Abstract
Obesity is an increasingly prevalent disease worldwide. While genetic and environmental factors are known to regulate the development of obesity and associated metabolic diseases, emerging studies indicate that innate and adaptive immune cell responses in adipose tissue have critical roles in the regulation of metabolic homeostasis. In the lean state, type 2 cytokine-associated immune cell responses predominate in white adipose tissue and protect against weight gain and insulin resistance through direct effects on adipocytes and elicitation of beige adipose. In obesity, these metabolically beneficial immunologic pathways become dysregulated, and adipocytes and other factors initiate metabolically deleterious type 1 inflammation that impairs glucose metabolism. This review discusses our current understanding of the functions of different types of adipose tissue, how immune cells regulate adipocyte function and metabolic homeostasis in the context of health and disease, and highlights the potential of targeting immuno-metabolic pathways as a therapeutic strategy to treat obesity and associated diseases.
INTRODUCTION
Obesity is in an increasingly prevalent metabolic disease characterized by excess accumulation of adipose tissue. Obesity increases the risk of developing a wide variety of diseases including but not limited to type 2 diabetes, cardiovascular diseases and multiple forms of cancer, and has been strongly associated with increased mortality (Collaboration, 2009; Flegal et al., 2013; Oliveros and Villamor, 2008; Pi-Sunyer, 1999; Pontiroli and Morabito, 2011; Reilly and Kelly, 2011; Rodriguez et al., 2001). In the past few decades, the prevalence of obesity has risen dramatically in both industrialized and less industrialized nations across all continents (Kelly et al., 2008; Ng et al., 2014), and this has been associated with high healthcare expenditures (Withrow and Alter, 2011). For example, in the United States (U.S.) in 2009–2010, obesity afflicted 36% of adults (Flegal et al., 2012; Ogden et al., 2012, 2014) and accounted for approximately $190 billion in annual healthcare costs, representing nearly 20% of total national healthcare expenditures that year (Cawley and Meyerhoefer, 2012; Finkelstein et al., 2009). More recent statistics indicate that 35% of adults in the U.S. were obese in 2011–2012 but was as high as 48% in some segments of the population (Ogden et al., 2014). Therefore, obesity is a critical problem with major health and economic consequences. Increasing our understanding of the pathways involved in the development of obesity will be critical for the development of new intervention strategies to prevent or treat this disease and its associated co-morbidities.
As in many chronic inflammatory diseases, genetic and environmental factors are important for the development of obesity and associated diseases (Bouchard, 2008; Brestoff and Artis, 2013; McCarthy, 2010; Walley et al., 2009). In addition, emerging studies have implicated various cell types of the immune system as critical regulators of metabolic homeostasis (Jin et al., 2013; Lumeng and Saltiel, 2011; Odegaard and Chawla, 2011, 2013b; Osborn and Olefsky, 2012). Seminal studies connecting the immune system to metabolic dysfunction in obesity indicated that tumor necrosis factor-α (TNF-α) production was upregulated in obese mice and that neutralization of TNF-α improved glucose uptake in murine obesity (Hotamisligil et al., 1993). Subsequent studies revealed that mice lacking TNF-α were protected from high fat diet-induced insulin resistance (Uysal et al., 1997). Increased TNF- α production was also observed in human obesity, and weight loss in humans was associated with decreased TNF-α levels (Hotamisligil et al., 1995; Kern et al., 1995). Later it was discovered that proinflammatory macrophages accumulate in adipose of obese mice and that these cells were dominant sources of TNF- α to promote insulin resistance (Weisberg et al., 2003; Xu et al., 2003). Collectively, these studies revealed that obesity is associated with chronic low-grade inflammation and suggested that inflammatory responses had detrimental metabolic consequences. It is now appreciated that in obesity chronic low-grade inflammation occurs in many organs including but not limited to white adipose tissue (WAT), brown adipose tissue (BAT), pancreas, liver, brain, muscle and intestine (Cildir et al., 2013). Of these, WAT is the most studied organ in terms of immune-metabolic interactions in obesity.
In white adipose tissue (WAT), which coordinates metabolism at distant tissues such as the brain, liver, pancreas and muscle, there is a diverse set of immune cells at steady state (Exley et al., 2014; Ibrahim, 2010; McNelis and Olefsky, 2014; Mraz and Haluzik, 2014). This network of immune cells appears to be poised to recognize, integrate and respond to environmental signals including bacterial products, endogenous lipid species and hormones in order to coordinate metabolism (Odegaard and Chawla, 2013a). Changes in immune cell composition and function in WAT have been closely associated with obesity and the regulation of metabolic homeostasis, and disruption of this network of immune cells can have either detrimental or beneficial effects on mammalian health (Exley et al., 2014; Lumeng and Saltiel, 2011; Mraz and Haluzik, 2014; Odegaard and Chawla, 2013b; Osborn and Olefsky, 2012). In addition, recent work has demonstrated that immune system-associated transcription factors including but not limited to Nuclear factor-κ B (NK-κB), c-Jun kinase (JNK) and Interferon regulatory factor 4 (IRF4) are key regulators of metabolic homeostasis (Lumeng and Saltiel, 2011; Osborn and Olefsky, 2012). Conversely, metabolite-sensing receptors such as Peroxisome proliferator-activated receptor (PPAR)-γ, Farnesoid X receptor (FXR), Liver X receptor (LXR), G protein coupled receptor 120 (GPR120) and Carbohydrate-responsive element binding protein (ChREBP) among others have been shown to regulate immune responses (Brestoff and Artis, 2013; Glass and Saijo, 2010; Hotamisligil and Erbay, 2008; Shoelson et al., 2007; Zelcer and Tontonoz, 2006). Therefore, dissecting the complex interactions between immune and metabolic systems will provide important insights into the biology underlying obesity and have implications for understanding how current and future therapeutics might influence metabolism.
The purpose of this review is to describe our current understanding of how immune cells in adipose tissue regulate metabolism. First, we will summarize recent advances in understanding the roles of white, beige and brown adipose tissues in the regulation of weight gain. Second, we will describe the immune cell composition of adipose tissue at steady state and discuss how these immune cell pathways interact and contribute to the maintenance of metabolic homeostasis. Third, we will discuss immunologic changes that occur in adipose in the setting of obesity and highlight how these changes contribute to metabolic dysfunction. Finally, we will discuss potential therapeutic implications of targeting the immune system to treat obesity and its associated diseases.
Roles of adipose tissues in the regulation of obesity
Mammals possess multiple types of adipose tissues including white, brown and beige adipose. These tissues are found in distinct anatomic locations and are comprised of different adipocyte cell types – white, beige and/or brown – that have unique developmental and functional properties that are critical for host metabolism (Fig. 1) (Bartelt and Heeren, 2014; Cannon and Nedergaard, 2004; Harms and Seale, 2013; Ibrahim, 2010; Peirce et al., 2014; Pfeifer and Hoffmann, 2014; Wu et al., 2013). This section describes white, brown and beige adipocyte cell types and summarizes their roles in regulating weight gain.
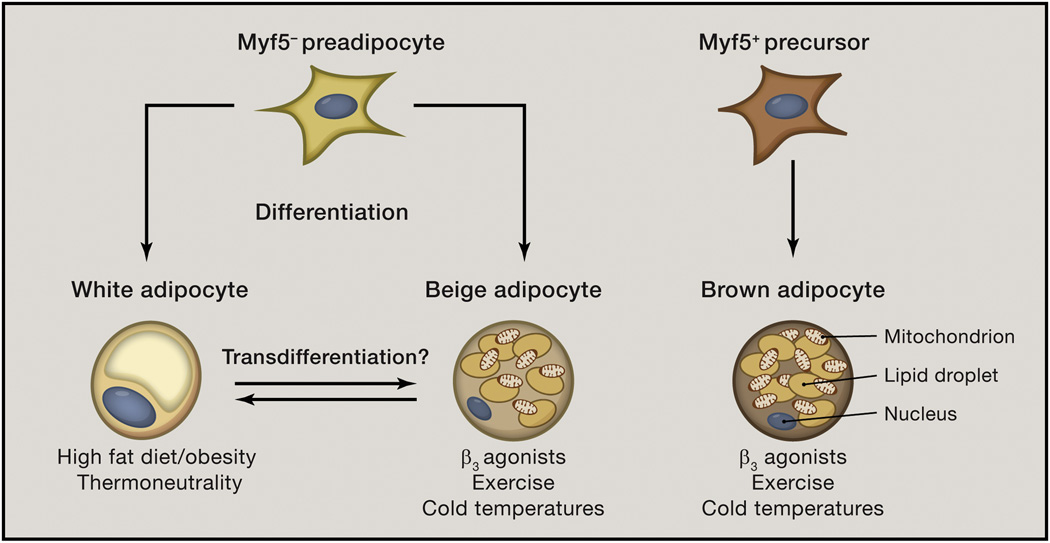
Figure 1. White, beige and brown adipocytes are developmentally and functionally distinct cell populations.
White and beige adipocytes arise from a Myf5− precursor cell population that is bipotent. These pre-adipocytes give rise to white or beige adipocytes depending on the stimulus and physiologic setting. White adipocytes are promoted by high fat diet feeding or obesity and by thermoneutrality (30°C in mice). Beige adipocytes are elicited by β3 adrenergic receptor agonists such as norepinephrine or epinephrine, and are recruited within white adipose tissue in the settings of chronic exercise or exposure to cold environmental temperatures. Although white and beige adipocytes emerge from pre-adipocytes via cell differentiation, mature white and beige adipocytes might undergo a process called transdifferentiation, in which one cell type acquires phenotypic characteristics of the other. There is uncertainty about whether transdifferentiation occurs. In contrast, brown adipocytes arise from a Myf5+ precursor cell population and are present in discrete brown adipose tissue depots. Despite being developmentally distinct cell populations, beige and brown adipocytes are activated by similar physiologic stimuli, including exercise- and cold temperature-induced hormones and metabolites.
White adipocytes
White adipose tissue (WAT) is distributed throughout the mammalian body in subcutaneous depots and in association with organs, where it has important roles in insulation and physical protection of the viscera, and is compromised predominantly of white adipocytes (Peirce et al., 2014; Pfeifer and Hoffmann, 2014). These specialized cell types arise from a Myf5− pre-adipocyte lineage and store large amounts of triglycerides in a single large lipid droplet (Pfeifer and Hoffmann, 2014; Sanchez-Gurmaches and Guertin, 2014). In addition to their ability to store triglycerides, white adipocytes respond to hormonal signals to induce lipolysis and release free fatty acids (FFA) into the circulation for oxidation or storage by other cell types (Arner et al., 2011; Bartness et al., 2010). Therefore white adipocytes are critical for regulating both fat storage and release. Beyond this function, white adipocytes produce various adipocyte-specific hormones (also known as adipokines) including but not limited to leptin, resistin, retinol binding protein 4 (RBP4), fibroblast grown factor 21 (FGF21) and adiponectin that regulate metabolic homeostasis by acting on distant organs such as the brain, kidney, liver, pancreas and skeletal muscle (Allison and Myers, 2014; Itoh, 2014; Kadowaki et al., 2006; Kershaw and Flier, 2004; Kotnik et al., 2011; Lazar, 2007; Ouchi et al., 2011).
In the context of obesity, mature white adipocytes undergo proliferation and become hypertrophic through accumulation of triglycerides to increase the number and size of the adipocyte pool in WAT (Foster and Bartness, 2006; Hausman et al., 2001; Kubota et al., 1999; Spalding et al., 2008). As WAT expands, adipocytes increase production of leptin to suppress food intake and therefore limit the rate of triglyceride accumulation and adipocyte expansion (Allison and Myers, 2014). In obesity, this food intake-suppressive effect of upregulated leptin production is blunted by the development of resistance to leptin action (Park and Ahima, 2015). Leptin also has pro-inflammatory effects by eliciting type 1 effector cytokine production by immune cells (Attie and Scherer, 2009; Gregor and Hotamisligil, 2011; Mraz and Haluzik, 2014; Ouchi et al., 2011). In addition to leptin, RBP4 and resistin production by white adipocytes are increased in obesity, and both of these factors promote insulin resistance in mice (Kotnik et al., 2011; Lazar, 2007; Moraes-Vieira et al., 2014; Norseen et al., 2012; Steppan et al., 2001; Yang et al., 2005). Upregulation of RBP4 expression has been linked to inappropriate sensing of low glucose levels by adipocytes (Yang et al., 2005), whereas resistin production is increased due to elevated levels of insulin and TNF-α among other factors (Lazar, 2007; Song et al., 2002). Both of these factors appear to promote insulin resistance in mice, at least in part, through elicitation of type 1 immune responses in WAT (Jung and Choi, 2014; Ouchi et al., 2011). Thus, leptin, resistin and RBP4 are pro-inflammatory factors that are increased in obesity and that stimulate type 1 immunity in WAT.
In contrast, adiponectin has anti-inflammatory and insulin-sensitizing effects (Gregor and Hotamisligil, 2011; Kadowaki et al., 2006; Lumeng and Saltiel, 2011). Adiponectin production by white adipocytes is decreased in the context of obesity (Ouchi et al., 2011), and loss of this anti-inflammatory signal may be one factor that contributes to the development of inflammation in WAT in obesity. Other adipokines are also emerging as potential anti-inflammatory factors. In particular, recent work suggests that FGF21 might have immunosuppressive effects through inhibition of the transcription factor Nuclear factor-κ B (NF-κB) (Yu et al., 2015), a master regulator of inflammatory gene expression. Additional research is needed to determine whether FGF21 regulates inflammation in obesity and to determine whether the immune system regulates expression of FGF21. Other adipokines such as Fatty acid binding protein 4 (also known as Adipocyte protein 2) and Neuregulin 4 (NRG4) that have important roles in regulating metabolic responses in the liver and other tissues have also been associated with inflammatory markers (Cao et al., 2013; Terra et al., 2011; Wang et al., 2014), however future research will be needed to determine whether these adipokines are direct pro- or anti-inflammatory mediators in WAT in obesity. Therefore white adipocytes appear to link metabolic status of mammals to immune responses in WAT.
Brown adipocytes
Although brown adipocytes can also produce adipokines, these cells differ from white adipocytes developmentally and functionally and in their anatomic distribution. In mice, brown adipocytes arise from a Myf5+ lineage and are developmentally more closely related to skeletal muscle cells than to white adipocytes (Kajimura et al., 2009; Seale et al., 2008). The primary function of brown adipocytes in both mice and humans is to convert chemical energy into heat via Uncoupling protein 1 (UCP1) (Cannon and Nedergaard, 2004). UCP1 is a long chain fatty acid/proton symporter that dissipates the mitochondrial electrochemical gradient, thereby uncoupling energy substrate oxidation from ATP synthesis and resulting in the generation of heat (Fedorenko et al., 2012; Matthias et al., 2000). This process is critical for maintaining core body temperature and in the regulation of weight gain by increasing whole body energy expenditure. In the latter case, for example, mice that lack UCP1 develop obesity when housed at thermoneutrality (30°C) as compared to UCP1-sufficient controls (Feldmann et al., 2009). Although global deletion of UCP1 impairs thermogenesis in both brown and beige adipocytes (see below), interscapular brown adipose tissue (BAT) is believed to be an important regulator of metabolic rate and obesity susceptibility in mice.
Brown adipocytes are found in discrete BAT depots. In mice, the largest BAT depot is present in the interscapular space, and smaller depots are present in the axilla and along the cervical column (Bartelt and Heeren, 2014). In humans, brown adipocytes are abundant in newborns including in the interscapular space (Hondares et al., 2014), whereas in lean adult humans brown adipocytes are reported to be present in the supraclavicular space of the neck and adjacent to the cervical column and heart (van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009). Subsequent studies have determined that supraclavicular neck BAT in healthy adults exhibits a gene expression profile and has morphologic characteristics consistent with those of beige adipocytes (Lee et al., 2014b; Sharp et al., 2012; Wu et al., 2012).
Beige adipocytes
Like brown adipocytes, beige adipocytes (also known as brown-like or brite adipocytes) express high levels of UCP1 and store triglyceride in one or more lipid droplets, giving rise to a paucilocular or multilocular cell morphology. Although beige and brown adipocytes have similar UCP1-dependent functional characteristics, these cell types differ from each other developmentally and anatomically. Brown adipocytes arise from a Myf5+ precursor and are developmentally related to skeletal muscle cells (Kajimura et al., 2009; Seale et al., 2008), whereas beige adipocytes arise from Myf5− precursors within WAT and are developmentally related to smooth muscle cells (Long et al., 2014) and/or white adipocytes (Sanchez-Gurmaches and Guertin, 2014). Beige adipocytes may arise from multiple developmental pathways, including differentiation from committed beige pre-adipocytes as well as white-to-beige transdifferentiation in which mature white adipocytes are converted into beige adipocytes (Fig. 1) (Barbatelli et al., 2010; Harms and Seale, 2013; Moisan et al., 2014; Sanchez-Gurmaches and Guertin, 2014; Wang et al., 2013b; Wu et al., 2012). While differentiation is a well-accepted developmental pathway for beige adipocytes, white-to-beige transdifferentiation is a controversial topic, and further research is needed to determine whether this process contributes to beige adipogenesis in different physiologic contexts or in response to different stimuli. In mice, beige adipocytes are enriched in subcutaneous WAT compared to visceral fat. However, beige adipocytes are elicited in both types of WAT (Qiu et al., 2014), and future studies will be required to determine whether beige adipocytes in subcutaneous and visceral fat differ from each other developmentally and/or functionally.
Beige adipocytes are elicited in response to environmental stimuli including exposure to cold environmental temperatures or in response to exercise, and these cells are activated or induced to differentiate in response to a range of small molecules and hormones. Factors that stimulate beige adipocyte development or activation include norepinephrine (NE) (Qiu et al., 2014; Rao et al., 2014), adenosine (Gnad et al., 2014), lactate (Carriere et al., 2014), β-aminoisobutyric acid (BAIBA) (Roberts et al., 2014), prostaglandin E2 (PGE2) (Madsen et al., 2010), parathyroid hormone-related protein (PTHrp) (Fisher et al., 2012), bone morphogenetic protein 4 (BMP4) (Qian et al., 2013), BMP7 (Schulz et al., 2011), fibroblast growth factor 21 (FGF21) (Fisher et al., 2012), irisin (Wu et al., 2012) and meteorin-like (Rao et al., 2014). Although many factors are known to promote beige adipocyte differentiation or function, it remains unknown whether these factors act on different subsets of beige adipocytes or whether they elicit developmentally or functionally distinct subsets of beige adipocytes. In addition, it is unclear to what extent these factors coordinately regulate beige adipocyte function.
Nonetheless, recent studies indicate that selective deletion of beige but not brown adipocytes results in impaired glucose homeostasis and increased susceptibility to weight gain in response to high fat diet (HFD) feeding (Cohen et al., 2014). Conversely, mice with increased beige adipocytes exhibit increased metabolic rate and decreased obesity (Wang et al., 2013a). Consistent with this finding, 129Sv mice that are resistant to diet-induced obesity exhibit increased beiging of WAT compared to C57BL/6 mice that are susceptible to diet-induced obesity (Shabalina et al., 2013). Inhibition of beige adipocytes by supplementing HFD chow with indomethacin resulted in increased weight gain following HFD feeding in 129Sv mice (Madsen et al., 2010). Collectively, these data indicate that impaired beiging is associated with increased weight gain and suggest that elicitation of beige adipocytes can combat obesity in mice.
Recent studies support the relevance of these findings in humans. In the context of human obesity, the incidence of brown/beige adipose tissue (Sharp et al., 2012) is significantly decreased in obese patients compared to lean patients (Saito et al., 2009). In addition, UCP1 expression was found to be higher in cultured beige adipocytes from lean patients as compared to obese patients, suggesting that beige adipocyte differentiation or activation is impaired in human obesity (Carey et al., 2014). Consistent with this, ephedrine-induced activation of brown/beige adipose tissue is impaired in human obesity (Carey et al., 2013). While the role of brown/beige adipocytes in the regulation of energy expenditure in humans is unclear, these studies suggest that obesity is associated with loss of beige fat function and raise the possibility that defective beige fat may have deleterious metabolic effects including increased susceptibility to weight gain.
Immune cell composition and function in lean white adipose tissue
White adipose is a heterogeneous tissue comprised of multiple cell types including a diverse array of immune cells. In the steady state, these immune cells tend to be associated with the type 2 immune axis and include alternatively activated macrophages (AAMacs), eosinophils, group 2 innate lymphoid cells (ILC2s), invariant natural killer T (iNKT) cells, T helper type 2 (Th2) cells, regulatory T (Treg) cells and other immune cell populations that communicate with each other and participate in cross-talk with adipocytes. As discussed below, the interface between immune cells and adipocytes contributes to the regulation of lipid storage, glucose utilization, redox balance and energy expenditure (Fig. 2). However, in the context of obesity, type 2 immune cells in WAT become dysregulated and, in some cases, acquire a proinflammatory phenotype that exacerbates adipose tissue inflammation with deleterious effects on metabolism. In this section, we discuss emerging concepts in the composition and function of immune cells in WAT at steady state and how these cells contribute to protection from diet-induced obesity.
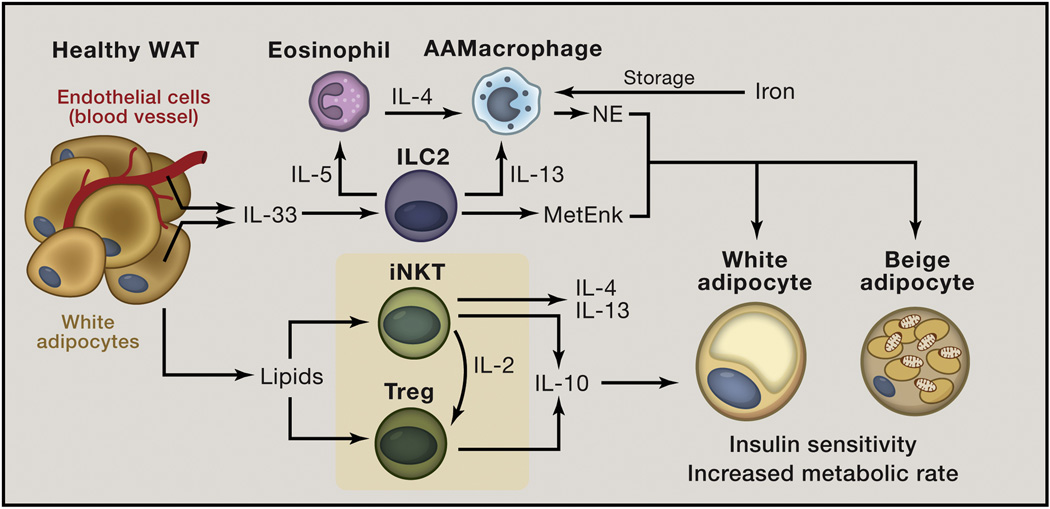
Figure 2. Healthy white adipose tissue (WAT) is enriched in type 2 cytokine-associated immune cells.
In the lean state, adipocytes and endothelial cells in WAT constitutively produce interleukin (IL)-33 that can act on Group 2 innate lymphoid cells (ILC2s) to induce production of IL-5 and IL-13 that sustain eosinophil (Eos) and alternatively activated macrophage (AAMac) responses, respectively, in WAT. In addition, eosinophils produce IL-4 that is necessary to maintain AAMac responses in WAT. AAMacs have multiple functions to maintain metabolic homeostasis, including storing large amounts of iron, leading to sequestration of this pro-oxidative metal cation from adipocytes to prevent lipid peroxidation, oxidative damage to proteins and mitochondrial dysfunction. In addition, AAMacs produce norepinephrine (NE) that acts on both white and beige adipocytes via the β3 adrenergic receptor to stimulate lipolysis and elicit beige adipocytes that increase metabolic rate. ILC2s can also promote beiging through production of methionine-enkpehalin (MetEnk) peptides. In addition, adipocytes present lipid antigen to invariant natural killer T (iNKT) cells to elicit a unique tissue-specific anti-inflammatory phenotype characterized by increased production of IL-4 and IL-13, which may act on AAMacs, and IL-10. Lipids from adipocytes are also believed to promote regulatory T (Treg) responses in WAT to induce production of IL-10. iNKT cells are critical sources of IL-2 and are necessary to sustain Tregs in WAT. IL-10 production by iNKT cells, Tregs and other cell types promote insulin action in white adipocytes to facilitate maintenance of an insulin-sensitive state. Together, these pathways contribute to metabolically healthy WAT.
The IL-4/Alternatively activated macrophage pathway
Alternatively activated macrophages (AAMac) are IL-4- and IL-13-dependent cells that require Signal transducer and activator of transcription 6 (STAT6) to maintain an alternative activation state that is distinct from the classical activation state induced by interferon-γ (IFNγ) (Gordon and Martinez, 2010; Hill et al., 2014; Martinez et al., 2009; Odegaard and Chawla, 2011; Olefsky and Glass, 2010). AAMacs are critical for protective immunity against helminth pathogens and are associated with pathologic allergic inflammation in the lung (Gordon and Martinez, 2010; Martinez et al., 2009). Recent studies have also implicated AAMacs in the regulation of metabolic homeostasis (Hill et al., 2014; Odegaard and Chawla, 2011; Olefsky and Glass, 2010). Early studies linking AAMacs to metabolism demonstrated that macrophage-specific deletion of Peroxisome proliferator-associated receptor (PPAR)-γ results in decreased AAMacs in WAT in association with increased weight gain, elevated fat accumulation, impaired glucose uptake and insulin resistance following HFD feeding (Odegaard et al., 2007). In addition, deletion of PPAR-δ in leukocytes also impaired AAMac responses and was associated with increased adiposity and insulin resistance (Odegaard et al., 2008). These loss-of-function studies are complemented by gain-of-function approaches indicating that administration of exogenous IL-4 to mice to boost AAMac responses protected mice from HFD-induced obesity and insulin resistance (Chang et al., 2012; Ricardo-Gonzalez et al., 2010). Together, these studies identified a critical role for the IL-4/AAMac axis in the regulation of metabolic homeostasis.
Emerging work has revealed that AAMacs employ a diverse functional repertoire to regulate metabolism. For example, a subset of AAMacs in WAT can express genes involved in iron handling and has the capacity to store large amounts of iron in lean mice (Orr et al., 2014). The capacity of these specialized AAMacs to store iron is diminished in the setting of obesity, leading to abnormal accumulation of iron in adipocytes (Orr et al., 2014) that may lead to iron-initiated production of highly toxic lipid aldehydes that damage cellular proteins, impair adipocyte insulin sensitivity and lead to mitochondrial dysfunction (Curtis et al., 2010; Curtis et al., 2012). Therefore it appears that specialized iron-storing AAMacs have an essential role in regulating iron homeostasis in WAT to maintain optimal glucose metabolism and mitochondrial function at steady state, and dysregulation of these cells in obesity may have deleterious effects on glucose and energy homeostasis. Consistent with this, treatment of obese mice with the iron chelator deferoxamine is associated with improved glucose tolerance, decreased body weight and fat mass, and less inflammation in fat compared to vehicle-treated obese controls (Tajima et al., 2012).
In addition, AAMacs from adipose are capable of producing catecholamines such as norepinephrine (NE) that act on adipocytes via the β3 adrenergic receptor (β3AR) to stimulate lipolysis, activate mitochondrial biogenesis and upregulate expression of UCP1 (Fig. 2 and fig. 3) (Liu et al., 2014; Nguyen et al., 2011; Qiu et al., 2014; Rao et al., 2014). AAMac-derived catecholamine production was described in the setting of brown adipocyte activation (Nguyen et al., 2011). Mice that lack IL-4 and IL-13 (Il4/13−/−); the shared receptor for these cytokines, IL-4Rα (Il4ra−/−); or STAT6, a transcription factor downstream of IL-4Rα signaling (Stat6−/−), had defective AAMac responses (Martinez et al., 2009) and exhibited decreased UCP1 expression in BAT (Nguyen et al., 2011). This defect in UCP1 expression in BAT was associated with impaired maintenance of core body temperature following acute exposure to cold environmental temperatures over a 6 hour period compared to wild-type controls (Nguyen et al., 2011). These findings suggested that IL-4/IL-4Rα-dependent activation of AAMacs stimulated production of NE that was necessary for brown adipocyte activation in the setting of short-term cold exposure over 6 hours. However, subsequent studies indicated that IL-4/IL-4Rα-dependent AAMacs were dispensable for brown adipocyte activation in the setting of more prolonged cold exposure over 72 hours (Qiu et al., 2014). This suggests that there may be time-dependent factors that influence whether the IL-4/AAMac/NE pathway activates brown adipocytes, a topic that should be investigated further.
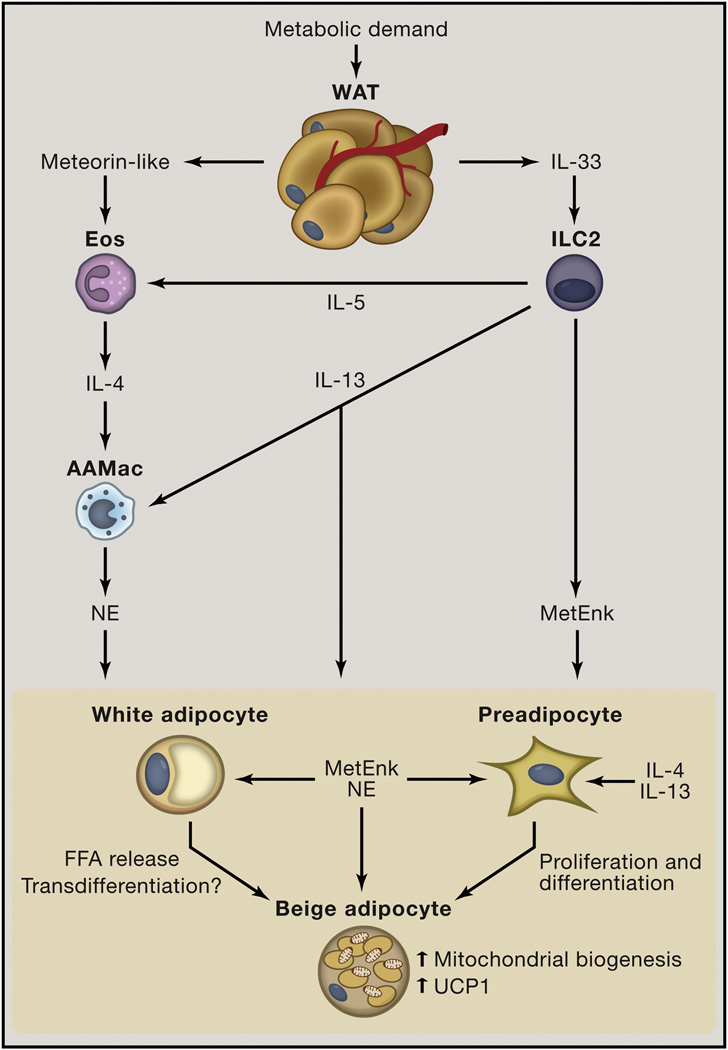
Figure 3. Immunologic mechanisms that regulate beiging.
In the context of chronic exposure to cold environmental temperatures or chronic exercise, white adipose tissue (WAT) and muscle produce the adipokine/myokine meteorin-like. This hormone promotes eosinophil (Eos) accumulation in WAT. Whether other factors contribute to increased Eos in WAT to promote beiging remains unknown. Meteorin-like induces interleukin (IL)-4 and IL-13 production by Eos and possibly other cell types. IL-4 and perhaps IL-13 act on AAMacs to stimulate norepinephrine (NE) production. NE acts to stimulate beiging via differentiation and/or transdifferentiation pathways and to activate existing beige adipocytes, resulting in mitochondrial biogenesis, Uncoupling protein 1 (UCP1) upregulation and UCP1-dependent increases in energy expenditure. In addition, WAT produces the cytokine IL-33 that is critical for maintaining Group 2 innate lymphoid cell (ILC2) responses in WAT. IL-33 stimulates ILC2s to produce IL-5 and IL-13 that sustain the eosinophil/AAMac pathways that can contribute to beiging. In addition, IL-13 can act on pre-adipocytes to promote their proliferation and induce differentiation to beige adipocytes. Further, IL-33 stimulates ILC2s to produce methionine-enkephalin (MetEnk) peptides that can directly promote beiging. Therefore AAMacs and ILC2s both contribute to beiging through production of distinct effector molecules.
The IL-4/AAMac/NE pathway is also critical for activating beige adipocytes in WAT (Fig. 3) (Qiu et al., 2014; Rao et al., 2014). Mice lacking IL-4/IL-13, IL-4Rα or STAT6 failed to undergo optimal beiging of WAT and had decreased metabolic rate compared to wildtype controls after 72 hours of exposure to cold environmental temperatures (Qiu et al., 2014; Rao et al., 2014). This phenotype appears to be mediated by macrophage-intrinsic production of norepinephrine, as mice that lack the rate-limiting step of catecholamine synthesis, tyrosine hydroxylase (TH), in LysM-Cre-expressing cells such as macrophages exhibit impaired beige adipose development and exhibit a 10–15% decrease in whole body oxygen consumption in the setting of chronic exposure to mild (room temperature) or severe (5°C) cold environmental temperatures (Qiu et al., 2014). This defect in metabolic rate suggests that AAMac-derived catecholamines might also be important for limiting weight gain. Consistent with this, mice that lack Receptor interacting protein 140 (RIP140), a transcriptional co-regulator that regulates metabolism in various tissues and that suppresses mitochondrial uncoupling (Rosell et al., 2011), had increased AAMac responses in WAT and increased beiging that was associated with protection from diet-induced obesity (Liu et al., 2014). In addition, treatment of mice with exogenous IL-4 elicits beiging and increases oxygen consumption levels in an UCP1-dependent manner, effects that are associated with decreased severity of diet-induced obesity (Chang et al., 2012; Qiu et al., 2014; Ricardo-Gonzalez et al., 2010). Together, these data suggest that IL-4 in WAT is critical for AAMac-mediated beiging and regulation of metabolic rate through UCP1-dependent thermogenesis.
Sources of IL-4 in WAT: eosinophils and invariant natural killer T (iNKT) cells
The appreciation of AAMacs as critical regulators of metabolic homeostasis sparked interest in determining the cellular sources of IL-4 in WAT. Using the 4get mouse, a reporter strain that identifies IL-4-competent cells, eosinophils were identified as an abundant immune cell population in WAT and an important source IL-4 in this compartment at steady state (Wu et al., 2011). Eosinophils are granulocytes that are developmentally dependent on the cytokine IL-5 and the transcription factor GATA-binding protein 1 (GATA-1), and these cells contribute to type 2 immune responses by producing a variety of effector molecules including IL-4 (Rosenberg et al., 2013; Rothenberg and Hogan, 2006). Eosinophils are decreased in WAT of HFD-fed and genetically obese ob/ob mice (Wu et al., 2011). ΔDblGata1 mutants or IL-5-deficient mice, both of which lack eosinophils (Rosenberg et al., 2013; Rothenberg and Hogan, 2006), develop more severe obesity and insulin resistance than wildtype controls in association with dysregulated AAMac responses (Wu et al., 2011). Further, mice with elevated numbers of eosinophils in WAT due to constitutive overexpression of IL-5 or infection with helminth pathogens were protected from diet-induced obesity and insulin resistance (Wu et al., 2011; Yang et al., 2013). Eosinophils are also recruited to WAT in the setting of chronic exposure to cold environmental temperatures, and eosinophil-deficient ΔDblGata1 mice exhibit defective beiging of WAT (Qiu et al., 2014; Rao et al., 2014). These beneficial metabolic effects of eosinophils appear to be mediated by IL-4-dependent AAMac responses in WAT (Wu et al., 2011; Qiu et al., 2014), however whether eosinophils have other functions in WAT remains unknown.
In addition to eosinophils, invariant natural killer T (iNKT) cells that express lipid antigen-specific T cell receptor variants appear to be an important source of IL-4 in WAT (Hams et al., 2013; Lynch et al., 2012). iNKT cells in WAT produce more IL-4 and IL-10 and less IFN-γ than do iNKT cells in the liver and spleen (Lynch et al., 2012), suggesting that iNKT cells in WAT are in an “alternative activation” state. Consistent with this, recent work has shown that mammals produce endogenous lipid antigens such as α-galactosylceramides (αGalCer) that promote production of type 2 cytokines by iNKT cells (Kain et al., 2014), and that adipocytes can present lipid antigens to iNKT cells via CD1d (Huh et al., 2013; Rakhshandehroo et al., 2014). iNKT cells are decreased in murine and human obesity, and surgical or dietary treatment of obesity restores iNKT cells in WAT (Lynch et al., 2012). Genetic studies in mice provide further support for the hypothesis that iNKT cells play a critical role in maintenance of body weight and metabolism. Jα18-deficient mice that lack iNKT cells exhibit increased body weight and adiposity when fed a HFD compared to wildtype controls, and CD1d-deficient mice that cannot present lipid antigen to iNKT cells develop spontaneous obesity on a low fat diet (Lynch et al., 2012). In addition, mice lacking iNKT cells had more severe glucose intolerance and increased pro-inflammatory macrophages in WAT (Lynch et al., 2012), suggesting that iNKT cells are essential for limiting inflammatory responses in the setting of obesity. Adoptive transfer of iNKT cells induces weight loss and decreases adipocyte size while improving insulin resistance in HFD-fed mice (Hams et al., 2013; Lynch et al., 2012), and treatment of HFD mice with αGalCer recapitulates these effects in an iNKT-dependent manner by increasing IL-4 and IL-10 production by iNKT cells (Lynch et al., 2012). Therefore, eosinophils and iNKT cells may be important sources of IL-4 or other factors that support AAMac function or that directly regulate metabolic homeostasis to limit obesity and insulin resistance.
The IL-33/Group 2 innate lymphoid cell (ILC2) pathway
ILC2s are recently described immune cells (Moro et al., 2009; Price et al., 2010; Neill et al. 2010) that control eosinophil and AAMac responses (Molofsky et al., 2013; Nussbaum et al., 2013). ILC2s are members of a broader family of ILCs that comprise T-bet-dependent Group 1 ILCs that produce IFN-γ; GATA-3-dependent ILC2s that produce IL4, IL-5, IL-9, IL-13 and amphiregulin; and RORγt-dependent ILC3s that produce IL-17A and IL-22 (Kim, 2014; Monticelli et al., 2012; Sonnenberg et al., 2013; Spits et al., 2013; Spits and Cupedo, 2012). At barrier surfaces such as the gut, lung and skin, ILC2s respond to epithelial cell-derived cytokines IL-33, IL-25 and thymic stromal lymphopoietin (TSLP) to initiate type 2 immune responses that protect against helminth infection or that promote pathologic allergic inflammation (Kim, 2014; Monticelli et al., 2012; Sonnenberg et al., 2013; Spits et al., 2013; Spits and Cupedo, 2012). ILC2s were recently shown to be present in murine WAT, where these cells constitutively produce the effector cytokines IL-5 and IL-13 to maintain eosinophil and AAMac responses, respectively, in WAT (Molofsky et al., 2013). Strikingly, IL-5-deficient mice exhibit decreased energy expenditure and increased obesity compared to IL-5-sufficient controls (Molofksy et al., 2013; Wu et al., 2011). Recent studies showed that antibody-mediated depletion of ILC2s is associated with increased weight gain and more severe insulin resistance in mice fed a HFD (Hams et al., 2013), and transferring IL-25-elicited ILC2s was sufficient to promote weight loss in diet-induced obese mice (Hams et al., 2013). These data suggest that ILC2s negatively regulate the development of obesity.
Although ILC2s can regulate metabolic homeostasis through their effects on eosinophils and AAMacs, they also have direct effects on metabolism through production of IL-13 and enkephalin peptides (Brestoff et al., 2014; Lee et al., 2014a). In the context of exposure to cold environmental temperatures, ILC2-derived IL-13 promotes pre-adipocyte proliferation and differentiation into beige adipocytes in an IL-4Rα-dependent manner (Lee et al., 2014a). The ILC2/IL-13/beiging pathway may also be related to young age-associated increases in beiging that may be critical for maintaining core body temperature during mammalian development (Lee et al., 2014a). However, whether ILC2-derived IL-13 regulates obesity remains unknown. In this disease context a distinct ILC2-dependent pathway can promote beiging independently of eosinophils or IL-4Rα (Brestoff et al., 2014). Specifically, ILC2s produce methionine-enkephalin (MetEnk) peptides in response to IL-33 stimulation, and MetEnk can act directly on adipocytes from WAT to upregulate Ucp1 expression levels in vitro and drive the formation of functional beige adipose tissue in vivo (Brestoff et al., 2014). MetEnk was previously shown to stimulate lipolysis in adipocytes (Nencini and Paroli, 1981), a process that is critical for beige adipocyte responses and UCP1 function (Fedorenko et al., 2012; Harms and Seale, 2013; Rosen and Spiegelman, 2014; Wu et al., 2013). Therefore, ILC2s directly contribute to beiging under various physiologic settings by producing IL-13 and MetEnk.
It is possible that ILC2-derived IL-13 and MetEnk, coupled with AAMac-derived NE, cooperatively support optimal beiging to regulate metabolic homeostasis. Supporting this hypothesis, previous studies have suggested that NE and MetEnk signaling pathways might interact to cooperatively stimulate lipolysis in adipocytes (Nencini and Paroli, 1981). Another possibility is that IL-13, MetEnk and NE elicit distinct populations of beige adipocytes involved in the regulation of metabolic homeostasis in different physiologic contexts or following different environmental stressors. Future studies focused on IL-13, MetEnk and NE interactions in adipocytes are warranted to better understand the mechanisms by which ILC2s, eosinophils and AAMacs contribute to the regulation of beiging and metabolic rate.
ILC2-mediated regulation of metabolic processes may be dysregulated in the context of obesity. The cytokine IL-33 is critical for stimulating proliferation and activation of ILC2s (Kim et al., 2014; Molofsky et al., 2013; Monticelli et al., 2011; Moro et al., 2010; Neill et al., 2010; Price et al., 2010) and is expressed at higher levels in WAT of obese mice and humans compared to non-obese controls (Zeyda et al., 2013). However, ILC2s are decreased in murine and human obesity (Brestoff et al., 2014; Molofsky et al., 2013), suggesting that in the obese state ILC2s may be hypo-responsive to IL-33 or have altered cell death, proliferation or migration. IL-33 was recently shown to be critical for protecting mice from obesity (Brestoff et al., 2014; Miller et al., 2010). Mice lacking IL-33 or the IL-33R exhibit increased obesity and exacerbated impairments in glucose homeostasis, and treatment of obese mice with recombinant IL-33 limited adiposity and improved glucose metabolism in association with enhanced ILC2 and AAMac responses in WAT (Brestoff et al., 2014; Miller et al., 2010). This suggests that targeting the IL-33/ILC2 axis therapeutically could have beneficial metabolic effects that limit obesity and/or associated diseases such as type 2 diabetes.
CD4+ T helper cells and regulatory T cells
In addition to regulating the ILC2/eosinophil/AAMac pathway, IL-33 also acts on adaptive immune cells such as Th2 cells (Molofsky et al., 2013) and regulatory T cells (Tregs) (Schiering et al., 2014). Although Th2 cells are relatively rare in WAT (Molofsky et al 2013) and remain poorly understood in the context of obesity, Tregs in WAT have been shown to be unique members of the Treg pool exhibiting uniquely elevated expression of PPAR-γ, Foxp3 and IL-10 (Cipolletta et al., 2012; Deiuliis et al., 2011; Feuerer et al., 2009). Tregs in WAT contribute to the maintenance of insulin sensitivity in WAT by limiting inflammation and producing insulin-sensitizing factors such as IL-10 (Cipolletta et al., 2012; Feuerer et al., 2009; Ilan et al., 2010). In obesity, Tregs are decreased in both mice and humans (Cipolletta et al., 2012; Feuerer et al., 2009; Wagner et al., 2013), which may be due to aberrant iNKT cell responses in obese WAT (Lynch et al., 2014). iNKT cells in WAT produce IL-2 to sustain Tregs and are an additional source of IL-10 (Lynch et al., 2014; Lynch et al., 2012). IL-10 suppresses Monocyte chemotactic protein-1 (MCP-1) expression by adipocytes to limit inflammatory macrophage infiltration of WAT and inhibits the ability of TNF-α to down-regulate Glucose transporter 4 (GLUT-4) expression and impair insulin action in adipocytes (Lumeng et al., 2007). Interestingly, recent studies indicate that the anti-diabetic class of drugs known as thiazoladinediones (TZD), which are PPAR-γ agonists, ameliorate insulin resistance in obese mice in part via their effects on Tregs (Cipolletta et al., 2012). Therefore promoting Treg responses may be a useful strategy to treat or prevent type 2 diabetes.
Immune cell responses in white adipose tissue in obesity
In obesity, WAT undergoes metabolic and inflammatory changes. As white adipocytes accumulate triglycerides and become hypertrophic, the vasculature in WAT becomes rarified leading to hypoxia and oxidative stress (Curtis et al., 2010; Pasarica et al., 2009). In addition, increased oxygen consumption by adipocytes undergoing hypertrophy results in relative local hypoxia that triggers hypoxia-inducible factor 1 α (HIF-1α) activation (Lee et al., 2014c). These changes are associated with increased adipocyte cell death and elevated production of adipocyte-derived inflammatory mediators, including the adipokines leptin, resistin and RBP4 (Attie and Scherer, 2009; Greenberg and Obin, 2006; Lazar, 2007; McNelis and Olefsky, 2014; Osborn and Olefsky, 2012; Ouchi et al., 2011). As discussed below, these and other proinflammatory factors initiate a type 1 immune response in obese WAT that is characterized by increased accumulation of CD4+ T helper type 1 (Th1) cells, cytotoxic CD8+ T cells and pro-inflammatory classically activated macrophages (Fig. 4). These changes are discussed in this section.
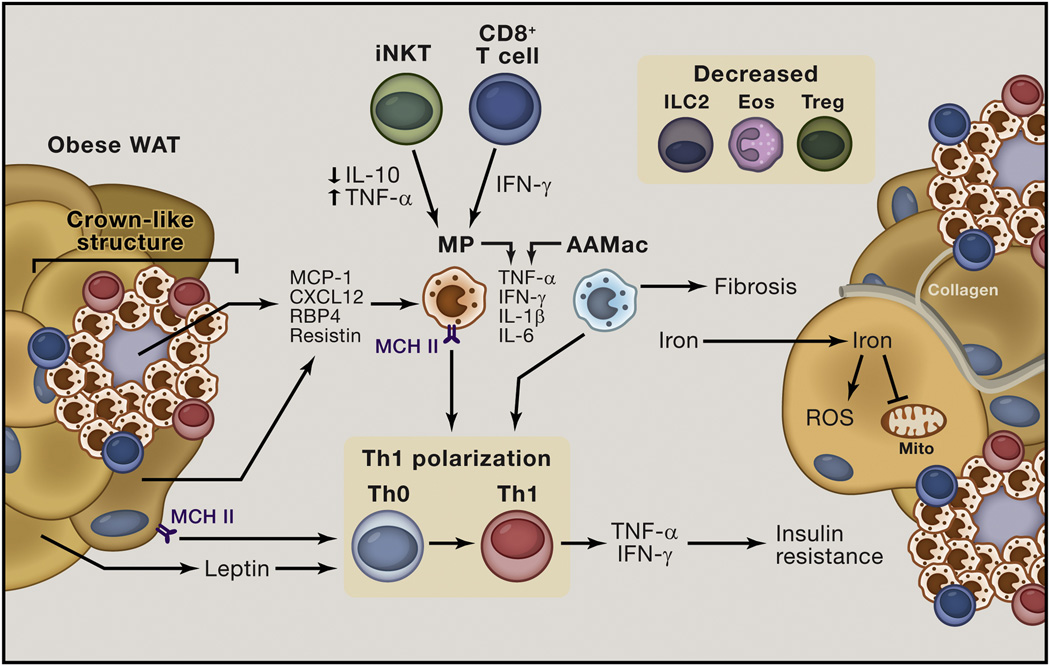
Figure 4. Obese white adipose tissue (WAT) is characterized by type 1 cytokine-associated immune responses.
In obese WAT, adipocyte hypertrophy is associated with hypoxia and adipocyte cell death. Dead and neighboring adipocytes produce pro-inflammatory signals such as Monocyte chemotractant protein 1 (MCP1), C-X-C motif chemokine 12 (CXCL12), Retinol binding protein 4 (RBP4) and Resistin that are associated with the recruitment of classically activated macrophages (MP) into WAT and MP activation. MP accumulation in WAT is also mediated by pro-inflammatory invariant natural killer T (iNKT) cells that exhibit impaired production of interleukin (IL)-10 and upregulated production of Tumor necrosis factor-α (TNF-α), and by CD8+ cytotoxic T cells that produce Interferon (IFN)-γ. These factors also promote MP activation and upregulate Major histocompatibility complex class II (MHC II) on MP and adipocytes. MHC II-mediated antigen presentation by MP and adipocytes stimulates polarization of CD4+ T cells towards a T helper type 1 (Th1) phenotype. Supporting this process are TNF-α, IFN-γ, IL-1β and IL-6 produced by MP cells, dysregulated alternatively activated macrophages (AAMacs), iNKT cells and CD8+ T cells. In addition, leptin is upregulated in obese WAT, and this factor also promotes Th1 cell polarization. MP cells, CD8+ T cells and Th1 cells collectively interact to form crown-like structures (CLS) to facilitate phagocytosis of dead adipocytes. This process further promotes antigen presentation and type 1 immune responses, establishing a vicious cycle. Type 1 cytokines such as TNF-α and IFN-γ act directly on adipocytes to impair insulin action, leading to insulin resistance. Dysregulated AAMacs also produce type 1 cytokines in the setting of obesity to contribute to insulin resistance. AAMacs also lose their capacity to store iron, resulting in redistribution of iron to adipocytes. This results in iron-initiated lipid peroxidation that causes reactive oxygen species (ROS) production, insulin resistance and mitochondrial dysfunction. In addition, AAMacs in obese WAT promote collagen deposition in WAT and fibrosis, leading ultimately to exacerbated hypoxia and inflammation and potentiation of the type 1 immune response. These processes occur in the setting of decreased abundance of regulatory T cell (Treg), Group 2 innate lymphoid cell (ILC2) and eosinophils (Eos) that promote insulin sensitivity and metabolic homeostasis in WAT in the steady state.
Adipocytes, macrophages and type 1 cytokine-associated T cells participate in a proinflammatory positive-feedback loop in obesity
In obesity adipocyte-derived inflammatory mediators such as monocyte chemotactic protein (MCP-1), C-X-C motif chemokine 12 (CXCL12), prostaglandins, leukotrienes and other factors are increased and promote classically activated monocyte/macrophage activation, proliferation and infiltration of WAT (Amano et al., 2014; Nomiyama et al., 2007; Oh et al., 2012; Weisberg et al., 2003). These cells engulf dying or dead adipocytes, forming crown-like structures (CLS) that are characterized morphologically as a ring of macrophages and other immune cells surrounding an adipocyte (Murano et al., 2008; Ouchi et al., 2011). The formation of CLS may be an adaptive mechanism to scavenge cellular debris or to limit the release of toxic lipid species when adipocytes undergo cell death, via phagocytosis. However, in addition to their phagocytic roles in CLS, classically activated macrophages in WAT of obese mice produce IL-1β, TNF-α, IFN-γ and IL-6 among other factors that potentiate the type 1 inflammatory response (Lumeng et al., 2007; Nguyen et al., 2007; Zeyda et al., 2010). These cytokines act directly on adipocytes and other cell types in distant tissues such as skeletal muscle to inhibit insulin-dependent glucose uptake (Exley et al., 2014; Gregor and Hotamisligil, 2011; Jin et al., 2013; Osborn and Olefsky, 2012). This pro-inflammatory process is therefore associated with the development of insulin resistance by promoting chronic low-grade type 1 inflammation.
The accumulation of macrophages in obese WAT also appears to be regulated by CD8+ T cells (Rausch et al., 2008). These adaptive immune cells are recruited to WAT before infiltration by pro-inflammatory macrophages in the setting of HFD feeding (Nishimura et al., 2009). Deletion of CD8+ T cells decreases macrophage accumulation in WAT of obese mice and ameliorates obesity-associated insulin resistance, and adoptive transfer of CD8+ T cells to mice fed a HFD is sufficient to promote macrophage infiltration of WAT and exacerbate insulin resistance (Nishimura et al., 2009). In the setting of HFD feeding, CD8+ T cells produce IFN-γ that has multiple effects on immune cells in WAT (Revelo et al., 2014). IFN-γ acts on macrophages to upregulate expression of pro-inflammatory effector cytokines and to increase expression of Major histocompatibility complex class II (MHCII) (Schroder et al., 2004). This promotes macrophage antigen presentation to CD4+ T cells and induces Th1 cell polarization and proliferation (Cho et al., 2014; Morris et al., 2013). T-bet-dependent Th1 cells produce TNF-α and IFN-γ to further potentiate insulin resistance in WAT (Cho et al., 2014; Morris et al., 2013; Stolarczyk et al., 2013). Thus, the CD8+ T cell/classically activated macrophage pathway in WAT appears to be critical for establishing the pro-inflammatory environment that influences Th1 cell responses. However, the precise factors that spur this cytotoxic CD8+ T cell-dependent response remain to be determined.
Adipocytes also appear to directly contribute to Th1 cell responses in obesity. With increased triglyceride deposition, adipocytes upregulate their expression of leptin, a hormone that limits food intake, as a compensatory mechanism to guard against overly rapid weight gain (Allison and Myers, 2014). While this is a beneficial response to maintain energy homeostasis, in WAT leptin acts on CD4+ T cells to induce Th1 polarization and IFN-γ expression to drive a pro-inflammatory immune response that limits insulin sensitivity (Deng et al., 2013). In turn, IFN-γ upregulates CIITA and MHCII expression in adipocytes (Deng et al., 2013) and macrophages (Cho et al., 2014; Morris et al., 2013) to promote adipocyte-mediated antigen presentation to Th1 cells. These MHCII-mediated inflammatory changes in obesity are critical drivers of insulin resistance (Cho et al., 2014; Deng et al., 2013). Therefore it appears that adipocytes, macrophages, CD8+ T cells and CD4+ Th1 cells participate in a pro-inflammatory positive-feedback loop with deleterious consequences for WAT inflammation and glucose metabolism.
Alternatively-activated macrophages acquire a classical activate state in obesity and potentiate type 1 inflammation in WAT
As discussed above, AAMacs have critical roles for regulating metabolic homeostasis in WAT at steady state. In obesity, however, AAMacs appear to acquire a pro-inflammatory M1-like phenotype that contributes to the development of type 1 inflammatory responses in WAT. For example, in obesity, AAMacs decrease their expression of IL-10 and upregulate expression of TNF-α, IFN-γ, IL-6 and IL-1β (Han et al., 2013; Lumeng et al., 2007; Moraes-Vieira et al., 2014). This phenotypic switch in AAMacs appears to be driven, at least in part, by RBP4 that is upregulated in obesity and that impairs glucose homeostasis (Graham et al., 2006; Norseen et al., 2012). AAMacs from transgenic mice that overexpress RBP4 exhibit upregulated expression of antigen presentation machinery, TNF-α and IL-1β, and polarize CD4+ T cells in WAT towards a Th1 phenotype characterized by increased expression of T-bet and IFN-γ (Moraes-Vieira et al., 2014). Interestingly, this effect was not observed in the liver, suggesting a tissue-specific effect of RBP4 on antigen presenting cells and T cell activation (Moraes-Vieira et al., 2014). In addition, transfer of RBP4-activated bone marrow-derived DCs (BMDCs) to lean recipient mice was sufficient to promote Th1 cell polarization in WAT and insulin resistance compared to unactivated BMDCs (Moraes-Vieira et al., 2014). This suggests that AAMacs in WAT become dysregulated in the setting of obesity and acquire a pro-inflammatory classical activation state that supports Th1 cell polarization and the development of insulin resistance.
Perspectives and Conclusions
Adipose tissues are diverse in their structure and function and have multiple roles in the regulation of energy balance and weight gain (Fig. 1). WAT is essential for triglyceride storage and regulation of glucose homeostasis, and white adipocytes appear to link mammalian metabolic status to immune cell responses. In addition, WAT contains beige adipocytes that have been shown to be key regulators of energy expenditure and the development of obesity. In the lean state WAT is populated by type 2 cytokine-associated immune cells including AAMacs, eosinophils, ILC2s, Th2 and iNKT cells as well as anti-inflammatory cells such as Tregs. These immune cells participate in a complex dialog to maintain optimal immune and adipocyte function (Fig. 2). Although the precise mechanisms by which type 2 immune cells in WAT regulate each other, it appears that elicitation of these cell pathways is associated with increased insulin sensitivity, optimal adipocyte mitochondrial function and in some cases elicitation of beige adipocytes within WAT (Fig. 3). Conversely, disruption of these immunologic pathways results in impaired adipocyte function characterized by insulin resistance, oxidative stress, impaired respiratory capacity and triglyceride deposition resulting in adipocyte hypertrophy and weight gain. Therefore, type 2 immune pathways in WAT appear to have protective roles that support maintenance of metabolic homeostasis and limit the development of obesity. This implies that eliciting type 2 immune cell pathways may be a useful strategy to treat or prevent obesity.
However in the context of obesity, the immunologic milieu of WAT undergoes a dramatic shift from a type 2 to type 1 cytokine-associated inflammatory environment (Fig. 4). Type 2 immune cells are decreased or dysregulated (e.g., ILC2s and eosinophils), and in some cases acquire a pro-inflammatory phenotype (e.g., iNKT cells and AAMacs). As type 2 immune cells tend to be associated with protection against obesity, these alterations in type 2 cytokine-associated immunologic pathways may contribute to the development of obesity and subsequent type 1 inflammatory responses. In addition, in obesity there is recruitment of various granulocytes, monocytes and lymphocytes to WAT. These cell types produce cytokines such as TNF-α, IFN-γ and IL-1β among others that potentiate type 1 immune responses and enhance antigen presentation to CD4+ T cells, polarizing these cells towards a Th1 cell phenotype. In turn, Th1 cells produce additional TNF-α and IFN-γ, establishing a positive-feedback loop resulting in chronic low-grade type 1 inflammation and dysregulated glucose homeostasis. The precipitating factors that initiate type 1 immune responses in WAT are not well understood but may be related to adipocyte cell death (Spalding et al., 2008), hypoxia (Lee et al., 2014c; Sun et al., 2011), the generation of toxic lipid species (Muoio and Newgard, 2006), direct effects of dietary lipids or carbohydrates (Calder, 2002), and translocation of commensal bacteria to WAT (Amar et al., 2011; Cani et al., 2007) among other factors. The apparent multifactorial nature of the type 1 immune response in WAT suggests that targeting downstream inflammatory mediators such as IFN-γ, TNF-α or IL-1β might have beneficial therapeutic effects in obesity-associated insulin resistance.
Finally, emerging studies have revealed complex immunomodulatory cross-talk between the type 1 and type 2 immune systems, where type 2 inflammation impairs type 1 responses and vice versa (Osborne et al., 2014; Reese et al., 2014; Stelekati and Wherry, 2012). Given the complex, dramatic shift in the immunologic landscape within obese WAT from a type 2 to type 1 cytokine-associated response, targeting a single immunologic factor may not be sufficient to treat obesity and restore a normal immunologic profile in WAT. Clinical trials employing biologic agents that inhibit IL-1 (anakinra, canakinumab) or TNF-α (infliximab) in patients with type 2 diabetes or metabolic syndrome have been shown to produce moderate or no improvements in glucose metabolism, as assessed by glycated hemoglobin levels or indices of insulin resistance (Larsen et al., 2007; Ridker et al., 2012; Wascher et al., 2011). However the effects of these therapies on body weight or adiposity is not clear. Further research on immunomodulatory biologic therapy to treat obesity should be considered. In addition, the effectiveness of “two-factor” immunomodulatory therapies (e.g., neutralizing TNF-α antibody plus recombinant IL-33) should be explored as potential anti-obesity regimens. Targeting the immune system with biologics could represent a new strategy to limit food intake and/or increase energy expenditure to treat or prevent obesity and related metabolic diseases such as type 2 diabetes. In addition, understanding how current and future treatments for obesity (e.g. diet, exercise, drugs and surgery) influence the immune system will be important for understanding their mechanisms of action and the potential side effects of treatment. Therefore, a deeper understanding of how the immune and metabolic systems interact to support metabolic homeostasis will be critical for understanding the biology of obesity and for the development of novel treatment and prevention strategies against this disease.
ACKNOWLEDGEMENTS
The authors wish to thank members of the Artis laboratory for the critical reading of this manuscript. Research in the Artis lab is supported by the National Institutes of Health (AI061570, AI074878, AI095466, AI095608, AI102942, and AI097333 to D.A.), the Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease Award (D.A.) and Crohn’s & Colitis Foundation of America (D.A.). Additional funding was provided by NIH F30-AI112023 and T32-AI060516 to J.R.B.
Footnotes
AUTHOR INFORMATION
The authors declare no competing financial interests.
BIBLIOGRAPHY
- 1.Allison MB, Myers MG., Jr 20 years of leptin: connecting leptin signaling to biological function. The Journal of endocrinology. 2014;223:T25–T35. doi: 10.1530/JOE-14-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell metabolism. 2014;19:162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, Smirnova N, Berge M, Sulpice T, Lahtinen S, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO molecular medicine. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, Buchholz BA, Eriksson M, Arner E, Hauner H, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 2011;478:110–113. doi: 10.1038/nature10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attie AD, Scherer PE. Adipocyte metabolism and obesity. Journal of lipid research. 2009;(50 Suppl):S395–S399. doi: 10.1194/jlr.R800057-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. American journal of physiology Endocrinology and metabolism. 2010;298:E1244–E1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 7.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nature reviews Endocrinology. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 8.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Molecular and cellular endocrinology. 2010;318:34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchard C. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obesity. 2008;(16 Suppl 3):S5–S10. doi: 10.1038/oby.2008.528. [DOI] [PubMed] [Google Scholar]
- 10.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nature immunology. 2013;14:676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2014 doi: 10.1038/nature14115. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calder PC. Dietary modification of inflammation with lipids. The Proceedings of the Nutrition Society. 2002;61:345–358. doi: 10.1079/pns2002166. [DOI] [PubMed] [Google Scholar]
- 13.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 14.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 15.Cao H, Sekiya M, Ertunc ME, Burak MF, Mayers JR, White A, Inouye K, Rickey LM, Ercal BC, Furuhashi M, et al. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell metabolism. 2013;17:768–778. doi: 10.1016/j.cmet.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey AL, Formosa MF, Van Every B, Bertovic D, Eikelis N, Lambert GW, Kalff V, Duffy SJ, Cherk MH, Kingwell BA. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia. 2013;56:147–155. doi: 10.1007/s00125-012-2748-1. [DOI] [PubMed] [Google Scholar]
- 17.Carey AL, Vorlander C, Reddy-Luthmoodoo M, Natoli AK, Formosa MF, Bertovic DA, Anderson MJ, Duffy SJ, Kingwell BA. Reduced UCP-1 content in in vitro differentiated beige/brite adipocytes derived from preadipocytes of human subcutaneous white adipose tissues in obesity. PloS one. 2014;9:e91997. doi: 10.1371/journal.pone.0091997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carriere A, Jeanson Y, Berger-Muller S, Andre M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014;63:3253–3265. doi: 10.2337/db13-1885. [DOI] [PubMed] [Google Scholar]
- 19.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. Journal of health economics. 2012;31:219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Chang YH, Ho KT, Lu SH, Huang CN, Shiau MY. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. International journal of obesity. 2012;36:993–998. doi: 10.1038/ijo.2011.168. [DOI] [PubMed] [Google Scholar]
- 21.Cho KW, Morris DL, DelProposto JL, Geletka L, Zamarron B, Martinez-Santibanez G, Meyer KA, Singer K, O'Rourke RW, Lumeng CN. An MHC II-Dependent Activation Loop between Adipose Tissue Macrophages and CD4(+) T Cells Controls Obesity-Induced Inflammation. Cell reports. 2014;9:605–617. doi: 10.1016/j.celrep.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cildir G, Akincilar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends in molecular medicine. 2013;19:487–500. doi: 10.1016/j.molmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collaboration PS. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtis JM, Grimsrud PA, Wright WS, Xu X, Foncea RE, Graham DW, Brestoff JR, Wiczer BM, Ilkayeva O, Cianflone K, et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59:1132–1142. doi: 10.2337/db09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtis JM, Hahn WS, Stone MD, Inda JJ, Droullard DJ, Kuzmicic JP, Donoghue MA, Long EK, Armien AG, Lavandero S, et al. Protein carbonylation and adipocyte mitochondrial function. The Journal of biological chemistry. 2012;287:32967–32980. doi: 10.1074/jbc.M112.400663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deiuliis J, Shah Z, Shah N, Needleman B, Mikami D, Narula V, Perry K, Hazey J, Kampfrath T, Kollengode M, et al. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PloS one. 2011;6:e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, Ren Y, Yin Z, Hamilton DJ, Reardon PR, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell metabolism. 2013;17:411–422. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Exley MA, Hand L, O'Shea D, Lynch L. Interplay between the immune system and adipose tissue in obesity. The Journal of endocrinology. 2014;223:R41–R48. doi: 10.1530/JOE-13-0516. [DOI] [PubMed] [Google Scholar]
- 31.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell metabolism. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature medicine. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health affairs. 2009;28:w822–W831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 35.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 37.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. Jama. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster MT, Bartness TJ. Sympathetic but not sensory denervation stimulates white adipocyte proliferation. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291:R1630–R1637. doi: 10.1152/ajpregu.00197.2006. [DOI] [PubMed] [Google Scholar]
- 39.Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nature reviews Immunology. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 40.Gnad T, Scheibler S, von Kugelgen I, Scheele C, Kilic A, Glode A, Hoffmann LS, Reverte-Salisa L, Horn P, Mutlu S, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A receptors. Nature. 2014;516:395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- 41.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. The New England journal of medicine. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 43.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. The American journal of clinical nutrition. 2006;83:461S–S465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 44.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 45.Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. Journal of immunology. 2013;191:5349–5353. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nature medicine. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 48.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 49.Hill AA, Reid Bolus W, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunological reviews. 2014;262:134–152. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hondares E, Gallego-Escuredo JM, Flachs P, Frontini A, Cereijo R, Goday A, Perugini J, Kopecky P, Giralt M, Cinti S, et al. Fibroblast growth factor-21 is expressed in neonatal and pheochromocytoma-induced adult human brown adipose tissue. Metabolism: clinical and experimental. 2014;63:312–317. doi: 10.1016/j.metabol.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 51.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of clinical investigation. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nature reviews Immunology. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 54.Huh JY, Kim JI, Park YJ, Hwang IJ, Lee YS, Sohn JH, Lee SK, Alfadda AA, Kim SS, Choi SH, et al. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Molecular and cellular biology. 2013;33:328–339. doi: 10.1128/MCB.00552-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 56.Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itoh N. FGF21 as a Hepatokine, Adipokine, and Myokine in Metabolism and Diseases. Frontiers in endocrinology. 2014;5:107. doi: 10.3389/fendo.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin C, Henao-Mejia J, Flavell RA. Innate immune receptors: key regulators of metabolic disease progression. Cell metabolism. 2013;17:873–882. doi: 10.1016/j.cmet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 59.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. International journal of molecular sciences. 2014;15:6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. The Journal of clinical investigation. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kain L, Webb B, Anderson BL, Deng S, Holt M, Constanzo A, Zhao M, Self K, Teyton A, Everett C, et al. The Identification of the Endogenous Ligands of Natural Killer T Cells Reveals the Presence of Mammalian alpha-Linked Glycosylceramides. Immunity. 2014;41:543–554. doi: 10.1016/j.immuni.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. International journal of obesity. 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 64.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. The Journal of clinical investigation. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. The Journal of clinical endocrinology and metabolism. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 66.Kim BS. Innate Lymphoid Cells in the Skin. The Journal of investigative dermatology. 2014 doi: 10.1038/jid.2014.401. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, Noti M, Tait Wojno ED, Fung TC, Kubo M, et al. Basophils promote innate lymphoid cell responses in inflamed skin. Journal of immunology. 2014;193:3717–3725. doi: 10.4049/jimmunol.1401307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kotnik P, Fischer-Posovszky P, Wabitsch M. RBP4: a controversial adipokine. European journal of endocrinology / European Federation of Endocrine Societies. 2011;165:703–711. doi: 10.1530/EJE-11-0431. [DOI] [PubMed] [Google Scholar]
- 69.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Molecular cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 70.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. The New England journal of medicine. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 71.Lazar MA. Resistin- and Obesity-associated metabolic diseases. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2007;39:710–716. doi: 10.1055/s-2007-985897. [DOI] [PubMed] [Google Scholar]
- 72.Lee M, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated Type 2 Innate Lymphoid Cells Regulate Beige Fat Biogenesis. Cell. 2014a doi: 10.1016/j.cell.2014.12.011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee P, Werner CD, Kebebew E, Celi FS. Functional thermogenic beige adipogenesis is inducible in human neck fat. International journal of obesity. 2014b;38:170–176. doi: 10.1038/ijo.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee YS, Kim JW, Osborne O, Oh da Y, Sasik R, Schenk S, Chen A, Chung H, Murphy A, Watkins SM, et al. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell. 2014c;157:1339–1352. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu PS, Lin YW, Lee B, McCrady-Spitzer SK, Levine JA, Wei LN. Reducing RIP140 Expression in Macrophage Alters ATM Infiltration, Facilitates White Adipose Tissue Browning, and Prevents High-Fat Diet-Induced Insulin Resistance. Diabetes. 2014;63:4021–4031. doi: 10.2337/db14-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, et al. A smooth muscle-like origin for beige adipocytes. Cell metabolism. 2014;19:810–820. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. The Journal of clinical investigation. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, Besra G, Vomhof-Dekrey EE, Tighe M, Koay HF, et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T cells and macrophages in adipose tissue. Nature immunology. 2014 doi: 10.1038/ni.3047. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O'Shea D, O'Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Madsen L, Pedersen LM, Lillefosse HH, Fjaere E, Bronstad I, Hao Q, Petersen RK, Hallenborg P, Ma T, De Matteis R, et al. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PloS one. 2010;5:e11391. doi: 10.1371/journal.pone.0011391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annual review of immunology. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 83.Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. The Journal of biological chemistry. 2000;275:25073–25081. doi: 10.1074/jbc.M000547200. [DOI] [PubMed] [Google Scholar]
- 84.McCarthy MI. Genomics, type 2 diabetes, and obesity. The New England journal of medicine. 2010;363:2339–2350. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- 85.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Miller AM, Asquith DL, Hueber AJ, Anderson LA, Holmes WM, McKenzie AN, Xu D, Sattar N, McInnes IB, Liew FY. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circulation research. 2010;107:650–658. doi: 10.1161/CIRCRESAHA.110.218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moisan A, Lee YK, Zhang JD, Hudak CS, Meyer CA, Prummer M, Zoffmann S, Truong HH, Ebeling M, Kiialainen A, et al. White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nature cell biology. 2014 doi: 10.1038/ncb3075. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. The Journal of experimental medicine. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature immunology. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Monticelli LA, Sonnenberg GF, Artis D. Innate lymphoid cells: critical regulators of allergic inflammation and tissue repair in the lung. Current opinion in immunology. 2012;24:284–289. doi: 10.1016/j.coi.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moraes-Vieira PM, Yore MM, Dwyer PM, Syed I, Aryal P, Kahn BB. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell metabolism. 2014;19:512–526. doi: 10.1016/j.cmet.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 93.Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, Singer K, Lumeng CN. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. The Journal of endocrinology. 2014;222:R113–R127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 95.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annual review of biochemistry. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 96.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. Journal of lipid research. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 97.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nencini P, Paroli E. The lipolytic activity of met-enkephalin, leu-enkephalin, morphine, methadone and naloxone in human adipose tissue. Pharmacological research communications. 1981;13:535–540. doi: 10.1016/s0031-6989(81)80023-9. [DOI] [PubMed] [Google Scholar]
- 99.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. The Journal of biological chemistry. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 102.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature medicine. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 103.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschop MH, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. The Journal of clinical investigation. 2007;117:2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Norseen J, Hosooka T, Hammarstedt A, Yore MM, Kant S, Aryal P, Kiernan UA, Phillips DA, Maruyama H, Kraus BJ, et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Molecular and cellular biology. 2012;32:2010–2019. doi: 10.1128/MCB.06193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annual review of pathology. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Odegaard JI, Chawla A. The immune system as a sensor of the metabolic state. Immunity. 2013a;38:644–654. doi: 10.1016/j.immuni.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013b;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell metabolism. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. Jama. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61:346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual review of physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 115.Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta-analysis. Obesity. 2008;16:515–521. doi: 10.1038/oby.2007.102. [DOI] [PubMed] [Google Scholar]
- 116.Orr JS, Kennedy A, Anderson-Baucum EK, Webb CD, Fordahl SC, Erikson KM, Zhang Y, Etzerodt A, Moestrup SK, Hasty AH. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes. 2014;63:421–432. doi: 10.2337/db13-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nature medicine. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 118.Osborne LC, Monticelli LA, Nice TJ, Sutherland TE, Siracusa MC, Hepworth MR, Tomov VT, Kobuley D, Tran SV, Bittinger K, et al. Coinfection. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science. 2014;345:578–582. doi: 10.1126/science.1256942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature reviews Immunology. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism: clinical and experimental. 2015;64:24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature. 2014;510:76–83. doi: 10.1038/nature13477. [DOI] [PubMed] [Google Scholar]
- 123.Pfeifer A, Hoffmann LS. Brown, Beige, and White: The New Color Code of Fat and Its Pharmacological Implications. Annual review of pharmacology and toxicology. 2015;55:207–227. doi: 10.1146/annurev-pharmtox-010814-124346. [DOI] [PubMed] [Google Scholar]
- 124.Pi-Sunyer FX. Comorbidities of overweight and obesity: current evidence and research issues. Medicine and science in sports and exercise. 1999;31:S602–S608. doi: 10.1097/00005768-199911001-00019. [DOI] [PubMed] [Google Scholar]
- 125.Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. a systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Annals of surgery. 2011;253:484–487. doi: 10.1097/SLA.0b013e31820d98cb. [DOI] [PubMed] [Google Scholar]
- 126.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, Xue RD, Yu HY, Guo L, Gao HD, et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E798–E807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rakhshandehroo M, Gijzel SM, Siersbaek R, Broekema MF, de Haar C, Schipper HS, Boes M, Mandrup S, Kalkhoven E. CD1d–mediated presentation of endogenous lipid antigens by adipocytes requires microsomal triglyceride transfer protein. The Journal of biological chemistry. 2014;289:22128–22139. doi: 10.1074/jbc.M114.551242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. International journal of obesity. 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 132.Reese TA, Wakeman BS, Choi HS, Hufford MM, Huang SC, Zhang X, Buck MD, Jezewski A, Kambal A, Liu CY, et al. Coinfection. Helminth infection reactivates latent gamma-herpesvirus via cytokine competition at a viral promoter. Science. 2014;345:573–577. doi: 10.1126/science.1254517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. International journal of obesity. 2011;35:891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 134.Revelo XS, Tsai S, Lei H, Luck H, Ghazarian M, Tsui H, Shi SY, Schroer S, Luk C, Lin GH, et al. Perforin is a Novel Immune Regulator of Obesity Related Insulin Resistance. Diabetes. 2014 doi: 10.2337/db13-1524. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 135.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, Mukundan L, Wu D, Locksley RM, Chawla A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T, Group CPI. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 137.Roberts LD, Bostrom P, O'Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell metabolism. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:345–353. [PubMed] [Google Scholar]
- 139.Rosell M, Jones MC, Parker MG. Role of nuclear receptor corepressor RIP140 in metabolic syndrome. Biochimica et biophysica acta. 2011;1812:919–928. doi: 10.1016/j.bbadis.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nature reviews Immunology. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rothenberg ME, Hogan SP. The eosinophil. Annual review of immunology. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 143.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sanchez-Gurmaches J, Guertin DA. Adipocyte lineages: tracing back the origins of fat. Biochimica et biophysica acta. 2014;1842:340–351. doi: 10.1016/j.bbadis.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. Journal of leukocyte biology. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 147.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell reports. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 150.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PloS one. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 152.Song H, Shojima N, Sakoda H, Ogihara T, Fujishiro M, Katagiri H, Anai M, Onishi Y, Ono H, Inukai K, et al. Resistin is regulated by C/EBPs, PPARs, and signal-transducing molecules. Biochemical and biophysical research communications. 2002;299:291–298. doi: 10.1016/s0006-291x(02)02551-2. [DOI] [PubMed] [Google Scholar]
- 153.Sonnenberg GF, Mjosberg J, Spits H, Artis D. SnapShot: innate lymphoid cells. Immunity. 2013;39:622–622. doi: 10.1016/j.immuni.2013.08.021. e621. [DOI] [PubMed] [Google Scholar]
- 154.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 155.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nature reviews Immunology. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 156.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annual review of immunology. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 157.Stelekati E, Wherry EJ. Chronic bystander infections and immunity to unrelated antigens. Cell host & microbe. 2012;12:458–469. doi: 10.1016/j.chom.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 159.Stolarczyk E, Vong CT, Perucha E, Jackson I, Cawthorne MA, Wargent ET, Powell N, Canavan JB, Lord GM, Howard JK. Improved insulin sensitivity despite increased visceral adiposity in mice deficient for the immune cell transcription factor T-bet. Cell metabolism. 2013;17:520–533. doi: 10.1016/j.cmet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. The Journal of clinical investigation. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tajima S, Ikeda Y, Sawada K, Yamano N, Horinouchi Y, Kihira Y, Ishizawa K, Izawa-Ishizawa Y, Kawazoe K, Tomita S, et al. Iron reduction by deferoxamine leads to amelioration of adiposity via the regulation of oxidative stress and inflammation in obese and type 2 diabetes KKAy mice. American journal of physiology Endocrinology and metabolism. 2012;302:E77–E86. doi: 10.1152/ajpendo.00033.2011. [DOI] [PubMed] [Google Scholar]
- 162.Terra X, Quintero Y, Auguet T, Porras JA, Hernandez M, Sabench F, Aguilar C, Luna AM, Del Castillo D, Richart C. FABP 4 is associated with inflammatory markers and metabolic syndrome in morbidly obese women. European journal of endocrinology / European Federation of Endocrine Societies. 2011;164:539–547. doi: 10.1530/EJE-10-1195. [DOI] [PubMed] [Google Scholar]
- 163.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 164.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 165.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 166.Wagner NM, Brandhorst G, Czepluch F, Lankeit M, Eberle C, Herzberg S, Faustin V, Riggert J, Oellerich M, Hasenfuss G, et al. Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity. 2013;21:461–468. doi: 10.1002/oby.20087. [DOI] [PubMed] [Google Scholar]
- 167.Walley AJ, Asher JE, Froguel P. The genetic contribution to non-syndromic human obesity. Nature reviews Genetics. 2009;10:431–442. doi: 10.1038/nrg2594. [DOI] [PubMed] [Google Scholar]
- 168.Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nature medicine. 2014;20:1436–1443. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang J, Liu R, Wang F, Hong J, Li X, Chen M, Ke Y, Zhang X, Ma Q, Wang R, et al. Ablation of LGR4 promotes energy expenditure by driving white-to-brown fat switch. Nature cell biology. 2013a;15:1455–1463. doi: 10.1038/ncb2867. [DOI] [PubMed] [Google Scholar]
- 170.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature medicine. 2013b;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wascher TC, Lindeman JH, Sourij H, Kooistra T, Pacini G, Roden M. Chronic TNF-alpha neutralization does not improve insulin resistance or endothelial function in "healthy" men with metabolic syndrome. Molecular medicine. 2011;17:189–193. doi: 10.2119/molmed.2010.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12:131–141. doi: 10.1111/j.1467-789X.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 174.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes & development. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 179.Yang Z, Grinchuk V, Smith A, Qin B, Bohl JA, Sun R, Notari L, Zhang Z, Sesaki H, Urban JF, Jr, et al. Parasitic nematode-induced modulation of body weight and associated metabolic dysfunction in mouse models of obesity. Infection and immunity. 2013;81:1905–1914. doi: 10.1128/IAI.00053-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yu Y, Li S, Liu Y, Tian G, Yuan Q, Bai F, Wang W, Zhang Z, Ren G, Zhang Y, et al. Fibroblast growth factor 21 (FGF21) ameliorates collagen-induced arthritis through modulating oxidative stress and suppressing nuclear factor-kappa B pathway. International immunopharmacology. 2015 doi: 10.1016/j.intimp.2015.01.005. pii: S1567-5769(15)00012-0. [DOI] [PubMed] [Google Scholar]
- 181.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. The Journal of clinical investigation. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zeyda M, Gollinger K, Kriehuber E, Kiefer FW, Neuhofer A, Stulnig TM. Newly identified adipose tissue macrophage populations in obesity with distinct chemokine and chemokine receptor expression. International journal of obesity. 2010;34:1684–1694. doi: 10.1038/ijo.2010.103. [DOI] [PubMed] [Google Scholar]
- 183.Zeyda M, Wernly B, Demyanets S, Kaun C, Hammerle M, Hantusch B, Schranz M, Neuhofer A, Itariu BK, Keck M, et al. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. International journal of obesity. 2013;37:658–665. doi: 10.1038/ijo.2012.118. [DOI] [PubMed] [Google Scholar]