript SK+ | High-copy-no. cloning vector; Ampr | Stratagene |
| pBSΔcpxRA | ΔcpxRA deletion construct in pBluescript SK+ | This study |
| pRE112 | Sucrose-sensitive (sacB1) suicide vector; Cmr | 50 |
| pΔcpxRA | ΔcpxRA deletion construct in pRE112 | This study |
| pGP-Tn7-Gm | pGP704::Tn7-Gm; Ampr Gentr | 33 |
| pcpxRA | C. rodentium DBS100 cpxRA cloned into pGP-Tn7-Gm | This study |
| pFUSE | Suicide vector, lacZYA, mob+ (RP4), ori R6K, Cmr | 34 |
| pspy::lacZ | spy promoter fusion to lacZ in pFUSE | This study |
TABLE 2.
Primers used in this studya
| Primer | Sequence | Usage |
|---|---|---|
| cpxRA1 | GCTCTAGAGCTGTTAAGGCGAGAGTGGATGA | ΔcpxRA 5′ F XbaI |
| cpxRA2 | CCGCTCGAGCTCTCGGTCATCATCAACTAA | ΔcpxRA 5′ R XhoI |
| cpxRA3 | CCGCTCGAGTAATAACGCTGAGTTGCCGGATGG | ΔcpxRA 3′ F XhoI |
| cpxRA4 | GGGGTACCGAATGTCTTTATCGGTGATATCC | ΔcpxRA 3′ R KpnI |
| cpxRA5 | CCGCTCGAGCACTTGCTCCCAAAATCTTTTCTG | pcpxRA F XhoI |
| cpxRA6 | GAGGTACCCGTTTACGCCGCCATCCGGCAA | pcpxRA R KpnI |
| spy_F | CCGTCTAGAGCTGGCATCTGGATCTTCACAC | pspy::lacZ F XbaI |
| spy_R | CCGCCCGGGCGGTTTTGCGTCTGCCGGC | pspy::lacZ R SmaI |
| qσ701 | ATCAAAGCGAAAGGTCGTAGCCAC | qPCR σ70 F |
| qσ702 | CCATCATCACGCGCATACTGTTCA | qPCR σ70 R |
| qcpxR1 | GGAACAGGCGCTGGAGCTTC | qPCR cpxR 5′ F |
| qcpxR2 | ACGGGGGTCTGGTGTGTCTG | qPCR cpxR 3′ R |
| qcpxA1 | AACGCCGTTGACCCGCTTAC | qPCR cpxA 5′ F |
| qcpxA2 | GCGCGACATCACCAACAGGT | qPCR cpxA 3′ R |
| qcpxP1 | AAGCCATGCTGCTGAAGTCGATAC | qPCR cpxP 5′ F |
| qcpxP2 | CGCATCTGTTGACGTTGATGTTCG | qPCR cpxP 3′ R |
Restriction sites are underlined. F indicates forward, and R indicates reverse.
Single-copy chromosomal complementation of the ΔcpxRA strain.
The ΔcpxRA::cpxRA strain with cpxRA integrated at the att Tn7 site was generated as previously described (33). Briefly, genomic DNA from C. rodentium was used as a template to PCR amplify both the promoter and open reading frame of cpxRA (primer pair cpxRA5/cpxRA6) (Table 2). The resultant PCR product was treated with XhoI and KpnI and then ligated into pGP-Tn7-Gm that had been treated with the same restriction enzymes, generating pcpxRA. The sequence of plasmid pcpxRA was verified by sequencing (Genome Quebec). The complementation plasmid, pcpxRA, was conjugated into the ΔcpxRA(pSTNSK) strain at 30°C by using E. coli χ7213 as the donor strain. Integration of the cpxRA DNA fragment into the chromosome at the att Tn7 site and loss of pSTNSK were selected for by plating bacteria onto LB agar supplemented with Gent and incubating the cells for 4 h at 42°C, followed by 18 h at 37°C. Gent-resistant transformants of C. rodentium were then patched onto LB agar supplemented with either Gent, Amp, or Kan. Colonies that were resistant to Gent but sensitive to Amp and Kan were screened by PCR to ensure the integration of the cpxRA fragment at the att Tn7 site.
Construction of C. rodentium strains with chromosomal transcriptional spy::lacZ fusions.
Chromosomal transcriptional fusions between the spy promoter and the lacZ reporter gene were generated in C. rodentium strains by using the suicide vector pFUSE (34). The spy::lacZ fusion was constructed by PCR amplifying the spy promoter using C. rodentium genomic DNA and primers spyF and spyR. The PCR product was digested with XbaI and SmaI and cloned into the corresponding sites of pFUSE. The resulting construct was transferred into the C. rodentium wild-type, ΔcpxRA, and ΔcpxRA::cpxRA strains by conjugation and integrated by homologous recombination as described above.
β-Galactosidase assays.
β-Galactosidase assays were performed as previously described, with minor modifications (35). Briefly, cultures of C. rodentium strains grown overnight were diluted 1:100 into 3 ml of LB medium buffered with 100 mM sodium phosphate at pH 5.5 or 8.5 and grown for 3 h at 37°C with aeration. Cells (2 ml) were recovered by centrifugation and resuspended in Z-buffer (2 ml) (60 mM Na2HPO4·7H2O, 40 mM Na2H2PO4·H2O, 10 mM KCl, 1 mM MgSO4, 50 mM β-mercaptoethanol). A volume (500 μl) of resuspended cells was combined with reaction buffer (650 μl) (Z-buffer with 0.008% [wt/vol] SDS and 15% [vol/vol] chloroform), vortexed, and incubated for 5 min at 28°C. The reaction was started by the addition of ortho-nitrophenyl-β-galactoside (200 μl; 4 mg/ml) to each reaction tube and allowed to develop at 28°C. Reactions were stopped by the addition of Na2CO3 (500 μl; 1 M) to the mixture, cell debris was removed by centrifugation, and the optical densities at 420, 540, and 595 nm were measured. All experiments were performed at least three times in triplicate. Miller units were calculated as previously described (36).
Secretion assays.
Bacterial secretion assays were performed as previously described (37). Briefly, cultures of bacteria grown overnight were subcultured 1:50 into 6-well tissue culture plates containing Dulbecco's modified Eagle medium (DMEM; Gibco). After a 6-h incubation at 37°C with 5% CO2, whole cells were separated from the culture medium by centrifugation. The supernatant was transferred into a new tube, and the bacterial pellets, now referred to as whole-cell lysates, were resuspended in electrophoresis sample buffer (ESB) (0.0625 M Tris-HCl [pH 6.8], 1% [wt/vol] SDS, 10% glycerol, 2% [vol/vol] 2-mercaptoethanol, 0.001% [wt/vol] bromphenol blue), boiled, and stored at −20°C. Contaminating cells in the supernatant were removed by centrifugation (13,000 × g for 2 min). The proteins in the supernatant, now referred to as secreted proteins, were precipitated in sterile glass tubes with 10% (vol/vol) 6 M trichloroacetic acid and incubated for 1 h on ice. Secreted proteins were separated by centrifugation (13,000 × g for 30 min at 4°C) and washed overnight with acetone at −80°C. The next day, secreted proteins were collected by centrifugation (13,000 × g for 30 min at 4°C), supernatants were removed, and the pellets were air dried. Secreted-protein pellets were resuspended in ESB, boiled, separated on 10% SDS-PAGE gels, and visualized by Coomassie blue staining. Aliquots (2 μl) of whole-cell lysate proteins were separated on 10% SDS-PAGE gels and transferred onto polyvinylidene difluoride (PVDF) membranes, and DnaK and EspB were detected by Western blotting using anti-DnaK (Stressgen) and anti-EspB (clone 2A11) antibodies (38).
qPCR.
Quantitative PCR (qPCR) was performed as previously described (39). Briefly, the terminal centimeter of colon from infected C3H/HeJ or C57BL/6J mice was collected, homogenized by using a Polytron homogenizer, and stored at −80°C in TRIzol. Total RNA was isolated by using TRIzol reagents (Invitrogen) and treated with a DNA-free kit (Ambion) to remove any remaining DNA. The absence of contaminating DNA was confirmed by qPCR using primers qCR16SF and qCR16SR (40). RNA was reverse transcribed by using Superscript III (Invitrogen). As a negative control, a reaction mixture without Superscript III was also included (NRT). qPCRs were performed with a Rotor-Gene 3000 thermal cycler (Corbett Research), using the Maxima SYBR green PCR kit (Fermentas), according to the manufacturer's instructions. Primers used are listed in Table 2. The level of gene transcript was normalized to σ70 values and analyzed by using the 2−ΔCT (where CT is threshold cycle) method (41). Reverse transcription (RT) was performed three times independently, and the NRT sample was used as a negative control.
In vivo C. rodentium infections.
All animal experiments were performed under conditions specified by the Canadian Council on Animal Care and were approved by the McGill University Animal Care Committee. C57BL/6J and C3H/HeJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained in a specific-pathogen-free facility at McGill University. Four-week-old mice were orally inoculated with C. rodentium strains. For oral inoculations, bacteria were grown overnight in 3 ml of LB broth with aeration. Mice were infected by oral gavage of 0.1 ml of LB broth containing 2 × 108 to 3 × 108 CFU of C. rodentium. The infectious dose was verified by plating serial dilutions of the inoculum onto MacConkey agar (Difco). For survival analysis of C3H/HeJ mice, the mice were monitored daily and were killed if they met any of the following clinical endpoints: 20% body weight loss, hunching and shaking, inactivity, or body condition score of <2. To monitor bacterial colonization in C3H/HeJ and C57BL/6J mice, fecal pellets or the terminal centimeter of colon was collected and homogenized in phosphate-buffered saline by using a Polytron homogenizer. Homogenates were serially diluted in sterile phosphate-buffered saline, and 0.1-ml aliquots of each serial dilution were plated onto MacConkey agar. C. rodentium was distinguished by its characteristic colony morphology on this medium, as previously described (42). Plates containing between 30 and 300 colonies were counted. When bacterial loads were low, leading to the undiluted-sample plate having <30 colonies, the number of colonies on this plate was counted. Spleens were removed and weighed, and splenic indexes were calculated [√(weight of spleen × 100/weight of mouse)]. For histological analysis, the last 0.5 cm of the colon of infected mice was fixed in 10% neutral buffered formalin, processed, cut into 3-μm sections, and stained with hematoxylin and eosin. Pathological scoring was performed in a blind fashion by a board-certified veterinary pathologist using the scoring matrix provided in Table S2 in the supplemental material. Crypt heights were measured by using Infinity Analyze software on a Zeiss A1 microscope, with 10 well-oriented crypts being measured per mouse.
RESULTS
Expression of cpxRA during intestinal infection of C57BL/6J mice.
To assess the role of the CpxRA TCS during intestinal infection with C. rodentium, we first performed qPCR on cDNA samples prepared from the distal colon of C57BL/6J mice on day 9 of infection. At this time point, bacterial loads in the colon were high enough to obtain reproducible signals for bacterial gene expression. As shown in Fig. 1, both cpxA and cpxR were expressed in infected colons. To determine if CpxRA is active under these conditions, we also examined the expression of cpxP, which has previously been shown to be strongly upregulated by CpxRA as part of a negative feedback loop (10). We found that cpxP was highly expressed, suggesting that CpxRA was activated (Fig. 1). To ensure that the signal detected was not the result of nonspecific amplification of bacterial or murine cDNA found in the colon of mice, we performed control qPCR experiments on uninfected mice with our rpoD, cpxA, cpxR, and cpxP primers. These primers did not amplify any products in the absence of C. rodentium (see Fig. S1 in the supplemental material). These data provide evidence that cpxRA is expressed during intestinal infection.
FIG 1.
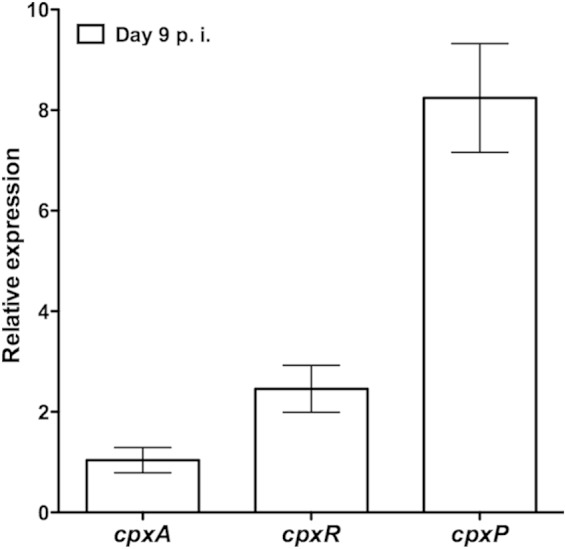
qPCR analysis of cpx gene expression during infection of C57BL/6J mice. Data were normalized to rpoD expression levels. Relative expression is indicative of 2−ΔCT. Data shown are the means ± standard errors of the means from 5 biological samples, in triplicate. p.i., postinfection.
The C. rodentium CpxRA TCS is activated under in vitro conditions similar to those of its E. coli homologue.
To further analyze CpxRA in C. rodentium, an in-frame cpxRA deletion strain (ΔcpxRA) and the ΔcpxRA::cpxRA strain, consisting of ΔcpxRA with a chromosomal insertion of cpxRA, were generated (Table 1). For E. coli, several in vitro growth conditions, including alkaline pH, have been shown to activate the CpxRA TCS (35). Under this condition, the spy promoter was the second most activated promoter by the CpxRA TCS (35). To determine whether the C. rodentium CpxRA TCS is activated by alkaline pH, we generated chromosomal spy::lacZ transcriptional fusions within the wild-type, ΔcpxRA, and ΔcpxRA::cpxRA strains and grew these strains under both CpxRA-repressing (pH 5.5) and -activating (pH 8.5) conditions. As shown in Fig. 2, there was an increase in β-galactosidase activity in the wild-type strain at pH 8.5 compared to bacteria grown at pH 5.5. These data indicate that the spy promoter is activated in wild-type C. rodentium at alkaline pH, which is similar to what had been previously observed for E. coli. In contrast, there was no difference in the β-galactosidase activity of the ΔcpxRA strain when grown at either pH. Complementation of the ΔcpxRA strain restored β-galactosidase activity to wild-type levels. Altogether, these data show that the C. rodentium CpxRA TCS is activated by growth at alkaline pH; they also suggest that the C. rodentium and E. coli CpxRA TCSs fulfill similar functions.
FIG 2.
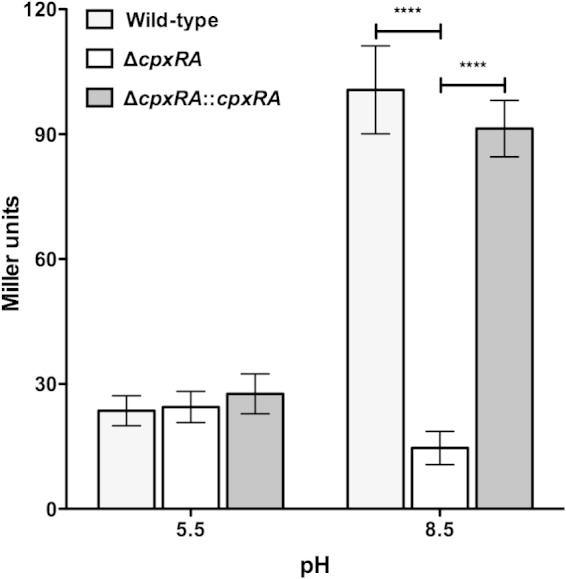
CpxRA is activated by alkaline pH. The indicated bacterial strains containing a chromosomal spy::lacZ fusion were grown for 2.5 h in LB medium buffered with sodium phosphate to pH 5.5 or 8.5, followed by determination of β-galactosidase activity, expressed as Miller units. Data are from 5 different experiments performed in triplicate. Data shown are means ± standard errors of the means. Asterisks indicate statistical significance (P < 0.0001), as determined by analysis of variance followed by Bonferroni's multiple-comparison post hoc analysis.
CpxRA impacts colonization and disease in C57BL/6J mice.
We next assessed whether cpxRA is required for bacterial fitness and/or virulence by comparing infections of C57BL/6J mice with the wild-type, ΔcpxRA, and ΔcpxRA::cpxRA strains. As shown in Fig. 3A, the ΔcpxRA strain displayed a significant defect in intestinal colonization (P < 0.001) compared to wild-type C. rodentium. There was a significant increase in colonization with the complemented strain compared to the ΔcpxRA strain (P < 0.001). A large spread in the bacterial loads of mice infected with the ΔcpxRA strain compared to mice infected with the wild-type and ΔcpxRA::cpxRA strains was observed, with 7 out of 15 ΔcpxRA-infected mice having no detectable C. rodentium present in the colon (Fig. 3A). These data indicate that, at the peak of infection, intestinal colonization levels of the ΔcpxRA strain are significantly decreased and that complementation restores colonization to wild-type levels. We next determined the splenic indexes for these mice. As shown in Fig. 3B, the wild-type-infected mice had significantly larger spleens than did the ΔcpxRA-infected mice at day 12 postinfection, indicating that there is less inflammation during infection with the ΔcpxRA strain (P < 0.001). The spleens of mice infected with the complemented strain were significantly larger than those infected with the ΔcpxRA strain and were similar to those of wild-type-infected mice (Fig. 3B).
FIG 3.
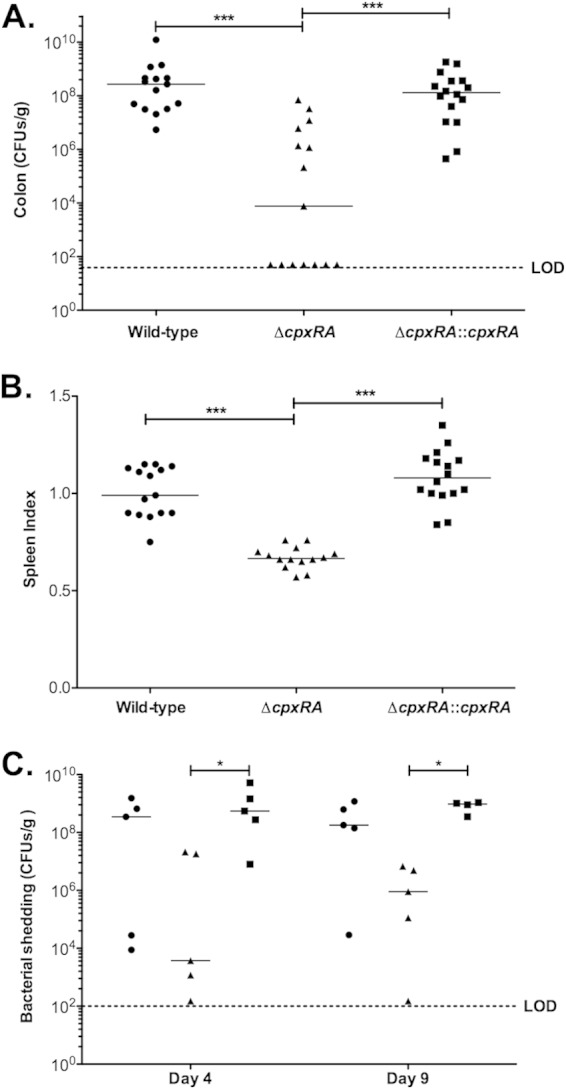
CpxRA is essential for efficient colonization and infection of C57BL/6J mice. C57BL/6J mice were infected with the indicated C. rodentium strains. Mice were sacrificed at 12 days postinfection. Each mouse in the experiment is represented by a single point. The median for each group is indicated on the graph by horizontal bars. Asterisks indicate statistical significance (*, P < 0.05; ***, P < 0.001), as determined by Kruskall-Wallis analysis and Dunn's multiple-comparison post hoc analysis. (A) Numbers of CFU per gram of colon from each animal were determined by serial dilution plating. (B) Prior to dissection, each animal was weighed, the spleens were then removed and weighed individually, and spleen indexes were calculated [√(weight of spleen × 100/weight of mouse)]. (C) Numbers of CFU per gram of feces were determined for mice infected with the wild-type (circles), ΔcpxRA (triangles), or ΔcpxRA::cpxRA (squares) strain by serial dilution plating. LOD indicates the limit of detection.
To investigate the origin of the lower colonization levels at the peak of infection with the ΔcpxRA strain, we monitored bacterial colonization with C. rodentium at earlier time points by measuring its shedding into the feces of mice (Fig. 3C). These experiments indicated that whereas both the wild-type and ΔcpxRA::cpxRA strains had already colonized to very high levels by day 4 postinfection, the ΔcpxRA strain was already displaying a comparative attenuation in colonization at this time point. Decreased colonization by the ΔcpxRA strain was also evident at day 9 postinfection (Fig. 3C). Altogether, these data indicate that C. rodentium ΔcpxRA cells have an early colonization defect.
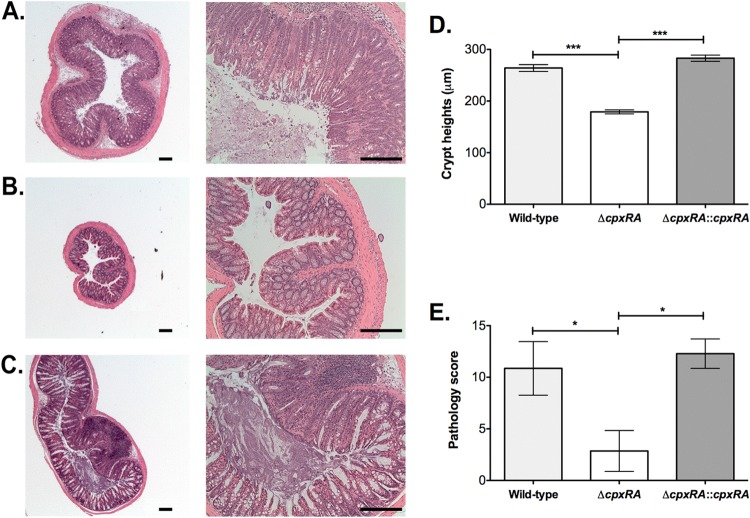
Histological analyses of biopsy specimens taken from the distal colon of mice revealed that all animals infected with the ΔcpxRA strain had significantly lower pathology scores than did mice infected with either the wild-type or ΔcpxRA::cpxRA strain, whereas the pathology scores for the wild-type- and ΔcpxRA::cpxRA-infected mice were similar (Fig. 4E). Colon sections from wild-type- and ΔcpxRA::cpxRA-infected mice displayed a loss of normal tissue architecture, with signs of epithelial hyperplasia, inflammation, goblet cell loss, and shedding of cells in the intestinal lumen, whereas all ΔcpxRA-infected mice showed well-preserved tissue morphology, with few signs of inflammation (Fig. 4A to C). Additionally, mice infected with the wild-type and ΔcpxRA::cpxRA strains had colonic crypt heights that were approximately double those of mice infected with the ΔcpxRA strain, indicating that mice infected with the latter strain suffer less hyperplasia (Fig. 4D).
FIG 4.
Histological analysis of infected C57BL/6J mouse colons. (A to C) Mice were infected with either the wild-type (A), ΔcpxRA (B), or ΔcpxRA::cpxRA (C) C. rodentium strain and sacrificed at 12 days postinfection. Hematoxylin- and eosin-stained tissue sections for all mice were observed and photographed by using 2.5× (left) and 10× (right) objectives. Typical results are shown. Bar, 200 μm. (D) Crypt heights in mice infected with the indicated C. rodentium strains (n = 7) were measured. Data shown are the means ± standard errors of the means. Asterisks indicate statistical significance (***, P < 0.001), as determined by a Kruskal-Wallis test followed by Dunn's post hoc analysis. (E) A pathology score was determined for mice infected with the indicated C. rodentium strains (n = 7). Asterisks indicate statistical significance (*, P < 0.05), as determined by analysis of variance followed by Bonferroni's multiple-comparison post hoc analysis.
The colonization defect of the ΔcpxRA strain is due to neither deficient TTS nor an altered growth rate.
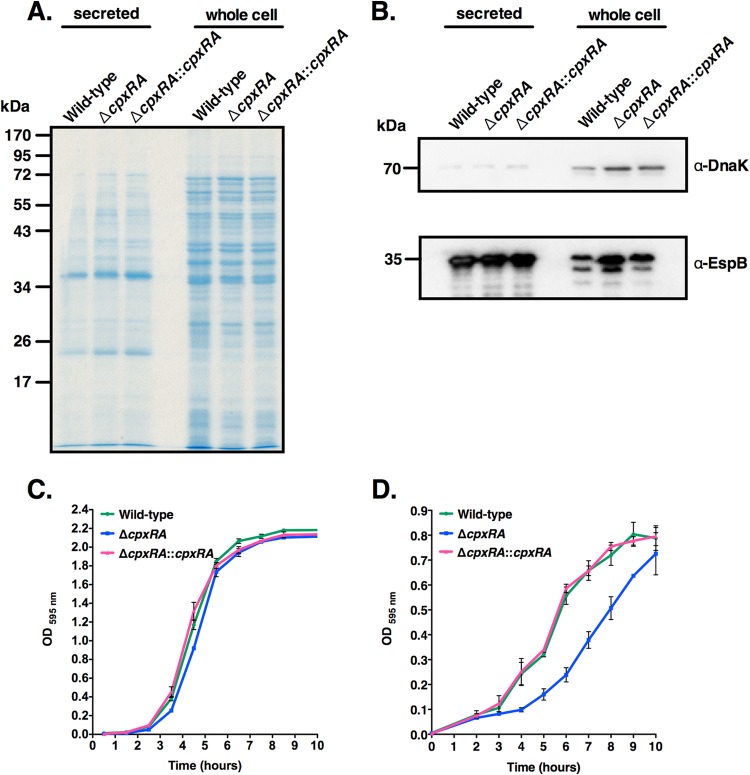
Since CpxRA has been implicated in the regulation of TTS in other bacterial species (26), and since TTS is a key virulence mechanism of C. rodentium (16), we next asked whether the ΔcpxRA strain was deficient in TTS, which would provide an underlying mechanism for its colonization defect. The wild-type, ΔcpxRA, and ΔcpxRA::cpxRA strains were grown under TTS-inducing conditions (37), and the secreted protein and whole-cell fractions were analyzed by Coomassie staining and Western blotting. As shown in Fig. 5A and B, the TTSS was functional in all three strains. Western blotting for the abundant cytoplasmic, nonsecreted bacterial protein DnaK was used to monitor the amount of bacterial lysis or leakage of cytoplasmic components into the secreted protein fractions. This revealed small amounts of DnaK in the secreted protein fractions of all strains, suggesting that small amounts of bacterial lysis or leakage had occurred. The levels of the type III secreted protein EspB detected in the secreted protein fractions were equivalent for all strains (Fig. 5B). There was a slightly elevated amount of EspB detected in the whole-cell fraction of the ΔcpxRA strain, but this is unlikely to indicate an increased amount of EspB produced, as it correlates with an increase in DnaK levels, suggesting that slightly more whole-cell protein extract was loaded into this well (Fig. 5B). Growth curve experiments with the wild-type, ΔcpxRA, and ΔcpxRA::cpxRA strains also indicated that the ΔcpxRA strain does not have a noticeable growth defect in LB broth (Fig. 5C). In DMEM, with the exception of a longer lag phase for the ΔcpxRA strain, there were no significant differences in exponential growth rates and final cell densities between strains (Fig. 5D). Taken together, these results provide evidence that the colonization defect of the ΔcpxRA strain is not due to deficient TTS or a change in the bacterial growth rate.
FIG 5.
Deletion of the cpxRA genes does not affect TTS or bacterial growth. (A) Coomassie blue-stained SDS-PAGE gel showing secreted and whole-cell proteins from the indicated C. rodentium strains. (B) Western blot analyses of secreted and whole-cell proteins from the indicated C. rodentium strains, using DnaK and EspB antibodies. (C and D) Growth curves of the indicated C. rodentium strains in LB medium (C) or DMEM (D). This experiment was repeated at least twice in triplicate. OD, optical density.
CpxRA is required for virulence in a fatal infection in susceptible mice.
To gain further insight into the role of CpxRA during intestinal C. rodentium infection, we tested the wild-type, ΔcpxRA, and ΔcpxRA::cpxRA strains in a lethal intestinal infection model in susceptible mice (16). Many inbred mouse strains suffer self-limiting disease during infection with C. rodentium, whereas some inbred strains such as C3H/HeJ, AKR/J, and FVB/J suffer more severe disease and high mortality rates (43). In C3H/HeJ mice, 100% of animals infected with either the wild-type or cpxRA::cpxRA strain succumbed to the infection. In contrast, animals infected with the ΔcpxRA strain did not develop overt signs of disease, and 100% of them survived up to 30 days postinfection, when the experiment was terminated (Fig. 6A). To determine if the decreased mortality was associated with altered levels of bacterial colonization in C3H/HeJ mice, we assessed shedding of C. rodentium strains into the feces of infected mice. As shown in Fig. 6B, at day 3 postinfection, there was significantly (P < 0.05 and 0.01) higher levels of colonization by wild-type and ΔcpxRA::cpxRA bacteria than by ΔcpxRA bacteria. Furthermore, whereas the levels of wild-type and ΔcpxRA::cpxRA C. rodentium strains increased >10-fold between days 3 and 6 postinfection, the amount of bacteria shed by mice infected with the ΔcpxRA strain remained stable. The lower level of colonization by the ΔcpxRA strain was also evident in the colons of infected C3H/HeJ mice at day 9 postinfection (Fig. 6B). Lower levels of colonization by the ΔcpxRA strain were not attributed to a delay in colonization, since C3H/HeJ mice infected with the ΔcpxRA strain remained healthy up to day 28 postinfection and displayed complete clearance of C. rodentium at this time point (see Fig. S2 in the supplemental material). These data clearly show that CpxRA is required for C. rodentium virulence in both the lethal and nonlethal infection models.
FIG 6.
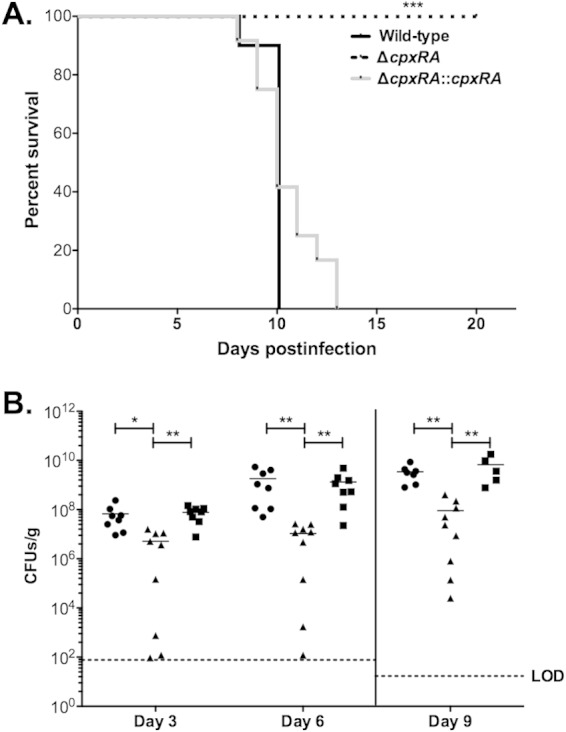
CpxRA is required for efficient infection of C3H/HeJ mice. C3H/HeJ mice were infected by oral gavage with 2 × 108 CFU of the wild-type, ΔcpxRA, or ΔcpxRA::cpxRA C. rodentium strain. (A) Each data point represents the percentage of mice still surviving from an initial population of 9 to 12 mice. Statistical analyses were performed by using a Gehan-Breslow-Wilcoxon test, and asterisks indicate statistical significance (***, P < 0.001). (B) Numbers of CFU per gram of feces (day 3 and day 6) or colon (day 9) were determined for mice infected with the wild-type (circles), ΔcpxRA (triangles), or ΔcpxRA::cpxRA (squares) strain by serial dilution plating. LOD indicates the limit of detection. The median for each group is indicated on the graph by horizontal bars. Asterisks indicate statistical significance (**, P < 0.05; **, P < 0.01), as determined by a Kruskall-Wallis test and Dunn's multiple-comparison post hoc analysis.
Dynamic CpxRA expression during infection of susceptible mice.
To examine when cpxRA is expressed during intestinal infection of susceptible mice with C. rodentium, we performed qPCR on cDNA prepared from the colon of C3H/HeJ mice on days 4 and 9 of infection. At these time points, bacterial loads in the distal colon of C3H/HeJ mice were high enough to obtain reproducible signals for expression of bacterial genes. As shown in Fig. 7, cpxA, cpxR, and cpxP were significantly more expressed in infected colons on day 4 postinfection than on day 9 postinfection. These data indicate that cpxRA expression is temporally regulated, with higher expression levels occurring during the initial stages of C. rodentium infection of susceptible mice.
FIG 7.
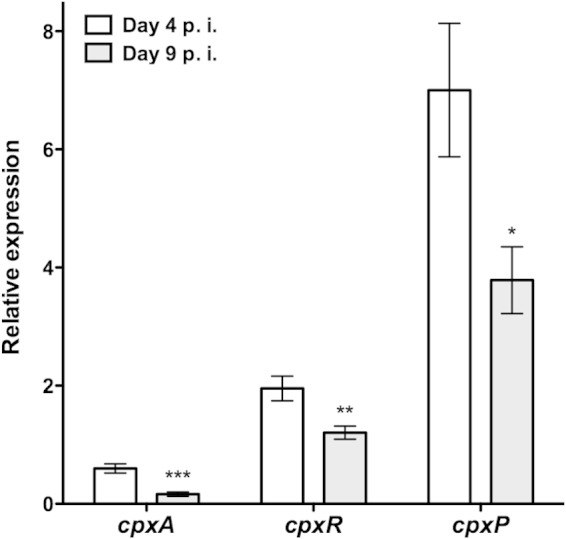
qPCR analysis of cpx gene expression during infection of C3H/HeJ mice. Data were normalized to rpoD expression levels. Relative expression is indicative of 2−ΔCT. Data shown are the means ± standard errors of the means from 5 biological samples, in triplicate. p.i., postinfection. Asterisks indicate statistical significance between data obtained on days 4 and 9 postinfection (*, P < 0.05; **, P < 0.01; ***, P < 0.001), as determined by using an unpaired t test.
DISCUSSION
TCSs are activated in response to changing environmental cues, resulting in the transcriptional regulation of gene expression that promotes bacterial adaptation (1). The CpxRA TCS responds to cell envelope stress and is thought to modulate virulence gene expression (3, 4, 26). In this study, we show that the C. rodentium CpxRA TCS plays an important role during intestinal infection. Using two different murine infection models, we consistently observed striking defects in colonization and virulence in the ΔcpxRA strain, which were restored to wild-type levels when the strain was complemented with a chromosomal insertion of cpxRA.
Our results are in good agreement with data from previous studies demonstrating a role for CpxRA in the in vivo virulence of some Gram-negative pathogens, including uropathogenic E. coli (19) and X. nematophila (44). Specifically, our results are consistent with studies that have shown that CpxRA activation has a beneficial effect on bacteria in times of envelope stress. In EPEC, CpxRA is required to adapt to envelope stress; however, CpxRA activation leads to decreased TTS gene expression; these apparently contrasting roles suggest that cpxRA expression is under tight control in vivo (13). In support of this, it was previously shown that both inhibition and constitutive activation of CpxRA in EPEC negatively affect virulence in the wax worm larva model (30). Consistent with these data, attempts to complement the C. rodentium CpxRA TCS by using low-copy-number plasmids were successful in vitro but resulted in only inconsistent complementation in vivo, further suggesting that fine-tuning of cpxRA expression is important for infection (data not shown). In addition, the consistent decreases in cpxA, cpxR, and cpxP expression levels over the course of infection in susceptible mice suggest that the temporal expression of this TCS is critical for C. rodentium virulence (Fig. 7).
The CpxRA regulon affects the expression of hundreds of genes, which results in the modulation of a myriad of functions (13). In various bacterial species, CpxRA has been implicated in the modulation of protein folding and degradation, TTS, adhesion and biofilm formation, motility, and chemotaxis. Since C. rodentium is nonmotile (45), the loss of virulence of the ΔcpxRA strain is not due to a disruption of motility. Additionally, we showed that the virulence defect of the C. rodentium ΔcpxRA strain is not due to a major defect in TTS or an altered growth rate (Fig. 5). The ΔcpxRA strain colonized the intestines of both C57BL/6J and C3H/HeJ mice to a significantly lesser extent than did the wild-type strain (Fig. 3A and C and 6B). In agreement with this observation, the murine commensal E. coli MP1 strain lacking cpxR was shown to have a marked colonization defect (46). Levels of all indicators of inflammation, pathology, and hyperplasia were significantly lower in mice infected with the ΔcpxRA strain than in mice infected with the wild-type and complemented strains (Fig. 3B and 4). We hypothesize that these altered levels of infection severity are directly related to the lower levels of colonization by the ΔcpxRA strain.
Collectively, our data suggest that C. rodentium CpxRA is required to respond to envelope stress encountered in the intestine. Possible sources of envelope stress during intestinal infection are host-derived antimicrobial peptides and proteins, complement, or pH changes. Furthermore, microbe-derived factors such as bacteriocins or other microbiota-associated antagonistic factors might also contribute to stress (47). Future experiments examining the virulence of C. rodentium ΔcpxRA strains in mutant mice lacking some of these specific host components or germfree mice that lack microbiota might lead to further insights into the specific stimuli leading to the activation of CpxRA during intestinal infection. Moreover, CpxRA may play a role in C. rodentium by modulating the expression of pili required for intestinal colonization. More work is required to identify the C. rodentium genes downstream of CpxRA that affect colonization and virulence in vivo.
In conclusion, this is the first study to identify a TCS that is essential for C. rodentium virulence. This TCS, CpxRA, appears to be involved in the early stages of intestinal colonization, as evidenced by temporal cpxRA expression during in vivo intestinal infection. In addition, the deletion of cpxRA nullified the pathogenic potential of C. rodentium in its natural host. By using single-copy chromosomal complementation, we restored the virulence of the ΔcpxRA strain. Future studies should focus on confirming the importance of fine-tuned cpxRA expression during infection by EPEC and EHEC, as this TCS presents a promising potential target for therapeutics.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by an award from the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT). S.G. is supported by a Canada Research Chair. J.-L.T. was supported by a Hugh Burke fellowship awarded from the Faculty of Medicine, McGill University. J.G. was supported by a Natural Sciences and Engineering Research Council (NSERC) undergraduate student research award.
We are grateful to Charles Dozois (INRS, Institut Armand Frappier) for providing the pSTNSK and pGP-Tn7-Gm plasmids and to Jean-Martin Lapointe (McGill University) for performing the pathology scoring. We also acknowledge Genome Quebec and the McGill Goodman Cancer Research Center histology core for sequencing and histology services, respectively. We thank B. Brett Finlay (University of British Columbia) and Tracy Raivio (University of Alberta) for helpful discussions.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00194-15.
REFERENCES
- 1.Krell T, Lacal J, Busch A, Silva-Jimenez H, Guazzaroni ME, Ramos JL. 2010. Bacterial sensor kinases: diversity in the recognition of environmental signals. Annu Rev Microbiol 64:539–559. doi: 10.1146/annurev.micro.112408.134054. [DOI] [PubMed] [Google Scholar]
- 2.Mascher T, Helmann JD, Unden G. 2006. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol Mol Biol Rev 70:910–938. doi: 10.1128/MMBR.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosma CL, Danese PN, Carlson JH, Silhavy TJ, Snyder WB. 1995. Mutational activation of the Cpx signal transduction pathway of Escherichia coli suppresses the toxicity conferred by certain envelope-associated stresses. Mol Microbiol 18:491–505. doi: 10.1111/j.1365-2958.1995.mmi_18030491.x. [DOI] [PubMed] [Google Scholar]
- 4.Danese PN, Snyder WB, Cosma CL, Davis LJ, Silhavy TJ. 1995. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev 9:387–398. doi: 10.1101/gad.9.4.387. [DOI] [PubMed] [Google Scholar]
- 5.Danese PN, Silhavy TJ. 1997. The sigma(E) and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev 11:1183–1193. doi: 10.1101/gad.11.9.1183. [DOI] [PubMed] [Google Scholar]
- 6.Pogliano J, Lynch AS, Belin D, Lin EC, Beckwith J. 1997. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev 11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- 7.Raivio TL, Silhavy TJ. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol 179:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raivio TL, Popkin DL, Silhavy TJ. 1999. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J Bacteriol 181:5263–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tschauner K, Hornschemeyer P, Muller VS, Hunke S. 2014. Dynamic interaction between the CpxA sensor kinase and the periplasmic accessory protein CpxP mediates signal recognition in E. coli. PLoS One 9:e107383. doi: 10.1371/journal.pone.0107383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danese PN, Silhavy TJ. 1998. CpxP, a stress-combative member of the Cpx regulon. J Bacteriol 180:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder WB, Davis LJ, Danese PN, Cosma CL, Silhavy TJ. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J Bacteriol 177:4216–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otto K, Silhavy TJ. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc Natl Acad Sci U S A 99:2287–2292. doi: 10.1073/pnas.042521699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raivio TL. 2014. Everything old is new again: an update on current research on the Cpx envelope stress response. Biochim Biophys Acta 1843:1529–1541. doi: 10.1016/j.bbamcr.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Gangaiah D, Zhang X, Fortney KR, Baker B, Liu Y, Munson RS Jr, Spinola SM. 2013. Activation of CpxRA in Haemophilus ducreyi primarily inhibits the expression of its targets, including major virulence determinants. J Bacteriol 195:3486–3502. doi: 10.1128/JB.00372-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raivio TL, Leblanc SK, Price NL. 2013. The Escherichia coli Cpx envelope stress response regulates genes of diverse function that impact antibiotic resistance and membrane integrity. J Bacteriol 195:2755–2767. doi: 10.1128/JB.00105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. 2005. Citrobacter rodentium of mice and man. Cell Microbiol 7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 17.Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. 2012. Fucose sensing regulates bacterial intestinal colonization. Nature 492:113–117. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitobe J, Arakawa E, Watanabe H. 2005. A sensor of the two-component system CpxA affects expression of the type III secretion system through posttranscriptional processing of InvE. J Bacteriol 187:107–113. doi: 10.1128/JB.187.1.107-113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debnath I, Norton JP, Barber AE, Ott EM, Dhakal BK, Kulesus RR, Mulvey MA. 2013. The Cpx stress response system potentiates the fitness and virulence of uropathogenic Escherichia coli. Infect Immun 81:1450–1459. doi: 10.1128/IAI.01213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbert Tran EE, Andersen AW, Goodrich-Blair H. 2009. CpxRA influences Xenorhabdus nematophila colonization initiation and outgrowth in Steinernema carpocapsae nematodes through regulation of the nil locus. Appl Environ Microbiol 75:4007–4014. doi: 10.1128/AEM.02658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Loughlin JL, Spinner JL, Minnich SA, Kobayashi SD. 2010. Yersinia pestis two-component gene regulatory systems promote survival in human neutrophils. Infect Immun 78:773–782. doi: 10.1128/IAI.00718-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labandeira-Rey M, Dodd D, Fortney KR, Zwickl B, Katz BP, Janowicz DM, Spinola SM, Hansen EJ. 2011. A Haemophilus ducreyi CpxR deletion mutant is virulent in human volunteers. J Infect Dis 203:1859–1865. doi: 10.1093/infdis/jir190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinola SM, Fortney KR, Baker B, Janowicz DM, Zwickl B, Katz BP, Blick RJ, Munson RS Jr. 2010. Activation of the CpxRA system by deletion of cpxA impairs the ability of Haemophilus ducreyi to infect humans. Infect Immun 78:3898–3904. doi: 10.1128/IAI.00432-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphreys S, Rowley G, Stevenson A, Anjum MF, Woodward MJ, Gilbert S, Kormanec J, Roberts M. 2004. Role of the two-component regulator CpxAR in the virulence of Salmonella enterica serotype Typhimurium. Infect Immun 72:4654–4661. doi: 10.1128/IAI.72.8.4654-4661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slamti L, Waldor MK. 2009. Genetic analysis of activation of the Vibrio cholerae Cpx pathway. J Bacteriol 191:5044–5056. doi: 10.1128/JB.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacRitchie DM, Ward JD, Nevesinjac AZ, Raivio TL. 2008. Activation of the Cpx envelope stress response down-regulates expression of several locus of enterocyte effacement-encoded genes in enteropathogenic Escherichia coli. Infect Immun 76:1465–1475. doi: 10.1128/IAI.01265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nevesinjac AZ, Raivio TL. 2005. The Cpx envelope stress response affects expression of the type IV bundle-forming pili of enteropathogenic Escherichia coli. J Bacteriol 187:672–686. doi: 10.1128/JB.187.2.672-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giron JA, Ho AS, Schoolnik GK. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- 29.MacRitchie DM, Acosta N, Raivio TL. 2012. DegP is involved in Cpx-mediated posttranscriptional regulation of the type III secretion apparatus in enteropathogenic Escherichia coli. Infect Immun 80:1766–1772. doi: 10.1128/IAI.05679-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leuko S, Raivio TL. 2012. Mutations that impact the enteropathogenic Escherichia coli Cpx envelope stress response attenuate virulence in Galleria mellonella. Infect Immun 80:3077–3085. doi: 10.1128/IAI.00081-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 32.Donnenberg MS, Kaper JB. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun 59:4310–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crepin S, Harel J, Dozois CM. 2012. Chromosomal complementation using Tn7 transposon vectors in Enterobacteriaceae. Appl Environ Microbiol 78:6001–6008. doi: 10.1128/AEM.00986-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bäumler AJ, Tsolis RM, van der Velden AW, Stojiljkovic I, Anic S, Heffron F. 1996. Identification of a new iron regulated locus of Salmonella typhi. Gene 183:207–213. doi: 10.1016/S0378-1119(96)00560-4. [DOI] [PubMed] [Google Scholar]
- 35.DiGiuseppe PA, Silhavy TJ. 2003. Signal detection and target gene induction by the CpxRA two-component system. J Bacteriol 185:2432–2440. doi: 10.1128/JB.185.8.2432-2440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller JH. 1992. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 37.Thomassin JL, He X, Thomas NA. 2011. Role of EscU auto-cleavage in promoting type III effector translocation into host cells by enteropathogenic Escherichia coli. BMC Microbiol 11:205. doi: 10.1186/1471-2180-11-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gauthier A, Puente JL, Finlay BB. 2003. Secretin of the enteropathogenic Escherichia coli type III secretion system requires components of the type III apparatus for assembly and localization. Infect Immun 71:3310–3319. doi: 10.1128/IAI.71.6.3310-3319.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomassin JL, Brannon JR, Gibbs BF, Gruenheid S, Le Moual H. 2012. OmpT outer membrane proteases of enterohemorrhagic and enteropathogenic Escherichia coli contribute differently to the degradation of human LL-37. Infect Immun 80:483–492. doi: 10.1128/IAI.05674-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Sage V, Zhu L, Lepage C, Portt A, Viau C, Daigle F, Gruenheid S, Le Moual H. 2009. An outer membrane protease of the omptin family prevents activation of the Citrobacter rodentium PhoPQ two-component system by antimicrobial peptides. Mol Microbiol 74:98–111. doi: 10.1111/j.1365-2958.2009.06854.x. [DOI] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 42.Diez E, Zhu L, Teatero SA, Paquet M, Roy MF, Loredo-Osti JC, Malo D, Gruenheid S. 2011. Identification and characterization of Cri1, a locus controlling mortality during Citrobacter rodentium infection in mice. Genes Immun 12:280–290. doi: 10.1038/gene.2010.76. [DOI] [PubMed] [Google Scholar]
- 43.Vallance BA, Deng W, Jacobson K, Finlay BB. 2003. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect Immun 71:3443–3453. doi: 10.1128/IAI.71.6.3443-3453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herbert EE, Cowles KN, Goodrich-Blair H. 2007. CpxRA regulates mutualism and pathogenesis in Xenorhabdus nematophila. Appl Environ Microbiol 73:7826–7836. doi: 10.1128/AEM.01586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petty NK, Bulgin R, Crepin VF, Cerdeno-Tarraga AM, Schroeder GN, Quail MA, Lennard N, Corton C, Barron A, Clark L, Toribio AL, Parkhill J, Dougan G, Frankel G, Thomson NR. 2010. The Citrobacter rodentium genome sequence reveals convergent evolution with human pathogenic Escherichia coli. J Bacteriol 192:525–538. doi: 10.1128/JB.01144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lasaro M, Liu Z, Bishar R, Kelly K, Chattopadhyay S, Paul S, Sokurenko E, Zhu J, Goulian M. 2014. Escherichia coli isolate for studying colonization of the mouse intestine and its application to two-component signaling knockouts. J Bacteriol 196:1723–1732. doi: 10.1128/JB.01296-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buffie CG, Pamer EG. 2013. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schauer DB, Falkow S. 1993. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect Immun 61:4654–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roland K, Curtiss R III, Sizemore D. 1999. Construction and evaluation of a Δcya Δcrp Salmonella typhimurium strain expressing avian pathogenic Escherichia coli O78 LPS as a vaccine to prevent airsacculitis in chickens. Avian Dis 43:429–441. doi: 10.2307/1592640. [DOI] [PubMed] [Google Scholar]
- 50.Edwards RA, Keller LH, Schifferli DM. 1998. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207:149–157. doi: 10.1016/S0378-1119(97)00619-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.